Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid synthesis of hydrogen bond templated handcuff rotaxanes†
Sean R.
Barlow
 ,
David
Tomkinson
,
Nathan R.
Halcovitch
,
David
Tomkinson
,
Nathan R.
Halcovitch
 and
Nicholas H.
Evans
and
Nicholas H.
Evans
 *
*
Department of Chemistry, Lancaster University, Lancaster, LA1 4YB, UK. E-mail: n.h.evans@lancaster.ac.uk
First published on 10th June 2024
Abstract
The rapid synthesis of hydrogen bond templated handcuff rotaxanes is described. The isolated rotaxanes were characterized by NMR and IR spectroscopies and high resolution mass spectrometry. This report represents a rare demonstration of preparing (2)handcuff [2]rotaxanes by covalently linking separate axles threaded through the rings of a bis-macrocycle by use of the copper catalyzed azide–alkyne cycloaddition (CuAAC) reaction.
Introduction
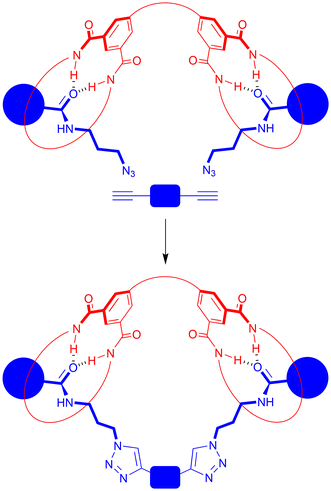
Demand for synthetic routes that rapidly lead to [2]rotaxanes (a macrocyclic ring trapped on a stoppered axle) and [2]catenanes (two interlocked macrocyclic rings) – the archetypal classes of mechanically interlocked molecule (MIM)1 – is driven by their deployment in a range of arena including host–guest recognition,2 catalysis3 and biological systems4 exploiting their unusual three-dimensional molecular structures and (on occasion) controlled motion of their interlocked components. However, by incorporating more interlocked components and/or installing covalent linkages, an exotic range of MIM architectures exists. Amongst these are mechanically interlocked molecular “handcuffs”.5 (2)Handcuff [2]rotaxanes consist of an axle threaded through two covalently linked macrocycles. They are typically prepared by stoppering a single axle component passing through both rings of a bis-macrocycle6 but have also been synthesized by linking the rings of a [3]rotaxane7 and the linking of separate axles passing through each ring of a bis-macrocycle.8,9 Hinting at potential nanotechnological applications, the relative motion of the interlocked components of a few (2)handcuff [2]rotaxanes has been studied.6a,d,eOur group has reported on the rapid synthesis of [2]rotaxanes by copper catalyzed azide–alkyne cycloaddition (CuAAC) stoppering of a hydrogen bond templated pseudorotaxane consisting of a simple aromatic amide azide threading through an isophthalamide macrocycle.10,11 We envisioned that adapting such a methodology by use of an analogous bis-macrocycle and a bis-alkyne rather than an alkyne stopper could afford (2)handcuff [2]rotaxanes in a short synthetic pathway (Fig. 1). This approach would represent an example of one of the rarely used strategies to prepare (2)handcuff [2]rotaxanes, namely covalently linking separate axles passing through each ring of a bis-macrocycle.8 Here we report on the successful preparation and characterization of such handcuff rotaxanes.
Results and discussion
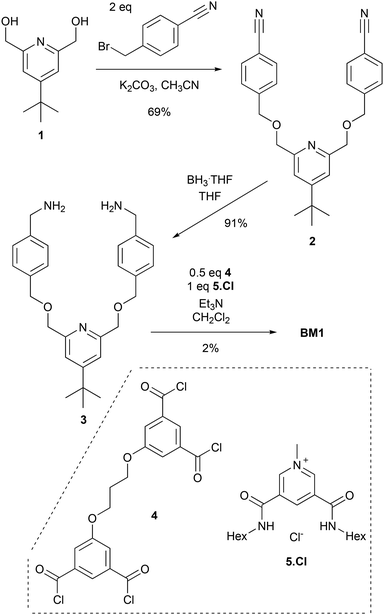
The design of a suitable bis-macrocycle BM1, consisting of two linked isophthalamide macrocycles, is presented in Fig. 2. Initial experiments had indicated that the analogous glycol bis-macrocycle BM0 was highly insoluble.12 However, the recent report13 of the synthesis of 4-(1,1-dimethylethyl)-2,6-pyridinedimethanol 1, inspired us to prepare BM1, in the hope that the t-butyl substituted pyridyl rings would enhance solubility of the bis-macrocycle.We initially targeted preparation of BM1 by a “one-pot” double cyclization strategy using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of novel bis-amine precursor 3 to tetra-acid chloride unit 414 (Scheme 1). A solution containing equimolar equivalents of bis-amine 2 and methyl pyridinium template 5.Cl15 and excess Et3N in CH2Cl2 was prepared. To this solution was added dropwise a solution with 0.5 equivalents of tetra-acid chloride 4 in CH2Cl2. Upon aqueous work-up and chromatographic purification, the target bis-macrocycle BM1 was isolated in a very low 2% yield. A variety of reaction conditions were screened (see ESI, p. S2†), but without any improvement in the yield of BM1.
1 ratio of novel bis-amine precursor 3 to tetra-acid chloride unit 414 (Scheme 1). A solution containing equimolar equivalents of bis-amine 2 and methyl pyridinium template 5.Cl15 and excess Et3N in CH2Cl2 was prepared. To this solution was added dropwise a solution with 0.5 equivalents of tetra-acid chloride 4 in CH2Cl2. Upon aqueous work-up and chromatographic purification, the target bis-macrocycle BM1 was isolated in a very low 2% yield. A variety of reaction conditions were screened (see ESI, p. S2†), but without any improvement in the yield of BM1.
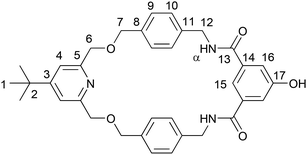
Considering the extremely low yield of the final step of the previous synthesis, attention towards an alternative synthesis of bis-macrocycle BM1. To begin, equimolar equivalents of bis-amine 3, methyl pyridinium template 5.Cl, with excess Et3N were dissolved in CH2Cl2. To this solution was added dropwise a solution of TBDMS-protected 5-hydroxyisophthaloyl chloride 616 in CH2Cl2. Upon aqueous work-up and chromatographic purification the TBDMS-protected macrocycle 7 was afforded in a moderate 15% yield. Deprotection of the TBDMS group was performed by reacting macrocycle 7 with excess TBAF, affording the deprotected phenol macrocycle 8 in almost quantitative – 96% – yield (Scheme 2).
With macrocycle 8 in hand, attempts at the synthesis of the bis-macrocycle BM1 were undertaken. Phenol macrocycle 8, 1,3-dibromopropane and K2CO3 were added to DMF, the mixture then being heated to 80 °C for 16 hours. Following aqueous work-up and purification by column chromatography it was found the reaction did not afford the desired bis-macrocycle, but a mixture of mono-alkylated 9 and mono-alkylated elimination product 10 (Scheme 3).17
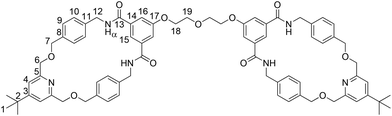
In response to this setback a new bis-macrocycle BM2 was targeted (Scheme 4). Phenol macrocycle 8, diethylene glycol di(p-toluenesulfonate) 918 and K2CO3 were added to DMF. After heating the reaction to 80 °C for 16 hours, aqueous work-up and chromatographic purification, target BM2 was isolated in a pleasing 67% yield. A single crystal suitable for X-ray crystallography was grown from a chloroform solution, the solved structure confirming the anticipated bond connectivity (see inset in Scheme 4).
The synthesis of (2)handcuff [2]rotaxanes was then attempted (Scheme 5). This involved dissolving each bis-macrocycle with 2.4 equivalents of azide 1010a in CH2Cl2 and allowing to stir for 10 minutes, so the pseudorotaxane complex could form. Then, 1.2 equivalents of bis-alkyne 1119 Cu(CH3CN)4BF4, TBTA and DIPEA were added to facilitate the CuAAC reaction, and after stirring overnight, aqueous work-up and chromatographic separation by preparative TLC handcuff rotaxanes HR1 and HR2 were isolated in ∼30%20 and 15% yields respectively.
The novel handcuff rotaxanes were characterized by 1H and 13C NMR spectroscopy, IR spectroscopy and electrospray high resolution mass spectrometry. The 1H NMR spectra of handcuff rotaxane HR2, along with those of bis-macrocycle BM2 and non-threaded axle Ax21 for comparison are shown in Fig. 3. Upon studying the stacked spectra, evidence for the formation of handcuff rotaxane is observed. The upfield shift and splitting of aromatic protons 9 and 10 in the interlocked molecule compared to non-interlocked bis-macrocycle BM2 is consistent with the axle passing between the aromatic rings of each macrocyclic ring. The downfield shift of proton 15 and the amide protons α of the bis-macrocycle in the handcuff rotaxane are indicative of interactions with a hydrogen bond acceptor on the threaded axle. In addition, the splitting of protons 7 and 12 arises from the desymmetrization caused by the axle component threaded through the two macrocyclic cavities. The interlocked architecture of the handcuff rotaxane HR2 was further supported by multiple through-space correlations between signals arising from protons in the two components as observed in the 1H–1H ROESY NMR spectrum (see ESI, p. S20†). In addition, the molecular ion peak for the target rotaxane is also observed in the electrospray HRMS (see ESI, p. S19†). Similar observations and conclusions regarding the formation of handcuff rotaxane HR1 can also be seen by, e.g., comparing its spectra with its analogous bis-macrocycle BM1 and axle Ax (see ESI, pp. S15–S17 and S23†).
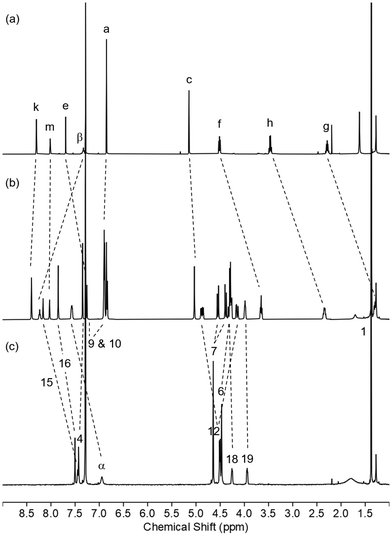 | ||
| Fig. 3 1H NMR spectra of (a) axle Ax, (b) handcuff rotaxane HR2 and (c) bis-macrocycle BM2 (CDCl3, 400 MHz, 298 K). For atom labels see Schemes 4 and 5. | ||
Conclusions
We have successfully synthesized novel hydrogen bond templated (2)handcuff [2]rotaxanes by a rare application of the strategy to covalently link axles threaded through each ring of a bis-macrocycle. Indeed, careful comparison to the only previously reported example of this synthetic strategy to prepare (2)handcuff [2]rotaxanes,8 reveals a unique feature of our design – namely the use of a separate molecule to link the two axle components. Successful preparation of the rotaxanes was confirmed by NMR spectroscopy and electrospray HRMS. Investigations into deploying our hydrogen bond templation methodologies to prepare – and functionally deploy – exotic interlocked structures are continuing in our laboratories.Experimental
General information
All reagents and solvents were used as obtained from commercial suppliers, unless otherwise stated. Dry solvents, Et3N and DIPEA were purchased dry and stored under an inert atmosphere. Cu(CH3CN)4BF4 was stored in a desiccator over P4O10. Petrol refers to the fractions of petroleum that boil between 40 °C and 60 °C. Deionized water was used in all cases. All aqueous solutions are saturated unless otherwise stated.Silica gel with a 60 Å particle size was used as the stationary phase for column chromatography. Analytical TLC was used to monitor the progress of column chromatography, with analytical TLC plates examined under short wavelength (254 nm) UV light or staining with potassium permanganate and/or phosphomolybdic acid solutions. Preparatory TLC was carried out on silica gel possessing a fluorescent indicator to allow for examination with short wavelength UV light.
IR spectra were recorded on an Agilent Technologies Cary 640 FTIR spectrometer. NMR spectra were recorded on a Bruker AVANCE III 400 at 298 K (unless otherwise stated). Mass spectra were recorded on a Shimadzu LCMS IT ToF instrument at Lancaster University and Bruker Compact ToF coupled to an Agilent 1260 Infinity LC at the University of York. Melting points were recorded on a Gallenkamp capillary melting point apparatus and are uncorrected.
4-(1,1-Dimethylethyl)-2,6-pyridinedimethanol 1,13 tetra-acid chloride 4,14 methyl pyridinium chloride template 5.Cl,15 acid chloride 6,16 diethylene glycol di(p-toluenesulfonate) 9,18 azide 10,10a bis-alkyne 1119 and axle Ax21 were synthesized by adaptation of previously reported procedures.
Experimental procedures
Bis-nitrile 2
4-(1,1-Dimethylethyl)-2,6-pyridinedimethanol 1 (7.17 g, 36.7 mmol) was dissolved in dry THF (100 mL) under argon and cooled to 0 °C. NaH (5.15 g, 128.7 mmol, 60% dispersion in mineral oil) was added portion wise and left to stir until foaming subsided. 4-(Bromomethyl)benzonitrile (18.0 g, 91.9 mmol) was then added and the reaction warmed to room temperature and stirred under argon for 18 hours. The reaction was then quenched with water and extracted with CH2Cl2 (2 × 100 mL). The combined organic layers were dried (MgSO4) and concentrated in vacuo. The crude material was purified by silica gel column chromatography (EtOAc/Petrol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the title product as a yellow solid (10.7 g, 69%).
1) to afford the title product as a yellow solid (10.7 g, 69%).
R
f
: 0.22 [EtOAc/Petrol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]. m.p. 85–87 °C. νmax/cm−1(neat): 2961 (C–H), 2230 (C
1]. m.p. 85–87 °C. νmax/cm−1(neat): 2961 (C–H), 2230 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1604 (C–O), 1347 (C–O), 1123 (C–H). δH(400 MHz, CDCl3): 7.67 (4H, d, J = 8.2 Hz, H10), 7.51 (4H, d, J = 8.2 Hz, H9), 7.39 (2H, s, H4), 4.72 (4H, s, H7), 4.69 (4H, s, H6), 1.34 (9H, s, H1). δC(100 MHz, CDCl3): 161.8 (C3), 157.1 (C5), 143.6 (C8), 132.2 (C10), 127.8 (C9), 118.7 (C12), 117.4 (C4), 111.4 (C11), 73.8 (C6), 71.9 (C7), 34.9 (C2), 30.6 (C1). m/z (ESI): 448.1982 [M + Na]+, C27H27N3O2 requires 448.1995.
N), 1604 (C–O), 1347 (C–O), 1123 (C–H). δH(400 MHz, CDCl3): 7.67 (4H, d, J = 8.2 Hz, H10), 7.51 (4H, d, J = 8.2 Hz, H9), 7.39 (2H, s, H4), 4.72 (4H, s, H7), 4.69 (4H, s, H6), 1.34 (9H, s, H1). δC(100 MHz, CDCl3): 161.8 (C3), 157.1 (C5), 143.6 (C8), 132.2 (C10), 127.8 (C9), 118.7 (C12), 117.4 (C4), 111.4 (C11), 73.8 (C6), 71.9 (C7), 34.9 (C2), 30.6 (C1). m/z (ESI): 448.1982 [M + Na]+, C27H27N3O2 requires 448.1995.
Bis-amine 3
Bis-nitrile 2 (1.00 g, 2.35 mmol) was dissolved in dry THF (20 mL) under nitrogen and cooled to 0 °C. BH3·THF (1 M in THF, 13 mL, 11.7 mmol) was added dropwise, then the reaction was allowed to stir for 1 hour. The reaction was then warmed to 70 °C and stirred for a further 18 hours. Upon cooling to room temperature, the reaction was quenched with conc. HCl (4 mL). All volatiles were then removed under reduced pressure to afford a white solid. The crude material was re-dissolved in water (50 mL) and washed with CH2Cl2 (50 mL). The aqueous layer was basified to pH 12 with 10% NaOH (aq.) and extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried (MgSO4) and concentrated in vacuo to afford the title product (925 mg, apparent yield 91%) as a light green oil. NMR spectral analysis reveals some impurities, but material taken on without further purification.ν max /cm −1 (neat): 2957 (C–H), 2857 (C–H), 2372 (N–H), 1604 (C–O), 1347 (C–O), 1097 (C–O). δH(400 MHz, CDCl3): 7.41–7.36 (6H, m, H4 & H9), 7.32 (4H, d, J = 8.2 Hz, H10), 4.66 (4H, s, H6), 4.65 (4H, s, H7), 3.88 (4H, s, H12), 1.34 (9H, s, H1). δC(100 MHz, CDCl3): 161.4 (C3), 157.6 (C5), 142.9 (C11), 136.5 (C8), 128.2 (C9), 127.1 (C10), 117.1 (C4), 73.2 (C6), 72.7 (C7), 46.3 (C12), 34.9 (C2), 30.6 (C1). m/z (ESI): 456.2619 [M + Na]+, C27H35N3O2 requires 456.2621.
Bis-macrocycle BM1
To a solution of bis-amine 3 (750 mg, 1.73 mmol) and methyl-pyridinium template 5.Cl (662 mg, 1.73 mmol) in dry CH2Cl2 (50 mL) under argon was added Et3N (1.20 mL, 8.65 mmol), followed immediately by a dropwise solution of tetra-acid chloride 4 (350 mg, 0.86 mmol) in dry CH2Cl2 (20 mL). The reaction was stirred for 1.5 hours at room temperature, then washed with 10% citric acid (2 × 50 mL) and brine (1 × 50 mL). The reaction mixture was then dried (MgSO4) and concentrated in vacuo. The crude material was purified by silica gel column chromatography (CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), followed by preparative TLC (CH2Cl2/CH3OH 98
2), followed by preparative TLC (CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–96
2–96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to afford the title product as a colourless film (18 mg, 2%).
4) to afford the title product as a colourless film (18 mg, 2%).
R
f
: 0.32 [CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2]. νmax/cm−1(neat): 3330 (N–H), 2922 (C–H), 2860 (C–H), 1645 (C
2]. νmax/cm−1(neat): 3330 (N–H), 2922 (C–H), 2860 (C–H), 1645 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1515 (C–O), 1066 (C–O). δH(400 MHz, CDCl3): 7.57 (4H, s, H16), 7.42 (2H, s, H15), 7.36 (4H, s, H4), 7.29 (16H, s, H9 & H10), 6.93 (4H, bs, Hα), 4.64 (8H, s, H7), 4.55 (8H, d, J = 5.4 Hz, H12), 4.40 (8H, s, H6), 4.22 (4H, t, J = 5.5 Hz, H18), 2.26 (2H, bs, H19), 1.36 (18H, s, H1). δC(100 MHz, CDCl3): 166.7 (C13), 161.5 (C3), 159.2 (C17), 157.1 (C5), 137.5 (C11), 137.0 (C8), 136.2 (C14), 128.9 (C9), 128.6 (C10), 117.3 (C4), 116.8 (C16), 116.5 (C15), 72.2 (C7), 71.7 (C6), 64.4 (C18), 44.2 (C12), 34.9 (C2), 30.6 (C1), 29.0 (C19). m/z (ESI): 1199.5873 [M + H]+, C73H79N6O10 requires 1199.5852.
O), 1515 (C–O), 1066 (C–O). δH(400 MHz, CDCl3): 7.57 (4H, s, H16), 7.42 (2H, s, H15), 7.36 (4H, s, H4), 7.29 (16H, s, H9 & H10), 6.93 (4H, bs, Hα), 4.64 (8H, s, H7), 4.55 (8H, d, J = 5.4 Hz, H12), 4.40 (8H, s, H6), 4.22 (4H, t, J = 5.5 Hz, H18), 2.26 (2H, bs, H19), 1.36 (18H, s, H1). δC(100 MHz, CDCl3): 166.7 (C13), 161.5 (C3), 159.2 (C17), 157.1 (C5), 137.5 (C11), 137.0 (C8), 136.2 (C14), 128.9 (C9), 128.6 (C10), 117.3 (C4), 116.8 (C16), 116.5 (C15), 72.2 (C7), 71.7 (C6), 64.4 (C18), 44.2 (C12), 34.9 (C2), 30.6 (C1), 29.0 (C19). m/z (ESI): 1199.5873 [M + H]+, C73H79N6O10 requires 1199.5852.
TBDMS-protected macrocycle 7
To a solution of bis-amine 3 (860 mg, 1.98 mmol) and methyl-pyridinium template 5.Cl (758 mg, 1.98 mmol) in dry CH2Cl2 (60 mL) under argon was added Et3N (0.68 mL, 4.95 mmol), followed immediately by a dropwise solution of TBDMS-protected acid chloride 6 (589 mg, 1.99 mmol) in dry CH2Cl2 (20 mL). The reaction was stirred for 1.5 hours at room temperature, then washed with 0.5 M HCl (2 × 40 mL) and brine (1 × 40 mL). The reaction mixture was then dried (MgSO4) and concentrated in vacuo. The crude material was purified by silica gel column chromatography (CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford the title product as a colourless solid (240 mg, 17%).
2) to afford the title product as a colourless solid (240 mg, 17%).
R
f
: 0.41 [CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2]. m.p. 145–147 °C. νmax/cm−1(neat): 3300 (N–H), 2952 (C–H), 2857 (C–H), 1649 (C–O), 1101 (C–O), 836 (Si–C). δH(400 MHz, CDCl3): 7.52 (2H, d, J = 1.3 Hz, H16), 7.41 (3H, app s, H4 & H15), 7.32 (8H, s, H9 & H10), 6.41 (2H, bs, Hα), 4.67 (4H, s, H7), 4.58 (4H, d, J = 5.6 Hz, H12), 4.45 (4H, s, H6), 1.37 (9H, s, H1), 1.02 (9H, s, H20), 0.27 (6H, s, H18). δC(100 MHz, CDCl3): 166.3 (C13), 162.1 (C3), 156.5 (C17), 137.5 (C11), 137.1 (C8), 136.3 (C14), 129.0 (C9), 128.7 (C10), 122.4 (C16), 117.5 (C4), 116.4 (C15), 72.2 (C7), 71.5 (C6), 44.2 (C12), 35.0 (C2), 30.6 (C1), 25.6 (C20), 18.1 (C19), −4.3 (C18). m/z (ESI): 716.3511 [M + Na]+, C41H51N3NaO5Si requires 716.3490.
2]. m.p. 145–147 °C. νmax/cm−1(neat): 3300 (N–H), 2952 (C–H), 2857 (C–H), 1649 (C–O), 1101 (C–O), 836 (Si–C). δH(400 MHz, CDCl3): 7.52 (2H, d, J = 1.3 Hz, H16), 7.41 (3H, app s, H4 & H15), 7.32 (8H, s, H9 & H10), 6.41 (2H, bs, Hα), 4.67 (4H, s, H7), 4.58 (4H, d, J = 5.6 Hz, H12), 4.45 (4H, s, H6), 1.37 (9H, s, H1), 1.02 (9H, s, H20), 0.27 (6H, s, H18). δC(100 MHz, CDCl3): 166.3 (C13), 162.1 (C3), 156.5 (C17), 137.5 (C11), 137.1 (C8), 136.3 (C14), 129.0 (C9), 128.7 (C10), 122.4 (C16), 117.5 (C4), 116.4 (C15), 72.2 (C7), 71.5 (C6), 44.2 (C12), 35.0 (C2), 30.6 (C1), 25.6 (C20), 18.1 (C19), −4.3 (C18). m/z (ESI): 716.3511 [M + Na]+, C41H51N3NaO5Si requires 716.3490.
Phenol macrocycle 8
To a solution of TBDMS-protected macrocycle 7 (260 mg, 0.353 mmol) in dry THF (10 mL) was added TBAF (185 mg, 0.706 mmol). The reaction was stirred at room temperature for 2 hours then quenched with the addition of NH4Cl (aq.). The aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried (MgSO4) and concentrated in vacuo. The crude material was purified by silica gel column chromatography (CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–95
2–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford the title product as a colourless solid (196 mg, 96%).
5) to afford the title product as a colourless solid (196 mg, 96%).
R
f
: 0.28 [CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2]. m.p. 178–180 °C. νmax/cm−1(neat): 3552 (N–H), 3281 (O–H), 2927 (C–H), 2338 (C–H), 1651 (C
2]. m.p. 178–180 °C. νmax/cm−1(neat): 3552 (N–H), 3281 (O–H), 2927 (C–H), 2338 (C–H), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1101 (C–O). δH(400 MHz, 1
O), 1101 (C–O). δH(400 MHz, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 CDCl3/CD3OD): 7.52 (1H, t, J = 1.5 Hz, H15), 7.48 (2H, d, J = 1.5 Hz, H16), 7.42 (2H, s, H4), 7.31 (8H, s, H9 & H10), 4.65 (4H, s, H7), 4.55 (4H, s, H12), 4.41 (4H, s, H6), 1.36 (9H, s, H1). δC(100 MHz, 1
1 CDCl3/CD3OD): 7.52 (1H, t, J = 1.5 Hz, H15), 7.48 (2H, d, J = 1.5 Hz, H16), 7.42 (2H, s, H4), 7.31 (8H, s, H9 & H10), 4.65 (4H, s, H7), 4.55 (4H, s, H12), 4.41 (4H, s, H6), 1.36 (9H, s, H1). δC(100 MHz, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 CDCl3/CD3OD): 167.7 (C13), 162.3 (C3), 157.8 (C17), 156.8 (C5), 137.7 (C11), 136.6 (C8), 135.7 (C14), 128.6 (C9), 128.1 (C10), 118.0 (C4), 117.8 (C16), 115.4 (C15), 72.1 (C7), 71.2 (C6), 43.6 (C12), 34.8 (C2), 30.1 (C1). m/z (ESI): 602.2638 [M + Na]+, C35H37N3NaO5 requires 602.2625.
1 CDCl3/CD3OD): 167.7 (C13), 162.3 (C3), 157.8 (C17), 156.8 (C5), 137.7 (C11), 136.6 (C8), 135.7 (C14), 128.6 (C9), 128.1 (C10), 118.0 (C4), 117.8 (C16), 115.4 (C15), 72.1 (C7), 71.2 (C6), 43.6 (C12), 34.8 (C2), 30.1 (C1). m/z (ESI): 602.2638 [M + Na]+, C35H37N3NaO5 requires 602.2625.
Bis-macrocycle BM2
To a solution of phenol macrocycle 8 (50 mg, 0.086 mmol) in dry DMF (5 mL) was added K2CO3. The solution was stirred for 5 minutes then diethylene glycol di(p-toluenesulfonate) 9 (18 mg, 0.043 mmol) was added. The reaction was heated at 80 °C under argon for 16 hours. The reaction was then cooled to room temperature and excess DMF removed in vacuo. The crude residue was dissolved in CH2Cl2 (15 mL) and washed with water (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried (MgSO4) and concentrated in vacuo. The crude material was purified by silica gel column chromatography (CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–96
2–96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to afford the title product as a colourless solid (35 mg, 67%).
4) to afford the title product as a colourless solid (35 mg, 67%).
R
f
: 0.35 [CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2]. m.p. 156–158 °C. νmax/cm−1(neat): 3296 (N–H), 2968 (C–H), 2853 (C–H), 1647 (C
2]. m.p. 156–158 °C. νmax/cm−1(neat): 3296 (N–H), 2968 (C–H), 2853 (C–H), 1647 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1535 (C–O), 1101 (C–O). δH(400 MHz, CDCl3): 7.45 (4H, s, H16), 7.41 (2H, s, H15), 7.36 (4H, s, H4), 7.30–7.23 (16H, m, H9 & H10), 7.03 (4H, bt, J = 5.3 Hz, Hα), 4.62 (8H, s, H7), 4.48 (8H, d, J = 5.3 Hz, H12), 4.41 (8H, s, H6), 4.17 (4H, s, H18), 3.88 (4H, s, H19), 1.38 (18H, s, H1). δC(100 MHz, CDCl3): 166.9 (C13), 161.6 (C3), 159.2 (C17), 157.1 (C5), 137.5 (C11), 137.0 (C8), 136.1 (C14), 128.9 (C9), 128.4 (C10), 117.5 (C4), 117.0 (C16), 116.6 (C15), 72.1 (C7), 71.8 (C6), 69.6 (C19), 67.9 (C18), 44.0 (C12), 34.9 (C2), 30.6 (C1). m/z (ESI): 1251.5850 [M + Na]+, C74H80N6NaO11 requires 1251.5777.
O), 1535 (C–O), 1101 (C–O). δH(400 MHz, CDCl3): 7.45 (4H, s, H16), 7.41 (2H, s, H15), 7.36 (4H, s, H4), 7.30–7.23 (16H, m, H9 & H10), 7.03 (4H, bt, J = 5.3 Hz, Hα), 4.62 (8H, s, H7), 4.48 (8H, d, J = 5.3 Hz, H12), 4.41 (8H, s, H6), 4.17 (4H, s, H18), 3.88 (4H, s, H19), 1.38 (18H, s, H1). δC(100 MHz, CDCl3): 166.9 (C13), 161.6 (C3), 159.2 (C17), 157.1 (C5), 137.5 (C11), 137.0 (C8), 136.1 (C14), 128.9 (C9), 128.4 (C10), 117.5 (C4), 117.0 (C16), 116.6 (C15), 72.1 (C7), 71.8 (C6), 69.6 (C19), 67.9 (C18), 44.0 (C12), 34.9 (C2), 30.6 (C1). m/z (ESI): 1251.5850 [M + Na]+, C74H80N6NaO11 requires 1251.5777.
Handcuff rotaxane HR1
Bis-macrocycle BM1 (9 mg, 0.007 mmol) and azide 10 (6 mg, 0.016 mmol) were dissolved in dry CH2Cl2 (1 mL) under an argon atmosphere. Then alkyne 11 (1.5 mg, 0.008 mmol), [Cu(CH3CN)4BF4] (1 mg, 0.0007 mmol), TBTA (∼1.5 mg, 0.0007 mmol) and dry DIPEA (1 μL, 0.007 mmol) were added. The reaction was stirred at RT for 18 hours maintaining the argon atmosphere. Then, the reaction was diluted to 10 mL, washed with 0.02 M EDTA in aq. 1 M NH3 solution (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried (MgSO4), filtered and solvent removed in vacuo. The crude material was purified by preparative TLC (CH2Cl2/CH3OH 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–97.5
2–97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) to afford the title product (∼5 mg, ∼30%) as a colourless film.
2.5) to afford the title product (∼5 mg, ∼30%) as a colourless film.
R
f
: 0.42 [CH2Cl2/CH3OH 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3]. νmax/cm−1(neat): 3295 (N–H), 2950 (C–H), 2920 (C–H), 2851 (C–H), 1645 (C
3]. νmax/cm−1(neat): 3295 (N–H), 2950 (C–H), 2920 (C–H), 2851 (C–H), 1645 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1274 (C–N), 1131 (C–O). δH(400 MHz, CDCl3): 8.39 (4H, s, Hk), 8.22 (2H, t, J = 5.2 Hz, Hβ), 8.15 (2H, s, H15), 8.03 (2H, s, Hm), 7.86 (4H, s, H16), 7.57–7.53 (4H, m, Hα), 7.36 (2H, s, He), 7.34 (4H, s, H4), 6.90 (8H, d, J = 8.1 Hz, H10), 6.87 (4H, s, Ha), 6.84 (8H, d, J = 8.1 Hz, H9), 5.02 (4H, s, Hc), 4.88 (4H, dd, J = 14.3 Hz, 6.8 Hz, H12), 4.55 (4H, d, J = 11.1 Hz, H7), 4.39 (4H, d, J = 11.1 Hz, H7′), 4.34–4.25 (12H, m, H6 & H18), 4.16 (4H, dd, J = 14.3 Hz, 6.8 Hz, H12′), 3.68 (4H, t, J = 7.1 Hz, Hf), 2.39–2.30 (6H, m, H19 & Hh), 1.37 (18H, s, H1), 1.33–1.28 (4H, m, Hg). δC(100 MHz, CDCl3): 165.9 (C13), 164.2 (Ci), 162.5 (C3), 159.6 (C17), 156.6 (C5), 152.6 (Cb), 144.1 (Cd), 138.0 (C11), 135.8 (C8), 135.5 (C14), 135.2 (Cj), 129.3 (C9), 128.5 (Ck), 128.3 (C10), 124.6 (Cm), 123.1 (Ce), 118.3 (C4), 117.9 (C16), 116.0 (C15), 115.8 (Ca), 73.4 (C7), 71.9 (C6), 65.0 (C18), 62.3 (Cc), 47.5 (Cf), 44.1 (C12), 37.2 (Ch), 35.0 (C2), 30.5 (C1), 28.8 (Cg). δF(377 MHz, CDCl3): −62.7. m/z (ESI): 2087.7909 [M + Na]+, C109H108F12N14NaO14 requires 2087.7870.
O), 1274 (C–N), 1131 (C–O). δH(400 MHz, CDCl3): 8.39 (4H, s, Hk), 8.22 (2H, t, J = 5.2 Hz, Hβ), 8.15 (2H, s, H15), 8.03 (2H, s, Hm), 7.86 (4H, s, H16), 7.57–7.53 (4H, m, Hα), 7.36 (2H, s, He), 7.34 (4H, s, H4), 6.90 (8H, d, J = 8.1 Hz, H10), 6.87 (4H, s, Ha), 6.84 (8H, d, J = 8.1 Hz, H9), 5.02 (4H, s, Hc), 4.88 (4H, dd, J = 14.3 Hz, 6.8 Hz, H12), 4.55 (4H, d, J = 11.1 Hz, H7), 4.39 (4H, d, J = 11.1 Hz, H7′), 4.34–4.25 (12H, m, H6 & H18), 4.16 (4H, dd, J = 14.3 Hz, 6.8 Hz, H12′), 3.68 (4H, t, J = 7.1 Hz, Hf), 2.39–2.30 (6H, m, H19 & Hh), 1.37 (18H, s, H1), 1.33–1.28 (4H, m, Hg). δC(100 MHz, CDCl3): 165.9 (C13), 164.2 (Ci), 162.5 (C3), 159.6 (C17), 156.6 (C5), 152.6 (Cb), 144.1 (Cd), 138.0 (C11), 135.8 (C8), 135.5 (C14), 135.2 (Cj), 129.3 (C9), 128.5 (Ck), 128.3 (C10), 124.6 (Cm), 123.1 (Ce), 118.3 (C4), 117.9 (C16), 116.0 (C15), 115.8 (Ca), 73.4 (C7), 71.9 (C6), 65.0 (C18), 62.3 (Cc), 47.5 (Cf), 44.1 (C12), 37.2 (Ch), 35.0 (C2), 30.5 (C1), 28.8 (Cg). δF(377 MHz, CDCl3): −62.7. m/z (ESI): 2087.7909 [M + Na]+, C109H108F12N14NaO14 requires 2087.7870.
Handcuff rotaxane HR2
Bis-macrocycle BM2 (53 mg, 0.043 mmol) and azide 10 (35 mg, 0.103 mmol) were dissolved in dry CH2Cl2 (3 mL) under an argon atmosphere. Then alkyne 11 (9.6 mg, 0.051 mmol), [Cu(CH3CN)4BF4] (1.3 mg, 0.004 mmol), TBTA (2.2 mg, 0.004 mmol) and dry DIPEA (7 μL, 0.043 mmol) were added. The reaction was stirred at RT for 18 hours maintaining the argon atmosphere. Then, the reaction was diluted to 10 mL, washed with 0.02 M EDTA in aq. 1 M NH3 solution (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried (MgSO4), filtered and solvent removed in vacuo. The crude material was purified by silica gel column chromatography (CH2Cl2/CH3OH 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford rotaxane with a small amount of axle component. The material was further purified by preparative TLC (CH2Cl2/acetone/CH3OH 88.5
3) to afford rotaxane with a small amount of axle component. The material was further purified by preparative TLC (CH2Cl2/acetone/CH3OH 88.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) to afford the title product (14 mg, 15%) as a colourless film.
1.5) to afford the title product (14 mg, 15%) as a colourless film.
R
f
: 0.49 [CH2Cl2/CH3OH 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3]. νmax/cm−1(neat): 3322 (N–H), 2924 (C–H), 2857 (C–H), 1647 (C
3]. νmax/cm−1(neat): 3322 (N–H), 2924 (C–H), 2857 (C–H), 1647 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1276 (C–N), 1129 (C–O). δH(400 MHz, CDCl3): 8.40 (4H, s, Hk), 8.23 (2H, t, J = 5.5 Hz, Hβ), 8.16 (2H, s, H15), 8.02 (2H, s, Hm), 7.84 (4H, s, H16), 7.60–7.53 (4H, s, Hα), 7.34 (4H, s, H4), 7.25 (2H, s, He), 6.91–6.87 (12H, m, Ha & H10), 6.86 (8H, d, J = 8.1 Hz, H9), 5.03 (4H, s, Hc), 4.87 (4H, dd, J = 13.9 Hz, 7.0 Hz, H12), 4.54 (4H, d, J = 11.0 Hz, H7), 4.39 (4H, d, J = 11.0 Hz, H7′), 4.34–4.24 (12H, m, H6 & H18), 4.15 (4H, dd, J = 13.9 Hz, 7.0 Hz, H12′), 3.98 (4H, t, J = 4.4 Hz, H19), 3.65 (4H, t, J = 6.9 Hz, Hf), 2.34 (4H, app quart, Hh), 1.37 (18H, s, H1), 1.29 (4H, app quint, Hg). δC(100 MHz, CDCl3): 165.9 (C13), 164.2 (Ci), 162.4 (C3), 159.5 (C17), 156.6 (C5), 152.6 (Cb), 144.0 (Cd), 138.0 (C11), 135.8 (C8), 135.6 (C14), 135.2 (Cj), 131.5 (Cl, q, J = 33 Hz), 129.3 (C9), 128.5 (Ck), 128.3 (C10), 124.6 (Cm), 123.1 (Ce), 123.0 (Cn, q, J = 273 Hz), 118.3 (C4), 117.9 (C16), 116.2 (C15), 115.8 (Ca), 73.4 (C7), 71.9 (C6), 69.8 (C19), 68.1 (C18), 62.2 (Cc), 47.5 (Cf), 44.0 (C12), 37.2 (Ch), 35.0 (C2), 30.5 (C1), 28.9 (Cg). δF(377 MHz, CDCl3): −62.7. m/z (ESI): 2117.8048 [M + Na]+, C110H110F12N14NaO15 requires 2117.7976.
O), 1276 (C–N), 1129 (C–O). δH(400 MHz, CDCl3): 8.40 (4H, s, Hk), 8.23 (2H, t, J = 5.5 Hz, Hβ), 8.16 (2H, s, H15), 8.02 (2H, s, Hm), 7.84 (4H, s, H16), 7.60–7.53 (4H, s, Hα), 7.34 (4H, s, H4), 7.25 (2H, s, He), 6.91–6.87 (12H, m, Ha & H10), 6.86 (8H, d, J = 8.1 Hz, H9), 5.03 (4H, s, Hc), 4.87 (4H, dd, J = 13.9 Hz, 7.0 Hz, H12), 4.54 (4H, d, J = 11.0 Hz, H7), 4.39 (4H, d, J = 11.0 Hz, H7′), 4.34–4.24 (12H, m, H6 & H18), 4.15 (4H, dd, J = 13.9 Hz, 7.0 Hz, H12′), 3.98 (4H, t, J = 4.4 Hz, H19), 3.65 (4H, t, J = 6.9 Hz, Hf), 2.34 (4H, app quart, Hh), 1.37 (18H, s, H1), 1.29 (4H, app quint, Hg). δC(100 MHz, CDCl3): 165.9 (C13), 164.2 (Ci), 162.4 (C3), 159.5 (C17), 156.6 (C5), 152.6 (Cb), 144.0 (Cd), 138.0 (C11), 135.8 (C8), 135.6 (C14), 135.2 (Cj), 131.5 (Cl, q, J = 33 Hz), 129.3 (C9), 128.5 (Ck), 128.3 (C10), 124.6 (Cm), 123.1 (Ce), 123.0 (Cn, q, J = 273 Hz), 118.3 (C4), 117.9 (C16), 116.2 (C15), 115.8 (Ca), 73.4 (C7), 71.9 (C6), 69.8 (C19), 68.1 (C18), 62.2 (Cc), 47.5 (Cf), 44.0 (C12), 37.2 (Ch), 35.0 (C2), 30.5 (C1), 28.9 (Cg). δF(377 MHz, CDCl3): −62.7. m/z (ESI): 2117.8048 [M + Na]+, C110H110F12N14NaO15 requires 2117.7976.
Author contributions
NHE proposed the study. SRB and DT conducted the synthesis and characterization of materials (with contribution from NRH). NHE supervised the work. SRB and NHE wrote the manuscript. All authors discussed and commented on the manuscript.Data availability
Underlying data for this paper are provided in the Experimental section and ESI.† Electronic copies of NMR spectra (including fid files) will be available upon publication from: https://doi.org/10.17635/lancaster/researchdata/663. Crystallographic data for BM2 has been deposited at CCDC (https://ccdc.cam.ac.uk) under structure number 2343052.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. R. B. is grateful for provision of EPSRC DTP PhD funding from the Engineering and Physical Sciences Research Council [EP/T518037/1] and a Sydney Andrew Scholarship from the Society of Chemical Industry. We acknowledge Emily Mason (Lancaster University) for synthetic efforts attempting to prepare bis-macrocycle BM0. We thank Dr David Rochester and Harry Mills (Lancaster University) for the recording of mass spectrometry data of compounds 2 and 3. All other mass spectrometry data were recorded by Karl Heaton of the mass spectrometry service at the University of York.References
- C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines, Wiley & Sons, 2017 Search PubMed.
- K. M. Bąk, K. Porfyrakis, J. J. Davis and P. D. Beer, Mater. Chem. Front., 2020, 4, 1052–1073 RSC.
- (a) C. Kwamen and J. Niemeyer, Chem. – Eur. J., 2021, 27, 175–186 CrossRef CAS PubMed; (b) A. W. Heard, J. M. Suárez and S. M. Goldup, Nat. Rev. Chem., 2022, 6, 182–196 CrossRef PubMed.
- S. R. Beeren, C. T. McTernan and F. Schaufelberger, Chem, 2023, 9, 1378–1412 CAS.
- N. Pearce, M. Tarbowska, N. J. Andersen, A. Wahrhaftig-Lewis, B. S. Pilgrim and N. R. Champness, Chem. Sci., 2022, 13, 3915–3941 RSC.
- (a) Z.-J. Zhang, M. Han, H.-Y. Zhang and Y. Liu, Org. Lett., 2013, 15, 1698–1701 CrossRef CAS PubMed; (b) R. Ciao, C. Talotta, C. Gaeta, L. Margarucci, A. Casapullo and P. Neri, Org. Lett., 2013, 15, 5694–5697 CrossRef CAS PubMed; (c) L. Kaufmann, N. L. Traulsen, A. Springer, H. V. Schröder, T. Mäkelä, K. Rissanen and C. A. Schalley, Org. Chem. Front., 2014, 1, 521–531 RSC; (d) Y.-X. Ma, Z. Meng and C.-F. Chen, Org. Lett., 2014, 16, 1860–1863 CrossRef CAS PubMed; (e) H. Li, X. Li, Y. Wu, H. Ågren and D.-H. Qu, J. Org. Chem., 2014, 79, 6996–7004 CrossRef CAS PubMed; (f) H. V. Schröder, H. Hupatz, A. J. Achazi, S. Sobottka, B. Sarkar, B. Paulus and C. A. Schalley, Chem. – Eur. J., 2017, 23, 2960–2967 CrossRef PubMed; (g) T. Tskuamoto, R. Sasahara, A. Muranaka, Y. Miura, Y. Suzuki, M. Kimura, S. Miyagawa, T. Kawasaki, N. Kobayashi, M. Uchiyama and Y. Tokunaga, Org. Lett., 2018, 20, 4745–4748 CrossRef PubMed; (h) L. Yang, P. Langer, E. S. Davies, M. Balodoni, K. Wickham, N. A. Besley, E. Besley and N. R. Champness, Chem. Sci., 2019, 10, 3723–3732 RSC; (i) S. Tajima, A. Muranaka, M. Naito, N. Taniguch, M. Harada, S. Miyagawa, M. Ueda, H. Takaya, N. Kobayashi, M. Uchiyama and Y. Tokunaga, Org. Lett., 2021, 23, 8678–8682 CrossRef CAS PubMed.
- (a) M. R. Kishan, A. Parham, F. Schelhase, A. Yoneva, G. Silva, X. Chen, Y. Okamoto and F. Vögtle, Angew. Chem., Int. Ed., 2006, 45, 7296–7299 CrossRef CAS PubMed; (b) T. Sato and T. Takata, Tetrahedron Lett., 2007, 48, 2797–2801 CrossRef CAS.
- (a) I. Hajime, Y. Yukimi, F. Yoshimasa and H. Takeharu, Chem. Lett., 2010, 39, 24–25 CrossRef; (b) H. Iwamoto, Y. Yawata, Y. Fukazawa and T. Haino, Supramol. Chem., 2010, 22, 812–826 CrossRef.
- Other examples of multiple threading of bis- and tris-macrocycles highlighted by one of the reviewers include: (a) R. Ciao, C. Talotta, C. Gaeta and P. Neri, Supramol. Chem., 2014, 26, 569–578 CrossRef CAS; (b) V. Iuliano, R. Ciao, E. Vignola, C. Talotta, P. Iannece, M. De Rosa, A. Soriente, C. Gaeta and P. Neri, Beilstein J. Org. Chem., 2019, 15, 2092–2104 CrossRef CAS PubMed.
- (a) B. E. Fletcher, M. J. G. Peach and N. H. Evans, Org. Biomol. Chem., 2017, 15, 2797–2803 RSC; (b) C. E. Gell, T. A. McArdle-Ismaguilov and N. H. Evans, Chem. Commun., 2019, 55, 1576–1579 RSC.
- More recently we have demonstrated the synthesis of related [2]rotaxanes using isophthalamide, urea and squaramide threading components: (a) S. R. Barlow, G. R. Akien and N. H. Evans, Org. Biomol. Chem., 2023, 21, 402–414 RSC; (b) R. L. Spicer, C. C. Shearman and N. H. Evans, Chem. – Eur. J., 2023, 29, e2022030502 CrossRef PubMed.
- (a) E. J. Mason, MChem thesis, Lancaster University, 2018; (b) N. H. Evans, unpublished results.
- L. M. Thierer, Q. Wang, S. H. Brooks, P. Cui, J. Qi, M. R. Gau, B. C. Manor, P. J. Carroll and N. C. Tomson, Polyhedron, 2021, 198, 115044 CrossRef CAS PubMed.
- V. Berl, M. Schmutz, M. J. Kirsche, R. G. Khoury and J.-M. Lehn, Chem. – Eur. J., 2002, 8, 1227–1244 CrossRef CAS PubMed.
- (a) J. A. Wisner, P. D. Beer and M. G. B. Drew, Angew. Chem., Int. Ed., 2001, 40, 3606–3609 CrossRef CAS; (b) L. M. Hancock, DPhil thesis, University of Oxford, 2011.
- E. N. Parker, J. Song, G. D. K. Kumar, S. O. Odutola, G. E. Chavarria, A. K. Charlton-Sevcik, T. E. Strecker, A. L. Brnes, D. R. Sudhan, T. R. Wittenborn, D. W. Siemann, M. R. Horsman, D. J. Chaplin, M. L. Trawick and K. F. Pinney, Bioorg. Med. Chem., 2015, 23, 6974–6992 CrossRef CAS PubMed.
- Taking the mixture of 9 and 10 and attempting to alkylate remaining 9 with further macrocycle 8 failed to generate any bis-macrocycle BM1.
- E. Bednářová, S. Hybelbauerová and J. Jindřich, Beilstein J. Org. Chem., 2016, 12, 349–352 CrossRef PubMed.
- J. Bi, X. Zeng, D. Tian and H. Li, Org. Lett., 2016, 18, 1092–1095 CrossRef CAS PubMed.
- The challenges in preparing BM1 meant that the reaction to synthesize HR1 was run on a scale that only allows for the reporting of an approximate yield.
- N. H. Evans and G. R. Akien, Supramol. Chem., 2018, 30, 758–764 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Further detail of synthetic investigations; copies of characterization spectra; crystallographic data for bis-macrocycle BM2. CCDC 2343052. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00672k |
| This journal is © The Royal Society of Chemistry 2024 |