Open Access Article
Open Access ArticleRapid in situ generation of 2-(halomethyl)-5-phenylfuran and nucleophilic addition in a microflow reactor†
Yuma
Matsuura
and
Shinichiro
Fuse
 *
*
Department of Basic Medicinal Sciences, Graduate School of Pharmaceutical Sciences, Nagoya University, Nagoya 464-8601, Japan. E-mail: fuse.shinichiro.z3@f.mail.nagoya-u.ac.jp
First published on 10th April 2024
Abstract
2,5-Disubstituted furans are frequently found in pharmaceuticals and bioactive natural products. Nucleophilic substitution reactions on the carbon atom adjacent to the furan ring are useful for producing various furan derivatives. However, the formation of 5-substituted 2-halomethylfuran and the subsequent nucleophilic substitution reactions are often limited by severe undesired reactions caused by the highly reactive halomethylfurans. This paper reports the successful rapid synthesis of various 2,5-disubstituted furans using microflow technology, which suppresses undesired reactions including dimerization and ring opening of the furans. We observed that Brønsted acids had a significant effect on the nucleophilic substitution reaction and the use of HBr and HI gave the best results. A plausible mechanism of the Brønsted acid-mediated nucleophilic substitutions in the developed approach was proposed.
The furan ring has been recognized as an important structure,1 ranking as the 29th most frequently used among the 378 ring systems found in marketed drugs,2 and is frequently found in bioactive natural products.3 In particular, 2,5-disubstituted furans are frequently found in pharmaceuticals.4 Heteroatom alkylations and arylations have been reported to be the most widely used organic transformations in the pharmaceutical field over the past four decades.5 Therefore, the development of heteroatom alkylations for the synthesis of various 2,5-disubstituted furans is an important pursuit.
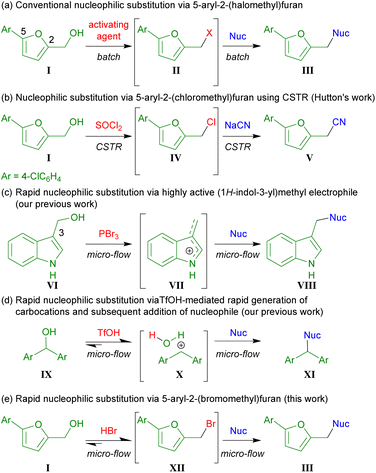
The in situ generation of 5-aryl-2-(halomethyl) furan II from I and an activating agent such as PBr3 and subsequent heteroatom alkylations have been reported (Scheme 1a).6 However, the desired products were obtained in moderate yields because of the severe undesired reactions caused by the highly reactive II. Continuous synthesis is a scalable, cost- and energy-efficient approach.7 In 1979, Hutton et al. reported that the chlorination of furfuryl alcohol derivative I (Ar = 4-ClC6H4) using SOCl2, followed by the addition of sodium cyanide to the unstable alkyl chloride IV using a continuous stirred-tank reactor (CSTR) consists of two 10 mL flasks (Scheme 1b).8 This pioneering work demonstrated the high-yielding synthesis of desired V (ca. 10 kg scale preparation of V per week) by controlling the residence time (ca. 1 min) of the unstable IV.
Microflow syntheses have attracted considerable attention owing to their superiority in controlling short reaction times (<1 s) and temperatures compared to those under batch conditions.9 Earlier, we had developed efficient microflow syntheses for generating valuable organic compounds.10 We recently reported the rapid generation of highly active indolylmethyl electrophile VII from alcohol VI and PBr3, its nucleophilic substitution in a microflow reactor (Scheme 1c).11 We also demonstrated the rapid formation of highly electrophilic carbocation X from diarylmethanol derivatives IX and TfOH, and the subsequent addition of nucleophiles (Scheme 1d).12
In this study, we synthesized various furan derivatives III from alcohols I and HBr using microflow to prevent undesired reactions by the highly reactive XII (Scheme 1e). The generated XII was rapidly used in subsequent nucleophilic substitution reactions. The undesired reactions caused by the coexistence of I and XII were avoided in the developed process.
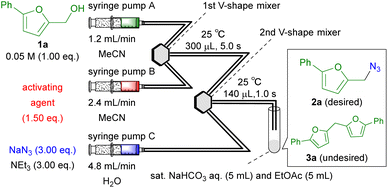
The synthesis of azide 2a from 5-phenyl-2-furanmethanol (1a) was investigated (Table 1). Two V-shape mixers with better mixing ability than that of the T-shaped mixer,10c were connected by a Teflon® tube, and the reactor was immersed in a water bath (25 °C). A solution of alcohol 1a was introduced into the first mixer using syringe pump A, followed by the introduction of a solution containing the activating agent using syringe pump B and alcohol 1a was rapidly converted into an electrophile. In the second mixer, an aqueous solution of NaN3 was introduced to initiate nucleophilic substitution. The resulting mixture containing azide 2a was poured into a mixture of aqueous solution of sodium bicarbonate and ethyl acetate at 25 °C. The effectiveness of halogenating agents PBr3 and SOCl2 used in our previous study11 (Scheme 1c) and Hutton's study8 (Scheme 1b) were examined (entries 1 and 2). PBr3 produced desired product 2a in high yield (entry 1), whereas SOCl2 did not (entry 2). Subsequently, we examined the effectiveness of Brønsted acids (entries 3–8) including TfOH, which had afforded the best results (entry 8) for activating diarylmethanols in our previous study (Scheme 1d).12 When hydrogen halides were used, the yield of the desired 2a increased as the acidity increased (entries 4, 6, and 7). However, neither H2SO4 (pKa = 8.7) nor TfOH (pKa = 0.7) gave satisfactory results, irrespective of the acidity (entries 5 and 8). Although the highest yield was obtained with HI aq. (entry 7), HBr aq. was used for subsequent experiments, because it also gave a high yield, and HBr aq. is less expensive and more stable than HI aq.
| Entry | Activating agent (pKa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a) a) |
Yieldf [%] | ||
|---|---|---|---|---|
| 2a (desired) | 1a (sub) | 3a (undesired) | ||
| a pKa in MeCN. b 0.500 eq. of PBr3 were used. c Using HCl aqueous solution (36 w/w%). d Using aqueous solution of HBr (47 w/w%). e Using aqueous solution of HI (57 w/w%). f Yields were determined by 1H NMR using 1,1,2-trichloroethane. TfOH = trifluoromethanesulfonic acid. | ||||
| 1 | PBr3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
87 | <1 | 9 |
| 2 | SOCl2 | 2 | 97 | <1 |
| 3 | AcOH (23.5)13 | <1 | 97 | <1 |
| 4 | HClc (10.3)13 | 8 | 66 | 26 |
| 5 | H2SO4 (8.7)14 | <1 | 5 | 72 |
| 6 | HBrd (5.5)13 | 92 | 5 | 3 |
| 7 | HIe (2.8)13 | 95 | 1 | 1 |
| 8 | TfOH (0.7)13 | <1 | <1 | <10 |
The significant influence of the Brønsted acids on the reaction was observed. The plausible mechanism of Brønsted acid-mediated nucleophilic substitutions is shown in Scheme 2. We speculated that the use of highly acidic HBr (and HI) rapidly converts 1a to the corresponding oxonium ion 4a (Scheme 2a). Nucleophilic Br− smoothly attacked highly electrophilic and unstable 4a to generate metastable alkyl bromide 5a.15 This avoided the undesired coexistence of 1a and 4a generating undesired dimer 3a; the nucleophilic substitution of 5a afforded the desired 2a in high yield. In contrast, less acidic HCl (pKa = 10.3) could not rapidly convert 1a to 4a (Scheme 2b). In addition, the generation of alkyl chloride 5a was slow due to the lower nucleophilicity of Cl− compared to that of Br−.16 Thus, the undesired coexistence of 1a and 4a caused the generation of 3a. The low yield obtained using H2SO4, despite its higher acidity than that of HCl, is attributable to the poor nucleophilicity of HSO4− (Scheme 2c).17 This caused the undesired coexistence of 1a and 4a. The poor yield obtained using TfOH, which gave the best results in our previously reported activation of diarylmethanols12 is attributable to the polymerization of 4a and undesired reactions involving the opening of the furan ring caused by the high acidity of TfOH (Scheme 2d). Murkovic et al. and Dumesic et al. reported that furfuryl alcohol derivatives undergo polymerization and furan ring-opening reactions in the presence of strong acid.18
 | ||
| Scheme 2 Plausible mechanisms of (a) HCl-mediated, (b) HBr-mediated, (c) H2SO4-mediated, and (d) TfOH-mediated nucleophilic substitutions. | ||
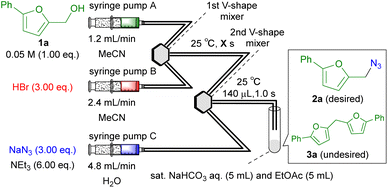
The reaction time for the HBr-mediated activation of 1a was then investigated (Table 2); the results indicated that decreasing the reaction time to 1.0 s decreased the yield of 2a (entries 1 vs. 2), whereas increasing the reaction time to 10 s slightly improved the yield (entries 2 vs. 3). Further extension of the reaction time to 60 s did not improve the yield of 2a (entries 3 vs. 5). Additional examination (for details, see the ESI†) enabled the determination of the optimal conditions of the reaction (entry 4). The desired product 2a was obtained in 97% NMR yield (95% isolated yield) under the optimal conditions. To verify the importance of the microflow conditions, we examined comparative batch conditions, except for the reaction time for the nucleophilic attack of N3− (10 s), because performing the batch reaction in 1.0 s (entry 4) was impossible. (Caution: As the reaction is accompanied by the highly exothermic neutralization and potential generation of toxic and explosive HN3, batch reaction should be performed with extreme caution. Particularly in scale-up synthesis, the use of batch synthesis should be avoided). Both the yield and reproducibility under batch conditions were lower than those under flow conditions (entries 4 vs. 6). These results clearly indicate the importance of the microflow technology in the developed approach.
| Entry | X sc | Yielde [%] | ||
|---|---|---|---|---|
| 2a (desired) | 1a (sub) | 3a (undesired) | ||
| a Three independent experiments were performed. b Reaction mixture was magnetically stirred (1000 rpm) under batch conditions. c The volume of the tubes for the reaction of 1a with HBr was 60 μL (X = 1.0 s), 300 μL (X = 5.0 s), 600 μL (X = 10 s), and 3600 μL (X = 60 s). d Isolated yield. e Yields were determined by 1H NMR using 1,1,2-trichloroethane. | ||||
| 1 | 1.0 | 64 | 34 | 2 |
| 2 | 5.0 | 96 | 2 | 2 |
| 3 | 10 | 97 | <1 | 2 |
| 4a | 10 | 97 ± 1 (95)d | <1 | <1 |
| 5 | 60 | 97 | <1 | 2 |
| 6a,b | 10 | 51 ± 5 | <1 | 43 ± 1 |
Subsequently, the scope of the nucleophiles in the developed approach was examined (Fig. 1). The use of pyrrolidine as a nucleophile produced the desired 2b in good yield (71%), whereas the use of 2-bromoaniline with weak nucleophilicity produced the desired 2c in low yield (33%). The use of sulfur nucleophiles afforded the desired 2d–2f in good to high yields (64–90%). The synthesis of 2f was scaled-up by extending the pumping time (960 s) under higher concentration conditions (0.200 M) to increase productivity. The desired 2f (0.72 g) was obtained in 63% yield. Although the use of phenol as an oxygen nucleophile afforded a complex mixture, the use of KCN and Meldrum's acid as carbon nucleophiles afforded the desired 2h19 and 2i in acceptable yields (59 and 55%).
The scope of electrophiles was examined (Fig. 2). Compounds 2j and 2k containing electron-donating OMe or electron-withdrawing Cl groups on the benzene ring, were obtained in high to excellent yields (86–94%), whereas 2l containing a strong electron-withdrawing NO2 group, was obtained in a low yield (34%), presumably due to the slow generation of the electrophilic intermediate. The synthesis of 2m containing a benzene ring at the 4-position of the furan ring was examined. However, the desired product was not obtained; conversely, a complex mixture was generated. We speculated that an undesired reaction occurred at the 5-position of the furan ring.20 The 2n and 2o containing phenyl and ethyl groups at the 2′-position, respectively were obtained in moderate to good yields (76% and 43%). The moderate yield of 2o was presumably due to competitive dehydrative alkene formation. Although the compounds 2d–2f, 2h, and 2i derived from S- and C-nucleophiles are stable, the compounds 2a–2c and 2j–2o containing amino and N3 groups, respectively are relatively unstable. In particular, the compound 2o containing the secondary N3 group is unstable.
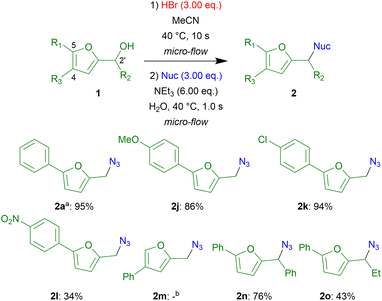 | ||
Fig. 2 Examination of the scope of electrophiles a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The yield of 2a was re-shown to clarify the scope and limitation of electrophiles. b The yield of 2a was re-shown to clarify the scope and limitation of electrophiles. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Complex mixture was obtained. Complex mixture was obtained. | ||
In general, highly basic nucleophiles cannot be used in the acid-mediated activation of alcohols and subsequent nucleophilic substitution reactions because the acid deactivates the nucleophiles. Therefore, low-basicity nucleophiles, such as amides, imines, and anilines, have often been employed. However, the developed approach can introduce basic amines, rendering it a complementary approach to conventional methods. In addition, although preparations21 and uses22 of 5-nonsubstituted furfurylbromides have been reported, the preparation and use of 5-aryl-substituted furfurylbromides have been challenging, as previously described. From this perspective too, the developed approach complements conventional approaches.
Conclusions
We developed a method for the rapid (11 or 20 s) nucleophilic substitution reaction at the adjacent carbon of the furan ring. Brønsted acids significantly influence the nucleophilic substitution and the use of HI and HBr afforded the best results. We speculate that the strong acid-induced rapid generation of highly electrophilic and unstable intermediates and the nucleophilic Br− and I− smoothly attacked the intermediate to generate a metastable alkyl halide intermediate. This avoids the undesired coexistence of the alcohol and electrophilic intermediate, thus preventing the generation of undesired dimers. Interestingly, TfOH, which afforded the best results in our previously reported activation of diarylmethanols, did not yield the desired product. This is probably because the strongly acidic TfOH induces the decomposition of the furan ring. We demonstrate the importance of microflow technology in the developed approach. In addition, the developed approach complements existing conventional approaches. The developed approach could be utilized for producing various 2,5-disubstituted furans, which could lead to the potential development of furan-based drug candidates and functional materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP22ama121044 and JP23ama121044.References
- (a) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G.-Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939–9953 CrossRef CAS; (b) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef CAS PubMed; (c) M. S. Butler, J. Nat. Prod., 2004, 67, 2141–2153 CrossRef CAS PubMed.
- J. Shearer, J. L. Castro, A. D. G. Lawson, M. MacCoss and R. D. Taylor, J. Med. Chem., 2022, 65, 8699–8712 CrossRef CAS PubMed.
- (a) F.-J. Gamo, L. M. Sanz, J. Vidal, C. Cozar, E. Alvarez, J.-L. Lavandera, D. E. Vanderwall, D. B. S. Green, V. Kumar, S. Hasan, J. R. Brown, C. E. Peishoff, L. R. Cardon and J. F. Garcia-Bustos, Nature., 2010, 798, 305–310 CrossRef PubMed; (b) S. Alnabulsi, E. Santina, I. Russo, B. Hussein, M. Kadirvel, A. Chadwick, E. V. Bichenkova, R. A. Bryce, K. Nolan, C. Demonacos and I. J. Stratford, Eur. J. Med. Chem., 2016, 111, 33–45 CrossRef CAS PubMed.
- (a) S. J. Pridmore, P. A. Slatford and J. M. J. Williams, Tetrahedron Lett., 2007, 48, 5111–5114 CrossRef CAS; (b) A. V. Butin, T. A. Nevolina, V. A. Shcherbinin, M. G. Uchuskin, O. V. Serdyuk and I. V. Trushkov, Synthesis, 2010, 2969–2978 CrossRef CAS; (c) S. H. Krake, P. D. G. Martinez, J. McLaren, E. Ryan, G. Chen, K. White, S. A. Charman, S. Campbell, P. Willis and L. C. Dias, Eur. J. Med. Chem., 2017, 126, 929–936 CrossRef CAS PubMed; (d) Y.-S. Li, M. He, T.-S. Zhou, Q. Wang, L. He, S.-J. Wang, B. Hu, B. Wei, H. Wang and Z.-N. Cui, Eur. J. Med. Chem., 2021, 216, 113322–113331 CrossRef CAS PubMed.
- N. Schneider, D. M. Lowe, R. A. Sayle, M. A. Tarselli and G. A. Landrum, J. Med. Chem., 2016, 59, 4385–4402 CrossRef CAS PubMed.
- (a) J. E. Zanetti and J. T. Bashour, J. Am. Chem. Soc., 1939, 61, 2249–2251 CrossRef CAS; (b) J. S. Kaltenbronn and T. O. Rhee, J. Med. Chem., 1968, 11, 902 CrossRef CAS PubMed; (c) A. Krutosikoca, J. Kovac and R. Frimm, Chem. Zvesti, 1973, 27, 107–111 Search PubMed.
- For selected recent reviews of continuous-flow syntheses, see: (a) M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS PubMed; (b) C. A. Shukla and A. A. Kulkarni, Beilstein J. Org. Chem., 2017, 13, 960–987 CrossRef CAS PubMed; (c) F. Fanelli, G. L. Parisi, L. Degennaro and R. Luisi, Beilstein J. Org. Chem., 2017, 13, 520–542 CrossRef CAS PubMed; (d) J. Britton and C. L. Raston, Chem. Soc. Rev., 2017, 46, 1250–1271 RSC; (e) J. M. De Souza, R. Galaverna, A. A. N. De Souza, T. J. Brocksom, J. C. Pastre, R. O. M. A. De Souza and K. T. De Oliveira, An. Acad. Bras. Cienc., 2018, 90, 1131–1174 CrossRef CAS PubMed; (f) B. T. Ramanjaneyulu, N. K. Vishwakarma, S. Vidyacharan, P. R. Adiyala and D.-P. Kim, Bull. Korean Chem. Soc., 2018, 39, 757–772 CrossRef CAS; (g) P. L. Suryawanshi, S. P. Gumfekar, B. A. Bhanvase, S. H. Sonawane and M. S. Pimplapure, Chem. Eng. Sci., 2018, 189, 431–448 CrossRef CAS; (h) M. Colella, A. Nagaki and R. Luisi, Chem. – Eur. J., 2020, 26, 19–32 CrossRef CAS PubMed; (i) R. Gérardy, N. Emmanuel, T. Toupy, V.-E. Kassin, N. N. Tshibalonza, M. Schmitz and J.-C. M. Monbaliu, Eur. J. Org. Chem., 2018, 2301–2351 CrossRef; (j) M. Guidi, P. H. Seeberger and K. Gilmore, Chem. Soc. Rev., 2020, 49, 8910–8932 RSC for selected recent reviews of continuous syntheses using continuous stirred tank reactors, see: (k) N. G. Anderson, Org. Process Res. Dev., 2012, 16, 852–869 CrossRef CAS; (l) N. Cherkasov, S. J. Adams, E. G. A. Bainbridge and J. A. M. Thornton, React. Chem. Eng., 2023, 8, 266–277 RSC.
- J. A. Foulkes and J. Hutton, Synth. Commun., 1979, 9, 625–630 CrossRef CAS.
- Representative publications for “flash chemistry” enabled by flow micro technologies reported by Yoshida et al., see: (a) J.-i. Yoshida, A. Nagaki and T. Yamada, Chem. – Eur. J., 2008, 14, 7450–7459 CrossRef CAS PubMed; (b) J.-i. Yoshida, Flash Chemistry: Fast Organic Synthesis in Micro Systems, Wiley-VCH, Weinheim, 2008 CrossRef; (c) J.-i. Yoshida, Chem. Rec., 2010, 10, 332–341 CrossRef CAS PubMed; (d) J.-i. Yoshida, Y. Takahashi and A. Nagaki, Chem. Commun., 2013, 49, 9896–9904 RSC ; for pioneering and recent works on flow microsynthesis using highly reactive carbocations, see: ; (e) S. Suga, M. Okajima, K. Fujiwara and J.-i. Yoshida, J. Am. Chem. Soc., 2001, 123, 7941–7942 CrossRef CAS PubMed; (f) M. Takumi, H. Sakaue and A. Nagaki, Angew. Chem., Int. Ed., 2022, 61, e202116177 CrossRef CAS PubMed.
- (a) S. Fuse, Y. Mifune and T. Takahashi, Angew. Chem., Int. Ed., 2014, 53, 851–855 CrossRef CAS PubMed; (b) S. Fuse, Y. Mifune, H. Nakamura and H. Tanaka, Nat. Commun., 2016, 7, 13491 CrossRef CAS PubMed; (c) Y. Otake, H. Nakamura and S. Fuse, Angew. Chem., Int. Ed., 2018, 57, 11389–11393 CrossRef CAS PubMed; (d) N. Sugisawa, A. Ando and S. Fuse, Chem. Sci., 2023, 14, 6986–6991 RSC.
- (a) H. Masui, S. Kanda and S. Fuse, Commun. Chem., 2023, 6, 47 CrossRef CAS PubMed; (b) S. Fuse, S. Kanda and H. Masui, Chem. – Asian J., 2023, e202300909 Search PubMed.
- Y. Matsuura and S. Fuse, Chem. Commun., 2024, 60, 2497–2500 RSC.
- E. Raamata, K. Kaupmeesa, G. Ovsjannikova, G. Trummalb, A. Kütta, J. Saamea, I. Koppela, I. Kaljuranda, L. Lippinga, T. Rodimaa, V. Pihla, I. A. Koppela and I. Leitoa, J. Phys. Org. Chem., 2013, 26, 162–170 CrossRef.
- A. Kütt, T. Rodima, J. Saame, E. Raamat, V. Mäemets, I. Kaljurand, L. A. Koppel, R. Y. Garlyauskayte, Y. L. Yagupolskii, L. M. Yagupolskii, E. Bernhardt, H. Willner and I. Leito, J. Org. Chem., 2011, 76, 391–395 CrossRef PubMed.
- To confirm the generation of alkyl bromide 5a, additional experiments were performed (for details, see ESI†).
- The nucleophilicity of Br− (N, Parameter: 13.80) is higher than that of Cl− (N Parameter: 12.00) in a 1: 1 water-MeCN mixed solvent from Mayr's nucleophilicity parameter see: S. Minegishi, R. Los, S. Kobayashi and H. Mayr, J. Am. Chem. Soc., 2005, 127, 2641–2649 CrossRef CAS PubMed.
- (a) S. Kiyooka, D. Kaneno and R. Fujiyama, Tetrahedron, 2013, 69, 4247–4258 CrossRef CAS; (b) S. Kiyooka, D. Kaneno and R. Fujiyama, Tetrahedron Lett., 2013, 54, 339–342 CrossRef CAS.
- (a) Y. R. Swasti and M. Murkovic, Food Funct., 2012, 3, 965–969 RSC; (b) P. Azadi, R. Carrasquillo-Flores, Y. J. Pagán-Torres, E. I. Gürbüz, R. Farnood and J. A. Dumesic, Green Chem., 2012, 14, 1573–1576 RSC.
- The compound 2h containing CN group has been known as a precursor of an anti-inflammatory agent. J. S. Kaltenbronn and A. A. Mich, 1971, US3560525.
- S. Divald, M. C. Chun and M. M. Joullie, Tetrahedron Lett., 1970, 10, 777–780 CrossRef.
- (a) L. Khazdooz, A. Zarei, H. Aghaei, G. Azizi and M. M. Gheisari, Tetrahedron Lett., 2016, 57, 168–171 CrossRef CAS; (b) Y. Guo, T. Quan, Y. Lu and T. Luo, J. Am. Chem. Soc., 2017, 139, 6815–6818 CrossRef CAS PubMed; (c) M. N. Alam, S. R. Dash, A. Mukherjee, S. Pandole, U. K. Marelli, K. Vanka and P. Maity, Org. Lett., 2021, 23, 890–895 CrossRef CAS PubMed; (d) Z. Feng, J.-A. Ma and C. W. Cheung, Org. Chem. Front., 2022, 9, 3869–3875 RSC; (e) E. Joseph, R. D. Hernandez and J. A. Tunge, Chem. – Eur. J., 2023, 29, e20230217 Search PubMed.
- (a) X. Zhang, W. Zeng, Y. Yang, H. Huang and Y. Liang, Org. Lett., 2014, 16, 876–879 CrossRef CAS PubMed; (b) Y. Xie, D. Xu, B. Huang, X. Ma, W. Qi, F. Shi, X. Liu, Y. Zhang and W. Xu, J. Med. Chem., 2014, 57, 8445–8458 CrossRef CAS PubMed; (c) L. Leng and J. M. Ready, ACS Catal., 2020, 10, 13196–13201 CrossRef CAS; (d) C.-L. Wang, J. Wang, J.-K. Jin, B. Li, Y. L. Phang, F.-L. Zhang, T. Ye, H.-M. Xia, L.-W. Hui, J.-H. Su, Y. Fu and Y.-F. Wang, Science, 2023, 382, 1056–1065 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob00358f |
| This journal is © The Royal Society of Chemistry 2024 |