Open Access Article
Open Access ArticleUse of ionic liquids in amidation reactions for proteolysis targeting chimera synthesis†
Michela
Eleuteri
 *,
Jenny
Desantis
*,
Jenny
Desantis
 ,
Gabriele
Cruciani
,
Raimondo
Germani
and
Laura
Goracci
,
Gabriele
Cruciani
,
Raimondo
Germani
and
Laura
Goracci

Department of Chemistry, Biology, and Biotechnology, University of Perugia, Italy. E-mail: michela.eleuteri1@studenti.unipg.it
First published on 11th April 2024
Abstract
Selective degradation of disease-causing proteins using proteolysis targeting chimeras (PROTACs) has gained great attention, thanks to its several advantages over traditional therapeutic modalities. Despite the advances made so far, the structural chemical complexity of PROTACs poses challenges in their synthetic approaches. PROTACs are typically prepared through a convergent approach, first synthesizing two fragments separately (target protein and E3 ligase ligands) and then coupling them to produce a fully assembled PROTAC. The amidation reaction represents the most common coupling exploited in PROTACs synthesis. Unfortunately, the overall isolated yields of such synthetic procedures are usually low due to one or more purification steps to obtain the final PROTAC with acceptable purity. In this work, we focused our attention on the optimization of the final amidation step for the synthesis of an anti-SARS-CoV-2 PROTAC by investigating different amidation coupling reagents and a range of alternative solvents, including ionic liquids (ILs). Among the ILs screened, [OMIM][ClO4] emerged as a successful replacement for the commonly used DMF within the HATU-mediated amidation reaction, thus allowing the synthesis of the target PROTAC under mild and sustainable conditions in very high isolated yields. With the optimised conditions in hand, we explored the scalability of the synthetic approach and the substrate scope of the reaction by employing different E3 ligase ligand (VHL and CRBN)-based intermediates containing linkers of different lengths and compositions or by using different target protein ligands. Interestingly, in all cases, we obtained high isolated yields and complete conversion in short reaction times.
Introduction
In the last two decades, proteolysis targeting chimeras (PROTACs) technology has emerged as a new therapeutic approach with the potential to revolutionize drug discovery.1,2 Since the synthesis of the first PROTAC in 2001 by Crews and Deshaies,3 PROTACs have received considerable attention from both academic and industrial laboratories worldwide due to their abilities to induce targeted degradation of pathogenic proteins.4Structurally, PROTACs are heterobifunctional molecules comprising a ligand targeting a protein of interest (POI), a ligand binding an E3 ligase, and a connecting linker.1 The formation of a ternary complex between the POI, PROTAC molecule, and the recruited E3 ligase brings the two proteins in spatial proximity to each other, thus facilitating ubiquitin transfer from the E3 ligase to the lysine residues of the POI. Once the POI is ubiquitinated, it is recognized and degraded by the 26S proteasome.1 Among the three components, the linker moiety plays a key role in the biological activity of PROTACs. In fact, the length, composition, and flexibility of the linker are pivotal for the formation of a productive ternary complex and degradation activity. Additionally, linker–protein interactions can also significantly impact the stability of the POI–PROTAC–E3 ternary complex.5,6 In general, the modular nature of PROTACs makes them well suited for parallel synthesis, enabling a rapid and efficient generation of PROTAC libraries in order to identify a promising degrader.5
Thanks to their event-driven mechanism of action, PROTACs possess several advantages over traditional occupancy-driven small molecule inhibitors, including overcoming potential resistance to therapeutic treatments.7,8 To date, 26 PROTAC degraders have entered clinical studies.9 Among them, PROTACs ARV-110 and ARV-471, both developed by Arvinas (for prostate cancer and breast cancer), are in phase II and III clinical trials, respectively.10 So far, PROTAC technology has been successfully used to degrade several distinct target proteins associated with various diseases, such as cancer, immune disorders, neurodegenerative conditions, cardiovascular diseases and viral infections.7,11–13 Given the growing success of PROTAC technology, there is a lot of attention being paid to the optimization of their current synthetic strategies. In particular, the large size of these molecules (molecular weight of 600–1900 Da) coupled with their intricate structures demands increased synthetic efforts.
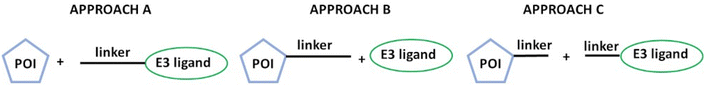
From a synthetic chemistry point of view, PROTAC derivatives are typically prepared through three main approaches: (1) coupling of the linker to the E3 ligand before attaching it to the POI (Approach A, Fig. 1), (2) functionalization of the POI with the linker followed by its coupling to the E3 ligand (Approach B, Fig. 1), and (3) binding of two linker fragments on both the POI and E3 ligands, followed by their final coupling (Approach C, Fig. 1).5,14–16
Notably, the amide bond is not only one of the most fundamental functional group linkages in biomolecules and pharmaceuticals but also the most exploited linkage in the design and synthesis of PROTACs. In fact, PROTAC components are often combined via late-stage amide couplings, due to the reliability and robustness of amide bond formation. According to the statistics made on the largest web-accessible database dedicated to PROTACs (PROTAC-DB 2.0, https://cadd.zju.edu.cn/protacdb/)17 (Fig. 2), among the total of 3270 reported PROTACs (data accessed on 27th November 2023), 83% contain an amide bond as a connection between modules. More in detail, among them, the amide linkage is variously exploited to connect the linker moiety to the POI ligand (31%), the E3 ligase ligand (22%), both (16%), or even two E3 ligase ligands (1%). Additionally, to a lesser extent, it has also been used as a connection for two linker fragments following Approach C (13%).
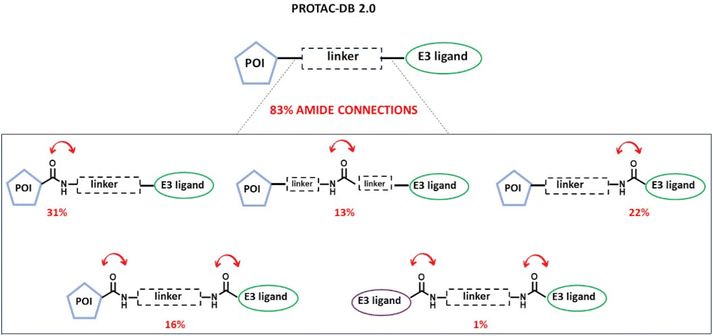 | ||
| Fig. 2 Overview of the presence of the amide bond as a connection at different positions of a PROTAC molecule based on the statistics of the available structures deposited in PROTAC-DB 2.0 (data accessed on 27th November 2023).17 Percentages indicate the relative abundance of the total of 3270 PROTACs endowed with one or two amide connections. | ||
Despite the wide use of amidation reactions, when the last coupling reaction for PROTACs synthesis is amidation, the isolated yields are commonly low, probably due to the formation of by-products and impurities. Thus, the purification step might be difficult and tricky, and sometimes two or three successive purifications are needed to obtain the final PROTAC compounds with acceptable purity for biological evaluation (>95%). Furthermore, only a few conditions for the amide coupling in the synthesis of PROTACs have been reported so far in the literature (Fig. 3).5,14,18 The use of 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU) as an amidation coupling reagent in the presence of N,N-diisopropylethylamine (DIPEA) as the base remains the most dominant, followed, to a lesser extent, by other coupling agents and combinations of bases such as N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC*HCl)/1-hydroxybenzotriazole (HOBt) with N-methylmorpholine (NMM), dicyclohexylcarbodiimide (DCC) with DIPEA, and O-(1H-benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) with triethylamine (Et3N).5,15,18 Concerning the reaction solvent, these reactions are typically conducted in hazardous dipolar aprotic solvents such as anhydrous N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), dichloromethane (DCM) or tetrahydrofuran (THF) depending on the solubility of PROTAC building blocks. Among them, DMF is the most exploited one, thanks to its high solvency power.
 | ||
| Fig. 3 Typical reported conditions in the literature for the last amide coupling for PROTACs synthesis. | ||
To date, our group's interest in targeted protein degradation applied to different research fields19–22 led us to synthesize a wide structurally heterogeneous library of more than 100 PROTACs. During the preparation of this library, we obtained consistently low yields, particularly in the last coupling synthetic amidation step, thus recognizing the need for exploring and identifying better reaction parameters/conditions.
In this work, we report an in-depth study aimed at exploring and optimizing the final amidation step for the synthesis of PROTAC derivatives. Through the evaluation of alternative solvents, we identified an ionic liquid as a valid replacement for DMF within the HATU-mediated amidation reaction, thus allowing the synthesis of PROTACs under mild and sustainable conditions with very high yields.
Results and discussion
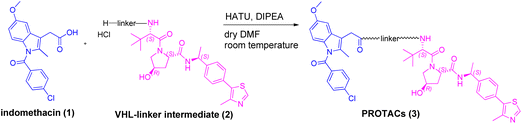
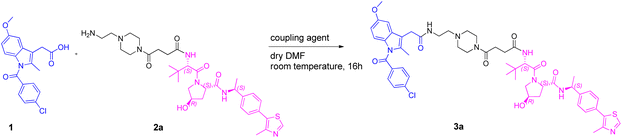
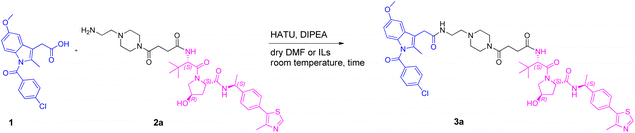
We began our investigation on PROTACs synthesis by focusing on the amidation reaction between indomethacin (INM) (1) and the suitable E3 ligase ligand-linker intermediate (Von Hippel–Lindau (VHL) as the E3 ligase) (2) to prepare anti-SARS-CoV-2 INM-based PROTACs (3), previously synthesized and reported by us (Table 1).21,22 In particular, by following common Approach A (Fig. 1), PROTACs 3a–3g were synthesized by an amidation reaction in the presence of HATU and DIPEA in anhydrous DMF at room temperature in poor isolated yields (17–32%) (Table 1).21,22| INM-based PROTACs | Linker | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reactions were performed on a 0.059–0.139 mmol scale with 1 (1.0 equiv.), 1.0 equiv. of 2, 1.25 equiv. of HATU, 3.0 equiv. of DIPEA, in dry DMF at 0.059–0.139 M concentration. b Isolated yields. | |||
| 3a |
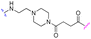
|
16 | 24 |
| 3b |

|
16 | 28 |
| 3c |
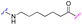
|
16 | 31 |
| 3d |
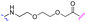
|
5 | 17 |
| 3e |
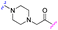
|
18 | 19 |
| 3f |
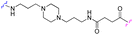
|
3 | 28 |
| 3g |
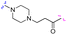
|
4 | 32 |
Indeed, in PROTACs synthesis, HATU is the coupling agent most used for amidation coupling (as reported in the literature, it reduces the risk of epimerization in the coupling of chiral compounds),23,24 while DMF is the preferred solvent due to the high solubility that PROTAC building blocks have in it.5,25 Initially, we selected the synthesis of INM-based PROTAC 3a, emerged as one of the most active compounds in inhibiting SARS-CoV-2 in vitro, as a case study to investigate and optimize the reaction conditions.
In our first attempt to explore the reaction conditions, the amidation reaction step for the synthesis of PROTAC 3a was carried out using other coupling agents than HATU (Table 2).26 In particular, five of the most common amide coupling agents or reagent combinations were selected, i.e., (1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate (COMU),27–29 dicyclohexylcarbodiimide/4-dimethylaminopyridine (DCC/DMAP),30,31 EDC*HCl/HOBt,31,32 (benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP)33 and HBTU26 (Table 2, entries 1–5). All reactions were stirred in dry DMF at room temperature using DIPEA or Et3N as the base and stopped, in the first evaluation, after 16 h. For comparison purposes, the reaction was re-run under the same conditions in the presence of HATU (Table 2, entry 6). All reactions proceeded until the complete conversion of both starting materials (as monitored by TLC), but the isolated yields (Table 2, entries 1–6) were anyway low (<34%) and in the same range as that of our case study (Table 2, entry 6).
| Entry | Coupling agent (1.25 eq.) | Base (3.0 eq.) | Solvent (0.056 M) | Time (h) | Conversionb (%) | Yield of 3ac (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.056 mmol, 1 equiv.), 2a (0.056 mmol, 1 equiv.), coupling agent (0.070 mmol, 1.25 equiv.), DIPEA (0.168 mmol, 3 equiv.), dry DMF (0.056 M), room temperature, 16 h. b Monitored by TLC with respect to consumption of both starting materials. c Isolated yield. | ||||||
| 1 | HBTU | Et3N | DMF | 16 | 100 | 25 |
| 2 | DCC/DMAP | DIPEA | DMF | 16 | 100 | 31 |
| 3 | HOBt/EDC*HCl | DIPEA | DMF | 16 | 100 | 23 |
| 4 | PyBOP | DIPEA | DMF | 16 | 100 | 26 |
| 5 | COMU | DIPEA | DMF | 16 | 100 | 34 |
| 6 | HATU | DIPEA | DMF | 16 | 100 | 28 |
Although COMU provided PROTAC 3a in a slightly higher yield than HATU, we decided to use HATU for subsequent investigations as it is the ‘gold standard’ reagent23 for amide formation and PROTACs synthesis in both liquid and solid phases showing good versatility and reliability. Additionally, it is known to reduce the risk of racemization in compounds that have multiple chiral centers.34,35
Thus, by maintaining HATU as the coupling agent and DIPEA as the base, different solvents than the hazardous DMF were evaluated, including emerging greener alternatives such as CPME and 2-Me–THF. All reactions were conducted at room temperature and stopped after 16 h. While DCM gave the same results as DMF (Table 3, entry 1), in the case of CPME and 2-Me–THF (Table 3, entries 2 and 3), the conversion was below 100% due to incomplete solubilization of the starting materials, thus affording lower final yields.
| INM-based PROTACs | Coupling agent (1.25 eq.) | Base (3.0 eq.) | Solvent (0.056 M) | Time (h) | Conversion (%) | Yield of 3ad (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.056 mmol, 1 equiv.), 2a (0.056 mmol, 1 equiv.), HATU (0.070 mmol, 1.25 equiv.), DIPEA (0.168 mmol, 3 equiv.), solvent (0.056 M), room temperature, 16 h. b As monitored by TLC with respect to consumption of both starting materials. c As monitored by HPLC with respect to consumption of 1. d Isolated yield. | ||||||
| 1 | HATU | DIPEA | DCM | 16 | 100b | 28 |
| 2 | HATU | DIPEA | CPME | 16 | 85.54c | 14 |
| 3 | HATU | DIPEA | 2-Me–THF | 16 | 82.13c | 17 |
| 4 | HATU | DIPEA | [OMIM][NTf2] | 16 | 100b | 55 |
| 5 | HATU | DIPEA | [OMIM][PF6] | 16 | 100b | 68 |
| 6 | HATU | DIPEA | [OMIM][ClO4] | 16 | 100b | 75 |
| 7 | HATU | DIPEA | [BMIM][BF4] | 16 | 100b | 68 |
| 8 | HATU | DIPEA | [BMIM][PF6] | 16 | 100b | 73 |
| 9 | HATU | DIPEA | [BMIM][NTf2] | 16 | 72.48c | 30 |
| 10 | HATU | DIPEA | [TBMA][MsO] | 16 | 71.9c | 32 |
| 11 | — | — | [TBMA][PF6] | — | — | — |
Aiming at exploring further sustainable and safe solvents able to replace DMF, we decided to explore the use of ionic liquids (ILs). Indeed, their unique features, such as low volatility, non-flammability, good thermal and chemical stability, and the ability to dissolve both organic and inorganic compounds,36,37 represent intriguing advantages over conventional organic volatile, flammable, and toxic solvents.38–40 The high efficiency of ILs as solvents and/or catalysts for the direct amidation of carboxylic acid and amine has already been reported in the literature, arousing considerable interest.41–48 Additionally, ILs have been extensively used in pharmaceutical fields as reaction media for the synthesis of pharmaceutical compounds,36,49,50 including non-steroidal anti-inflammatory drugs51 and antiviral,52,53 antimicrobial,54,55 antimalarial,56 and antitumor agents.57,58 Here, a set of differently structured ILs were explored as reaction media for the synthesis of PROTAC 3a, including room-temperature ionic liquids (RTILs), such as 1-methyl-3-octylimidazolium bis(trifluoromethylsulfonyl)imide ([OMIM][NTf2]), 1-methyl-3-octyl-imidazolium-hexafluorophosphate ([OMIM][PF6]), 1-methyl-3-octyl-imidazolium-perchlorate ([OMIM][ClO4]), 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]), 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]) and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMIM][NTf2]) (Table 3, entries 4–9), and solid-state ionic liquids (SSILs), such as tributylmethylammonium methanesulfonate ([TBMA][MsO]) (Table 3, entry 10). Among them, [OMIM][ClO4] is the only IL that has never been reported before; conversely, the anion [ClO4]− has already been widely reported in the literature for the synthesis of ILs used for different purposes.59–64
Overall, as reported in Table 3, the use of RTILs proved beneficial for the synthesis of 3a achieved under mild reaction conditions with an impressive improvement in the reaction yield, as compared to those obtained with DMF or other organic solvents. In fact, the replacement of DMF with [OMIM][PF6] (entry 5), [OMIM][ClO4] (entry 6), [BMIM][BF4] (entry 7) and [BMIM][PF6] (entry 8) afforded the highest yields, reaching up to 68–75%. Conversely, the reactions performed in [OMIM][NTf2] (entry 4), [BMIM][NTf2] (entry 9), and [TBMA][MsO] (entry 10, in this case, the reaction was performed at 75 °C) gave lower yields. Moreover, the use of [BMIM][NTf2] or [TBMA][MsO] led to an incomplete conversion of 1.
Since reactions performed in ILs with [PF6]− anions provided high yields (Table 3, entries 5 and 8), we also synthesized [TBMA][PF6] (entry 11). Unfortunately, [TBMA][PF6] provided a solid at room temperature, with a high melting point (129.6–131.1 °C) (Table 4), thus not compatible with the mild reaction conditions applied to PROTAC synthesis.
| Ionic liquid | Structure | Molecular weight (g mol−1) | Viscosityb (cP) | Densityc (g cm−3) | Melting pointd (°C) | Physical state (25 °C) |
|---|---|---|---|---|---|---|
| a Values determined experimentally in house. b Data reported by the seller Iolitec; values reported for ILs that have a purity >99% and a water content <250 ppm for [BMIM][BF4], [BMIM][PF6], [OMIM][PF6], and <100 ppm for [BMIM][NtF2], [OMIM][NtF2]. c Data reported by the seller Iolitec; values reported for ILs that have a purity >99% and a water content <250 ppm for [BMIM][BF4], [BMIM][PF6], [OMIM][PF6], and <100 ppm for [BMIM][NtF2], [OMIM][NtF2]. d Data reported by the seller Iolitec; values reported for ILs that have a purity >99% and a water content <250 ppm for [BMIM][BF4], [BMIM][PF6], [OMIM][PF6], and <100 ppm for [BMIM][NtF2], [OMIM][NtF2]. | ||||||
| [OMIM][NTf2] |
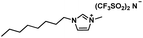
|
475.47 | 86 (25 °C) | 1.32 (25 °C) | <RT | Liquid |
| [OMIM][PF6] |
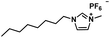
|
340.29 | 608 (25 °C) | 1.24 (24 °C) | −74 | Liquid |
| [OMIM][ClO4] |
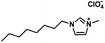
|
294.77 | 432.1 (20 °C)a | 1.07 ± 0.02 (20 °C)a | −57/−58a | Liquid |
| [BMIM][BF4] |
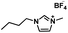
|
226.02 | 233 (25 °C) | 1.20 (25 °C) | −82 | Liquid |
| [BMIM][PF6] |
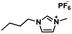
|
284.19 | 400 (25 °C) | 1.37 (25 °C) | −8 | Liquid |
| [BMIM][NTf2] |
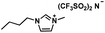
|
419.36 | 52 (25 °C) | 1.42 (25 °C) | −4 | Liquid |
| [TBMA][MsO] |
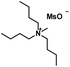
|
295.48 | — | 1.02 (80 °C) | 70–72 | Solid |
| [TBMA][PF6] |
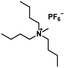
|
345.35 | — | — | 129.6–131.1a | Solid |
By analyzing the structural properties of the ILs used for the synthesis of 3a, it can be observed that RTILs with the large anion [NTf2]− performed poorly (Table 3, entries 4 and 9) when compared to those with smaller anions, such as [PF6]−, [ClO4]− and [BF4]− (Table 3, entries 5–8). Although the overall properties of ILs result from the composite properties of the cation and anion, the latter plays a fundamental role in determining the physicochemical properties of the ILs including the melting point, viscosity and density.65–69 According to the literature, the [NTf2]− anion offers imidazolium-based ionic liquids with low viscosity, while the [BF4]−, [PF6]− and [ClO4]− anions contribute significantly to increasing the viscosity. By comparing the length of the alkyl chains on the imidazolium ring, explored in our study, with the anions, the viscosity can be broadly depicted as [BMIM][NTf2] < [OMIM][NTf2] < [BMIM][BF4] < [BMIM][PF6] < [OMIM][ClO4] < [OMIM][PF6] (Table 4). A different pattern can be observed for the density of the described RTILs, which is closely related to the mass of the anion. In particular, for the same cation, an increase in the mass of the anion corresponds to an increase in density as follows: [OMIM][ClO4] < [OMIM][PF6] < [OMIM][NTf2] and [BMIM][BF4] < [BMIM][PF6] < [BMIM][NTf2] (Table 4).
By analysing the structural and physicochemical properties of the ILs used for the synthesis of 3a, it can be noted that among the RTILs explored, [OMIM][PF6], [OMIM][ClO4], [BMIM][BF4], and [BMIM][PF6] (Table 3 entries 5, 6, 7 and 8, respectively) allowed us to achieve the highest yields, and were all characterized by higher values of viscosity and lower values of density at 20–25 °C (Table 4).
To further explore the reaction conditions with the four best performing ILs, the amidation reaction for the synthesis of 3a in [OMIM][PF6], [OMIM][ClO4], [BMIM][BF4], and [BMIM][PF6] was repeated, and each reaction was stopped once the total conversion of the starting materials was achieved (Table 5, entries 1–4). Interestingly, as shown in Table 5, all four RTILs afforded PROTAC 3a in high yields (68–77%) in a short reaction time from 1 h to 2 h. Among them, [OMIM][ClO4] (Table 5, entry 1) furnished the target compound in the shortest reaction time (1 h), while a slightly longer reaction time (1.5–2 h) was required for the reactions in [OMIM][PF6], [BMIM][BF4] and [BMIM][PF6] (Table 5, entries 2–4).
| INM-based PROTACs | Coupling agent (1.25 eq.) | Base (3.0 eq.) | Solvent (0.056 M) | Time (h) | Conversionb (%) | Yield of 3ac (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.056 mmol, 1 equiv.), 2a (0.056 mmol, 1 equiv.), HATU (0.070 mmol, 1.25 equiv.), DIPEA (0.168 mmol, 3 equiv.), ILs (0.056 M), room temperature, 1–2 h. b As monitored by TLC with respect to consumption of both starting materials. c Isolated yield. | ||||||
| 1 | HATU | DIPEA | [OMIM][ClO4] | 1 | 100 | 75 |
| 2 | HATU | DIPEA | [OMIM][PF6] | 2 | 100 | 69 |
| 3 | HATU | DIPEA | [BMIM][BF4] | 1.5 | 100 | 68 |
| 4 | HATU | DIPEA | [BMIM][PF6] | 2 | 100 | 77 |
To deeply ascertain the reaction performance in RTILs [OMIM][PF6], [OMIM][ClO4], [BMIM][BF4], and [BMIM][PF6], each reaction was also analysed by HPLC at different time points (up to 120 minutes) to give a conversion curve, which could be directly compared across the solvent selection (Fig. 4). In particular, given the rapid solubilization of our PROTAC building blocks (1 and 2a) in [OMIM][ClO4], the amidation reaction in this medium proceeded very quickly exhibiting the fastest conversion rate. Notably, in the case of [BMIM][BF4] and [OMIM][PF6], we observed a sudden jump in the conversion rate only after 30 minutes and 1 hour, respectively, which is due to the delay necessary to achieve a complete solubilization of the starting materials in the selected reaction medium.
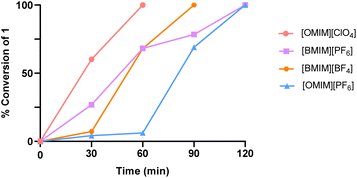 | ||
| Fig. 4 Representation of conversion data as determined by HPLC: amidation reaction for the synthesis of 3a using HATU with four different ILs. | ||
Among the screened ILs, [OMIM][ClO4] and [BMIM][PF6] were selected for further studies considering their favourable balance in terms of conversion rate and yield. In particular, we evaluated the impact of the addition of a small amount of water (1–2% (v/v)) to [OMIM][ClO4] and [BMIM][PF6] on the final yield and reaction time. As illustrated in Table 6, the addition of 1% of water had no effect on the conversion rates as well as on the yields of the reactions (Table 6, entries 2 and 5), compared to the ones conducted in the absence of water (Table 6, entries 1 and 4), although in the case of [OMIM][ClO4], an increased reaction time was required (Table 6, entry 2). Conversely, when 2% of water was added, both reactions (Table 6, entries 3 and 6) did not show complete conversion even after 24 h of reaction, thus affording lower yields.
| INM-based PROTACs | Coupling agent (1.25 eq.) | Base (3.0 eq.) | Solvent (0.056 M) | Time (h) | Conversion (%) | Yield of 3ad (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.056 mmol, 1 equiv.), 2a (0.056 mmol, 1 equiv.), HATU (0.070 mmol, 1.25 equiv.), DIPEA (0.168 mmol, 3 equiv.), IL (0.056 M) or IL with 1 or 2% H2O (0.056 M), room temperature, 1–24 h. b As monitored by TLC with respect to consumption of both starting materials. c As monitored by HPLC with respect to consumption of 1. d Isolated yield. | ||||||
| 1 | HATU | DIPEA | [OMIM][ClO4] | 1 | 100b | 75 |
| 2 | HATU | DIPEA | [OMIM][ClO4] + 1% H2O | 2 | 100b | 73 |
| 3 | HATU | DIPEA | [OMIM][ClO4] + 2% H2O | 24 | 90.08c | 55 |
| 4 | HATU | DIPEA | [BMIM][PF6] | 2 | 100b | 77 |
| 5 | HATU | DIPEA | [BMIM][PF6] + 1% H2O | 2 | 100b | 66 |
| 6 | HATU | DIPEA | [BMIM][PF6] + 2% H2O | 24 | 81.68c | 37 |
Successively, we evaluated the impact of concentrations on the performance of the amidation reaction in both [OMIM][ClO4] and [BMIM][PF6]. As shown in Table 7, the doubling or halving of the final concentration of both ILs did not affect the reaction time or the conversion rate (Table 7, for [OMIM][ClO4], compare entries 2 and 3 with entry 1, while for [BMIM][PF6], compare entries 5 and 6 with entry 4). However, while halving the final IL concentration to 0.028 M showed a reduction in terms of yields compared to a concentration of 0.056 M (Table 7, entries 3 and 6 vs. entries 1 and 4, respectively), the higher concentration of ILs at 0.112 M slightly improved the final reaction yields (Table 7, entries 2 and 5 vs. entries 1 and 4, respectively).
| INM-based PROTACs | Coupling agent (1.25 eq.) | Base (3.0 eq.) | Solvent | Time (h) | Conversionb (%) | Yield of 3ac (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.056 mmol, 1 equiv.), 2a (0.056 mmol, 1 equiv.), HATU (0.070 mmol, 1.25 equiv.), DIPEA (0.168 mmol, 3 equiv.), IL (0.112 M or 0.028 M), room temperature, 1–2 h. b As monitored by TLC with respect to consumption of both starting materials. c Isolated yield. | ||||||
| 1 | HATU | DIPEA | [OMIM][ClO4] | 1 | 100 | 75 |
| 0.056 M | ||||||
| 2 | HATU | DIPEA | [OMIM][ClO4] | 1 | 100 | 83 |
| 0.112 M | ||||||
| 3 | HATU | DIPEA | [OMIM][ClO4] | 1 | 100 | 61 |
| 0.028 M | ||||||
| 4 | HATU | DIPEA | [BMIM][PF6] | 2 | 100 | 77 |
| 0.056 M | ||||||
| 5 | HATU | DIPEA | [BMIM][PF6] | 2 | 100 | 81 |
| 0.112 M | ||||||
| 6 | HATU | DIPEA | [BMIM][PF6] | 2 | 100 | 73 |
| 0.028 M | ||||||
Although both [OMIM][ClO4] and [BMIM][PF6] emerged as good alternatives to DMF in the HATU-mediated amidation for the synthesis of 3a with improved isolated final yields, we decided to select [OMIM][ClO4] for further investigations due to its superior performance, lower density (1.07 ± 0.02 g cm−3) than other conventional ILs (Table 4), excellent solvency power and also considering that the presence of fluorine atoms in the anion with respect to [BMIM][PF6] can decompose and form toxic volatiles such as HF and phosphorus oxyfluoride.
Next, the optimized reaction conditions identified so far for the synthesis of PROTAC 3a (1.0 equiv. of 1, 1.0 equiv. of 2a, 1.25 equiv. of HATU, 3.0 equiv. of DIPEA, 0.056 M [OMIM][ClO4], and room temperature) were applied to explore the scalability of the synthetic approach. In particular, by employing the same reagent ratio, the reaction was performed on a 0.28 mmol scale affording PROTAC 3a in high isolated yield (68%) with only a slight increase in the reaction time (2.5 h) compared to a smaller scale (1 h, Table 5, entry 1).
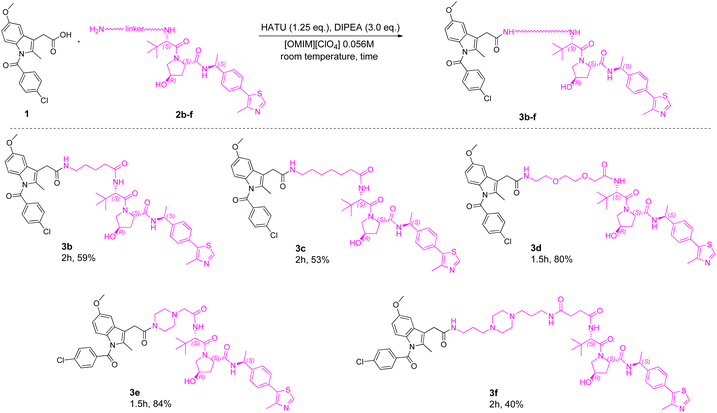
Thus, with the established optimal reaction conditions, the substrate scope of the reaction was successively explored. In particular, different intermediates bearing ligands for two E3 ligases (VHL and Cereblon) functionalized with linkers of different lengths and compositions were used as amine derivatives (Schemes 1 and 2) in the amidation coupling with INM. Moreover, different POI ligands (already exploited in the literature for PROTAC synthesis) were used as acid derivatives in the coupling with intermediate 2a (Scheme 3).
Initially, 1 was coupled with a set of different VHL-linker intermediates (2b–f) to afford PROTACs 3b–f (Scheme 1), previously synthesized with the DMF-based protocol21,22 (Table 1). Overall, [OMIM][ClO4] was confirmed to be a good solvent in amidation coupling, affording target PROTACs 3b–f in good to excellent yields (40–84%) and short reaction times (1.5–2 h). Additionally, compared with the yields obtained with DMF as the solvent, (Table 1), in all cases, the use of [OMIM][ClO4] gave higher isolated yields.
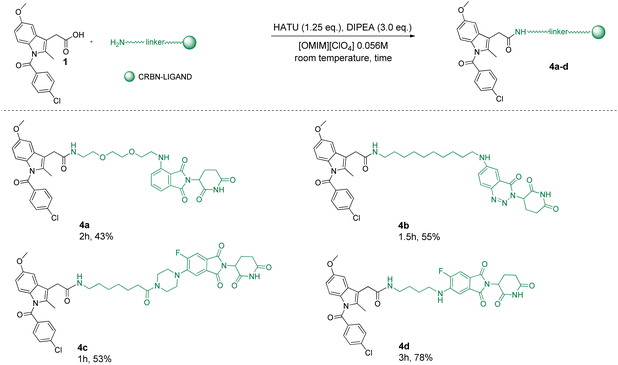
Then, to further expand the substrate chemical space, we decided to include Cereblon (CRBN) E3 ligase ligands, which together with VHL represent the most exploited E3 ligase in the synthesis of PROTACs. In particular, 1 was coupled with various CRBN binders functionalized with linkers of different lengths and compositions leading to new PROTACs 4a–4d (Scheme 2). Also in this case, all PROTACs were obtained in short reaction times (1–3 h) with high isolated yields, all above 50% (4b–4d) except for 4a (43%). Notably, despite the observed 100% conversion rate, the isolated yields for these PROTACs were low. This discrepancy can be ascribed to the known hydrolytic degradation at the glutarimide and/or phthalimide rings of the thalidomide moiety that can occur not during the reaction itself but during the reaction work-up in aqueous media.19,70–72 Nevertheless, it is important to note that, for example, compound 4d was obtained in a significantly higher yield (78%) compared to the yield of the same reaction but with DMF as the reaction medium (38%).73 Therefore, in this specific case, with a reference compound, we can confidently state that the use of [OMIM][ClO4] as the reaction medium led to successful optimization.
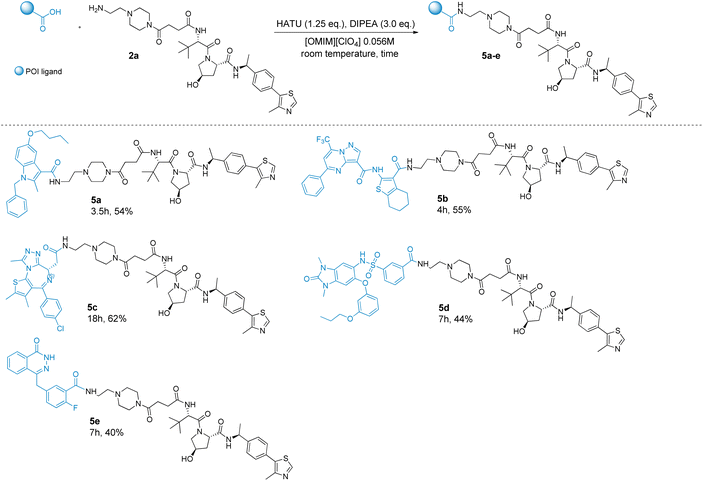
Finally, as shown in Scheme 3, by maintaining the VHL-linker intermediate 2a, different POI ligands than indomethacin 1 have been exploited leading to PROTACs 5a–e. In particular, we decided to expand the POI ligand chemical space including common ligands used for PROTACs synthesis such as JQ1 (bromo-and-extra-terminal (BET) inhibitor),74 olaparib (PARP1/2 inhibitor)75 and IACs-7e (TRIM24 inhibitor).76 Moreover, the 5,6-dihydro-4H-cyclopenta[b]thiophen-pyrazolo[1,5-a]pyrimidine-3-carboxylic acid derivative (anti-influenza compound)77 and 1-benzyl-5-butoxy-2-methyl-1H-indole-3-carboxylic acid derivative (efflux pump inhibitor)78 were also included for investigation purposes. For all the synthesized PROTACs 5a–5e, we observed complete conversion, which occurred within a short reaction time for 5a and 5b (3.5–4 h). A longer reaction time was instead required in the case of 5c–5e (7–18 h), due to the initial lower solubility of the selected POI ligands in the reaction medium (Scheme 3). Overall, while the yields for 5d and 5e were slightly lower (44% and 40%, respectively), compounds 5a–5c were obtained in good to high yields (54–62%). Notably, although the reactions for the synthesis of PROTACs 5d and 5e proceeded with the complete conversion of the starting materials, the final isolated yield was low, due to the formation of traces of more than one side product/degradation product, which made the purification step more complicated and trickier, in turn affecting the rate of the isolated yield.
Overall, the results obtained herein confirmed that [OMIM][ClO4], in addition to the general advantages related to the use of ILs, can be considered a suitable replacement for the classic DMF as the medium for amidation reactions during PROTACs synthesis.
Indeed, being characterized by very low density and high solvency power, [OMIM][ClO4] permitted us to achieve generally high isolated yields through a complete conversion rate, a short reaction time, and mild reaction conditions.
Conclusions
In this work, we have evaluated several coupling agents and solvents in the amidation reaction for PROTACs synthesis. Among the coupling agents screened, HATU was selected for further evaluations in several solvents. In particular, we tried to replace the environmentally undesirable organic solvent commonly used in this reaction step, namely DMF, with potential greener alternatives. We found that ILs generally proved to be suitable replacements for DMF when compared to other organic solvents. Indeed, in most cases, the use of RTILs as solvents in the amidation coupling for the synthesis of our case study PROTAC 3a resulted in an impressive improvement in isolated yields (up to 75–77%) with complete conversion (100%), mild reaction conditions (anhydrous conditions not required, room temperature), and short reaction time (up to 2 h). Among the ILs screened, [OMIM][ClO4] was selected for further studies because of the favourable balance between results (times and yields) and its good physicochemical properties.A scale-up experiment and the exploration of the reaction scope for PROTACs synthesis further confirmed that the employment of HATU/DIPEA in [OMIM][ClO4] was a reliable protocol to obtain variously functionalized PROTACs in good to high isolated yields and short reaction times. Moreover, to the best of our knowledge, there have been no reports on the use of ILs for the synthesis of PROTACs. Given the promising results obtained in the present study, successive exploration will be focused on the possible application of ionic liquids as solvents in further types of reactions commonly used for PROTACs synthesis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Ministero dell'Università e della Ricerca (MIUR), Italy, with PRIN 2022 – cod. 20223RYYFC (to G. Cruciani and L. G.), Project “ZODIAC” (to J. D.), and Molecular Horizon (Italy) within the PRO-CURA project (to L. G.).References
- K. Li and C. M. Crews, PROTACs: past, present and future, Chem. Soc. Rev., 2022, 51, 5214–5236 RSC.
- M. Bekes, D. R. Langley and C. M. Crews, PROTAC targeted protein degraders: the past is prologue, Nat. Rev. Drug Discovery, 2022, 21, 181–200 CrossRef CAS PubMed.
- K. M. Sakamoto, K. B. Kim, A. Kumagai, F. Mercurio, C. M. Crews and R. J. Deshaies, Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8554–8559 CrossRef CAS PubMed.
- X. Sun, H. Gao, Y. Yang, M. He, Y. Wu, Y. Song, Y. Tong and Y. Rao, PROTACs: great opportunities for academia and industry, Signal Transduction Targeted Ther., 2019, 4, 64 CrossRef PubMed.
- N. A. Zografou-Barredo, A. J. Hallatt, J. Goujon-Ricci and C. Cano, A beginner's guide to current synthetic linker strategies towards VHL-recruiting PROTACs, Bioorg. Med. Chem., 2023, 88–89, 117334 CrossRef CAS PubMed.
- R. I. Troup, C. Fallan and M. G. J. Baud, Current strategies for the design of PROTAC linkers: a critical review, Explor. Targeted Anti-Tumor Ther., 2020, 1, 273–312 Search PubMed.
- R. P. Bhole, P. R. Kute, R. V. Chikhale, C. G. Bonde, A. Pant and S. S. Gurav, Unlocking the potential of PROTACs: A comprehensive review of protein degradation strategies in disease therapy, Bioorg. Chem., 2023, 139, 106720 CrossRef CAS PubMed.
- N. I. Sincere, K. Anand, S. Ashique, J. Yang and C. You, PROTACs: Emerging Targeted Protein Degradation Approaches for Advanced Druggable Strategies, Molecules, 2023, 28, 4014 CrossRef CAS PubMed.
- X. Liu and A. Ciulli, Proximity-Based Modalities for Biology and Medicine, ACS Cent. Sci., 2023, 9, 1269–1284 CrossRef CAS PubMed.
- D. Chirnomas, K. R. Hornberger and C. M. Crews, Protein degraders enter the clinic - a new approach to cancer therapy, Nat. Rev. Clin Oncol., 2023, 20, 265–278 CrossRef CAS PubMed.
- Z. Liu, M. Hu, Y. Yang, C. Du, H. Zhou, C. Liu, Y. Chen, L. Fan, H. Ma, Y. Gong and Y. Xie, An overview of PROTACs: a promising drug discovery paradigm, Mol. Biomed., 2022, 3, 46 CrossRef CAS PubMed.
- J. Desantis and L. Goracci, Proteolysis targeting chimeras in antiviral research, Future Med. Chem., 2022, 14, 459–462 CrossRef CAS PubMed.
- R. M. Espinoza-Chavez, A. Salerno, A. Liuzzi, A. Ilari, A. Milelli, E. Uliassi and M. L. Bolognesi, Targeted Protein Degradation for Infectious Diseases: from Basic Biology to Drug Discovery, ACS Bio Med Chem Au, 2023, 3, 32–45 CrossRef CAS PubMed.
- C. Cao, M. He, L. Wang, Y. He and Y. Rao, Chemistries of bifunctional PROTAC degraders, Chem. Soc. Rev., 2022, 51, 7066–7114 RSC.
- A. Bricelj, C. Steinebach, R. Kuchta, M. Gutschow and I. Sosic, E3 Ligase Ligands in Successful PROTACs: An Overview of Syntheses and Linker Attachment Points, Front. Chem., 2021, 9, 707317 CrossRef CAS PubMed.
- K. C. Carmony and K. B. Kim, PROTAC-induced proteolytic targeting, Methods Mol. Biol., 2012, 832, 627–638 CrossRef CAS PubMed.
- G. Weng, X. Cai, D. Cao, H. Du, C. Shen, Y. Deng, Q. He, B. Yang, D. Li and T. Hou, PROTAC-DB 2.0: an updated database of PROTACs, Nucleic Acids Res., 2023, 51, D1367–D1372 CrossRef PubMed.
- O. Bakulina, A. Sapegin, A. S. Bunev and M. Krasavin, Synthetic approaches to constructing proteolysis targeting chimeras (PROTACs), Mendeleev Commun., 2022, 32, 419–432 CrossRef CAS.
- L. Goracci, J. Desantis, A. Valeri, B. Castellani, M. Eleuteri and G. Cruciani, Understanding the Metabolism of Proteolysis Targeting Chimeras (PROTACs): The Next Step toward Pharmaceutical Applications, J. Med. Chem., 2020, 63, 11615–11638 CrossRef CAS PubMed.
- B. Castellani, M. Eleuteri, S. Di Bona, G. Cruciani, J. Desantis and L. Goracci, VHL-Modified PROteolysis TArgeting Chimeras (PROTACs) as a Strategy to Evade Metabolic Degradation in In Vitro Applications, J. Med. Chem., 2023, 66, 13148–13171 CrossRef CAS PubMed.
- J. Desantis, B. Mercorelli, M. Celegato, F. Croci, A. Bazzacco, M. Baroni, L. Siragusa, G. Cruciani, A. Loregian and L. Goracci, Indomethacin-based PROTACs as pan-coronavirus antiviral agents, Eur. J. Med. Chem., 2021, 226, 113814 CrossRef CAS PubMed.
- J. Desantis, A. Mammoli, M. Eleuteri, A. Coletti, F. Croci, A. Macchiarulo and L. Goracci, PROTACs bearing piperazine-containing linkers: what effect on their protonation state?, RSC Adv., 2022, 12, 21968–21977 RSC.
- A. El-Faham and F. Albericio, Peptide coupling reagents, more than a letter soup, Chem. Rev., 2011, 111, 6557–6602 CrossRef CAS PubMed.
- E. Valeur and M. Bradley, Amide bond formation: beyond the myth of coupling reagents, Chem. Soc. Rev., 2009, 38, 606–631 RSC.
- J. R. Dunetz, J. Magano and G. A. Weisenburger, Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals, Org. Process Res. Dev., 2016, 20, 140–177 CrossRef CAS.
- I. Abdelmoty, F. Albericio, L. A. Carpino, B. M. Foxman and S. A. Kates, Structural studies of reagents for peptide bond formation: Crystal and molecular structures of HBTU and HATU, Lett. Pept. Sci., 1994, 1, 57–67 CrossRef CAS.
- A. El-Faham, R. Subiros Funosas, R. Prohens and F. Albericio, COMU: a safer and more effective replacement for benzotriazole-based uronium coupling reagents, Chemistry, 2009, 15, 9404–9416 CrossRef CAS PubMed.
- A. El-Faham and F. Albericio, Morpholine-based immonium and halogenoamidinium salts as coupling reagents in Peptide synthesis1, J. Org. Chem., 2008, 73, 2731–2737 CrossRef CAS PubMed.
- R. Subiros-Funosas, R. Prohens, R. Barbas, A. El-Faham and F. Albericio, Oxyma: an efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion, Chemistry, 2009, 15, 9394–9403 CrossRef CAS PubMed.
- C. K. Z. Andrade, R. O. Rocha, O. E. Vercillo, W. A. Silva and R. A. F. Matos, DCC/DMAP-mediated coupling of carboxylic acids with oxazolidinones and thiazolidinethiones, Synlett, 2003, 2351–2352 CrossRef CAS.
- A. K. Ghosh and D. Shahabi, Synthesis of amide derivatives for electron deficient amines and functionalized carboxylic acids using EDC and DMAP and a catalytic amount of HOBt as the coupling reagents, Tetrahedron Lett., 2021, 63, 152719 CrossRef CAS PubMed.
- K. A. Mahmoud, Y. T. Long, G. Schatte and H. B. Kraatz, Rearrangement of the active ester intermediate during HOBt/EDC amide coupling, Eur. J. Inorg. Chem., 2005, 2005, 173–180 CrossRef.
- J. Coste, D. Lenguyen and B. Castro, PyBOP®: A new peptide coupling reagent devoid of toxic by-product, Tetrahedron Lett., 1990, 31, 205–208 CrossRef CAS.
- L. A. Carpino, 1-Hydroxy-7-Azabenzotriazole - an Efficient Peptide Coupling Additive, J. Am. Chem. Soc., 1993, 115, 4397–4398 CrossRef CAS.
- M. M. Joullié and K. M. Lassen, Evolution of amide bond formation, ARKIVOC, 2010, 2010, 189–250 Search PubMed.
- R. M. Moshikur, M. R. Chowdhury, M. Moniruzzaman and M. Goto, Biocompatible ionic liquids and their applications in pharmaceutics, Green Chem., 2020, 22, 8116–8139 RSC.
- Z. G. Lei, B. H. Chen, Y. M. Koo and D. R. MacFarlane, Introduction: Ionic Liquids, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Tools and techniques for solvent selection: green solvent selection guides, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- D. Prat, J. Hayler and A. Wells, A survey of solvent selection guides, Green Chem., 2014, 16, 4546–4551 RSC.
- R. Stuart, Lessons Learned from a Short-Term Exposure to DMF, ACS Chem. Health Saf., 2023, 30, 44–48 CrossRef CAS.
- N. Galy, M. R. Mazières and J. C. Plaquevent, Toward waste-free peptide synthesis using ionic reagents and ionic liquids as solvents, Tetrahedron Lett., 2013, 54, 2703–2705 CrossRef CAS.
- S. M. Baghbanian and M. Farhang, Protic [TBD][TFA] ionic liquid as a reusable and highly efficient catalyst for N-formylation of amines using formic acid under solvent-free condition, J. Mol. Liq., 2013, 183, 45–49 CrossRef CAS.
- A. A. Tietze, P. Heimer, A. Stark and D. Imhof, Ionic Liquid Applications in Peptide Chemistry: Synthesis, Purification and Analytical Characterization Processes, Molecules, 2012, 17, 4158–4185 CrossRef CAS PubMed.
- S. Majumdar, J. De, J. Hossain and A. Basak, Formylation of amines catalysed by protic ionic liquids under solvent-free condition, Tetrahedron Lett., 2013, 54, 262–266 CrossRef CAS.
- M. Konwar, N. D. Khupse, P. J. Saikia and D. Sarma, A potential greener protocol for peptide coupling reactions using recyclable/reusable ionic liquid [C4-DABCO][N(CN)2], J. Chem. Sci., 2018, 130, 1–8 CrossRef CAS.
- R. M. N. Kalla, J. Lim, J. Bae and I. Kim, Sulfated choline ionic liquid-catalyzed acetamide synthesis by grindstone method, Tetrahedron Lett., 2017, 58, 1595–1599 CrossRef CAS.
- L. Zhang, J. Jiang, L. Li, Q. Chen, L. Zhang, H. Sun and C. Li, Sustainable Synthesis of Amides from Carboxylic Acids and Equivalent Amounts of Amines Using a Reusable Brønsted Acidic Ionic Liquid as a Catalyst and a Solvent, ACS Sustainable Chem. Eng., 2022, 10, 8433–8442 CrossRef CAS.
- P. Petiot, C. Charnay, J. Martinez, L. Puttergill, F. Galindo, F. Lamatya and E. Colacino, Synthesis of a new hydrophilic poly(ethylene glycol)-ionic liquid and its application in peptide synthesis, Chem. Commun., 2010, 46, 8842–8844 RSC.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- S. N. Pedro, R. F. Cs, A. J. D. Silvestre and M. G. Freire, The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications, Int. J. Mol. Sci., 2020, 21, 8298 CrossRef CAS PubMed.
- S. Goindi, R. Kaur and R. Kaur, An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex vivo and in vivo evaluation, Int. J. Pharm., 2015, 495, 913–923 CrossRef CAS PubMed.
- V. Kumar and S. V. Malhotra, Ionic liquid mediated synthesis of 5-halouracil nucleosides: key precursors for potential antiviral drugs, Nucleosides, Nucleotides Nucleic Acids, 2009, 28, 821–834 CrossRef CAS PubMed.
- S. V. M. Vineet Kumar, Synthesis of nucleoside-based antiviral drugs in ionic liquids, Bioorg. Med. Chem. Lett., 2008, 18, 5640–5642 CrossRef PubMed.
- I. V. Kapitanov, A. Jordan, Y. Karpichev, M. Spulak, L. Perez, A. Kellett, K. Kümmerer and N. Gathergood, Synthesis, self-assembly, bacterial and fungal toxicity, and preliminary biodegradation studies of a series of L-phenylalanine-derived surface-active ionic liquids, Green Chem., 2019, 21, 1777–1794 RSC.
- N. Rezki, S. A. Al-Sodies, H. E. A. Ahmed, S. Ihmaid, M. Messali, S. Ahmed and M. R. Aouad, A novel dicationic ionic liquids encompassing pyridinium hydrazone-phenoxy conjugates as antimicrobial agents targeting diverse high resistant microbial strains, J. Mol. Liq., 2019, 284, 431–444 CrossRef CAS.
- S. Y. Choi, H. Rodríguez, A. Mirjafari, D. F. Gilpin, S. McGrath, K. R. Malcolm, M. M. Tunney, R. D. Rogers and T. McNally, Dual functional ionic liquids as plasticisers and antimicrobial agents for medical polymers, Green Chem., 2011, 13, 1527–1535 RSC.
- M. R. Chowdhury, R. M. Moshikur, R. Wakabayashi, Y. Tahara, N. Kamiya, M. Moniruzzaman and M. Goto, Ionic-Liquid-Based Paclitaxel Preparation: A New Potential Formulation for Cancer Treatment, Mol. Pharm., 2018, 15, 2484–2488 CrossRef CAS PubMed.
- J. Tang, H. Song, X. Feng, A. Yohannes and S. Yao, Ionic Liquid-Like Pharmaceutical Ingredients and Applications of Ionic Liquids in Medicinal Chemistry: Development, Status and Prospects, Curr. Med. Chem., 2019, 26, 5947–5967 CrossRef CAS PubMed.
- D. Landini and A. Maia, Anion nucleophilicity in ionic liquids: a comparison with traditional molecular solvents of different polarity, Tetrahedron Lett., 2005, 46, 3961–3963 CrossRef CAS.
- M. Schmeisser, P. Keil, J. Stierstorfer, A. König, T. M. Klapötke and R. van Eldik, An Ionic Liquid Designed for Coordination Chemistry Revisited: Synthetic Routes and Safety Tests for 1-Ethyl-3-methylimidazolium Perchlorate ([emim][ClO4]), Eur. J. Inorg. Chem., 2011, 2011, 4862–4868 CrossRef CAS.
- I. M. Saaid, S. Q. A. Mahat, B. Lal, M. I. Abd Mutalib and K. M. Sabil, Experimental Investigation on the Effectiveness of 1-Butyl-3-methylimidazolium Perchlorate Ionic Liquid as a Reducing Agent for Heavy Oil Upgrading, Ind. Eng. Chem. Res., 2014, 53, 8279–8284 CrossRef.
- X. D. Wang, W. Y. Wu, G. F. Tu and K. X. Jiang, Synthesis and physico-chemical properties of new green electrolyte 1-butyl-3-methylimidazolium perchlorate, Trans. Nonferrous Met. Soc. China, 2010, 20, 2032–2036 CrossRef CAS.
- F. Shirini, M. S. N. Langarudi, N. Daneshvar, N. Jamasbi and M. Irankhah-Khanghah, Preparation and characterization of [H-DABCO][ClO4]2 as a new member of DABCO-based ionic liquids for the synthesis of pyrimido [4,5-b]-quinoline and pyrimido[4,5-d]pyrimidine derivatives, J. Mol. Struct., 2018, 1161, 366–382 CrossRef CAS.
- P. Biswas, Y. J. Wang, E. Hagen and M. R. Zachariah, Electrochemical Modulation of the Flammability of Ionic Liquid Fuels, J. Am. Chem. Soc., 2023, 145, 16318–16323 CrossRef CAS PubMed.
- A. Yadav, A. Guha, A. Pandey, M. Pal, S. Trivedi and S. Pandey, Densities and dynamic viscosities of ionic liquids having 1-butyl-3-methylimidazolium cation with different anions and bis(trifluoromethylsulfonyl)imide anion with different cations in the temperature range (283.15 to 363.15) K, J. Chem. Thermodyn., 2018, 116, 67–75 CrossRef CAS.
- A. Ahosseini and A. M. Scurto, Viscosity of imidazolium-based ionic liquids at elevated pressures: Cation and anion effects, Int. J. Thermophys., 2008, 29, 1222–1243 CrossRef CAS.
- P. Barthen, W. Frank and N. Ignatiev, Development of low viscous ionic liquids: the dependence of the viscosity on the mass of the ions, Ionics, 2015, 21, 149–159 CrossRef CAS.
- D. Santos, M. Santos, E. Franceschi, C. Dariva, A. Barison and S. Mattedi, Experimental Density of Ionic Liquids and Thermodynamic Modeling with Group Contribution Equation of State Based on the Lattice Fluid Theory, J. Chem. Eng. Data, 2016, 61, 348–353 CrossRef CAS.
- J. O. Valderrama and R. A. Campusano, Melting properties of molten salts and ionic liquids. Chemical homology, correlation, and prediction, C. R. Chim., 2016, 19, 654–664 CrossRef CAS.
- H. Schumacher, R. L. Smith and R. T. Williams, The metabolism of thalidomide: the fate of thalidomide and some of its hydrolysis products in various species, Br. J. Pharmacol. Chemother., 1965, 25, 338–351 CrossRef CAS PubMed.
- H. Schumacher, R. L. Smith and R. T. Williams, The metabolism of thalidomide: the spontaneous hydrolysis of thalidomide in solution, Br. J. Pharmacol. Chemother., 1965, 25, 324–337 CrossRef CAS PubMed.
- J. Min, A. Mayasundari, F. Keramatnia, B. Jonchere, S. W. Yang, J. Jarusiewicz, M. Actis, S. Das, B. Young, J. Slavish, L. Yang, Y. Li, X. Fu, S. H. Garrett, M. K. Yun, Z. Li, S. Nithianantham, S. Chai, T. Chen, A. Shelat, R. E. Lee, G. Nishiguchi, S. W. White, M. F. Roussel, P. R. Potts, M. Fischer and Z. Rankovic, Phenyl-Glutarimides: Alternative Cereblon Binders for the Design of PROTACs, Angew. Chem., Int. Ed., 2021, 60, 26663–26670 CrossRef CAS PubMed.
- J. Desantis, A. Bazzacco, M. Eleuteri, S. Tuci, E. Bianconi, A. Macchiarulo, B. Mercorelli, A. Loregian and L. Goracci, Design, synthesis, and biological evaluation of first-in-class indomethacin-based PROTACs degrading SARS-CoV-2 main protease and with broad-spectrum antiviral activity, Eur. J. Med. Chem., 2024, 268, 116202 CrossRef CAS PubMed.
- P. Filippakopoulos, J. Qi, S. Picaud, Y. Shen, W. B. Smith, O. Fedorov, E. M. Morse, T. Keates, T. T. Hickman, I. Felletar, M. Philpott, S. Munro, M. R. McKeown, Y. Wang, A. L. Christie, N. West, M. J. Cameron, B. Schwartz, T. D. Heightman, N. La Thangue, C. A. French, O. Wiest, A. L. Kung, S. Knapp and J. E. Bradner, Selective inhibition of BET bromodomains, Nature, 2010, 468, 1067–1073 CrossRef CAS PubMed.
- C. C. Gunderson and K. N. Moore, Olaparib: an oral PARP-1 and PARP-2 inhibitor with promising activity in ovarian cancer, Future Oncol., 2015, 11, 747–757 CrossRef CAS PubMed.
- J. Bennett, O. Fedorov, C. Tallant, O. Monteiro, J. Meier, V. Gamble, P. Savitsky, G. A. Nunez-Alonso, B. Haendler, C. Rogers, P. E. Brennan, S. Muller and S. Knapp, Discovery of a Chemical Tool Inhibitor Targeting the Bromodomains of TRIM24 and BRPF, J. Med. Chem., 2016, 59, 1642–1647 CrossRef CAS PubMed.
- S. Lepri, G. Nannetti, G. Muratore, G. Cruciani, R. Ruzziconi, B. Mercorelli, G. Palu, A. Loregian and L. Goracci, Optimization of small-molecule inhibitors of influenza virus polymerase: from thiophene-3-carboxamide to polyamido scaffolds, J. Med. Chem., 2014, 57, 4337–4350 CrossRef CAS PubMed.
- F. Buonerba, S. Lepri, L. Goracci, B. D. Schindler, S. M. Seo, G. W. Kaatz and G. Cruciani, Improved Potency of Indole-Based NorA Efflux Pump Inhibitors: From Serendipity toward Rational Design and Development, J. Med. Chem., 2017, 60, 517–523 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental and characterization data, including 1H NMR, 13C NMR and LC-HRMS spectra for all synthesized compounds. See DOI: https://doi.org/10.1039/d4ob00304g |
| This journal is © The Royal Society of Chemistry 2024 |