 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent progress on nanosystems for nucleic acid delivery
Shanka
Walia
a and
Mohit J.
Mehta
 *bcd
*bcd
aSwami Sahajanand College of Computer Science, Plot No. 639, ISCON Mega City, Gujarat 364002, India
bNanomedicine Lab, Department of Biological Sciences and Bioengineering, Inha University, 100 Inha-ro, Michuhol-gu, Incheon, 22212, Republic of Korea
cAdult Stem Cell Group, Faculty of Medicine and Health Technology, Tampere University, Tampere, 33520, Finland
dTays Research Services, Wellbeing Services County of Pirkanmaa, Tampere University, Hospital, Tampere, 33520, Finland. E-mail: mohitjmehta@gmail.com; mohit.mehta@tuni.fi
First published on 7th August 2024
Abstract
Nucleic acid (NA) based therapeutics have witnessed tremendous progress and breakthroughs in treating pathological conditions, including viral infections, neurological disorders, genetic diseases, and metabolic disorders. NAs such as plasmid DNA (pDNA), short interfering RNA (siRNA), microRNA (miRNA), and antisense oligonucleotides (ASOs) can be modified to revolutionize personalized medicine. Despite the great potential of NA-based therapeutics, their clinical transformation is significantly hampered by instability, degradation, and inefficient delivery to the targeted site in the in vivo system. Lipid-based delivery systems hold great potential to overcome these shortcomings to enhance the delivery and bioavailability, improve stability, and increase the therapeutic effect of the NAs by delivering them to the active site. This review emphasized various nucleic acid-based therapeutics and their enhanced and improved delivery using different nanocarriers. Ultimately, the importance of lipid-based nanocarriers for delivering NAs is discussed and provides perspective in this field.
1. Introduction
Nucleic acids (NAs) are immensely recognized for their therapeutic potential during the COVID-19 pandemic. NAs, including DNA, antisense oligonucleotides (ASO), aptamers, short interfering RNA (siRNA), microRNA (miRNA), and messenger RNA (mRNA), provide a highly versatile platform at the underlying level of transcription and translation for the treatment of diseases as compared with traditional drugs.1,2 Nucleic acids’ high specificity and pharmacological effect offered unprecedented opportunities to combat viral infections and complicated diseases, including cancer, diabetes, cardiovascular diseases, genetic disorders, and acquired conditions.3 The majority of the NAs bind selectively to their target molecules via complementary Watson–Crick base pairing, making target molecule detection simple.4 NAs are good candidates for the personalized medicinal approach because genes and their mutations can be easily questioned. Hence, NAs can target patient-specific genes with minimal off-target effects.5 In contrast, pharmaceutical drug molecules require extensive screening and medicinal chemical optimization.However, the intracellular uptake of NAs is quite challenging because of their anionic nature and poor stability, which makes the intracellular uptake of NAs difficult. Due to their highly hydrophilic and polyvalent anionic properties, NAs are degraded by extracellular enzymes and, thus, poorly absorbed by cells. When injected intravenously, they tend to accumulate in the liver and kidneys.6 All these factors reduced the efficiency of the delivered cargo to elicit the desired response.7 Furthermore, due to their high molecular weight, negative zeta potential, and hydrophilicity, NAs cannot penetrate the cellular membrane. However, clinical application is the primary goal of any therapeutic molecule; hence, nanovehicles should ensure adequate delivery of drugs to the targeted cell or organ without causing any harmful effect on the healthy cells. The therapeutic payload is delivered to the targeted cells via two main approaches, namely, passive targeting and active targeting. The foundation of passive targeting is the enhanced permeability and retention (EPR) effect. For example, tumor vasculature is often leaky compared with healthy blood vessels. This helps nanoparticles to penetrate the cancer cells more efficiently.8 In contrast to this, active targeting facilitates surface modifications of nanovehicles with specific ligands, resulting in enhanced binding to the receptors of targeted cells. Presently, a structure capable of improving the bio-efficacy and controlled release of NAs at the desired site is of the utmost importance. Nucleic acids possess versatile physicochemical properties and tunability. These features allow them to be easily functionalized with various biomolecules and nanoparticles (NPs) for enhanced therapeutic effect with selective binding to the targeted molecules. Different nanoparticle-based platforms such as metal NPs, polymers, dendrimers, proteins, lipids, and other biomolecules are widely exploited in conjugation with NAs.9,10 For instance, liposomes and micelles, which have now received FDA approval, were part of the first generation of nanoparticle-based therapy.11 Lipid-based nanocarriers were introduced as promising tools to retain the structural integrity and therapeutic potential of NAs. Lipid-based nanocarriers offer a versatile platform for NA encapsulation, which resulted in the clinical translation of several NA therapeutics. This review aims to provide an in-depth discussion on various nanosystems for nucleic acid delivery and therapeutics. They offer a powerful approach to address the limitations of traditional treatments, and hence can potentially overcome these challenges.
2. Nucleic acid-based therapeutics
In the last decade, nucleic acid-based therapies have emerged as promising approaches for treating multiple disorders by regulating molecular pathways.12 NAs target disease-causing genes in a specific manner, enabling precise and personalized treatment for life-threatening diseases.13 NA-based therapeutics knock down, upregulate, or alter targeted gene expression. Currently, several NA-based therapeutics are used for the treatment of severe ailments (Table 1).| S. no. | Nucleic acid | Biomedical application | Ref. |
|---|---|---|---|
| 1 | Plasmid DNA | Gene cloning, gene therapy, DNA vaccine, protein expression, gene editing | 14 and 15 |
| 2 | Antisense oligonucleotides (ASOs) | Targeting RNA functioning of genetic disorders, gene silencing | 16 and 17 |
| 3 | Aptamers | Cell tracking, bacterial and viral protein sensing | 18 |
| 4 | siRNA and miRNA | Gene therapy, antiviral therapy, drug discovery | 19–21 |
| Biomarkers, cancer treatment | |||
| 5 | mRNA | Vaccine production, cancer immunotherapy, gene editing | 22 and 23 |
| 6 | CpG DNA | Immunotherapy, vaccine adjuvant, gene therapy, anti-inflammatory | 24 and 25 |
2.1 Plasmids
Plasmids are circular, double-stranded, high molecular weight (<1000 to >200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bp) DNA constructs. They are often used as vectors to introduce a foreign gene into a target cell for various purposes such as gene therapy, vaccine production, and protein expression.14,15 The plasmid backbone contains the gene for antibiotic resistance controlled by a prokaryotic promoter (a prokaryotic origin of replication for plasmid replication),14 and an expression cassette with a promoter to initiate the transcription of a transgene that encodes a therapeutic protein.15 Plasmid DNA molecules typically contain several regulatory signals, such as promoter and enhancer sequences, responsible for regulating gene expression.14 Promoters (cytomegalovirus (CMV) and Rous sarcoma virus or human alpha-actin, human beta-actin promoter) commence gene transcription by providing a recognition site for the RNA polymerase. Enhancers play a vital role in the large-scale production of a gene with specificity. They are localized either downstream or upstream from the promoter site. Transcription efficiency can be elevated by selecting the promoter and enhancer of interest; for example incorporating SV40 enhancer in the expression plasmid enhanced muscle-specific gene expression.26 Plasmids also play an essential role in generating vaccines for genetic immunization. Plasmid-derived vaccines are easy to produce if a genetic sequence of the concerned variant is identified, and are also known to control the infection.27 Pardridge et al. (2020) developed a plasmid DNA approach for the treatment of Niemann–Pick C1 (NPC1), a lysosomal cholesterol storage disorder affecting the brain.28 Plasmid DNA was encapsulated in pegylated liposomes encoding the functional human NPC1 gene. Quantitative PCR has shown the successful delivery of pDNA and NPC1 mRNA expression in the brain, liver, and spleen. Malardo and coworkers (2012) demonstrated that injecting a low dose of pDNA (pcDNA3) in the Wistar rat endotoxemia model has increased plasma vasopressin and regulates blood pressure.29 The rats treated with 10–20 μg pDNA showed lower levels of inflammatory cytokines, namely, IL-6 and TNF-α.
000 bp) DNA constructs. They are often used as vectors to introduce a foreign gene into a target cell for various purposes such as gene therapy, vaccine production, and protein expression.14,15 The plasmid backbone contains the gene for antibiotic resistance controlled by a prokaryotic promoter (a prokaryotic origin of replication for plasmid replication),14 and an expression cassette with a promoter to initiate the transcription of a transgene that encodes a therapeutic protein.15 Plasmid DNA molecules typically contain several regulatory signals, such as promoter and enhancer sequences, responsible for regulating gene expression.14 Promoters (cytomegalovirus (CMV) and Rous sarcoma virus or human alpha-actin, human beta-actin promoter) commence gene transcription by providing a recognition site for the RNA polymerase. Enhancers play a vital role in the large-scale production of a gene with specificity. They are localized either downstream or upstream from the promoter site. Transcription efficiency can be elevated by selecting the promoter and enhancer of interest; for example incorporating SV40 enhancer in the expression plasmid enhanced muscle-specific gene expression.26 Plasmids also play an essential role in generating vaccines for genetic immunization. Plasmid-derived vaccines are easy to produce if a genetic sequence of the concerned variant is identified, and are also known to control the infection.27 Pardridge et al. (2020) developed a plasmid DNA approach for the treatment of Niemann–Pick C1 (NPC1), a lysosomal cholesterol storage disorder affecting the brain.28 Plasmid DNA was encapsulated in pegylated liposomes encoding the functional human NPC1 gene. Quantitative PCR has shown the successful delivery of pDNA and NPC1 mRNA expression in the brain, liver, and spleen. Malardo and coworkers (2012) demonstrated that injecting a low dose of pDNA (pcDNA3) in the Wistar rat endotoxemia model has increased plasma vasopressin and regulates blood pressure.29 The rats treated with 10–20 μg pDNA showed lower levels of inflammatory cytokines, namely, IL-6 and TNF-α.
2.2 Antisense oligonucleotide (ASO)
Antisense oligonucleotides (ASOs) of therapeutic importance comprise single-stranded nucleic acids and are 18–30 base pairs in length.30 ASOs form complementary base pairs with their target RNA by classical Watson and Crick base pairing. After binding with the target RNA, ASOs resulted in gene silencing by altering or degrading the expression of the target RNA via cleavage or blockage.16,17 The various mechanisms adopted by ASOs to degrade target RNA include (1) modified splicing by skipping and inclusion of exons, and inhibition of 5′ cap formation, (2) steric blockage of ribosomal functions as they inhibit the binding of target RNA with ribosomes through the translation arrest of the target RNA, and (3) induction of RNAse H that recognizes ASO–RNA hybrids and degrade the target RNA present in the hybrid.31,32 ASOs are primarily administered via transfection and transduction in vitro and in vivo.33 For example, Passini et al. (2011) used 2′-O-2-methoxyethyl-modified ASOs for the treatment of severe spinal muscular atrophy (SMA).34 SMA is an autosomal recessive neuromuscular disorder related to the deficiency of survival motor neuron (SMN) protein caused by mutations in the SMN1 gene. ASO injection resulted in increased levels of SMN protein in SMA-infected mice. In vivo studies suggested improved therapeutic effects on muscle physiology, functioning, and the survival rate of motor neurons. Some examples of FDA-approved ASO-based drugs include eteplirsen, golodirsen, and nusinersen.4 Several others are under clinical trials for the treatment of neurological disorders, hepatitis B virus infections, solid tumors, and renal diseases.35Although ASOs might have shown enhanced efficacy in vitro and in vivo, they showed drawbacks related to low cellular uptake and poor stability in body fluids that hindered their potential from bench to bedside.
2.3 Aptamers
Aptamers are short single-stranded synthetic oligonucleotides of 10–100 nucleotides folded into three-dimensional shapes.36,37 Aptamers possess a remarkable tendency to bind non-covalently to their target with high selectivity and specificity. Aptamers are widely explored to target biomolecules of interest, including proteins, peptides, carbohydrates, antibodies, small molecules, toxins, live cells, and even heavy metals.18 Thus, aptamers are potential candidates for cell tracking, bacterial and viral protein sensing, medicine, and analytical chemistry. Aptamers are primarily generated in vitro from the systematic evolution of ligands by exponential enrichment (SELEX).38 Aptamers are often compared with antibodies because of their similar function in binding the proteins with specificity. Unfortunately, antibodies are fraught with significant shortcomings, such as limited and unreproducible synthesis, being expensive, and being prone to generating immunogenicity. However, aptamers with versatile properties have gained much attention in the research community since their discovery in 1990.39,40 Aptamers are a fascinating alternative to conventional antibodies due to their facile and reproducible production, small size, reduced immunogenic effect, physicochemical stability, longer shelf life, and in vitro chemical synthesis.412.4 Small interfering RNA and microRNA
Small interfering RNA (siRNA) and microRNA (miRNAs) are representative modulators of the interference RNA (RNAi) mechanism. RNAi comprises a group of agents that use double-stranded RNA containing homologous sequences complementary to the target gene and perform sequence-specific gene silencing.19 The primary function of RNAi is to build a robust defense mechanism for protecting the genome from mobile genetic material released from viruses, which, on activation, produce abnormal dsRNA or RNA.42 For example, stable RNAi nanocomplexes with redox-sensitive glycol chitosan derivatives were synthesized based on rolling circle transcription. This nanocomplex ensured systemic and targeted siRNA delivery with enhanced therapeutic effects in vivo via the EPR effect.43 A nanomedicine platform based on AuNPs covalently functionalized with siRNA duplexes was efficiently used to neutralize oncogene expression in glioblastoma multiforme.44In vivo studies indicated that the AuNP–siRNA nanocomplex penetrates the blood–brain barrier. AuNP–siRNA nanocomplex effectively knocked down endogenous Bcl2L12 mRNA and protein levels and sensitized glioma cells toward therapy-induced apoptosis by targeting the oncoprotein Bcl2L12. Elbashir and coworkers (2001), demonstrated that 21-nucleotide siRNA duplexes mediated the knockdown of endogenous and heterologous genes in human embryonic kidney (293) and HeLa cells.45 In 2018, the FDA approved the first siRNA-based drug, patisiran (Onpattro), to treat familial amyloid polyneuropathy.46 Other siRNA-based medications approved by the FDA for clinical use include givosiran (to treat acute hepatic porphyria), lumasiran (for the treatment of primary hyperoxaluria type 1), and inclisiran (to treat atherosclerotic cardiovascular disease).47 Vir Biotechnology and Alnylam Pharmaceuticals recently developed ALN-COV (VIR 2703) based on siRNA therapeutics to cure SARS-CoV and SARS-CoV-2 infections.20microRNA (miRNA) is an attractive therapeutic tool, particularly in cancer treatment. miRNA interacts with the 3′ or 5′ untranslated region of the targeted mRNA, resulting in degradation and translational suppression.21 Li et al. (2021) reported intracellular miRNA imaging and gene silencing using a let-7a miRNA-activated DNA nanomachine.48 Multifunctional miRNA-515 sponge-loaded magnetic nanodroplets combined with ultrasound and magnetism were used to treat hepatocellular carcinoma (HCC).49In vivo, studies showed the suppression of xenograft HCC because miRNA-515 upregulated the expression of anti-oncogenes, namely CD22, P21, TIMP1, NFKB, and E-cadherin in cancerous cells. However, no miRNA drug is available and approved by the FDA for therapeutic purposes. Most of the miRNA drugs are still under clinical trials; for example, miravirsen (miR-122 inhibitor) for treating hepatitis (Hep) C has completed Phase II clinical trials. Similarly, lademirsen, or RG-012 (miR-21 inhibitor), is in Phase II clinical trials for treating Alport syndrome (NCT02855268). MRG-110 has completed phase I clinical trials, and further studies are underway to treat impaired wounds. MRG-110 (miRNA-92a inhibitor) improved wound healing in preclinical models and can effectively treat impaired wound healing conditions in diabetic patients. In short, several miRNA drugs are under preclinical and clinical trials to treat various disorders.
2.5 Messenger RNA (mRNA)
Messenger RNA (mRNA) is a type of genetic material that contains the information for producing proteins from DNA, the genetic code found in the nucleus of a cell.50 In recent years, scientists have investigated the use of mRNA as a therapeutic agent, specifically in the medical field. mRNA therapeutics, commonly termed mRNA vaccines or mRNA drugs, are designed from synthetic or modified mRNA molecules to stimulate the production of specific proteins in the body to treat diseases.51 The Pfizer-BioNTech and Moderna COVID-19 vaccines, which employ mRNA to guide cells to generate the spike protein present on the surface of the SARS-CoV-2 virus, are among the most noteworthy milestones of mRNA therapies.22 The immune system then recognizes these spike proteins as foreign moieties and stimulates an immune response against the virus. This method has proved to be efficient in preventing critical illness and death from COVID-19, and regulatory agencies around the globe have permitted its emergency usage.51,52Additionally, mRNA therapies can cure many ailments, including cancer, genetic abnormalities, and viral infections.23,53 For instance, mRNA is used in cancer immunotherapy to engineer T cells and natural killer (NK) cells with antigen receptors and as a template for immunologically active proteins in various immune and non-immune cells.23 mRNA therapeutics also have the potential to treat genetic disorders by providing cells with functional copies of genes that are missing or non-functional. Researchers have already demonstrated the ability to use mRNA to correct genetic mutations in animal models of certain diseases, such as inherited retinal diseases. They are working on advancing these therapies to clinical trials.54 mRNA therapeutics have several advantages over traditional drugs. Because mRNA does not integrate into the genome, it does not have the potential to cause permanent genetic changes.55 Moreover, mRNA can be easily synthesized, thus allowing the rapid development and production of new therapeutics. Despite these advantages, some challenges still need to be overcome to fully realize the potential of mRNA therapeutics. One of the main challenges is ensuring mRNA's safe and effective delivery to the targeted cells. Researchers are developing various delivery methods, such as NPs and viral vectors, to overcome this challenge.56 Furthermore, mRNA therapeutics are relatively new, and more research is needed to fully understand their safety and efficacy.55 Overall, mRNA therapeutics represent a promising new approach to treating and preventing various diseases and conditions. With continued research and development, mRNA therapeutics have the potential to transform the way we think about medicine and improve the lives of millions of people worldwide.
2.6 Cytosine-phosphate-guanine (CpG) DNA
Cytosine-phosphate-guanine (CpG) is a synthetic oligonucleotide studied as a therapeutic agent for several undruggable conditions. CpG activates the immune system by binding to Toll-like receptor 9 (TLR9), expressed in immune cells such as dendritic and B cells.57 Consequently, these cells are activated, and an immunological response is initiated. Preclinical investigations demonstrated that CpG has antitumor properties and enhances the immune response to cancer cells, increasing tumor cell death.24,58 It has been reported that CpG has anti-inflammatory properties, making it a suitable candidate for treating autoimmune illnesses such as rheumatoid arthritis and multiple sclerosis.59 CpG has been shown to enhance the immune response to viral infections, including influenza and herpes simplex virus, HIV,60,61 hepatitis B and C,62,63 and bacterial infections such as Streptococcus pneumoniae.25,64,65 In addition, CpG has been studied as a potential treatment for allergies. In preclinical studies, CpG has been found to reduce the severity of allergic reactions and is currently being investigated as a treatment for allergies such as asthma and allergic rhinitis.66–68 Overall, CpG is a promising therapeutic agent with many potential applications. However, additional research is required to completely comprehend its mechanism of action and determine its human safety and efficacy.3. Classification of spherical particles/nanoparticles for potential complexation with nucleic acids
The development of NPs provides diverse platforms for delivering drugs with enhanced effects. However, it is reported that NP morphology (viz., shape and size) can directly affect their response in biological systems.69 Amidst them, spherical NPs such as metallic NPs, polymeric NPs, protein, nucleic acids, and lipid-based complexes having a shape in common are the most promising drug delivery agents and therapeutic probes. It has been reported that under in vivo conditions, the NPs behave differently from biomolecules in therapeutic applications, particularly in vaccine delivery.70 (1) The larger surface area to volume ratio and permeability of these nanocomplexes allow efficient and targeted delivery of the cargo (DNA/RNA etc.) and reduce unwanted side effects.71 (2) Nanocarriers can be tailored to slowly release loaded their cargo, providing controlled and sustained release within the targeted tissue or organ. (3) In biological systems, small molecules face challenges such as degradation by enzymes and other biological processes. Nanostructures can encapsulate the therapeutic molecules, protecting them from degradation and ensuring their effective and targeted delivery. This is especially important for fragile molecules like DNA and RNA used in nucleic acid vaccines.72 (4) Naked DNA or RNA can sometimes trigger unwanted immune responses. Nanocarriers can shield the nucleic acids, overcoming such reactions and promoting a more focused immune response toward the target antigen.73,74 Overall, under in vivo conditions, nanostructures provide distinct advantages over small molecules. Their ability to deliver drugs directly to target sites, offer controlled release, and protect sensitive molecules makes them a powerful tool for improved treatment efficacy and reduced side effects.Table 2 shows the merits and demerits of these drug delivery vehicles. One can choose the ideal carrier to deliver particular drugs/genes based on these advantages and disadvantages. A perfect carrier transports the drug to its target site and releases it at the site of action. Several conditions must be addressed, including specific and selective interactions, sufficient drug delivery, and sustained drug release at the targeted area. For example, nanoparticles can be made from various materials to encapsulate hydrophobic and hydrophilic drugs with increased stability. Similarly, polymeric NPs can be used to encapsulate hydrophobic and hydrophilic drugs and can be designed to release drugs in a controlled manner over time. Lipid-based drug delivery agents can encapsulate hydrophobic drugs, protecting them from degradation and helping them reach the targeted area in the body.
| S. no | Delivery agent | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| 1 | Inorganic NPs | ✓ Ease of synthesis and surface functionalization | • Intracellular toxicity | 75 |
| ✓ Enhanced intracellular uptake | • Rapid elimination by the reticuloendothelial system | |||
| ✓ Able to entrap both hydrophilic and hydrophobic drugs | • Lack of clinical trials | |||
| ✓ Enabled active and passive targeting | • Poor biodegradability | |||
| ✓ Photoluminescence properties | • May trigger the immune system | |||
| ✓ Stability (w.r.t. wide range of pH and temperatures) | ||||
| Nobel metal (AuNPs, AgNPs) NPs | ✓ Strong biocompatibility | • Expensive for large-scale applications | 76 | |
| ✓ Tunable optical properties | • Potential toxicity concern | |||
| ✓ High surface-to-volume ratio | • Stability issues due to aggregation at elevated temperatures | |||
| ✓ High binding affinity | ||||
| Quantum dots (QDs) | ✓ High quantum yield | • High cytotoxicity | 77 | |
| ✓ Resistance to photobleaching as compared with traditional organic dyes | • Exponential decrease in fluorescence and blinking of different QDs | |||
| ✓ Size-dependent emission wavelength from visible to NIR region | • Instability and elevated hydrodynamic diameter on interaction with serum proteins | |||
| Silicon NPs (SiNPs) | ✓ Good biocompatibility and biodegradable | • Low quantum yield | 78 | |
| ✓ Tunable optical properties | • Potential toxicity concerns | |||
| ✓ Ease of functionalization | ||||
| ✓ Thermal stability | ||||
| Carbon nanomaterials (CMs) | ✓ Tunable photoluminescence | • Toxicity concerns due to ROS generation, inflammation, DNA damage | 79 | |
| ✓ Photostability | • Limited understanding of the long-term effect on human health | |||
| ✓ Ease of fabrication | • Limited control over size and structure | |||
| ✓ Economical | ||||
| ✓ Eco-friendly, and biocompatible | ||||
| Iron oxide NPs | ✓ Biocompatibility | • Toxicity concerns | 80 | |
| ✓ Magnetic properties | • Stability issues due to aggregation | |||
| ✓ High surface-to-volume ratio | • Long-term effects | |||
| ✓ Tunable properties | • Costly production | |||
| 2 | Polymeric NPs | ✓ Biocompatible and biodegradable | • Burst effect | 81–83 |
| ✓ Able to entrap both hydrophilic and hydrophobic drugs | • Limited drug loading | |||
| ✓ Ease of surface functionalization | • Deep knowledge of polymer–receptor molecular interactions required | |||
| ✓ Controlled and sustained release | • Limited knowledge about long-term side effects | |||
| ✓ Protect the drug from metabolic degradation | ||||
| ✓ Prolonged residence time | ||||
| 3 | Dendrimers | ✓ Increasing solubility of highly lipophilic drugs | • Not a suitable candidate carrier for hydrophilic drugs | 84 |
| ✓ Tunable physicochemical properties | • Cellular toxicity | |||
| ✓ Ease of surface functionalization for targeted drug delivery | • Elimination and metabolism depend on the generation and materials | |||
| ✓ Covalently associating drugs | • High cost for their synthesis | |||
| 4 | Nanofibers | ✓ Large surface area | • Toxicity | 85 and 86 |
| ✓ Biocompatibility | • Degradation | |||
| ✓ Encapsulation and targeted delivery | • Expensive for large-scale production | |||
| ✓ Tissue engineering | ||||
| 5 | Proteins/peptides | ✓ Various anticancer effects | • Size influenced pharmacokinetics | 83 |
| ✓ High cell permeability | • Short half-life | |||
| ✓ Low systemic toxicity | ||||
| ✓ Improved target selectivity | ||||
| ✓ Bypass biological barriers | ||||
| 6 | SNAs | ✓ High specificity and potency | • Low yield and high cost of synthesis | 87 and 88 |
| ✓ High drug loading efficiency | • Low stability w.r.t. temperature and UV exposure | |||
| ✓ Biocompatibility and prolonged circulation time | • Limited targeting ability | |||
| ✓ Dense packing and delivery of therapeutics | • Limited knowledge about their behavior in in vivo systems | |||
| ✓ Resistant to nuclear degradation | ||||
| ✓ Effective gene regulation | ||||
| 7 | Liposomes | ✓ Biocompatible and biodegradable | • Poor stability | 83 |
| ✓ Able to entrap both hydrophilic and hydrophobic drugs | • Short shelf life and instability in circulation | |||
| ✓ Controlled release | • A special storage system is needed | |||
| ✓ Protect the drug from metabolic degradation. Prolonged circulation time | • Batch-to-batch variation in the size of liposomes | |||
| ✓ Low systemic toxicity | ||||
| 8 | Lipid-based nanocomplexes (LNPs and SLNs) | ✓ Production on a large-scale industry level | • Limited drug loading for SLNs | 84 |
| ✓ Easy sterilization | • Instability in the bloodstream | |||
| ✓ Able to entrap both hydrophilic and hydrophobic drugs | • SLNs tend to gelation | |||
| ✓ Able to target specific cells or tissues | • Polymorphic transition for SLNs | |||
| ✓ Modulated and controlled release | • Particle size growth and drug expulsion during storage | |||
| ✓ Low toxicity due to their biocompatible and biodegradable components and the absence of organic solvents in their process | ||||
| ✓ Protecting drugs from environmental conditions | ||||
| ✓ Low cost compared with liposomes |
3.1 Inorganic NPs
Inorganic NPs are vital in biomedical applications, viz., bioimaging, therapy, diagnosis, and drug delivery.89,90 Inorganic NPs are mainly metal oxides, semiconductors, and noble metals with sizes ranging from 1–100 nm. These NPs possessed intrinsic physical, optical, magnetic, and electrical properties due to the characteristics of the core material. Additionally, these properties can be controlled by tailoring the size, shape, structure, and composition to achieve enhanced sensing and therapeutic effects.91 NPs gain positive responses as nanocarriers because of their tunable properties, improved efficacy, and decreased side effects by boosting their targeting ability.92,93 The loading can be done via four methods: physical adsorption, electrostatic interaction, encapsulation inside the NP core, or covalent binding. For example, gold NPs (AuNPs) are well known for their therapeutic and delivery capabilities due to their stability, inertness, high binding affinity to thiols, amines, and disulfides, and ease of functionalization with ligands such as drugs, proteins, and nucleic acids. Excellent fluorescence due to the surface plasmon resonance effect resulted in absorption in the visible and NIR regions. Therefore, it is essential in diagnosis and sensing applications.94 Also, depending upon the shape and size of the AuNPs, the free electrons on the surface of AuNPs oscillate continually, granting them photothermal properties.95,96 Thiolate NAs can be easily conjugated to AuNPs via covalent and electrostatic interactions between sulfur and gold.9 Gracezyk et al. (2021) developed an AuNP carrier to deliver siRNA by functionalizing AuNPs with a thiol-modified tectoRNAs trimer (structural RNA). They applied this conjugate to regulate the CopGFP expression in MDA-MB-231 GFP/RFP cells (Fig. 1a).97 The cellular uptake of AuNP: to tectoRNA conjugate was determined by TEM studies, showing their presence in the cytoplasm both inside the cytosol and membrane structure. Also, the prepared AuNP:tecto RNA conjugates effectively regulated gene expression, as demonstrated by GFP expression studies using fluorescence techniques. Kyriazi et al. (2018) synthesized a DNA–AuNP dimer-based multifunctional platform for mRNA sensing and targeted drug delivery.98 The synthesized DNA–AuNP dimer specifically recognizes keratin 8 and vimentin mRNAs in keratin 8-expressing 16HBE (epithelial cells), vimentin-expressing MRC 5 (mesenchymal cells), and A549, showing the expression of both keratin 8 and vimentin respectively. Furthermore, two anticancer drugs, doxorubicin (Dox), which detects keratin 8 mRNA, and mitoxantrone (MXT), which detects vimentin mRNA, were intercalated into a DNA duplex, resulting in cell death in response to specific mRNA signatures. Contrary to this, the scrambled NPs, without having recognition sites for targeted mRNA, failed to release the loaded drugs (Fig. 1b).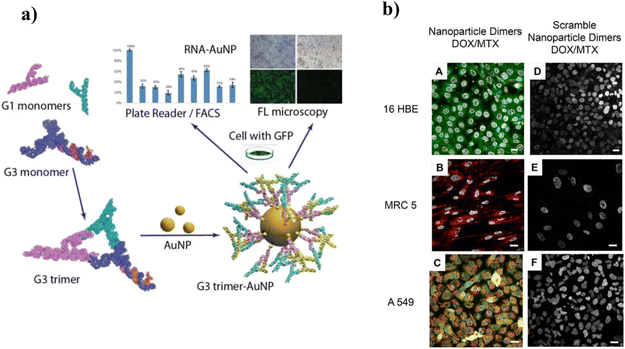 | ||
| Fig. 1 (a) Impact of AuNP:structural RNA complex on MDA-MB-231 GFP/RFP cells. In vitro studies show the regulation of CopGFP expression in the targeted cells. Adapted with permission from ref. 97. Copyright 2021 ACS Chemical Society. (b) Confocal microscopy images of cells incubated with (A–C) nanoparticle dimers and (D−F) “non-targeting” scrambled nanoparticle dimers. Sixteen HBE cells express only keratin 8, and MRC 5 cells express only vimentin. A549 expresses both keratin 8 and vimentin. A fluorescence signal corresponding to the presence of keratin 8 mRNA (A), vimentin mRNA (B), and both vimentin and keratin 8 mRNA (C) are observed. When incubated with nanoparticle dimers designed with “non-targeting” scramble sequences, all three cell lines display no response. Adapted with permission from ref. 98. Copyright 2018 ACS Chemical Society. | ||
Another class of inorganic NPs includes semiconductor quantum dots (QDs) due to their nanosize showing size-tunable 3D quantum confinement effects, thus giving rise to exquisite optical properties.99–101 QDs are typically made of semiconducting materials such as group II–VI elements (CdS, CdSe, CdTe, ZnS, ZnSe, ZnTe), group III–V elements (InP or InAs), group I–III–VI2 elements (CuInS2, AgInS2), group IV–VI elements (PbS, PbSe, PbTe), or group IV elements (C, Si, Ge).102–105 QDs are important fluorophores as they provide emission in the UV, visible, and near-infrared ranges with an impressive quantum yield compared with traditional organic dyes, with drawbacks including photobleaching and poor signal intensity.106,107 All these properties of QDs make them suitable probes for optical bioimaging and targeted molecular sensing. For instance, Ma et al. (2019) designed a CdTe:Zn2+ QD nanobeacon conjugated with black hole quencher (BHQ1) and phosphorothioate co-modified DNA by hydrothermal synthesis for single RNA detection and imaging (Fig. 2a(A)).108 This nanobeacon was highly sensitive and efficiently detected low-abundance nucleic acids in live cells via FRET. QDs functionalized with BHQ1, and single DNA were applied to detect and image single HIV-1 RNAs in live HIV-1 integrated cells (Fig. 2a(B, C)). Similarly, gene silencing in tumor cells of the central nervous system was done using siRNA-loaded polyethylenimine (PEI) functionalized CdSSe/ZnS QD-based nanocarriers.109 siRNA-loaded PEI-CdSSe/ZnS QDs efficiently target human telomerase reverse transcriptase (TERT). Two glioblastoma cell lines, U87 and U251, after transfection showed a decrease in gene and protein expression levels of TERT with a high level of gene transfection efficiency within 48 h.
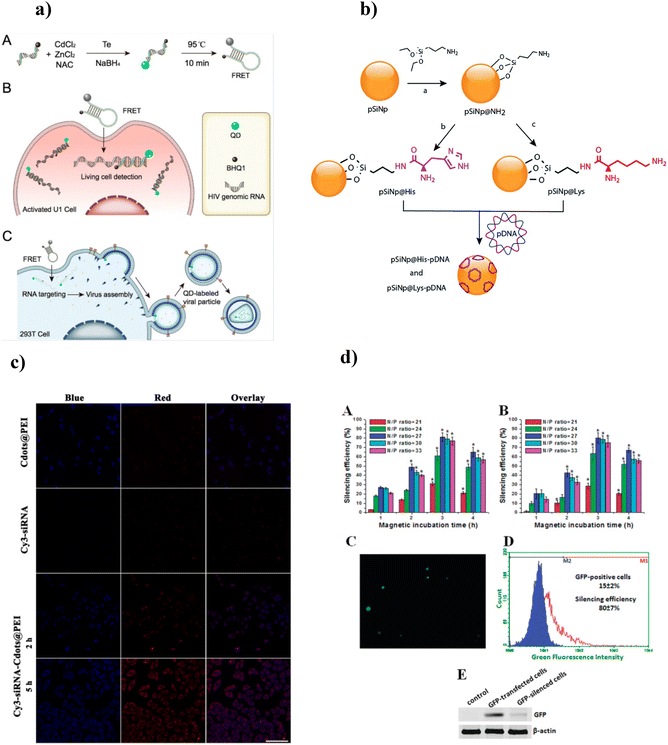 | ||
| Fig. 2 (a) Schematic illustration of QD-NBs for HIV-1 genomic RNA detection. (A) Schematic diagram of the QD-NBs’ preparation; schematic illustration of QD-NBs for (B) HIV-1 genomic RNA detection in living cells, and (C) fluorescence labeling of single virus particles. Adapted with permission from ref. 108. Copyright 2019 American Chemical Society. (b) Reaction scheme for the chemical functionalization of SiNPs with histidine and lysine and the complexation of pDNA. Adapted with permission from ref. 114. Copyright 2019 Royal Society of Chemistry. (c) Confocal laser scanning microscopic images of MGC-803 cells incubated with Cdots@PEI or Cy3-siRNA for 2 h, or Cy3-siRNA-Cdots@PEI complexes for 2 and 5 h. Adapted with permission from ref. 117. Copyright 2014 Springer Nature. (d) GFP silencing by PEI-coated SPMNs/GFP shRNA in GFP-transfected 3D cell cultures. Adapted with permission from ref. 123. Copyright 2010 American Chemical Society. | ||
The rapid development of silicon nanostructures provides a potential class of sensitive sensors and therapeutic agents for real-time diagnosis and therapeutic applications.110–112 Silicon NPs (SiNPs) are indirect band gap semiconductors and thus exhibit longer excited-state lifetimes; after entering the cellular system, SiNPs are biodegraded into silicic acid (nontoxic compound) and easily excreted out of the body without showing any sign of toxicity.78,113 Chaix et al. (2019) reported amine-functionalized porous SiNPs for the loading and improved delivery of pDNA114 (Fig. 2b). In vitro studies suggested that the SiNPs showed better biocompatibility and successful transfection of up to 107 RLU mg−1 proteins in HEK 293 cells.
Carbon nanomaterials (CNMs), also called green NMs, are admirable fluorescent NMs with fascinating characteristics such as tunable photoluminescence, photostability, ease of fabrication, economical production, eco-friendliness, and biocompatibility.115 In contrast to QDs, the mechanism behind the fluorescence property of CNMs involves π-plasmon and surface defects generated from radiative recombination of the surface-confined electrons and holes.116 Wang et al. (2014) synthesized fluorescent carbon dots (CDs) for simultaneous imaging and efficient siRNA delivery for cancer therapy.117 PEI-functionalized CDs were applied for the adsorption of survivin siRNA. After transfection for two hours with siRNA-loaded CDs/PEI complexes, MGC-803 gastric cancer cells showed blue fluorescence in the cytoplasm, thus suggesting the internalization of loaded siRNA into the cancerous cells (Fig. 2c). Furthermore, the expression of survivin mRNA in MGC-803 cells was downregulated to 96.4 ± 8.7 when exposed to siRNA-loaded CD/PEI complexes. Iron oxide NPs (IONPs), with their remarkable superparamagnetic properties and excellent surface-to-volume ratio, have attracted worldwide interest.80 Magnetic NPs are widely used for magnetic particle imaging, magnetic resonance imaging, hyperthermia, cell tracking, targeted genes, and drug delivery.118,119 Iron oxide NPs are comparatively less toxic and cytocompatible, thus allowing clinical translation. IONPs are usually synthesized from iron oxides such as maghemite (γ-Fe2O3) and magnetite (Fe3O4), metal alloys (e.g., FeCo and FePt), as well as the doping of magnetically susceptible elements (e.g., MnFe2O4 and CoFe2O4).120,121 Efficient gene (pDNA) delivery to mesenchymal stem cells was accomplished using positively charged, PEI-coated IONPs.122 The uniform and narrow-sized (15 nm) IONPs exhibit good magnetic properties. In vitro studies suggested that IONPs under the external magnetic field efficiently delivered 99% of the loaded pDNA within 30 min, followed by nuclear importing of the carried genes, resulting in enhanced gene expression of the treated cells. The caveolin-mediated pathway facilitated the internalization of pDNA-loaded IONPs into the cytoplasm. Zhang et al. (2010) designed PEI-coated IONPs and applied them to deliver interfering RNA (siRNA) GFP plasmid via an external magnetic force in 3D cell culture (NIH 3T3 cells).123 The NPs facilitated 64% and 77% transfection efficiency for siRNA and GFP plasmid, respectively. These transfection complexes significantly reduced the GFP-expressed cells’ growth, with 80–82% silencing efficiency. Furthermore, these complexes delivered four toxic shRNA to the 3D cell culture, enhancing cell death (41–51%) (Fig. 2d).
Other commonly used inorganic NPs include silica NPs, which are hydrophilic and porous, have access to surface functionalization due to silane groups, and are widely used for drug delivery, biomolecule conjugation, and many more applications.
However, for in vivo applications, the toxicity of these inorganic NPs is an inevitable issue among researchers. Additionally, the cellular uptake, circulation, and clearance of NPs depend upon the physicochemical properties of the NPs. After entering the bloodstream, NPs come across the biological barrier composed of lipids, proteins, and other components. The interaction of NPs with biomolecules resulted in the formation of biomolecule coronas around the NPs before reaching the target site, thus influencing the biological fate of the NPs.100 To circumvent these issues, researchers developed biocompatible surface modification approaches to inorganic NPs to avoid or decrease the nonspecific interactions of these NPs with biomolecules and increase prolonged accumulation at the site of interest.
3.2 Polymeric NPs
Polymeric nanoparticles have shown great potential in recent years due to their small size, ranging from 1–1000 nm. Polymeric nanoparticles are mostly spherical with a solid structure. The main advantages of polymeric NPs (PNPs) include (1) the high molecular weight and polyvalent nature of these molecules, which enable the encapsulation and delivery of bulky and long-chain NAs,124 (2) biocompatibility and biodegradability: PNPs easily break down inside the body into water and carbohydrates and hence can be quickly eliminated from the body,125 (3) facilitation of successful loading and controlled drug delivery,126,127 (4) retention of the bioactivity of drugs or biomolecules by evading the immune system, thus enhancing their bioavailability and therapeutic potential, and (5) the provision of a platform for ligand functionalization, resulting in the targeted and stealthy delivery of drugs.96 Polymeric nanoparticles can be synthesized by natural and synthetic polymers.128 Natural polymers, namely chitosan, hyaluronic acid, starch, alginate, cellulose, and lignin, and synthetic polymers, viz., polylactide-co-glycolide (PLGA), polylactides (PLA), polyethylenimine (PEI), polyethylene glycol (PEG), polycaprolactones, and polyacrylates, have been widely explored for the synthesis of PNPs.125,126,128 The most common polymeric NPs include nanocapsules (polymeric capsules surrounding a cavity) and nanospheres (solid matrix). For instance, a nanovehicle based on cationic cyclodextrin-polyethyleneimine 2k conjugate delivered mRNA encoding HIV glycoprotein120 for treating HIV-1.129 The delivery system enhanced the intranasal delivery of mRNA by crossing the nasal epithelial barrier through intracellular pathways, thus resulting in a solid anti-HIV immune response. Biodegradable chitosan-alginate 3D porous injectable gel was designed for in vivo mRNA vaccine delivery.130 Increased levels of IFN-α secretion, luciferase reporter protein expression, and T-cell proliferation were observed from mRNA lipoplex-loaded gel scaffolds compared with the systemic injection of naked mRNA and mRNA:lipoplex.A nanovehicle based on PLGA-encapsulated antisense microRNA-21 (miRNA-21), known to be overexpressed in glioblastoma cells, was applied for the improved therapeutic effect of temozolomide (TMZ) on glioblastoma cells (Fig. 3a).131 Enhanced drug delivery and sustained gene silencing of miRNA 21 were observed in glioblastoma cells, namely U87 MG, LN229, and T98G cells. This nanovehicle also showed a significant decrease in cell viability (p < 0.001) with a 1.6-fold increase in cell arrest at the G2/M phase in the TMZ-treated cells (Fig. 3b–d). Furthermore, the intracellular co-delivery of the nanocomplex and TMZ in glioblastoma cells resulted in a 67% and 15% enhancement in the expression of miRNA-21 targeted phosphatase and tension homologue (PTEN) genes and apoptosis-associated caspase-3 respectively (Fig. 3e). The PLGA–SNA complex was designed to accommodate the chemotherapeutic drug coumarin spatially and nucleic acid to independently improve the controlled loading and tunable release of encapsulated moieties.132In vitro studies performed on RAW blue cells to confirm the immunotherapeutic response of PLGA–SNAs depicted dose- and time-dependent activation of TLR9. PLGA–SNAs were cytocompatible at concentrations ranging from 10 × 10−9 M to 2 × 10−6 M. Also, the cellular uptake efficiency of PLGA–SNAs was tenfold higher than their linear counterpart and at a shorter time of 0.5 h. However, PNPs also suffer from drawbacks, including toxicity and a high risk of particle aggregation. The FDA approves very few PNP-based drugs for clinical applications.133
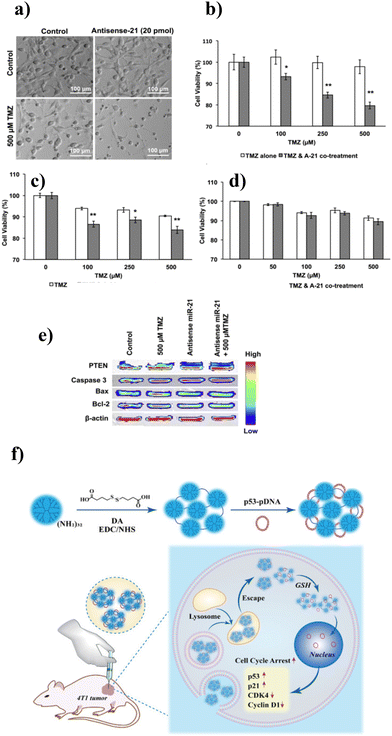 | ||
| Fig. 3 (a) Phase contrast microscopic images of control and antisense miR-21 transfected cells treated with 500 μM TMZ. In vitro cell viability MTT assays on control and antisense miR-21 transfected (b) U87 MG cells, (c) LN 229 cells, and (d) T98G cells treated with different concentrations of TMZ. (e) Cellular pathway analysis of antisense miR-21 and TMZ co-treatment on U87 MG cells. Adapted with permission from ref. 131. Copyright 2015 American Chemical Society. (f) Schematic representation of PAMAM dendrimer/pDNA-p53 nanocarrier preparation for cancer gene therapy. Adapted with permission from ref. 139. Copyright 2021, American Chemical Society. | ||
Dendrimers or “dense stars” are globular, hyperbranched 3D structures, and their physicochemical properties can be controlled for the desired application. Dendrimers can be synthesized by divergent or convergent techniques.134,135 The remarkable properties of dendrimers make them suitable vehicles for the delivery of nucleic acids. For instance, the polycation nature of dendrimers provides multiple sites for the electrostatic binding of the negatively charged phosphate backbone of NAs, resulting in solid DNA complexation. Also, the formation of the dendrimer–NA complex protects against nuclease degradation of the attached NAs. It is speculated that the presence of tertiary amines in the structure of dendrimers enhanced the cellular uptake and delivery of NAs through endosome escape via the sponge effect.136 Other merits include the attachment of biomolecules or ligands to the functional groups available on the exterior surface. In contrast, drugs and small cargo can be encapsulated in the interior of the dendrimer.133 Various dendrimers are studied for the delivery of NAs, such as poly L-lysine, triazine, polyglycerol, poly(propyleneimine), and poly(amidoamine) (PAMAM) based dendrimers. At the same time, PAMAM is the most investigated nanocarrier because of facile synthesis and functionalization.137 Palombarini and coworkers (2021) demonstrated the targeted delivery of miRNA to myeloid leukemia cells (which are otherwise challenging to transfect) by incorporating nucleic acid with ferritin-poly(amidoamine) (PAMAM) dendrimer NPs.138 The cellular internalization of this nanocomplex drives morphological changes and enhances the expression of the retinoic acid receptor, alpha (RARα), an early hallmark of granulocytic differentiation. Mekuria et al. (2021) developed a nanocarrier by covalently binding PAMAM dendrimer with 4,4′-dithiodibutryic acid (DA) to successfully deliver p53-pDNA. The nanocarrier reflected a 2.3- and 2.1-fold increased gene transfection in 4T1 and mouse breast cancer cells.139 Furthermore, in vitro and in vivo studies showed an upregulated expression of mRNA and p53 and p21 protein and downregulation of cyclin-D1 and CDK-4 protein, thus facilitating cell cycle arrest in the G1 phase (Fig. 3f).
Dendrimers are ideal for delivering NAs with enhanced stability and improved cellular internalization compared with other therapeutics. However, regarding the safety concerns, the toxicity of these dendrimers is still unaddressed. The strong cationic amine groups on the surface of dendrimers resulted in solid binding with the negatively charged cell membrane, which led to destabilization, disruption of cell components, and eventually lysis.140
Nanofibers are a promising class of biomaterials for nucleic acid delivery, offering several advantages including a large surface area, biocompatibility, encapsulation and targeted delivery (gene, growth factors, protein, and peptide delivery), and scaffolding for tissue engineering.85 Nanofibers can be synthesized by different methods, namely, phase separation, electrospinning, and physical and chemical fabrication. Various natural, semisynthetic, and synthetic polymers are widely used for their synthesis. Furono et al. (2022) reported plasmid DNA delivery using horseradish peroxidase cross-linked gelatin nanofibers.141 Nanofibers immobilized with Lipofectamine/pDNA resulted in the transfection of pDNA delivery in HEK293 cells. Furthermore, genome-editing molecules including Cas9 protein and guide RNA (gRNA) were expressed in nanofiber-treated HEK293 cells, resulting in gene knock-in and knock-out. Polycaprolactone nanofiber-encapsulated siRNA showed controlled release for up to 28 days with successful transfection of the treated HEK 293 cells.142 The cells showed enhanced cellular uptake and GAPDH gene silencing of 61–81%. Nanofibrous scaffolds made up of collagen type 1 were used for the controlled and long-term delivery (at least 5 months) of siRNA/silica NPs.143In vivo studies revealed that the nanofiber-based scaffolds showed more effective gene silencing (p < 0.05) as compared with traditional bolus delivery. An in vivo biodistribution study revealed that siRNA stayed confined up to ∼290 μm from the implants. As compared with negative scrambled siRNA therapy, a reduction in fibrous capsules of ∼45.8% was observed after 4 weeks.
Along with the advantages, nanofibers do have some drawbacks that researchers are working to overcome.86 (1) Nanofibers may have inherent toxicity or cause inflammatory responses in the body. (2) Depending on the material, nanofibers may degrade too quickly or too slowly, affecting the release profile of the drugs they carry. (3) Manufacturing nanofibers for large-scale clinical use can be complex and expensive.
3.3 Proteins/peptides
Proteins, a class of natural biomolecules, stand out as an attractive biocompatible substitute for synthetic polymers in nanomedicine due to their biodegradability, natural abundance, mild synthesis, and fast metabolization.144,145 Additionally, proteins showed remarkable chemical modification properties due to their surface abundance of functional groups such as carboxyl, amine, and hydroxyl. The amphiphilic nature makes them amenable for small molecules, metallic NPs, and hydrophobic and hydrophilic drugs.145,146 Protein-based NPs, when administered inside the body, showed weak immunogenicity, were efficiently degraded by the enzymes and were eliminated through hepatic clearance. Abraxane is an FDA-approved drug composed of paclitaxel-bound albumin-based NPs used to treat metastatic breast cancer by inhibiting the mitosis of cancer cells.147,148 The drug avoids hypersensitivity due to albumin protein, a major drawback of traditional anticancer drugs. Protein NPs can be easily synthesized via electrospray, emulsion, and desolvation processes using natural proteins, namely albumin, fibroins, 30Kc19, gelatin, lipoprotein, legumin, zein, gliadin, and ferritin proteins.144,149Peptides are short chains of amino acids linked by a covalent amide bond (peptide bond). They exhibit remarkable sequence and functional diversity, merits employed to design spherical NPs via a self-assembly approach.150 Several peptides are currently used for biomarker imaging, targeted drug and gene delivery, bioprinting, wound healing, and tissue engineering.151,152 Different peptides such as nuclear localization, tumor-targeted, and cell penetration peptide sequences are extensively reported for biomedical applications. Jia et al. (2020) reported enhanced siRNA delivery and improved gene silencing using hyaluronic acid (HA)-modified transmembrane peptide octa-arginine (R8)-based R8-bipolar (HA-bibola/siRNA) and R8-monobola (HA-bola/siRNA) amphiphilic nanocomplexes.153 Cell viability studies reflected better cytocompatibility of HA-bibola/siRNA compared with HA-bola/siRNA and control samples (PEI/siRNA) in 4T1 cells. Additionally, HA-bibola/siRNA showed enhanced cell uptake efficiency and down-regulation of Bcl-2 protein expression due to the presence of cell-penetrating peptides on the surface of the nanocomplex. In vivo, studies reflected higher antitumor efficacy, improved targeted ability, and increased Bcl-2 gene suppression in 4T1 tumor-bearing Balb/c mice.
3.4 Spherical nucleic acids (SNAs)
The primary function of nucleic acids includes storing and transduction of genetic information. Nucleic acid-based (DNA, RNA, CRISPR/Cas9 gene editing system) spherical NPs hold great significance in the biosensing, therapy, and silencing of various diseases ranging from viral infections to neurological disorders, cardiovascular diseases, and cancer by taking advantage of cellular pathways.154–156 In the present era, therapeutic technologies related to nucleic acids are at the forefront of fighting the COVID-19 pandemic around the globe.157,158 They consist of highly compact self-assembled oligonucleotide layers oriented in 3D geometries based on typical Watson–Crick base-pairing.119,120 Selective and precise base-pairing of DNA/RNA NPs differentiates them from traditional biomolecules, which might be otherwise difficult to design with such ease.159 Various chemical modifications (nucleotide sequence, sugar, or phosphate backbone modification), biocompatibility, and programmable therapeutic approaches are other fascinating properties of these nanostructures.160 Most nucleic acid-based NPs interact via complementary base pairing with their target molecules, thus resulting in specific and rapid action.161 Spherical nucleic acids (SNAs) comprise (1) the outer shell of densely packed nucleic acid radially encasing the contents, and (2) an inner core of NPs. They were initially made of DNA shells and AuNP cores.161,162 Since then, various inorganic nanoparticles such as Ag,163 QDs, magnetic NPs,164 silica,165 organic nanocomposites such as polymers, proteins, and liposomes,166,167 and hybrid structures of inorganic–organic materials were used to explore different biomedical applications. The three-dimensional architecture of SNAs provides them with unique physicochemical properties and makes them superior to their linear counterparts.87 The spherical shape allows the dense packing of the oligonucleotide into a limited space.168 This compact structure of SNAs leads to an enhanced electric charge, increasing the stability of the SNAs and providing resistance to nuclease degradation and prolonged cell accumulation via scavenger receptor engagement and endocytosis.169 SNAs can easily evade the immune system's attack due to strong electric charges because of the dense packing. Also, the interaction of SNAs with receptors on the cell surface resulted in easy penetration through the cell, tissue membranes, and even the blood–brain barrier.170 Melamed et al. (2018) suggested that the spherical architecture of SNAs functionalized with polyethylenimine has improved siRNA-mediated GFP gene silencing 10-fold.171 Wang et al. (2019) reported an SNA-based vaccine for treating a mouse tumor.172 SNA co-delivered CpG oligonucleotide (adjuvant) and peptide (antigen) to generate an antitumor immune response. The results suggested that the vaccine could increase the survival rate to 31 days, also delaying tumor growth by 15 days. SNAs efficiently promote gene regulation for the treatment of skin diseases.173 Randeria et al. (2015) showed the application of siRNA-encapsulated SNAs in impaired wound healing by downregulating ganglioside GM3 synthase (GM3S) in diabetic mice.174 The expression of GM3S was reduced by >80% at the wound site via the siRNA pathway, and the wound was observed to heal within 12 days in the SNA-treated mice.174Although nucleic acid-based drugs are in the infant stages of clinical trials, they have revolutionized therapeutics’ fate in recent years. Despite the remarkable merits of SNA-based therapeutics, challenges still hinder their further clinical and translational applications.
4. Classification of lipid nanocomplexes for efficient delivery of NAs
The clinical application of NPs depends on their successful delivery to the disease site. The effectiveness of synthesized NPs in treating diseases depends upon their therapeutic response, which can be achieved by delivering a required dose to the targeted site. During their journey to a target site, NPs must evade multiple biological barriers at different sites, including (1) phagocytosis by liver cells while NPs are circulating in the blood.175 (2) Even after reaching the target site, NP entry into the disease site is restricted by the endothelial cell walls. (3) The immune system also recognizes and destroys foreign components (NPs) and vectors (spherical DNA/RNA containing genetic information).176 (4) The internalization of NPs in the cell membrane or nucleus enhanced the effect of the former. The overall effect of these barriers restricted the penetration of most NPs into the targeted cell or organ and thus reduced their therapeutic efficiency. Recently, remarkable progress has been made in designing and developing delivery systems that enhance the therapeutic trajectory of drugs, inorganic NPs, and DNA/RNA-based genetic drugs.Lipid-based delivery cargos, namely micelles, liposomes, and lipid nanoparticles (LNPs), are promising delivery agents as they can protect nanomaterials from degradation and enhance circulation by avoiding early clearance by the immune system.177,178 Lipids are widely used nonviral delivery agents and are fascinating because of their easy synthesis, characterization, greater payload, homogeneity, biodegradability, and marginal toxicity profile. A broad range of lipid-based NPs is utilized to deliver NAs, including cationic lipids, ionizable lipids, zwitterionic lipids, LNPs, liposomes, and solid lipid NPs (SLNs) (Table 3).
| S. no | Type of lipid vehicle | Composition of vehicle | Cargo | Method of synthesis | Treatment | Ref. |
|---|---|---|---|---|---|---|
| 1 | Cationic | DOTAP/cholesterol | mRNA, pDNA, and oligonucleotide | Thin-film evaporation | Ovarian cancer | 179 |
| 2 | Cationic | 9322-O16B/Chol/DOPE | mRNA | Chemical | B-cell lymphoma | 180 |
| 3 | Cationic | DOPE-stearylated octaarginine (STR-R8), DOTMA-YSK05, cholesterol-GALA peptide | pDNA | Chemical | Efficient and selective delivery of pDNA to the lungs | 181 |
| 4 | Ionizable cationic | DLin-MC3-DMA/DLin-KC2-DMA/DODAP/DSPC/PEG-DSPE, cholesterol | siRNA | Microfluidic | pDNA transfection | 182 |
| 5 | Ionizable cationic | C-12-200 (IL)/DOPE, cholesterol/PEG-lipid conjugate | pDNA | Microfluidic | Cardiovascular diseases | 183 |
| 6 | Ionizable cationic | DLin-MC3-DMA, DSPC, cholesterol, DMG-PEG2K | mRNA | Chemical | Hepatic reticuloendothelial diseases | 184 |
| 7 | Ionizable | DSPC, CHO, DMG-PEG2000 | siRNA | Chemical | Hyperlipidemia | 185 |
| 8 | Zwitterionic | Phosphatidylcholine, DPPC, cholesterol | pDNA | Chemical | pDNA transfection | 186 |
| 9 | Liposome | DOTAP/DOPE/DSPE-PEG | 6-Carboxyfluorescein-labeled 14-mer oligonucleotide | Ethanol dilution | In vivo labeling of human microbiota | 187 |
| 10 | Cationic liposome | DPPC/DOTAP/cholesterol | GFP-mRNA | Lipid film hydration | Neurodegenerative diseases | 188 |
| 11 | Protamine liposome | DOTAP/cholesterol | mRNA | Chemical | Colorectal cancer gene therapy | 189 |
| 12 | Lipid nanoparticles | C-14-4 IL/DOPE/cholesterol/PEG | mRNA | Microfluidic | Engineering of CAR T cells to kill cancer cells | 190 |
| 13 | Lipid nanoparticles | DLin-MC3-DMA/DAP/phospholipid/cholesterol/PEG | Spherical DNA/RNA | Ethanol dilution | Organ-specific delivery of nucleic acid | 191 |
| 14 | Solid lipid NPs | DOTAP/lecithin/cholesterol/lipopolysaccharide | TNF-α siRNA | Ultrasonication | Rheumatoid arthritis | 192 |
| 15 | Solid lipid NPs | Stearic acid, soya lecithin | pDNA, Dox | Solvent displacement | Lung cancer therapy | 193 |
| 16 | Cationic solid lipid NPs | Peptide-cationic lipid CDO14 | siRNA, upconversion NPs | Thin-film dispersion | Bioimaging and gene therapy | 194 |
Lipid-based delivery agents, viz., micelles, liposomes, and lipid NPs, have been extensively studied to encapsulate and deliver NAs, primarily due to their ease of synthesis and interaction with NAs (Fig. 4a).195 The essential components of FDA-approved LNPs for NA delivery developed by pharmaceutical companies include cationic or ionizable lipids, cholesterol, a helper lipid, and a PEG-lipid (Fig. 4b and c), for example DLin-MC3-DMA, PEG: PEG-2000-C-DMG (Alnylam), SM-102, PEG-2000-DMG (Moderna), and ALC-0315(Pfizer/BioNTech) ALC-0159 (Pfizer/BioNTech/Acuitas), along with DSPC and cholesterol (Fig. 4d).
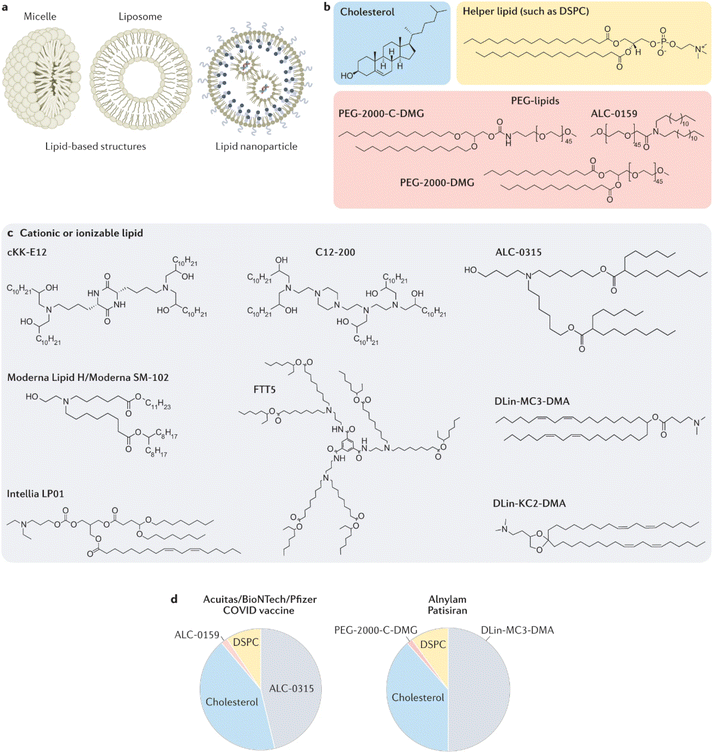 | ||
| Fig. 4 FDA-approved lipid-based structures contain some variation of the four essential components: cholesterol, a helper lipid, a PEG-lipid, and a cationic or ionizable lipid. (a) Lipid-based structures can include micelles, which consist of a lipid monolayer, or liposomes, which consist of a bilayer. Lipid nanoparticles comprise multiple lipid layers and lipid and nucleic acid microdomains. (b) and (c) In addition to the RNA payload, LNPs often consist of cholesterol, a helper lipid, a PEG-lipid (all shown in part b), and a cationic or ionizable lipid (part c). (d) The molar ratios of the four components constitute the FDA-approved Acuitas/BioNTech/Pfizer COVID-19 vaccine and patisiran, which delivers siRNA to the liver. Adapted with permission from ref. 195. Copyright 2022, Springer Nature. | ||
Moreover, lipid-based nanovehicles can be surface functionalized to achieve the targeted delivery and release of therapeutics in a specific tissue or cell.196,197 However, it is important to consider the chemical properties of the lipids used in NA delivery for efficient drug development. One of the critical parameters of lipids is their surface charge. Differently charged lipids can have varying degrees of compatibility with nucleic acids. In general, positively charged lipids tend to have a higher degree of compatibility with negatively charged nucleic acids, such as DNA and RNA, as electrostatic interactions between the positive charges on the lipids and the negative charges on the nucleic acids can help to stabilize the interactions between the two. Conversely, negatively charged lipids may have reduced compatibility with NAs, as the negative charges on the lipids may repel the negative charges on the NAs. Also, it is well known that the charge of NPs alters their tissue selectivity after intravenous administration. Taking advantage of this approach, different tissue-targeted LNPs have been designed by incorporating cationic or anionic lipids into the general composition of LNPs to tune their charge.198 For example, using zwitterionic lipids, which have both a positive and negative charge, has been shown to enhance the stability of NPs and improve in vivo efficacy.199 Additionally, using lipids with a high melting point can help improve the NPs’ stability and enhance their ability to penetrate cell membranes.200 Another critical factor to consider is the size of the NPs formed by the lipids. Smaller NPs have been shown to penetrate cell membranes more effectively and deliver NAs to target cells.201 However, it is also important to note that the size of the NPs should not be so small that they are rapidly cleared from the body by the immune system. Besides considering the lipids’ structural and chemical properties, the NPs’ composition is also an essential consideration for NA delivery.202 For example, using PEG as a surface coating on lipids has been shown to improve the stability of the particles and reduce their clearance from the body.203 Additionally, using other polymers, such as PEI or PLA, can also help improve the stability and efficacy of the lipid-based delivery agents. Overall, the design of lipids for NA delivery is a complex process that requires a thorough understanding of the structural, chemical, and composition-based factors that influence their efficacy. Through the use of structure–activity relationships and careful consideration of the properties of the lipids, it is possible to design nanoparticles that can effectively deliver NAs to target cells and improve the efficacy of gene therapy.
4.1 Cationic lipids
Cationic lipids (CLs) are well characterized by their positively charged hydrophilic head (such as quaternary ammonium, amines), linker (such as ester, ether, disulfide), and negatively charged hydrophobic tail (cholesterol, tocopherol). CLs are well-explored for the delivery of negatively charged nucleic acids. Cationic lipid, namely N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTMA) bearing a quaternary ammonium head group and unsaturated phosphatidylethanolamine (DOPE), was first employed for nucleic acid delivery for gene therapy by Felgner's group in 1987.204 The lipid formed is still commercially available with Lepofectin and is extensively used for the in vitro delivery of nucleic acids.205 CLs formed complexes with negatively charged phosphate groups in the polynucleotides’ DNA. The complexes are formed through positively charged head groups via electrostatic interactions, whereas hydrophobic tails exist as bilayers around DNA.206 This lipid envelope surrounding DNA provides resistance against omnipresent nucleases and overcomes immunogenicity and mutagenicity. Additionally, it can easily combine with other anionic sulfate groups of cell membrane proteoglycans, thus ensuring cell uptake. 1,2-Dioleoyl-3-(trimethylammonium) propane (DOTAP) and polyethylene-glycol-2000-1,2-distearyl-3-sn-phosphatidylethanolamine (PEG-DSPE) based cationic lipid was used to coat hydrophobic IONPs via self-assembly synthesis.207 The lipid-coated IONPs showed an average size of 46 nm and improved delivery in three cell lines: HeLa, PC-3, and Neuro-2a. Furthermore, in vivo, studies revealed that the growth status of the tumor cells in Balb/c mice was efficiently monitored through MR images, and the magnetic property of IONPs was retained up to 15 days after administration. Sun et al. (2022) demonstrated the efficacy of DOTAP/cholesterol-based CL NPs for successfully delivering mRNA, pDNA, and oligonucleotide.208 The nanocomplex was stable for up to 60 days at 4 °C storage conditions without affecting transfection efficacy. The effective DOTAP/cholesterol CL NP:mRNA ratio for in vitro studies was 62.5 μM lipid to 1 μg mRNA. It was speculated that a lower lipid concentration carried a lower surface charge, resulting in decreased cell interaction and endosome escape. In contrast, a higher concentration might lead to cytotoxicity and inhibition of mRNA dissociation from the nanocomplex.Based on the chemical structure of a positively charged head group, lipids can be classified into six classes, namely quaternary ammoniums, amines (primary to quaternary), amino acids or peptides, guanidiniums, heterocyclic headgroups, and some uncommon headgroups.177,209 Headgroups containing charge and dimensions impact cell interaction, endosome escape, and access to the target cell.210
Linkers typically comprise non-biodegradable (e.g., ethers and carbamates) and widely used biodegradable (e.g., esters, amides, and thiols) functional moieties. Linkers form a junction between the headgroup and tails.211 The linker affects the stability, biodegradability, cytotoxicity, and transfection efficiency of LNPs.
The negatively charged, lipophilic aliphatic chains of sterols form the last part of cationic lipids. The hydrophobic tails elicit NP formation and potency by maintaining fluidity, hydrophobicity, and fusion with the cell membrane.209
Nevertheless, despite the advantages CLs offer for the delivery of therapeutic cargo, researchers are motivated to continue their development and optimization in preclinical research. Their drawbacks include reduced in vivo efficacy, low circulation time, rapid elimination by RES, off-target accumulation in the negatively charged cellular system, and unacceptable toxicity related to inflammatory responses, which has challenged their clinical acceptance.212–214
4.2 Ionizable lipids
Ionizable lipids (ILs) or ionizable cationic lipids are small amphiphilic vesicles containing an ionizable protonatable tertiary-amino head group, a spacer/linker, and a hydrophobic moiety.215 ILs showed the unique feature of electrostatic charge dependency on the lipid pKa and pH of their environmental surroundings and, thus, become positively charged at acidic pH, ensuring the binding of therapeutic cargos while maintaining a neutral charge at physiological pH, reducing toxicity effects.216,217 ILs with a suitable pKa can modify their electrostatic charge to ensure prolonged circulation and cytosolic release of therapeutics, elevated transfection efficiency, access to targeted sites, and reduced toxicity. The transfection efficiency of ILs depends mainly on the pKa, with an optimal pKa value of 6.2–6.5.176,218Ionizable lipids can be categorized into various subclasses based on their structure.
(1) Unsaturated ILs: the degree of tail unsaturation significantly impacts the fluidity and delivery efficiency of ILs. The high unsaturation of lipid tails leads to nonbilayer lipid formation, enhancing membrane disruption and cargo release. Some common unsaturated ILs include DLin-MC3-DMA (MC3), A18-iso5-2DC18, OF02, etc.
(2) Polymeric ILs: the structural basis of polymeric ILs is the substitution of free amines on cationic polymers with alkyl tails, and their hydrophobic aggregation results in improved particle formation. Common examples include (1,2 bis(triclosan-10,12-diynoyl)-snglycero-3-phosphocholine) (DC8,9PC), 7C1, G0-C14 etc.
(3) Biodegradable ILs: the main objective of using different materials for biomedical applications includes improved biocompatibility and biodegradability with reduced toxicity and inappropriate accumulation inside the body.202 Adding biocleavable ester bonds (simple structure, chemical stability) to ILs provides biocompatibility via undergoing enzymatic hydrolysis in vivo. Maier et al. (2013) synthesized a library of biodegradable ILs by incorporating ester bonds at different lipid positions.202
Recently, anti-SARS-CoV-2 vaccines have also used ILs for their formation.219 The vaccines mainly comprise mRNA motifs and LNPs composed of pH-sensitive ILs. Kim et al. (2021) reported on the target delivery of RNA using engineered ILs into specific liver cells, namely hepatocytes and liver sinusoidal endothelial cells.220 The delivery of ILs to the targeted site was ensured by controlling the size and PEG:lipid ratio (Fig. 5a). Moreover, active targeting was achieved by adding mannose to the ILs. In vivo, gene silencing studies showed the selectivity of the engineered ILs towards the targeted liver cells (Fig. 5b).
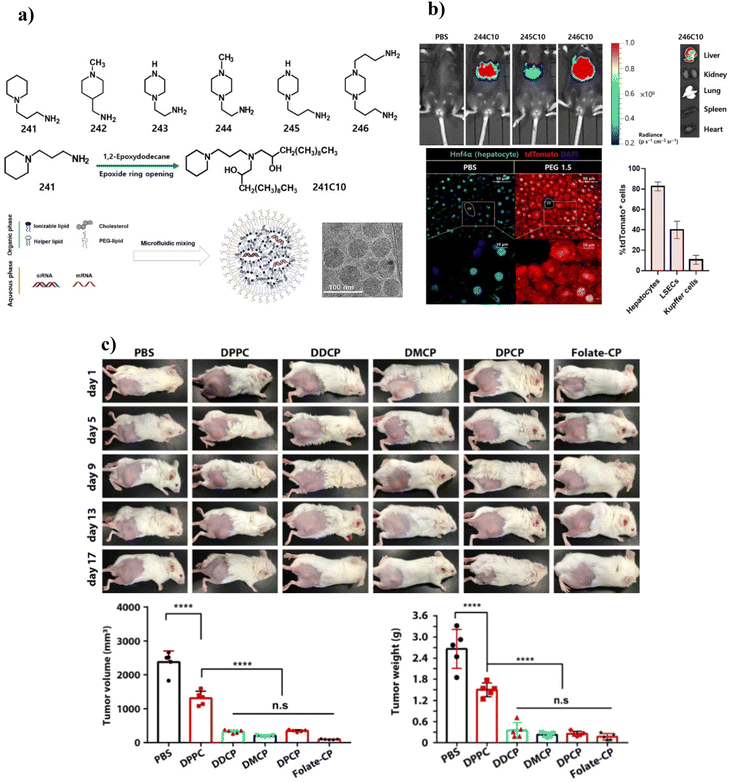 | ||
| Fig. 5 (a) Preparation of LNPs derived from ionizable lipids. (b) In vivo evaluation of ILs showed potent luciferase expression. Ex vivo organ images showed that LNPs were mostly taken up into the liver. Liver histology image and transfection efficiency showed significant tdTomato fluorescence in the hepatocyte. cv, central vein. Adapted with permission from ref. 220. Copyright 2021, American Association for the Advancement of Science. (b) Synthesis and in vivo application of CP liposomes. (c) Tumor therapy efficacy of CP liposomes (DDCP-c, DMCP-c, DPCP-c, and folate-CP) with DPPC as the control. Adapted with permission from ref. 228. Copyright 2021, American Chemical Society. | ||
ILs are widely explored by modifying their domains, including hydrophobic tails, hydrophilic heads, or linkers. Walsh et al. (2013) reported a new class of IL with a lysine head group linked to long-chain dialkylamine via an amide linker for siRNA delivery.221 The resulting pH-dependent ILs contain a carboxylate group and two ionizable amines. This ionizable lipid exhibits electrostatic charge-dependent membrane disruption advantages, successful in vitro siRNA transfection, and enhanced siRNA-mediated knockdown in transfected HeLa cells. Nucleic acid delivery was also accomplished using different aminoglycoside-derived ILs.222,223
Lipid-based delivery systems are widely used for improved chemotherapy for cancer treatment. Broma et al. (2019) reported ILs as a Trojan horse in delivering AuNPs with a size range of 5 nm for enhanced outcomes in the radiation therapy of triple-negative MDA-MB-231 cells.224 The ILs with the composition DLin-MC3-DMA/DSPC/cholesterol/PEG were used to coat AuNPs. The complex of IL–AuNPs showed a ∼73-fold increase in the uptake of small-sized AuNPs in cancerous cells.
4.3 Zwitterionic lipids
The zwitterionic lipids (ZILs) contain an equal number of covalently bonded anionic and cationic moieties.225 ZILs have gained the utmost attention in biomedical applications, including drug delivery and increased uptake of loaded molecules by reducing the adsorption of proteins on nanocarriers in serum.226 Obata et al. (2010) designed endosomal-pH-responsive liposomes functionalized with glutamic acid-based zwitterionic lipids for enhanced drug delivery applications.227 The liposomes showed a positive zeta potential at lower pH and became negatively charged at basic pH due to the carboxyl group moiety in the glutamic acid. Furthermore, synthesized pH-responsive lipids reflected high fusogenic potential with the anionic membrane of cancer cells. Thus, it ensures the improved release of encapsulated Dox in HeLa cells and high antitumor activity in vivo against a xenograft breast cancer tumor. Cancer treatment with targeted and enhanced drug delivery was ensured using a novel biorthogonal zwitterionic lipid (choline phosphate (CP)) based liposome functionalized with folic acid using a click reaction.228 Furthermore, compared with phosphatidylcholine (PC) based liposomes, supramolecular ionic pair interactions of zwitterionic lipid exhibit adhesive characteristics with the cell membrane of cancer cells, enhanced biocompatibility in normal cells, significantly enhanced cytotoxicity and inhibition of tumor growth (Fig. 5c).4.4 Liposomes
Liposomes are small spherical-shaped artificial vesicles synthesized from cholesterol and nontoxic phospholipids. Liposomes are widely explored as delivery agents due to their size and ability to load both hydrophilic and hydrophobic molecules.229 Doxil was the first liposomal formulation approved in 1995 by the FDA of the USA for the treatment of refractory acquired immune deficiency syndrome (AIDS)-related to Kaposi's sarcoma.230 The remarkable journey of liposomes as delivery agents includes ligand-targeted delivery, nucleic acid/gene delivery, and delivery of active drugs, polymers, anesthetics, and antimicrobial agents.231 Spherical liposomes are readily taken up by irregular and distorted tumor cells via an enhanced permeability and retention effect, resulting in elevated drug distribution at the tumor site.232 Curcumin and metformin-loaded DSPE-PEG2000-hyaluronic acid liposomes were designed to target hepatocellular tumors and drug resistance.233In vitro and in vivo studies revealed that this formulation exhibits more potent antiproliferation and antimetastasis. The inhibition of drug resistance and tumor growth was attributed to the down-regulation of multidrug resistance-related P-glycoprotein and the inducing epithelial–mesenchymal transformation of tumor cells. Michel et al. (2017) developed cationic liposomes to load mRNA and improve cell transfection to treat alpha-1-antitrypsin deficiency.234 Liposomes showed a prolonged transfection effect with negligible cytotoxicity in A549 cells. Liposomes had a long-acting transfection effect on cells, resulting in increased expression of a functional alpha-1-antitrypsin protein. Dhaliwal et al. (2020) developed a cationic liposome-based nanovehicle for intranasal delivery and potent mRNA transfection to a murine model's brain.188 The incubation of mRNA-loaded cationic liposomes with J774.1 macrophage cells showed stable GFP expression in the cytosol up to 24 h (Fig. 6a and b). Furthermore, intranasal administration of mRNA-loaded cationic liposomes in mice compared with control (GFP-mRNA) and vehicle (liposome)-treated groups showed significantly higher GFP expression by ∼15% (p < 0.05) up to 24 h (Fig. 6c).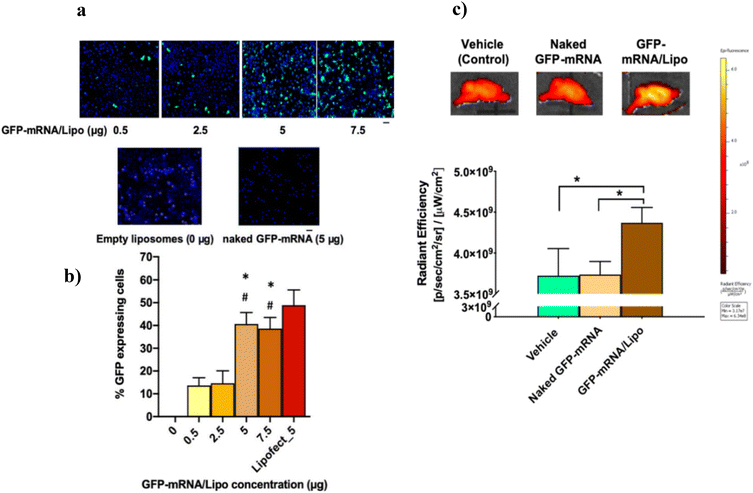 | ||
| Fig. 6 (a) GFP-mRNA transfection studies in J774A.1 macrophages using cationic liposomes at different concentrations. (b) Quantification of % GFP-expressing cells. Lipofect_5 represents Lipofectamine (with 5 μg GFP-mRNA)-treated cells as a positive control, and the 0 μg GFP-mRNA/Lipo group represents empty liposome-treated cells as a negative control (showed no GFP signal). (c) GFP-mRNA expression in mouse brain, 24 h post intranasal administration of GFP-mRNA-loaded cationic liposomes. Adapted with permission from ref. 188. Copyright 2020, American Chemical Society. | ||
Lei et al. (2020) demonstrated colorectal cancer gene therapy using a protamine/liposome-based delivery carrier loaded with IL-15 mRNA (CLPP-mIL-5 complex).189 Cytokine IL-15 can be a cancer gene therapy due to its immune stimulation characteristics. In vitro studies showed the successful delivery of mRNA in C26 cells. The accumulation of mRNA in cancer cells induced cytotoxicity and lymphocyte stimulation. Following systemic delivery in three C26 murine colon cancer-bearing mice, CLPP-mIL-5 complex inhibits cancer rates up to 70%, 55%, and 69% in abdominal cavity metastasis tumor, subcutaneous, and pulmonary metastasis models, respectively.
4.5 Lipid nanoparticles (LNPs)
Lipid NPs are the highly advanced clinically viable vector for NA delivery with Onpattro (patisiran) approval for amyloidosis treatment and their application in COVID-19 vaccine around the globe.177,235 Lipid NPs are composed of four different lipids, namely (1) ionizable cationic lipid, (2) three neutral helper lipids, namely phospholipid, (3) cholesterol, and (4) PEGylated lipid.236 In general, ionizable cationic lipids form an electrostatic complex with anionic nucleic acid, enabling intracellular uptake and endosomal escape. The stability and fluidity of the complex are maintained by cholesterol. Furthermore, phospholipids enhance the structural integrity of the complex. PEGylated lipid is an important module that maintains stability, enables cellular uptake, and protects the lipid–NA complex from being captured by the protein corona.237,238 Billingsley et al. (2022) developed a sequential library of biocompatible lipid NPs via microfluidic mixing to successfully deliver mRNA to T cells (Fig. 7a and b).190 Their study revealed that lipid NPs composed of C14-4 ionizable lipid improved the delivery of mRNA by a 3-fold increase in primary human T cells. Additionally, lipid NPs with minimal cytotoxicity induced chimeric antigen receptor (CAR) expression and effectively killed cancer cells (Fig. 7c). Ly et al. (2022) developed lipid NPs using FDA-approved ionizable lipids, namely, MC3, ALC-0315, and SM-102, and applied them for the loading and delivery of self-amplifying mRNA (saRNA), protein expression, and cytokine activation in vitro (triggering different levels of IL-6 response).236 Furthermore, it was found that PEG facilitates the encapsulation and stability of saRNA, which is otherwise complicated to preserve as compared with mRNA. The type of ionizable lipid highly influenced the protein expression. Protein expression was highest in ALC-0315, followed by SM-102, whereas MC3 failed to show potent protein expression. The study provides an insight into lipid NPs required for specific applications, including the delivery of heavy RNA molecules (saRNA, Cas9), therapies related to protein replacement, and vaccine production. | ||
| Fig. 7 (a) Schematic of LNP synthesis, including the components used to make LNPs via microfluidic mixing and the expected resulting structure. (b) Visualization of the design process used to generate libraries A and B with library A resulting from orthogonal DOE screening of a wide range of excipient molar ratios, and library B examining more formulations within a narrowed range of excipient ratios based on the results from the library A screen. (c) Schematic of the CAR T cell production utilizing either LNPs or EP for mRNA delivery to T cells. The treated T cell populations may differ in viability and CAR potency depending on the transfection method. Still, both can generate functional CAR T cells to induce targeted cancer cell killing. Adapted with permission from ref. 190. Copyright 2022, American Chemical Society. | ||
4.6 Solid lipid nanoparticles
Solid lipid NPs (SLNs) have been extensively studied over the past decade because they provide the combinatorial effect of various carrier systems, including polymeric nanoparticles, emulsions, and liposomes.239 SLNs have changed the dimensions of gene and drug delivery because of their advantages, including biocompatibility and safety profile, avoidance of organic solvents, ease of large-scale synthesis, water-based technology, physicochemical stability, ability to encapsulate proteins and nucleic acids along with hydrophilic, hydrophobic drugs and bioactive compounds, and controlled and sustained release of loaded components.240–242 SLNs are spherical nanoparticles with diameters of 50–1000 nm.239,242 SLNs remain solid at room temperature because of the core of SLNs. The core of SLNs is composed of solid lipids such as glycerides, steroids, fatty acids, or waxes, whereas LNPs comprise solid and liquid crystalline lipids.201,243 SLNs are superior to lipid nanoparticles in protecting nucleic acids from degradation and leakage during storage. SLNs are synthesized using physiological, biodegradable, and biocompatible lipids in a solid state at room temperature, emulsifiers, or a combination of pharmaceutical agents and solvents. Different methods have been extensively investigated for the synthesis of SLNs, such as emulsion-solvent evaporation,244 microemulsion,245 high-shear homogenization,246 ultrasonication,247 solvent injection methods,248 nanoprecipitation (solvent displacement technique) and so on.193 SLNs possess a solid lipid core loaded with the active ingredient and stabilized with an outer surface of surfactant. Nonviral delivery of plasmid DNA was efficiently done using SLP-based cargos.249 The key ingredients of the SLNs prepared via the solvent-emulsification method include cholesteryl oleate glycerol trioleate, and DOPE cholesterol3β-[N-(dimethylaminoethane)carbamoyl (DC)-cholesterol]. Furthermore, plasmid DNA was incorporated into SLNPs. In vitro studies suggested improved transfection efficiency of plasmid DNA in dendritic cells. Song et al. (2017) synthesized cationic solid lipid NPs (CSLNs) for the delivery of siRNA and β-NaYF4:Yb,Er upconversion nanoparticles (UCNPs) and applied these for bioimaging and gene therapy in A549 cells.194 CSLPs were synthesized via the thin-film dispersion method using a peptide-based cationic lipid, CDO14, and further conjugated to UCNPs and siRNA by electrostatic interaction. Gene silencing, bioimaging, and cytocompatibility studies demonstrated that encapsulated siRNA and UCNPs could efficiently be delivered and internalized into the cells and displayed superior cellular imaging and gene silencing with CSLPs (Fig. 8a & b). Cytotoxicity studies performed on A549 cells with an incubation of 48 h showed that CLSPs were cytocompatible, with cell viability higher than 90% (Fig. 8c).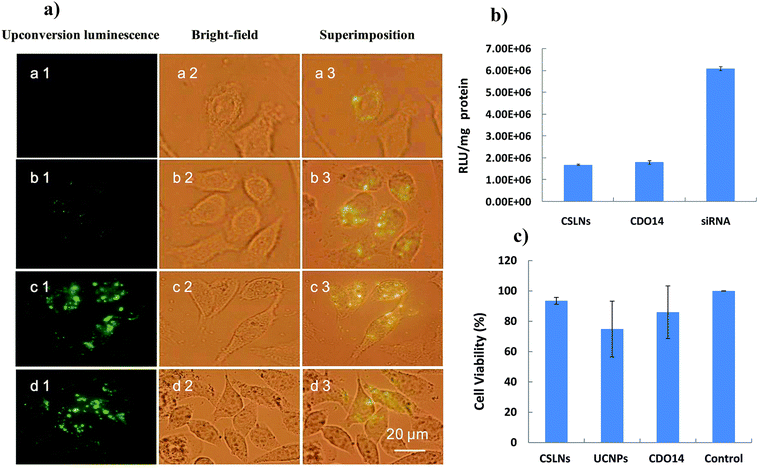 | ||
| Fig. 8 (a) Uptake of CSLNs into A549 cells. The fluorescence microscope images after treatment with CSLNs (excited by 980 nm light) at concentrations of (a) 5 μg μL−1, (b) 10 μg μL−1, (c) 15 μg μL−1 and (d) 20 μg μL−1. (b) Gene silencing mediated by CSLNs. (c) Cytotoxicity of CSLNs/siRNA and UCNPs/siRNA in A549 cells by MTT assay. Adapted with permission from ref. 194. Copyright 2017, The Royal Society of Chemistry. | ||
4.7 Commercial history of lipid-based drug delivery agents
The commercial history of lipidic formulations for drug and gene delivery can be traced back to the late 20th century when the first liposomal drug, Doxil (doxorubicin hydrochloride liposome injection), was approved by the US FDA in 1995.250 Since then, lipid formulations have become a popular and effective means of delivering a wide range of therapeutic agents, including cancer drugs, anti-inflammatory agents, and nucleic acids for gene therapy. Liposomes, LNPs, and spherical structures can encapsulate drugs and protect them from rapid clearance and degradation, enabling targeted delivery to cells and tissues. The market for lipid-based drug delivery systems has snowballed in recent years, with several new drugs and gene therapies using lipidic formulations in development and commercialization. One of the main advantages of lipidic formulations is their ability to enhance the efficacy of drugs and reduce their toxic side effects. All these advantages have led to the approval of several lipid-based drugs for the treatment of cancer, including Doxil,250 Camptosar (lipid NPs),200 and DaunoXome (daunorubicin citrate liposome injection).251 In recent years, the use of lipidic formulations for gene delivery has also become increasingly popular, leading to the development of new treatments for genetic diseases and other disorders. During the outbreak of SARS-CoV-2, several LNP mRNA vaccines were developed to fight the disease, such as Comirnaty and Spikevax.177,252 Lipid-based delivery systems are widely accepted in clinical applications and have been approved by the FDA to deliver various therapeutics, including drugs and nucleic acids (Table 4).| S. no. | Commercial name | Developer | Date of approval | Lipid | Drug | Treatment | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Diprivan | Crucell | Feb 23, 1989 | Liposome | Propofol | Induction and maintenance of anesthesia | 251 |
| 2 | Doxil® | Janssen | Nov 17, 1995 | Liposome | Dox | Ovarian and breast cancer | 250 |
| 3 | Daunoxome® | Galen | Apr 8, 1996 | Liposome | Daunorubicin | HIV-associated Kaposi's sarcoma | 251 |
| 4 | AmBisome | Gilead Sciences | Nov 8, 1997 | Liposome | Amphotericin B | Invasive fungal infections | 251 |
| 5 | Cytosar-U® | Pfizer | Jan 8, 1999 | Lipid NPs | Cytarabine | Leukemia and lymphoma | 200 |
| 6 | Visudyne | Bausch and Lomb | Apr 30, 2002 | Liposomal | Verteporfin | Macular degeneration, pathologic, or ocular histoplasmosis | 251 |
| 7 | Camptosar® | Pfizer | June 24, 2004 | Lipid NPs | Irinotecan | Metastatic colon or rectum cancer | 200 |
| 8 | Vinlon™ | Talon Therapeutics, Inc. | Sept 8, 2012 | Liposome | Vincristine | Acute lymphoblastic leukemia | 200 |
| 9 | Onivyde® | Merrimack | Oct 22, 2015 | Liposome | Irinotecan | Various metastatic cancers, including breast, pancreatic, sarcomas, or brain | 251 |
| 10 | Nocita® | Aratana therapeutics | August 12, 2016 | Liposome | Bupivacaine | Anesthetic | 253 |
| 11 | Vyxeos CPX-351® | Jazz Pharmaceuticals | Aug 3, 2017 | Liposome | Cytarabine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :daunorubicin :daunorubicin |
Acute myeloid leukemia | 251 |
| 12 | Onpattro® (patisiran) | Alnylam Pharmaceuticals Inc. | Aug 8, 2018 | Lipid NPs | siRNA | Hereditary transthyretin amyloidosis | 235 |
| 13 | Givosiran (Givlaari®) | Alnylam Pharmaceuticals Inc. | Nov 20, 2019 | Lipid NPs | siRNA | Hepatic porphyria | 254 |
| 14 | Comirnaty® | BioNTech, Pfizer | Aug 23, 2021 | Lipid NPs | mRNA | COVID-19 | 177 |
| 15 | Spikevax® | Moderna | Jan 31, 2022 | Lipid NPs | mRNA | COVID-19 | 252 |
5. Nanomaterial delivery vehicles for nucleic acids: promise and challenges
Nucleic acids are widely explored for the treatment of a broad spectrum of diseases including cancer, heart diseases, genetic disorders, and viral and bacterial infections. However, the applicability of nucleic acid in therapeutics is challenging due to enzymatic degradation, and unfavorable physiocochemical properties (hydrophilicity and high molecular weight, negative charge) resulting in reduced cellular uptake, poor in vivo stability, rapid excretion or uptake by non-targeted cells.72,255 Suitable vectors that can overcome inadequate efficacy, susceptibility to enzymatic degradation, low bioavailability, and off-target side effects are required to increase the therapeutic efficacy of nucleic acids. Nanomaterials offer exciting possibilities for the therapeutic delivery of nucleic acids, like DNA or RNA.223 These nanocarriers hold immense potential for the pharmaceutical landscape as they revolutionized gene therapy, RNA interference, and vaccine development. Compared with traditional methods of delivering nucleic acids into cells, nanomaterials offer a multitude of advantages. (1) Nucleic acids on their own have difficulty penetrating cell membranes and reaching their target sites. Nanomaterials can encapsulate and protect nucleic acids from enzymatic degradation, facilitating their cellular uptake and delivery across biological barriers.234 (2) Nanocarriers can be engineered with specific molecules on their surface that recognize and bind to particular cell types. This targeted delivery allows researchers to focus the effects of nucleic acids on the desired cells, reducing side effects on healthy tissues.249 (3) Nanocarriers can be designed to release their nucleic acid cargo in a controlled manner over time. This sustained release can be crucial for some therapies, allowing for longer-lasting effects.256 (4) Some nanocarriers can be engineered to combine functionalities like delivering therapeutic molecules and imaging capabilities, offering a more comprehensive approach to treatment and monitoring.257 (5) Nanocarriers can be designed to release their cargo in response to specific stimuli like pH changes or light exposure.237 This allows for a more controlled and localized release of nucleic acids within the body.While nanomaterials offer exciting possibilities for nucleic acid delivery, there are also some limitations and potential downsides to consider. (1) Despite targeting strategies, there is a risk that nanocarriers could deliver nucleic acids to unintended cell types. This can lead to unwanted side effects or even toxicity.74 (2) The body's immune system may recognize nanocarriers as foreign invaders and trigger an inflammatory response. This can limit the effectiveness of the therapy or cause complications.258 (3) Developing and manufacturing safe and effective nanocarriers for nucleic acid delivery can be complex and expensive. This can hinder the accessibility and affordability of these therapies. (4) Some nanomaterials may not be efficiently cleared from the body after delivering their payload. This can raise concerns about potential long-term effects.259
6. Future strategies
Researchers are actively exploring several strategies to further improve nanomaterial-based delivery vehicles for nucleic acids, addressing the current limitations and unlocking their full potential. (1) Attaching targeting ligands to the surface of nanocarriers can significantly improve their specificity. These ligands can bind to receptors present on specific cell types, directing the delivery of nucleic acids to the desired location. (2) Developing nanocarriers that respond to specific environmental cues within the body. For example, carriers could release their cargo in response to changes in pH or temperature, allowing for targeted release at the disease site.260 (3) Modifying the surface properties of nanocarriers to minimize interactions with the immune system and reduce the risk of inflammatory responses.261 This can involve using biocompatible coatings or manipulating surface charges. (4) Developing nanocarriers from biodegradable polymers that are naturally broken down and eliminated by the body, reducing long-term accumulation concerns.259 (4) Optimizing the rate at which nucleic acids are released from nanocarriers. This can help to minimize off-target effects and improve the overall therapeutic efficacy. (5) Developing nanocarriers that can co-deliver nucleic acids with other therapeutic agents, such as drugs or imaging molecules, for combination therapy and improved treatment outcomes.262 (6) Creating nanocarriers that can be tracked within the body using imaging techniques, allowing researchers to monitor their delivery pathway and optimize targeting strategies. (7) Developing efficient and cost-effective production methods for well-defined and consistent nanocarriers, facilitating their wider clinical application.263 By addressing these challenges and focusing on these future strategies, researchers can unlock the full potential of nanomaterial-based delivery vehicles for nucleic acids. This has the potential to revolutionize how we treat various diseases, offering more targeted, effective, and safer therapies based on gene therapy, RNAi, and DNA vaccines.7. Conclusion
Nucleic acid-based therapeutics has experienced the transformation from concept into clinical reality in recent years. The first FDA-approved siRNA-based drug, Onpattro (2018), provides a platform for further developing NA therapeutics to cure rare conditions, cancers, and infectious diseases.235,264 Since then, significant progress has been made to improve their therapeutic effect, further amplified in the development of the SARS-Cov-2 vaccine worldwide.157 However, clinical availability usually requires modification of NAs and their carriers to increase nuclease resistance and enhance cellular uptake. SNAs with densely packed 3D structures have overcome some challenges without further modification. SNAs have minimized the nuclease degradation of delivered NAs.191 Despite this, significant issues still need to be addressed, most notably in effectively providing therapeutic NAs to the targeted site in the required quantity. The stability of SNA structures can be improved by tuning them with other NPs, resulting in tissue/organ-specific delivery. Among all the classes of delivery agents, lipid-based nanocomplexes have shown ideal delivery efficiency at the clinical level.265 The clinical trials of the different diseases have proved the importance of lipid-based NPs in NA therapeutics. Over the past decade, several NA therapeutics have been in clinical trials, and only a few enjoyed commercial successes. These points highlight the challenges in translating new drugs from animals to humans. With the success story of Onpattro, lipid-based technology is accepted worldwide and is ready to uplift the next wave of NAs therapeutics for vaccination, gene therapy, and protein production. Undoubtedly, the field of NAs is undergoing a massive expansion, and their potential applications in the treatment of chronic disorders, immunotherapy, and personalized medicine will ensure their development to revolutionize the biomedical field in the near future.Abbreviations
| DOTAP | 1,2-Dioleoyl-3-(trimethylammonium) propane |
| ASO | Antisense oligonucleotides |
| CNMs | Carbon nanomaterials |
| CLs | Cationic lipids |
| FDA | Food and drug administration |
| AuNPs | Gold NPs |
| GFP | Green fluorescent protein |
| ILs | Ionizable lipids |
| IONPs | Iron oxide NPs |
| LNPs | Lipid NPs |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| NPs | Nanoparticles |
| NAs | Nucleic acids |
| pDNA | Plasmid DNA |
| PAMAM | Poly(amidoamine) |
| PEG | Polyethylene glycol |
| PEG-DSPE | Polyethylene-glycol-2000-1,2-distearyl-3-sn-phosphatidylethanolamine |
| PEI | Polyethyleneimine |
| PLGA | Polylactide-co-glycolide |
| PNPs | Polymeric NPs |
| QDs | Quantum dots |
| siRNA | Short interfering RNA |
| SiNPs | Silicon NPs |
| SLNPs | Solid lipid NPs |
| SNAs | Spherical nucleic acids |
| UCNPs | Upconversion nanoparticles |
| ZILs | Zwitterionic lipids. |
Author contributions
All the authors read and approved the manuscript.Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This project has received funding from the European Union's Horizon Europe research and innovation program under Marie Skłodowska-Curie grant agreement No. 101103113. M. J. Mehta was supported by the National Research Foundation of Korea grant (No. 2021R1A2C1007668 and RS-2024-00398030) funded by the Korean government.References
- C. E. Dunbar, K. A. High, J. K. Joung, D. B. Kohn, K. Ozawa and M. Sadelain, Gene therapy comes of age, Science, 2018, 359(6372), eaan4672 CrossRef PubMed.
- A. Gupta, J. L. Andresen, R. S. Manan and R. Langer, Nucleic acid delivery for therapeutic applications, Adv. Drug Delivery Rev., 2021, 178, 113834 CrossRef CAS PubMed.
- B. B. Mendes, J. Conniot, A. Avital, D. Yao, X. Jiang and X. Zhou, et al., Nanodelivery of nucleic acids, Nat. Rev. Methods Primers, 2022, 2(1), 1–21 CrossRef.
- T. C. Roberts, R. Langer and M. J. A. Wood, Advances in oligonucleotide drug delivery, Nat. Rev. Drug Discovery, 2020, 19(10), 673–694 CrossRef CAS PubMed.
- J. Kim, C. Hu, C. Moufawad El Achkar, L. E. Black, J. Douville and A. Larson, et al., Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease, N. Engl. J. Med., 2019, 381(17), 1644–1652 CrossRef CAS PubMed.
- Y. Yamada, Nucleic Acid Drugs—Current Status, Issues, and Expectations for Exosomes, Cancers, 2021, 13(19), 5002 CrossRef CAS PubMed.
- C. H. Jones, C. K. Chen, A. Ravikrishnan, S. Rane and B. A. Pfeifer, Overcoming Nonviral Gene Delivery Barriers: Perspective and Future, Mol. Pharmaceutics, 2013, 10(11), 4082–4098 CrossRef CAS PubMed.
- M. Chehelgerdi, M. Chehelgerdi, O. Q. B. Allela, R. D. C. Pecho, N. Jayasankar and D. P. Rao, et al., Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation, Mol. Cancer, 2023, 22, 169 CrossRef PubMed.
- A. Graczyk, R. Pawlowska, D. Jedrzejczyk and A. Chworos, Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery, Molecules, 2020, 25(1), 204 CrossRef CAS PubMed.
- M. J. Mehta, H. J. Kim, S. B. Lim, M. Naito and K. Miyata, Recent Progress in the Endosomal Escape Mechanism and Chemical Structures of Polycations for Nucleic Acid Delivery, Macromol. Biosci., 2024, 24(4), 2300366 CrossRef CAS PubMed.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. P. Rodriguez-Torres del and L. S. Acosta-Torres, et al., Nano based drug delivery systems: recent developments and future prospects, J. Nanobiotechnol., 2018, 16(1), 71 CrossRef PubMed.
- T. De Serres-Bérard, S. Ait Benichou, D. Jauvin, M. Boutjdir, J. Puymirat and M. Chahine, Recent Progress and Challenges in the Development of Antisense Therapies for Myotonic Dystrophy Type 1, Int. J. Mol. Sci., 2022, 23(21), 13359 CrossRef PubMed.
- B. Hu, L. Zhong, Y. Weng, L. Peng, Y. Huang and Y. Zhao, et al., Therapeutic siRNA: state of the art, Signal Transduction Targeted Ther., 2020, 5(1), 1–25 CrossRef PubMed.
- P. D. Williams and P. A. Kingston, Plasmid-mediated gene therapy for cardiovascular disease, Cardiovasc. Res., 2011, 91(4), 565–576 CrossRef CAS PubMed.
- C. Uherek and W. Wels, DNA-carrier proteins for targeted gene delivery, Adv. Drug Delivery Rev., 2000, 44(2–3), 153–166 CrossRef CAS PubMed.
- C. Rinaldi and M. J. A. Wood, Antisense oligonucleotides: the next frontier for treatment of neurological disorders, Nat. Rev. Neurol., 2018, 14(1), 9–21 CrossRef CAS PubMed.
- A. J. Ward, M. Norrbom, S. Chun, C. F. Bennett and F. Rigo, Nonsense-mediated decay as a terminating mechanism for antisense oligonucleotides, Nucleic Acids Res., 2014, 42(9), 5871–5879 CrossRef CAS PubMed.
- A. Ruscito and M. C. DeRosa, Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications, Front. Chem., 2016, 4 DOI:10.3389/fchem.2016.00014.
- A. Levanova and M. M. Poranen, RNA Interference as a Prospective Tool for the Control of Human Viral Infections, Front. Microbiol., 2018, 9 DOI:10.3389/fmicb.2018.02151.
- P. P. Mehta and V. S. Dhapte-Pawar, Novel and Evolving Therapies for COVID-19 Related Pulmonary Complications, Am. J. Med. Sci., 2021, 361(5), 557–566 CrossRef PubMed.
- D. P. Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell, 2004, 116(2), 281–297 CrossRef CAS PubMed.
- Z. Wang, F. Schmidt, Y. Weisblum, F. Muecksch, C. O. Barnes and S. Finkin, et al., mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants, Nature, 2021, 592(7855), 616–622 CrossRef CAS PubMed.
- J. D. Beck, D. Reidenbach, N. Salomon, U. Sahin, Ö Türeci and M Vormehr, , et al., mRNA therapeutics in cancer immunotherapy, Mol. Cancer, 2021, 20(1), 69 CrossRef CAS PubMed.
- B. Jahrsdörfer and G. J. Weiner, CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy, Curr. Opin. Investig. Drugs, 2003, 4(6), 686–690 Search PubMed.
- A. Dalpke, S. Zimmermann and K. Heeg, CpG DNA in the Prevention and Treatment of Infections, BioDrugs, 2002, 16(6), 419–431 CrossRef CAS PubMed.
- S. Li, F. C. MacLaughlin, J. G. Fewell, M. Gondo, J. Wang and F. Nicol, et al., Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid, Gene. Ther., 2001, 8(6), 494–497 CrossRef CAS PubMed.
- R. Lassaunière, C. Polacek, G. J. Gram, A. Frische, J. L. Tingstedt and M. Krüger, et al., Preclinical evaluation of a candidate naked plasmid DNA vaccine against SARS-CoV-2, npj Vaccines, 2021, 6(1), 1–13 CrossRef PubMed.
- D. Jiang, H. Lee and W. M. Pardridge, Plasmid DNA gene therapy of the Niemann-Pick C1 mouse with transferrin receptor-targeted Trojan horse liposomes, Sci. Rep., 2020, 10(1), 13334 CrossRef CAS PubMed.
- T. Malardo, M. E. Batalhão, A. Panunto-Castelo, L. P. Almeida, E. Padilha and I. C. Fontoura, et al., Low-dose plasmid DNA treatment increases plasma vasopressin and regulates blood pressure in experimental endotoxemia, BMC Immunol., 2012, 13(1), 59 CrossRef CAS PubMed.
- F. Wada, T. Yamamoto, T. Kobayashi, K. Tachibana, K. R. Ito and M. Hamasaki, et al., Drug discovery and development scheme for liver-targeting bridged nucleic acid antisense oligonucleotides, Mol. Ther.–Nucleic Acids, 2021, 26, 957–969 CrossRef CAS PubMed.
- D. Di Fusco, V. Dinallo, I. Marafini, M. M. Figliuzzi, B. Romano and G. Monteleone, Antisense Oligonucleotide: Basic Concepts and Therapeutic Application in Inflammatory Bowel Disease, Front. Pharmacol., 2019, 10 DOI:10.3389/fphar.2019.00305.
- D. R. Scoles, E. V. Minikel and S. M. Pulst, Antisense oligonucleotides: A primer, Neurol.: Genet., 2019, 5(2), e323 CAS.
- T. P. Prakash, An Overview of Sugar-Modified Oligonucleotides for Antisense Therapeutics, Chem. Biodiversity, 2011, 8(9), 1616–1641 CrossRef CAS PubMed.
- M. A. Passini, J. Bu, A. M. Richards, C. Kinnecom, S. P. Sardi and L. M. Stanek, et al., Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy, Sci. Transl. Med., 2011, 3(72), 72ra18 Search PubMed.
- M. Amanat, C. L. Nemeth, A. S. Fine, D. G. Leung and A. Fatemi, Antisense Oligonucleotide Therapy for the Nervous System: From Bench to Bedside with Emphasis on Pediatric Neurology, Pharmaceutics, 2022, 14(11), 2389 CrossRef CAS PubMed.
- A. D. Keefe, S. Pai and A. Ellington, Aptamers as therapeutics, Nat. Rev. Drug Discovery, 2010, 9(7), 537–550 CrossRef CAS PubMed.
- R. Piyush, K. Rajarshi, A. Chatterjee, R. Khan and S. Ray, Nucleic acid-based therapy for coronavirus disease 2019, Heliyon, 2020, 6(9), e05007 CrossRef CAS PubMed.
- B. N. Gawande, J. C. Rohloff, J. D. Carter, I. von Carlowitz, C. Zhang and D. J. Schneider, et al., Selection of DNA aptamers with two modified bases, Proc. Natl. Acad. Sci. U. S. A., 2017, 114(11), 2898–2903 CrossRef CAS PubMed.
- C. Tuerk and L. Gold, Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase, Science, 1990, 249(4968), 505–510 CrossRef CAS PubMed.
- A. D. Ellington and J. W. Szostak, In vitro selection of RNA molecules that bind specific ligands, Nature, 1990, 346(6287), 818–822 CrossRef CAS PubMed.
- J. Zhang, L. P. Smaga, N. S. R. Satyavolu, J. Chan and Y. Lu, DNA Aptamer-Based Activatable Probes for Photoacoustic Imaging in Living Mice, J. Am. Chem. Soc., 2017, 139(48), 17225–17228 CrossRef CAS PubMed.
- A. Fire, S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver and C. C. Mello, Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans, Nature, 1998, 391(6669), 806–811 CrossRef CAS PubMed.
- H. Nam, S. H. Ku, H. Y. Yoon, K. Kim, I. C. Kwon and S. H. Kim, et al., Enhancing Systemic Delivery of Enzymatically Generated RNAi Nanocomplexes for Cancer Therapy, Adv. Ther., 2019, 2(6), 1900014 CrossRef.
- S. A. Jensen, E. S. Day, C. H. Ko, L. A. Hurley, J. P. Luciano and F. M. Kouri, et al., Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma, Sci. Transl. Med., 2013, 5(209), 209ra152–209ra152 Search PubMed.
- S. M. Elbashir, J. Harborth, W. Lendeckel, A. Yalcin, K. Weber and T. Tuschl, Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells, Nature, 2001, 411(6836), 494–498 CrossRef CAS PubMed.
- Y. Weng, H. Xiao, J. Zhang, X. J. Liang and Y. Huang, RNAi therapeutic and its innovative biotechnological evolution, Biotechnol. Adv., 2019, 37(5), 801–825 CrossRef CAS PubMed.
- M. I. Sajid, M. Moazzam, Y. Cho, S. Kato, A. Xu and J. J. Way, et al., siRNA Therapeutics for the Therapy of COVID-19 and Other Coronaviruses, Mol. Pharmaceutics, 2021, 18(6), 2105–2121 CrossRef CAS PubMed.
- L. Li, Y. Ren, X. Wen, Q. Guo, J. Wang and S. Li, et al., Endogenous miRNA-Activated DNA Nanomachine for Intracellular miRNA Imaging and Gene Silencing, Anal. Chem., 2021, 93(41), 13919–13927 CrossRef CAS PubMed.
- W. Dong, P. Wu, M. Qin, S. Guo, H. Liu and X. Yang, et al., Multipotent miRNA Sponge-Loaded Magnetic Nanodroplets with Ultrasound/Magnet-Assisted Delivery for Hepatocellular Carcinoma Therapy, Mol. Pharmaceutics, 2020, 17(8), 2891–2910 CrossRef CAS PubMed.
- S. Qin, X. Tang, Y. Chen, K. Chen, N. Fan and W. Xiao, et al., mRNA-based therapeutics: powerful and versatile tools to combat diseases, Signal Transduction Targeted Ther., 2022, 7, 166 CrossRef CAS PubMed.
- N. Chaudhary, D. Weissman and K. A. Whitehead, mRNA vaccines for infectious diseases: principles, delivery and clinical translation, Nat. Rev. Drug Discovery, 2021, 20(11), 817–838 CrossRef CAS PubMed.
- E. V. Bailey and F. A. Wilson, Vaccine Uptake in the US After Full Food and Drug Administration Approval of the BNT162b2 mRNA COVID-19 Vaccine, JAMA Netw. Open, 2022, 5(4), e226108 CrossRef PubMed.
- C. Zhang, G. Maruggi, H. Shan and J. Li, Advances in mRNA Vaccines for Infectious Diseases, Front. Immunol., 2019, 10 DOI:10.3389/fimmu.2019.00594.
- J. Devoldere, K. Peynshaert, H. Dewitte, C. Vanhove, L. De Groef and L. Moons, et al., Non-viral delivery of chemically modified mRNA to the retina: Subretinal versus intravitreal administration, J. Controlled Release, 2019, 307, 315–330 CrossRef CAS PubMed.
- N. Pardi, M. J. Hogan, F. W. Porter and D. Weissman, mRNA vaccines—a new era in vaccinology, Nat. Rev. Drug Discovery, 2018, 17(4), 261–279 CrossRef CAS PubMed.
- S. Xu, K. Yang, R. Li and L. Zhang, mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection, Int. J. Mol. Sci., 2020, 21(18), 6582 CrossRef CAS PubMed.
- J. E. Wooldridge and G. J. Weiner, CpG DNA and cancer immunotherapy: orchestrating the antitumor immune response, Curr. Opin. Oncol., 2003, 15(6), 440–445 CrossRef CAS PubMed.
- A. M. Krieg, Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides, Curr. Oncol. Rep., 2004, 6(2), 88–95 CrossRef PubMed.
- P. P. Ho, P. Fontoura, P. J. Ruiz, L. Steinman and H. Garren, An immunomodulatory GpG oligonucleotide for the treatment of autoimmunity via the innate and adaptive immune systems, J. Immunol., 2003, 171(9), 4920–4926 CrossRef CAS PubMed.
- S. Alipour, A. Mahdavi and A. Abdoli, The effects of CpG-ODNs and Chitosan adjuvants on the elicitation of immune responses induced by the HIV-1-Tat-based candidate vaccines in mice, Pathogens and Disease, 2017, 75(2), ftx013 CrossRef PubMed.
- H. Kim, W. Zhang, J. Hwang, E. K. An, Y. K. Choi and E. Moon, et al., Carrier-free micellar CpG interacting with cell membrane for enhanced immunological treatment of HIV-1, Biomaterials, 2021, 277, 121081 CrossRef CAS PubMed.
- H. Zhao, Q. Han, A. Yang, Y. Wang, G. Wang and A. Lin, et al., CpG-C ODN M362 as an immunoadjuvant for HBV therapeutic vaccine reverses the systemic tolerance against HBV, Int. J. Biol. Sci., 2022, 18(1), 154–165 CrossRef CAS PubMed.
- J. G. McHutchison, B. R. Bacon, S. C. Gordon, E. Lawitz, M. Shiffman and N. H. Afdhal, et al., Phase 1B, randomized, double-blind, dose-escalation trial of CPG 10101 in patients with chronic hepatitis C virus, Hepatology, 2007, 46(5), 1341–1349 CrossRef CAS PubMed.
- N. Hanagata, CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies, Int. J. Nanomed., 2017, 12, 515–531 CrossRef CAS PubMed.
- T. H. Kim, D. Kim, H. Lee, M. H. Kwak, S. Park, Y. Lee and H. J. Kwon, CpG-DNA induces bacteria-reactive IgM enhancing phagocytic activity against Staphylococcus aureus infection, BMB Rep., 2019, 52(11), 635–640 CrossRef CAS PubMed.
- R. Q. Prado, T. B. Bertolini, A. R. Piñeros, A. F. Gembre, S. G. Ramos and C. L. Silva, et al., Attenuation of experimental asthma by mycobacterial protein combined with CpG requires a TLR9-dependent IFN-γ-CCR2 signalling circuit, Clin. Exp. Allergy, 2015, 45(9), 1459–1471 CrossRef CAS PubMed.
- H. T. Li, Z. G. Chen, H. Liu, J. Ye, X. L. Zou and Y. H. Wang, et al., Treatment of allergic rhinitis with CpG oligodeoxynucleotides alleviates the lower airway outcomes of combined allergic rhinitis and asthma syndrome via a mechanism that possibly involves in TSLP, Exp. Lung Res., 2016, 42(6), 322–333 CrossRef CAS PubMed.
- D. E. Fonseca and J. N. Kline, Use of CpG oligonucleotides in treatment of asthma and allergic disease, Adv. Drug Delivery Rev., 2009, 61(3), 256–262 CrossRef CAS PubMed.
- H. Hadji and K. Bouchemal, Effect of micro- and nanoparticle shape on biological processes, J. Controlled Release, 2022, 342, 93–110 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Strategies in the design of nanoparticles for therapeutic applications, Nat. Rev. Drug Discovery, 2010, 9(8), 615–627 CrossRef CAS PubMed.
- F. U. Din, W. Aman, I. Ullah, O. S. Qureshi, O. Mustapha and S. Shafique, et al., Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors, Int. J. Nanomed., 2017, 12, 7291–7309 CrossRef PubMed.
- Y. Qin, L. Ou, L. Zha, Y. Zeng and L. Li, Delivery of nucleic acids using nanomaterials, Mol. Biomed., 2023, 4, 48 CrossRef CAS PubMed.
- A. Baker, J. Lorch, D. VanderWeele and B. Zhang, Smart Nanocarriers for the Targeted Delivery of Therapeutic Nucleic Acid for Cancer Immunotherapy, Pharmaceutics, 2023, 15(6), 1743 CrossRef CAS PubMed.
- H. J. Vaughan, J. J. Green and S. Y. Tzeng, Cancer-Targeting Nanoparticles for Combinatorial Nucleic Acid Delivery, Adv. Mater., 2020, 32(13), e1901081 CrossRef PubMed.
- Y. Jiang, S. Huo, J. Hardie, X. J. Liang and V. M. Rotello, Progress and perspective of inorganic nanoparticle-based siRNA delivery systems, Expert Opin. Drug Delivery, 2016, 13(4), 547–559 CrossRef CAS PubMed.
- R. Zhao, J. Xiang, B. Wang, L. Chen and S. Tan, Recent Advances in the Development of Noble Metal NPs for Cancer Therapy, in Bioinorganic Chemistry and Applications, ed. V. De Matteis, 2022, vol. 2022, pp. 1–14 Search PubMed.
- C. Amri, A. K. Shukla and J. H. Lee, Recent Advancements in Nanoparticle-Based Optical Biosensors for Circulating Cancer Biomarkers, Materials, 2021, 14(6), 1339 CrossRef CAS PubMed.
- S. Walia, A. Guliani and A. Acharya, A Theragnosis Probe Based on BSA/HSA-Conjugated Biocompatible Fluorescent Silicon Nanomaterials for Simultaneous in Vitro Cholesterol Effluxing and Cellular Imaging of Macrophage Cells, ACS Sustainable Chem. Eng., 2017, 5(2), 1425–1435 CrossRef CAS.
- D. Holmannova, P. Borsky, T. Svadlakova, L. Borska and Z. Fiala, Carbon Nanoparticles and Their Biomedical Applications, Appl. Sci., 2022, 12(15), 7865 CrossRef CAS.
- A. Ali, H. Zafar, M. Zia, I. ul Haq, A. R. Phull and J. S. Ali, et al., Synthesis, characterization, applications, and challenges of iron oxide nanoparticles, Nanotechnol. Sci. Appl., 2016, 9, 49–67 CrossRef CAS PubMed.
- A. Gagliardi, E. Giuliano, E. Venkateswararao, M. Fresta, S. Bulotta and V. Awasthi, et al., Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors, Front. Pharmacol., 2021, 12 DOI:10.3389/fphar.2021.601626.
- S. Adepu and S. Ramakrishna, Controlled Drug Delivery Systems: Current Status and Future Directions, Molecules, 2021, 26(19), 5905 CrossRef CAS PubMed.
- S. Burgio, L. Noori, A. Marino Gammazza, C. Campanella, M. Logozzi and S. Fais, et al., Extracellular Vesicles-Based Drug Delivery Systems: A New Challenge and the Exemplum of Malignant Pleural Mesothelioma, Int. J. Mol. Sci., 2020, 21(15), 5432 CrossRef CAS PubMed.
- E. Kahraman, S. Güngör and Y. Özsoy, Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery, Ther. Delivery, 2017, 8(11), 967–985 CrossRef CAS PubMed.
- A. Hiwrale, S. Bharati, P. Pingale and A. Rajput, Nanofibers: A current era in drug delivery system, Heliyon, 2023, 9(9), e18917 CrossRef CAS PubMed.
- S. M. S. Shahriar, J. Mondal, M. N. Hasan, V. Revuri, D. Y. Lee and Y. K. Lee, Electrospinning Nanofibers for Therapeutics Delivery, Nanomaterials, 2019, 9(4), 532 CrossRef CAS PubMed.
- Y. Song, W. Song, X. Lan, W. Cai and D. Jiang, Spherical nucleic acids: Organized nucleotide aggregates as versatile nanomedicine, Aggregate, 2022, 3(1), e120 CrossRef CAS PubMed.
- F. Xu, Q. Xia and P. Wang, Rationally Designed DNA Nanostructures for Drug Delivery, Front. Chem., 2020, 8, 751 CrossRef CAS PubMed.
- Y. Wei, X. Mo, P. Zhang, Y. Li, J. Liao and Y. Li, et al., Directing Stem Cell Differentiation via Electrochemical Reversible Switching between Nanotubes and Nanotips of Polypyrrole Array, ACS Nano, 2017, 11(6), 5915–5924 CrossRef CAS PubMed.
- Z. Gao, T. Ma, E. Zhao, D. Docter, W. Yang and R. H. Stauber, et al., Small is Smarter: Nano MRI Contrast Agents – Advantages and Recent Achievements, Small, 2016, 12(5), 556–576 CrossRef CAS PubMed.
- M. Jiao, P. Zhang, J. Meng, Y. Li, C. Liu and X. Luo, et al., Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications, Biomater. Sci., 2018, 6(4), 726–745 RSC.
- Á. Artiga, I. Serrano-Sevilla, L. D. Matteis, S. G. Mitchell and J. M. de la Fuente, Current status and future perspectives of gold nanoparticle vectors for siRNA delivery, J. Mater. Chem. B, 2019, 7(6), 876–896 RSC.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet and H. F. Dvorak, et al., Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater., 2016, 1(5), 1–12 Search PubMed.
- J. Ou, Z. Zhou, Z. Chen and H. Tan, Optical Diagnostic Based on Functionalized Gold Nanoparticles, Int. J. Mol. Sci., 2019, 20(18), E4346 CrossRef PubMed.
- J. Wang, A. M. Potocny, J. Rosenthal and E. S. Day, Gold Nanoshell-Linear Tetrapyrrole Conjugates for Near Infrared-Activated Dual Photodynamic and Photothermal Therapies, ACS Omega, 2020, 5(1), 926–940 CrossRef CAS PubMed.
- From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), World Stroke Organization (WSO), D. Sacks, B. Baxter, B. C. V. Campbell, J. S. Carpenter and C. Cognard, et al., Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke, Int. J. Stroke, 2018, 13(6), 612–632 Search PubMed.
- A. Graczyk, R. Pawlowska and A. Chworos, Gold Nanoparticles as Carriers for Functional RNA Nanostructures, Bioconjugate Chem., 2021, 32(8), 1667–1674 CrossRef CAS PubMed.
- M. E. Kyriazi, D. Giust, A. H. El-Sagheer, P. M. Lackie, O. L. Muskens and T. Brown, et al., Multiplexed mRNA Sensing and Combinatorial-Targeted Drug Delivery Using DNA-Gold Nanoparticle Dimers, ACS Nano, 2018, 12(4), 3333–3340 CrossRef CAS PubMed.
- K. D. Wegner, P. T. Lanh, T. Jennings, E. Oh, V. Jain and S. M. Fairclough, et al., Influence of Luminescence Quantum Yield, Surface Coating, and Functionalization of Quantum Dots on the Sensitivity of Time-Resolved FRET Bioassays, ACS Appl. Mater. Interfaces, 2013, 5(8), 2881–2892 CrossRef CAS PubMed.
- I. V. Martynenko, A. P. Litvin, F. Purcell-Milton, A. V. Baranov, A. V. Fedorov and Y. K. Gun'ko, Application of semiconductor quantum dots in bioimaging and biosensing, J. Mater. Chem. B, 2017, 5(33), 6701–6727 RSC.
- S. Walia, C. Sharma and A. Acharya, Biocompatible Fluorescent Nanomaterials for Molecular Imaging Applications, in Nanomaterial - Based Biomedical Applications in Molecular Imaging, Diagnostics and Therapy, ed. A. Acharya, Springer, Singapore, 2020. p. 27–53. DOI:10.1007/978-981-15-4280-0_3.
- D. Aldakov, A. Lefrançois and P. Reiss, Ternary and quaternary metal chalcogenide nanocrystals: synthesis, properties and applications, J. Mater. Chem. C, 2013, 1(24), 3756–3776 RSC.
- Y. Lou, Y. Zhao, J. Chen and J. J. Zhu, Metal ions optical sensing by semiconductor quantum dots, J. Mater. Chem. C, 2013, 2(4), 595–613 RSC.
- S. Liu and X. Su, The synthesis and application of I–III–VI type quantum dots, RSC Adv., 2014, 4(82), 43415–43428 RSC.
- G. Xu, S. Zeng, B. Zhang, M. T. Swihart, K. T. Yong and P. N. Prasad, New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine, Chem. Rev., 2016, 116(19), 12234–12327 CrossRef CAS PubMed.
- O. Chen, H. Wei, A. Maurice, M. Bawendi and P. Reiss, Pure colors from core–shell quantum dots, MRS Bull., 2013, 38(9), 696–702 CrossRef CAS.
- M. A. Cotta, Quantum Dots and Their Applications: What Lies Ahead?, ACS Appl. Nano Mater., 2020, 3(6), 4920–4924 CrossRef CAS.
- Y. Ma, G. Mao, W. Huang, G. Wu, W. Yin and X. Ji, et al., Quantum Dot Nanobeacons for Single RNA Labeling and Imaging, J. Am. Chem. Soc., 2019, 141(34), 13454–13458 CrossRef CAS PubMed.
- G. Lin, T. Chen, J. Zou, Y. Wang, X. Wang and J. Li, et al., Quantum Dots-siRNA Nanoplexes for Gene Silencing in Central Nervous System Tumor Cells, Front. Pharmacol., 2017, 8 DOI:10.3389/fphar.2017.00182.
- X. Ji, H. Wang, B. Song, B. Chu and Y. He, Silicon Nanomaterials for Biosensing and Bioimaging Analysis, Front. Chem., 2018, 6, 38 CrossRef PubMed.
- Y. Park, J. Yoo, M. H. Kang, W. Kwon and J. Joo, Photoluminescent and biodegradable porous silicon nanoparticles for biomedical imaging, J. Mater. Chem. B, 2019, 7(41), 6271–6292 RSC.
- F. Peng, Y. Su, Y. Zhong, C. Fan, S. T. Lee and Y. He, Silicon Nanomaterials Platform for Bioimaging, Biosensing, and Cancer Therapy, Acc. Chem. Res., 2014, 47(2), 612–623 CrossRef CAS PubMed.
- C. J. T. Robidillo and J. G. C. Veinot, Functional Bio-inorganic Hybrids from Silicon Quantum Dots and Biological Molecules, ACS Appl. Mater. Interfaces, 2020, 12(47), 52251–52270 CrossRef CAS PubMed.
- A. Chaix, E. Cueto-Diaz, A. Delalande, N. Knezevic, P. Midoux and J. O. Durand, et al., Amino-acid functionalized porous silicon nanoparticles for the delivery of pDNA, RSC Adv., 2019, 9(55), 31895–31899 RSC.
- T. Y. Wang, C. Y. Chen, C. M. Wang, Y. Z. Tan and W. S. Liao, Multicolor Functional Carbon Dots via One-Step Refluxing Synthesis, ACS Sens., 2017, 2(3), 354–363 CrossRef CAS PubMed.
- G. E. LeCroy, S. K. Sonkar, F. Yang, L. M. Veca, P. Wang and K. N. Tackett, et al., Toward Structurally Defined Carbon Dots as Ultracompact Fluorescent Probes, ACS Nano, 2014, 8(5), 4522–4529 CrossRef CAS PubMed.
- Q. Wang, C. Zhang, G. Shen, H. Liu, H. Fu and D. Cui, Fluorescent carbon dots as an efficient siRNA nanocarrier for its interference therapy in gastric cancer cells, J. Nanobiotechnol., 2014, 12(1), 58 CrossRef PubMed.
- S. M. Dadfar, D. Camozzi, M. Darguzyte, K. Roemhild, P. Varvarà and J. Metselaar, et al., Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance, J. Nanobiotechnol., 2020, 18(1), 22 CrossRef CAS PubMed.
- G. Tang, J. He, J. Liu, X. Yan and K. Fan, Nanozyme for tumor therapy: Surface modification matters, Exploration, 2021, 1(1), 75–89 CrossRef PubMed.
- Y. W. Jun, J. H. Lee and J. Cheon, Chemical Design of Nanoparticle Probes for High-Performance Magnetic Resonance Imaging, Angew. Chem., Int. Ed., 2008, 47(28), 5122–5135 CrossRef CAS PubMed.
- Y. Jun, J. Seo and J. Cheon, Nanoscaling Laws of Magnetic Nanoparticles and Their Applicabilities in Biomedical Sciences, Acc. Chem. Res., 2008, 41(2), 179–189 CrossRef CAS PubMed.
- Q. Xu, T. Zhang, Q. Wang, X. Jiang, A. Li and Y. Li, et al., Uniformly sized iron oxide nanoparticles for efficient gene delivery to mesenchymal stem cells, Int. J. Pharm., 2018, 552(1), 443–452 CrossRef CAS PubMed.
- H. Zhang, M. Y. Lee, M. G. Hogg, J. S. Dordick and S. T. Sharfstein, Gene Delivery in Three-Dimensional Cell Cultures by Superparamagnetic Nanoparticles, ACS Nano, 2010, 4(8), 4733–4743 CrossRef CAS PubMed.
- P. S. Kowalski, A. Rudra, L. Miao and D. G. Anderson, Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery, Mol. Ther., 2019, 27(4), 710–728 CrossRef CAS PubMed.
- N. Kamaly, B. Yameen, J. Wu and O. C. Farokhzad, Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release, Chem. Rev., 2016, 116(4), 2602–2663 CrossRef CAS PubMed.
- A. Zielińska, F. Carreiró, A. M. Oliveira, A. Neves, B. Pires and D. N. Venkatesh, et al., Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology, Molecules, 2020, 25(16), E3731 CrossRef PubMed.
- R. A. Youness, A. M. Al-Mahallawi, F. H. Mahmoud, H. Atta, M. Braoudaki and S. A. Fahmy, Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells, Polymers, 2023, 15(6), 1464 CrossRef CAS PubMed.
- A. Fabozzi, F. D. Sala, M. di Gennaro, N. Solimando, M. Pagliuca and A. Borzacchiello, Polymer based nanoparticles for biomedical applications by microfluidic techniques: from design to biological evaluation, Polym. Chem., 2021, 12(46), 6667–6687 RSC.
- M. Li, M. Zhao, Y. Fu, Y. Li, T. Gong and Z. Zhang, et al., Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways, J. Controlled Release, 2016, 228, 9–19 CrossRef CAS PubMed.
- J. Yan, R. Chen, H. Zhang and J. D. Bryers, Injectable Biodegradable Chitosan-Alginate 3D Porous Gel Scaffold for mRNA Vaccine Delivery, Macromol. Biosci., 2019, 19(2), e1800242 CrossRef PubMed.
- J. S. Ananta, R. Paulmurugan and T. F. Massoud, Nanoparticle-Delivered Antisense MicroRNA-21 Enhances the Effects of Temozolomide on Glioblastoma Cells, Mol. Pharmaceutics, 2015, 12(12), 4509–4517 CrossRef CAS PubMed.
- S. Zhu, H. Xing, P. Gordiichuk, J. Park and C. A. Mirkin, PLGA Spherical Nucleic Acids, Adv. Mater., 2018, 30(22), 1707113 CrossRef PubMed.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Engineering precision nanoparticles for drug delivery, Nat. Rev. Drug Discovery, 2021, 20(2), 101–124 CrossRef CAS PubMed.
- M. A. Hutnick, S. Ahsanuddin, L. Guan, M. Lam, E. D. Baron and J. K. Pokorski, PEGylated Dendrimers as Drug Delivery Vehicles for the Photosensitizer Silicon Phthalocyanine Pc 4 for Candidal Infections, Biomacromolecules, 2017, 18(2), 379–385 CrossRef CAS PubMed.
- V. Percec, M. Peterca, A. E. Dulcey, M. R. Imam, S. D. Hudson and S. Nummelin, et al., Hollow spherical supramolecular dendrimers, J. Am. Chem. Soc., 2008, 130(39), 13079–13094 CrossRef CAS PubMed.
- L. Palmerston Mendes, J. Pan and V. P. Torchilin, Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy, Molecules, 2017, 22(9), 1401 CrossRef PubMed.
- J. Wu, W. Huang and Z. He, Dendrimers as Carriers for siRNA Delivery and Gene Silencing: A Review, Sci. World J., 2013, 2013, e630654 CrossRef PubMed.
- F. Palombarini, S. Masciarelli, A. Incocciati, F. Liccardo, E. Di Fabio and A. Iazzetti, et al., Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells, J. Nanobiotechnol., 2021, 19(1), 172 CrossRef CAS PubMed.
- S. L. Mekuria, J. Li, C. Song, Y. Gao, Z. Ouyang and M. Shen, et al., Facile Formation of PAMAM Dendrimer Nanoclusters for Enhanced Gene Delivery and Cancer Gene Therapy, ACS Appl. Bio Mater., 2021, 4(9), 7168–7175 CrossRef CAS PubMed.
- M. Labieniec-Watala and C. Watala, PAMAM Dendrimers: Destined for Success or Doomed to Fail? Plain and Modified PAMAM Dendrimers in the Context of Biomedical Applications, J. Pharm. Sci., 2015, 104(1), 2–14 CrossRef CAS PubMed.
- K. Furuno, K. Suzuki and S. Sakai, Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery, Biomolecules, 2022, 12(11), 1638 CrossRef CAS PubMed.
- H. Cao, X. Jiang, C. Chai and S. Y. Chew, RNA interference by nanofiber-based siRNA delivery system, J. Controlled Release, 2010, 144(2), 203–212 CrossRef CAS PubMed.
- C. Pinese, J. Lin, U. Milbreta, M. Li, Y. Wang and K. W. Leong, et al., Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing, Acta Biomater., 2018, 76, 164–177 CrossRef CAS PubMed.
- S. Hong, D. W. Choi, H. N. Kim, C. G. Park, W. Lee and H. H. Park, Protein-Based Nanoparticles as Drug Delivery Systems, Pharmaceutics, 2020, 12(7), 604 CrossRef CAS PubMed.
- A. Jain, S. K. Singh, S. K. Arya, S. C. Kundu and S. Kapoor, Protein Nanoparticles: Promising Platforms for Drug Delivery Applications, ACS Biomater. Sci. Eng., 2018, 4(12), 3939–3961 CrossRef CAS PubMed.
- F. F. An and X. H. Zhang, Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery, Theranostics, 2017, 7(15), 3667–3689 CrossRef CAS PubMed.
- I. Cucinotto, L. Fiorillo, S. Gualtieri, M. Arbitrio, D. Ciliberto and N. Staropoli, et al., Nanoparticle Albumin Bound Paclitaxel in the Treatment of Human Cancer: Nanodelivery Reaches Prime-Time?, J. Drug Delivery, 2013, 2013, 905091 Search PubMed.
- W. Ma, A. Saccardo, D. Roccatano, D. Aboagye-Mensah, M. Alkaseem and M. Jewkes, et al., Modular assembly of proteins on nanoparticles, Nat. Commun., 2018, 9(1), 1489 CrossRef PubMed.
- M. Mendes, J. Sousa, A. Pais and C. Vitorino, 4 - Clinical applications of nanostructured drug delivery systems: From basic research to translational medicine, in Core-Shell Nanostructures for Drug Delivery and Theranostics, ed. M. L. Focarete and A. Tampieri, Woodhead Publishing, 2018. p. 43–116. (Woodhead Publishing Series in Biomaterials). Available from: https://www.sciencedirect.com/science/article/pii/B9780081021989000041 Search PubMed.
- G. Wei, Y. Wang, X. Huang, H. Hou and S. Zhou, Peptide-Based Nanocarriers for Cancer Therapy, Small Methods, 2018, 2(9), 1700358 CrossRef.
- K. Han, Z. Ma and H. Han, Functional peptide-based nanoparticles for photodynamic therapy, J. Mater. Chem. B, 2017, 6(1), 25–38 RSC.
- M. Abbas, H. H. Susapto and C. A. E. Hauser, Synthesis and Organization of Gold-Peptide Nanoparticles for Catalytic Activities, ACS Omega, 2022, 7(2), 2082–2090 CrossRef CAS PubMed.
- N. Jia, J. Ma, Y. Gao, H. Hu, D. Chen and X. Zhao, et al., HA-Modified R8-Based Bola-Amphiphile Nanocomplexes for Effective Improvement of siRNA Delivery Efficiency, ACS Biomater. Sci. Eng., 2020, 6(4), 2084–2093 CrossRef CAS PubMed.
- M. Chandler and K. A. Afonin, Smart-Responsive Nucleic Acid Nanoparticles (NANPs) with the Potential to Modulate Immune Behavior, Nanomaterials, 2019, 9(4), 611 CrossRef CAS PubMed.
- A. J. Mukalel, R. S. Riley, R. Zhang and M. J. Mitchell, Nanoparticles for nucleic acid delivery: Applications in cancer immunotherapy, Cancer Lett., 2019, 458, 102–112 CrossRef CAS PubMed.
- G. Huang, C. Su, L. Wang, Y. Fei and J. Yang, The Application of Nucleic Acid Probe–Based Fluorescent Sensing and Imaging in Cancer Diagnosis and Therapy, Front. Chem., 2021, 9 DOI:10.3389/fchem.2021.705458.
- J. A. Kulkarni, D. Witzigmann, S. B. Thomson, S. Chen, B. R. Leavitt and P. R. Cullis, et al., The current landscape of nucleic acid therapeutics, Nat. Nanotechnol., 2021, 16(6), 630–643 CrossRef CAS PubMed.
- W. Ma, Y. Zhan, Y. Zhang, C. Mao, X. Xie and Y. Lin, The biological applications of DNA nanomaterials: current challenges and future directions, Sig. Transduct. Target Ther., 2021, 6, 351 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/34620843/.
- S. Walia, A. R. Chandrasekaran, B. Chakraborty and D. Bhatia, Aptamer-Programmed DNA Nanodevices for Advanced, Targeted Cancer Theranostics, ACS Appl. Bio Mater., 2021, 4(7), 5392–5404 CrossRef CAS PubMed.
- U. Singh, V. Morya, A. Rajwar, A. R. Chandrasekaran, B. Datta, C. Ghoroi and D. Bhatia, DNA-Functionalized Nanoparticles for Targeted Biosensing and Biological Applications, ACS Omega, 2020, 5(48), 30767–30774 CrossRef CAS PubMed , Available from: https://pubmed.ncbi.nlm.nih.gov/33324786/.
- N. L. Rosi, D. A. Giljohann, C. S. Thaxton, A. K. R. Lytton-Jean, M. S. Han and C. A. Mirkin, Oligonucleotide-modified gold nanoparticles for intracellular gene regulation, Science, 2006, 312(5776), 1027–1030 CrossRef CAS PubMed.
- H. Li, B. Zhang, X. Lu, X. Tan, F. Jia and Y. Xiao, et al., Molecular spherical nucleic acids, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(17), 4340–4344 CrossRef CAS PubMed.
- Y. W. Cao, R. Jin and C. A. Mirkin, DNA-Modified Core−Shell Ag/Au Nanoparticles, J. Am. Chem. Soc., 2001, 123(32), 7961–7962 CrossRef CAS PubMed.
- Y. Ji and J. B. Lee, DNA microsponge-templated growth of metal nanoparticles for signal-enhanced colorimetric detection, Appl. Surf. Sci., 2021, 569, 151028 CrossRef CAS.
- K. L. Young, A. W. Scott, L. Hao, S. E. Mirkin, G. Liu and C. A. Mirkin, Hollow spherical nucleic acids for intracellular gene regulation based upon biocompatible silica shells, Nano Lett., 2012, 12(7), 3867–3871 CrossRef CAS PubMed.
- B. Deng, B. Ma, Y. Ma, P. Cao, X. Leng and P. Huang, et al., Doxorubicin and CpG loaded liposomal spherical nucleic acid for enhanced Cancer treatment, J. Nanobiotechnol., 2022, 20(1), 140 CrossRef CAS PubMed.
- C. E. Callmann, C. D. Kusmierz, J. W. Dittmar, L. Broger and C. A. Mirkin, Impact of Liposomal Spherical Nucleic Acid Structure on Immunotherapeutic Function, ACS Cent. Sci., 2021, 7(5), 892–899 CrossRef CAS PubMed.
- C. A. Mirkin, C. Laramy and K. Skakuj, The Power of Spheres, Nature, 2019, 576(7785), S3–S7 CrossRef CAS.
- C. H. J. Choi, L. Hao, S. P. Narayan, E. Auyeung and C. A. Mirkin, Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(19), 7625–7630 CrossRef CAS PubMed.
- S. C. P. Williams, Spherical nucleic acids: A whole new ball game, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(33), 13231–13233 CrossRef CAS PubMed.
- J. R. Melamed, N. L. Kreuzberger, R. Goyal and E. S. Day, Spherical Nucleic Acid Architecture Can Improve the Efficacy of Polycation-Mediated siRNA Delivery, Mol. Ther.–Nucleic Acids, 2018, 12, 207–219 CrossRef PubMed.
- S. Wang, L. Qin, G. Yamankurt, K. Skakuj, Z. Huang and P. C. Chen, et al., Rational vaccinology with spherical nucleic acids, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(21), 10473–10481 CrossRef CAS PubMed.
- H. Tr and P. As, Gene Regulation Using Spherical Nucleic Acids to Treat Skin Disorders, Pharmaceuticals, 2020, 13(11) Search PubMed , Available from: https://pubmed.ncbi.nlm.nih.gov/33147737/.
- P. S. Randeria, M. A. Seeger, X. Q. Wang, H. Wilson, D. Shipp and C. A. Mirkin, et al., siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(18), 5573–5578 CrossRef CAS PubMed.
- W. Poon, B. R. Kingston, B. Ouyang, W. Ngo and W. C. W. Chan, A framework for designing delivery systems, Nat. Nanotechnol., 2020, 15(10), 819–829 CrossRef CAS PubMed.
- P. R. Cullis and M. J. Hope, Lipid Nanoparticle Systems for Enabling Gene Therapies, Mol. Ther., 2017, 25(7), 1467–1475 CrossRef CAS PubMed.
- Y. Eygeris, M. Gupta, J. Kim and G. Sahay, Chemistry of Lipid Nanoparticles for RNA Delivery, Acc. Chem. Res., 2022, 55(1), 2–12 CrossRef CAS PubMed.
- L. Miao, L. Li, Y. Huang, D. Delcassian, J. Chahal and J. Han, et al., Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation, Nat. Biotechnol., 2019, 37(10), 1174–1185 CrossRef CAS PubMed.
- M. Sun, U. J. Dang, Y. Yuan, A. M. Psaras, O. Osipitan and T. A. Brooks, et al., Optimization of DOTAP/chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery, AAPS PharmSciTech, 2022, 23(5), 135 CrossRef CAS PubMed.
- Z. Ye, J. Chen, X. Zhao, Y. Li, J. Harmon and C. Huang, et al., In Vitro Engineering Chimeric Antigen Receptor Macrophages and T Cells by Lipid Nanoparticle-Mediated mRNA Delivery, ACS Biomater. Sci. Eng., 2022, 8(2), 722–733 CrossRef CAS PubMed.
- Y. Hagino, I. A. Khalil, S. Kimura, K. Kusumoto and H. Harashima, GALA-Modified Lipid Nanoparticles for the Targeted Delivery of Plasmid DNA to the Lungs, Mol. Pharmaceutics, 2021, 18(3), 878–888 CrossRef CAS PubMed.
- A. Algarni, E. H. Pilkington, E. J. A. Suys, H. Al-Wassiti, C. W. Pouton and N. P. Truong, In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression, Biomater. Sci., 2022, 10(11), 2940–2952 RSC.
- S. Scalzo, A. K. Santos, H. A. Ferreira, P. A. Costa, P. H. Prazeres and N. J. da Silva, et al., Ionizable Lipid Nanoparticle-Mediated Delivery of Plasmid DNA in Cardiomyocytes, IJN, 2022, 17, 2865–2881 CrossRef PubMed.
- R. Pattipeiluhu, G. Arias-Alpizar, G. Basha, K. Y. T. Chan, J. Bussmann and T. H. Sharp, et al., Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System, Adv. Mater., 2022, 34(16), 2201095 CrossRef CAS PubMed.
- B. Hu, B. Li, K. Li, Y. Liu, C. Li and L. Zheng, et al., Thermostable ionizable lipid-like nanoparticle (iLAND) for RNAi treatment of hyperlipidemia, Sci. Adv., 2022, 8(7), eabm1418 CrossRef CAS PubMed.
- M. R. Mohammadabadi, M. El-Tamimy, R. Gianello and M. R. Mozafari, Supramolecular assemblies of zwitterionic nanoliposome-polynucleotide complexes as gene transfer vectors: Nanolipoplex formulation and in vitro characterisation, J. Liposome Res., 2009, 19(2), 105–115 CrossRef CAS PubMed.
- L. Moreira, N. M. Guimarães, S. Pereira, R. S. Santos, J. A. Loureiro and M. C. Pereira, et al., Liposome Delivery of Nucleic Acids in Bacteria: Toward In Vivo Labeling of Human Microbiota, ACS Infect. Dis., 2022, 8(7), 1218–1230 CrossRef CAS PubMed.
- H. K. Dhaliwal, Y. Fan, J. Kim and M. M. Amiji, Intranasal Delivery and Transfection of mRNA Therapeutics in the Brain Using Cationic Liposomes, Mol. Pharmaceutics, 2020, 17(6), 1996–2005 CrossRef CAS PubMed.
- S. Lei, X. Zhang, K. Men, Y. Gao, X. Yang and S. Wu, et al., Efficient Colorectal Cancer Gene Therapy with IL-15 mRNA Nanoformulation, Mol. Pharmaceutics, 2020, 17(9), 3378–3391 CrossRef CAS PubMed.
- M. M. Billingsley, A. G. Hamilton, D. Mai, S. K. Patel, K. L. Swingle and N. C. Sheppard, et al., Orthogonal Design of Experiments for Optimization of Lipid Nanoparticles for mRNA Engineering of CAR T Cells, Nano Lett., 2022, 22(1), 533–542 CrossRef CAS PubMed.
- A. J. Sinegra, M. Evangelopoulos, J. Park, Z. Huang and C. A. Mirkin, Lipid Nanoparticle Spherical Nucleic Acids for Intracellular DNA and RNA Delivery, Nano Lett., 2021, 21(15), 6584–6591 CrossRef CAS PubMed.
- A. M. Aldayel, H. L. O'Mary, S. A. Valdes, X. Li, S. G. Thakkar and B. E. Mustafa, et al., Lipid nanoparticles with minimum burst release of TNF-α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate, J. Controlled Release, 2018, 283, 280–289 CrossRef CAS PubMed.
- Y. Han, P. Zhang, Y. Chen, J. Sun and F. Kong, Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy, Int. J. Mol. Med., 2014, 34(1), 191–196 CrossRef CAS PubMed.
- C. Song, S. Zhang, Q. Zhou, L. Shi, L. Du and D. Zhi, et al., Bifunctional cationic solid lipid nanoparticles of β-NaYF4:Yb,Er upconversion nanoparticles coated with a lipid for bioimaging and gene delivery, RSC Adv., 2017, 7(43), 26633–26639 RSC.
- K. Paunovska, D. Loughrey and J. E. Dahlman, Drug delivery systems for RNA therapeutics, Nat. Rev. Genet., 2022, 23(5), 265–280 CrossRef CAS PubMed.
- A. G. Niculescu, A. C. Bîrcă and A. M. Grumezescu, New Applications of Lipid and Polymer-Based Nanoparticles for Nucleic Acids Delivery, Pharmaceutics, 2021, 13(12), 2053 CrossRef CAS PubMed.
- C. Zeng, C. Zhang, P. G. Walker and Y. Dong, Formulation and Delivery Technologies for mRNA Vaccines, Curr. Top. Microbiol. Immunol., 2022, 440, 71–110 Search PubMed.
- Q. Cheng, T. Wei, L. Farbiak, L. T. Johnson, S. A. Dilliard and D. J. Siegwart, Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing, Nat. Nanotechnol., 2020, 15(4), 313–320 CrossRef CAS PubMed.
- J. Kim, A. Jozic and G. Sahay, Naturally Derived Membrane Lipids Impact Nanoparticle-Based Messenger RNA Delivery, Cell. Mol. Bioeng., 2020, 13(5), 463–474 CrossRef CAS PubMed.
- X. Hou, T. Zaks, R. Langer and Y. Dong, Lipid nanoparticles for mRNA delivery, Nat. Rev. Mater., 2021, 6(12), 1078–1094 CrossRef CAS PubMed.
- C. Zhang, Y. Ma, J. Zhang, J. C. T. Kuo, Z. Zhang and H. Xie, et al., Modification of Lipid-Based Nanoparticles: An Efficient Delivery System for Nucleic Acid-Based Immunotherapy, Molecules, 2022, 27(6), 1943 CrossRef CAS PubMed.
- M. A. Maier, M. Jayaraman, S. Matsuda, J. Liu, S. Barros and W. Querbes, et al., Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics, Mol. Ther., 2013, 21(8), 1570–1578 CrossRef CAS PubMed.
- A. Spadea, M. Jackman, L. Cui, S. Pereira, M. J. Lawrence and R. A. Campbell, et al., Nucleic Acid-Loaded Lipid Nanoparticle Interactions with Model Endosomal Membranes, ACS Appl. Mater. Interfaces, 2022, 14(26), 30371–30384 CrossRef CAS PubMed.
- P. L. Felgner, T. R. Gadek, M. Holm, R. Roman, H. W. Chan and M. Wenz, et al., Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure, Proc. Natl. Acad. Sci. U. S. A., 1987, 84(21), 7413–7417 CrossRef CAS PubMed.
- M. Schlich, R. Palomba, G. Costabile, S. Mizrahy, M. Pannuzzo and D. Peer, et al., Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles, Bioeng. Transl. Med., 2021, e10213 CrossRef CAS PubMed.
- K. A. Mislick and J. D. Baldeschwieler, Evidence for the role of proteoglycans in cation-mediated gene transfer, Proc. Natl. Acad. Sci. U. S. A., 1996, 93(22), 12349–12354 CrossRef CAS PubMed.
- H. C. Huang, P. Y. Chang, K. Chang, C. Y. Chen, C. W. Lin and J. H. Chen, et al., Formulation of novel lipid-coated magnetic nanoparticles as the probe for in vivo imaging, J. Biomed. Sci., 2009, 16(1), 86 CrossRef PubMed.
- M. Sun, U. J. Dang, Y. Yuan, A. M. Psaras, O. Osipitan and T. A. Brooks, et al., Optimization of DOTAP/chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery, AAPS PharmSciTech, 2022, 23(5), 135 CrossRef CAS PubMed.
- Y. Zhang, C. Sun, C. Wang, K. E. Jankovic and Y. Dong, Lipids and Lipid Derivatives for RNA Delivery, Chem. Rev., 2021, 121(20), 12181–12277 CrossRef CAS PubMed.
- D. Zhi, S. Zhang, S. Cui, Y. Zhao, Y. Wang and D. Zhao, The headgroup evolution of cationic lipids for gene delivery, Bioconjugate Chem., 2013, 24(4), 487–519 CrossRef CAS PubMed.
- F. Ponti, M. Campolungo, C. Melchiori, N. Bono and G. Candiani, Cationic lipids for gene delivery: many players, one goal, Chem. Phys. Lipids, 2021, 235, 105032 CrossRef CAS PubMed.
- S. Cui, Y. Wang, Y. Gong, X. Lin, Y. Zhao and D. Zhi, et al., Correlation of the cytotoxic effects of cationic lipids with their headgroups, Toxicol. Res., 2018, 7(3), 473–479 CrossRef CAS PubMed.
- S. J. H. Soenen, A. R. Brisson and M. De Cuyper, Addressing the problem of cationic lipid-mediated toxicity: The magnetoliposome model, Biomaterials, 2009, 30(22), 3691–3701 CrossRef CAS PubMed.
- R. J. C. Bose, Y. Arai, J. C. Ahn, H. Park and S. H. Lee, Influence of cationic lipid concentration on properties of lipid-polymer hybrid nanospheres for gene delivery, Int. J. Nanomed., 2015, 10, 5367–5382 CAS.
- A. L. Bailey and P. R. Cullis, Modulation of membrane fusion by asymmetric transbilayer distributions of amino lipids, Biochemistry, 1994, 33(42), 12573–12580 CrossRef CAS PubMed.
- L. Miao, J. Lin, Y. Huang, L. Li, D. Delcassian and Y. Ge, et al., Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver, Nat. Commun., 2020, 11(1), 2424 CrossRef CAS PubMed.
- M. M. Żak and L. Zangi, Lipid Nanoparticles for Organ-Specific mRNA Therapeutic Delivery, Pharmaceutics, 2021, 13(10), 1675 CrossRef PubMed.
- S. C. Semple, S. K. Klimuk, T. O. Harasym, N. Dos Santos, S. M. Ansell and K. F. Wong, et al., Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures, Biochim. Biophys. Acta, 2001, 1510(1–2), 152–166 CrossRef CAS PubMed.
- M. Paloncýová, P. Čechová, M. Šrejber, P. Kührová and M. Otyepka, Role of Ionizable Lipids in SARS-CoV-2 Vaccines As Revealed by Molecular Dynamics Simulations: From Membrane Structure to Interaction with mRNA Fragments, J. Phys. Chem. Lett., 2021, 12(45), 11199–11205 CrossRef PubMed.
- M. Kim, M. Jeong, S. Hur, Y. Cho, J. Park and H. Jung, et al., Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver, Sci. Adv., 2021, 7(9), eabf4398 CrossRef CAS PubMed.
- C. L. Walsh, J. Nguyen, M. R. Tiffany and F. C. Szoka, Synthesis, characterization, and evaluation of ionizable lysine-based lipids for siRNA delivery, Bioconjugate Chem., 2013, 24(1), 36–43 CrossRef CAS PubMed.
- X. Yu, S. Liu, Q. Cheng, T. Wei, S. Lee and D. Zhang, et al., Lipid-Modified Aminoglycosides for mRNA Delivery to the Liver, Adv. Healthcare Mater., 2020, 9(7), 1901487 CrossRef CAS PubMed.
- T. Colombani, P. Peuziat, L. Dallet, T. Haudebourg, M. Mével and M. Berchel, et al., Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery, J. Controlled Release, 2017, 249, 131–142 CrossRef CAS PubMed.
- K. Bromma, K. Rieck, J. Kulkarni, C. O'Sullivan, W. Sung and P. Cullis, et al., Use of a lipid nanoparticle system as a Trojan horse in delivery of gold nanoparticles to human breast cancer cells for improved outcomes in radiation therapy, Cancer Nanotechnol., 2019, 10(1), 1 CrossRef.
- A. Erfani, J. Seaberg, C. P. Aichele and J. D. Ramsey, Interactions between Biomolecules and Zwitterionic Moieties: A Review, Biomacromolecules, 2020, 21(7), 2557–2573 CrossRef CAS PubMed.
- D. Montizaan, K. Yang, C. Reker-Smit and A. Salvati, Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells, Nanomedicine, 2020, 30, 102300 CrossRef CAS PubMed.
- Y. Obata, S. Tajima and S. Takeoka, Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo, J. Controlled Release, 2010, 142(2), 267–276 CrossRef CAS PubMed.
- W. Wang, S. Jiang, S. Li, X. Yan, S. Liu and X. Mao, et al., Functional Choline Phosphate Lipids for Enhanced Drug Delivery in Cancer Therapy, Chem. Mater., 2021, 33(2), 774–781 CrossRef CAS.
- M. Zhang, A. Hussain, H. Yang, J. Zhang, X. J. Liang and Y. Huang, mRNA-based modalities for infectious disease management, Nano Res., 2023, 16(1), 672–691 CrossRef CAS PubMed.
- T. M. Allen and P. R. Cullis, Liposomal drug delivery systems: from concept to clinical applications, Adv. Drug Delivery Rev., 2013, 65(1), 36–48 CrossRef CAS PubMed.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami and Y. Hanifehpour, et al., Liposome: classification, preparation, and applications, Nanoscale Res. Lett., 2013, 8(1), 102 CrossRef PubMed.
- M. N. Vu, P. Rajasekhar, D. P. Poole, S. Y. Khor, N. P. Truong and C. J. Nowell, et al., Rapid assessment of nanoparticle extravasation in a microfluidic tumor model, ACS Appl. Nano Mater., 2019, 2(4), 1844–1856 CrossRef CAS.
- X. Gong, J. Wu, J. Wen, X. Ding, N. Xu and M. Sun, et al., Dual-Ligand-Modified Nanoscale Liposomes Loaded with Curcumin and Metformin Inhibit Drug Resistance and Metastasis of Hepatocellular Carcinoma, ACS Appl. Nano Mater., 2022, 5(5), 7063–7077 CrossRef CAS.
- T. Michel, D. Luft, M. K. Abraham, S. Reinhardt, M. L. Salinas Medina and J. Kurz, et al., Cationic Nanoliposomes Meet mRNA: Efficient Delivery of Modified mRNA Using Hemocompatible and Stable Vectors for Therapeutic Applications, Mol. Ther.–Nucleic Acids, 2017, 8, 459–468 CrossRef CAS PubMed.
- H. Wood, FDA approves patisiran to treat hereditary transthyretin amyloidosis, Nat. Rev. Neurol., 2018, 14(10), 570–570 Search PubMed.
- H. H. Ly, S. Daniel, S. K. V. Soriano, Z. Kis and A. K. Blakney, Optimization of Lipid Nanoparticles for saRNA Expression and Cellular Activation Using a Design-of-Experiment Approach, Mol. Pharmaceutics, 2022, 19(6), 1892–1905 CrossRef CAS PubMed.
- A. R. Ferhan, S. Park, H. Park, H. Tae, J. A. Jackman and N. J. Cho, Lipid Nanoparticle Technologies for Nucleic Acid Delivery: A Nanoarchitectonics Perspective, Adv. Funct. Mater., 2022, 2203669 CrossRef CAS.
- B. L. Mui, Y. K. Tam, M. Jayaraman, S. M. Ansell, X. Du and Y. Y. C. Tam, et al., Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles, Mol. Ther.–Nucleic Acids, 2013, 2, e139 CrossRef CAS PubMed.
- Y. Duan, A. Dhar, C. Patel, M. Khimani, S. Neogi and P. Sharma, et al., A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems, RSC Adv., 2020, 10(45), 26777–26791 RSC.
- S. Scioli Montoto, G. Muraca and M. E. Ruiz, Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects, Front. Mol. Biosci., 2020, 7 DOI:10.3389/fmolb.2020.587997.
- P. Ghasemiyeh and S. Mohammadi-Samani, Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages, Res. Pharm. Sci., 2018, 13(4), 288–303 CrossRef PubMed.
- R. K. Shirodkar, L. Kumar, S. Mutalik and S. Lewis, Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems, Pharm. Chem. J., 2019, 53(5), 440–453 CrossRef CAS.
- R. Tenchov, R. Bird, A. E. Curtze and Q. Zhou, Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement, ACS Nano, 2021, 15(11), 16982–17015 CrossRef CAS PubMed.
- S. Beg, A. K. Malik, M. J. Ansari, A. A. Malik, A. M. A. Ali and A. Theyab, et al., Systematic Development of Solid Lipid Nanoparticles of Abiraterone Acetate with Improved Oral Bioavailability and Anticancer Activity for Prostate Carcinoma Treatment, ACS Omega, 2022, 7(20), 16968–16979 CrossRef CAS PubMed.
- M. Kotmakçı, H. Akbaba, G. Erel, G. Ertan and G. Kantarcı, Improved Method for Solid Lipid Nanoparticle Preparation Based on Hot Microemulsions: Preparation, Characterization, Cytotoxicity, and Hemocompatibility Evaluation, AAPS PharmSciTech, 2017, 18(4), 1355–1365 CrossRef PubMed.
- E. B. Souto, S. Doktorovova, A. Zielinska and A. M. Silva, Key production parameters for the development of solid lipid nanoparticles by high shear homogenization, Pharm. Dev. Technol., 2019, 24(9), 1181–1185 CrossRef CAS PubMed.
- H. B. Abo-zalam, E. S. El-Denshary, R. M. Abdelsalam, I. A. Khalil, M. M. Khattab and M. A. Hamzawy, Therapeutic advancement of simvastatin-loaded solid lipid nanoparticles (SV-SLNs) in treatment of hyperlipidemia and attenuating hepatotoxicity, myopathy and apoptosis: Comprehensive study, Biomed. Pharmacother., 2021, 139, 111494 CrossRef CAS PubMed.
- V. A. Duong, T. T. L. Nguyen and H. J. Maeng, Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method, Molecules, 2020, 25(20), E4781 CrossRef PubMed.
- A. Penumarthi, D. Parashar, A. N. Abraham, C. Dekiwadia, I. Macreadie and R. Shukla, et al., Solid lipid nanoparticles mediate non-viral delivery of plasmid DNA to dendritic cells, J. Nanopart. Res., 2017, 19(6), 210 CrossRef.
- Y. C. Barenholz, Doxil®—The first FDA-approved nano-drug: Lessons learned, J. Controlled Release, 2012, 160(2), 117–134 CrossRef CAS PubMed.
- A. C. Anselmo and S. Mitragotri, Nanoparticles in the clinic: An update, Bioeng. Transl. Med., 2019, 4(3), e10143 CrossRef PubMed.
- Commissioner O of the. Moderna COVID-19 Vaccines, FDA, 2023, Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccines.
- T. T. H. Thi, E. J. A. Suys, J. S. Lee, D. H. Nguyen, K. D. Park and N. P. Truong, Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines, Vaccines, 2021, 9(4), 359 CrossRef CAS PubMed.
- A. J. Debacker, J. Voutila, M. Catley, D. Blakey and N. Habib, Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug, Mol. Ther., 2020, 28(8), 1759–1771 CrossRef CAS PubMed.
- A. I. S. van den Berg, C. O. Yun, R. M. Schiffelers and W. E. Hennink, Polymeric delivery systems for nucleic acid therapeutics: Approaching the clinic, J. Controlled Release, 2021, 331, 121–141 CrossRef CAS PubMed.
- Y. Guo, X. Cao, X. Zheng, S. J. Abbas, J. Li and W. Tan, Construction of nanocarriers based on nucleic acids and their applications in nanobiology delivery systems, Natl. Sci. Rev., 2022, 9(5), nwac006 CrossRef CAS PubMed.
- H. Sabit, M. Abdel-Hakeem, T. Shoala, S. Abdel-Ghany, M. M. Abdel-Latif and J. Almulhim, et al., Nanocarriers: A Reliable Tool for the Delivery of Anticancer Drugs, Pharmaceutics, 2022, 14(8), 1566 CrossRef CAS PubMed.
- S. Khatun, C. L. Putta, A. Hak and A. K. Rengan, Immunomodulatory nanosystems: An emerging strategy to combat viral infections, Biomater. Biosyst., 2023, 9, 100073 CAS.
- S. Su and P. M. Kang, Systemic Review of Biodegradable Nanomaterials in Nanomedicine, Nanomaterials, 2020, 10(4), 656 CrossRef CAS PubMed.
- J. M. Benns, J. S. Choi, R. I. Mahato, J. S. Park and S. W. Kim, pH-Sensitive Cationic Polymer Gene Delivery Vehicle: N-Ac-poly(l-histidine)-graft-poly(l-lysine) Comb Shaped Polymer, Bioconjugate Chem., 2000, 11(5), 637–645 CrossRef CAS PubMed.
- Y. Huang, X. Guo, Y. Wu, X. Chen, L. Feng and N. Xie, et al., Nanotechnology's frontier in combatting infectious and inflammatory diseases: prevention and treatment, Signal Transduction Targeted Ther., 2024, 9(1), 1–50 CrossRef PubMed.
- L. Sun, H. Liu, Y. Ye, Y. Lei, R. Islam and S. Tan, et al., Smart nanoparticles for cancer therapy, Signal Transduction Targeted Ther., 2023, 8, 418 CrossRef CAS PubMed.
- M. Chehelgerdi, M. Chehelgerdi, O. Q. B. Allela, R. D. C. Pecho, N. Jayasankar and D. P. Rao, et al., Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation, Mol. Cancer, 2023, 22, 169 CrossRef PubMed.
- Z. Hejdankova, V. Vanek, F. Sedlak, J. Prochazka, A. Diederichs and S. Kereïche, et al., Lipid Nanoparticles for Broad-Spectrum Nucleic Acid Delivery, Adv. Funct. Mater., 2021, 31(47), 2101391 CrossRef CAS.
- E. Samaridou, J. Heyes and P. Lutwyche, Lipid nanoparticles for nucleic acid delivery: Current perspectives, Adv. Drug Delivery Rev., 2020, 154–155, 37–63 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
