Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Au(III)/TPPMS-catalyzed Friedel–Crafts benzylation of deactivated N-alkylanilines in water†
Hidemasa
Hikawa
 *,
Akane
Fukuda
,
Kazuma
Kondo
,
Taku
Nakayama
*,
Akane
Fukuda
,
Kazuma
Kondo
,
Taku
Nakayama
 ,
Tomokatsu
Enda
,
Shoko
Kikkawa
,
Tomokatsu
Enda
,
Shoko
Kikkawa
 and
Isao
Azumaya
and
Isao
Azumaya
 *
*
Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan. E-mail: hidemasa.hikawa@phar.toho-u.ac.jp; isao.azumaya@phar.toho-u.ac.jp
First published on 29th August 2024
Abstract
The Friedel–Crafts reaction of electronically deactivated anilines including those with strong electron-withdrawing NO2, CN or CO2H groups is challenging due to the reduced electron density of the aromatic ring. Here, we demonstrate the Au(III)/TPPMS-catalyzed Friedel–Crafts benzylation of deactivated anilines in water. This reaction exhibits operational simplicity and a broad substrate scope with high regioselectivity, enabling rapid access to 2-benzylanilines.
Introduction
The Friedel–Crafts reaction is one of the oldest and most powerful approaches for creating a C–C bond from an unactivated aromatic C–H bond of an electron-rich arene, which is a fundamental synthetic protocol for the construction of organic molecules. However, this transformation using unprotected anilines is notoriously difficult, leading to the formation of N-substituted anilines instead.1,2 Furthermore, anilines tend to capture the Lewis acid catalyst and prevent its turnover. In 1978, Sugasawa et al. first developed an ortho-acylation of unprotected anilines with nitriles as the acylating agents by employing a Lewis acid (Scheme 1A).3,4 Recently, the Gandon and Bour group demonstrated In(I)-catalyzed C-alkylations of para-substituted anilines via a tandem hydroamination/Hofmann–Martius rearrangement (Scheme 1B, left).5 Despite these tremendous advancements for the direct transformation of anilines,6 highly electron-poor arenes such as 4-nitroanilines are more challenging substrates due to the electron density of the aromatic ring which is reduced by the strong electron-withdrawing group.5–7 Therefore, only a few studies with these substrates have been reported to date.8,9 For example, the Ghorai group demonstrated a dehydrative ortho C–H benzylation of 4-nitroaniline with benzhydrols employing a Re2O7 catalyst (Scheme 1B, right).8aOur group previously reported a new dehydrative C3-benzylation of indoles utilizing the combination of Lewis acidic Au(III)10 with a water-soluble phosphine ligand, Ph2P(m-C6H4SO3Na) (TPPMS), in water.11 In contrast, no reaction occurred when using NaAuCl4·2H2O in organic solvents such as CH2Cl2, 1,4-dioxane, and toluene, suggesting that strong π nucleophilic indole substrates suppress the Lewis acidity of gold(III) catalysts in organic solvents.12 Notably, our Lewis acidic Au(III)/TPPMS system in water provided a unique strategy to enable catalytic reactions in systems that would otherwise be inert. Based on this encouraging result, we aimed to develop a novel synthetic strategy for the catalytic dehydrative Friedel–Crafts benzylation in water.
We herein present an efficient, green, and convenient protocol for the dehydrative ortho C–H selective benzylation of deactivated anilines utilizing the Au(III)/TPPMS catalytic system in water. Our design approach involves a tandem reaction via an initial dehydrative N-benzylation followed by Hofmann–Martius rearrangement,13 resulting in the synthesis of a series of novel 2-benzylaniline derivatives with good functional group tolerance. In general, the conventional Friedel–Crafts reaction relies heavily on anhydrous organic solvents.14,15 Therefore, our catalytic reaction in water should attract a great deal of research interest from synthetic organic chemists. This environmentally friendly approach reduces the use of harmful organic solvents, promoting sustainability in chemical processes.16
Results and discussion
We first examined the reaction between nitroaniline 1a and alcohol 2a as model substrates (Table 1). Recent literature reports have shown that the rhenium(VII) catalyst can be efficient in the dehydrative Friedel–Crafts benzylation.8 However, the reaction between 1a and 2a using the Re2O7 catalyst in water afforded the N-benzylated product 4a in 45% yield along with only 8% of the desired 2-benzylaniline 3a (entry 1). Similar results were obtained when using FeCl2, CuCl2 or HCl (entries 2–4). Other Lewis acids such as ZnCl2, CoCl2, and MnCl2 exhibited no catalytic activities.| Entry | Catalyst | Ligand | Solvent | Yieldb (%) | |
|---|---|---|---|---|---|
| 3a | 4a | ||||
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a (1 mmol), catalysts (5 mol%), ligand (10 mol%), 2a (1.2 mmol), and solvent (4 mL) were used at 90 °C for 18 h. All the reactions were performed in a sealed tube under air.
b 5 mol% was used.
c P(C6H4-m-SO3Na)3.
d At 100 °C. 1a (1 mmol), catalysts (5 mol%), ligand (10 mol%), 2a (1.2 mmol), and solvent (4 mL) were used at 90 °C for 18 h. All the reactions were performed in a sealed tube under air.
b 5 mol% was used.
c P(C6H4-m-SO3Na)3.
d At 100 °C.
|
|||||
| 1 | Re2O7 | TPPMS | H2O | 8 | 45 |
| 2 | FeCl2 | TPPMS | H2O | 2 | 60 |
| 3 | CuCl2 | TPPMS | H2O | 3 | 55 |
| 4 | HCl | None | H2O | 7 | 54 |
| 5 | NaAuCl 4 ·2H 2 O | TPPMS | H 2 O | 87 | 5 |
| 6 | AuBr3 | TPPMS | H2O | 79 | 11 |
| 7 | HAuCl4·4H2O | TPPMS | H2O | 79 | 18 |
| 8 | AuCl3 | TPPMS | H2O | 23 | 26 |
| 9 | AuCl | TPPMS | H2O | 31 | 30 |
| 10 | NaAuCl4·2H2O | None | H2O | 0 | 34 |
| 11 | NaAuCl4·2H2O | TPPMSb | H2O | 17 | 13 |
| 12 | NaAuCl4·2H2O | TPPTSc | H2O | 0 | 54 |
| 13 | NaAuCl4·2H2O | TPPMS | Toluene | 20 | 43 |
| 14 | NaAuCl4·2H2O | TPPMS | DMF | 0 | 11 |
| 15 | NaAuCl4·2H2O | PPh3 | DMF | 10 | 14 |
| 16d | NaAuCl 4 ·2H 2 O | TPPMS | H 2 O | 96 | 4 |
To our delight, the dehydrative coupling utilizing a NaAuCl4·2H2O catalyst with the TPPMS ligand gave the desired 2-benzylaniline 3a as the major product in contrast to the predominance of the N-benzylated product 4a in similar catalytic scenarios (entry 5 vs. entries 1–4). Replacing NaAuCl4·2H2O with other gold(III) salts such as AuBr3 or HAuCl4·4H2O led to similar yields of 3a (entries 6 and 7). However, AuCl3 and AuCl were less efficient (entries 8 and 9). Notably, the use of the TPPMS ligand was essential for achieving the Friedel–Crafts reaction (entry 10). Decreasing the amount of TPPMS to 5 mol% also significantly reduced the yield of 3a (entry 11). When replacing TPPMS with another water-soluble phosphine ligand, P(C6H4-m-SO3Na)3 (TPPTS),17 product 3a was not obtained (entry 12). When using organic solvents such as toluene or DMF instead of water, either a low yield of 3a or no reaction occurred (entries 13 and 14). The reaction did not proceed in DMF when using PPh3 (entry 15). The dehydrative Friedel–Crafts benzylation proceeded to near completion upon increasing the temperature to 100 °C (entry 16).
With the optimized reaction conditions in hand, we explored the scope of alcohols 2 in the coupling of 4-nitroaniline 1a (Scheme 2). Both electron-withdrawing and electron-donating groups on the benzene ring were well tolerated, furnishing the C-benzylated products 3a–g in moderate to excellent yields (50–83%). The structure of the desired product 3a was unambiguously confirmed by X-ray single-crystal diffraction analysis. Allylic alcohol 2, trans-1,3-diphenyl-2-propen-1-ol, was also a viable coupling partner to afford the desired product 3h in 82% yield. In contrast, the desired C-benzylated products were not obtained when using 9-fluorenol 2A, propargylic alcohol 2B and benzyl alcohol 2C. Furthermore, bis(4-diethylaminophenyl)methanol 2D was also not applicable to the Friedel–Crafts benzylation due to its basicity.
Next, we explored the reaction scope of anilines 1 capable of yielding the C-benzylated product 3. Aniline bearing an electron-withdrawing cyano group was a suitable substrate, leading to the formation of C-benzylated aniline 3i in excellent yield (98%). Advantageously, using allylic alcohol 2, the aniline substrate containing an unprotected carboxylic acid functional group could be converted to the desired product 3j in moderate yield (58%) while keeping the acid moiety intact. The ester and acetyl moieties were also well tolerated under the standard conditions (3k, 67%; 3l, 61%). In contrast, when N-methyl-m-toluidine was used instead of deactivated N-alkylanilines 1, the reaction did not proceed efficiently, and the unreacted starting material was recovered. Sterically hindered N-alkylanilines with alkyl side chains including ethyl, n-butyl and isopropyl exhibited good reactivities (3m–o, 70–95%). Interestingly, substitution with the bulky isopropyl group led to the formation of the desired product 3o in excellent yield. This is likely due to increased steric hindrance around the nitrogen atom, which promotes the cleavage of the C–N bond and enhances the rearrangement reaction. The reaction of N-alkylaniline 1p with an alcohol moiety afforded C-benzylated product 3p and C,O-dibenzylated product 3p′ in 54% and 26% yields, respectively.
A Hammett study was performed to investigate the substituent effects of benzhydrols 2X (X = OMe, Me, F, Cl) (Fig. 1).18 Hammett plots were obtained against log(kX/kH) and the substitution constant σp and showed a linear slope (R2 = 0.96) with a ρ value of −2.0. The negative slope indicates the involvement of a charged transition state in which the positive charge is stabilized inductively.19 Therefore, the presence of electron-donating groups on the benzhydrols 2X accelerates the reaction compared to electron-withdrawing substituents. These results are consistent with the Hofmann–Martius rearrangement process with the in situ generated benzyl cation species from N-benzylated intermediate 4a involved in the rate-determining step.
 | ||
| Fig. 1 Hammett plot for the gold-catalyzed C-benzylation of 1a using para-substituted benzhydrols 2X. | ||
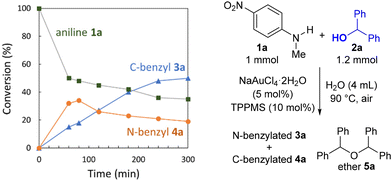
The time course reaction profile for the Friedel–Crafts benzylation between 4-nitroaniline 1a and alcohol 2a (1.2 equiv.) in water is shown in Fig. 2. The formation of N-benzylaniline 4a was observed in the initial phase of the reaction along with the slow formation of C-benzylated product 3a. After 60 min, further consumption of 4a was observed, ultimately forming 2-benzylaniline 3a. These observations are in excellent agreement with a cascade reaction involving N-benzylation of 1a, followed by the Hofmann–Martius rearrangement of N-benzylaniline 4a in the rate-determining step. When the conversion of alcohol 2a was monitored, its rapid depletion and the formation of a small amount of ether 5a were observed in the early stages of the reaction (Fig. S2B in the ESI†).20 This result suggests that upon activation of alcohol 2a, ether 5a was formed via homocoupling of 2a due to the nucleophilicity of the oxygen atom. However, ether 5a did not decay, suggesting that it did not serve as a coupling partner. We recently reported the Au(III)/TPPMS-catalyzed dehydrative N-benzylation of 2-aminopyridines with benzhydrols in water.21 Indeed, no reaction occurred when using ether 5a instead of benzhydrol 2a as a coupling partner in a control experiment.
Next, we conducted solvent isotope effect studies for the catalytic Friedel–Crafts benzylation using H2O and D2O as solvents. In our previous work, the dehydrative N-benzylation of 4-nitroaniline with benzhydrol employing the Au(III)/TPPMS system showed an inverse kinetic solvent isotope effect (KSIE, kH2O/kD2O) of 0.6,19 which is consistent with the specific acid catalysis mechanism (Scheme 3A). In contrast, the time course reaction profiles in the Friedel–Crafts benzylation showed almost the same results in H2O and D2O (Fig. S3 in the ESI†). Although the Lewis acidity of gold catalysts is significantly suppressed by water hydration, the basic nitrogen of N-benzylaniline 4a can coordinate with the Au(III)/TPPMS catalyst without the influence of water molecules (Scheme 3B). Therefore, the resulting active gold complex 4a′ is converted to the 2-benzylated product 3avia the rate-determining Hofmann–Martius rearrangement step.
The use of water as a reaction medium is essential for achieving the catalytic Friedel–Crafts benzylation (see Table 1). The transition state TS of the Hofmann–Martius rearrangement step involves benzyl cation A and aniline moiety B linked by the gold catalyst, which is stabilized by hydrophobic interactions between the phenyl rings in water to promote C–C bond formation.22
We ran several control experiments to elucidate the reaction mechanism for the dehydrative Friedel–Crafts benzylation in water. As expected, the rearrangement of N-benzylated substrate 4a proceeded successfully to form the desired C-benzylated aniline 3a (Scheme 4A). Additionally, debenzylation of intermediate 4a occurred, leading to the regeneration of 4-nitroaniline 1a in 34% yield. This result suggests that the Lewis acidic Au(III)/TPPMS cation enables the cleavage of the sp3 C–N bond of intermediate 4a′ to generate the benzyl cation A with gold(III)-amide complex B. Cation A is simultaneously trapped by the gold-amide complex B to give the desired 3a as the thermodynamically controlled product. Furthermore, the gold(III) complex B reacts with water as a solvent to regenerate aniline 1a. No dibenzylated product 6 was obtained from N-benzylaniline 4a and alcohol 2a (Scheme 4B), nor did direct C-benzylation of N,N-dimethylaniline 7 occur (Scheme 4C).23 These results exclude the reaction pathway via direct coupling of the N,N-disubstituted aniline substrates 4a or 7 with the in situ generated benzyl cation species A. A crossover experiment between intermediate 4a and 4-cyanoaniline 1i led to the formation of crossover product 3i along with the rearrangement product 3a in 40% and 18% yields, respectively (Scheme 4D). This indicates that the Hofmann–Martius rearrangement proceeds through an intermolecular pathway via the formation of a carbocation intermediate.
Based on the results from several mechanistic studies, a plausible mechanism for the ortho-C–H benzylation of deactivated aniline 1a with alcohol 2a employing the Au(III)/TPPMS catalyst is outlined in Scheme 5. Initially, the Lewis acidic gold catalyst promotes the cleavage of the sp3 C–O bond of alcohol 2a to form the benzyl cation A (step 1). Subsequently, aniline 1a attacks the electrophilically active cation A, generating N-benzylaniline 4a as the kinetically controlled product (step 2). This then undergoes an unprecedented sp3 C–N bond cleavage of the gold(III) cation complex 4a′ to regenerate the benzylcation species A along with the gold-amide complex B. The benzyl group is then introduced into the benzene ring of complex B to give 2-benzylaniline 3a as the thermodynamically controlled product. The cation species B is quenched by water, regenerating the aniline substrate 1a (see Scheme 4A). A Hammett ρ value of −2.0 in the Friedel–Crafts benzylation is consistent with the Hofmann–Martius rearrangement pathway that generates a cationic charge at the benzylic position of the TS.
Conclusions
In summary, we have established a dehydrative Friedel–Crafts benzylation of poorly nucleophilic aromatic amines such as 4-nitroanilines with benzhydrols under environmentally friendly reaction conditions in water. This protocol offers an efficient route for the selective synthesis of a new family of 2-benzylanilines from easily available deactivated anilines and benzhydrols, featuring high regioselectivity and a broad substrate scope. Notably, the Lewis acidic Au(III)/TPPMS catalyst was revealed to be highly efficient for the N-benzylation of deactivated anilines with benzhydrols, followed by the Hofmann–Martius rearrangement of the resulting N-benzylated intermediates.Experimental
General procedure
A mixture of anilines 1 (1 mmol), alcohols 2 (1.2 mmol), NaAuCl4·2H2O (5 mol%) and TPPMS (10 mol%) in water (4 mL) was stirred at 90–140 °C for 18 h in a sealed tube under air. After cooling, the reaction mixture was extracted with EtOAc. The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash column chromatography (silica gel, n-hexane/EtOAc) to give the desired product 3.Author contributions
H. H. designed the studies and prepared the original draft of the manuscript. A. F., K. K., T. N., and T. E. performed the synthesis and characterization of the compounds. S. K. contributed to reviewing and editing the manuscript. I. A. supervised the project, conceptualized the idea, and contributed to reviewing and editing the manuscript. The manuscript was written through the contributions of all authors.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI grant number 23K06033. The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing.References
- For reviews on the N-alkylation of anilines, see: (a) E. Podyacheva, O. I. Afanasyev, D. V. Vasilyev and D. Chusov, ACS Catal., 2022, 12, 7142–7198 CrossRef CAS; (b) A. Trowbridge, S. M. Walton and M. J. Gaunt, Chem. Rev., 2020, 120, 2613–2692 CrossRef CAS PubMed; (c) T. Irrgang and R. Kempe, Chem. Rev., 2019, 119, 2524–2549 CrossRef CAS PubMed; (d) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed; (e) G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed.
- (a) F. Chen, H. Geng, Y. Zhao, C. Li, B. Guo, L. Tang and Y. Yanga, Adv. Synth. Catal., 2024, 366, 502–507 CrossRef CAS; (b) J. Yang, Y.-D. Wu and M. Pu, J. Org. Chem., 2023, 88, 7172–7178 CrossRef CAS PubMed; (c) R. Upadhyay and S. K. Maurya, J. Org. Chem., 2023, 88, 16960–16966 CrossRef CAS PubMed; (d) Y.-Z. Sun, Z.-Y. Ren, Y.-X. Yang, Y. Liu, G.-Q. Lin and Z.-T. He, Angew. Chem., Int. Ed., 2023, 62, e202314517 CrossRef CAS PubMed; (e) M. M. Guru, P. R. Thorve and B. Maji, J. Org. Chem., 2020, 85, 806–819 CrossRef CAS PubMed; (f) S.-S. Meng, X. Tang, X. Luo, R. Wu, J.-L. Zhao and A. S. C. Chan, ACS Catal., 2019, 9, 8397–8403 CrossRef CAS; (g) Q. Xu, H. Xie, E.-L. Zhang, X. Ma, J. Chen, X.-C. Yu and H. Li, Green Chem., 2016, 18, 3940–3944 RSC; (h) H. Hikawa, H. Suzuki, Y. Yokoyama and I. Azumaya, J. Org. Chem., 2013, 78, 6714–6720 CrossRef CAS PubMed.
- A. W. Douglas, N. L. Abramson, I. N. Houpis, S. Karady, A. Molina, L. C. Xavier and N. Yasuda, Tetrahedron Lett., 1994, 35, 6807–6810 CrossRef CAS.
- T. Sugasawa, T. Toyoda, M. Adachi and K. Sasakura, J. Am. Chem. Soc., 1978, 100, 4842–4852 CrossRef CAS.
- Z. Li, S. Yang, G. Thiery, V. Gandon and C. Bour, J. Org. Chem., 2020, 85, 12947–12959 CrossRef CAS PubMed.
- (a) M. Albert-Sorianoa and I. M. Pastora, Adv. Synth. Catal., 2020, 362, 2494–2502 CrossRef; (b) C. K. Rank, B. Özkaya and F. W. Patureau, Org. Lett., 2019, 21, 6830–6834 CrossRef CAS PubMed; (c) W. Zhu, Q. Sun, Y. Wang, D. Yuan and Y. Yao, Org. Lett., 2018, 20, 3101–3104 CrossRef CAS PubMed; (d) M. Amézquita-Valencia and H. Alper, Chem. – Eur. J., 2016, 22, 16774–16778 CrossRef PubMed; (e) S. Kobayashi, I. Komoto and J.-I. Matsuo, Adv. Synth. Catal., 2001, 343, 71–74 CrossRef CAS.
- (a) K. Chen, Y. Li, S. A. Pullarkat and P.-H. Leunga, Adv. Synth. Catal., 2012, 354, 83–87 CrossRef CAS; (b) L. L. Anderson, J. Arnold and R. G. Bergman, J. Am. Chem. Soc., 2005, 127, 14542–14543 CrossRef CAS PubMed.
- (a) R. Nallagonda, M. Rehan and P. Ghorai, J. Org. Chem., 2014, 79, 2934–2943 CrossRef CAS PubMed; (b) K. Chen, H. J. Chen, J. Wong, J. Yang and S. A. Pullarkat, ChemCatChem, 2013, 5, 3882–3888 CrossRef CAS.
- For the Friedel–Crafts alkylation of deactivated arenes with benzylic alcohols, see: (a) H. T. Ang, J. P. G. Rygus and D. G. Hall, Org. Biomol. Chem., 2019, 17, 6007–6014 RSC; (b) G. Schäfer and J. W. Bode, Angew. Chem., Int. Ed., 2011, 50, 10913–10916 CrossRef PubMed.
- For reviews on gold catalysis, see: Z. Lu, T. Li, S. R. Mudshinge, B. Xu and G. B. Hammond, Chem. Rev., 2021, 121, 8452–8477 CrossRef CAS PubMed.
- (a) H. Hikawa, H. Suzuki and I. Azumaya, J. Org. Chem., 2013, 78, 12128–12135 CrossRef CAS PubMed; (b) H. Hikawa, H. Suzuki, Y. Yokoyama and I. Azumaya, J. Org. Chem., 2013, 78, 6714–6720 CrossRef CAS PubMed.
- A. Arcadi, G. Bianchi, M. Chiarini, G. D'Anniballe and F. Marinelli, Synlett, 2004, 944–950 CrossRef CAS.
- (a) P. Magnus and R. Turnbull, Org. Lett., 2006, 8, 3497–3499 CrossRef CAS PubMed; (b) A. G. Giumanini, S. Roveri and D. D. Mazza, J. Org. Chem., 1975, 40, 1677–1678 CrossRef CAS; (c) Y. Ogata and K. Takagi, J. Org. Chem., 1970, 35, 1642–1645 CrossRef CAS; (d) A. D. Site, J. Org. Chem., 1966, 31, 3413–3415 CrossRef; (e) G. J. Russelll, R. D. Topsom and J. Vaughan, Chem. Commun., 1965, 529–530 RSC; (f) H. Hart and J. R. Kosak, J. Org. Chem., 1962, 27, 116–121 CrossRef CAS; (g) A. Fischer, R. Topsom and J. Vaughan, J. Org. Chem., 1960, 25, 463–464 CrossRef CAS.
- For reviews on the Friedel–Crafts reactions, see: (a) H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. Norwood IV and J. Aubé, Chem. Rev., 2022, 122, 12544–12747 CrossRef CAS PubMed; (b) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS PubMed; (c) P. H. Gore, Chem. Rev., 1955, 55, 229–281 CrossRef CAS; (d) N. O. Calloway, Chem. Rev., 1935, 17, 327–392 CrossRef CAS.
- For Friedel–Crafts reactions in water, see: (a) C. Wang, M. Hao, Q. Qi, J. Dang, X. Dong, S. Lv, L. Xiong, H. Gao, G. Jia, Y. Chen, J. S. Hartig and C. Li, Angew. Chem., Int. Ed., 2020, 59, 3444–3449 CrossRef CAS; (b) J. Sun, Y. Gui, Y. Huang, J. Li, Z. Zha, Y. Yang and Z. Wang, ACS Omega, 2020, 5, 11962–11970 CrossRef CAS PubMed; (c) P. L. Manna, A. Soriente, M. D. Rosa, A. Buonerba, C. Talotta, C. Gaeta and P. Neri, ChemSusChem, 2019, 12, 1673–1683 CrossRef PubMed; (d) S. Cao, H. Yuan, Y. Yang, M. Wang, X. Zhang and J. Zhang, J. Comput. Chem., 2017, 38, 2268–2275 CrossRef CAS PubMed; (e) X.-L. Shi, H. Lin, P. Li and W. Zhang, ChemCatChem, 2014, 6, 2947–2953 CrossRef CAS.
- G. Zhu, Z.-C. Duan, H. Zhu, D. Ye and D. Wang, Chin. Chem. Lett., 2022, 33, 266–270 CrossRef CAS.
- (a) G. Verspui, I. I. Moiseev and R. A. Sheldon, J. Organomet. Chem., 1999, 586, 196–199 CrossRef CAS; (b) G. Verspui, G. Papadogianakis and R. A. Sheldon, Catal. Today, 1998, 42, 449–458 CrossRef CAS.
- J. Li, A. Mao, W. Yao, H. Zhua and D. Wang, Green Chem., 2022, 24, 2602–2612 RSC.
- A negative Hammett ρ value of 2.8 was obtained in the reaction of para-substituted benzhydryl alcohols with 4-nitroaniline. H. Hikawa, M. Matsumoto, S. Tawara, S. Kikkawa and I. Azumaya, Synthesis, 2019, 51, 2729–2736 CrossRef CAS.
- For the Friedel–Crafts alkylation of arenes with benzylic alcohols via ether intermediates, see: H. T. Ang, J. P. G. Rygus and D. G. Hall, Org. Biomol. Chem., 2019, 17, 6007–6014 RSC.
- H. Hikawa, T. Nakayama, S. Nakamura, S. Kikkawa and I. Azumaya, Org. Biomol. Chem., 2022, 20, 4183–4188 RSC.
- For reviews on hydrophobic interactions, see: (a) M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237–4266 RSC; (b) M. F. Ruiz-Lopez, J. S. Francisco, M. T. C. Martins-Costa and J. M. Anglada, Nat. Rev. Chem., 2020, 4, 459–475 CrossRef CAS PubMed.
- H. Wu, T. Zhao and X. Hu, Sci. Rep., 2018, 8, 11449 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for all compounds. CCDC 2358605. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob01234h |
| This journal is © The Royal Society of Chemistry 2024 |