Open Access Article
Open Access ArticleExpanding the scope of the successive ring expansion strategy for macrocycle and medium-sized ring synthesis: unreactive and reactive lactams†
Zhongzhen
Yang
 ab,
Marion
Arnoux
c,
Damien
Hazelard
ab,
Marion
Arnoux
c,
Damien
Hazelard
 c,
Owen R.
Hughes
c,
Owen R.
Hughes
 ad,
Joe
Nabarro
ad,
Adrian C.
Whitwood
ad,
Joe
Nabarro
ad,
Adrian C.
Whitwood
 a,
Martin A.
Fascione
a,
Martin A.
Fascione
 ad,
Christopher D.
Spicer
ad,
Christopher D.
Spicer
 ad,
Philippe
Compain
ad,
Philippe
Compain
 *c and
William P.
Unsworth
*c and
William P.
Unsworth
 *a
*a
aDepartment of Chemistry, University of York, Heslington, York YO10 5DD, UK. E-mail: william.unsworth@york.ac.uk
bDepartment of Pharmacy, West China Hospital, Sichuan University, Chengdu 610041, China
cLaboratoire d'Innovation Moléculaire et Applications (LIMA), Univ. de Strasbourg, Univ. de Haute-Alsace, CNRS (UMR 7042), Equipe de Synthèse Organique et Molécules Bioactives (SYBIO), ECPM, 25 Rue Becquerel, 67000 Strasbourg, France. E-mail: philippe.compain@unistra.fr
dYork Biomedical Research Institute, University of York, Heslington, YO10 5DD, UK
First published on 20th March 2024
Abstract
New methods are described that expand the scope of the Successive Ring Expansion (SuRE) with respect to synthetically challenging lactams. A protocol has been developed for use with ‘unreactive’ lactams, enabling SuRE reactions to be performed on subsrates that fail under previously established conditions. Ring expansion is also demonstarted on ‘reactive’ lactams derived from iminosugars for the first time. The new SuRE methods were used to prepare a diverse array of medium-sized and macrocyclic lactams and lactones, which were evaluted in an anti-bacterial assay against E. coli BW25113WT.
Introduction
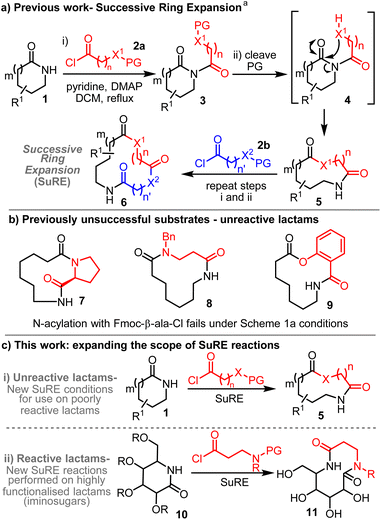
Macrocycles (12 + membered rings) and medium-sized rings (8–11-membered) are important ring systems in medicinal chemistry, with many drugs based on these large-ring frameworks on the market.1 New methods to synthesise large rings are therefore of value, especially those that can be performed on scale and avoid the problems typically associated with end-to-end macrocyclisation reactions,2,3 most notably competing intermolecular coupling. Ring expansion reactions4,5 are important in this regard, as they allow macrocycles and medium-sized rings to be prepared without the need to perform a discrete end-to-end macrocyclisation step; in well-designed cases, large ring products can be obtained without resorting to high-dilution conditions, via rearrangements that operate solely via ‘normal-sized ring’ (5–7-membered) cyclisation reactions.4eA major focus of our group in recent years has been the development of side-chain insertion type ring expansion reactions,4a for the synthesis of medium-sized and macrocyclic lactams,6 lactones,7 thiolactones,8 sulfonamides9 and phosphonate esters.10 Within this programme, the development of ‘Successive Ring Expansion’ (SuRE) methods has been a major driver. The general SuRE strategy is summarised in Scheme 1a, which depicts a method for the ring expansion of lactams 1, via N-acylation (1 → 3, X can be NR, O or S), protecting group cleavage (3 → 4) and ring expansion (4 → 5).11 A key design principle in SuRE is that the ring expanded product (in this case 5) can itself be expanded again via another iteration of the same sequence; thus, the reaction of 5 with acid chloride 2b in the same way can enable successive ring expansion to form lactam 6.
 | ||
Scheme 1 Expanding the scope of the Successive Ring Expansion strategy for macrocycle and medium-sized ring synthesis. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X = NR, O or S; PG = Fmoc, CBz, Bn or Fm; n = 1–2, m = various ring sizes. X = NR, O or S; PG = Fmoc, CBz, Bn or Fm; n = 1–2, m = various ring sizes. | ||
A range of lactam derivatives have been demonstrated to undergo SuRE ring expansions of the type summarised in Scheme 1a. Nonetheless, the requirement to N-acylate a lactam – a comparatively weak nitrogen nucleophile – can be challenging. Our published N-acylation conditions involve heating the lactam and acyl chloride with pyridine and DMAP in refluxing DCM. In some cases, N-acylation using this method fails; for example, lactams 7–9 (prepared using published SuRE reactions)6 all failed to undergo N-acylation with Fmoc-β-Ala-Cl under the standard conditions.
The discovery of alternative conditions that enable N-acylation of unreactive lactam starting materials therefore would represent an important extension to the SuRE method. The successful development of such conditions for use with ‘unreactive’ lactams is reported herein, with the application of the new conditions in the synthesis of a range of macrocyclic lactam and lactone products described in Results and Discussion Section i. We were also keen to extend the scope of the SuRE method to more functionalised, ‘reactive’ lactams. This work – which focuses on the ring expansion of iminosugar derivatives of the type 10 – is described in Results and discussion section ii. Both series permitted the biological evaluation of a selection of ring expanded products for their potential to be used as anti-bacterial agents (Results and discussion section iii).
Results and discussion
(i) Unreactive lactams: alternative conditions for the N-acylation of poorly reactive lactams
The search for new lactam N-acylation conditions started by exploring the reaction of 12-membered ring lactam 9 with propionyl chloride 12. Lactam 9 was already shown not to react with Fmoc-β-Ala-Cl under the standard conditions (0% conversion),6 and similarly, none of the acylated product 13 was isolated when the same conditions were tested using propionyl chloride 12 (Scheme 2 Table, entry 1). Extensive optimisation of this N-acylation reaction was therefore undertaken. Variation of the reaction solvent, temperature, time, the base/nucleophilic catalyst and the addition of Lewis acidic additives was explored. Full details of these optimisation experiments can be found in the ESI (see ESI, Tables S1–4†) with selected results summarised in Scheme 2. This optimisation culminating in the discovery of the optimised conditions described in entry 8 of the Scheme 2 Table. These N-acylation conditions are radically different to those used in the established SuRE methodology; the temperature (80 °C) is higher, the solvent (benzene) and the base (N-methyl morpholine; NMM) have both been changed and catalytic CuO was also found to improve the reaction. We are unable to fully rationalise why all of these changes combine to make such a difference to the reaction conversion. However, in general we think that enabling the N-acylation rate to be increased without also promoting degradation of the sensitive acyl chloride moiety is important. The best conditions are presumably those that achieve the most favourable balance between these competing pathways. It is possible that ketene formation from the acid chloride may play some part in effecting the change in reactivity.12 However, the observation that optically active products were obtained in all cases in which enantiopure Fmoc-Pro-Cl was used (see later) suggests that if ketene-formation does play a role, it is not the sole reaction pathway.13 Pleasingly, lactams 7 and 8 – another two lactams that react poorly under the standard N-acylation conditions – reacted well using the same method, to afford imides 14 and 15 each in good yield.Attention then turned to testing the new N-acylation conditions using three representative acid chloride types that typically work well in SuRE reactions (16–18, a β-amino, α-amino and β-phenolic acid chloride) and six lactams (7–9, 29, 36, 43) that are known to react poorly using the standard conditions (Scheme 3). These six lactams were themselves prepared using published SuRE reactions,6 with the fragment inserted in the first SuRE reaction indicated in red. This series of reactions was therefore focused on the more challenging second SuRE reaction, with the linear fragment inserted indicated in blue.
First, lactam 7 was reacted with Fmoc-Pro-Cl 16 and N-acylation proceeded smoothly, with imide 19 being isolated in 60% yield. Subsequent reaction with DBU then promoted Fmoc cleavage and ring expansion to afford 14-membered ring bis-proline adduct 20. Notably, product 20 was found to have a non-zero optical rotation, confirming that racemisation of the proline stereogenic centre did not take place under the reaction conditions (although this does not necessarily rule out partial epimerisation).13 Similarly, 12-membered lactam 8 was acylated successfully with Fmoc-Pro-Cl 16, and the same lactam was also acylated with Fmoc protected β-alanine derived acid chloride 17 and benzyl protected phenolic acid chloride 18, to afford imides 21, 23 and 25 respectively. Each imide then went on to undergo protecting group cleavage and ring expansion, upon reaction with DBU (for Fmoc derivatives 21 and 23) or hydrogenolysis (for OBn derivative 25), to form ring expanded products 22, 24 and 26 in good overall yields. Four more lactams (9, 29, 36, 43) were treated in the same way with some/all of acid chlorides 16–18, to form a range of macrocyclic lactam and lactone products (28, 31, 33, 35, 38, 40, 42 and 45). The expected ring expansion products were obtained in all cases tested, with the exception of lactone 47; in this case, the N-acylation proceeded as expected to form imide 46, but failed at the hydrogenolysis step.
Thus, the new N-acylation conditions were shown to work well across a range of lactams that perform poorly using the published method. It is important to note that the published conditions still work well for many lactam substrates and certainly should not be discounted.6 Nonetheless, in cases in which N-acylation is found to be sluggish, the new conditions represent a very useful alternative.
(ii) Reactive lactams: ring expansion of iminosugar lactams
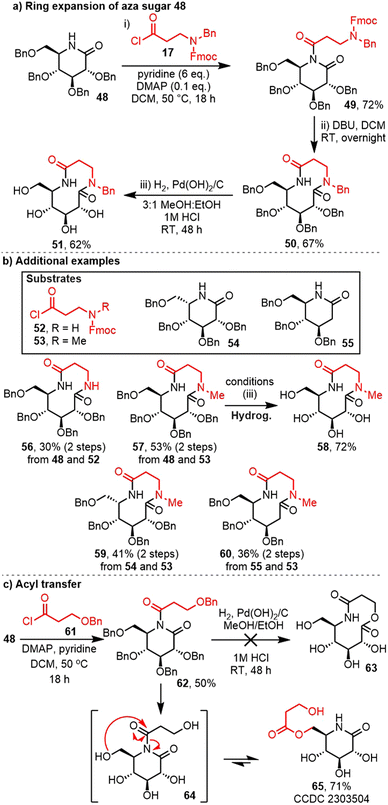
Having established new SuRE conditions compatible with poorly reactive lactams, attention then turned to demonstrating that SuRE reactions can also be performed on more reactive, and more functionalised lactams. This was done using sugar-derived lactam starting materials. The high degree of oxygenation and steric crowding in lactams like 48 was expected to provide a significant synthetic challenge to our established SuRE protocols. Of further interest, nitrogen-containing sugars and carbohydrate mimetics are well known for their useful biological properties, for example as potent inhibitors of glycosidases and glycosyltransferases.14,15aWe started by examining glucose-derived lactam 48 (Scheme 4a).15 In this case, the lactam was sufficiently reactive to undergo N-acylation with acid chloride 17 using our standard, published SuRE conditions, with pyridine and DMAP in refluxing DCM. The resulting imide 49 was then treated with DBU in DCM at RT, which duly promoted Fmoc cleavage and ring expansion as expected, to afford 10-membered bislactam 50 in 67% yield. Cleavage of the benzyl ether protecting groups was also achieved, by hydrogenolysis under acidic conditions, without cleaving the N–Bn bond, to afford 10-membered ring cyclic tetrol 51.
The same protocol was also used for the ring expansion of lactam 48 with alternative β-amino acid chlorides 52 and 53, resulting in the formation of ring expanded bislactams 56 and 57 (Scheme 4b). Debenzylation of lactam 57 was also achieved, using the same hydrogenolysis method as before. Alternative sugar-derived lactam starting materials 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 and 55
16 and 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 are also compatible with the SuRE protocol, affording bislactams 59 and 60, respectively.
17 are also compatible with the SuRE protocol, affording bislactams 59 and 60, respectively.
The challenge of working with these highly functionalised substrates was highlighted during the attempted ring expansion of lactam 48 using OBn-containing acid chloride 61 (Scheme 4c). In this case, N-acylation went relatively smoothly, with imide 62 isolated in 50% yield. Next, the hope then was that hydrogenolysis would promote concomitant debenzylation of all five protected alcohols and enable ring expansion in situ to form lactone 63. However, while cleavage of the five benzyl groups was successful, an alternative acyl transfer reactions took place instead, with migration of the β-hydroxy acid fragment on the primary alcohol (i.e.64 → 65) taking place. Given the known difficulty of ‘normal-to-medium’ sized ring expansion,4e it is likely that this alternative rearrangement is simply a lower energy, more kinetically favourable pathway. The structure of the product 65 was confirmed by X-ray crystallography.18
Thus, the efficacy of SuRE reactions on functionalised sugar-derived lactams has been confirmed. A small series of 10-membered ring bislactams have been assembled, via the overall insertion of a β-amino acid fragment into a 6-membered ring iminosugar. This is potentially important, given the known efficacy of related iminosugar derivatives to inhibit glycosidases and glycosyltransferases, as noted above.14,15a Furthermore, analogy can be made between these products and biologically important macrocycles; for example, related compounds are known to have human renin inhibitor activity,19 can be used for the immunomodulation of α-galactosylceramides related to KRN 7000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and could potentially be used as building blocks for the synthesis novel classes of biomaterials.21
20 and could potentially be used as building blocks for the synthesis novel classes of biomaterials.21
(iii) Biological evaluation
Due to the large current interest in the evaluation of macrocycles as novel anti-bacterial agents,22 we screened 17 of the reported medium-sized rings and macrocycles in a minimum inhibitory concentration (MIC) growth assay against E. coli BW25113WT. Unfortunately, no appreciable growth inhibition was observed for any of the compounds screened in this assay, at a range of concentrations up to 1 mM. More information on the compounds screened, the assay protocol and the results are included in section 5 of the ESI.†Conclusions
In summary, the scope of the SuRE method has been expanded through the development of a new protocol for the N-acylation of ‘unreactive’ lactams. This new approach enabled SuRE reactions to be performed on substrates that failed to react using previously published SuRE methods. Furthermore, the ring expansion of iminosugar derived lactams has been demonstrated for the first time, showing that the methodology is compatible with these oxygen-rich, highly functionalised ‘reactive’ lactams. Through both series, a range of novel medium-sized ring and macrocyclic products was synthesised, and a selection of these products was evaluated in an E. coli BW25113WT inhibition growth assay. Unfortunately, none of the compounds tested showed significant anti-bacterial activity against this target. Nonetheless, the value of SuRE method to prepare diverse libraries of medium-sized rings and macrocycles for bioassay has been demonstrated. The synthesis of larger compound libraries for biological evaluation, and testing against a wider range of targets, will be a major focus of our future work.Author contributions
The project was conceived by WPU and PC. Initial method development and all of the ring expansion chemistry was done by ZY. Synthesis of iminosugar starting materials was done by MA and DH. Bioassays were performed by ORH and JN, with guidance from MAF and CDS. X-ray crystallography was done by ACW. The manuscript was written through contributions from all authors, led by WPU. The project was directed and managed by WPU.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the China Scholarship Council for funding the PhD studentship of Z. Y.References
- For medium-sized rings and macrocycles in medicinal chemistry, see: (a) F. Kopp, C. F. Stratton, L. B. Akella and D. S. Tan, A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion, Nat. Chem. Biol., 2012, 8, 358 CrossRef CAS PubMed; (b) C. Zhao, Z. Ye, Z.-X. Ma, S. A. Wildman, S. A. Blaszczyk, L. Hu, I. A. Guizei and W. Tang, A general strategy for diversifying complex natural products to polycyclic scaffolds with medium-sized rings, Nat. Commun., 2019, 10, 4015 CrossRef PubMed; (c) E. M. Driggers, S. P. Hale, J. Lee and N. K. Terrett, The exploration of macrocycles for drug discovery - an underexploited structural class, Nat. Rev. Drug Discovery, 2008, 7, 608 CrossRef CAS PubMed; (d) E. Marsault and M. L. Peterson, Macrocycles Are Great Cycles: Applications, Opportuni-ties, and Challenges of Synthetic Macrocycles in Drug Discovery, J. Med. Chem., 2011, 54, 1961 CrossRef CAS PubMed; (e) F. Giordanetto and J. Kihlberg, Macrocyclic Drugs and Clinical Candidates: What Can Medicinal Chemists Learn from Their Properties?, J. Med. Chem., 2014, 57, 278 CrossRef CAS PubMed.
- For discussion on the influence of ring size on end-to-end cyclisation reactions, see: (a) G. Illuminati and L. Mandolini, Ring closure reactions of bifunctional molecules, Acc. Chem. Res., 1981, 14, 95 CrossRef CAS; (b) J. Fastrez, Macrocyclization versus polymerization in polycondensation reactions under high-dilution conditions: a theoretical study, J. Phys. Chem., 1989, 93, 2635 CrossRef CAS; (c) J. C. Collins and K. James, Emac – a comparative index for the assessment of macro-cyclization efficiency, MedChemComm, 2012, 3, 1489 RSC; (d) H. Kurouchi and T. Ohwada, Synthesis of Medium-Ring-Sized Benzolactams by Using Strong Electrophiles and Quantitative Evaluation of Ring-Size Dependency of the Cyclization Reaction Rate, J. Org. Chem., 2020, 85, 876 CrossRef CAS PubMed.
- For general perspective on macrocycle synthesis, see: (a) A. K. Yudin, Macrocycles: lessons from the distant past, recent developments, and future directions, Chem. Sci., 2015, 6, 30 RSC; (b) K. T. Mortensen, T. J. Osberger, T. A. King, H. F. Sore and D. R. Spring, Strategies for the Diversity-Oriented Synthesis of Macro-cycles, Chem. Rev., 2019, 119, 10288 CrossRef CAS PubMed; (c) S. D. Appavoo, S. Huh, D. B. Diaz and A. K. Yudin, Conformational Control of Macrocycles by Remote Structural Modification, Chem. Rev., 2019, 119, 9724 CrossRef CAS PubMed; (d) I. V. Smolyar, A. K. Yudin and V. G. Nenajdenko, Heteroaryl Rings in Peptide Macrocycles, Chem. Rev., 2019, 119, 10032 CrossRef CAS PubMed.
- For reviews of ring expansion chemistry, see: (a) M. Hesse, in Ring Enlargement in Organic Chemistry, Wiley-VCH, Weinheim, 1991 Search PubMed; (b) W. P. Unsworth and J. R. Donald, Ring expansion reactions in the synthesis of macrocycles and medium sized rings, Chem. – Eur. J., 2017, 23, 8780 CrossRef PubMed; (c) K. Prantz and J. Mulzer, Synthetic Applications of the Carbonyl Generating Grob Fragmentation, Chem. Rev., 2010, 110, 3741 CrossRef CAS PubMed; (d) T. C. Stephens and W. P. Unsworth, Consecutive Ring-Expansion Reactions for the Iterative Assembly of Medium-Sized Rings and Macrocycles, Synlett, 2020, 133 CAS; (e) A. K. Clarke and W. P. Unsworth, A happy medium: the synthesis of medicinally important medium-sized rings via ring expansion, Chem. Sci., 2020, 11, 2876 RSC; (f) B. Biletskyi, P. Colonna, K. Masson, J.-L. Parrain, L. Commeiras and G. Chouraqui, Small rings in the bigger picture: ring expansion of three- and four-membered rings to access larger all-carbon cyclic systems, Chem. Soc. Rev., 2021, 50, 7513 RSC.
- For prominent recent examples, see: (a) L. Li, Z.-L. Li, F.-L. Wang, Z. Guo, Y.-F. Cheng, N. Wang, X.-W. Dong, C. Fang, J. Liu, C. Hou, B. Tan and X.-Y. Liu, Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings, Nat. Commun., 2016, 7, 13852 CrossRef PubMed; (b) R. Costil, Q. Lefebvre and J. Clayden, Medium-Sized-Ring Analogues of Dibenzodiazepines by a Conformationally Induced Smiles Ring Expansion, Angew. Chem., Int. Ed., 2017, 56, 14602 CrossRef CAS PubMed; (c) A. Lawer, J. A. Rossi-Ashton, T. C. Stephens, B. J. Challis, R. G. Epton, J. M. Lynam and W. P. Unsworth, Internal Nucleophilic Catalyst Mediated Cyclisation/Ring Expansion Cascades for the Synthesis of Medium-Sized Lactones and Lactams, Angew. Chem., Int. Ed., 2019, 58, 13942 CrossRef CAS PubMed; (d) Y. Yuan, Z. Guo, Y. Mu, Y. Wang, M. Xu and Y. Li, Synthesis of Spiro[5.n (n=6–8)]heterocycles through Successive Ring-Expansion/Indole C-2 Functionalization, Adv. Synth. Catal., 2020, 362, 1298 CrossRef CAS; (e) J. Shang, V. J. Thombare, C. L. Charron, U. Wille and C. Hutton, Ring Expansion of Thiolactams via Imide Intermediates: An Amino Acid Insertion Strategy, Chem. – Eur. J., 2021, 27, 1620 CrossRef CAS PubMed; (f) I. Zalessky, J. M. Wootton, J. K. F. Tam, D. E. Spurling, W. C. Glover-Humphreys, J. R. Donald, W. E. Orukotan, L. C. Duff, B. J. Knapper, A. C. Whitwood, T. F. N. Tanner, A. H. Miah, J. M. Lynam and W. P. Unsworth, A Modular Strategy for the Synthesis of Macrocycles and Medium-Sized Rings via Cyclization/Ring Expansion Cascade Reactions, J. Am. Chem. Soc., 2024, 146, 5702 CrossRef CAS PubMed.
- (a) C. Kitsiou, J. J. Hindes, P. I'Anson, P. Jackson, T. C. Wilson, E. K. Daly, H. R. Felstead, P. Hearnshaw and W. P. Unsworth, The Synthesis of Structurally Diverse Macrocycles by Successive Ring Expansion, Angew. Chem., Int. Ed., 2015, 54, 15794 CrossRef CAS PubMed; (b) L. G. Baud, M. A. Manning, H. L. Arkless, T. C. Stephens and W. P. Unsworth, Ring expansion approaches to medium sized lactams and analysis of their medicinal lead like properties, Chem. – Eur. J., 2017, 23, 2225 CrossRef CAS PubMed; (c) T. C. Stephens, M. Lodi, A. Steer, Y. Lin, M. Gill and W. P. Unsworth, Synthesis of cyclic peptide mimetics by the successive ring expansion of lactams, Chem. – Eur. J., 2017, 23, 13314 CrossRef CAS PubMed; (d) A. Lawer, R. G. Epton, T. C. Stephens, K. Y. Palate, M. Lodi, E. Marotte, K. J. Lamb, J. K. Sangha, J. Lynam and W. P. Unsworth, Evaluating the viability of successive ring expansion reactions based on amino acid and hydroxyacid side chain insertion, Chem. – Eur. J., 2020, 26, 12674 CrossRef CAS PubMed; (e) K. Y. Palate, Z. Yang, A. C. Whitwood and W. P. Unsworth, Synthesis of medium-ring lactams and macrocyclic peptide mimetics via conjugate addition/ring expansion cascade reactions, RSC Chem. Biol., 2022, 3, 334 RSC.
- T. C. Stephens, A. Lawer, T. French and W. P. Unsworth, Iterative assembly of macrocyclic lactones using successive ring expansion reactions, Chem. – Eur. J., 2018, 24, 13947 CrossRef CAS PubMed.
- K. Y. Palate, R. G. Epton, A. C. Whitwood, J. M. Lynam and W. P. Unsworth, Synthesis of macrocyclic and medium-sized ring thiolactones via the ring expansion of lactams, Org. Biomol. Chem., 2021, 19, 1404 RSC.
- Z. Yang, I. Zalessky, R. G. Epton, A. C. Whitwood, J. M. Lynam and W. P. Unsworth, Ring Expansion Strategies for the Synthesis of Medium Sized Ring and Macrocyclic Sulfonamides, Angew. Chem., Int. Ed., 2023, 62, e202217178 CrossRef CAS PubMed.
- Z. Yang, J. K. F. Tam, J. M. Wootton, J. M. Lynam and W. P. Unsworth, Ring expansion reactions of P=O-containing Molecules, Chem. Commun., 2023, 59, 7927 RSC.
- For a complementary ring expansion method developed by another group at the same time, see: R. Mendoza-Sanchez, V. B. Corless, Q. N. N. Nguyen, M. Bergeron-Brlek, J. Frost, S. Adachi, D. J. Tantillo and A. K. Yudin, Cyclols Revisited: Facile Synthesis of Medium-Sized Cyclic Peptides, Chem. – Eur. J., 2017, 23, 13319 CrossRef CAS PubMed.
- (a) E. Wedekind, Ber. Dtsch. Chem. Ges., 1901, 34, 2070 CrossRef CAS; (b) G. Cevasco and S. Thea, Mechanism of Alkaline Hydrolysis of Some HO-π-COOAr Acyl Derivatives, J. Org. Chem., 1999, 64, 5422 CrossRef CAS PubMed; (c) H. R. Smallman, J. A. Leitch, T. McBride, S. V. Ley and D. L. Browne, Formation and utility of reactive ketene intermediates under continuous flow conditions, Tetrahedron, 2021, 93, 132305 CrossRef CAS.
- Non-zero optical rotation ([α]D) data were obtained for all of the ring expansion products in this manuscript derived from Fmoc-Pro-Cl 16, specifically products 7, 20, 22, 31, 38, 43 and 45.
- (a) D. D'Alonzo, A. Guaragna and G. Palumbo, Glycomimetics at the mirror: medicinal chemistry of L-iminosugars, Curr. Med. Chem., 2009, 16, 473 CrossRef PubMed; (b) P. Compain and O. R. Martin, Carbohydrate mimetics-based glycosyltransferase inhibitors, Bioorg. Med. Chem., 2001, 9, 3077 CrossRef CAS PubMed; (c) G. Horne, F. X. Wilson, J. Tinsley, D. H. Williams and R. Storer, Iminosugars past, present and future: medicines for tomorrow, Drug Discovery Today, 2011, 16, 107 CrossRef CAS PubMed; (d) R. J. Nash, A. Kato, C. Y. Yu and G. W. Fleet, Iminosugars as therapeutic agents: recent advances and promising trends, Future Med. Chem., 2011, 3, 1513 CrossRef CAS PubMed; (e) J. Boisson, A. Thomasset, E. Racine, P. Cividino, T. Banchelin Sainte-Luce, J. F. Poisson, J. B. Behr and S. Py, Hydroxymethyl-Branched Polyhydroxylated Indolizidines: Novel Selective α-Glucosidase Inhibitors, Org. Lett., 2015, 17, 3662 CrossRef CAS PubMed; (f) Q. Foucart, Y. Shimadate, J. Marrot, A. Kato, J. Désiré and Y. Blériot, Synthesis and glycosidase inhibition of conformationally locked DNJ and DMJ derivatives exploiting a 2-oxo-C-allyl iminosugar, Org. Biomol. Chem., 2019, 17, 7204 RSC; (g) C. Loukou, M. Tosin, H. Müller-Bunz and P. V. Murphy, Synthesis of Sugar-Lactams from Azides of Glucuronic Acid, Carbohydr. Res., 2007, 342, 1953 CrossRef CAS PubMed; (h) Iminosugars: from Synthesis to Therapeutic Applications, ed. P. Compain and O. R. Martin, Wiley & Sons, Chichester, 2007 Search PubMed.
- (a) P. Compain, C. Decroocq, J. Iehl, M. Holler, D. Hazelard, T. M. Barragán, C. O. Mellet and J. F. Nierengarten, Glycosidase Inhibition with Fullerene Iminosugar Balls: A Dramatic Multivalent Effect, Angew. Chem., Int. Ed., 2010, 49, 5753 CrossRef CAS PubMed; (b) F. Stauffert, M. L. Lepage, M. M. Pichon, D. Hazelard, A. Bodlenner and P. Compain, A Convenient, Gram-Scale Synthesis of 1-Deoxymannojirimycin, Synthesis, 2016, 1177 CAS.
- D. Hazelard, M. L. Lepage, J. P. Schneider, M. M. Pichon, F. Massicot and P. Compain, in Carbohydrates Chemistry: Proven Synthetic Methods, ed. C. Vogel & and P. Murphy, CRC Press, 2018, vol. 4, p. 303 Search PubMed.
- M. M. Pichon, F. Stauffert, L. G. Addante-Moya, A. Bodlenner and P. Compain, Metal-Free Iodine-Mediated Deoxygenation of Alcohols in the Position α to Electron-Withdrawing Groups, Eur. J. Org. Chem., 2018, 1538 CrossRef CAS.
- CCDC 2303504 (compound 65) contains the crystallographic data, see: https://www.ccdc.cam.ac.uk/data_request/cif.†.
- A. E. Weber, T. A. Halgren, J. J. Doyle, R. J. Lynch, P. K. S. Siegl, W. H. Parsons, W. J. Greenlee and A. A. Patchett, Design and synthesis of P2-P1′-linked macrocyclic human renin inhibitors, J. Med. Chem., 1991, 34, 2692 CrossRef CAS PubMed.
- J. Janssens, J. Van der Eycken and S. Van Calenbergh, Ceramide-Templated Macrolactams: Total Synthesis and Biological Evaluation of Macrocyclic α-Galactosylceramide Analogues and their Aglycons, Eur. J. Org. Chem., 2019, 2253 CrossRef CAS.
- (a) A. A. Edwards, G. W. J. Fleet, B. A. Mayes, S. J. Hunter and G. E. Tranter, Spectroscopic studies of carbopeptoid-cyclodextrins, Chirality, 2005, 17, S114 CrossRef CAS PubMed; (b) B. A. Mayes, A. R. Cowley, C. W. G. Ansell and G. W. J. Fleet, Mixed sugar-nylon 14-, 28- and 42-membered ring macrocyclic lactams, Tetrahedron Lett., 2004, 45, 163 CrossRef CAS.
- (a) I. B. Seiple, Z. Zhang, P. Jakubec, A. Langlois-Mercier, P. M. Wright, D. T. Hog, K. Yabu, S. R. Allu, T. Fukuzaki, P. N. Carlsen, Y. Kitamura, X. Zhou, M. L. Condakes, F. T. Szczypiński, W. D. Green and A. G. Myers, A platform for the discovery of new macrolide antibiotics, Nature, 2016, 533, 338 CrossRef CAS PubMed; (b) R. Shukla, A. J. Peoples, K. C. Ludwig, S. Maity, M. G. N. Derks, S. De Benedetti, A. M. Krueger, B. J. A. Vermeulen, T. Harbig, F. Lavore, R. Kumar, R. V. Honorato, F. Grein, K. Nieselt, Y. Liu, A. M. J. J. Bonvin, M. Baldus, U. Kubitscheck, E. Breukink, C. Achorn, A. Nitti, C. J. Schwalen, A. L. Spoering, L. L. Ling, D. Hughes, M. Lelli, W. H. Roos, K. Lewis, T. Schneider and M. Weingarth, An antibiotic from an uncultured bacterium binds to an immutable target, Cell, 2023, 186, 4059 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2303504. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00285g |
| This journal is © The Royal Society of Chemistry 2024 |