 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reduction of sulfoxides catalyzed by the commercially available manganese complex MnBr(CO)5†
Daniel L.
Lourenço
and
Ana C.
Fernandes
 *
*
Centro de Química Estrutural, Institute of Molecular Sciences, Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisboa, Portugal. E-mail: anacristinafernandes@tecnico.ulisboa.pt; Tel: +351 218419220
First published on 16th April 2024
Abstract
A new methodology for the reduction of a wide variety of aliphatic and aromatic sulfoxides catalyzed by the air-stable, cheap and commercially available manganese catalyst MnBr(CO)5 with excellent yields is reported in this work. The catalytic system MnBr(CO)5/PhSiH3 is highly chemoselective, allowing the effective reduction of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the presence of different functional groups.
O bond in the presence of different functional groups.
Introduction
The reduction of sulfoxides to the corresponding sulfides is one of the most important chemical transformations in organic chemistry, due to the relevance of sulfides as intermediates in the synthesis of many biological, pharmaceutical and natural active molecules. Over the years, several methods have been developed to synthesize sulfides by reducing sulfoxides using a variety of catalysts containing different metals, including Mo,1–7 Re,8–12 Fe,13,14 Zn,15–17 Cu,18 Ti,19 Nb,20,21 Co,22 B,23,24etc.,25–27 by employing silanes, boranes, phosphites and H2 as reducing agents.The development of methodologies using inexpensive, non-toxic and commercially available catalysts containing Earth-abundant metals is highly desirable. Manganese, as one of the most abundant metals in the Earth's crust, is cheap and less toxic and has been applied as a catalyst in a variety of organic reductions,28–31 including the reduction of aldehydes and ketones,32–38 CO2,39–47 esters,48–56 amides,57 and imines.58–60
MnBr(CO)5 is also an inexpensive and commercially available air-stable manganese compound that has been used as a starting material for the preparation of several manganese catalysts containing different ligands, including triazole or N-heterocyclic carbene ligands, widely used in different organic transformations.32–37,41,43,44,46,47,49,50,53,55–57,59
More recently, this manganese compound has also attracted attention as an effective catalyst for the hydrogenation of N-heteroarenes,61 reduction of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 or carboxylic acids,63 and the depolymerization of plastic waste.64 The deoxygenation of sulfoxides catalyzed by manganese catalysts has been reported by Royo and coworkers using a Mn–NHC complex.65
62 or carboxylic acids,63 and the depolymerization of plastic waste.64 The deoxygenation of sulfoxides catalyzed by manganese catalysts has been reported by Royo and coworkers using a Mn–NHC complex.65
With the goal of developing methodologies for the reduction of sulfoxides using simple, inexpensive and commercially available catalysts based on Earth-abundant metals, in this work, we evaluated the efficiency of the manganese catalyst MnBr(CO)5 in the reduction of sulfoxides.
Results and discussion
Initially, we investigated the reduction of diphenyl sulfoxide using silanes and boranes catalyzed by the commercial available catalyst MnBr(CO)5 under an air atmosphere (Table 1). The best yield (97%) was obtained in the presence of 5 mol% of MnBr(CO)5 and an equimolar amount of PhSiH3 in toluene at reflux temperature after 15 minutes (Table 1, entry 1). The reaction carried out at room temperature gave the product in 62% yield after 24 h (Table 1, entry 2). When using a lower amount of MnBr(CO)5 (3 mol%), the reduction produced the sulfide in only 65% yield after 24 h (Table 1, entry 3). The reduction carried out with diphenylsilane also produced the sulfide in good yields after 30 minutes (Table 1, entry 4). In THF, the reduction did not occur, even when using 150 mol% of PhSiH3 (Table 1, entry 5). Several silanes, namely (EtO)2MeSiH, Pr3SiH, Et3SiH, Me2PhSiH, Ph3SiH, TMDS and PMHS, were also evaluated in the reduction of diphenyl sulfoxide in the presence of 5 mol% of MnBr(CO)5 in toluene at reflux temperature. Moderate to low yields or no reactions were observed with these silanes (Table 1, entries 6–12).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | MnBr(CO)5 (mol%) | Reducing agent | Reducing agent (mol%) | Solvent | Temp. (°C) | Time | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a The reactions were carried out using 0.5 mmol of sulfoxide in 3 mL of toluene. b Yields were determined by 1H NMR spectroscopy using mesitylene as the internal standard. | |||||||
| 1 | 5 | PhSiH3 | 100 | Toluene | 110 | 15 min | 97 |
| 2 | 5 | PhSiH3 | 100 | Toluene | r.t. | 24 h | 62 |
| 3 | 3 | PhSiH3 | 100 | Toluene | 110 | 24 h | 65 |
| 4 | 3 | Ph2SiH2 | 100 | Toluene | 110 | 30 min | 90 |
| 5 | 5 | PhSiH3 | 150 | THF | 70 | 24 h | No reaction |
| 6 | 5 | (EtO)2MeSiH | 120 | Toluene | 110 | 24 h | 29 |
| 7 | 5 | Pr3SiH | 120 | Toluene | 110 | 24 h | 35 |
| 8 | 5 | Et3SiH | 120 | Toluene | 110 | 24 h | No reaction |
| 9 | 5 | Me2PhSiH | 120 | Toluene | 110 | 24 h | No reaction |
| 10 | 5 | Ph3SiH | 120 | Toluene | 110 | 24 h | No reaction |
| 11 | 5 | TMDS | 120 | Toluene | 110 | 24 h | 29 |
| 12 | 5 | PMHS | 120 | Toluene | 110 | 24 h | 40 |
| 13 | 5 | HBpin | 120 | Toluene | 110 | 6 h | 75 |
| 14 | 5 | HBcat | 120 | Toluene | 110 | 24 h | 65 |
| 15 | — | PhSiH3 | 100 | Toluene | 110 | 24 h | No reaction |
| 16 | 5 | — | 100 | Toluene | 110 | 24 h | No reaction |
The deoxygenation of diphenyl sulfoxide was also evaluated by employing boranes as the reducing agent. From the reaction with 120 mol% of pinacolborane (HBpin) in the presence of 5 mol% of MnBr(CO)5 in toluene at reflux temperature, the corresponding sulfide was obtained in 75% yield after 6 h (Table 1, entry 13). Under the same reaction conditions, the reduction performed with catecholborane (HBcat) gave phenyl sulfide in 65% yield after 24 h (Table 1, entry 14).
Finally, no reduction of phenyl sulfoxide was observed when the reaction was performed in the absence of a catalyst or reducing agent (Table 1, entries 15 and 16).
We also evaluated the efficiency of the commercially available manganese catalysts Mn(OTf)2, MeCpMn(CO)3 and Mn2(CO)10 in the reduction of phenyl sulfoxide using PhSiH3 as the reducing agent in toluene at reflux temperature. After 3 h, no reduction was observed in the reactions carried out with 5 mol% of Mn(OTf)2 or MeCpMn(CO)3 and a small amount of diphenyl sulfide (15%) was formed in the reaction with Mn2(CO)10.
The applicability of the catalytic system MnBr(CO)5 (5 mol%)/PhSiH3 (100 mol%) was evaluated in the deoxygenation of a wide variety of sulfoxides in toluene at reflux temperature under an air atmosphere (Table 2). Generally, this methodology proved to be very efficient, allowing the deoxygenation of sulfoxides into the corresponding sulfides with excellent yields in a few minutes, including sulfoxides bearing electron-withdrawing groups. From the analysis of Table 2, it is possible to conclude that this catalytic system is equally applicable to diaryl, aryl alkyl, and dialkyl sulfoxides.
| Entry | Sulfoxide | Product | Time | Yieldb (%) |
|---|---|---|---|---|
| a The reactions were carried out using 0.5 mmol of sulfoxide, 0.5 mmol of PhSiH3, and 5 mol% of MnBr(CO)5 in 3 mL of toluene at reflux temperature. b Yields were determined by 1H NMR spectroscopy using mesitylene as the internal standard. | ||||
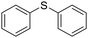
| 1 |

|
|
15 min | 97 |
| 2 |

|

|
30 min | 97 |
| 3 |

|

|
15 min | 95 |
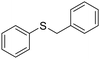
| 4 |

|
|
15 min | 94 |
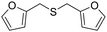
| 5 |

|
|
1 h | 91 |
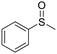
| 6 |
|

|
1 h | 83 |
| 7 |

|

|
15 min | 97 |
| 8 |
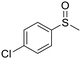
|

|
15–30 min | 96 |
| 9 |

|
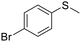
|
1 h 15 min | 95 |
| 10 |

|

|
15 min | 95 |
| 11 |

|

|
15 min | 97 |
| 12 |

|

|
30 min | 89 |
| 13 |
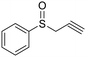
|

|
1 h | 92 |
| 14 |

|

|
2 h 30 min | 87 |
| 15 |

|

|
1 h | 77 |
| 16 |
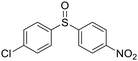
|

|
1 h | 79 |
| 17 |

|

|
35 min | 95 |
| 18 |
|

|
30 min | 96 |
The reduction of substituted diaryl and dibenzyl sulfoxides was successfully achieved within a few minutes (15–30 minutes) at 110 °C with excellent yields (94–97%) (Table 2, entries 1–4). The deoxygenation of furfuryl sulfoxide was also efficiently carried out, giving the corresponding sulfide in 91% yield after 1 h (Table 2, entry 5).
This methodology is very chemoselective, tolerating the presence of several functional groups. For example, halogen atoms (Cl– and Br–) in the aromatic ring or in the aliphatic chain were not affected under these reaction conditions (Table 2, entries 2, 8, 9, 11 and 16). This method also tolerates double and triple bonds as observed in the selective reduction of phenyl vinyl sulfoxide and phenyl propargyl sulfoxide giving 89% and 92% yields, respectively (Table 2, entries 12 and 13), which is confirmed by the analysis of 1H NMR spectra of the products (ESI†). The deoxygenation of methyl phenylsulfonylacetate was also possible in the presence of an ester group (Table 2, entry 14), which was confirmed by the presence of the signal at δ 170.2 ppm in the 13C NMR spectrum of sulfide corresponding to the CO2Me group (ESI†). The reduction of 4-nitrophenyl phenyl sulfoxide and 4-chloro-4'-nitrodiphenyl sulfoxide, containing a NO2 group, was carried out, which gave good yields (Table 2, entries 15 and 16); however, the formation of a small amount of the amino products (15–18% yields) was also observed.
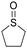
Finally, we decided to study the efficiency of the catalytic system PhSiH3/MnBr(CO)5 in the reduction of aliphatic sulfoxides. The reaction of butyl sulfoxide led to the formation of butyl sulfide in 95% yield after 35 minutes (Table 2, entry 17) and the deoxygenation of tetrahydrothiophene 1-oxide gave tetrahydrothiophene in 96% yield after 30 minutes (Table 2, entry 18).
To further evaluate the chemoselectivity of the catalytic system MnBr(CO)5/PhSiH3, we also investigated the reduction of phenyl sulfoxide in the presence of 4-chlorobenzonitrile, benzamide, acetophenone or furfuryl alcohol. These reactions were carried out using 0.5 mmol of the substrates, 5 mol% of MnBr(CO)5 and 0.5 mmol of PhSiH3 in toluene at reflux temperature. From the reaction of phenyl sulfoxide and 4-chlorobenzonitrile, we observed the formation of diphenyl sulfide with a yield of 85% after 15 minutes and a small amount of sulfoxide that did not react. No reduction products of 4-chlorobenzonitrile were detected. Likewise, the reaction of phenyl sulfoxide in the presence of benzamide only led to the selective reduction of sulfoxide in 95% yield after 15 minutes. In contrast, when the reduction of phenyl sulfoxide was carried out in the presence of acetophenone, the simultaneous reduction of the sulfoxide and the acetophenone occurs giving good yields after 15 minutes. A similar result was also observed in the reaction of phenyl sulfoxide in the presence of furfuryl alcohol, which led to the complete reduction of phenyl sulfoxide and the partial deoxygenation of the alcohol. These results demonstrated the chemoselectivity of this catalytic system in the reduction of sulfoxide in the presence of cyano and amide functional groups.
The catalytic system MnBr(CO)5/PhSiH3 was also successfully applied to the reduction of 1 g of phenyl sulfoxide in 15 minutes with a yield of 95%.
In another experiment, we evaluated the reduction of phenyl sulfone with MnBr(CO)5 (5 mol%) and PhSiH3 in toluene at reflux temperature, but we did not observe the reduction of this substrate even after 24 h.
The reduction of sulfoxides using the MnBr(CO)5/HSiR3 system should involve the activation of the Si–H bond of the silane by the catalyst, generating a hydride species. This species then promotes the hydrosilylation of the sulfur–oxygen double bond present in the sulfoxide, leading to the formation of sulfide and silanol (Scheme 1).
Conclusion
This work reports a novel methodology for the reduction of a large variety of aliphatic and aromatic sulfoxides using an air-stable, cheap and commercially available manganese catalyst. The use of a catalyst based on an Earth-abundant metal is very relevant, making the reduction of sulfoxides more eco-friendly and economical.Excellent yields were obtained with both aliphatic and aromatic sulfoxides, in many cases in just a few minutes, with good chemoselectivity. The method has also the advantage of using a very simple catalyst without containing complex ligands, which is beneficial from a scale-up point of view. Other advantages of this methodology include the air stability of the catalyst and easy work-up, allowing the reaction to be carried out under an air atmosphere. All these features make this method a useful and practical alternative to the conventional methods for the deoxygenation of sulfoxides to sulfides.
The high efficiency of the MnBr(CO)5/PhSiH3 catalytic system suggests its future application in the reduction of other functional groups.
Experimental section
General procedure for the reduction of sulfoxides with the catalytic system PhSiH3/MnBr(CO)5
To an open-air flask containing a sulfoxide (0.5 mmol) and MnBr(CO)5 (5 mol%, 0.006 g) in toluene (3 mL), PhSiH3 (0.5 mmol, 0.062 mL) was added and the reaction mixture was stirred at 110 °C. The progress of the reaction was monitored by TLC and 1H NMR spectroscopy. Upon completion, the product was purified by silica gel column chromatography using an appropriate mixture of hexane/ethyl acetate, affording the corresponding pure sulfides, which are all known products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Fundação para a Ciência e Tecnologia (FCT) through projects https://doi.org/10.54499/PTDC/QUI-QOR/0490/2020, https://doi.org/10.54499/UIDP/00100/2020 and https://doi.org/10.54499/LA/P/0056/2020. DLL thanks FCT for the grant (2022.11513.BD).References
- R. Sanz, J. Escribano, R. Aguado, M. R. Pedrosa and F. J. Arnáiz, Synthesis, 2004, 1629–1632 CrossRef CAS.
- A. C. Fernandes and C. C. Romao, Tetrahedron, 2006, 62, 9650–9654 CrossRef CAS.
- A. C. Fernandes and C. C. Romao, Tetrahedron Lett., 2007, 48, 9176–9179 CrossRef CAS.
- M. Bagherzadeh and S. Ghazali-Esfahan, New. J. Chem., 2012, 36, 971–976 RSC.
- N. Garcia, P. Garcia-Garcia, M. A. Fernandez-Rodriguez, R. Rubio, M. R. Pedrosa, F. J. Arnaiz and R. Sanz, Adv. Synth. Catal., 2012, 354, 321–327 CrossRef CAS.
- N. Garcia, P. Garcia-Garcia, M. A. Fernandez-Rodriguez, R. Rubio, M. R. Pedrosa, F. J. Arnaiz and R. Sanz, Green Chem., 2013, 15, 999–1005 RSC.
- N. Garcia, M. A. Fernandez-Rodriguez, P. Garcia-Garcia, M. R. Pedrosa, F. J. Arnaiz and R. Sanz, RSC Adv., 2016, 6, 27083–27086 RSC.
- S. C. A. Sousa and A. C. Fernandes, Tetrahedron Lett., 2009, 50, 6872–6876 CrossRef CAS.
- A. C. Fernandes, J. A. Fernandes, C. C. Romão, L. F. Veiros and M. J. Calhorda, Organometallics, 2010, 29, 5517–5525 CrossRef CAS.
- I. Cabrita, S. C. A. Sousa and A. C. Fernandes, Tetrahedron Lett., 2010, 51, 6132–6135 CrossRef CAS.
- S. C. A. Sousa, J. R. Bernardo, C. C. Romao and A. C. Fernandes, Tetrahedron, 2012, 68, 8194–8197 CrossRef CAS.
- S. C. A. Sousa, J. R. Bernardo, M. Wolff, B. Machura and A. C. Fernandes, Eur. J. Org. Chem., 2014, 1855–1859 CrossRef CAS.
- S. Enthaler, ChemCatChem, 2011, 3, 666–670 CrossRef CAS.
- J. M. S. Cardoso and B. Royo, Chem. Commun., 2012, 48, 4944–4946 RSC.
- S. Enthaler, Catal. Sci. Technol., 2011, 1, 104–110 RSC.
- S. Enthaler, S. Krackl, E. Irran and S. Inoue, Catal. Lett., 2012, 142, 1003–1010 CrossRef CAS.
- S. Enthaler, Catal. Lett., 2012, 142, 1306–1311 CrossRef CAS.
- S. Enthaler and M. Weidauer, Catal. Lett., 2011, 141, 833–838 CrossRef CAS.
- B. W. Yoo, K. H. Choi, D. Y. Kim, K. I. Choi and J. H. Kim, Synth. Commun., 2003, 33, 53–57 CrossRef CAS.
- B. W. Yoo, H. M. Kim and D. Kim, Synth. Commun., 2013, 43, 2057–2061 CrossRef CAS.
- K. Oh and W. E. Knabe, Tetrahedron Lett., 2009, 65, 2966–2974 CrossRef CAS.
- S. Yakabe and M. T. Morimoto, Synth. Commun., 2011, 41, 2251–2255 CrossRef CAS.
- F. Ding, Y. Jiang, S. Gan, R. L. Y. Bao, K. Lin and L. Shi, Eur. J. Org. Chem., 2017, 3427–3430 CrossRef CAS.
- D. Porwal and M. Oestreich, Synthesis, 2017, 4698–4702 CAS.
- M. Madesclaire, Tetrahedron, 1988, 44, 6537–6580 CrossRef CAS.
- L. Shiri and M. Kazemi, Res. Chem. Intermed., 2017, 43, 6007–6041 CrossRef CAS.
- H. Lin, L. Wu and M. Kazemi, Synth. Commun., 2021, 51, 1609–1635 CrossRef CAS.
- P. Schlichter and C. Werlé, Synthesis, 2022, 517–534 CAS.
- K. Azouzi, D. A. Valyaev, S. Bastin and J.-B. Sortais, Curr. Opin. Green Sustainable Chem., 2011, 31, 100511 CrossRef.
- K. R. Rohit, S. Radhika, S. Saranya and G. Anilkuma, Adv. Synth. Catal., 2020, 362, 1602–1650 CrossRef CAS.
- J. R. Carney, B. R. Dillon and S. P. Thomas, Eur. J. Org. Chem., 2016, 3912–3929 CrossRef CAS.
- W. Yang, I. Y. Chernyshov, R. K. A. van Schendel, M. Weber, C. Müller, G. A. Filonenko and E. A. Pidko, Nat. Commun., 2021, 12, 12 CrossRef CAS PubMed.
- S. Weber, J. Brünig, L. F. Veiros and K. Kirchner, Organometallics, 2021, 40, 1388 CrossRef CAS PubMed.
- S. Vijjamarri, T. M. O'Denius, B. Yao, A. Kubátová and G. Du, Organometallics, 2020, 39, 3375–3383 CrossRef CAS.
- R. Buhaibeh, O. A. Filippov, A. Bruneau-Voisine, J. Willot, C. Duhayon, D. A. Valyaev, N. Lugan, Y. Canac and J.-B. Sortais, Angew. Chem., Int. Ed., 2019, 58, 6727–6731 CrossRef CAS PubMed.
- M. Pinto, S. Friães, F. Franco, J. Lloret-Fillol and B. Royo, ChemCatChem, 2018, 10, 2734–2740 CrossRef CAS.
- A. Bruneau-Voisine, D. Wang, V. Dorcet, T. Roisnel, C. Darcel and J.-B. Sortais, Org. Lett., 2017, 19, 3656–3659 CrossRef CAS PubMed.
- V. K. Chidara and G. Du, Organometallics, 2013, 32, 5034–5037 CrossRef CAS.
- B. Siritanaratkul, C. Eagle and A. J. Cowan, Acc. Chem. Res., 2022, 55, 955–965 CrossRef CAS PubMed.
- Y. Yang, Z. Zhang, X. Chang, Y.-Q. Zhang, R.-Z. Liao and L. Duan, Inorg. Chem., 2010, 59, 10234–10242 CrossRef PubMed.
- S. E. Tignor, H.-Y. Kuo, T. S. Lee, G. D. Scholes and A. B. Bocarsly, Organometallics, 2019, 38, 1292–1299 CrossRef CAS.
- B. Zhang, J. Zhang, J. Shi, D. Tan, L. Liu, F. Zhang, C. Lu, Z. Su, X. Tan, X. Cheng, B. Han, L. Zheng and J. Zhang, Nat. Commun., 2019, 10, 2980 CrossRef PubMed.
- M. Stanbury, J.-D. Compain and S. Chardon-Noblat, Coord. Chem. Rev., 2018, 361, 120–137 CrossRef CAS.
- S. Kar, A. Goeppert, J. Kothandaraman and G. K. S. Prakash, ACS Catal., 2017, 7, 6347–6351 CrossRef CAS.
- M. D. Sampson, A. D. Nguyen, K. A. Grice, C. E. Moore, A. L. Rheingold and C. P. Kubiak, J. Am. Chem. Soc., 2014, 136, 5460–5471 CrossRef CAS PubMed.
- H. A. Petersen, T. H. T. Myren and O. R. Luca, Inorganics, 2020, 8, 62 CrossRef CAS.
- M. McKinnon, V. Belkina, K. T. Ngo, M. Z. Ertem, D. C. Grills and J. Rochford, Front. Chem., 2019, 7, 628 CrossRef CAS PubMed.
- T. K. Mukhopadhyay, M. Flores, T. L. Groy and R. J. Trovitch, J. Am. Chem. Soc., 2014, 136, 882–885 CrossRef CAS PubMed.
- S. Elangovan, M. Garbe, H. Jiao, A. Spannenberg, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 15364–15368 CrossRef CAS PubMed.
- R. van Putten, E. A. Uslamin, M. Garbe, C. Liu, A. Gonzalez-de-Castro, M. Lutz, K. Junge, E. J. M. Hensen, M. Beller, L. Lefort and E. A. Pidko, Angew. Chem., Int. Ed., 2017, 56, 7531–7534 CrossRef CAS PubMed.
- N. A. Espinosa-Jalapa, A. Nerush, L. J. W. Shimon, G. Leitus, L. Avram, Y. Bem-David and D. Milstein, Chem. – Eur. J., 2017, 23, 5934–5938 CrossRef CAS PubMed.
- C. M. Kelly, R. McDonald, O. L. Sydora, M. Stradiotto and L. Turculet, Angew. Chem., Int. Ed., 2017, 56, 15901–15904 CrossRef CAS PubMed.
- O. Martínez-Ferraté, B. Chatterjee, C. Werlé and W. Leitner, Catal. Sci. Technol., 2019, 9, 6370–6378 RSC.
- C. L. Oates, M. B. Widegren and M. L. Clarke, Chem. Commun., 2020, 56, 8635–8638 RSC.
- S. C. A. Sousa, S. Realista and B. Royo, Adv. Synth. Catal., 2020, 362, 2437–2443 CrossRef CAS.
- R. R. Behera, R. Ghosh, S. Panda, S. Khamari and B. Bagh, Org. Lett., 2020, 22, 3642–3648 CrossRef CAS PubMed.
- V. Papa, J. R. Cabrero-Antonino, E. Alberico, A. Spanneberg, K. Junge, H. Junge and M. Beller, Chem. Sci., 2017, 8, 3576–3585 RSC.
- D. A. Roa and J. J. Garcia, Inorg. Chim. Acta, 2017, 516, 120167 CrossRef.
- D. Wei, A. Bruneau-Voisine, M. Dubois, S. Bastin and J.-B. Sortais, ChemCatChem, 2019, 11, 5256–5259 CrossRef CAS.
- D. Wei, A. Bruneau-Voisine, D. A. Valyaev, N. Luganb and J.-B. Sortais, Chem. Commun., 2018, 54, 4302–4305 RSC.
- V. Papa, Y. Cao, A. Spannenberg, K. Junge and M. Beller, Nat. Catal., 2020, 3, 135–142 CrossRef CAS.
- T. González and J. García, Polyhedron, 2021, 203, 115242 CrossRef.
- E. Antico, P. Schlichter, C. Werlé and W. Leitner, JACS Au, 2021, 1, 742–749 CrossRef CAS PubMed.
- D. L. Lourenço, D. F. Oliveira and A. C. Fernandes, Adv. Sustainable Syst., 2024, 8, 2300381 CrossRef.
- S. C. A. Sousa, C. J. Carrasco, M. F. Pinto and B. Royo, ChemCatChem, 2019, 11, 3839–3843 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob00204k |
| This journal is © The Royal Society of Chemistry 2024 |



