Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iodine/Oxone® oxidative system for the synthesis of selenylindoles bearing a benzenesulfonamide moiety as carbonic anhydrase I, II, IX, and XII inhibitors†
Martina
Palomba‡
a,
Andrea
Angeli‡
b,
Riccardo
Galdini
a,
Alexandra Joana
Hughineata
a,
Gelson
Perin
c,
Eder João
Lenardão
 c,
Francesca
Marini
c,
Francesca
Marini
 a,
Claudio
Santi
a,
Claudio
Santi
 *a,
Claudiu T.
Supuran
*a,
Claudiu T.
Supuran
 b and
Luana
Bagnoli
b and
Luana
Bagnoli
 *a
*a
aDepartment of Pharmaceutical Sciences (Group of Catalysis, Synthesis and Organic Green Chemistry), University of Perugia, Via del Liceo, 1-06123 Perugia, Italy. E-mail: luana.bagnoli@unipg.it; claudio.santi@unipg.it
bUniversity of Florence, NEUROFARBA Dept., Sezione di Scienze Farmaceutiche, Via Ugo Schiff 6, 50019 Sesto Fiorentino, Italy
cLaboratório de Síntese Orgânica Limpa (LASOL), Centro de Ciências Químicas, Farmacêuticas e de Alimentos (CCQFA), Universidade Federal de Pelotas (UFPel), P.O. Box 354, CEP: 96010-900 Pelotas, RS, Brazil
First published on 22nd July 2024
Abstract
A wide range of 3-selenylindoles were synthesized via an eco-friendly approach that uses Oxone® as the oxidant in the presence of a catalytic amount of iodine. This mild and economical protocol showed broad functional group tolerance and operational simplicity. A series of novel selenylindoles bearing a benzenesulfonamide moiety were also synthesized and evaluated as carbonic anhydrase inhibitors of the human (h) isoforms hCa I, II, IX, and XII, which are involved in pathologies such as glaucoma and cancer. Several derivatives showed excellent inhibitory activity towards these isoforms in the nanomolar range, lower than that shown by acetazolamide.
Introduction
N-Heterocycles play pivotal roles in chemistry and biology because they are ubiquitous in nature and are privileged scaffolds in medicinal chemistry.1 For example, indoles are considered highly significant molecules in the medical field due to their prevalence in drugs approved for the treatment of various diseases.2 In the past years, great attention has been paid to the incorporation of selenium moieties into bioactive heterocycles and natural products.3 Xu also tried to correlate the chemical properties of selenium with the pharmacological activities of selenobioactive compounds.3c These derivatives have emerged as potential therapeutic agents for the treatment of a range of diseases, acting as antitumoral, antiviral, and antimicrobial agents in addition to presenting a series of antioxidant properties.4 In this field, 3-selenylindoles are currently receiving increasing attention because of their pharmacological properties. As highlighted in Fig. 1, 3-selenylindoles and their corresponding selenoxides show antiproliferative properties and are active in vitro against cancer cells, acting as combretastatin A-4 analogs.5 Other derivatives presented antidepressant-like activity in mice6 and anti-inflammatory properties with potential application in the treatment of diseases associated with oxidative damage.7,4d Due to the important applications of 3-chalcogenylindoles, the development of methods for the synthesis of selenylindoles has increased in the past few years.In the case of the formation of C–Se bonds, one traditional method involves direct C(sp2)–H functionalization of indoles with diorganoyl diselenides catalysed by metals.8 Several approaches based on the use of transition metal-free conditions are also available in the literature. Commonly, they involve direct selenylations of the indole nucleus with various electrophilic reagents generated in the presence of oxidants.9 Base-promoted10 and photo11 or electrochemical12 reactions have also been investigated (Fig. 2). In continuation of our interest in the development of new C(sp2)–H functionalized eco-friendly processes and considering our long-standing interest in the indole nucleus,13 in this paper, we describe an alternative and general procedure for the regioselective synthesis of 3-selenylindoles. This sustainable approach uses Oxone® as the oxidant in the presence of a catalytic amount of iodine. Novel 3-selenylindoles bearing a benzenesulfonamide moiety could be prepared as potential inhibitors of human carbonic anhydrase (hCA). CAs are metalloenzymes that catalyse a very simple reaction, the hydration of carbon dioxide to bicarbonate and protons.14 These enzymes are involved in a variety of diseases such as glaucoma, retinitis pigmentosa, epilepsy, and arthritis, and in the development of tumours. Recently, some of us reported that selenols, selenoureas, diselenides, and selenides containing a benzenesulfonamide moiety show relevant hCA-inhibitory activity.15
Results and discussion
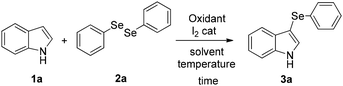
Initially, we started our exploration focusing on the reaction between the 1H-indole 1a and the diphenyl diselenide 2a, aiming to prepare the 3-(phenylselanyl)-1H-indole 3a. Preliminary investigations to produce electrophilic selenium species were carried out using various oxidants, such as phenyliodine diacetate (PID, Table 1, entries 1–4), ammonium persulfate (entry 5), and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, entry 6), in stoichiometric or over-stoichiometric amounts, and the expected 3-selenylindole 3a was obtained only in low yields. Recently, some authors have explored the use of Oxone® as the oxidant in selenocyclization reactions to prepare seleno-functionalized heterocycles.16 Oxone® (2KHSO5·KHSO4·K2SO4) is a stable, easy to handle, eco-friendly and cheap triple salt.17 Unfortunately, when Oxone® was used in acetonitrile, the reaction did not proceed well (entry 7). However, in the presence of iodine (20 mol%) under an air atmosphere, the selenenylated product 3a was isolated in good yield (entry 8), comparable to that obtained with stoichiometric iodine9f (entry 9), but in a shorter reaction time. In recent years, iodine has emerged as a catalyst of choice in numerous selenylation reactions, including those involving alkenes, aromatic, and (hetero)cyclic compounds.18 The influence of temperature, solvents or loading of catalyst was then investigated. As shown in Table 1, increasing the temperature from room temperature to 50 °C resulted in a decrease in the yield (entry 10). Furthermore, decreasing the catalyst loading to 10 mol% and 5 mol% reduced the yield to 66% (entry 11) and 42% (entry 12), respectively. Some polar and apolar solvents (entries 13–17) were then screened. Good yields of product 3a were obtained using polar solvents with respect to nonpolar ones, but acetonitrile remains the most efficient for this process. Moreover, when the reaction was performed under an argon atmosphere, a decrease in the yield was observed (44% yield, entry 18), demonstrating the beneficial effect of the air atmosphere.| Entrya | Oxidant (eq.) | I2 (% mol) | T (°C) | Time (h) | Solvent | Yield (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: indole 1a (1 eq.), diphenyl diselenide 2a (0.5 eq.), Oxone® (0.5 eq.), solvent (5.0 mL), in air. b Indole 1a was used in excess (5 eq.). c Iodine amount (50, 20, 10, and 5 mol%) with respect to indole 1a. d Reaction was carried out in an argon atmosphere. | ||||||
| 1 | PID (0.8) | — | r.t. | 24 | MeOH | 13 |
| 2 | PID (2) | — | r.t. | 36 | MeOH | 7 |
| 3b | PID (2) | — | r.t | 24 | CH3CN | 10 |
| 4 | PID (0.8) | — | 80 | 28 | CH3CN | 15 |
| 5 | (NH4)2S2O8 (0.6) | — | 80 | 24 | CH3CN | 25 |
| 6 | DDQ (2) | — | 80 | 24 | MeOH | 18 |
| 7 | Oxone (0.5) | — | r.t. | 18 | CH3CN | 20 |
| 8 | Oxone (0.5) | 20 | r.t. | 4 | CH 3 CN | 70 |
| 9 | 50c | r.t. | 28 | CH3CN | 67 | |
| 10 | Oxone (0.5) | 20c | 50 | 5 | CH3CN | 50 |
| 11 | Oxone (0.5) | 10c | r.t. | 4 | CH3CN | 66 |
| 12 | Oxone (0.5) | 5c | r.t. | 24 | CH3CN | 42 |
| 13 | Oxone (0.5) | 20c | r.t. | 5 | MeOH | 60 |
| 14 | Oxone (0.5) | 20c | r.t. | 7 | DMF | 55 |
| 15 | Oxone (0.5) | 20c | r.t. | 8 | THF | 63 |
| 16 | Oxone (0.5) | 20c | r.t. | 27 | CH2Cl2 | 45 |
| 17 | Oxone (0.5) | 20c | r.t. | 20 | Toluene | 43 |
| 18d | Oxone (0.5) | 20c | r.t. | 4 | CH3CN | 44 |
With the optimized conditions in hand (Table 1, entry 8), a small library of 3-selenylindoles was created to evaluate the scope and the limitation of the protocol. The scope of the reaction was examined with indoles containing various functional groups and with differently substituted diselenides (Table 2). Several 5-substituted indoles, including chloro, bromo, iodo and methoxy, are suitable substrates, affording the corresponding products 3b–e in 51–80% yields. The effect of the substitution at the 2- and 4-positions of the indole was also evaluated. The electronic and steric effects of the amide groups at the 2-position of the indole were tolerated, and novel 2-carboxamide-3-selenylindoles 3f and 3g were obtained in satisfactory yields. When a cyano group was present at the C4 position, the corresponding 3-selenylindole 3h was obtained in a high yield. If the C3-position of indole ring is occupied by a methyl group, only 28% of the 2-selenylindole 3i was formed, while the reaction failed in the case of compound 3j, in which both 2- and 3-positions are substituted. The N-acetyl indole was also tested but only a 5% yield of product 3k was obtained. Next, several diorganoyl diselenides were tested to evaluate the influence of different groups linked to selenium. Electron-donating or -accepting substituents attached at the para-position of the aromatic ring of the diselenide did not affect the performance and the corresponding 3-selenylindoles 3l–n were obtained in good yields. Notably, the presence of acidic hydrogen, such as in the case of the carboxyl group at the ortho-position of the diselenide, also did not significantly influence the reaction, furnishing the corresponding product 3o in an acceptable yield. The reaction also worked well with a heterocyclic diselenide, containing pyridine, affording the corresponding 3-selenylindole 3p in 78% yield. In contrast, when the reaction was carried out using an aliphatic diselenide, such as didodecyl diselenide, the corresponding 3-selenylindole 3q was obtained in only 28% yield, even after 24 h of reaction. Moreover, no significant changes in the yields were observed when the loading of the catalyst was decreased to 10 mol% or 5 mol% or increased to 40 mol% (Table 2, compound 3q). In the literature it is reported that aliphatic diselenides are less reactive with respect to aromatic ones for this type of transformation.9a In order to expand the substrate scope to other heteroaromatic compounds, the indole was replaced with the pyrazole ring (Table 2). The 4-selenyl-1H-pyrazoles 3r–s were synthesized in acceptable yields. The preparation of 3-sulfenylindoles was further explored using the same conditions. As shown in Table 2, the mono- and bis-sulfenylindoles 3t and 3t′ were isolated in pure form by column chromatography in equal yields of 28%.
| a Using 5 mol% of iodine. |
|---|

|
Double sulfenylations of indoles have already been observed in iodine-catalyzed oxidative sulfenylation processes.19 Furthermore, to clarify the reaction mechanism, a radical trapping experiment was conducted by the addition of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) using indole 1a and diselenide 2a under the optimized reaction conditions, but no influence in the formation of product 3a was observed. This result suggests that radical intermediates are not involved (Scheme 1).
Based on this experimental result and previous relevant studies,9a,b a plausible reaction mechanism has been proposed (Scheme 2). Initially, diorganyl diselenide reacts with molecular iodine to produce the electrophilic species arylselenyl iodide (ArSeI). Subsequently, the Friedel–Crafts reaction of the indole with the electrophilic species ArSe+ provides the desired 3-selenylindole, with concomitant formation of HI. Finally, HI is oxidized by Oxone® to regenerate molecular iodine, thus completing the catalytic cycle.
Moreover, with the aim of synthesizing new types of selenyl derivatives as potential inhibitors of human carbonic anhydrase (hCA), we sought to exploit this C(sp2)–H bond selenylation reaction with a diselenide containing a sulfonamide moiety. As highlighted in Table 3, novel 3-selenylindoles were synthesized employing 4,4′-diselane diyldibenzenesulfonamide (2i), which was prepared according to the literature.15a These molecules contain three bioactive cores, indole, selenium, and sulfonamide, which are multivalent molecules20 that may improve potency and selectivity when compared with the monovalent entity. Mono and di-substituted indoles containing various electron-donating or electron-withdrawing groups at different positions were employed (Table 3).
Halides (4b–e, and h) or methoxy (4f), alkyl (4g–4h), cyano (4i), ester (4j) and amide (4k) groups at the C5, C4 and C2 positions were well tolerated, and the N-methyl protected indole 4l was successfully employed. It is important to note that these densely functionalized compounds offer the opportunity for further elaboration, allowing the expansion of the range of possible derivatives.
Biological activities
All 3-selenylindoles containing benzensulfonamides 4a–l were tested in vitro for their inhibitory activity towards the physiologically relevant hCA isoforms I, II, IX, and XII using the stopped-flow carbon dioxide hydration assay,21 after a period of 15 min of incubation of the enzyme and inhibitor solutions. Their activities were compared with that of the standard CAI acetazolamide AAZ (Table 4).
K
i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (nM) a (nM) |
||||
|---|---|---|---|---|
| Compound | hCA I | hCA II | hCA IX | hCA XII |
| a Mean from three different assays, via a stopped-flow technique (errors are in the range of ±5–10% of the reported values). | ||||
| 4a | 73.6 | 23.6 | 3.1 | 6.8 |
| 4b | 491.9 | 59.1 | 2.9 | 451.3 |
| 4c | 59.9 | 43.1 | 24.0 | 60.5 |
| 4d | 7356 | 948.2 | 25.4 | 92.5 |
| 4e | 65.0 | 6.9 | 69.6 | 32.3 |
| 4f | 519.7 | 187.2 | 14.6 | 52.2 |
| 4g | 467.7 | 83.9 | 2.1 | 367.3 |
| 4h | 573.4 | 6.2 | 20.8 | 87.3 |
| 4i | 51.1 | 37.2 | 17.2 | 68.8 |
| 4j | 8488 | 6915 | 77.1 | 323.7 |
| 4k | 80.8 | 46.2 | 24.2 | 9.8 |
| 4l | 41.0 | 6.0 | 24.5 | 70.0 |
| AAZ | 250 | 12.1 | 25.8 | 5.7 |
The following preliminary structure–activity relationship (SAR) may be noted from the inhibition data shown in Table 4.
(i) The ubiquitous cytosolic hCA I was inhibited by compounds 4a, 4c, 4e, 4i, 4k, and 4l, each with inhibitory constants (Ki) in the nanomolar range (41.0 nM to 80.8 nM), which were lower than that of AAZ. Notably, compound 4d, with a bromine atom at position C5 displayed a significantly lower potency with a Ki of 7356 nM. Conversely, introducing a methyl group at the position C2, as seen in compound 4h, enhanced the potency by 12 fold (Ki 573.4 nM). The presence of an ethyl ester at position C2 in the indole scaffold, as in compound 4j, resulted in a high inhibitory constant with a Ki of 8488 nM, indicating a detrimental effect on activity.
(ii) The dominant cytosolic human isoform hCA II was effectively inhibited by compounds 4e, 4h, and 4l, all of which exhibited inhibitory constants in the nanomolar range (Ki 6.0 to 6.9 nM), lower than that of AAZ. Notably, the introduction of a methyl group at position C2 in compound 4h significantly enhanced its inhibitory potency by two orders of magnitude compared to compound 4d (Ki 6.2 nM versus 948.2 nM, respectively), without this moiety, and also improved its selectivity for this isoform. Another compound demonstrating selectivity for hCA II was 4l (Ki = 6.0 nM), which had a methyl group at position N-1 of the indole scaffold. Conversely, the presence of an ethyl ester at position C2, as in the previous case, resulted in poor inhibitory activity towards this isoform (Ki = 6915 nM).
(iii) All the evaluated compounds effectively inhibited the transmembrane tumor-associated hCA IX, with Kis in the nanomolar range spanning from 2.1 nM to 77.1 nM. Simple substitutions on the indole ring significantly influenced selectivity. Compounds 4b and 4g, which contain a fluoride and a methyl group at position C5 of the indole, demonstrated potent and selective activity towards this isoform, with inhibition constants of 2.9 nM and 2.1 nM, respectively. Notably, a decrease in inhibitory activity towards the isoform hCA IX was observed with progression down the halogen series; the fluorine derivative 4b exhibited the highest selectivity for isoform IX, with progressively less activity noted with chlorine (4c), bromine (4d), and iodine (4e) substitutions.
(iv) The second transmembrane isoform hCA XII was inhibited by all compounds in the medium nanomolar range (Kis spanning from 6.8 nM to 92.5 nM), except for three compounds, 4b, 4g, and 4j that exhibited high nanomolar inhibition constants (Kis spanning from 323.7 nM to 451.3 nM). Generally, simple substituents at position C5 of the indole scaffold were detrimental to activity compared to the unsubstituted derivative 4a. Similarly, substitutions at position C2 also generally decreased the activity, with the exception of compound 4k, which displayed a similar Ki value to 4a (Ki 9.8 nM and 6.8 nM, respectively). Additionally, substitution at N-1, as in the case of 4l, proved to be less effective in inhibiting potency than 4a (Ki 70 nM and 6.8 nM, respectively).
Conclusions
In conclusion, a general alternative methodology for the regioselective synthesis of 3-selenylindoles, which uses Oxone® as the oxidant in the presence of a catalytic amount of iodine in acetonitrile at room temperature, has been reported, through direct C(sp2)–H bond selenylation. This environmentally benign approach is operationally simple, inexpensive, and compatible with a wide array of substrates. Furthermore, several novel 3-selenylbenzensulfonamide indoles were synthesized and tested as inhibitors of four human carbonic anhydrase (hCA) isoforms of pharmacologic relevance, i.e., hCA I, II, IX, and XII. These isoforms are targeted by drugs for glaucoma (hCA I and II) and anticancer therapies (hCA IX and XII). The 3-selenylbenzensulfonamide indoles 4e, 4h, and 4l demonstrated high and selective inhibition towards the isoform hCA II. Conversely, derivatives 4b and 4g showed selective activity towards hCA IX. These findings position 3-selenylbenzensulfonamide indoles as promising initial candidates for the development of more potent and selective hCA-inhibitors.Experimental section
Chemistry
Commercial reagents and solvents were purchased from Sigma Aldrich, Alfa Aesar or VWR International and used without further purification. The starting products indole carboxamides 1f–1g and diselenides 2b–2g and 2i were prepared according to the literature procedures.4c,11d,15a,22 Thin layer chromatography (TLC) was performed using 60 F254 (Merck, KGaA, Darmstadt, Germany) silica gel supported on aluminium sheets. Reaction products were purified by column chromatography on Merck 60 (70–230 mesh) silica gel. Yields correspond to isolated compounds. Purity was estimated to be ≥95% based on 1H NMR spectroscopic analysis. NMR experiments were carried out at 25 °C on a Bruker Avance NEO 400 MHz spectrometer equipped with a sample case operating at 400 MHz for 1H NMR and 100.62 MHz for 13C NMR or on a Bruker Avance NEO 600 MHz spectrometer equipped with a Prodigy™BBO-Cryoprobe operating at 600.13 MHz for 1H NMR and 150.90 MHz for 13C NMR in CDCl3, CD3OD, DMSO-d6 and CD3COCD3. 77Se NMR spectra are referenced to a diphenyl diselenide external standard (PhSe)2 and were recorded at 114 MHz.23 Coupling constants J are expressed in Hz. The following abbreviations are used to indicate multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, sext = sextet, m = multiplet, and brs = broad singlet. High-resolution mass spectrometry (HRMS) measurements were performed using a Synapt G2-Si mass spectrometer (Waters) equipped with an APCI/ESI source and a quadrupole-time-of-flight mass analyzer. The mass spectrometer was operated in the positive ion detection mode. To ensure accurate mass measurements, data were collected in a centroid mode and mass was corrected during acquisition using leucine enkephalin solution as an external reference. The results of the measurements were processed using MassLynx 4.1 software (Waters) incorporated with the instrument. Melting points were determined using a Kofler melting apparatus and values are uncorrected.General procedure for the synthesis of 3-selenylindoles 3a–q, 4-selenylpyrazoles 3r–s, mono- and bis-sulphenylindoles 3t–3t′, and 3-selenyl-benzensulfonammideindoles 4a–l
The indoles 1a–k and m–q (1 eq., 0.5 mmol) or the pyrazole 1l (1 eq., 0.5 mmol), the diselenides 2a–g and i (0.5 eq., 0.25 mmol) or disulfide 2h (0.5 eq., 0.25 mmol), Oxone® (0.5 eq., 0.25 mmol) and iodine (20% mol) were dissolved in 5.0 mL of CH3CN. The reaction mixture was vigorously stirred at room temperature for the time indicated in Tables 2 and 3. Then the reaction was quenched with a saturated solution of Na2S2O3, extracted with ethyl acetate (3 × 10 mL), dried with Na2SO4, and filtered and the solvent was evaporated under reduced pressure. The crude mixture was purified by column chromatography on silica gel to obtain the corresponding products. Physical and spectroscopic data of the 3-selenylindoles 3a–q, 4-selenylpyrazoles 3r–s, mono- and di-sulphenylindoles 3t–t′, and 3-selenylindoles bearing benzene solfonamides 4a–l are reported below. The 1H NMR spectra registered in CD3OD did not show signals for the exchangeable protons of indoles NH and SO2NH2. Such protons are visible in DMSO-d6.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a white solid (mp 135–137 °C, lit.11a 134–135 °C) in 70% yield. Rf = 0.51 (PE/EtOAc 8
20) and afforded as a white solid (mp 135–137 °C, lit.11a 134–135 °C) in 70% yield. Rf = 0.51 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.44 (brs, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.50 (d, J = 1.9 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.32–7.10 (m, 7H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.44 (brs, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.50 (d, J = 1.9 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.32–7.10 (m, 7H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a white solid (mp 109–111 °C, lit.11a 112–115 °C) in 51% yield. Rf = 0.33 (PE/EtOAc 8
20) and afforded as a white solid (mp 109–111 °C, lit.11a 112–115 °C) in 51% yield. Rf = 0.33 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.50 (brs, 1H), 7.68 (s, 1H), 7.49 (s, 1H), 7.36 (d, J = 7.6 Hz, 1H), 7.30–7.17 (m, 6H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.50 (brs, 1H), 7.68 (s, 1H), 7.49 (s, 1H), 7.36 (d, J = 7.6 Hz, 1H), 7.30–7.17 (m, 6H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a white solid (mp 136–138 °C, lit.11a 135–138 °C) in 80% yield. Rf = 0.36 (PE/EtOAc 8
20) and afforded as a white solid (mp 136–138 °C, lit.11a 135–138 °C) in 80% yield. Rf = 0.36 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.51 (brs, 1H), 7.76 (s, 1H), 7.46 (d, J = 2.6 Hz, 1H), 7.39–7.09 (m, 7 H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.51 (brs, 1H), 7.76 (s, 1H), 7.46 (d, J = 2.6 Hz, 1H), 7.39–7.09 (m, 7 H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a white solid (mp 128–131 °C, lit.11a 125–128 °C) in 61% yield. Rf = 0.36 (PE/EtOAc 8
20) and afforded as a white solid (mp 128–131 °C, lit.11a 125–128 °C) in 61% yield. Rf = 0.36 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.47 (brs, 1H), 8.00 (s, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.45 (d, J = 2.2 Hz, 1H), 7.29–7.10 (m, 6H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.47 (brs, 1H), 8.00 (s, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.45 (d, J = 2.2 Hz, 1H), 7.29–7.10 (m, 6H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a yellow oil in 55% yield. Rf = 0.44 (PE/EtOAc 1
20) and afforded as a yellow oil in 55% yield. Rf = 0.44 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.38 (brs, 1H), 7.45 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.26–7.23 (m, 2H), 7.18–7.09 (m, 4H), 6.94 (dd, J = 2.2, 8.8 Hz, 1H), 3.82 (s, 3H).
1); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.38 (brs, 1H), 7.45 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.26–7.23 (m, 2H), 7.18–7.09 (m, 4H), 6.94 (dd, J = 2.2, 8.8 Hz, 1H), 3.82 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to light petroleum/ethyl acetate 85
5 to light petroleum/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and afforded as a white solid (mp 184–187 °C) in 65% yield. Rf = 0.38 (PE/EtOAc 8
15) and afforded as a white solid (mp 184–187 °C) in 65% yield. Rf = 0.38 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.30 (brs, 1H), 8.79 (brs, 1H), 7.65–7.38 (m, 2H), 7.37–7.07 (m, 12H), 4.56 (d, J = 5.3 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 160.9, 138.9, 136.1, 134.6, 132.6, 130.4, 129.6 (2C), 129.1 (2C), 128.6 (2C), 127.3 (2C), 127.1, 126.6, 124.6, 121.3, 120.9, 113.1, 96.6, 42.8; 77Se NMR (76.27 MHz, DMSO-d6): δ (ppm) = 198.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H19N2Ose: 407.0663; found: 407.0673.
2); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.30 (brs, 1H), 8.79 (brs, 1H), 7.65–7.38 (m, 2H), 7.37–7.07 (m, 12H), 4.56 (d, J = 5.3 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 160.9, 138.9, 136.1, 134.6, 132.6, 130.4, 129.6 (2C), 129.1 (2C), 128.6 (2C), 127.3 (2C), 127.1, 126.6, 124.6, 121.3, 120.9, 113.1, 96.6, 42.8; 77Se NMR (76.27 MHz, DMSO-d6): δ (ppm) = 198.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H19N2Ose: 407.0663; found: 407.0673.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to light petroleum/ethyl acetate 85
5 to light petroleum/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and afforded as a white solid (mp 224–228 °C) in 48% yield. Rf = 0.58 (PE/EtOAc 8
15) and afforded as a white solid (mp 224–228 °C) in 48% yield. Rf = 0.58 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.44 (brs, 1H) 10.38 (brs, 1H), 7.71 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.2 Hz, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.37 (t, J = 7.5 Hz, 2H), 7.30 (dt, J = 1.1, 7.0 Hz, 1H), 7.23–7.07 (m, 7H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 159.3, 138.5, 136.4, 135.1, 132.7, 130.2, 129.7 (2C), 129.3 (2C), 129.2 (2C), 126.6, 124.9, 124.3, 121.4, 120.9, 119.8 (2C), 113.1, 97.9; 77Se NMR (76.27 MHz, DMSO-d6): δ (ppm) = 204.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H17N2OSe 393.0506; found: 393.0516.
2); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.44 (brs, 1H) 10.38 (brs, 1H), 7.71 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.2 Hz, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.37 (t, J = 7.5 Hz, 2H), 7.30 (dt, J = 1.1, 7.0 Hz, 1H), 7.23–7.07 (m, 7H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 159.3, 138.5, 136.4, 135.1, 132.7, 130.2, 129.7 (2C), 129.3 (2C), 129.2 (2C), 126.6, 124.9, 124.3, 121.4, 120.9, 119.8 (2C), 113.1, 97.9; 77Se NMR (76.27 MHz, DMSO-d6): δ (ppm) = 204.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H17N2OSe 393.0506; found: 393.0516.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a yellow solid (mp 165–167 °C, lit.11a 166–168 °C) in 79% yield. Rf = 0.14 (PE/EtOAc 8
20) and afforded as a yellow solid (mp 165–167 °C, lit.11a 166–168 °C) in 79% yield. Rf = 0.14 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, DMSO-d6, 25 °C):11aδ (ppm) = 12.27 (brs, 1H), 7.99 (d, J = 2.2 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.57 (d, J = 7.3 Hz, 1H), 7.30 (t, J = 7.9 Hz, 1H), 7.22–7.10 (m, 5H).
2); 1H NMR (400 MHz, DMSO-d6, 25 °C):11aδ (ppm) = 12.27 (brs, 1H), 7.99 (d, J = 2.2 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.57 (d, J = 7.3 Hz, 1H), 7.30 (t, J = 7.9 Hz, 1H), 7.22–7.10 (m, 5H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to light petroleum/ethyl acetate 90
5 to light petroleum/ethyl acetate 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and afforded as a white solid (mp 96–98 °C, lit.8c 97–98 °C) in 28% yield. Rf = 0.72 (PE/EtOAc 8
10) and afforded as a white solid (mp 96–98 °C, lit.8c 97–98 °C) in 28% yield. Rf = 0.72 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.47 (brs, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.43–7.05 (m, 8H), 2.46 (s, 3H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.47 (brs, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.43–7.05 (m, 8H), 2.46 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and afforded as a yellow oil in 5% yield. Rf = 0.46 (PE/EtOAc 9
5) and afforded as a yellow oil in 5% yield. Rf = 0.46 (PE/EtOAc 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (600 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.48 (d, J = 7.6 Hz, 1H), 7.72 (s, 1H), 7.55 (d, J = 7.6 Hz, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.36–7.32 (m, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.23–7.18 (m, 3H), 2.60 (s, 3H); 13C NMR (150 MHz, CDCl3): δ (ppm) = 163.2, 136.0, 131.7, 131.3, 130.9, 129.9 (2C), 129.3 (2C), 126.5, 125.9, 124.3, 120.7, 116.7, 116.6, 106.9, 23.9; 77Se NMR (114 MHz, CDCl3): δ (ppm) = 230.9.
1); 1H NMR (600 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.48 (d, J = 7.6 Hz, 1H), 7.72 (s, 1H), 7.55 (d, J = 7.6 Hz, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.36–7.32 (m, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.23–7.18 (m, 3H), 2.60 (s, 3H); 13C NMR (150 MHz, CDCl3): δ (ppm) = 163.2, 136.0, 131.7, 131.3, 130.9, 129.9 (2C), 129.3 (2C), 126.5, 125.9, 124.3, 120.7, 116.7, 116.6, 106.9, 23.9; 77Se NMR (114 MHz, CDCl3): δ (ppm) = 230.9.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 85
10 to light petroleum/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and afforded as a white solid (mp 114–112 °C, lit.11a 113–115 °C) in 55% yield. Rf = 0.44 (PE/EtOAc 8
15) and afforded as a white solid (mp 114–112 °C, lit.11a 113–115 °C) in 55% yield. Rf = 0.44 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.37 (brs, 1H), 7.70 (d, J = 7.5 Hz, 1H), 7.45–7.38 (m, 2H), 7.34–7.14 (m, 4H), 6.79–6.68 (m, 2H), 3.75 (s, 3H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.37 (brs, 1H), 7.70 (d, J = 7.5 Hz, 1H), 7.45–7.38 (m, 2H), 7.34–7.14 (m, 4H), 6.79–6.68 (m, 2H), 3.75 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 85
10 to light petroleum/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and afforded as a white solid (mp 108–110 °C, lit.11a 105–106 °C) in 72% yield. Rf = 0.70 (PE/EtOAc 8
15) and afforded as a white solid (mp 108–110 °C, lit.11a 105–106 °C) in 72% yield. Rf = 0.70 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.29 (brs, 1H), 7.71 (d, J = 7.8 Hz, 1H), 7.43–7.39 (m, 2H), 7.35–7.17 (m, 4H), 7.05–6.95 (m, 2H), 2.29 (s, 3H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.29 (brs, 1H), 7.71 (d, J = 7.8 Hz, 1H), 7.43–7.39 (m, 2H), 7.35–7.17 (m, 4H), 7.05–6.95 (m, 2H), 2.29 (s, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 80
10 to light petroleum/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a white solid (mp 122–124 °C, lit.11a 117–120 °C) in 61% yield. Rf = 0.47 (PE/EtOAc 8
20) and afforded as a white solid (mp 122–124 °C, lit.11a 117–120 °C) in 61% yield. Rf = 0.47 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.47 (brs, 1H), 7.60 (d, J = 7.7 Hz, 1H), 7.51 (d, J = 2.2 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.31–7.25 (m, 1H), 7.21–7.07 (m, 5H).
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.47 (brs, 1H), 7.60 (d, J = 7.7 Hz, 1H), 7.51 (d, J = 2.2 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.31–7.25 (m, 1H), 7.21–7.07 (m, 5H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to dichloromethane/methanol 96
2 to dichloromethane/methanol 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and afforded as a brown solid (mp 155–158 °C, lit.11a 152–154 °C) in 52% yield. Rf = 0.39 (CH2Cl2/MeOH 8
4) and afforded as a brown solid (mp 155–158 °C, lit.11a 152–154 °C) in 52% yield. Rf = 0.39 (CH2Cl2/MeOH 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CD3COCD3, 25 °C): 10.74 (brs, 1H), 8.12 (dd, J = 2.0, 6.9 Hz, 1H), 7.67 (d, J = 2.6 Hz, 1H), 7.58 (d, J = 8.2 Hz, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.26–7.15 (m, 3H), 7.11 (t, J = 7.0 Hz, 1H), 6.96 (dd, J = 1.0, 7.6 Hz, 1H).
2); 1H NMR (400 MHz, CD3COCD3, 25 °C): 10.74 (brs, 1H), 8.12 (dd, J = 2.0, 6.9 Hz, 1H), 7.67 (d, J = 2.6 Hz, 1H), 7.58 (d, J = 8.2 Hz, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.26–7.15 (m, 3H), 7.11 (t, J = 7.0 Hz, 1H), 6.96 (dd, J = 1.0, 7.6 Hz, 1H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and afforded as a light yellow oil in 78% yield. Rf = 0.53 (CH2Cl2/MeOH 8
1) and afforded as a light yellow oil in 78% yield. Rf = 0.53 (CH2Cl2/MeOH 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) 11.81 (brs, 1H), 8.20 (d, J = 5.6 Hz, 2H), 7.77 (d, J = 2.5 Hz, 1H), 7.51 (d, J = 7.2 Hz, 1H), 7.35 (d, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.11–7.05 (m, 3H).
2); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) 11.81 (brs, 1H), 8.20 (d, J = 5.6 Hz, 2H), 7.77 (d, J = 2.5 Hz, 1H), 7.51 (d, J = 7.2 Hz, 1H), 7.35 (d, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.11–7.05 (m, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and afforded as a yellow oil in 28% yield. Rf = 0.62 (PE/EtOAc 8
20) and afforded as a yellow oil in 28% yield. Rf = 0.62 (PE/EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.18 (brs, 1H), 7.67 (d, J = 7.5 Hz, 1H), 7.32 (d, J = 7.5 Hz, 1H), 7.25 (d, J = 2.3 Hz, 1H), 7.18–7.09 (m, 3H), 2.60 (t, J = 7.4 Hz, 2H), 1.53 (quint, J = 7.2 Hz, 1H), 1.37–1.15 (m, 14H), 0.80 (t, J = 6.3 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) = 136.2, 130.3, 130.0, 122.5, 120.3, 120.2, 111.2, 98.9, 31.8, 30.5, 29.6, 29.5 (2C), 29.3, 29.1, 28.8, 22.6, 14.1; 77Se NMR (76.27 MHz, CDCl3): δ (ppm) = 85.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H28NSe: 338.1387; found: 338.1397.
2); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.18 (brs, 1H), 7.67 (d, J = 7.5 Hz, 1H), 7.32 (d, J = 7.5 Hz, 1H), 7.25 (d, J = 2.3 Hz, 1H), 7.18–7.09 (m, 3H), 2.60 (t, J = 7.4 Hz, 2H), 1.53 (quint, J = 7.2 Hz, 1H), 1.37–1.15 (m, 14H), 0.80 (t, J = 6.3 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) = 136.2, 130.3, 130.0, 122.5, 120.3, 120.2, 111.2, 98.9, 31.8, 30.5, 29.6, 29.5 (2C), 29.3, 29.1, 28.8, 22.6, 14.1; 77Se NMR (76.27 MHz, CDCl3): δ (ppm) = 85.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H28NSe: 338.1387; found: 338.1397.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) and afforded as a white solid (mp 92–94 °C; lit24 94–95 °C) in 69% yield. Rf = 0.50 (PE/EtOAc 5
40) and afforded as a white solid (mp 92–94 °C; lit24 94–95 °C) in 69% yield. Rf = 0.50 (PE/EtOAc 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.18 (brs, 1H), 7.78–7.74 (m, 2H), 7.33–7.25 (m, 2H), 7.24–7.12 (m, 3H).
5); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.18 (brs, 1H), 7.78–7.74 (m, 2H), 7.33–7.25 (m, 2H), 7.24–7.12 (m, 3H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) and afforded as a white solid (mp 130–133 °C) in 45% yield. Rf = 0.48 (PE/EtOAc 5
40) and afforded as a white solid (mp 130–133 °C) in 45% yield. Rf = 0.48 (PE/EtOAc 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 9.50 (brs, 1H), 7.82–7.70 (m, 2H), 7.23–7.11 (m, 4H); 13C NMR (100 MHz, CDCl3): δ (ppm) = 139.8, 132.2, 131.3, 130.5 (2C), 129.2 (2C), 100.9 (2C); 77Se NMR (76.27 MHz, CDCl3): δ (ppm) = 224.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C9H8N2ClSe: 258.9541; found: 258.9541.
5); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 9.50 (brs, 1H), 7.82–7.70 (m, 2H), 7.23–7.11 (m, 4H); 13C NMR (100 MHz, CDCl3): δ (ppm) = 139.8, 132.2, 131.3, 130.5 (2C), 129.2 (2C), 100.9 (2C); 77Se NMR (76.27 MHz, CDCl3): δ (ppm) = 224.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C9H8N2ClSe: 258.9541; found: 258.9541.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 85
10 to light petroleum/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and afforded as a white solid (mp 148–150 °C; lit.9a 150–151 °C) in 28% yield. Rf (PE/EtOAc 9
15) and afforded as a white solid (mp 148–150 °C; lit.9a 150–151 °C) in 28% yield. Rf (PE/EtOAc 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) = 0.63; 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ = 8.41 (brs, 1H), 7.64 (d, J = 7.9 Hz, 1H), 7.50–7.45 (m, 2H), 7.29 (t, J = 7.9 Hz, 1H,), 7.24–7.09 (m, 5H), 7.06 (t, J = 7.1 Hz, 1H).
1) = 0.63; 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ = 8.41 (brs, 1H), 7.64 (d, J = 7.9 Hz, 1H), 7.50–7.45 (m, 2H), 7.29 (t, J = 7.9 Hz, 1H,), 7.24–7.09 (m, 5H), 7.06 (t, J = 7.1 Hz, 1H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to light petroleum/ethyl acetate 85
10 to light petroleum/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and afforded as a yellow oil in 28% yield. Rf = 0.45 (PE/EtOAc 9
15) and afforded as a yellow oil in 28% yield. Rf = 0.45 (PE/EtOAc 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.37 (brs, 1H), 7.62 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.31–7.24 (m, 6H), 7.21–7.13 (m, 5H), 7.11–7.07 (m, 1H).
1); 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ (ppm) = 8.37 (brs, 1H), 7.62 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.31–7.24 (m, 6H), 7.21–7.13 (m, 5H), 7.11–7.07 (m, 1H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to light petroleum/ethyl acetate 50
30 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a white solid (mp 185–188 °C) in 70% yield. Rf = 0.40 (PE/EtOAc 1
50) and afforded as a white solid (mp 185–188 °C) in 70% yield. Rf = 0.40 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.64–7.59 (m, 3H), 7.50 (d, J = 8.1 Hz, 1H), 7.44 (d, J = 7.9 Hz, 1H), 7.28–7.25 (m, 2H), 7.21 (t, J = 7.6 Hz, 1H), 7.09 (t, J = 7.6 Hz, 1H); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 11.71 (brs, 1H), 7.72 (d, J = 2.5 Hz, 1H), 7.52 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 8.1 Hz, 1H), 7.29 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 8.4 Hz, 2H), 7.13 (t, J = 7.9 Hz, 1H), 7.01 (t, J = 7.2 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 141.0, 140.3, 137.0, 132.2, 129.4, 127.4 (2C), 125.9 (2C), 122.1, 119.9, 118.8, 111.5, 94.6; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 223.1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H13N2O2SSe: 352.9863; found: 352.9868.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.64–7.59 (m, 3H), 7.50 (d, J = 8.1 Hz, 1H), 7.44 (d, J = 7.9 Hz, 1H), 7.28–7.25 (m, 2H), 7.21 (t, J = 7.6 Hz, 1H), 7.09 (t, J = 7.6 Hz, 1H); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 11.71 (brs, 1H), 7.72 (d, J = 2.5 Hz, 1H), 7.52 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 8.1 Hz, 1H), 7.29 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 8.4 Hz, 2H), 7.13 (t, J = 7.9 Hz, 1H), 7.01 (t, J = 7.2 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 141.0, 140.3, 137.0, 132.2, 129.4, 127.4 (2C), 125.9 (2C), 122.1, 119.9, 118.8, 111.5, 94.6; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 223.1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H13N2O2SSe: 352.9863; found: 352.9868.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to light petroleum/ethyl acetate 50
30 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a brown solid (mp 62–64 °C) in 77% yield. Rf = 0.31 (PE/EtOAc 1
50) and afforded as a brown solid (mp 62–64 °C) in 77% yield. Rf = 0.31 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.65–7.58 (m, 3H), 7.45 (dd, 4JH–F = 4.3, 3JH–H = 8.8 Hz, 1H), 7.26–7.19 (m, 2H), 7.06 (dd, 4JH–H = 2.4, 3JH–F = 9.4 Hz, 1H), 6.95 (dt, 4JH–H = 2.4, 3JH–F, 3JH–H = 9.4 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 158.5 (d, 1JC–F = 233 Hz), 140.6, 140.5, 134.2, 133.6, 130.3 (d, 3JC–F = 10.0 Hz), 127.6 (2C), 126.1 (2C), 112.7 (d, 3JC–F = 9.5 Hz), 110.4 (d, 2JC–F = 26.0 Hz), 103.5 (d, 2JC–F = 24.0 Hz), 94.8 (d, 4JC–F = 4.8 Hz); 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 224.1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H12N2O2SSeF: 370.9769; found: 370.9771.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.65–7.58 (m, 3H), 7.45 (dd, 4JH–F = 4.3, 3JH–H = 8.8 Hz, 1H), 7.26–7.19 (m, 2H), 7.06 (dd, 4JH–H = 2.4, 3JH–F = 9.4 Hz, 1H), 6.95 (dt, 4JH–H = 2.4, 3JH–F, 3JH–H = 9.4 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 158.5 (d, 1JC–F = 233 Hz), 140.6, 140.5, 134.2, 133.6, 130.3 (d, 3JC–F = 10.0 Hz), 127.6 (2C), 126.1 (2C), 112.7 (d, 3JC–F = 9.5 Hz), 110.4 (d, 2JC–F = 26.0 Hz), 103.5 (d, 2JC–F = 24.0 Hz), 94.8 (d, 4JC–F = 4.8 Hz); 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 224.1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H12N2O2SSeF: 370.9769; found: 370.9771.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 50
20 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a white solid (mp 77–80 °C) in 54% yield. Rf = 0.40 (PE/EtOAc 1
50) and afforded as a white solid (mp 77–80 °C) in 54% yield. Rf = 0.40 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.70–7.63 (m, 3H), 7.49 (d, J = 8.6 Hz, 1H), 7.38 (d, J = 1.3 Hz, 1H), 7.31–7.24 (m, 2H), 7.19 (dd, J = 1.6, 8.6 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.3, 142.1, 137.2, 135.7, 132.5, 129.3 (2C), 127.9 (2C), 127.7, 124.1, 119.9, 114.6, 96.2; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 223.2; HRMS [M + H]+ calcd for C14H12N2O2SSeCl: 386.9473; found: 386.9475.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.70–7.63 (m, 3H), 7.49 (d, J = 8.6 Hz, 1H), 7.38 (d, J = 1.3 Hz, 1H), 7.31–7.24 (m, 2H), 7.19 (dd, J = 1.6, 8.6 Hz, 1H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.3, 142.1, 137.2, 135.7, 132.5, 129.3 (2C), 127.9 (2C), 127.7, 124.1, 119.9, 114.6, 96.2; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 223.2; HRMS [M + H]+ calcd for C14H12N2O2SSeCl: 386.9473; found: 386.9475.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to light petroleum/ethyl acetate 40
40 to light petroleum/ethyl acetate 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) and afforded as a white solid (mp 76–79 °C) in 86% yield. Rf = 0.31 (PE/EtOAc 1
60) and afforded as a white solid (mp 76–79 °C) in 86% yield. Rf = 0.31 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.72–7.65 (m, 3H), 7.56 (d, J = 1.2 Hz, 1H), 7.46 (d, J = 8.6 Hz, 1H), 7.34 (dd, J = 1.5, 8.7 Hz, 1H), 7.33–7.28 (m, 2H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.1, 141.7, 137.2, 135.3, 132.8, 128.9 (2C), 127.6 (2C), 126.4, 122.8, 114.8, 114.7, 95.9; 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 220.9; HRMS [M + H]+ calcd for C14H12N2O2SSeBr: 430.8968; found: 430.8966.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.72–7.65 (m, 3H), 7.56 (d, J = 1.2 Hz, 1H), 7.46 (d, J = 8.6 Hz, 1H), 7.34 (dd, J = 1.5, 8.7 Hz, 1H), 7.33–7.28 (m, 2H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.1, 141.7, 137.2, 135.3, 132.8, 128.9 (2C), 127.6 (2C), 126.4, 122.8, 114.8, 114.7, 95.9; 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 220.9; HRMS [M + H]+ calcd for C14H12N2O2SSeBr: 430.8968; found: 430.8966.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 50
20 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a white solid (mp 164–167 °C) in 66% yield. Rf = 0.38 (PE/EtOAc 1
50) and afforded as a white solid (mp 164–167 °C) in 66% yield. Rf = 0.38 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.74 (d, J = 1.3 Hz, 1H), 7.66–7.60 (m, 2H), 7.56 (s, 1H), 7.44 (dd, J = 1.4, 8.5 Hz, 1H), 7.31 (d, J = 8.5 Hz, 1H), 7.26–7.22 (m, 2H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.2, 142.1, 137.9, 135.1, 133.8, 132.3, 129.5, 129.2 (2C), 127.8 (2C), 115.4, 95.7, 84.9; 77Se NMR (76.27 MHz CD3OD): δ = 222.8; HRMS [M + H]+ calcd for C14H12N2O2SSeI: 478.8829; found: 478.8835.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.74 (d, J = 1.3 Hz, 1H), 7.66–7.60 (m, 2H), 7.56 (s, 1H), 7.44 (dd, J = 1.4, 8.5 Hz, 1H), 7.31 (d, J = 8.5 Hz, 1H), 7.26–7.22 (m, 2H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.2, 142.1, 137.9, 135.1, 133.8, 132.3, 129.5, 129.2 (2C), 127.8 (2C), 115.4, 95.7, 84.9; 77Se NMR (76.27 MHz CD3OD): δ = 222.8; HRMS [M + H]+ calcd for C14H12N2O2SSeI: 478.8829; found: 478.8835.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 50
20 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a brown solid (mp 179–182 °C) in 66% yield. Rf = 0.55 (PE/EtOAc 1
50) and afforded as a brown solid (mp 179–182 °C) in 66% yield. Rf = 0.55 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.66–7.59 (m, 2H), 7.56 (s, 1H), 7.41 (d, J = 8.8 Hz, 1H), 7.29–7.23 (m, 2H), 6.92 (d, J = 2.0 Hz, 1H), 6.88 (dd, J = 2.2, 8.8 Hz, 1H), 3.75 (s, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 156.6, 142.8, 142.1, 134.5, 133.8, 131.9, 129.1 (2C), 127.7 (2C), 114.2, 114.1, 101.9, 95.9, 56.3; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 222.6; HRMS [M + H]+ calcd for C15H15N2O3SSe: 382.9969; found: 382.9972.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.66–7.59 (m, 2H), 7.56 (s, 1H), 7.41 (d, J = 8.8 Hz, 1H), 7.29–7.23 (m, 2H), 6.92 (d, J = 2.0 Hz, 1H), 6.88 (dd, J = 2.2, 8.8 Hz, 1H), 3.75 (s, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 156.6, 142.8, 142.1, 134.5, 133.8, 131.9, 129.1 (2C), 127.7 (2C), 114.2, 114.1, 101.9, 95.9, 56.3; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 222.6; HRMS [M + H]+ calcd for C15H15N2O3SSe: 382.9969; found: 382.9972.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to light petroleum/ethyl acetate 50
30 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a brown solid in 65% yield. Rf = 0.45 (PE/EtOAc 1
50) and afforded as a brown solid in 65% yield. Rf = 0.45 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.64–7.59 (m, 2H), 7.53 (s, 1H), 7.37 (d, J = 8.3 Hz, 1H), 7.27–7.24 (m, 2H), 7.22 (s, 1H), 7.04 (d, J = 8.3 Hz, 1H), 2.37 (s, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.8, 141.8, 136.9, 133.8, 131.2, 130.9, 128.9 (2C), 127.5 (2C), 125.3, 119.9, 112.8, 95.6, 21.7; 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 220.9; HRMS [M + H]+ calcd for C15H15N2O2SSe: 367.0019; found: 367.0020.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.64–7.59 (m, 2H), 7.53 (s, 1H), 7.37 (d, J = 8.3 Hz, 1H), 7.27–7.24 (m, 2H), 7.22 (s, 1H), 7.04 (d, J = 8.3 Hz, 1H), 2.37 (s, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 142.8, 141.8, 136.9, 133.8, 131.2, 130.9, 128.9 (2C), 127.5 (2C), 125.3, 119.9, 112.8, 95.6, 21.7; 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 220.9; HRMS [M + H]+ calcd for C15H15N2O2SSe: 367.0019; found: 367.0020.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 60
20 to light petroleum/ethyl acetate 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) and afforded as a white solid (mp 126–127 °C) in 88% yield. Rf = 0.36 (PE/EtOAc 1
40) and afforded as a white solid (mp 126–127 °C) in 88% yield. Rf = 0.36 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.53–7.50 (m, 2H), 7.31 (s, 1H), 7.17 (d, J = 8.5 Hz, 1H), 7.11–7.06 (m, 3H), 2.38 (s, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 144.9, 141.9, 141.7, 136.5, 134.0, 128.8 (2C), 127.6 (2C), 125.6, 122.2, 114.5, 113.8, 94.2, 12.8; 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 196.9; HRMS [M + H]+ calcd for C15H14N2O2SSeBr: 444.9125; found: 444.9124.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.53–7.50 (m, 2H), 7.31 (s, 1H), 7.17 (d, J = 8.5 Hz, 1H), 7.11–7.06 (m, 3H), 2.38 (s, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 144.9, 141.9, 141.7, 136.5, 134.0, 128.8 (2C), 127.6 (2C), 125.6, 122.2, 114.5, 113.8, 94.2, 12.8; 77Se NMR (76.27 MHz CD3OD): δ (ppm) = 196.9; HRMS [M + H]+ calcd for C15H14N2O2SSeBr: 444.9125; found: 444.9124.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 50
20 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a white solid (mp 239–243 °C) in 72% yield. Rf = 0.36 (PE/EtOAc 7
50) and afforded as a white solid (mp 239–243 °C) in 72% yield. Rf = 0.36 (PE/EtOAc 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.3 (s, 1H), 7.96 (s, 1H), 7.79 (dd, J = 0.8, 8.3 Hz, 1H), 7.57–7.51 (m, 2H), 7.50 (dd, J = 0.8, 8.3 Hz, 1H), 7.26 (t, J = 8.1 Hz, 1H), 7.25–7.18 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 141.7, 140.5, 137.6, 137.5, 128.1 (2C), 128.0, 127.9 (2C), 126.6, 122.5, 118.1, 117.9, 101.8, 93.3; 77Se NMR (76.27 MHz DMSO-d6): δ (ppm) = 233.2; HRMS [M + H]+ calcd for C15H12N3O2SSe: 377.9815; found: 377.9822.
3); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.3 (s, 1H), 7.96 (s, 1H), 7.79 (dd, J = 0.8, 8.3 Hz, 1H), 7.57–7.51 (m, 2H), 7.50 (dd, J = 0.8, 8.3 Hz, 1H), 7.26 (t, J = 8.1 Hz, 1H), 7.25–7.18 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 141.7, 140.5, 137.6, 137.5, 128.1 (2C), 128.0, 127.9 (2C), 126.6, 122.5, 118.1, 117.9, 101.8, 93.3; 77Se NMR (76.27 MHz DMSO-d6): δ (ppm) = 233.2; HRMS [M + H]+ calcd for C15H12N3O2SSe: 377.9815; found: 377.9822.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 50
20 to light petroleum/ethyl acetate 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and afforded as a white solid (mp 126–127 °C) in 48% yield. Rf = 0.35 (PE/EtOAc 1
50) and afforded as a white solid (mp 126–127 °C) in 48% yield. Rf = 0.35 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.54–7.53 (m, 2H), 7.52 (d, J = 8.3 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H), 7.33–7.25 (m, 3H), 7.11 (t, J = 7.2 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 164.8, 144.6, 143.5, 140.8, 133.9, 133.4, 132.3 (2C), 129.9 (2C), 129.2, 125.0, 124.7, 116.2, 106.1, 64.7, 17.0; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 255.3; HRMS [M + H]+ calcd for C17H17N2O4SSe: 425.0074; found: 425.0076.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ (ppm) = 7.54–7.53 (m, 2H), 7.52 (d, J = 8.3 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H), 7.33–7.25 (m, 3H), 7.11 (t, J = 7.2 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD3OD): δ (ppm) = 164.8, 144.6, 143.5, 140.8, 133.9, 133.4, 132.3 (2C), 129.9 (2C), 129.2, 125.0, 124.7, 116.2, 106.1, 64.7, 17.0; 77Se NMR (76.27 MHz, CD3OD): δ (ppm) = 255.3; HRMS [M + H]+ calcd for C17H17N2O4SSe: 425.0074; found: 425.0076.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 to light petroleum/ethyl acetate 40
25 to light petroleum/ethyl acetate 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) and afforded as a white solid (mp 163–167 °C) in 65% yield. Rf = 0.36 (PE/EtOAc 1
60) and afforded as a white solid (mp 163–167 °C) in 65% yield. Rf = 0.36 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.48 (brs, 1H), 8.61 (brs, 1H), 7.70–7.58 (m, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.44 (d, J = 7.5 Hz, 1H), 7.39–7.17 (m, 11H), 7.17–7.07 (m, 1H), 4.53 (d, J = 4.6, 1H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 160.9, 142.0, 139.0, 138.4, 136.2, 135.4, 130.1, 128.6 (2C), 128.5 (2C), 127.3 (2C), 127.1, 126.7 (2C), 124.7, 121.5, 120.6, 113.2, 95.4, 42.8; 77Se NMR (76.27 MHz DMSO-d6): δ (ppm) = 217.2; HRMS [M + H]+ calcd for C22H20N3O3SSe: 486.0391; found: 486.0396.
1); 1H NMR (400 MHz, DMSO-d6, 25 °C): δ (ppm) = 12.48 (brs, 1H), 8.61 (brs, 1H), 7.70–7.58 (m, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.44 (d, J = 7.5 Hz, 1H), 7.39–7.17 (m, 11H), 7.17–7.07 (m, 1H), 4.53 (d, J = 4.6, 1H); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 160.9, 142.0, 139.0, 138.4, 136.2, 135.4, 130.1, 128.6 (2C), 128.5 (2C), 127.3 (2C), 127.1, 126.7 (2C), 124.7, 121.5, 120.6, 113.2, 95.4, 42.8; 77Se NMR (76.27 MHz DMSO-d6): δ (ppm) = 217.2; HRMS [M + H]+ calcd for C22H20N3O3SSe: 486.0391; found: 486.0396.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to light petroleum/ethyl acetate 60
20 to light petroleum/ethyl acetate 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) and afforded as a white solid (mp 159–161 °C) in 83% yield. Rf = 0.40 (PE/EtOAc 1
40) and afforded as a white solid (mp 159–161 °C) in 83% yield. Rf = 0.40 (PE/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD3OD, 25 °C): δ = 7.63–7.58 (m, 2H), 7.52 (s, 1H), 7.49 (d, J = 8.3 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.31–7.22 (m, 3H), 7.12 (t, J = 8.0 Hz, 1H), 3.89 (s, 3H); 13C NMR (100 MHz, CD3OD): δ = 140.8, 140.4, 137.7, 136.1, 130.0, 127.4 (2C), 125.9 (2C), 122.1, 120.1, 119.1, 109.6, 93.6, 31.7; 77Se NMR (76.27 MHz CD3OD): δ = 222.71; HRMS [M + H]+ calcd for C15H15N2O2SSe: 367.0019; found: 367.0028.
1); 1H NMR (400 MHz, CD3OD, 25 °C): δ = 7.63–7.58 (m, 2H), 7.52 (s, 1H), 7.49 (d, J = 8.3 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.31–7.22 (m, 3H), 7.12 (t, J = 8.0 Hz, 1H), 3.89 (s, 3H); 13C NMR (100 MHz, CD3OD): δ = 140.8, 140.4, 137.7, 136.1, 130.0, 127.4 (2C), 125.9 (2C), 122.1, 120.1, 119.1, 109.6, 93.6, 31.7; 77Se NMR (76.27 MHz CD3OD): δ = 222.71; HRMS [M + H]+ calcd for C15H15N2O2SSe: 367.0019; found: 367.0028.
Biological assay
Author contributions
Conceptualization, C. S., F. M., and L. B.; methodology, M. P., G. P., and L. B.; validation, A. A., M. P., and L. B.; formal analysis, M. P., A. A., and L. B.; investigation, M. P., A. A., R. G., and A. J. H.; resources, C. S.; data curation, M. P. and L. B.; writing—original draft preparation, A. A. and L. B.; writing—review and editing, G. P., E. J. L., F. M., C. S., C. T. S., and L. B.; supervision, F. M. and L. B.; and funding acquisition, C. S., C. T. S., and L. B. All authors have read and agreed to the published version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the University of Perugia, Fondo per il sostegno della Ricerca di Base 2018, Project “Sviluppo di metodologie innovative per la sintesi efficiente di composti eterociclici, molecole drug-like e intermedi sintetici ad alto valore aggiunto”. The work was undertaken as part of the scientific activity of the international multidisciplinary “SeS Redox and Catalysis” network and National Interuniversities Consortium C.I.N.M.P.I.S.References
- For selected reviews on N-heteroaromatic compounds in medicinal chemistry, see: (a) O. Ebenezer, M. A. Jordaan, G. Carena, T. Bono, M. Shapi and J. A. Tuszynski, Int. J. Mol. Sci., 2022, 23, 8117 CrossRef CAS PubMed; (b) M. M. Heravi and V. Zadsirjana, RSC Adv., 2020, 10, 44247 RSC.
- For selected reviews on the pharmacological importance of indoles, see: (a) A. Kumari and R. K. Singh, Bioorg. Chem., 2019, 89, 103021 CrossRef CAS PubMed; (b) N. Chadha and O. Silakari, Eur. J. Med. Chem., 2017, 134, 159 CrossRef CAS PubMed; (c) N. K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C. H. Kim, A. K. Verma and E. H. Choi, Molecules, 2013, 18, 6620 CrossRef CAS PubMed.
- (a) W. Hou, H. Dong, X. Zhang, Y. Wang, L. Su and H. Xu, Drug Discovery Today, 2022, 27, 2268 CrossRef CAS PubMed; (b) H. Chai, S.-Q. Zhang, H. Bai, J. Li, Y. Wang, J. Sun, E. Wen, J. Zhang and M. Xin, Eur. J. Med. Chem., 2021, 223, 113621 CrossRef PubMed; (c) W. Hou and H. Xu, J. Med. Chem., 2022, 65, 4436 CrossRef CAS PubMed; (d) Y. Wu, B. Li, L. Ying, Y. Chen, Y. Zhang, C. Hu, Y. Zhang, L. Yi, W. Xue, S. Huang and Z. Song, J. Med. Chem., 2024, 67(9), 7585 CrossRef CAS PubMed; (e) C. Morán-Serradilla, D. Plano, C. Sanmartín and A. K. Sharma, J. Med. Chem., 2024, 67(10), 7759 CrossRef PubMed.
- (a) W. Ali, R. Benedetti, J. Handzlik, C. Zwergel and C. Battistelli, Drug Discovery Today, 2021, 26, 256 CrossRef CAS PubMed; (b) F. Mangiavacchi, P. Botwina, E. Menichetti, L. Bagnoli, O. Rosati, F. Marini, S. F. Fonseca, L. Abenante, D. Alves, A. Dabrowska, A. Kula-Pacurar, D. Ortega-Alarcon, A. Jimenez-Alesanco, L. Ceballos-Laita, S. Vega, B. Rizzuti, O. Abian, E. J. Lenardão, A. Velazquez-Campoy, K. Pyrc, L. Sancineto and C. Santi, Int. J. Mol. Sci., 2021, 22, 7048 CrossRef CAS PubMed; (c) L. Sancineto, A. Mariotti, L. Bagnoli, F. Marini, J. Desanctis, N. Iraci, C. Santi, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2015, 58, 960 CrossRef PubMed; (d) B. M. Viera, S. Thurow, M. da Costa, A. M. Casaril, M. Domingues, R. F. Schumacher, G. Perin, D. Alves, L. Savegnago and E. J. Lenardão, Asian J. Org. Chem., 2017, 6, 1635 CrossRef; (e) D. de Souza, D. O. C. Mariano, F. Nedel, E. Schultze, V. F. Campos, F. Seixas, R. S. Da Silva, T. S. Munchen, V. Ilha, L. Dornelles, A. L. Braga, J. B. T. Rocha, T. Collares and O. E. D. Rodrigues, J. Med. Chem., 2015, 58, 3329 CrossRef CAS PubMed.
- (a) Z. Y. Wen, J. W. Xu, Z. W. Wang, H. Qi, Q. L. Xu, Z. S. Bai, Q. Zhang, K. Bao, Y. L. Wu and W. G. Zhang, Eur. J. Med. Chem., 2015, 90, 184 CrossRef CAS PubMed; (b) Q. Guan, C. Han, D. Zuo, M. Zhai, Z. Li, Q. Zhang, Y. Zhai, X. Jiang, K. Bao, Y. Wu and W. Zhang, Eur. J. Med. Chem., 2014, 87, 306 CrossRef CAS PubMed.
- P. T. Birmann, F. S. S. Sousa, D. H. de Oliveira, M. Domingues, B. M. Vieira, E. J. Lenardão and L. Savegnago, Eur. J. Pharmacol., 2018, 827, 71 CrossRef CAS PubMed.
- (a) A. M. Casaril, M. T. Igniasiak, C. Y. Chuang, B. Viera, N. B. Padilha, L. Carrol, E. J. Lenardão, L. Savegnano and M. J. Davies, Free Radicals Biol. Med., 2017, 113, 395 CrossRef CAS PubMed.
- (a) D. Luo, G. Wu, H. Yang, M. Liu, W. Gao, X. Huang, J. Chen and H. Wu, J. Org. Chem., 2016, 81, 4485 CrossRef CAS PubMed; (b) Y. Cao, J. Liu, F. Liu, L. Jiang and W. Yi, Org. Chem. Front., 2019, 6, 825 RSC; (c) E. Q. Luz, D. Seckler, J. S. Araújo, L. Angst, D. B. Lima, E. A. M. Rios, R. R. Ribeiro and D. S. Rampon, Tetrahedron, 2019, 75, 1258 CrossRef CAS; (d) T. Benchawan, J. Maneewong and R. Saeeng, ChemistrySelect, 2023, 8, e202301988 CrossRef CAS; (e) E. A. M. Rios, C. M. B. Gomes, G. L. Silverio, E. Q. Luz, S. Ali, C. D. R. M. D'Oca, B. Albach, R. B. Campos and D. S. Rampon, RSC Adv., 2023, 13, 914 RSC.
- (a) J. B. Azeredo, M. Godoi, G. M. Martins, C. C. Silveira and A. L. Braga, J. Org. Chem., 2014, 79, 4125 CrossRef CAS PubMed; (b) H. Li, X. Wang and J. Yan, Appl. Organomet. Chem., 2017, 31, e3864 CrossRef; (c) Y. H. Wang, Y. Q. Zhang, C. F. Zhou, Y. Q. Jiang, Y. Xu, X. Zeng and G. Q. Liu, Org. Biomol. Chem., 2022, 20, 5463 RSC; (d) J. Rafique, S. Saba, M. S. Franco, L. Bettanin, A. R. Schneider, L. T. Silva and A. L. Braga, Chem. – Eur. J., 2018, 24, 4173 CrossRef CAS PubMed; (e) J. R. Menezes, M. M. Gularte, F. C. dos Santos, J. A. Roehrs and J. B. Azeredo, Tetrahedron Lett., 2023, 120, 154446 CrossRef CAS; (f) S.-Q. Chen, Q.-M. Wang, P.-C. Xu, S.-P. Ge, P. Zhong and X.-H. Zhang, Phosphorus, Sulfur Silicon Relat. Elem., 2016, 191, 100 CrossRef CAS.
- (a) Y. Yu, Y. Zhou, Z. Song and G. Liang, Org. Biomol. Chem., 2018, 16, 4958 RSC; (b) N. L. Ferreira, J. B. Azeredo, B. L. Fiorentin and A. L. Braga, Eur. J. Org. Chem., 2015, 5070 CrossRef CAS; (c) S. K. Bhunia, P. Das and R. Jana, Org. Biomol. Chem., 2018, 16, 9243 RSC.
- (a) S. Saba, J. Rafique, M. S. Franco, A. R. Schneider, L. Espíndola, D. O. Silva and A. L. Braga, Org. Biomol. Chem., 2018, 16, 880 RSC; (b) V. Rathore and S. Kumar, Green Chem., 2019, 21, 2670 RSC; (c) I. D. Lemir, W. D. Castro-Godoy, A. A. Hereida, L. C. Schimidtand and J. E. Argüello, RSC Adv., 2019, 9, 22685 RSC; (d) Q. B. Zhang, Y. L. Ban, P. F. Yuan, S. J. Peng, J. G. Fang, L. Z. Wu and Q. Liu, Green Chem., 2017, 19, 5559 RSC.
- X. Zhang, C. Wang, H. Jiang and L. Sun, Chem. Commun., 2018, 54, 8781 RSC.
- (a) M. Palomba, I. F. Coelho Dias, M. Cocchioni, F. Marini, C. Santi and L. Bagnoli, Molecules, 2023, 28, 6026 CrossRef CAS PubMed; (b) M. Palomba, L. Sancineto, F. Marini, C. Santi and L. Bagnoli, Tetrahedron, 2018, 74, 7156 CrossRef CAS; (c) M. Palomba, S. Pompei, L. Roscini and L. Bagnoli, ARKIVOC, 2019, part ii, 163 Search PubMed; (d) M. Palomba, E. Vinti, F. Marini, C. Santi and L. Bagnoli, Tetrahedron, 2016, 72, 7059 CrossRef CAS; (e) L. Bagnoli, in Targets in Heterocyclic System, ed. O. A. Attanasi, P. Merino and D. Spinelli, 2019, vol. 23, ch. 11, pp. 220–236 Search PubMed.
- (a) C. T. Supuran, Nat. Rev. Drug Discovery, 2008, 7, 168 CrossRef CAS PubMed; (b) A. Nocentini and C. T. Supuran, Expert Opin. Drug Discovery, 2019, 14, 1175 CrossRef CAS PubMed.
- (a) A. Angeli, D. Tanini, A. Capperucci and C. T. Supuran, ACS Med. Chem. Lett., 2017, 8, 1213 CrossRef CAS PubMed; (b) A. Angeli, D. Tanini, C. Viglianisi, L. Panzella, A. Capperucci, S. Menichetti and C. T. Supuran, Bioorg. Med. Chem., 2017, 25, 2518 CrossRef CAS PubMed; (c) D. Tanini, A. Capperucci, M. Scopelliti, A. Milaneschi, A. Angeli and C. T. Supuran, Bioorg. Chem., 2019, 89, 102984 CrossRef CAS PubMed; (d) A. Angeli, L. di Cesare Mannelli, E. Trallori, T. S. Peat, C. Ghelardini, F. Carta and C. T. Supuran, ACS Med. Chem. Lett., 2018, 9, 462 CrossRef CAS PubMed; (e) A. Angeli, L. di Cesare Mannelli, E. Lucarini, T. S. Peat, C. Ghelardini and C. T. Supuran, Eur. J. Med. Chem., 2018, 154, 210 CrossRef CAS PubMed; (f) A. Angeli, D. Tanini, T. S. Peat, L. Di Cesare Mannelli, G. Bartolucci, A. Capperucci, C. Ghelardini, C. T. Supuran and F. Carta, ACS Med. Chem. Lett., 2017, 8, 963 CrossRef CAS PubMed; (g) A. Angeli, F. Carta, G. Bartolucci and C. T. Supuran, Bioorg. Med. Chem., 2017, 25, 3567 CrossRef CAS PubMed; (h) A. Angeli, D. Tanini, A. Nocentini, A. Capperucci, M. Ferraroni, P. Gratteri and C. T. Supuran, Chem. Commun., 2019, 55, 648 RSC.
- H. A. Goulart, D. R. Araujo, F. Penteado, R. G. Jacob, G. Perin and E. J. Lenardão, Molecules, 2021, 26, 7523 CrossRef CAS PubMed.
- H. Hussain, I. R. Green and I. Ahmed, Chem. Rev., 2013, 113, 3329 CrossRef CAS PubMed.
- P. Kumar and A. Bhalla, Top. Curr. Chem., 2024, 382(2), 12 CrossRef CAS PubMed.
- H. Zhang, X. Bao, Y. Song, J. Qu and B. Wang, Tetrahedron, 2015, 71, 8885 CrossRef CAS.
- S.-K. Choi, Synthetic Multivalent Molecules: Concepts and Biomedical Applications, Wiley Interscience, Hoboken NJ, 2004 Search PubMed.
- R. G. Khalifah, J. Biol. Chem., 1971, 246, 2561 CrossRef CAS PubMed.
- (a) S. K. Sharma, A. K. Mandadapu, B. Kumar and B. Kundu, J. Org. Chem., 2011, 76, 6798 CrossRef CAS PubMed; (b) A. Krief and M. Derock, Synlett, 2005, 1012 CrossRef CAS; (c) K. B. Sharpless and M. W. Young, J. Org. Chem., 1975, 40, 947 CrossRef CAS; (d) D. Singh, A. M. Deobald, L. R. S. Camargo, G. Tabarelli, O. E. D. Rodrigues and A. L. Braga, Org. Lett., 2010, 12, 3288 CrossRef CAS PubMed.
- R. H. Bartz, P. S. Hellwig, G. Perin, L. E. B. Iarocz, A. Madabeni, L. Orian and M. S. Silva, New J. Chem., 2024, 48, 2971 RSC.
- A. L. Belladona, R. Cervo, D. Alves, T. Barcellos, R. Cargnelutti and R. F. Schumacher, Tetrahedron Lett., 2020, 61, 152035 CrossRef CAS.
- A. Angeli, L. Micheli, R. Turnaturi, L. Pasquinucci, C. Parenti, V. Alterio, A. Di Fiore, G. De Simone, S. M. Monti, F. Carta, L. Di Cesare Mannelli, C. Ghelardini and C. T. Supuran, Eur. J. Med. Chem., 2023, 260, 115783 CrossRef CAS PubMed.
- A. Angeli, E. Trallori, F. Carta, L. Di Cesare Mannelli, C. Ghelardini and C. T. Supuran, ACS Med. Chem. Lett., 2018, 9, 947 CrossRef CAS PubMed.
- D. Tanini, A. Capperucci, M. Ferraroni, F. Carta, A. Angeli and C. T. Supuran, Eur. J. Med. Chem., 2020, 185, 111811 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob00826j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |