Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Borylation via iridium catalysed C–H activation: a new concise route to duocarmycin derivatives†
Marco M. D.
Cominetti
,
Zoë R.
Goddard
 ,
Bethany R.
Hood
,
Andrew M.
Beekman
,
Bethany R.
Hood
,
Andrew M.
Beekman
 ,
Maria A.
O'Connell
,
Maria A.
O'Connell
 and
Mark
Searcey
and
Mark
Searcey
 *
*
School of Pharmacy, University of East Anglia, Norwich Research Park, Norwich NR4 7TJ, UK. E-mail: m.searcey@uea.ac.uk
First published on 18th June 2024
Abstract
The synthesis of the ethyl ester analogue of the ultrapotent antitumour antibiotic seco-duocarmycin SA has been achieved in eleven linear steps from commercially available starting materials. The DSA alkylation subunit can be made in ten linear steps from the same precursor. The route involves C–H activation at the equivalent of the C7 position on indole leading to a borylated intermediate 9 that is stable enough for peptide coupling reactions but can be easily converted to the free hydroxyl analogue.
Introduction
CC-1065,1,2 the duocarmycins3 such as duocarmycin SA (1, Fig. 1) and yatakemycin4,5 are amongst the most potent natural antitumour antibiotics that have been discovered. The densely substituted pyrroloindoline core of the natural products continues to interest synthetic chemists and there have been numerous reports of the total synthesis of the natural compounds and synthetic analogues. Efforts to translate this activity to clinical advantage have tended to focus on targeted systems through prodrugs with bioreductive,6–8 bio-oxidative,9 esterase10,11 or glycosylase enzymes12–16 or through antibody,17 peptide18 or small molecule conjugates.19 Many of these efforts have focussed on the use of the synthetic duocarmycin alkylation subunit known as CBI (2, CBI = 1,2,9,9a-tetrahydroCyclopropa[c]Benzo[e]Indol-4-one), as it is considered to be synthetically accessible, available in six steps from commercially available 1,3-dihydroxynaphthalene 3,20 whereas the routes to the natural products tend to be significantly longer. In this paper, we describe a new synthesis to the ethyl ester analogue 4 of the duocarmycin SA alkylating subunit in only ten linear steps from commercially available ethyl 5-nitroindole-2-carboxylate 5. | ||
| Fig. 1 Duocarmycin SA (1). CBI (2) is often synthesised from 3. In this work, 4 is available from commercially available 5. | ||
In our efforts to generate bio-oxidatively activated prodrugs of the duocarmycins, we have previously shown that Cytochrome P450 (CYP) enzymes can oxidise de-hydroxylated chloromethylindoline derivatives such as 6 to their seco-CPI analogues (CPI = Cyclopropa[c]Pyrrolo[3,2-e]Indol-4(5H)one), which undergo spontaneous cyclisation and subsequent sequence selective DNA alkylation (Scheme 1).9,21–24 Direct hydroxylation via non-enzymatic routes to generate these derivatives is unreported, but we envisioned the possibility of introducing an intermediate boronic ester via C–H activation;25 this could be subsequently subjected to cross-coupling, or directly converted to the hydroxyl derivative at the final synthetic step (Scheme 1).26 Boronic acid derivatives are ideal coupling partners, suitable for Chan–Lam cross couplings, which allow convenient and mild C–O and C–N cross couplings,27 potentially opening possibilities for amino DSA derivatives (DSA = Duocarmycin SA alkylation subunit), but also direct conversion to hydroxyl groups. This transformation is mediated by different N-oxide derivatives and shows good conversions and functional group tolerance.28 Boronic esters have also been used within building blocks for peptide synthesis, demonstrating compatibility with the deprotections and amide couplings we intended to employ to make duocarmycin analogues.29,30
 | ||
| Scheme 1 (A) CYP-enzyme-mediated prodrug activation.5,11 (B) Biomimietic approach using CH activation to generate the correct, active alkylating group structure. | ||
Results
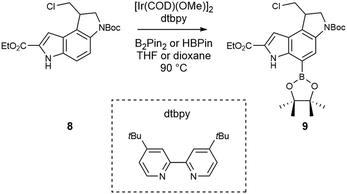
Previous reports have highlighted the ability of [Ir(COD)(OMe)]2 complexes to directly borylate the 7-position on indoles via C–H activation under reasonably mild conditions.31–33 Of particular note, is the accessibility of the route, with both catalyst and ligand being readily available through common suppliers. As a starting point, we used compound 7 (Scheme 2), which we previously reported.34 It can be obtained in 44% yield over 6 steps (with 3 chromatographic purifications) on a multigram scale starting from commercially available ethyl 5-nitroindole-2-carboxylate.34 The methyl ester, mirroring the natural product, is not commercially available as far as we are aware, but it was unlikely that ultimately the ethyl ester group would have significant impact on the biological activity of analogues.Due to the presence of the Boc group on the indole nitrogen and the suggested mechanism of C–H activation regioselectivity, which appears to be aided by coordination at the indole nitrogen,31 we decided to selectively deprotect this position. This can be readily achieved due to the unusual lability to nucleophilic bases of the Boc group on the indole nitrogen; the use of piperazine allows removal of the resulting Boc-protected piperazine by extraction, with quantitative recovery of product 8. Compound 8 was successfully resolved into two enantiomers using chiral flash chromatography (see ESI for methodology†).
The iridium catalysed borylation of indoles in the 7-position via C–H activation has been reported32,33 in either hexane, heptane, dioxane or THF, which led us to use THF to begin exploring optimal conditions, due to solubility limitations in the other solvents (Scheme 3). The reactions were carried out on 100–400 mg of starting material to evaluate isolated yields, and all starting material was extensively dried and stored in a desiccator. All reactions were set up in a glove box with anhydrous and degassed solvents in glass sealed vessels with PTFE lined septa; after preparation of the reaction mixture, the vials were transferred to a pre-heated block. We explored both pinacolborane and bis(pinacolato)diboron as donors, and both produced substantially identical results. In either case, and for all subsequent tests, we only observed product and unreacted starting material, without any noticeable side product. Interestingly, borane amount and catalyst loading did not affect the yield noticeably (Table 1). Doubling the reaction time also had limited effect on the yield, as did increasing the scale of the reaction. Dioxane was also tested as solvent, without any yield improvement. With only a variation of ∼10% in yield after these tests, it is apparent that different solvents, ligands and temperatures may have to be explored and further studies conducted to assess the cause of this limited conversion. Considering a 20–30% recovery of starting material that was then reusable, the reaction was then assessed on a preparative scale to complete the synthesis of the duocarmycin SA ethyl ester analogue.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yields are isolated yield after chromatographic separation
1. Yields are isolated yield after chromatographic separation
| Entry | Cat. (equiv) | B2Pin2 (equiv.) | HBPin (equiv.) | Time (h) | Yield (%) | Scale (mg SM) |
|---|---|---|---|---|---|---|
| a | 0.03 | 0.7 | 24 | 40 | 100 | |
| b | 0.03 | 1.5 | 24 | 38 | 100 | |
| c | 0.03 | 0.7 | 48 | 54 | 100 | |
| d | 0.03 | 1.4 | 48 | 43 | 100 | |
| e | 0.06 | 0.7 | 24 | 48 | 200 | |
| f | 0.06 | 0.7 | 48 | 52 | 200 | |
| g | 0.03 | 0.7 | 48 | 42 | 400 | |
| h | 0.03 | 0.7 | 48 | 42 | 100 |
With compound 9 in hand, we explored the hydroxylation of the boronic ester. Zhu et al. reported a convenient open flask method using different N-oxides, and highlighted how boronic esters reacted more sluggishly as compared to boronic acids.28 In our case, the reaction did not proceed at an appreciable rate with the evaporation of the solvent, CH2Cl2, being a major issue. The use of less volatile chloroform allowed longer reaction times, but still without appreciable conversion. Performing the reaction in a sealed NMR tube allowed the use of a higher temperature (60 °C) while monitoring the reaction progress via11B-NMR (Scheme 4). In anhydrous conditions, the reaction halts after the oxygen insertion into the C–B bond (Scheme 4, centre), as described by Xie and colleagues.35 The resulting borate ester has a clearly distinct shift and is rapidly hydrolysed in the presence of water and acid, which also limits spurious cyclisation of the seco-derivative.
 | ||
| Scheme 4 Monitoring the oxygen insertion into the C–B bond of 9 by 11B-NMR and subsequent conversion to compound 4 (90%) (NMO = N-methylmorpholine-N-oxide). | ||
After defining all required reactions, we proceeded to synthesise an analogue of duocarmycin SA. Following the previous C–H activation method, compound (S)-8 was converted to (S)-9 (Scheme 5) in 49% yield, which was subjected to Boc group deprotection and subsequent coupling with 5,6,7-trimethoxyindole-2-carboxylic acid (TMI-COOH) to give the product (S)-10 (33% yield compared with 48% for the racemic, no attempts were made to optimise yields). The main side product from this conversion was the hydrolysed boronic acid analogue (see 10A, ESI†). Hydroxylation of (S)-10 was effected as described for compound 9 to give the target ethyl ester analogue of seco-duocarmycin SA, compound (S)-11 (34% yield compared with 55% for racemic. No attempt was made to optimise yields).
The compounds were assessed for biological activity in HL-60 cells. Compounds 4, racemic 11 and (S)-11 demonstrated potent (nM to pM) activities in line with previously described derivatives (Table 2).36
| Compound | IC50 (nM) [95% CI] |
|---|---|
| 9 | 763.6 [665.4–892.4] |
| 4 | 26.88 [12.83–53.06] |
| Racemic 11 | 0.69 [0.61–0.79] |
| (S)-11 | 0.085 [0.078–0.094] |
Interestingly, compound 9 showed more potent, sub-micromolar activity than was expected based upon its structure. Boron has an empty p orbital and therefore lacks the ability to spirocyclise, a requirement for potent biological activity in this family of compounds. Compound 9 is remarkably stable, both in open air and in solution, without displaying signs of oxidation to compound 4 (the sample used for biological testing was analysed by LC-MS to identify the potential presence of seco- and spiro-derivatives). On the other hand, boronic acids and esters are known to be subject to oxidation in a biological context,37 with reactive oxygen species mimicking the reaction with NMO, and leading to the formation of the seco-product, which in turn cyclises and alkylates DNA. Preliminary data seems to support this view and we are currently investigating methods to monitor this process in cells (Scheme 6).
 | ||
| Scheme 6 Putative mechanism for the oxidative deborylation mediated by hydrogen peroxide to form the active seco-derivative 9. | ||
Conclusions
Trastuzumab duocarmazine, an antibody drug conjugate (ADC) that includes a duocarmycin as its payload, has progressed through Phase III clinical trials.38 It is apparent that the duocarmycin family of antitumour antibiotics have a role to play in the development of new therapeutics. Traditionally, analogues of the duocarmycins have focussed on CBI as the most accessible member of the family due to its synthetic tractability and similar potency to the natural products. The reported approach herein provides the shortest synthesis of a duocarmycin SA analogue to date and suggests that investigation of the natural product alkylation subunit as a central player in the design of new payloads for ADCs and for other targeted approaches is now accessible.All the reagents used in this approach are commercially available, making the route immediately attractive. The key C–H alkylation in the 7-position of the indole analogue 8 is a late stage modification which has been applied for the first time to this family of derivatives, and provides a boronic ester which can be converted to the active seco-duocarmycin in a final oxidative step. We have also shown that simple chiral flash chromatography can be used to separate 8 into its enantiomers (see ESI†). The boronic ester is also a potential new pro-drug derivative and will be investigated further.
Author contributions
MMDC: conceptualization, investigation, methodology, writing – original draft, writing – review and editing. ZRG: investigation, methodology, formal analysis, writing review and editing. BRH: investigation, formal analysis. AMB: conceptualization, methodology, writing – review and editing. MO'C: methodology, resources, writing – review and editing. MS: conceptualisation, funding acquisition, project administration, supervision, writing review and analysis.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
EPSRC for funding support (EP/S026563). Dr Rianne Lord for use of glovebox facilities. Mr Anthony Hinchcliffe for HR-MS and BBSRC for equipment funding (BB/T017708/1).References
- C. G. Chidester, W. C. Krueger, S. A. Mizsak, D. J. Duchamp and D. G. Martin, J. Am. Chem. Soc., 1981, 103, 7629–7635 CrossRef CAS
.
- L. J. Hanka, A. Dietz, S. A. Gerpheide, S. L. Kuentzel and D. G. Martin, J. Antibiot., 1978, 31, 1211–1217 CrossRef CAS PubMed
.
- D. L. Boger and D. S. Johnson, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 3642–3649 CrossRef CAS PubMed
.
- Y. Igarashi, K. Futamata, T. Fujita, A. Sekine, H. Senda, H. Naoki and T. Furumai, J. Antibiot., 2003, 56, 107–113 CrossRef CAS PubMed
.
- E. A. Mullins, R. Shi and B. F. Eichman, Nat. Chem. Biol., 2017, 13, 1002–1008 CrossRef CAS PubMed
.
- W. Jin, J. D. Trzupek, T. J. Rayl, M. A. Broward, G. A. Vielhauer, S. J. Weir, I. Hwang and D. L. Boger, J. Am. Chem. Soc., 2007, 129, 15391–15397 CrossRef CAS PubMed
.
- J. P. Lajiness, W. M. Robertson, I. Dunwiddie, M. A. Broward, G. A. Vielhauer, S. J. Weir and D. L. Boger, J. Med. Chem., 2010, 53, 7731–7738 CrossRef CAS PubMed
.
- A. L. Wolfe, K. K. Duncan, N. K. Parelkar, D. Brown, G. A. Vielhauer and D. L. Boger, J. Med. Chem., 2013, 5, 4104–4115 CrossRef
.
- K. Pors, P. M. Loadman, S. D. Shnyder, M. Sutherland, H. M. Sheldrake, M. Guino, K. Kiakos, J. A. Hartley, M. Searcey and L. H. Patterson, Chem. Commun., 2011, 47, 12062–12064 RSC
.
- A. L. Wolfe, K. K. Duncan, N. K. Parelkar, S. J. Weir, G. A. Vielhauer and D. L. Boger, J. Med. Chem., 2012, 55, 5878–5886 CrossRef CAS PubMed
.
- R. C. Elgersma, R. G. E. Coumans, T. Huijbregts, W. M. P. B. Menge, J. A. F. Joosten, H. J. Spijker, F. M. H. de Groot, M. M. C. van der Lee, R. Ubink and D. J. van den Dobbelsteen,
et al.
, Mol. Pharmaceutics, 2015, 12, 1813–1835 CrossRef CAS PubMed
.
- L. F. Tietze, R. Hannemann, W. Buhr, M. Lögers, P. Menningen, M. Lieb, D. Starck, T. Grote, A. Döring and I. Schuberth, Angew. Chem., Int. Ed., 1996, 35, 2674–2677 CrossRef CAS
.
- L. F. Tietze, T. Herzig, A. Fecher, F. Haunert and I. Schuberth, ChemBioChem, 2001, 2, 758–765 CrossRef CAS PubMed
.
- L. F. Tietze, F. Major and I. Schuberth, Angew. Chem., Int. Ed., 2006, 45, 6574–6577 CrossRef CAS PubMed
.
- L. F. Tietze, K. Schmuck, H. J. Schuster, M. Müller and I. Schuberth, Chem. – Eur. J., 2011, 17, 1922–1929 CrossRef CAS PubMed
.
- L. F. Tietze, H. J. Schuster, B. Krewer and I. Schuberth, J. Med. Chem., 2009, 52, 537–543 CrossRef CAS PubMed
.
-
A. M. Beekman, M. M. D. Cominetti and M. Searcey, Chapter, 9. Duocarmycins as Antibody–Drug Conjugate (ADC) Payloads, in Cytotoxic Payloads for Antibody–Drug Conjugates, The Royal Society of Chemistry, 2019, pp. 187–208 Search PubMed
.
- O. C. Cartwright, A. M. Beekman, M. M. D. Cominetti, D. A. Russell and M. Searcey, Bioconjugate Chem., 2020, 31, 1745–1749 CrossRef CAS PubMed
.
- A. M. Beekman, M. M. D. Cominetti, O. C. Cartwright, D. L. Boger and M. Searcey, MedChemComm, 2019, 10, 2170–2174 RSC
.
- D. L. Boger and J. A. McKie, J. Org. Chem., 1995, 60, 1271–1275 CrossRef CAS
.
- N. Ortuzar, K. Karu, D. Presa, G. R. Morais, H. M. Sheldrake, S. D. Shnyder, F. M. Barnieh, P. M. Loadman, L. H. Patterson, K. Pors and M. Searcey, Bioorg. Med. Chem., 2021, 40, 116167 CrossRef CAS PubMed
.
- M. Sutherland, J. H. Gill, P. M. Loadman, J. P. Laye, H. M. Sheldrake, N. A. Illingworth, M. N. Alandas, P. A. Cooper, M. Searcey, K. Pors, S. D. Shnyder and L. H. Patterson, Mol. Cancer Ther., 2013, 12, 27–37 CrossRef CAS PubMed
.
- H. M. Sheldrake, S. Travica, I. Johansson, P. M. Loadman, M. Sutherland, L. Elsalem, N. Illingworth, A. J. Cresswell, T. Reuillon, S. D. Shnyder, S. Mrktchian, M. Searcey, M. Ingelman-Sundberg, L. H. Patterson and K. Pors, J. Med. Chem., 2013, 56(15), 6273–6277 CrossRef CAS PubMed
.
- G. B. Aaron, M. Goreti, R. V. Venu, M. L. Paul, K. Pors; and E. E. Scott, Drug Metab. Dispos., 2022, 50, 49–57 CrossRef PubMed
.
- D. W. Robbins, T. A. Boebel and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 4068–4069 CrossRef CAS PubMed
.
- A. S. Eastbrook, C. Wang, E. K. Davison and J. Sperry, J. Org. Chem., 2015, 80, 1006–1017 CrossRef PubMed
.
- J.-Q. Chen, J.-H. Li and Z.-B. Dong, Adv. Synth. Catal., 2020, 362, 3311–3331 CrossRef CAS
.
- C. Zhu, R. Wang and J. R. Falck, Org. Lett., 2012, 14, 3494–3497 CrossRef CAS
.
- Y. Tan, J. Wu, L. Song, M. Zhang, C. J. Hipolito, C. Wu, S. Wang, Y. Zhang and Y. Yin, Int. J. Mol. Sci., 2021, 22, 12958 CrossRef CAS
.
- S. P. A. Hinkes, S. Kämmerer and C. D. P. Klein, Chem. Sci., 2020, 11, 9898–9903 RSC
.
- S. Paul, G. A. Chotana, D. Holmes, R. C. Reichle, R. E. Maleczka and M. R. Smith, J. Am. Chem. Soc., 2006, 128, 15552–15553 CrossRef CAS PubMed
.
- W. F. Lo, H. M. Kaiser, A. Spannenberg, M. Beller and M. K. Tse, Tetrahedron Lett., 2007, 48, 371–375 CrossRef CAS
.
- F. Shen, S. Tyagarajan, D. Perera, S. W. Krska, P. E. Maligres, M. R. Smith III and R. E. Maleczka Jr, Org. Lett., 2016, 18, 1554–1557 CrossRef CAS PubMed
.
- M. M. D. Cominetti, Z. R. Goddard, C. E. Howman, M. A. O'Connell and M. Searcey, Tetrahedron Lett., 2021, 72, 153058 CrossRef CAS
.
- Q. Xie and G. Dong, J. Am. Chem. Soc., 2022, 144, 8498–8503 CrossRef CAS PubMed
.
- M. J. Stephenson, L. A. Howell, M. A. O'Connell, K. R. Fox, C. Adcock, J. Kingston, H. E. Sheldrake, K. Pors, S. P. Collingwood and M. Searcey, J. Org. Chem., 2015, 80, 9454–9467 CrossRef CAS
.
- B. J. Graham, I. W. Windsor, B. Gold and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2013691118 CrossRef CAS
.
- P. G. Aftimos, N. Turner, J. O'Shaughnessy, E. van den Tweel, M. Oesterholt, S. Escrivá-de-Romaní, N. Quenel Tueux, T. J. Y. Tan, J. Lim, S. Ladoire, A. C. Armstronh, A. Stradella, G. V. Bianchi, M. A. Colleoni, E. Kaczmarek, K. Punie, X. Song, N. Koper and C. Saura Manich, Ann. Oncol., 2023, 34(S2), S340–S341 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob00814f |
| This journal is © The Royal Society of Chemistry 2024 |