 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Total synthesis of jamaicamide B†
Ryosuke
Shigeta
,
Takahiro
Suzuki
,
Kazuki
Kaneko
,
Hiroaki
Tanaka
,
Ibuki
Haishima
,
Kanata
Norio
,
Ayano
Tanaka-Yanuma
and
Toyonobu
Usuki
 *
*
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioicho, Chiyoda-ku, Tokyo 102-8554, Japan. E-mail: t-usuki@sophia.ac.jp
First published on 25th April 2024
Abstract
Jamaicamide B was isolated from the cyanobacterium Moorea producens in Jamaica and shows neurotoxicity. This unique mixed peptide–polyketide structure contains a pyrrolinone ring, a β-methoxy enone, an (E)-olefin, an undetermined stereocenter at C9, an (E)-chloroolefin, and a terminal alkyne. We report herein the first total synthesis and structural confirmation of the marine natural product (9R)-jamaicamide B.
Natural products are considered to be important resources for drug discovery and development.1 Jamaicamides A, B (1), and C (Fig. 1) are highly functionalized lipopeptides and were isolated by Gerwick and co-workers in 2004 from a dark green strain of the cyanobacterium Moorea producens found in Hector's Bay, Jamaica.2 These amide-type marine natural products have a unique peptide–polyketide mixed structure containing a pyrrolinone ring (C20–C23), a β-methoxy enone (C17–C19), an (E)-olefin (C10–C11), an (E)-chloroolefin (C6–C27), an undetermined stereocenter (C9), and an unusual terminal alkynyl bromide (C1–C2; only in jamaicamide A).
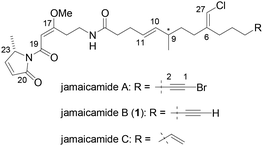 | ||
| Fig. 1 Structures of jamaicamides.2 | ||
Due to their intriguing structural features, biosynthesis of jamaicamides has been studied since their discovery.2–4 Recently, enzymatic halogenation of terminal alkynes has been found in JamD,4 which is one of the mixed peptide/polyketide synthetase. Moreover, the jamaicamide family exhibits multiple biological activities, including sodium channel-blocking activity and cytotoxicity against both H-460 human lung and Neuro-2a mouse neuroblastoma cell lines.2 It was also reported that jamaicamide B 1 shows anti-malarial activity and cytotoxicity to Vero cells.5
In synthetic studies of jamaicamides, the polyketide fragment of (S)-jamaicamide C was stereoselectively synthesized by Paige and co-workers employing Negishi cross-coupling and Johnson–Claisen rearrangement as the key steps.6 In contrast, we synthesized the N-di-tert-butoxycarbonyl [(Boc)2]-protected peptide moiety of the jamaicamides starting from chiral natural amino acids.7 We also reported the stereo- and regioselective synthesis of the polyketide unit, including construction of the (E)-chloroolefin moiety via Wittig reaction followed by separation of the (E) and (Z)-isomers, the chiral methyl stereocenter at C9 via Evans’ strategy, and the (E)-olefin via Julia–Kocienski coupling.8,9 However, the total synthesis of jamaicamides has to date not been accomplished, probably due to the unusual skeleton and the expected labile moieties such as the β-methoxy enone, pyrrolinone ring, and terminal alkynes. The related natural products biakamides A–D are chloro-olefines isolated from an Indonesian marine sponge and their total synthesis has been reported.10
Given their interesting chemical structures, attractive biological activities and unique biosynthesis as secondary metabolites, we embarked on and herein report the first total synthesis of jamaicamide B 1. Our approach starts with the condensation of peptide and polyketide moieties, followed by determination of the stereochemistry at C9 by comparison of the optical rotation values of synthetic (9S)- and (9R)-isomers with the natural product.
As illustrated in the retrosynthetic analysis shown in Scheme 1, 1 can be divided into two fragments: a pyrrolinone-containing peptide moiety and a polyketide moiety. Stereoselective synthesis of the N-[(Boc)2]-protected peptide moiety starting from L-alanine and N-Boc-β-alanine using Meldrum's acid has been reported,7 as has the stereo- and regioselective synthesis of the polyketide moiety.8,9 The 13-step synthesis of the latter moiety started from pentane-1,5-diol via Wittig reaction, followed by separation of the (E) and (Z) isomers for the (E)-chloroolefin, and Julia–Kocienski coupling to form the (E)-olefin with (9S) configuration by Evans’ chiral auxiliary. To determine the configuration of 1, a stereocenter at C9 would be installed by means of Evans’ oxazolidinone methodology to prepare both the (9S) and (9R)-polyketide intermediates.9
The synthesis commenced with the previously reported carboxylic acid 2 from pentane-1,5-diol in nine steps in 9% yield (Scheme 2).9 Acylation of 2 with pivaloyl chloride (PivCl) gave the ester intermediate, followed by conversion to the oxazolidinone-based chiral auxiliary using (5′S/5′R)-4-benzyl-2-oxazolidinone and n-butyllithium (nBuLi) to give (5′S)-3 and (5′R)-3 at −78 °C to room temperature in 91% and 94% yields in two steps, respectively. In our previous study, oxazolidinone was inserted at −78 °C to −40 °C to afford (5′S)-3 in 65% yield.9 The enolate of oxazolidinone 3 was then generated by treatment with lithium hexamethyldisilazide (LiHMDS), followed by the addition of iodomethane (MeI) to provide (9S)-4 in 75% yield and (9R)-4 in 80% yield, respectively.11,12 Removal of the oxazolidinone in 4 using lithium borohydride (LiBH4) led to the alcohol (9S)-5 in 74% yield and (9R)-5 in 70% yield, respectively. Next, 5 was oxidized with 2-iodoxybenzoic acid (IBX) to afford aldehyde (9S)-6 in 81% yield and (9R)-6 in 92% yield, respectively, whereas the previous conditions (TPAP/NMO) gave (9S)-6 in 74% yield.9 Transformation of the aldehyde 6 into the desired (E)-olefin was accomplished via a Julia–Kocienski coupling reaction.13,14 The prepared sulfone 78,9 was treated with potassium hexamethyldisilazide (KHMDS) to produce the corresponding anion, which was then reacted with 6 to give the desired olefin (9S)-8 and (9R)-8 in 94% and 76% yields, respectively. When NaHMDS was reacted with (9S)-6, the product (9S)-8 was obtained in 46% yield.9 The benzyl ester in 8 was converted to a carboxylic acid under basic hydrolysis conditions to afford carboxylic acid (9S)-9 (55%; E/Z = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) and (9R)-9 (58%; E/Z = 97
8) and (9R)-9 (58%; E/Z = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), respectively, and the E/Z ratios were determined by 1H NMR analysis.15 The moderate yield of the saponification is probably due to the partial cleavage of the TES group.
3), respectively, and the E/Z ratios were determined by 1H NMR analysis.15 The moderate yield of the saponification is probably due to the partial cleavage of the TES group.
The synthesis of peptide fragment 11a was first investigated by deprotection of the Boc group in N-[(Boc)2]-protected peptide moiety 10,7 which was synthesized previously (Table 1). Treatment of 10 with trifluoroacetic acid (TFA) in dichloromethane (CH2Cl2) solely gave the undesired product 11b in 93% yield upon removal of the β-methoxy enone moiety (entry 1). The same tendency was observed when 4 M hydrochloric acid solution in 1,4-dioxane was used, suggesting that acidic conditions promoted undesired demethylation (entry 2). Application of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of trifluoromethanesulfonate (TMSOTf)/2,6-lutidine gave a complex mixture (entry 3). These results suggested that the β-methoxy enone moiety in 10, attached to the electron-withdrawing pyrrolinone, is labile under acidic conditions.
1 mixture of trifluoromethanesulfonate (TMSOTf)/2,6-lutidine gave a complex mixture (entry 3). These results suggested that the β-methoxy enone moiety in 10, attached to the electron-withdrawing pyrrolinone, is labile under acidic conditions.
Our next target molecule was the peptide moiety 20′ (Scheme 3) bearing a 2-(trimethylsilyl)ethoxycarbonyl (Teoc) protecting group, since Teoc can be selectively removed using a fluorine anion such as tetrabutylammonium fluoride (TBAF). Starting from β-keto ester 12, synthesized from Boc-β-alanine in two steps,7 the removal of Boc using TFA followed by protection with Teoc via13 gave compound 14 in 78% yield in two steps. β-Methoxy enone 15 was then formed using dimethyl sulfone in 79% yield. The hydrolysis of 15 using LiOH gave 16 in 41% yield, followed by generation of the pentafluorophenol (Pfp) ester 17 in 92% yield. Condensation with six equivalents of pyrrolidone 18, obtained from L-alanine in five steps,7 afforded compound 19 in 30% yield using LiHMDS. However, treatment with lithium diisopropylamide (LDA) and PhSeBr failed to provide the desired 20, but rather gave a complex mixture. We therefore ruled out this synthetic route.
Thus, we turned our attention to taking advantage of the above obtained undesired β-ketoamide 11b. As shown in Scheme 4, N-[(Boc)2]-protected peptide moiety 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 was treated with TFA to give β-ketoamide 11b, which was further reacted with carboxylic acid 9 and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) in situ, successfully giving (9S)-21 in 63% yield and (9R)-21 in 46% yield, respectively. The ketone moiety of (9S)-21 was then methylated with Me2SO4 in acetone to give (9S)-22 in 17% yield. Removal of the triethysilyl (TES) group using TBAF in the presence of acetic acid (AcOH) produced (9S)-1 in 46% yield. The NMR spectrum of (9S)-1 showed excellent agreement with that of the natural product including the newly formed olefin geometry, but its optical rotation value was different {[α]D = +1.5 (synthetic) vs. +53 (natural)2}. Methylation of the ketone of (9R)-21 in N,N-dimethylformaldehyde (DMF) gave (9R)-22 in 47% yield, followed by removal of the terminal TES group using tris(dimethylamino)sulfonium difluorotrimethylsilicatetetrabutylammonium fluoride (TAS-F)16 in acetonitrile (AcCN) to afford (9R)-1 in 47% yield. The 1H and 13C NMR spectra and optical rotation of (9R)-1 were in excellent agreement with those of the natural product {[α]D = +43 (synthetic) vs. +53 (natural)2}, suggesting that the stereochemistry at C9 in jamaicamide B 1 is the (R)-configuration.
7 was treated with TFA to give β-ketoamide 11b, which was further reacted with carboxylic acid 9 and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) in situ, successfully giving (9S)-21 in 63% yield and (9R)-21 in 46% yield, respectively. The ketone moiety of (9S)-21 was then methylated with Me2SO4 in acetone to give (9S)-22 in 17% yield. Removal of the triethysilyl (TES) group using TBAF in the presence of acetic acid (AcOH) produced (9S)-1 in 46% yield. The NMR spectrum of (9S)-1 showed excellent agreement with that of the natural product including the newly formed olefin geometry, but its optical rotation value was different {[α]D = +1.5 (synthetic) vs. +53 (natural)2}. Methylation of the ketone of (9R)-21 in N,N-dimethylformaldehyde (DMF) gave (9R)-22 in 47% yield, followed by removal of the terminal TES group using tris(dimethylamino)sulfonium difluorotrimethylsilicatetetrabutylammonium fluoride (TAS-F)16 in acetonitrile (AcCN) to afford (9R)-1 in 47% yield. The 1H and 13C NMR spectra and optical rotation of (9R)-1 were in excellent agreement with those of the natural product {[α]D = +43 (synthetic) vs. +53 (natural)2}, suggesting that the stereochemistry at C9 in jamaicamide B 1 is the (R)-configuration.
In summary, the total synthesis of jamaicamide B 1 was achieved for the first time in 18 steps in 0.2% overall yield starting from pentane-1,4-diol. The synthesis involved Evans’ asymmetric methylation and a Julia–Kocienski coupling reaction. The unknown stereochemistry at C92 was determined to be the (R)-configuration by comparing the optical rotation values of both stereoisomers. This synthetic methodology will enable the synthesis of analogues of 1, such as jamaicamides A and C, facilitate their structure–activity relationship (SAR) studies, and shed light on the unexplored biological functions of these unique molecules.
Conflicts of interest
There are no conflicts to declare.Note added after first publication
This article replaces the version published on 8th May 2024 which contained errors in the stereochemical descriptors Scheme 1, Scheme 2 and Scheme 4.Acknowledgements
We are sincerely grateful to Professor William H. Gerwick (UC San Diego) for fruitful suggestions.References
- (a) J. Kobayashi and M. Ishibashi, in Comprehensive Natural Products Chemistry, ed. D. H. R. Barton and K. Nakanishi, Elsevier, New York, 1999 Search PubMed; (b) B. B. Mishra and V. K. Tiwari, Eur. J. Med. Chem., 2011, 46, 4769 CrossRef CAS PubMed; (c) W. H. Gerwick, B. S. Moore, Chem. Biol., 2012, 19, 85 CrossRef PubMed.
- D. J. Edwards, B. L. Marquez, L. M. Nogle, K. McPhail, D. E. Goeger, M. A. Roberts and W. H. Gerwick, Chem. Biol., 2004, 11, 817 CrossRef CAS PubMed.
- (a) L. Gu, B. Wang, A. Kulkarni, T. W. Geders, R. V. Grindberg, L. Gerwick, K. Håkansson, P. Wipf, J. L. Smith, W. H. Gerwick and D. H. Sherman, Nature, 2009, 459, 731 CrossRef CAS PubMed; (b) N. A. Moss, G. Seiler, T. F. Leão, G. Castro-Falcón, L. Gerwick, C. C. Hughes and W. H. Gerwick, Angew. Chem., Int. Ed., 2019, 58, 9027 CrossRef CAS PubMed.
- A. L. Lukowski, F. M. Hubert, T.-E. Ngo, N. E. Avalon, W. H. Gerwick and B. S. Moore, J. Am. Chem. Soc., 2023, 145, 18716 CrossRef CAS PubMed.
- K. L. McPhail, J. Correa, R. G. Linington, J. González, E. Ortega-Barría, T. L. Capson and W. H. Gerwick, J. Nat. Prod., 2007, 70, 984 CrossRef CAS PubMed.
- K. M. Graf, M. G. Tabor, M. L. Brown and M. Paige, Org. Lett., 2009, 11, 5382–5385 CrossRef CAS PubMed.
- A. Tanaka and T. Usuki, Tetrahedron Lett., 2011, 52, 5036 CrossRef CAS.
- S. Watanabe, S. Watanabe, N. Aoki and T. Usuki, Synth. Commun., 2013, 43, 1397–1403 CrossRef CAS.
- A. Tanaka-Yanuma, S. Watanabe, K. Ogawa, S. Watanabe, N. Aoki, T. Ogura and T. Usuki, Tetrahedron Lett., 2015, 56, 6777 CrossRef CAS.
- N. Kotoku, R. Ishida, H. Matsumoto, M. Arai, K. Toda, A. Setiawan, O. Muraoka and M. Kobayashi, J. Org. Chem., 2017, 82, 1705 CrossRef CAS PubMed.
- D. A. Evans, L. D. Wu, J. J. Wiener, J. S. Johnson, D. H. B. Ripin and J. S. Tedrow, J. Org. Chem., 1999, 64, 6411 CrossRef CAS.
- Use of sodium hexamethyldisilazide (NaHMDS) gave (9S)-4 in 45% yield (ref. 9).
- J. B. Baudin, G. Hareau, S. A. Julia and O. Ruel, Tetrahedron Lett., 1991, 32, 1175 CrossRef CAS.
- P. R. Blakemore, W. J. Cole, P. J. Kocieński and A. Morley, Synlett, 1998, 26 CrossRef CAS.
- Because chemical shifts of Bn overlapped with olefin peaks, the E/Z ratio was determined after hydrolysis.
- W. J. Middleton, Org. Synth., 1986, 64, 221 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and NMR spectra. See DOI: https://doi.org/10.1039/d4ob00580e |
| This journal is © The Royal Society of Chemistry 2024 |





