 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Cu(II)-Mediated aerobic oxidative synthesis of sulfonated chromeno[4,3-c]pyrazol-4(2H)-ones
Quan
Zhou
a,
Hai-Yan
Yu
a,
Yaqing
Zhou
b,
Jing-Ru
Wei
a and
Lei
Wang
*ac
aAdvanced Research Institute and Department of Chemistry, Taizhou University, Taizhou, Zhejiang 318000, P. R. China. E-mail: leiwang@chnu.edu.cn
bDepartment of Chemistry, Taizhou Jiaxin Metering and Testing Co. Ltd, Taizhou, Zhejiang 317000, P. R. China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, P. R. China
First published on 20th November 2024
Abstract
Correction for ‘Cu(II)-Mediated aerobic oxidative synthesis of sulfonated chromeno[4,3-c]pyrazol-4(2H)-ones’ by Quan Zhou et al., Org. Biomol. Chem., 2022, 20, 5575–5581, https://doi.org/10.1039/D2OB00639A.
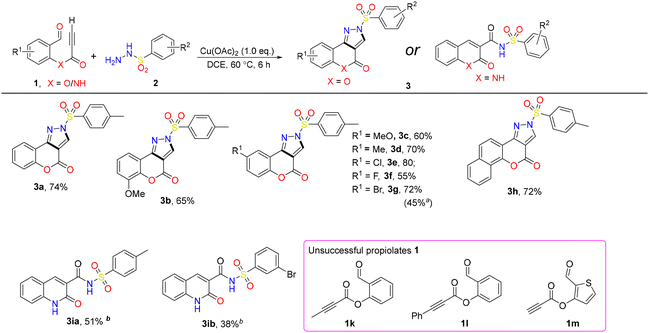
The authors regret that the molecules 3ia, 3ib in the original article (Scheme 2) were incorrectly reported. Instead, they are N-sulfonated quinolin-2(1H)-one-3-carboxamides, which was disclosed by X-ray diffraction and reported by the authors in a recent publication.1
The corrections to the article are detailed below.
1. The correct Scheme 2 is shown here incorporating the corrected structures for 3ia, 3ib.
2. In the Experimental section the corrected details for 3ia and 3ib are:
2-Oxo-N-tosyl-1,2-dihydroquinoline-3-carboxamide (3ia). White solid (51 mg, 49%); mp: 298–301 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.85 (s, 1H), 7.94 (d, J = 8.2 Hz, 3H), 7.76–7.65 (m, 1H), 7.45 (d, J = 8.2 Hz, 3H), 7.33 (t, J = 7.5 Hz, 1H), 2.39 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 167.3, 166.0, 151.5, 149.9, 145.1, 141.1, 139.4, 135.6, 134.9, 133.2, 128.7, 124.0, 123.9, 121.0, 26.3; HRMS (ESI) ([M + H]+) calcd for [C17H15N2O4S+]: 343.0747 (100.0%), found: 343.0745 (100%).
N-((3-Bromophenyl)sulfonyl)-2-oxo-1,2-dihydroquinoline-3-carboxamide (3ib). White solid (45 mg, 37%); mp: 265–270 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.93 (s, 1H), 8.27–8.20 (m, 1H), 8.11–8.06 (m, 1H), 7.98 (m, 2H), 7.75 (t, J = 7.8 Hz, 1H), 7.63 (t, J = 8.0 Hz, 1H), 7.48 (t, J = 8.7 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H); 13C NMR (101 MHz, DMSO) δ 162.6, 161.6, 147.0, 141.1, 140.4, 137.4, 134.8, 132.0, 130.9, 130.8, 127.4, 124.0, 122.3, 119.1, 116.3; HRMS (ESI) ([M + H]+) calcd for [C16H12BrN2O4S]: 406.9696 (100%), 408.9675 (97.3%), found: 406.9696 (100%), 408.9663 (97.3%).
3. In the abstract: “… we developed a concise and facile synthesis of 2-sulfonylated chromeno [4,3-c]pyrazol-4(2H)-ones or 2,5-dihydro-4H-pyrazolo[4,3-c]quinolin-4-ones via Cu(II)-promoted oxidative …” should read “… we developed a concise and facile synthesis of 2-sulfonylated chromeno[4,3-c]pyrazol-4(2H)-ones via Cu(II)-promoted oxidative …”
4. The table of contents graphic should be corrected to:
6. Conclusion: “Such strategy provides a quick avenue to develop a drug library of chromeno/quinolinone fused with pyrazole rings.” should read “Such a strategy provides a quick avenue to develop a drug library of chromeno fused with pyrazole rings.”
7. The corrected structures for compounds 3ia and 3ib have been included in the updated ESI.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- X. Chen, S.-J. Fang, Q. Zhou, W. Huang, Q.-L. Liu and L. Wang, Org. Biomol. Chem., 2024 10.1039/D4OB01071J.
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mmol scale, O2 (1 atm), 12 h;
4 mmol scale, O2 (1 atm), 12 h; 