Open Access Article
Open Access ArticleAntimicrobial nanoparticles: current landscape and future challenges
Suresh K.
Mondal
a,
Sourav
Chakraborty
a,
Sounik
Manna
b and
Santi M.
Mandal
 *a
*a
aDepartment of Bioscience and Biotechnology, Indian Institute of Technology Kharagpur, Kharagpur 721302, WB, India. E-mail: mandalsm@gmail.com; Fax: +91-3222-255303; Tel: +91-3222-282217
bMidnapore College (Autonomus), Midnapore 721101, WB, India
First published on 12th March 2024
Abstract
Antimicrobial resistance poses a serious threat to global health, necessitating the exploration of innovative solutions. Antimicrobial nanoparticles have emerged as a promising avenue, exhibiting unique properties by producing superoxide ions and hydroxyl radicals that efficiently kill bacteria. This article takes an in-depth look at state-of-the-art antimicrobial nanoparticles, their types, and modes of action. Metallic, polymeric, lipid, and carbon-based nanoparticles mostly exhibit antimicrobial actions by disrupting membranes, inhibiting enzymes, and producing different types of reactive oxygen species. Despite their promising potential, challenges and concerns surrounding cytotoxicity, biocompatibility, and environmental impact due to the development of resistance demand meticulous consideration and critical evaluation. This raises an urgent need for continuous research efforts, focusing on standardized regulatory outlines and advancements in the tunable synthesis of nanoparticles with optimized balance, large surface area, hydrophobicity, and cationic nature to harness their full potential in controlling antibiotic-resistant bacterial infections and wound management.
Introduction
Nanoscale particles (NPs) are increasingly used to control bacterial infections as an alternative to antibiotics. The utilization of NPs is very common in antimicrobial coatings over implantable devices, medical materials to prevent infection and promote wound healing, detection of bacteria for diagnostic purposes, and antibiotic delivery systems. Antimicrobial mechanisms and their real-life toxicity are poorly understood, but the currently accepted mechanisms include oxidative stress induction, metal ion release, and non-oxidative mechanisms. Multiple simultaneous mechanisms of action against microbes would require multiple simultaneous gene mutations in the same bacterial cell1 for antimicrobial resistance to develop, and therefore, it is difficult for bacteria to easily become resistant to metal NPs.Nanoparticles are used as antimicrobial agents.2,3 The composition of antimicrobial nanoparticles varies in physical and chemical properties. Some employ organic-based liposomes and capsules filled with conventional antibiotics or novel RNAs, termed nano-carriers, while others exploit cation leaching from metal colloid surfaces as the main antimicrobial agent.4,5 Further, metal colloids can be engineered to include different chemical components (such as silver or copper) and surface functionalities (such as aqueous suspension stabilizing agents or surface charges), with a primary particle size of <15 nm in diameter to enable passive diffusion across the bacterial cell wall and other intracellular membranes or >50 nm to enable extended duration of cation leaching in either biological or environmental matrices. All these properties are compiled into a nanoparticle profile and if the antimicrobial activity of each property can be measured, then antimicrobial nanoparticles can be designed in a safe and effective manner.
The advent of nanotechnology, particularly nanoparticle engineering, together with the accumulation of knowledge on infectious diseases, has allowed for significant advancement in the field of antimicrobial drug delivery. Major efforts have been devoted to developing various nanoparticle-based delivery platforms, including liposomes, polymeric nanoparticles, dendrimers, and inorganic nanoparticles. These nanoparticle approaches have shown excellent outcomes in treating and detecting bacterial pathogens by enabling targeted, responsive, and combinatorial delivery of antibiotics, effective antimicrobial vaccination, and rapid detection of bacteria. It is expected that nanotechnology will continue bringing improvements to antimicrobial delivery systems for efficacious, patient-compliant, and cost-effective therapeutics as well as for the specific and sensitive detection of various infectious diseases.
Antibiotic-resistant bacterial infections arising from acquired resistance and/or through biofilm formation necessitate the development of innovative outside-of-the-box therapeutics. Nanomaterial-based therapies are promising tools to combat bacterial infections that are difficult to treat, featuring the capacity to evade existing mechanisms associated with acquired drug resistance. In addition, the unique size and physical properties of nanomaterials give them the capability to target biofilms, overcoming recalcitrant infections. Here, we highlight the general mechanisms by which nanomaterials target bacteria to control infections associated with acquired antibiotic resistance or biofilms. We also address the design of elements and how their antimicrobial mechanisms extend to enhance the potency.
Historical development
In the late 19th century, the discovery of microorganisms by Antonie van Leeuwenhoek, followed by the work of Louis Pasteur and Robert Koch, laid the foundation for microbiology. This was followed by the discovery of antibiotics, with penicillin by Alexander Fleming in 1928 being a landmark. In the mid-20th century, the mass production of antibiotics revolutionized medicine as well as eventually led to the problem of antibiotic resistance.6 This initiated the search for new antimicrobial agents. Richard Feynman hypothesized that there is plenty of room at the bottom that is often cited as the conceptual beginning of nanotechnology. In the 1980s–1990s, the development of techniques such as scanning tunnelling microscopy allowed scientists to manipulate individual atoms and molecules, paving the way for nanotechnology research. In the late 20th century, researchers started to explore the antimicrobial properties of various nanoparticles such as silver, gold, and zinc oxide. Initial studies often focused on silver nanoparticles due to their well-known antimicrobial properties. In the early 21st century,7 with the advancement in nanoparticle synthesis and characterization techniques, a variety of nanoparticles were engineered and tested for antimicrobial properties. Studies extended from in vitro to in vivo models, and initial commercial applications began to appear. In the 2010s, there was acceleration in the application of antimicrobial nanoparticles in medical devices, water treatment, and consumer goods. Regulatory bodies started to pay attention to the safety aspects of nanoparticles.Types of antimicrobial nanoscale particles (AMNP)
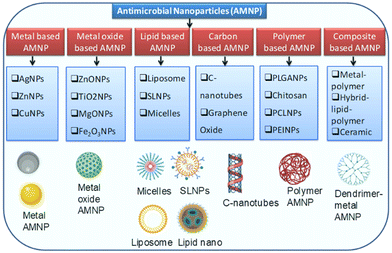
There are different forms of nanoparticles widely used in healthcare and medicine, particularly in antimicrobial chemotherapy. Metal-based nanoparticles have a wide range of applications from medicine and biotechnology to electronics and materials science. These nanoparticles often exhibit unique physical and chemical properties due to their small size and high surface area-to-volume ratio. Below are some details about metal-based nanoparticles, focusing on synthesis methods, applications, advantages, and challenges. Different types of antimicrobial nanoparticles are listed in Fig. 1, and they are summarized in detail below.Metal-based AMNPs
Metal-based nanoparticles are nanoscale particles composed primarily of metal atoms or metal compounds. These nanoparticles typically have dimensions in the range of 1 to 100 nanometres (nm), which is of the order of tens to hundreds of atoms across. Due to their small size and unique properties, metal-based nanoparticles exhibit a wide range of physical and chemical characteristics that distinguish them from their bulk counterparts. Antimicrobial nanoparticles come in various forms and are made from a range of materials, each with unique properties and mechanisms of action against microbial pathogens. Metal-based nanoparticles such as silver (Ag), zinc (Zn), and copper (Cu) are among the most studied and applied in the field of antimicrobial nanotechnology.Silver (Ag)-based AMNPs
Chemical reduction, physical vapour deposition, and biological methods are some of the common techniques used to prepare silver nanoparticles. The primary mode of action is through the release of silver ions that interfere with microbial cellular processes such as cell membrane disruption, enzyme deactivation, and inhibition of DNA/RNA synthesis.They are known for their remarkable properties such as high electrical and thermal conductivity, and strong antimicrobial activity. Their antimicrobial properties make them particularly useful in wound dressings, antimicrobial coatings, and water purification systems. Their size and shape can be controlled during synthesis to tailor their properties for specific applications such as broad-spectrum antimicrobial activities.8
Zinc (Zn)-based AMNPs
Sol–gel process, chemical precipitation, and green synthesis using plant extracts are some common methods to synthesize zinc nanoparticles. This primarily involves the generation of reactive oxygen species (ROS) that induce oxidative stress in microbial cells. They are used in protective coatings for corrosion resistance and antimicrobial properties. These particles are also used in sunscreens and cosmetics for their UV-blocking properties9 along with antimicrobial effects and also added to paint. They are used in pesticides and fertilizers to reduce microbial contamination. They have good antimicrobial and UV-blocking properties, and are safe for human skin.10Copper (Cu)-based AMNPs
Electrochemical methods and thermal decomposition are frequently used to synthesize Cu nanoparticles along with the common chemical reduction method. Like silver, copper nanoparticles release copper ions that disrupt microbial cell membranes and inhibit essential enzymes.11 They are generally used in food packaging industry and as surface coatings, which is applied to high-touch surfaces to reduce microbial contamination. Each of these metal-based nanoparticles offers a unique set of properties that make them suitable for specific applications. Their effectiveness can also vary depending on their size, shape, concentration, and the presence of stabilizing agents. As research progresses, these nanoparticles are continually optimized for greater efficacy, lower toxicity, and broader application. Copper nanoparticles have strong antimicrobial properties and are employed in coatings, textiles, and medical devices to prevent the growth of bacteria and other microorganisms.12Metal oxide AMNPs
Metal oxide nanoparticles are nanoscale particles composed of metal and oxygen atoms, forming metal–oxygen compounds. These nanoparticles have unique physical and chemical properties due to their small size and high surface area, setting them apart from their bulk metal oxide counterparts. Metal oxide nanoparticles are synthesized from various metal elements including transition metals such as iron, copper, and titanium, and non-metals such as silicon and aluminium.Zinc oxide nanoparticles
Zinc oxide nanoparticles (ZnO NPs) are composed of zinc and oxygen atoms, usually ranging from 1 to 100 nanometers in size. Zinc oxide itself is a compound with the combination of zinc cations (Zn2+) and oxide anions (O2−). At the nanoscale, the properties of materials can differ significantly from their bulk counterparts, and ZnO nanoparticles exhibit unique characteristics that make them valuable for various applications. Their activity is often attributed to the generation of reactive oxygen species (ROS) that cause oxidative stress in microbes. They are used in sunscreens and cosmetics for their UV-blocking properties along with antimicrobial effects. Zinc oxide nanoparticles are highly biocompatible and are extensively studied for use in medical and pharmaceutical applications including tissue engineering and drug delivery. Zinc oxide nanoparticles can act as photo catalysts when exposed to light, which makes them useful in water purification and air purification processes that rely on the degradation of organic pollutants.13Titanium dioxide nanoparticles
Titanium dioxide nanoparticles are often used in conjunction with UV light to enhance their antimicrobial activity. These nanoparticles also produce ROS that damage microbial cells. TiO2 nanoparticles exhibit photocatalytic activity when exposed to ultraviolet (UV) light.14 This property allows them to generate reactive oxygen species (ROS) such as superoxide ions and hydroxyl radicals, when irradiated with UV light. These ROS have strong oxidative properties that can damage the cell membranes, proteins, and DNA of microorganisms, leading to their inactivation and death. TiO2 nanoparticles have been found to have broad-spectrum antimicrobial activities against various microorganisms including bacteria, viruses, and fungi. This makes them valuable for disinfection and sterilization applications. Titanium dioxide nanoparticles are commonly used in sunscreens and other sun protection products as physical UV blockers. TiO2 nanoparticles serve as catalysts in chemical reactions due to their unique surface properties. They are employed in catalytic processes for organic synthesis and environmental remediation.15Magnesium oxide nanoparticles
Magnesium oxide nanoparticles exhibit unique physical and chemical properties due to their small size and high surface area, setting them apart from their bulk counterparts. These are less commonly used but have demonstrated some antimicrobial properties. MgO nanoparticles have shown the ability to inhibit the growth of a wide range of bacteria and fungi. They can disrupt the cell membranes and metabolic processes of microorganisms, leading to their inactivation.16Iron oxide nanoparticles
Iron oxide nanoparticles are nanoscale particles primarily composed of iron (Fe) and oxygen (O) atoms, forming various iron oxide compounds such as magnetite (Fe3O4), hematite (Fe2O3), and maghemite (γ-Fe2O3). These are mainly used for targeted drug delivery and they can also possess antimicrobial properties when coated or functionalized with antimicrobial molecules. Iron oxide nanoparticles, particularly magnetite and maghemite, are known for their magnetic properties. They are used in various magnetic applications including magnetic resonance imaging (MRI) contrast agents, magnetic drug delivery systems, and magnetic hyperthermia for cancer treatment.17 Iron oxide nanoparticles have demonstrated antimicrobial activity against a range of bacteria including both Gram-positive and Gram-negative bacteria, as well as various fungal species. This broad-spectrum activity is valuable for different applications. Iron oxide nanoparticles are extensively used in biomedical and healthcare applications. They can be coated with biocompatible materials and functionalized with ligands for targeted drug delivery, cell labelling, and imaging in the field of nanomedicine.18Polymer-based AMNPs
Polymer-based nanoparticles are nanoscale particles composed primarily of polymers, which are large molecules made up of repeating subunits called monomers. These nanoparticles are typically of the order of tens to hundreds of nanometres in size and are used in various fields due to their unique properties and versatility. Polymer-based nanoparticles are primarily made of synthetic or natural polymers. Synthetic polymers such as polyethylene, polyvinyl chloride (PVC), and polystyrene are commonly used. Natural polymers such as chitosan, alginate, and cellulose are also employed in nanoparticle formulations. Polymer-based nanoparticles have attracted significant attention in recent years for their potential in a wide array of applications including drug delivery, imaging, and as carriers for various bioactive agents.19 Below are some details about polymer-based nanoparticles, focusing on types, synthesis methods, applications, advantages, and challenges. Polymer nanoparticles are used in various biotechnological applications including DNA and RNA delivery, protein purification, and cell labelling.Common types of polymer-based nanoparticles
PEI nanoparticles are extensively used in gene delivery systems. They are capable of binding and condensing negatively charged nucleic acids (e.g., DNA or RNA) into nanoparticles, facilitating their entry into cells and allowing for gene transfection.32 PEI's cationic charge helps interact with the negatively charged cell membranes. PEI nanoparticles can be employed for drug delivery. They can encapsulate drugs, protect them from degradation, and promote controlled release. The cationic properties of PEI may also facilitate drug delivery to cells. PEI is a cationic polymer, which means it carries a positive charge. This charge allows it to interact with negatively charged molecules such as nucleic acids and cell membranes, facilitating gene delivery and cellular uptake.33
Carbon/graphene oxide-based nanoparticles
Carbon-based nanoparticles are nanoscale particles primarily composed of carbon atoms, and they come in various forms and structures. These nanoparticles have attracted considerable attention due to their unique properties and diverse applications across various fields.34 Graphene oxide (GO) nanomaterials show promising results as antimicrobial agents due to their ability to physically damage microbial cell membranes and serve as a carrier for other antimicrobial agents. GO possesses oxygen-containing functional groups on its surface, such as hydroxyl and carboxyl groups. These groups can interact with microbial cells, altering their membrane permeability and disrupting cellular processes.35Composite nanoparticles
Composite nanoparticles are composed of two or more different materials or components, typically combined at the nanoscale to achieve specific properties or functions that are not possible with individual materials alone.Metal–polymer composites
Metal and polymer composite nanomaterials have the advantage of enhancing antimicrobial efficacy and biocompatibility. Metal–polymer composites can slowly release metal ions over time. These metal ions are toxic to microorganisms and can disrupt their cellular processes, leading to cell death. Metal nanoparticles on the composite's surface can physically interact with microorganisms, disrupting cell membranes and interfering with microbial functions.36 Some metal–polymer composites exhibit broad-spectrum antimicrobial activities, and are effective against a wide range of bacteria, fungi, and other microorganisms.Metal–metal oxide composites
Combining different metals or metal oxides can sometimes offer synergistic effects that enhance antimicrobial activities. The combination of metal nanoparticles and metal oxide nanoparticles in these metal–metal oxide composites often results in synergistic antimicrobial effects. The metal component provides rapid antimicrobial action, while metal oxide nanoparticles can extend the antimicrobial activity over time and enhance the overall efficacy. Metal nanoparticles can release metal ions (e.g., Ag+ and Cu2+) over time. These ions are toxic to microorganisms and can disrupt microbial cell membranes, proteins, and DNA, leading to cell death.37 Metal oxide nanoparticles such as zinc oxide (ZnO) or titanium dioxide (TiO2) have photocatalytic properties. When exposed to light (UV or visible), they produce reactive oxygen species (ROS) that damage microbial cells.Hybrid lipid–polymer nanoparticles
These are designed to combine the benefits of both lipid- and polymer-based nanoparticles such as improved stability and controlled drug release. The combination of lipids and polymers can lead to synergistic antimicrobial effects. Lipids may enhance cellular uptake, while polymers can control the release kinetics and improve stability.38 Antimicrobial agents such as antibiotics, antimicrobial peptides, or other compounds with antimicrobial properties can be loaded into the lipid core of these nanoparticles. Hybrid nanoparticles offer controlled and sustained release of antimicrobial agents. The lipid core can protect the encapsulated agents from degradation and release them gradually, optimizing the therapeutic efficacy.Green synthesis of AMNPs
Green synthesis methods for the production of biologically active nanoparticles (NPs) have gained much interest due to their eco-friendly and sustainable nature. These approaches utilize natural resources and minimize environmental damage. These green synthesis approaches offer promising alternatives to traditional methods.39 The key principles and mechanisms of biogenic nanoparticle synthesis are reduction and stabilization, where biological agents such as plant extracts, biomolecules of fungi, algae, and bacteria act as reducing and capping agents that facilitate the reduction of metal ions to form nanoparticles. Further, nucleation occurs when metal ions aggregate, form small clusters and grow further into larger nanoparticles. The size and shape of the nanoparticles depend on the reaction conditions and the specific biomolecules involved.Role of nanoparticles in antimicrobial therapy
The use of nanoparticles in antimicrobial therapies opens a promising avenue for overcoming challenges associated with conventional antibiotic treatments including antibiotic resistance and limited drug penetration. Ongoing research aims to optimize nanoparticle design and delivery strategies for improved antimicrobial outcomes. Different advantages of nanoparticles to fight against bacterial pathogens are presented in Fig. 2, and the details are described below.Increased surface area
The increased surface area of nanoparticles is a key factor contributing to their enhanced antimicrobial activity. The increased surface area of nanoparticles provides more points of contact, higher drug loading capacity, and improved interactions with bacterial cells. These features contribute to the enhanced antimicrobial activity of nanoparticles, making them promising candidates for the development of more effective antimicrobial therapies.40 Nanoparticles have a much higher surface area than the larger particles of the same material. This increased surface area provides more opportunities for the nanoparticles to come into contact with bacterial cells.The larger surface area allows for more effective interactions between the nanoparticles and the bacterial cell membranes. This is particularly important for nanoparticles designed to deliver antimicrobial agents or disrupt the integrity of bacterial cells. Nanoparticles often have a high density of binding sites on their surfaces. These binding sites can interact with bacterial cell surfaces, facilitating the attachment of nanoparticles to the bacteria. The increased surface area also provides more space for loading antimicrobial agents.41 This means that a higher concentration of the therapeutic agents can be attached or encapsulated into the nanoparticles, leading to a more potent antimicrobial effect. When nanoparticles are used to deliver antimicrobial drugs, the increased surface area allows for a more efficient and targeted delivery of the drugs to the bacterial cells. This is particularly advantageous in achieving therapeutic concentrations at the site of infection. Increased surface area helps in quick and efficient penetration through the biofilm, which are communities of bacteria embedded in a self-produced matrix that often poses challenges to conventional antibiotics. This nanoparticle-aided penetration enables effective targeting of bacteria residing within the biofilm.42
Targeted antimicrobial delivery
The delivery of targeted antimicrobial agent using nanoparticles is a strategy designed to improve the precision and efficacy of antimicrobial therapies. By directing therapeutic agents specifically to the site of infection, targeted drug delivery minimizes damage to healthy tissues and enhances the antimicrobial activity in several ways as follows:Targeted nanoparticles can be engineered to recognize and bind specifically to bacterial cells or components, such as cell membranes or surface proteins. This specificity ensures that the therapeutic payload is delivered primarily to the site of infection, maximizing its impact on bacteria while minimizing exposure to healthy cells. By delivering drugs directly to the infection site, targeted drug delivery reduces the exposure of healthy tissues to the antimicrobial agents. This minimizes systemic toxicity and side effects associated with traditional antibiotic treatments.43
Targeted nanoparticles can enhance drug concentrations at the infection site. This is crucial for achieving effective antimicrobial activity, particularly in cases where the infection is located in hard-to-reach areas or deep-seated tissues. Bacterial biofilms, which are resistant to many conventional antibiotics, often form in chronic infections. Targeted nanoparticles can penetrate biofilms more effectively than free drug molecules, reaching bacteria embedded within the biofilm matrix and improving antimicrobial activity.44
Targeted drug delivery can help to reduce the emergence of drug resistance. By delivering drugs specifically to the bacterial cells, the risk of exposing bacteria to suboptimal drug concentrations is reduced, lowering the likelihood of resistance development. Controlled-release formulations of nanoparticles allow for a sustained and controlled delivery of antimicrobial agents over time. This ensures a continuous therapeutic effect at the infection site, reducing the chances of bacterial resurgence. Targeted drug delivery using nanoparticles can be adapted to various administration routes, including oral, intravenous, or local administration.45 This flexibility allows for personalized treatment approaches based on the nature and location of infection. Overall, targeted drug delivery with nanoparticles enhances the precision, efficiency, and safety of antimicrobial therapies by ensuring that therapeutic agents are delivered specifically to the infection site. This approach holds great promise for addressing challenges associated with bacterial infections, including antibiotic resistance and the limitations of conventional treatments.46
| COF-based NPs | Form to regulate COFs | Application |
|---|---|---|
| COF-909–Cu | Cu-Coordination | CDT and pyroptosis |
| COF–TiO2–HA | Hyaluronic acid | SDT |
| DPPN COF | DPP (aldehyde monomer) and TAPA (amino monomer) | PTT |
| RSL3@COF–Fe | FeCHO–RSL3 | CDT |
| CuS@COF–BDP | Cu coordination | PTT & PDT |
| Cu–DhaTph | Cu coordination | PTT/PDT |
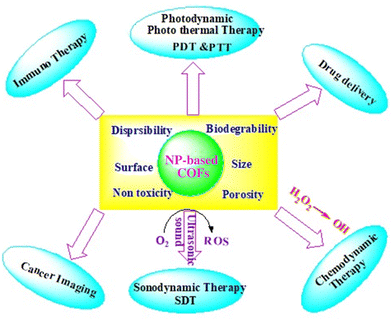
Therefore, advanced nanoparticles with high biocompatibility and enhanced therapeutic efficiency make them incredibly attractive for future nano medicines (Table 2). Different organic nanoparticles based on COFs are used in the medical field owing to their therapeutic efficiency (dendrimers, mesoporous polymers, liposomes, micelles, etc.), and several inorganic nanoparticles based COFs are also used in theranostic applications (i.e., drug delivery, cancer imaging, PDT, PTT, SDT, and immunotherapy) (Fig. 3).
| MOF-based NPs | Organic linker | Drug name |
|---|---|---|
| Fe–MOFs–MIL-89 (Fe) | Muconic acid | Ibuprofen, azidothymidine triphosphate |
| Zn–MOFs–ZnBDP_X | 1,4-Bis(1H-pyrazol-4-yl)-2-X-benzene, (H2BDP_X; X = H, NO2, NH2, OH) | Mitoxantrone |
| K–MOFs CD–MOF-1 | Cyclodextrins | Lansoprazole, azilsartan, valsartan |
| Cu–MOFs–HKUST-1 | 1,3,5-Benzenetricarboxylic acid | Ibuprofen |
| Zr–MOFs–UiO-66 | 1,4-Benzenedicarboxylic acid | Caffeine, dichloroacetate |
Activation of photothermal and photodynamic therapy
Nanoparticles can enhance antimicrobial activities through photothermal therapy (PTT) and photodynamic therapy (PDT), leveraging their unique properties in response to light. Both strategies involve the activation of nanoparticles by light to generate localized effects that can selectively destroy bacterial cells. Nanoparticles contribute to increased antimicrobial activity in photothermal and photodynamic therapies as follows:Nanoparticles, particularly those with plasmonic properties (e.g., gold or silver nanoparticles), can efficiently absorb light in the near-infrared (NIR) region. This absorption generates heat due to the plasmonic resonance of the nanoparticles. Upon exposure to light, nanoparticles convert light energy into heat, leading to localized hyperthermia in the vicinity of the nanoparticles. This elevated temperature is particularly effective at damaging bacterial cells.51 Elevated temperatures induced by PTT can lead to the disruption of bacterial cell membranes. This damage compromises the structural integrity of the bacteria, resulting in cell death. Nanoparticles can serve as carriers for photosensitizing agents. When exposed to specific wavelengths of light, these photosensitizers become activated and produce reactive oxygen species (ROS). ROS, such as singlet oxygen, generated during PDT can induce oxidative stress in bacterial cells. ROS cause damage to cellular components including proteins, lipids, and DNA, leading to bacterial cell death.52
Prevention of antibiotic resistance
Nanoparticles can contribute to the prevention of antibiotic resistance through various mechanisms, offering innovative solutions to address the challenges associated with the development of resistance in bacteria. Nanoparticles can help mitigate antibiotic resistance as follows:Nanoparticles can be designed to carry multiple antimicrobial agents or therapeutic payloads with different modes of action. This approach can address multiple targets in bacteria simultaneously, making it more challenging for bacteria to develop resistance against all mechanisms. Combining nanoparticles with conventional antibiotics can result in synergistic effects. The nanoparticles may enhance the efficacy of antibiotics, reducing the antibiotic concentrations, which can decrease the selective pressure for resistance development.
Bacteria often develop resistance by pumping out antibiotics through efflux pumps. Nanoparticles can be engineered to inhibit these efflux pumps,53 preventing the expulsion of antibiotics from bacterial cells and improving the effectiveness of antibiotics. Bacterial biofilms are resistant to antibiotics. Nanoparticles, due to their small size and unique properties, can penetrate through biofilms more effectively, reaching bacteria embedded within the biofilm matrix and overcoming one of the mechanisms contributing to antibiotic resistance.
Nanoparticles with distinct mechanisms of action may help prevent cross-resistance, where resistance to one antibiotic confers resistance to others with similar modes of action. This can be achieved by designing nanoparticles with diverse antimicrobial strategies. Targeted drug delivery using nanoparticles allows for the specific delivery of antimicrobial agents to the site of infection.54 This minimizes the exposure of bacteria in other parts of the body to antibiotics, reducing the overall selective pressure for resistance.
Nanoparticles can enhance the therapeutic efficacy of antibiotics, reducing antibiotic doses. Lower doses may decrease the likelihood of resistance development by reducing the intensity of selective pressure on bacteria. Nanoparticles can facilitate combination therapies where multiple antimicrobial agents including antibiotics and non-traditional antimicrobial agents are used simultaneously. This approach targets bacteria through different mechanisms, making it more challenging for them to develop resistance.55
Biofilm eradication by AMNPs
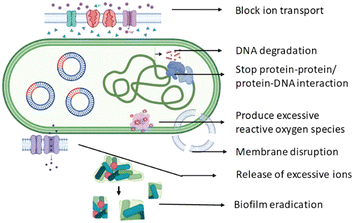
Biofilms are complex communities of microorganisms that adhere to surfaces and are embedded in a self-produced extracellular matrix. They pose a significant challenge in terms of microbial infections and biofouling, as biofilm formation enhances resistance to conventional antimicrobial agents. These biofilms can be found not only in infected zone but in various environments including medical devices, industrial pipelines, and natural aquatic systems. Biofilm formation in microbes involves a series of steps, from initial attachment to the development of the mature biofilm structure. Biofilm formation is a survival strategy for microbes, allowing them to thrive in diverse environments, resist antimicrobial agents, and interact with surfaces. Microbes initially attach to surfaces via weak physical forces such as van der Waals interactions and electrostatic forces. As the microbes continue to interact with the surface, irreversible attachment occurs through stronger forces including specific interactions between microbial adhesins and surface receptors.56 Then microbes start producing extracellular polymeric substances (EPSs), which play a crucial role in biofilm structure and stability. They form a matrix that encases microbial cells, providing mechanical strength and protection. Microbial cells within the biofilm multiply, leading to the formation of microcolonies. Chemical signalling molecules such as autoinducers are often involved in quorum sensing, a mechanism by which microbes communicate with each other to coordinate gene expression and regulate biofilm formation.57 The EPS matrix consolidates, creating a three-dimensional structure that encapsulates microbial cells. The EPS matrix acts as a physical barrier, limiting the penetration of antimicrobial agents, and also exhibits altered metabolic states, reducing susceptibility to antimicrobials.58 Understanding the interaction during biofilm formation is essential for developing strategies to prevent or disrupt biofilms in various settings. Researchers have developed different AMNPs and explore ways to interfere with the key steps in biofilm development, such as disrupting adhesion, targeting quorum sensing, and promoting biofilm dispersal, to combat antibiotic-resistant properties (Fig. 4).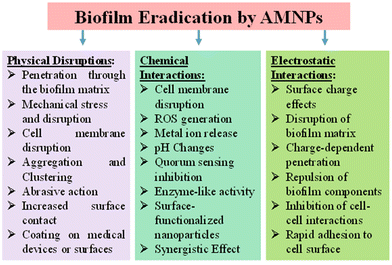 | ||
| Fig. 4 Schematic illustration of the detailed mechanism of AMNPs to disrupt and eradicate the microbial biofilm. | ||
Nanoparticles have a high surface area-to-volume ratio, which enhances their interaction with biofilm components. This increased surface area enables more effective contact with microbial cells. Nanoparticles can penetrate the biofilm matrix and disrupt microbial cells. Their small size facilitates penetration through the extracellular polymeric substance (EPS) that surrounds biofilm cells, allowing them to reach the microbial cells embedded within.59 AMNPs often exhibit multiple modes of action. They can disrupt cell membranes, interfere with cellular processes, generate reactive oxygen species (ROS), and interact with microbial DNA. The surface of nanoparticles can be modified to enhance their interaction with biofilms. Functionalization with molecules that have an affinity for biofilm components can improve the efficacy of the nanoparticles. The surface charge of nanoparticles can influence their interaction with microbial cells and the biofilm matrix. Positively charged nanoparticles may adhere to negatively charged microbial cell surfaces, disrupting the stability of the biofilm.60 Some nanoparticles can interfere with quorum sensing, a communication mechanism used by microbes in biofilms. By disrupting signalling molecules, nanoparticles can impede the coordination of biofilm formation.61
It is important to note that the efficacy of antimicrobial nanoparticles in biofilm disruption can depend on factors such as nanoparticle type, size, concentration, charge and the specific characteristics of the biofilm. Additionally, the potential toxicity of nanoparticles and their environmental impact should be carefully considered in practical applications. Ongoing research continues to explore and optimize the use of antimicrobial nanoparticles for biofilm control in various fields including healthcare, industry, and environmental management.
Removal of drug residue and drug toxicity
The overuse and improper disposal of antibiotics have led to the presence of antibiotic residues in the environment. These residues pose a serious threat due to the development of antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARGs). ARB and ARGs can compromise the effectiveness of antibiotic therapies and impact human health. In this context, antimicrobial nanoparticles can play a vital role by controlling the ARB- or ARG-associated pathogens. Moreover, bioremediation through biogenic nanoparticles may degrade antibiotics or toxic compounds to transform them into less toxic compounds.62Mechanism of ROS generation by AMNPs
ROS generated by CuNPs
CuNPs increase the rate of reduction: Cu2+ to Cu+.Cu+ increases the rate of formation of hydroxyl radicals (ROS).
ROS (1O2) generation by titanium-based nanomaterials
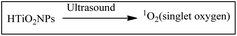
Titanium-based nanoparticles (TiNPs) are photosensitizers for PDT cancer treatment for their outstanding ROS-generating property in presence of light sources. (HTiO2 NP) that can be activated by ultrasound to generate reactive oxygen species (ROS). HTiO2 NPs use as a sensitizer for sonodynamic therapy in vivo.Generation of ROS iron oxide nanoparticles (IONPs)
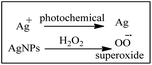
Superparamagnetic iron oxide nanoparticles have excellent ROS-generating abilities based on the Fenton and Haber–Weiss reactions. Through these reactions, iron ions lead to the production of highly reactive hydroxyl or hydroperoxy radicals. The catalytic activity of IONPs with H2O2 generates highly toxic hydroxyl radicals (˙OH), which can be used in cancer therapy.Generation of ROS by silver nanoparticles (AgNPs)
Silver nanoparticles (AgNPs) react with hydrogen peroxide to produce superoxide ions characterized by UV-visible spectroscopy under photochemical conditions. Silver nanoparticles (AgNPs) are one of the most commonly used nanomaterials because of their strong antimicrobial activities.
| O2˙− + O2˙− + 2H+ → H2O2 + O2 | (1) |
Formation of ROS by gold nanoparticles (AuNPs)
AuNPs generate reactive oxygen species by the formation of Au+ ions, which are very stable with unique optical properties. For this reason, AuNPs are widely used in ROS-based tumor therapeutics.Cerium oxide-based nanoparticles
Cerium oxide-based NPs are rare earth nanomaterials with high chemical activity. The catalytic activity of Ce3+ and Ce4+ is commonly used to generate reactive oxygen species and successfully used in the treatment of various cancers in vivo.Interaction of AMNPs with intracellular compounds and DNA
The interaction of AMNPs with intra/extracellular compounds and DNA is a complex process that depends on various factors including the physicochemical properties of nanoparticles, the cellular or extracellular environment, and the type of nanoparticles involved. When nanoparticles come into contact with biological fluids, they can form a protein corona, a layer of proteins adsorbed onto their surfaces. This protein corona can influence the nanoparticles’ interactions with cells and other biological components. Nanoparticles can be taken up by cells through various endocytic pathways such as phagocytosis, pinocytosis, or receptor-mediated endocytosis. The surface properties of nanoparticles including their size, charge, and surface chemistry play a role in their interaction with the cell membrane. Endosomal escape: once inside cells, nanoparticles may undergo endosomal trafficking.56 Some nanoparticles have mechanisms to escape from endosomes and release their cargo into the cytoplasm. Nanoparticles can interact with cellular organelles such as the mitochondria and endoplasmic reticulum influencing cellular functions. Nanoparticles can interact with DNA via surface binding. The surface charge and functional groups of nanoparticles influence this interaction. Nanoparticles can condense DNA into nanoscale complexes, protecting it from degradation and facilitating its delivery into cells. Certain nanoparticles may induce genotoxic effects by directly or indirectly causing DNA damage.63 This can occur via mechanisms such as the generation of reactive oxygen species (ROS) or interference with DNA repair processes.AMNPs regulate the expression of metabolic genes
Nanoparticles (NPs) can indeed influence the expression of metabolic genes, and the impact may vary depending on the type of nanoparticles, their size, surface properties, and the specific biological context. The interaction between nanoparticles and metabolic processes is an active area of research, and the findings are continually expanding our understanding. Some nanoparticles may induce oxidative stress within cells. This oxidative stress can activate signalling pathways that, in turn, influence the expression of genes involved in cellular metabolism. Inflammatory responses triggered by nanoparticles may also impact metabolic gene expression.64 Certain nanoparticles can affect mitochondrial functions influencing cellular energy metabolism. Mitochondrial dysfunction can lead to changes in the expression of genes involved in energy production and metabolism. AMNPs might induce epigenetic modifications, such as changes in DNA methylation or histone modifications. These modifications can affect the expression of metabolic genes.65 Some nanoparticles may cause stress to the endoplasmic reticulum (ER) within cells. ER stress can activate signalling pathways that impact gene expression, including genes related to metabolism. Nanoparticles may interact with transcription factors involved in the regulation of metabolic genes. This interaction can directly influence the transcriptional activity of these genes. AMNPs might interfere with cellular nutrient sensing pathways such as mTOR (mammalian target of rapamycin) signalling. These pathways play a key role in regulating metabolic gene expression in response to nutrient availability. It is important to note that the regulation of metabolic gene expression by nanoparticles can have implications for various physiological processes including energy metabolism, glucose homeostasis, and lipid metabolism. Additionally, understanding how nanoparticles influence metabolic gene expression is crucial for assessing the potential health risks associated with their use, particularly in the context of nanomedicine or consumer products.66Thus, AMNPs can kill bacteria by a variety of ways, including direct cell wall and/or membrane destruction, the formation of reactive oxygen species (ROS), and binding to intracellular components. Most antibiotics target cell walls or membranes or disturb intracellular activities. AMNPs can target these biological properties and provide advantages in treating antibiotic-resistant infections over small-molecule medications. Furthermore, AMNPs can be employed as nanocarriers to deliver other medicines. Nanomaterials’ processes are based on their unique physicochemical features, particularly multivalent interactions with bacterial cells. van der Waals forces, hydrophobic interactions, and electrostatic attractions all play roles at the interfaces of nanoparticles and bacteria (Fig. 2).
Toxicity and selection
Antimicrobial nanoparticles (AMNPs) have been extensively studied for their potential applications in various fields such as antimicrobial chemotherapy, medicine, and consumer products. However, concerns have been raised about the potential toxicity of silver nanoparticles, particularly when they are released into the environment or come into contact with living organisms. The size of nanoparticles is a crucial factor in determining their toxicity. Smaller particles generally have a larger surface area, which can lead to increased reactivity and potential toxicity. Studies have shown that smaller silver nanoparticles may exhibit higher toxicity than the larger ones.67 AMNPs can enter into cells and interact with cellular components, potentially leading to adverse effects. The particles may induce oxidative stress, damage cellular structures, and interfere with cellular functions. The mechanisms of toxicity can vary depending on factors such as nanoparticle size, shape, and surface coating.68 One of the mechanisms by which silver nanoparticles exert their antimicrobial effects is through the release of silver ions. While this property is desirable for certain applications, the release of silver ions can also contribute to toxicity. Silver ions can interact with proteins, enzymes, and DNA, leading to cellular damage.69 The use of silver nanoparticles in consumer products can result in their release into the environment. Once released, these nanoparticles may have ecotoxicological effects on aquatic organisms and other species. The long-term environmental impact of silver nanoparticles is an area of ongoing research. In the context of medical applications such as the use of silver nanoparticles in wound dressings or medical devices, there is a need to carefully assess potential risks to human health. While silver has a long history of use in medicine, the use of nanoparticles introduces new considerations, and studies continue to investigate the safety of these applications.Bacterial cell surfaces are highly negatively charged that enables them to interact preferentially with positively charged elements. In creating NPs to selectively disrupt the bacterial membranes, the charge density and hydrophobicity of the nanomaterial surface are critical considerations. Highly cationic NPs, as well as NPs with too hydrophobic surfaces, can attach to the surface of human cells, lowering the selectivity. Cationic nanomaterials with optimal amphiphilic balance (optimized balance of hydrophobicity and cationic charge) can deliver effective antimicrobial activities while exhibiting minimal levels of haemolysis and cytotoxicity due to the higher selectivity to bacterial cells than the human cell membrane. A variety of NPs target negatively charged planktonic bacterial surfaces. It is anticipated that the biodegradable cationic and amphiphilic polycarbonates were synthesized and self-assembled into cationic micellar nanoparticles that killed methicillin-resistant Staphylococcus aureus (MRSA). These polymeric nanoparticles interact with bacteria electrostatically, causing membrane rupture, resulting in cytoplasmic leakage and cell lysis.70 Understanding the interactions between bacteria and NPs is crucial for the safe design and use of nanoparticles. Researchers are actively exploring nanoparticle modifications and surface coatings to control these interactions and enhance the biocompatibility of nanoparticles. Additionally, assessing the potential genotoxicity of nanoparticles is an important aspect of their safety evaluation. It is important to note that the field of nanotoxicology is evolving, and ongoing research continues to provide insights into the complex interactions of nanoparticles with biological systems.
Antimicrobial nanoparticles in pharmaceutical applications
Antimicrobial nanoparticles such as silver, gold, and zinc oxide have attracted attention in the pharmaceutical and medical fields due to their unique properties that can combat microbial infections. In pharmaceutical applications such as wound dressings, antimicrobial nanoparticles can be incorporated into wound dressings to prevent or treat bacterial infections in the wound (Fig. 5). Nanofibers, hydrogels, hydrocolloids, and, nanohybrids, which are a combination of different nanotechnological systems, can aid in wound healing by carrying and delivering therapeutic agents in the wound bead or due to their inherent properties.71Further, nanoparticles with antimicrobial properties can be formulated into topical creams and ointments for skin infections. These formulations can provide targeted delivery of antimicrobial agents to the affected area, enhancing therapeutic efficacy. Nanoparticles have been used in a variety of applications including cosmetics and treatments for skin illnesses.72 In nanomedicine, liposomal systems for transdermal drug administration,73 contrast agents for disease diagnosis, and gene therapies have gained interest.74
They are engineered for inhalable drug delivery, making them suitable for treating respiratory infections. These nanoparticles can carry antimicrobial agents to the lungs, offering a more direct and efficient therapeutic approach. Sometimes, the antimicrobial properties of nanoparticles can be incorporated into oral care products such as toothpaste and mouthwash to combat bacterial growth and prevent oral infections.75
Antimicrobial nanoparticles in clinical trials
Clinical trials help assess the safety profile of antimicrobial nanoparticles when introduced into the human body. This includes understanding the potential side effects, toxicity levels, and any adverse reactions. This contributes to understanding how antimicrobial nanoparticles are absorbed, distributed, metabolized, and eliminated in the body (pharmacokinetics) and how they exert their effects on microorganisms (pharmacodynamics). This knowledge is crucial for refining treatment strategies. Ultimately, the successful development and deployment of antimicrobial nanoparticles can have a significant impact on patient outcomes, particularly in the context of antibiotic-resistant infections and the need for innovative solutions to combat infectious diseases.76 Therefore, clinical trials of antimicrobial nanoparticles are essential for bridging the gap between laboratory research and clinical applications, verifying whether these particles are safe, effective, and beneficial for patients or not.This review consolidates and summarizes the existing literature with a list of antimicrobial nanoparticles that are currently under clinical trial in different phases (Table 3). It also provides a comprehensive overview of the current state of knowledge, including trial outcomes, methodologies, and key findings. It may act as a valuable resource for various stakeholders involved in the development, regulation, and utilization of AMNPs.
| AMNPs | Study title | Gov. ID | Clinical trial | Intervention | Country |
|---|---|---|---|---|---|
| AgNPs | Topical silver nanoparticles for microbial activity | NCT03752424 | Phase 1 | Drug: silver nanoparticles/topical approved antimicrobial gel | Saudi Arabia |
| Evaluation of silver nanoparticles for the prevention of COVID-19 (COVID-19) | NCT04894409 | — | Device: mouthwash and nose rinse with the AgNPs solution/mouthwashes and nose rinse in a conventional way | Mexico | |
| Effect of metallic nanoparticles on nosocomial bacteria | NCT04775238 | — | Other: silver nanoparticles/copper nanoparticles | Egypt | |
| ZnONPs | Effectiveness and safety of Whitfield's solution, zinc oxide nanoparticles solution or combination for the treatment of fungal feet infection (WhitfieldZinc) | NCT05901961 | Phase 4 | Drug: Whitfield's solution/zinc oxide nanoparticles solution/combined Whitfield's and zinc oxide nanoparticles solution | — |
| TiO2NPs | The anti-microbial effect of titanium dioxide nano particles in complete dentures made for edentulous patients | NCT03666195 | — | Combination product: titanium dioxide nanoparticles | — |
| FeONPs | A new clinical use of ferumoxytol nanoparticles: an antibiofilm treatment | NCT06110494 | Phase 4 | Drug: iron oxide nanoparticles treatment ferumoxytol/H2O2/NaOCl/NaCl | United States |
| PLGANPs | PLGA nanoparticles entrapping ciprofloxacin to treat E. fecalis infections in endodontics | NCT05475444 | — | Device: chitosan coated PLGA nanoparticles entrapping ciprofloxacin incorporated in smart gels/ciprofloxacin paste and solution | Egypt |
| Modified surface of PLGA nanoparticles in smart hydrogel | NCT05442736 | Early phase 1 | Drug: ciprofloxacin | Egypt | |
| Chitosan AMNPs | Effectiveness of a novel respirator with chitosan nanoparticles | NCT04490200 | — | Device: VESTA respirator/conventional N95 respirator | Brazil |
| Liposomes | Amsterdam UMC clinical trial with a native-like HIV-1 envelope vaccine (ACTHIVE-001) | NCT03961438 | Phase 1 | Biological: ConM SOSIP.v7 gp140, adjuvanted with MPLA liposomes/ConM SOSIP.v7 gp140, adjuvanted with MPLA liposomes | Netherlands |
| Liposomal amphotericin b with or without sargramostim in treating patients with invasive fungal infection | NCT00003315 | Phase 3 | Biological: sargramostim | United Kingdom | |
| Drug: liposomal amphotericin B | |||||
| ProphyALL-study on the safety of liposomal amphotericin b to prevent antifungal infections in elderly patients with acute lymphoblastic leukemia | NCT00386997 | Phase 4 | Drug: liposomal amphotericin B (AmBisome®) | Germany | |
| Efficacy and safety of high-dose l-amb for disseminated histoplasmosis in AIDS (L-AmB_phase3) | NCT05814432 | Phase 3 | Drug: liposomal amphotericin B | ||
| Anidulafungin vs. amphotericin B safety in high risk hepatic transplant recipients (AVALTRA) | NCT01303549 | Phase 4 | Drug: anidulafungin/liposomal amphotericin B | Spain | |
| Randomized, double-blind, placebo-controlled safety, tolerability and immunogenicity study of candidate HIV-1 vaccines ChAdOx1.HTI and MVA.HTI with recombinant HIV-1 envelope protein ConM SOSIP.v7 gp140 vaccine, adjuvanted with MPLA liposomes in ART-suppressed HIV-1 positive individuals | NCT05208125 | Phase 1 | Biological: ChAdOx1.HTI at week 0, ConM SOSIP.v7 at weeks 4, 12 and 28, and MVA.HTI at week 22 (CSSMS) | Spain | |
| Other: normal saline solution | |||||
| Prevention of invasive fungal infections (IFIs) in subjects receiving chemotherapy for acute lymphoblastic leukemia (AmBiGuard) | NCT01259713 | Phase 3 | Drug: liposomal amphotericin B/placebo | United Kingdom | |
| SARS-COV-2-Spike-Ferritin-nanoparticle (SpFN) vaccine with ALFQ adjuvant for prevention of COVID-19 in healthy adults | NCT04784767 | Phase 1 | Biological: 25 μg SpFN_1B-06-PL + ALFQ (QS21 adjuvant) | United States | |
| Drug: sodium chloride, USP, for injection (0.9% NaCl) | |||||
| Biological: 50 μg SpFN_1B-06-PL + ALFQ (QS21 adjuvant) | |||||
| Efficacy and safety of oral encochleated amphotericin B for the treatment of cryptococcal infection (ORACLE) | NCT03196921 | Phase 1 | Drug: encochleated Amphotericin B | ||
| Phase 2 | |||||
| Micelles | The effect of micellized food supplements on health-related quality of life in patients with post-acute COVID-19 syndrome | NCT05150782 | — | Dietary supplement: curcumin/Boswellia Serrata/ascorbic acid mixture | — |
Future challenges
While antimicrobial nanoparticles hold promise for various applications, they also face several challenges that need to be overcome for their safe and effective use. Some of the future challenges associated with antimicrobial nanoparticles are as follows:i. Resistance development: there is a concern that prolonged and widespread use of antimicrobial nanoparticles could contribute to the development of microbial resistance. Research is needed to understand the mechanisms of resistance and strategies to mitigate this risk.
ii. Biocompatibility and toxicity: assessing the long-term biocompatibility and potential toxicity of antimicrobial nanoparticles is crucial. Understanding their impact on human health and the environment is necessary for safe use.
iii. Environmental impact: the release of antimicrobial nanoparticles into the environment, whether intentional or unintentional, raises concerns about their impact on ecosystems, aquatic life, and soil health. Studying their fate, transport, and ecological effects is essential.
iv. Nonspecific effects on microbiota: antimicrobial nanoparticles may not only target pathogenic microorganisms but also affect the beneficial microbiota. Maintaining a balance in microbial communities is important for overall health.
v. Optimization of formulations: fine-tuning the properties of antimicrobial nanoparticles, such as size, shape, surface charge, and coating materials, is necessary to enhance their efficacy while minimizing potential adverse effects.
vi. Delivery challenges: achieving targeted delivery of antimicrobial nanoparticles to specific sites within the body can be challenging. Designing delivery systems that allow controlled release and localization is essential.
vii. Regulatory framework: developing robust regulatory frameworks for the approval and monitoring of antimicrobial nanoparticles is necessary to ensure their safety and effectiveness in various applications including antimicrobial testing. Their disposal system should be followed properly and necessary to include as potential biohazards. Regulatory agencies in various countries are actively monitoring and assessing the safety of nanomaterials. Efforts to harmonize regulations at the international level are going on. Organizations such as the Organization for Economic Co-operation and Development (OECD) work towards establishing guidelines for the testing and assessment of nanomaterials. The OECD has specific guideline 129 that provide recommendations for the testing of nanomaterials, including considerations for cytotoxicity testing. The International Organization for Standardization (ISO) has a Technical Committee on Nanotechnologies (ISO/TC229) that works on developing standards for nanotechnology. These standards may include guidance on cytotoxicity testing method, their safe use in consumer products and medical applications.
viii. Nanoparticles can be engineered for specific targeting of bacterial cells. When these targeted nanoparticles accumulate at the bacterial infection site, the application of near infra-red light leads to selective heating of the nanoparticles and the surrounding bacteria, causing thermal damage, which needs to be optimized.
ix. Photothermal therapy (PTT) can be combined with photodynamic therapy (PDT) for a synergistic effect. The heat generated by PTT can enhance the effectiveness of photosensitizers used in PDT, leading to increased production of reactive oxygen species (ROS) during PDT. Combining PTT and PDT may provide unique properties for antimicrobial treatment with enhanced selectivity and reduced side effects.
x. Necessary to prevent horizontal gene transfer: nanoparticles can be designed to inhibit the transfer of antibiotic resistance genes between bacteria, reducing the spread of resistance within bacterial populations.
xi. Necessary to reduce the circulation time to avoid much immune response: surface modifications can help nanoparticles evade the immune system, leading to longer circulation times and improved therapeutic efficacy.
Conclusion
Antimicrobial nanoparticles represent an emerging area with enormous potential for impacting various sectors including healthcare, environmental science, and food safety. These nanoparticles offer several advantages such as enhanced surface area-to-volume ratios, unique chemical properties, and the ability to interact specifically with microbial cells, thus providing a more efficient therapeutic strategy of combating microbial infections than traditional antibiotics. The surface properties of nanoparticles can be tailored to optimize their interactions with bacterial cells, enhancing their antimicrobial efficacy while minimizing the potential for resistance development. In conclusion, nanoparticles offer a versatile platform for developing antimicrobial strategies that can help prevent or slow down the emergence of antibiotic resistance. By addressing multiple aspects of resistance development, nanoparticles may contribute to more effective and sustainable infection therapy and management.Conflicts of interest
Authors declared no conflict of interest.References
- M. M. Mamun, A. J. Sorinolu, M. Munir and E. P. Vejerano, Front. Chem., 2021, 9, 687660 CrossRef PubMed.
- R. K. Bera, S. M. Mandal and C. R. Raj, Lett. Appl. Microbiol., 2014, 58(6), 520–526 CrossRef CAS PubMed.
- R. Varshney, S. Bhadauria and M. S. Gaur, Nano Biomed. Eng., 2012, 4, 99–106 CAS.
- X. F. Zhang, Z. G. Liu, W. Shen and S. Gurunathan, Int. J. Mol. Sci., 2016, 17(9), 1534 CrossRef PubMed.
- G. Mi, D. Shi, M. Wang and T. J. Webster, Adv. Healthcare Mater., 2018, 7(13), 1800103 CrossRef PubMed.
- T. M. Uddin, A. J. Chakraborty, A. Khusro, B. R. Zidan, S. Mitra, T. B. Emran, K. Dhama, M. K. Ripon, M. Gajdács, M. U. Sahibzada and M. J. Hossain, J. Infect. Public Health, 2021, 14(12), 1750–1766 CrossRef PubMed.
- L. Xu, Y. Y. Wang, J. Huang, C. Y. Chen, Z. X. Wang and H. Xie, Theranostics, 2020, 10(20), 8996–9031 CrossRef CAS PubMed.
- W. Gao, Y. Chen, Y. Zhang, Q. Zhang and L. Zhang, Adv. Drug Delivery Rev., 2018, 127, 46–57 CrossRef CAS PubMed.
- T. G. Smijs and S. Pavel, Nanotechnol. Sci. Appl., 2011, 4, 95–112 CrossRef CAS PubMed.
- S. V. Gudkov, D. E. Burmistrov, D. A. Serov, M. B. Rebezov, A. A. Semenova and A. B. Lisitsyn, Front. Phys., 2021, 9, 641481 CrossRef.
- X. Ma, S. Zhou, X. Xu and Q. Du, Front. Surg., 2022, 9, 905892 CrossRef PubMed.
- M. L. Ermini and V. Voliani, ACS Nano, 2021, 15(4), 6008–6029 CrossRef CAS PubMed.
- D. De, S. M. Mandal, S. S. Gauri, R. Bhattacharya, S. Ram and S. K. Roy, J. Biomed. Nanotechnol., 2010, 6(2), 138–144 CrossRef CAS PubMed.
- C. Liao, Y. Li and S. C. Tjong, Nanomaterials, 2020, 10(1), 124 CrossRef CAS PubMed.
- N. Pangprasit, Y. Thammawong, A. Kulsirorat, P. Chuammitri, A. Kongkaew, M. Intanon, W. Suriyasathaporn, S. Pikulkaew and W. Chaisri, Animals, 2023, 13(17), 2688 CrossRef PubMed.
- J. Maji, S. Pandey and S. Basu, Bull. Mater. Sci., 2020, 43, 1–0 CrossRef.
- L. L. Israel, A. Galstyan, E. Holler and J. Y. Ljubimova, J. Controlled Release, 2020, 320, 45–62 CrossRef CAS PubMed.
- L. A. S. de Toledo, H. C. Rosseto and M. L. Bruschi, Pharm. Dev. Technol., 2018, 23(4), 316–323 CrossRef PubMed.
- H. Idrees, S. Z. J. Zaidi, A. Sabir, R. U. Khan, X. Zhang and S. U. Hassan, Nanomaterials, 2020, 10(10), 1970 CrossRef CAS PubMed.
- Y. Lu, D. Cheng, B. Niu, X. Wang, X. Wu and A. Wang, Pharmaceuticals, 2023, 16, 454 CrossRef CAS PubMed.
- J. Cruz Flórez, R. J. Torres, M. Urquiza, J. A. Gutiérrez, F. Guzmán and C. C. Ortiz, Nanotechnology, 2017, 28(13), 135102 CrossRef PubMed.
- K. Jafernik, A. Ładniak, E. Blicharska, K. Czarnek, H. Ekiert, A. E. Wiącek and A. Szopa, Molecules, 2023, 28, 1963, DOI:10.3390/molecules28041963.
- H. L. Loo, B. H. Goh, L. H. Lee and L. H. Chuah, Asian J. Pharm. Sci., 2022, 17(3), 299–332 CrossRef PubMed.
- K. Divya, S. Vijayan and T. K. George, Fibers Polym., 2017, 18, 221–230 CrossRef CAS.
- S. Ibrahim, M. E. El-Naggar and A. M. Youssef, J. Cluster Sci., 2020, 31, 1371–1382 CrossRef CAS.
- T. T. Hoang Thi, E. H. Pilkington, D. H. Nguyen, J. S. Lee, K. D. Park and N. P. Truong, Polymers, 2020, 12(2), 298 CrossRef PubMed.
- S. Roy Choudhury, S. Roy, A. Goswami and S. Basu, J. Antimicrob. Chemother., 2012, 67, 1134–1137 CrossRef CAS PubMed.
- A. Bhadran, T. Shah, G. K. Babanyinah, H. Polara, S. Taslimy, M. C. Biewer and M. C. Stefan, Pharmaceutics, 2023, 15(7), 1977 CrossRef CAS PubMed.
- A. Sharma, A. Gaur, V. Kumar, N. Sharma, S. A. Patil, R. K. Verma and A. K. Singh, Int. J. Pharm., 2021, 608, 121097 CrossRef CAS PubMed.
- A. Pamukçu, N. Erdoğan and D. Şen Karaman, J. Biomed. Mater. Res., Part B, 2022, 110(11), 2506–2520 CrossRef PubMed.
- I. Yudovin-Farber, N. Beyth, E. I. Weiss and A. J. Domb, J. Nanopart. Res., 2010, 12, 591–603 CrossRef CAS.
- A. Zakeri, M. A. J. Kouhbanani, N. Beheshtkhoo, V. Beigi, S. M. Mousavi, S. A. R. Hashemi, A. Karimi Zade, A. M. Amani, A. Savardashtaki, E. Mirzaei, S. Jahandideh and A. Movahedpour, Nano Rev. Exp., 2018, 9(1), 1488497 CrossRef PubMed.
- F. Masoud, D. Soodabeh, Z. Amir, A. Nasim, A. Abolfazl and R. Salehi, Nanomed. Biotechnol., 2018, 8, 1872–1891 Search PubMed.
- M. Ozdal and S. Gurkok, ADMET DMPK, 2022, 10(2), 115–129 Search PubMed.
- S. M. Mandal, S. Saha, J. Sengupta and S. Pratihar, Analyst, 2013, 138(18), 5197–5199 RSC.
- F. Perreault, A. F. De Faria, S. Nejati and M. Elimelech, ACS Nano, 2015, 9(7), 7226–7236 CrossRef CAS PubMed.
- M. Zahran and A. H. Marei, Int. J. Biol. Macromol., 2019, 136, 586–596 CrossRef CAS PubMed.
- Y. F. Mustafa, BioNanoScience, 2023, 13(2), 840–852 CrossRef.
- F. Khan, M. Shariq, M. Asif, M. A. Siddiqui, P. Malan and F. Ahmad, Nanomaterials, 2022, 12(4), 673 CrossRef CAS PubMed.
- P. Guo, B. A. Buttaro, H. Y. Xue, N. T. Tran and H. L. Wong, Eur. J. Pharm. Biopharm., 2020, 151, 189–198 CrossRef CAS PubMed.
- L. Wang, C. Hu and L. Shao, Int. J. Nanomed., 2017, 12, 1227–1249 CrossRef CAS PubMed.
- J. Tang, Q. Ouyang, Y. Li, P. Zhang, W. Jin, S. Qu, F. Yang, Z. He and M. Qin, Int. J. Mol. Sci., 2022, 23, 15738 CrossRef CAS PubMed.
- M. J. Mitchell, M. M. Billingsley and R. M. Haley, Nat. Rev. Drug Discovery, 2021, 101–124 CrossRef CAS PubMed.
- L. Kumar, M. Bisen, K. Harjai, S. Chhibber, S. Azizov, H. Lalhlenmawia and D. Kumar, ACS Omega, 2023, 8(24), 21391–21409 CrossRef CAS PubMed.
- Y. C. Yeh, T. H. Huang, S. C. Yang, C. C. Chen and J. Y. Fang, Front. Chem., 2020, 8, 286 CrossRef CAS PubMed.
- W. Gao, Y. Chen, Y. Zhang, Q. Zhang and L. Zhang, Adv. Drug Delivery Rev., 2018, 127, 46–57 CrossRef CAS PubMed.
- Y. Liu, S. Huang, X. Huang and D. Ma, Mater. Horiz., 2024, 11, 1395–1413 RSC.
- A. R. M. Silva, J. Y. N. H. Alexandre, J. E. S. Souza, J. G. L. Neto, P. G. de Sousa Júnior, M. V. P. Rocha and J. C. S. Dos Santos, Molecules, 2022, 27, 4529 CrossRef CAS PubMed.
- N. Singh, J. Kim, J. Kim, K. Lee, Z. Zunbul, I. Lee, E. Kim, S. G. Chi and J. S. Kim, Bioact. Mater., 2022, 21, 358–380 Search PubMed.
- S. He, L. Wu, X. Li, H. Sun, T. Xiong, J. Liu, C. Huang, H. Xu, H. Sun, W. Chen, R. Gref and J. Zhang, Acta Pharm. Sin. B, 2021, 11(8), 2362–2395 CrossRef CAS PubMed.
- N. Dey, C. Kamatchi, A. S. Vickram, K. Anbarasu, S. Thanigaivel, J. Palanivelu, A. Pugazhendhi and V. K. Ponnusamy, Environ. Res., 2022, 204, 111968 CrossRef CAS PubMed.
- I. Taylor, T. Sarah and L. P. Nicole, Int. J. Hyperthermia, 2018, 34(2), 144–156 CrossRef PubMed.
- R. Auten and J. Davis, Pediatr. Res., 2009, 66, 121–127 CrossRef CAS PubMed.
- N. Y. Lee, W. C. Ko and P. R. Hsueh, Front. Pharmacol., 2019, 10, 1153 CrossRef CAS PubMed.
- S. R. Elkin, A. M. Lakoduk and S. L. Schmid, Wien. Med. Wochenschr., 2016, 166(7–8), 196–204 CrossRef PubMed.
- R. K. Shukla, A. Badiye, K. Vajpayee and N. Kapoor, Front. Genet., 2021, 12, 728250 CrossRef CAS PubMed.
- M. H. Muhammad, A. L. Idris, X. Fan, Y. Guo, Y. Yu, X. Jin, J. Qiu, X. Guan and T. Huang, Front. Microbiol., 2020, 11, 928 CrossRef PubMed.
- V. G. Preda and O. Săndulescu, Discoveries, 2019, 7(3), e100 CrossRef PubMed.
- R. M. Pinto, F. A. Soares, S. Reis, C. Nunes and P. Van Dijck, Front. Microbiol., 2020, 11, 952 CrossRef PubMed.
- Y. Guo, Z. Mao, F. Ran, J. Sun, J. Zhang, G. Chai and J. Wang, Pharmaceutics, 2023, 15(11), 2582 CrossRef CAS PubMed.
- Y. Singh and M. K. Saxena, Front. Microbiol., 2022, 13, 982611 CrossRef PubMed.
- C. Sahli, S. E. Moya, J. S. Lomas, C. Gravier-Pelletier, R. Briandet and M. Hémadi, Theranostics, 2022, 12(5), 2383–2405 CrossRef CAS PubMed.
- S. Alghamdi, K. Khandelwal, S. Pandit, A. Roy, S. Ray, A. A. Alsaiari, A. Aljuaid, M. Almehmadi, M. Allahyani, R. Sharma and J. Anand, Prog. Biophys. Mol. Biol., 2023, 184, 13–31 CrossRef CAS PubMed.
- S. J. Cameron, J. Sheng, F. Hosseinian and W. G. Willmore, Int. J. Mol. Sci., 2022, 23(14), 7962 CrossRef CAS PubMed.
- R. Mazzone, C. Zwergel and M. Artico, Clin. Epigenet., 2019, 11, 34 CrossRef PubMed.
- W. Shim, M. Jeong Paik, D. T. Nguyen, J. K. Lee, Y. Lee, J. H. Kim, E. H. Shin, J. Seok Kang, H. S. Jung, S. Choi, S. Park, J. S. Shim and G. Lee, ACS Nano, 2012, 6(9), 7665–7680 CrossRef CAS PubMed.
- M. Akter, M. T. Sikder, M. M. Rahman, A. K. M. A. Ullah, K. F. B. Hossain, S. Banik, T. Hosokawa, T. Saito and M. Kurasaki, J. Adv. Res., 2017, 9, 1–16 Search PubMed.
- Y. Qing, L. Cheng, R. Li, G. Liu, Y. Zhang, X. Tang, J. Wang, H. Liu and Y. Qin, Int. J. Nanomed., 2018, 13, 3311–3327 CrossRef CAS PubMed.
- A. Roy, O. L. Franco and S. M. Mandal, Curr. Protein Pept. Sci., 2013, 14(7), 580–587 CrossRef CAS PubMed.
- P. J. J. Cypriyana, S. Saigeetha, A. V. Samrot, P. Ponniah and S. Chakravarthi, Biocatal. Agric. Biotechnol., 2021, 36, 102117 CrossRef.
- A. E. Stoica, C. Chircov and A. M. Grumezescu, Molecules, 2020, 25, 2699 CrossRef CAS PubMed.
- L. A. DeLouise, J. Invest. Dermatol., 2012, 132, 964–975 CrossRef CAS PubMed.
- S. Parveen, R. Misra and S. K. Sahoo, Nanomedicine, 2012, 8, 147–166 CrossRef CAS PubMed.
- G. G. Flores-Rojas, F. López-Saucedo, R. Vera-Graziano, E. Mendizabal and E. Bucio, Macromol, 2022, 2, 374–390 CAS.
- W. Song and S. Ge, Molecules, 2019, 24, 1033 CrossRef CAS PubMed.
- L. Wang, C. Hu and L. Shao, Int. J. Nanomed., 2017, 12, 1227–1249 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |