Synthesis of quinoline mimics via C–H bond functionalization of quinoline: a review on recent progress
Inder
Kumar†
 ab,
Ritika
Sharma†
ab,
Ritika
Sharma†
 ac and
Upendra
Sharma
ac and
Upendra
Sharma
 *a
*a
aChemical Technology Division, CSIR-Institute of Himalayan Bioresource Technology, Palampur, India. E-mail: upendraihbt@gmail.com
bDepartment of Chemistry, KDC Government College Jaisinghpur, Kangra, HP, India
cDepartment of Chemistry, University of Delhi, 110007, India
First published on 28th January 2025
Abstract
Functionalization of the quinoline ring has emerged as a transformative strategy in modern synthetic chemistry because of the medicinal potential of quinoline-based scaffolds. The precise and selective introduction of diverse functional groups significantly expands the chemical space and enhances the pharmacological profile of quinoline derivatives. By carefully selecting catalysts, reaction conditions, and directing groups, researchers have unlocked novel pathways for the efficient synthesis of quinoline-based compounds with improved efficacy, target selectivity, and safety. This approach accelerates drug discovery and broadens the therapeutic potential of quinoline scaffolds for treating various diseases, including cancer, infectious diseases, and neurological disorders. Over the past two decades, this field has experienced exponential growth, as evidenced by the increasing number of research publications and comprehensive review articles. This surge in interest is driven by the potential of quinoline functionalization to generate novel drug candidates with enhanced bioactivity and reduced side effects. This review summarizes the key advancements from January 2021 to 2024, focusing on the latest methodologies, catalytic systems, and applications in drug development.
Introduction
Quinoline is a double-ring structure consisting of a benzene ring fused with a N-containing pyridine ring. Quinine, including several other members of the quinoline family, was isolated from the Cinchona tree and used to treat malaria.1 Quinoline's status as a privileged scaffold is well justified, given its multifaceted therapeutic potential. Its derivatives continue to play a pivotal role in the fight against malaria, cancer, etc. (Fig. 1).2–6 Ongoing research and development efforts aim to refine these compounds, improve their efficacy, and minimize side effects. Functionalization of quinoline is a versatile and robust strategy to enhance its pharmacological properties and broaden its therapeutic applications.7–9 This approach has introduced a new phase into the traditional methods of organic synthesis.10 Different research groups have conducted ample amounts of work to develop environment-friendly methodologies, achieving better results with less waste generation.11–14Different modes of functionalization of quinoline
Among various approaches for the functionalization of quinoline, the most effective one encompasses catalyst-controlled C–H activation/functionalization, oxidative cascade annulations including (4 + 2) annulations, substitution, reductive aromatization, and dearomatization via cross-coupling reactions.The metal-free C–H bond functionalization strategy offers an effective tool for researchers, being economical and environment friendly. This includes functionalization via light-induced reactions, EDA complex-enabled functionalization, etc. Besides this, the utility of C–H activation at this juncture lies in its atom economy and ability to functionalize inert C–H bonds without needing pre-functionalized substrates, offering a more sustainable and streamlined synthesis.15 The use of transition-metal catalysts such as palladium, rhodium, and ruthenium has revolutionized the scope of C–H activation, allowing for a range of transformations, including arylation, alkylation, amination, and oxidative couplings.16,17 Additionally, emerging developments in ligand design and the use of Earth-abundant metals are pushing the boundaries of selectivity and reactivity, while minimizing environmental impact.18–20 Functionalization of quinoline via C–H activation is a powerful tool in synthetic chemistry, allowing for the efficient and selective introduction of various functional groups.21,22 The choice of catalyst, reaction conditions, and directing groups can significantly influence the outcome of these transformations.
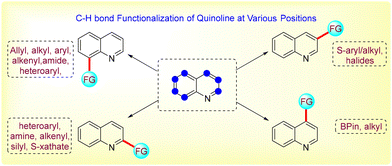
By modifying various positions on the quinoline ring, introducing heteroatoms, adding side chains, forming metal complexes, and utilizing photophysical properties, researchers can develop quinoline derivatives with improved efficacy, selectivity, and safety profiles.23,24 These modifications are essential for the continued success of quinoline-based drugs in treating a wide range of diseases. Consequently, different research groups disclosed modifications of the quinoline ring. In this review article, we have compiled the literature for the last five years and classified this research work based on the position of the quinoline ring's functionalization.
C-8 functionalization of quinoline
C-8 functionalization of quinoline represents a pivotal transformation in organic synthesis, enabling the diversification of quinoline derivatives. These compounds are relevant in diverse fields, such as medicinal chemistry, materials science, and catalysis. However, the inherent steric hindrance and electronic inaccessibility of the C-8 position present unique challenges, making the development of selective and efficient functionalization strategies advantageous. In light of this, transition-metal-catalyzed reactions remain at the forefront of C-8 functionalization due to their versatility and efficiency.25,26 This functionalization has already been achieved via cross-coupling or C–H activation. Radical-mediated processes, either via photocatalysis or direct radical addition, offer a promising route for functionalizing the C-8 position under mild conditions.In 2023, the Punniyamurthy group described rhodium-catalyzed C-8 allylation of quinoline N-oxide using vinylcyclopropanes as an allyl source (Scheme 1). The methodology proved to be the best fit for various vinylcyclopropanes (VCP) and substituted quinoline N-oxides. Interestingly, the reaction takes place at room temperature and affords good diastereoselectivity. A Rh(III)–Rh(I)–Rh(III) catalytic cycle facilitates the formation of an allylated product. This catalytic cycle allows the regeneration of the Rh(III) species, ensuring the reaction's efficiency. A successful gram-scale reaction further demonstrates the utility of this methodology.27
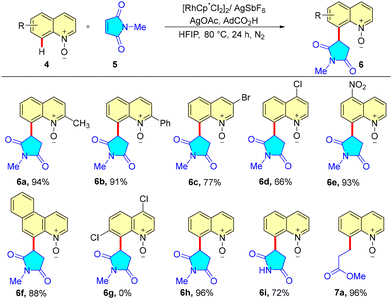
Continuing our efforts in functionalizing quinoline,28 a Rh(III)-catalyzed regioselective C-8 alkylation of quinoline N-oxides with maleimides was unveiled. An array of C-8 alkylated quinoline mimics was synthesized in excellent yields, tolerating both electron-donating and electron-withdrawing substituents. Benzo[f]quinoline-N-oxide afforded the desired product in excellent yield; however, 2,7-dichloroquinoline-N-oxide did not work, which may be attributed to steric hindrance. Besides maleimides, acrylates were also screened as alkylating agents to successfully obtain the expected products in excellent yields. Based on various screening experiments and literature reports, we also proposed a reaction mechanism that proceeds through the formation of a 5-membered metallacycle, alkene coordination, migratory insertion, and protodemetalation to yield the alkylated quinoline. Here, the authors proposed the lack of syn-periplanar β-hydrogen on the complex formed after migratory insertion that may limit the β-hydride elimination to afford the alkylated product, which otherwise would have furnished the alkenylated product (Scheme 2).29
Working in a similar direction, we uncovered C(sp2)-8 arylation of quinoline-N-oxide with bench-stable organoboranes using a Ru-catalyst, giving 70% arylated product. The use of rhodium(III) (isoelectronic species) instead of ruthenium(II) under similar conditions gave 8-arylquinoline-N-oxides. The substrate scope was widely explored with different quinoline-N-oxides as well as arylboronic acids. Distinctly substituted 8-aryl quinolines were synthesized in moderate to good yields under both conditions (Scheme 3).30 To explore the reaction mechanism, the standard reaction was performed with a Ru(I) catalyst which successfully provided the C-8 arylated product, though in a lower yield, thus confirming Ru(I) as the active catalyst. Moreover, control experiments not only confirmed its catalytic function but also suggested that ruthenium facilitates the deoxygenation of arylated quinoline-N-oxide. Based on these studies, the authors proposed a reaction pathway that involves the initial formation of Ru(p-cymene)(OTf)2 from the dimer catalyst by the action of triflic anhydride. This further reacts with quinoline-N-oxide to yield a five-membered metallacycle intermediate, which upon transmetalation followed by reductive elimination produces the arylated product (Fig. 2).
Building on our group's ongoing interest in quinoline functionalization, we developed a novel Rh-catalyzed C–H/C–H cross-coupling method between N-heterocycles and other heteroarenes to synthesize unsymmetrical heterobiaryl compounds. Various substituted benzoxazoles were successfully reacted with quinoline, affording the desired products in good to excellent yields. This method also proved effective for the C-2 benzoxazolylation of N-pyridyl and N-pyrimidyl indoles. Mechanistic studies revealed that a five-membered rhodacycle serves as the key intermediate in the catalytic cycle. Ag2O was found to activate another reactant, 12, facilitating its further participation in the reaction. Density Functional Theory (DFT) calculations were also conducted to elucidate the mechanism of the Cp*Rh-catalyzed directed C–H heteroarylation and to clarify the role of additives under the optimized reaction conditions. The oxidative coupling mechanism involves C–H activation of both 11 and 12, followed by reductive elimination and reoxidation of the Cp*Rh catalyst by the silver oxidant (Scheme 4).31
Considering the activity enhancement ability of the methyl group in biologically active compounds, our group proposed the methylation of 8-methylquinolines with alkyltrifluoroborates/alkylboronic acids. We devised a protocol for regioselective functionalization of the unactivated C(sp3)–H bond of 8-methylquinolines with bench-stable organoboron reagents using a Rh(III)-catalyst. Here, we reported the inaugural direct methylation of the C(sp3)–H bond of 8-methylquinolines using a rhodium catalyst. Under the optimized conditions, a variety of 8-methylquinolines were subjected to functionalization either with alkylboronates or alkyl boronic acids, thus affording alkylated products in good to excellent yields. 8-Methylquinoline gave 61% and 42% of 8-ethylquinoline with potassium trifluoromethylboronate and methylboronic acid, respectively. 8-Methylquinoline with methyl and other substituents at different positions produced 48–73% product yields. Sensitive groups such as thiophene and olefin endured the reaction conditions, leading to product formation with good yield. However, 8-methylquinoline bearing 2-methyl and 5-nitro substituents did not work under the developed conditions. Moreover, the reaction of 8-methylquinoline with butylboronate furnished the expected product, albeit in low yield, while phenylboronate was unsuccessful in providing the functionalized quinoline (Scheme 5).32
After performing several control experiments to get insight into the course of the reaction, the authors proposed a redox-neutral mechanism for the developed strategy. Here, AgSbF6 initially generates the active Rh(III)-species from the catalyst added, forming a five-membered rhodacycle upon insertion of 8-methylquinoline through C(sp3)–H activation. It's transmetalation with organoboranes generates another intermediate, which yields the alkylated product upon reductive elimination. The reduced catalyst so generated is further oxidized with silver to continue the catalytic process (Fig. 3).
Building upon earlier reports, our group advanced the study of transition-metal-catalyzed C-8 functionalization of quinolines. Specifically, we developed a cobalt-catalyzed C(8)–H-selective olefination and oxyarylation of quinoline-N-oxides using challenging terminal alkynes. During the substrate scope investigation, it was observed that quinoline N-oxides unsubstituted at the C-2 position yielded olefinated products, while C-2 substituted quinoline-N-oxides resulted in oxyarylated products. Steric and electronic factors governed the selectivity of product formation in this method. The practicality of this reaction was further demonstrated through a gram-scale synthesis of the oxyarylated product. Additionally, both oxyarylated and olefinated products were synthesized from quinoline-N-oxides containing natural molecules (Scheme 6).33
The proposed mechanistic pathway begins with the activation of the Co-catalyst, which undergoes C(8)–H cobaltation with quinoline-N-oxide to form intermediate A. This intermediate coordinates with the alkyne, leading to the insertion of the alkyne into the Co–C bond to produce intermediate B. Subsequent coordination with a ligand generates intermediate D, which undergoes protodemetalation in the presence of an acid to yield intermediate E and regenerate the active Co-species. Intermediate E is reduced to afford the olefinated product. In a parallel pathway, intermediate B undergoes oxyarylation to form intermediate C, which, after protodemetalation, delivers the oxyarylated product along with the active Co-catalyst (Fig. 4).
Recently, Sundararaju group uncovered an inventive Cp*Co(III)-catalyzed method for synthesizing bio-relevant hydrobenzofurans tethered at the C-8 position of quinoline, utilizing readily available quinoline-N-oxide and 1,6-enynes. The structure and stereochemistry of the product were established using X-ray crystallography. The catalyst analogues, i.e. Cp*Rh and Cp*Ir, were unsuccessful in furnishing the anticipated cascade product here. This protocol demonstrates excellent functional group tolerance, offering moderate to excellent yields, and applies to a broad spectrum of quinoline-N-oxide and 1,6-enyne substrates derived from phenol derivatives. Higher yields of the functionalized quinoline were obtained with electronically rich quinoline-N-oxides compared to electron-poor quinoline-N-oxides (Scheme 7).34
After performing a number of competitive, kinetic isotopic effect and deuterium scrambling experiments, the authors proposed the catalytic cycle for this cascade cyclization reaction. Silver salt-assisted ionization of the pre-catalyst Cp*Co(CO)I2 generates a cationic active catalyst, which then forms a cobaltacycle through successive coordination and cyclometalation with quinoline-N-oxide, which was detected in ESI-MS analysis. Furthermore, coordination via ligand exchange and regioselective insertion of the alkenyl substrate to the Co–C bond forms a 7-membered alkenyl intermediate. The addition of the nucleophilic carbon of the quinone ring to Co–C generates another intermediate, which, upon acid-assisted protodemetalation, furnishes the active catalysts besides forming the sought cascade cyclized product (Fig. 5).
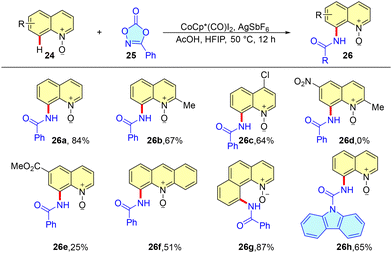
Working with cobalt catalysis, our group further explored the C-8 amidation of quinoline-N-oxides with dioxazolones as the amidation source. The reaction exhibits high efficiency and excellent compatibility with various functional groups. The product formed in this reaction is integrated with the N-oxide; hence, it could be easily used for further functionalization. Moreover, we also used this catalytic system for the C-7 amidation of N-substituted indolines besides the functionalization of 2-phenylpyridine and benzoquinolines. Substrates with alkyl, ester and halogen substituents delivered the expected products in good to excellent yields; however, with the -NO2 group, we could not obtain the desired product (Scheme 8).
We also proposed the mechanism of the reaction, which initiates through C(8)–H activation of quinoline-N-oxide with a Co(III) catalyst. Furthermore, coordination of dioxolone followed by nitrene insertion and elimination of CO2 delivers an intermediate. Migratory insertion generates an amido-inserted species, which upon protodemetalation yields C-8 amidated quinoline-N-oxide and generates the active cobalt catalyst for further reaction (Fig. 6).35
Palladium is renowned for its extraordinary catalytic abilities, and Pd-based catalysis is a versatile tool for the unorthodox activation/functionalization of C–H bonds. In this context, Wan's group uncovered the C(sp3)–H bond etherification of 8-methylquinolines with methanol using palladium catalysts. The author's explored the catalytic potential of a palladium nanocatalyst supported over N-doped TiO2-carbon composites to facilitate the oxidative coupling of methanol to 8-methylquinoline using phenyliodoacetate as an organo-oxidizing agent. The coupling reaction furnished 8-etheral quinolines with both electron-withdrawing and electron-donating groups in good to excellent yields (Scheme 9).36
The authors proposed a redox coupling mechanism for the reaction. The initial deposition of 8-methylquinoline on palladium yields a five-membered palladacycle, which upon oxidative addition of PhI(OAc)2 leads to the formation of a PdIV–diacetate complex of quinoline. Reductive elimination of this complex generates 8-etheral quinoline (Fig. 7).
In 2021, Rong's group unveiled a metal-free methodology for the C-8 functionalization of quinoline. A Brønsted acid-catalyzed Friedel–Crafts reaction between quinoline-N-oxide and ynamides afforded the final products in good yields. The reaction proceeds via the formation of an intermediate quinolyl enolonium ion. After optimizing the reaction conditions, the authors expanded the substrate scope and successfully obtained the desired products in good yields. Considering that 3-chlorobenzoic acid could act as a Brønsted acid, they attempted a one-pot reaction of quinoline with ynamides in the presence of m-CPBA, which afforded the final product, albeit in moderate yield (Scheme 10).37
C-2 functionalization of quinoline
Zhu et al. reported the nickel-catalyzed coupling of cyclic amines to quinoline, forming C-2 aminated quinoline derivatives. The authors circumvented the possibility of coordination of NH-amines with the transition metal, thus avoiding decreased catalytic activity. N-Oxide-directed C–H/N–H coupling delivered pivotal aminopyridine quinoline analogues in good yields. The reaction conditions favoured electron-rich pyridine rings, as the presence of electron-withdrawing groups led to decreased yields. Piperidine and pyrrolidine also furnished the aminated quinoline in moderate yields. Moreover, isoquinoline-N-oxide also led to aminated isomers, albeit in lower yields (Scheme 11).38The mechanism of the reaction involved proton-coupled electron transfer between morpholine and Ag2CO3. The morpholine N-radical thus generated attaches to the initially generated Ni(II) quinoline-N-oxide complex to form the Ni(III) alkyl amido complex. This complex furnishes the ortho-aminated quinoline-N-oxide through reductive C–N elimination, which is detected by mass spectrometry. Ni(I) reoxidation with functionalized N-oxide ultimately yields the C-2 aminated quinoline (Fig. 8).
The Wang group (2023) reported a phosphonium salt-promoted C(2)–H functionalization of various quinoline-N-oxides using primary or secondary amines, affording the desired products in moderate to excellent yields. As the activating group plays a crucial role in the functionalization of N-oxides, the authors discovered that phosphonium salts, due to the strength of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, provide a strong thermodynamic driving force for the completion of the reaction. Following an extensive optimization process, they identified the most suitable conditions for successfully carrying out the transformation, and the phosphonium salt was found to be essential for the reaction. Under the optimized conditions, a variety of quinoline-N-oxides were efficiently converted into the desired products with modest to excellent yields. Additionally, the methodology was shown to be applicable to other nucleophiles, such as phenols and benzene sulfinates, but not to butyl alcohol (Scheme 12).39
O bond, provide a strong thermodynamic driving force for the completion of the reaction. Following an extensive optimization process, they identified the most suitable conditions for successfully carrying out the transformation, and the phosphonium salt was found to be essential for the reaction. Under the optimized conditions, a variety of quinoline-N-oxides were efficiently converted into the desired products with modest to excellent yields. Additionally, the methodology was shown to be applicable to other nucleophiles, such as phenols and benzene sulfinates, but not to butyl alcohol (Scheme 12).39
The Vaccaro group investigated the catalytic properties of iron and used FeSO4 as a catalyst for direct C–H activation/functionalization of quinoline-N-oxide. The formation of H2O as the sole side product represents the greener aspect of this strategy. Quinoline-N-oxides with electron-withdrawing and electron-donating groups were regioselectively alkenylated. Olefination using acrylates and styrenes was successfully achieved with quinoline and quinoxaline-N-oxides (Scheme 13).40
The authors also proposed the mechanism of the reaction based on experimental and computational studies. It was proposed that the initial coordination of the metal catalyst with quinoline-N-oxide occurs, followed by C(2)–H activation through ligand-promoted deprotonation. This is followed by the oxidative insertion of the olefin into the C(2)–Fe bond, delivering the desired product through H-abstraction/double bond regeneration. The non-observed possibility of C(8)–H functionalization was ruled out on account of the high energy barriers shown by DFT calculations (Fig. 9).
Currently, a highly regioselective non-directed C(2)–H activation/functionalization of quinoline-N-oxides leading to the synthesis of 2-acetamidequinoline-N-oxides using a Cu(I) catalyst is reported by Zhang's research group. In 2020, Ankit et al. also used dioxazolones as the amidation source and prepared a library of 8-acetamidequinoline-N-oxides having substituents with different electronic effects.35 Under the current reaction conditions, Cu(CH3CN)4PF6 was explicitly more active in facilitating the amidation reaction compared to Cu(I) halides (Scheme 14).41 Additionally, to predict the course of transformation, the authors added radical quenchers like TEMPO, BHT, and 1,1-diphenylethylene and observed no significant decrease in product yield, thus ruling out the possibility of a radical pathway.
The reaction involves the coordination of CuI with dioxazolone, and the intermediate so formed reacts with quinoline-N-oxide in the presence of AgOAc to form a copper-nitrenoid species via CO2 elimination. Furthermore, reductive elimination of this generates a new copper-coordinated complex, which may react in two possible ways to yield the final product (Fig. 10).
Reckoning the importance of the C–Si bond, which is further susceptible to easy modification, the Chang group functionalized the C(2)–H bond of quinoline scaffolds to a C(2)–Si bond.42 The authors unveiled a metal-free strategy, 1,2-silaborylation of quinoline and similar heterocyclic moieties, using KOtBu, which affords a broad range of silylboronate derivatives. The products so formed being unstable were isolated as their N-acyl derivatives or rearomatized C(2)-silyl N-heterocyclic moieties. Defluorosilylation, which is often observed in C–F bond activation by alkali bases, was not observed under these conditions.43 Rearomatization of unstable N-boryl-2-silyl-dihydroquinoline compounds to 2-silylated quinolines was also successfully done by treating initially synthesized 2-slily enamines with methanol under ambient conditions. In the course of the reaction, the authors proposed a six-membered ion-pair transition state, demonstrating the activation of quinoline and silylborane through the use of KOtBu (Scheme 15).42
Anugu et al.'s protocol introduces an innovative variation of the Ugi reaction by replacing the typical carboxylic acid component with quinoline-N-oxides. This modification allows for the synthesis of α-quinolinamino amides, expanding the scope of the reaction and enhancing its functional group tolerance. Quinoline-N-oxides are significant as they enable a broader substrate range, facilitating the creation of diverse molecular structures with potential applications in medicinal chemistry and drug design. This approach may offer new pathways for complex molecule synthesis, particularly in heterocyclic chemistry (Scheme 16).44
The reaction begins with the condensation of benzaldehyde and aniline to form an imine intermediate. Protonation of the imine enhances its electrophilicity, facilitating the nucleophilic addition of isocyanide, which generates another intermediate. Subsequently, the oxygen atom of the N-oxide attacks the nitrilium ion, producing the next intermediate. The C-2 carbon of the activated quinoline then undergoes nucleophilic attack by aniline, leading to the formation of a bicyclic intermediate. Finally, a Mumm-type rearrangement followed by rearomatization yields the desired product (Fig. 11).
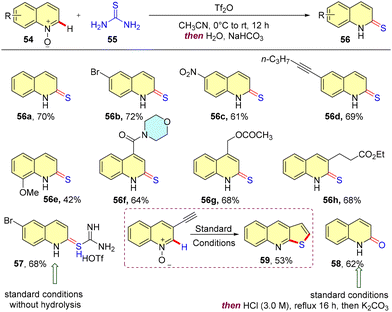
In 2023, Dmitri and coworkers reported the regioselective deoxygenative C(2)–H thiolation of quinoline-N-oxides with thiourea, leading to the synthesis of valuable quinoline-2-thiones. Here, the authors unveiled a one-pot, two-step method where the initial activation of a mixture of quinoline-N-oxide and thiourea with triflic anhydride furnishes an intermediate, 57, which upon basic hydrolysis yields the desired quinoline-2-thione. A range of quinoline-N-oxides with different electronic properties were subjected to the optimal reaction conditions, affording the C-2 thione products in good yields. The authors also characterized the intermediate salt 57, which could be obtained in high purity without isolation. Additionally, when N-oxides of 3-ethynylquinoline were selected as the substrate, it led to the formation of thieno[2,3-b]quinoline 59, owing to a cascade comprising a spontaneous 5-endo-dig cyclization (Scheme 17).45
On the basis of previous reports, the authors proposed the mechanism of the reaction, where triflic anhydride initially activates quinoline-N-oxide, thus making it more susceptible to nucleophilic attack. Addition of nucleophilic thiourea to this generates an intermediate, which upon subsequent aromatization leads to isothiuronium quinoline triflate. Hydrolysis of this salt under basic conditions generates quinoline-2-thiones;however, extensive hydrolysis of this salt 57 under acidic conditions yields 2-quinolinone 58 (Fig. 12).
The use of potassium O-alkyl xanthate as a nucleophilic source for the synthesis of S-quinoyl xanthates was developed under transition metal-free conditions at room temperature (Scheme 18).46 Interestingly, when potassium O-tert-butyl xanthate was employed as the nucleophile, more stable quinoline-2-thiones were obtained. These transformations were achieved using Ts2O as an activating agent for the deoxygenative C(2)–H dithiocarbonation of quinoline-N-oxides. The authors explored the substrate scope under the optimized conditions, examining both electron-donating and electron-withdrawing quinoline-N-oxides, and achieved good yields for the corresponding products. Several experiments were conducted, including a successful gram-scale reaction, demonstrating the synthetic utility of this method.
Control experiments were also performed to investigate the possible reaction mechanism. Initially, quinoline-N-oxide was activated by Ts2O, after which the intermediate was attacked by potassium O-alkyl xanthate, leading to re-aromatization and the formation of the final product (64). When potassium O-tert-butyl xanthate was used as the nucleophile, product 62 was further converted into a more stable compound (63) (Fig. 13).
C-3 functionalization of quinolone
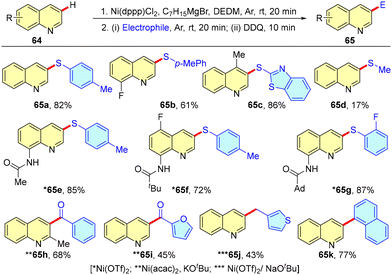
In 2023, Sheng et al. disclosed an electrophilic C(3)–H functionalization of quinolines using a Ni-catalyst and a Grignard reagent at room temperature (Scheme 19).47 The developed methodology enabled the coupling of quinoline with various electrophiles, including phenyl disulfides, alkyl disulfides, aldehydes, benzyl bromides, etc. This approach was demonstrated to be practical for gram-scale synthesis and was successfully applied to the synthesis of three biologically active molecules on a gram scale: an anti-fungal pathogen reagent,48 the potential Alzheimer's disease drug Intepirdine49 and a liver X receptor modulator.50 Control experiments were conducted, leading the authors to propose that nickel hydride species were formed through β-hydride elimination of alkyl nickel intermediates. These nickel hydride species then facilitated a 1,4-addition to quinolines, yielding 1,4-dihydroquinolines (DHQs). The DHQs subsequently underwent nucleophilic attack by electrophiles, followed by oxidative aromatization, leading to the formation of the final products.In 2023, Li et al. unveiled a groundbreaking strategy for the direct C(3)–H thiolation of quinolines, a traditionally challenging transformation. By employing an N-arylmethyl activation approach, this method allowed for efficient C-3 thiolation across a broad range of quinolines, including trifluoromethylthiolation, arylthiolation, alkylthiolation, and phenylselenylation, consistently delivering products in high yields. The versatility of this strategy was demonstrated through an extensive substrate scope, further emphasizing its broad applicability. To understand the underlying efficiency of the protocol, computational studies were performed. These investigations revealed that the N-activating groups play a crucial role in lowering the activation barrier for thiyl radical addition to the quinolinium salt, thus enhancing the reaction's efficiency. Furthermore, the high local nucleophilic indices at the C-3 position of the quinoline ring explained the remarkable regioselectivity observed. The practicality of this method was validated by a successful scale-up, and its utility was further demonstrated through the late-stage thiolation of a wide array of quinoline-based biologically active molecules (Scheme 20).51
In 2022, Cao et al. developed an innovative catalyst-free protocol for the C-3 functionalization of azaarenes, including pyridine, quinoline, and iso-quinoline. This methodology was particularly notable for enabling the late-stage functionalization of complex drug molecules, creating new opportunities in medicinal chemistry. The approach employed a de-aromative activation mode, which facilitated a wide range of transformations through radical and ionic pathways, providing a versatile platform for meta-selective reactions on azaarenes (Scheme 21).52
C-4 functionalization of quinoline
Catalytic C–H bond activation/functionalization to form C–B bonds offers a useful strategy, as functionalization provides a library of important molecules. Recently, Wang and Park reported the first dearomative reduction of quinolines to C-4 borylated N-protected tetrahydroquinolines through a rhodium-catalyzed C–H activation/functionalization approach. The authors used a preactivated cationic [Rh(cod)2]OTf catalyst along with the DPEPhos ligand to achieve borylated tetrahydroquinolines (THIQ) in good to excellent yields, with broad functional group tolerance. Wang et al. succeeded in combining the two-step strategy involving (i) dearomative reduction of quinoline to 1,2-dihydroquinoline and (ii) Cu/Ni-catalyzed hydroboration reported by Ito et.al. into a single-step dearomative hydroborylation under mild conditions.53,54 Here, the C-4 functionalized product is isolated after the stabilization of the initially formed double borylated isomer via the prevalent N-protection (Scheme 22).55Furthermore, NMR analysis of the reaction with time suggested the formation of a 1,2-dihydroquinoline intermediate in the initial hours, which was further directly consumed in the later stages of the reaction to yield the desired product. Additionally, mechanistic experimental studies predicted the continuous formation of the Rh(I)-bis-quinoline adduct [Rh-Q2] (confirmed by X-ray and NMR), serving as a prominent resting entity in the course of the reaction. The mechanism of the reaction suggests the oxidative coupling of one quinoline of Rh-Q2 with HBpin to form the RhIII–hydride complex, which furnishes N-Bpin-1,2- and 1,4-DHQ upon reductive elimination. Rh-Q1 so formed rapidly coordinates with quinoline to form Rh-Q2; however, it undergoes oxidative addition with HBpin to form Rh-(H)Bpin when quinoline is present in a low concentration. Regioselective hydroborylation of 1,2-DHQ with Rh-(H)Bpin generates the THQ-Rh-Bpin complex, and reductive elimination yields the diborylated quinoline derivative along with the generation of RhI. This coordinatively unsaturated RhI complex again coordinates with quinoline to continue the catalytic cycle until the completion of the reaction (Fig. 14).
Chen et al. recently reported an innovative catalytic reductive cyclization of N-azaarenium salts with aniline and formaldehyde, leading to the efficient synthesis of functionalized tetrahydroquinolines (THQs). This approach explored a broad scope of N-heteroarenium salts in conjunction with α-halo ketones and various nucleophiles to yield THQs bearing an azabicyclo[4.1.0]heptane core. The methodology is based on a carbonucleophilic 1,4-addition-triggered dearomatization of quinolinium salts, followed by intramolecular cyclopropanation with α-halo ketones, and subsequent α-nucleophilic addition with diverse nucleophiles. Under the catalytic influence of [HIrIIIX2], H2O, and TMSCN, the α-nucleophilic addition step facilitated the formation of both 2-unsubstituted and 2-functionalized THQs, while using ammonia as the nucleophile resulted in fused products via further condensation between the amino and carbonyl groups. This work opens new avenues for constructing complex, densely functionalized heterocycles, demonstrating the versatility of azaarenium salts in the synthesis of highly decorated tetrahydroquinolines, with potential applications in medicinal and synthetic chemistry (Scheme 23).56
Conclusions
In summary, the functionalization of the quinoline ring has become an essential strategy in contemporary synthetic chemistry, offering unparalleled efficiency and selectivity for the modification of quinoline scaffolds. These innovations have dramatically influenced the development of quinoline-based drugs, enabling the synthesis of compounds with enhanced pharmacological properties. By leveraging advanced catalytic systems, particularly transition-metal catalysis, along with cutting-edge ligand design and directing group strategies, researchers have expanded the chemical versatility of quinoline derivatives, allowing for a broad range of transformations with remarkable precision. While significant progress has been made, especially in the design of sustainable and selective methodologies, challenges such as improving site-specificity and reducing the environmental impact of these processes remain areas of ongoing research. As the field continues to advance, the potential for further breakthroughs in drug discovery is substantial, promising novel therapeutic agents capable of addressing a wide range of diseases. This continued innovation ensures that quinoline chemistry remains at the forefront of synthetic and medicinal chemistry for years to come.Data availability
No data are associated with this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Director, CSIR-IHBT, for continuous encouragement. This work is supported by CSIR (MMP035201 and FTT020503). CSIR-IHBT communication no. 5738.References
- J. Wiesner, R. Ortmann, H. Jomaa and M. Schlitzer, Angew. Chem., Int. Ed., 2003, 42, 5274–5293 CrossRef CAS PubMed.
- Y. Zhao, C. Zhang, W. Liu, Z. Guo, Y. Zhang, Y. Wu, C. Wei, J. Wu and X. Yang, Curr. Med. Chem., 2025, 32, 958–973 CrossRef CAS PubMed.
- X.-F. Shang, S. L. Morris-Natschke, Y.-Q. Liu, X. Guo, X.-S. Xu, M. Goto, J.-C. Li, G.-Z. Yang and K.-H. Lee, Med. Res. Rev., 2017, 38, 775–828 CrossRef.
- P. J. O’. Dwyer, F. P. LaCreta, N. B. Haas, T. Halbherr, H. Frucht, E. Goosenberg and K.-S. Yao, Cancer Chemother. Pharmacol., 1994, 34, S46–S52 CrossRef.
- G. R. Padmanabhan, Anal. Profiles Drug Subst., 1983, 12, 105–134 CAS.
- B. Lell, J. F. Faucher, M. A. Missinou, S. Borrmann, O. Dangelmaier, J. Horton and P. G. Kremsner, Lancet, 2000, 355, 2041–2045 CrossRef CAS PubMed.
- R. K. Mittal, M. Aggarwal, K. Khatana and P. Purohit, Med. Chem., 2023, 19, 31–46 CrossRef CAS.
- S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 24463–24476 RSC.
- V. Dhayalan, D. Sharma, R. Chatterjee and R. Dandela, Eur. J. Org. Chem., 2023, e202300285 CrossRef CAS.
- S. Atechian, N. Nock, R. D. Norcross, H. Ratni, A. W. Thomas, J. Verron and R. Masciadri, Tetrahedron, 2007, 63, 2811–2823 CrossRef CAS.
- V. V. Kouznetsov, L. Y. V. Méndez and C. M. M. Gómez, Curr. Org. Chem., 2005, 9, 141–161 CrossRef CAS.
- A. Weyesa and E. Mulugeta, RSC Adv., 2020, 10, 20784–20793 RSC.
- V. F. Batista, D. C. G. A. Pinto and A. M. S. Silva, ACS Sustainable Chem. Eng., 2016, 4, 4064–4078 CrossRef CAS.
- (a) R. S. Keri, S. Budagumpi and V. Adimule, ACS Omega, 2024, 42, 42630–42667 CrossRef PubMed; (b) S. Kumar, K. Padala and B. Maiti, ACS Omega, 2023, 8, 33058–33068 CrossRef CAS; (c) S. Kumar, R. Sivakumar, K. Padala and B. Maiti, ChemistrySelect, 2023, 8, e202303665 CrossRef CAS.
- J. A. Labinger and J. E. Bercaw, Nature, 2002, 417, 507–514 CrossRef CAS PubMed.
- J. J. W. Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740–4761 RSC.
- A. K. Dhiman, A. Thakur, R. Kumar and U. Sharma, Asian J. Org. Chem., 2020, 9, 1502–1518 CrossRef CAS.
- B. Su, Z.-C. Cao and Z.-J. Shi, Acc. Chem. Res., 2015, 48, 886–896 CrossRef CAS PubMed.
- P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed.
- J. Loup, U. Dhawa, F. Pesciaioli, J. W. Delord and L. Ackermann, Angew. Chem., Int. Ed., 2019, 58, 12803–12818 CrossRef CAS PubMed.
- R. Sharma, K. Thakur, R. Kumar, I. Kumar and U. Sharma, Catal. Rev., 2015, 57, 345–405 CrossRef CAS.
- A. Corio, C. G. Pelletier and P. Busca, Molecules, 2021, 27, 5467–5527 CrossRef.
- R. Kumar, A. Thakur, Sachin, D. Chandra, A. K. Dhiman, P. K. Verma and U. Sharma, Coord. Chem. Rev., 2024, 499, 215453–215526 CrossRef CAS.
- B. Prabagar, Y. Yang and Z. Shi, Chem. Soc. Rev., 2021, 50, 11249–11269 RSC.
- J. Kwak, M. Kim and S. Chang, J. Am. Chem. Soc., 2011, 133, 3780–3783 CrossRef CAS PubMed.
- R. Sharma, R. Kumar, I. Kumar and U. Sharma, Eur. J. Org. Chem., 2015, 2015, 7399–7611 CrossRef.
- S. Mandal, P. Karjee, S. Saha and T. Punniyamurthy, Chem. Commun., 2023, 59, 2823–2826 RSC.
- R. Sharma, I. Kumar, R. Kumar and U. Sharma, Adv. Synth. Catal., 2017, 359, 3022–3028 CrossRef CAS.
- A. Thakur, A. K. Dhiman, S. R. Kumar and U. Sharma, J. Org. Chem., 2021, 86, 6612–6621 CrossRef CAS.
- S. S. Gupta, R. Kumar and U. Sharma, ACS Omega, 2020, 5, 904–913 CrossRef CAS PubMed.
- D. Parmar, T. Sharma, A. K. Sharma and U. Sharma, Chem. Commun., 2023, 59, 9646–9649 RSC.
- R. Kumar, R. Sharma, R. Kumar and U. Sharma, Org. Lett., 2020, 22, 305–309 CrossRef CAS PubMed.
- D. Parmar, R. Kumar, R. Kumar and U. Sharma, J. Org. Chem., 2022, 87, 9069–9087 CrossRef CAS PubMed.
- B. Garai, M. R. Ali, R. Mandal and B. Sundararaju, Org. Lett., 2023, 25, 2018–2023 CrossRef CAS PubMed.
- A. K. Dhiman, A. Thakur, I. Kumar, R. Kumar and U. Sharma, J. Org. Chem., 2020, 85(14), 9244–9254 CrossRef CAS.
- X. Zhao, X. Zhu, K. Wang, J. Lv, S. Chen, G. Yao, J. Lang, F. Lv, Y. Pu, R. Yang, B. Zhang, Z. Jiang and Y. Wan, Nat. Commun., 2022, 13, 4180–4191 CrossRef CAS.
- W. Hu, F. Zhang, C. Chen, T. Qi, Y. Shen, G. Qian and Z. Rong, Chem. Commun., 2021, 57, 6995–6998 RSC.
- W. Z. Min, W. Y. Wang, G. Wang, J. Wang and H. Rao, Org. Lett., 2024, 26, 912–916 CrossRef PubMed.
- Q. Ma, Y. Shi and D. Wang, Org. Lett., 2023, 25, 9181–9185 CrossRef CAS PubMed.
- F. Ferlin, A. Zangarelli, S. Lilli, S. Santoro and L. Vaccaro, Green Chem., 2021, 23, 490–495 RSC.
- Z. Ren, T. Feng, T. Gao, B. Han, R. Guo, H. Ma, J.-J. Wang and Y. Zhang, Org. Lett., 2024, 26, 8532–8536 CrossRef CAS PubMed.
- E. Jeong, J. Heo, S. Jin, D. Kim and S. Chang, ACS Catal., 2022, 12, 4898–4905 CrossRef CAS.
- J. Zhou, B. Jiang, Y. Fujihira, Z. Zhao, T. Imai and N. Shibata, Nat. Commun., 2021, 12, 3749–3758 CrossRef CAS PubMed.
- N. K. Anugu, S. Thunga, S. Poshala and H. P. Kokatla, J. Org. Chem., 2022, 87, 10435–10440 CrossRef CAS PubMed.
- D. I. Bugaenko, O. A. Tikhanova and A. V. Karchava, J. Org. Chem., 2023, 88, 1018–1023 CrossRef CAS.
- M.-Y. Zhao, J.-J. Tang, Y.-J. Lin, Z.-Y. Tian, S. Peng and L.-Y. Xie, J. Org. Chem., 2024, 89, 5560–5572 CrossRef CAS PubMed.
- X. Sheng, M. Yan, B. Zhang, W.-Y. Wong, N. Kambe and R. Qiu, ACS Catal., 2023, 13, 9753–9765 CrossRef CAS.
- B. A. Bricker, M. K. Ashfaq, M. R. Jacob, S. I. Khan, L. A. Walker, S. Y. Ablordeppey, C. A. Boateng, S. V. K. Eyunni, X. Y. Zhu and J. R. Etukala, Bioorg. Med. Chem., 2011, 19, 458–470 CrossRef.
- F. M. Lang, Y. Mo, M. Sabbagh, P. Solomon, M. Boada, R. W. Jones, G. B. Frisoni, T. Grimmer, B. Dubois, M. Harnett, S. R. Friedhoff, S. Coslett and J. L. Cummings, Alzheimer's Dementia, 2021, 7, e12136 CrossRef.
- M. Kuriyama, N. Hamaguchi, K. Sakata and O. Onomura, Eur. J. Org. Chem., 2013, 16, 3378–3385 CrossRef.
- S. Li, J. Tang, Y.-H. Fu, X.-L. Zheng, M.-L. Yuan, R.-X. Li, Z.-S. Su, H.-Y. Fu and H. Chen, Org. Chem. Front., 2023, 10, 2324–2331 RSC.
- H. Cao, Q. Cheng and A. Studer, Science, 2022, 378, 779–785 CrossRef CAS.
- K. Kubota, Y. Watanabe and H. Ito, Adv. Synth. Catal., 2016, 358, 2379–2384 CrossRef CAS.
- K. Kubota, Y. Watanabe, K. Hayama and H. Ito, J. Am. Chem. Soc., 2016, 138, 4338–4341 CrossRef CAS PubMed.
- R. Wang and S. Park, ACS Catal., 2023, 13, 7067–7078 CrossRef CAS.
- J. Chen, J. Yang and M. Zhang, J. Org. Chem., 2024, 89, 8131–8136 CrossRef CAS.
Footnote |
| † Equal authorship. |
| This journal is © The Royal Society of Chemistry 2025 |