Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Construction of indolizine scaffolds from α,ω-alkynoic acids and α,ω-vinylamines via sequential-relay catalysis in “one pot”†
Jiami
Liu‡
,
Yi
Lu‡
,
Lingxuan
Zhu‡
and
Xinsheng
Lei
 *
*
School of Pharmacy, Fudan University, 826 Zhangheng Road, Pudong Zone, Shanghai 201203, China. E-mail: leixs@fudan.edu.cn
First published on 5th March 2024
Abstract
A simple and efficient method has been developed for the synthesis of a diverse range of aryl-fused indolizin-3-ones through sequential Au(I)-catalyzed hydrocarboxylation, aminolysis, and cyclization, followed by ruthenium-catalyzed ring-closing metathesis. Moderate to good yields were observed with satisfactory substrate scope and functional group tolerance. The developed protocol represents a practical strategy for the construction of bioactive aryl-fused indolizin-3-ones.
Introduction
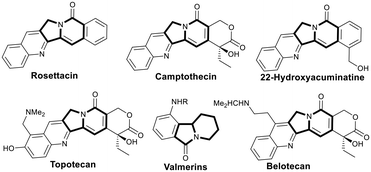
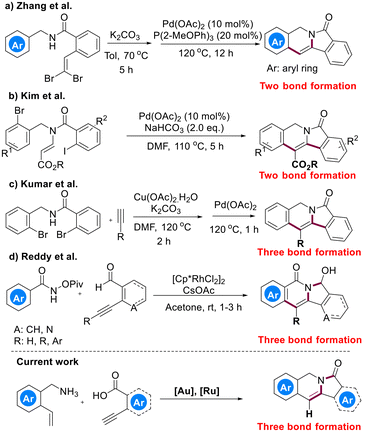
Nitrogen-containing heterocycles are prevalent structural motifs present in a large number of pharmaceuticals, bioactive compounds and natural products.1 Functionalized indolizines, particularly aryl-fused ones, broadly exist in a variety of natural and artificial compounds with diverse bioactivities (Fig. 1), representing a class of fundamentally important scaffolds.2 As useful precursors of indolizine scaffolds and versatile synthons,3 aryl-fused indolizin-3-ones have aroused significant interest among organic and medicinal chemists. Thus, exploring novel methodologies, particularly those guided by the principles of green chemistry,4 to construct these scaffolds is of prime importance. In this field, the “multicatalysis” protocol5 undoubtedly deserves merits due to its ability to convert relatively simple starting materials into more complex products and to significantly reduce time, waste and cost of synthetic processes.Recently, Zhang reported an efficient sequential relay catalysis to construct aryl-fused indolizinones from gem-dibromoolefin using K2CO3 and Pd[0] to execute debromination and C–H arylation, respectively (Scheme 1a).6 In 2016, Kim reported a domino catalysis using palladium-catalyzed intramolecular double Heck reactions to construct the 5-membered ring and 6-membered ring sequentially (Scheme 1b).7 In 2019, Kumar described a relay catalysis in which enamides were formed through copper-catalyzed Sonogashira coupling followed by intramolecular cyclization, and then obtained isoindolo[2,1-b]isoquinolin-7(5H)-one derivatives via a subsequent intramolecular Heck reaction (Scheme 1c).8 In 2018, Reddy reported a Rh(III)-catalyzed cascade annulation involving the combination of a [Cp*RhCl2]2-catalyzed domino catalysis and a base-mediated intramolecular addition of amide nitrogen to aldehyde (Scheme 1d).9
 | ||
| Scheme 1 The representative multicatalysis protocols for the construction of the aryl-fused indolizin-3-ones. | ||
In recent years, the bimetallic relay catalysis has become a useful strategy to activate and construct chemical bonds.10 This kind of catalytic model is a complement to the existing single catalytic technology.11 α,ω-Alkynoic acids are building blocks widely used to construct heterocyclic compounds through transition metal-catalyzed cascade reactions.12–26 We have recently disclosed a Ru-catalyzed cascade reaction of α,ω-alkynoic acids and arylethylamines,26 the result of which inspired us that if we use α,ω-vinylamines instead of arylethylamines, the corresponding enamide intermediates might be able to produce the desired indolizin-3-ones through ring-closing metathesis. We herein describe this multicatalytic reaction for the synthesis of aryl-fused indolizin-3-ones from α,ω-alkynoic acids and α,ω-vinylamines using Au(PPh3)Cl and Hoveyda–Grubbs II as catalysts.
Results and discussion
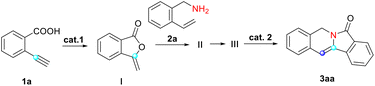
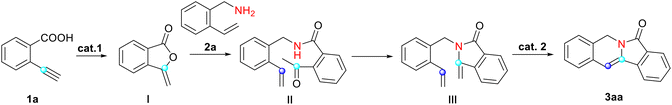
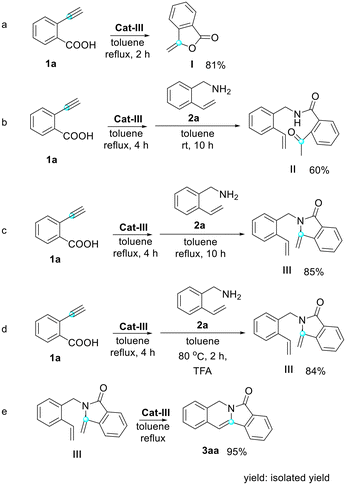
Our study was guided by the hypothesis that the selected model substrate 2-ethynylbenzoic acid would convert into the desired indolizin-3-one through hydrocarboxylation, aminolysis with 2-vinylbenzylamine, cyclization and ring-closing metathesis. To explore the feasibility of this strategy, we subjected 2-ethynylbenzoic acid (1a) and 2-vinylbenzylamine (2a) to similar conditions which we used for sequential reactions in our previous work,26 and used Hoveyda–Grubbs II (Cat-III)27 as the catalyst. After being heated with Cat-III for 2 hours in refluxed toluene, 1a converted into enol lactone (I) successfully. Fortunately, we also managed to produce intermediates II and III with satisfactory yields. (The yields of I and II were slightly lower than that of III due to the volatility of these products and resultant losses during purification.) All the intermediates were separated and elucidated using 1H-NMR, 13C-NMR and mass spectroscopic data. Finally, enamide III was heated with Cat-III in refluxed toluene and the desired indolizine 3aa was afforded in 95% isolated yield. This indicated that our strategy to construct indolizin-3-ones is very likely to be viable.Encouraged by the initial results, we decided to optimize the reaction conditions for the synthesis of 3aa by varying the catalyst, equivalent and solvent. The results are given in Tables 1–3.
| Entry | Catalyst 1 (mol%) | Catalyst 2 (mol%) | 1a/2a (mmol/mmol) | Solvent | Yield (I) | Yield (III)b | Yield (3aa)b |
|---|---|---|---|---|---|---|---|
| a Conditions: substrate 1a (quantity noted), 2a (quantity noted), anhydrous solvent (15 mL), refluxed overnight in a sealed tube. b Isolated yield. c Determined by crude NMR analysis. | |||||||
| 1 | Cat-III (10%) | Cat-III (10%) | 1.2/1.0 | Toluene | — | — | 66% |
| 2 | Cat-III (10%) | Cat-III (10%) | 1.0/1.0 | Toluene | — | — | 70% |
| 3 | Cat-III (10%) | Cat-III (10%) | 1.0/1.2 | Toluene | — | — | 68% |
| 4 | Cat-V (5%) | — | 1.0/1.0 | Toluene | 95%c | 90% | — |
| 5 | Cat-V (5%) | — | 1.0/1.0 | DCM | 90%c | 18% | — |
| 6 | Cat-V (5%) | — | 1.0/1.0 | DCE | 84%c | 47% | — |
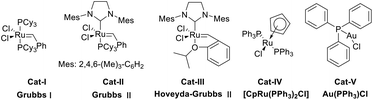
Three commercially available Grubbs’ ruthenium carbenes (Cat-I, Cat-II and Cat-III), one representative ruthenium complex (Cat-IV) along with a gold catalyst (Cat-V) reported for the hydrocarboxylation of terminal alkynes16–19,22–24 were used in the catalyst screening study (Fig. 2). All five catalysts were screened for their ability to accelerate the hydrocarboxylation of 1a. It turned out that 5 mol% of Cat-V is the most efficient catalyst among the five for the first step and less quantity (3 mol%) led to no significant variation in the yield of enol lactone I (Table 1). 5 mol% of Cat-III could also catalyze the transformation effectively, whereas increasing the amount of the catalyst (10 mol%) turned out to be a futile attempt to increase its efficiency (Table 1, entries 3 and 4). With the best candidate for catalyst 1 in hand, we tested the three ruthenium carbenes (Cat-I, Cat-II and Cat-III) for the fourth step, and 5 mol% of Cat-III (Hoveyda–Grubbs II) was proved to be the most efficient in catalyzing the ring-closing metathesis (Table 2). The result of catalyst screening enlightened us that we might be able to convert 1a into the desired indolizine 3aa in a “one pot” manner with Cat-III as the sole catalyst due to its ability to accelerate both the hydrocarboxylation and the ring-closing metathesis. However, this idea was soon proved infeasible, as this domino catalysis protocol with Cat-III as the catalyst failed to produce the desired indolizine 3aa, and increasing the quantity of Cat-III at the very beginning of the procedure made no difference to the disappointing result. A satisfactory yield of 3aa could only be achieved by adding fresh Cat-III into the reaction medium after the formation of enamide III. One probable explanation is that hydrocarboxylation is catalyzed by the decomposed product of Cat-III instead of Cat-III itself,28 therefore by the time the intermediate III was formed, there was little amount of Cat-III existing in the reaction medium and hence, 3aa could not be afforded in the desired quantity via ring-closing metathesis.
An equivalent screening study was then conducted. At the very beginning, we were worried that the remaining 2-vinylbenzylamine 2a in the reaction medium might hinder the ring-closing metathesis by poisoning the ruthenium catalyst Cat-III. To our delight, the result indicated that a slight modification of the 1a/2a ratio has no significant influence on the yield of 3aa (Table 3, entries 1–3). According to the result of the optimization study, we deemed it wiser to maintain the initial ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to achieve the best yield. We also examined the effect of various solvents and found that the yield of enamide III dropped significantly when DCM and DCE were used. The three solvents investigated in this study differed in their boiling points, which might explain why this protocol failed to produce intermediate III in the desired quantity in DCE and DCM. Given the fact that enol lactone I could be produced smoothly in both DCM and DCE (entries 16–18) and the formation of intermediate II did not require high temperature (Scheme 2), we assumed that high temperature might be fundamental for the cyclization of II and the low yield of III in DCM and DCE might be attributed to the comparatively low boiling points of DCM and DCE. To find proof for this hypothesis, we investigated the influence of reaction temperature in every step of the protocol using toluene as the solvent. The results (shown in Table S1†) further verified our assumption that the formation of III requires high temperature and revealed that the optimized temperature for this one pot reaction is 115 °C (temperature set for the oil bath). Nevertheless, we found that for volatile aliphatic alkynoic acids like pent-4-ynoic acid 1j (see Table S2†), it is difficult to fully convert them into the corresponding enol lactone I in refluxed toluene. DCM and DCE might be better solvents than toluene in the hydrocarboxylation step when the α,ω-alkynoic acid substrate is an aliphatic one, since pent-4-ynoic acid produced intermediate I in far better yields (95–96%) in DCM and DCE. Thus, for volatile aliphatic alkynoic acid substrates, it is wise to choose DCM or DCE as the solvent at the beginning, remove the solvent after the formation of intermediate II, and introduce toluene into the reacting system as it has a higher boiling point.
1 to achieve the best yield. We also examined the effect of various solvents and found that the yield of enamide III dropped significantly when DCM and DCE were used. The three solvents investigated in this study differed in their boiling points, which might explain why this protocol failed to produce intermediate III in the desired quantity in DCE and DCM. Given the fact that enol lactone I could be produced smoothly in both DCM and DCE (entries 16–18) and the formation of intermediate II did not require high temperature (Scheme 2), we assumed that high temperature might be fundamental for the cyclization of II and the low yield of III in DCM and DCE might be attributed to the comparatively low boiling points of DCM and DCE. To find proof for this hypothesis, we investigated the influence of reaction temperature in every step of the protocol using toluene as the solvent. The results (shown in Table S1†) further verified our assumption that the formation of III requires high temperature and revealed that the optimized temperature for this one pot reaction is 115 °C (temperature set for the oil bath). Nevertheless, we found that for volatile aliphatic alkynoic acids like pent-4-ynoic acid 1j (see Table S2†), it is difficult to fully convert them into the corresponding enol lactone I in refluxed toluene. DCM and DCE might be better solvents than toluene in the hydrocarboxylation step when the α,ω-alkynoic acid substrate is an aliphatic one, since pent-4-ynoic acid produced intermediate I in far better yields (95–96%) in DCM and DCE. Thus, for volatile aliphatic alkynoic acid substrates, it is wise to choose DCM or DCE as the solvent at the beginning, remove the solvent after the formation of intermediate II, and introduce toluene into the reacting system as it has a higher boiling point.
Now that we have known the cyclization step is the key step of the whole procedure, we tried to optimize the reaction conditions by introducing additives into the catalytic system. Considering the importance of Brønsted or Lewis acids in the cyclization step of this multicatalytic reaction, we tested TFA, a privileged additive reported in similar reactions,17,19,23 and found that TFA could indeed promote the subsequent transformation (see Table S3†). Other additives such as CH3COOH and Ac2O29 could also lower the temperature required for the formation of enamide III to 80 °C, but cannot match the efficiency of TFA. Therefore, we considered TFA as the best additive among all the acids tested. However, for aliphatic alkynoic acid substrates like 1j, this approach failed to give the corresponding enamide III at lower temperatures (see Table S3†). What's more, enamide III could not convert into the desired indolizine 3 in the desired quantity at 80 °C (Table S1†). As we intended to accomplish the synthesis of indolizine 3 in a “one pot” manner, we decided not to include TFA to our protocol and to maintain the initial optimized temperature for the cyclization step in order to simplify the catalytic system.
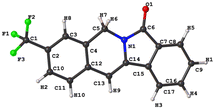
With the optimal reaction conditions in hand, we then examined the reaction of various amines (2) with 2-ethynylbenzoic acid (1a) to probe the generality of the protocol (Table 4). The performance of different amine substrates diverge from each other dramatically. Generally speaking, electron-withdrawing group-substituted vinylamines gave the corresponding indolizines (3) in higher yields than electron-donating group-substituted ones (3ab–3an). The structure of indolizine 3af was unambiguously confirmed by single crystal X-ray analysis (Fig. 3). For amines bearing the same substituents, the position of the substitution has a direct impact on the yield of the corresponding product 3, partly due to the steric hindrance hampering the ring-closing metathesis. A comparison between vinylamine substrate 2b–e indicated that the substitution at the ortho-position of the vinyl group is particularly undesirable. Even small substituents like fluorine at that position undermined the yield of the corresponding indolizine 3 dramatically, which offered an explanation to our failure in producing indolizine 3ao from vinylamine substrate 2o. Substitutions at other positions of the benzene ring, however, are well-tolerated. We even observed the formation of indolizine 3ap, indicating that the substitution at the benzyl position could be tolerated as well. We then explored whether this protocol could be applied to heterocyclic amines. To our dismay, electron-rich heterocyclic amines such as 2q suffered the same fate as electron-donating group-substituted phenylmethanamines, whereas nitrogen-containing heterocyclic amines such as 2r and 2s simply failed to give the expected indolizines, probably due to their complexion with the Cat-III catalyst. Our attempts to extend the substrate scope of this protocol to aniline (2t), phenethylamine (2v and 2w) and aliphatic amine (2u) also met with failure. Nevertheless, we observed the formation of enamide intermediate III in the case of 2u–w, indicating that ring-closing metathesis catalyzed by Cat-III might not be applicable for these enamide substrates under the given conditions.
| a Conditions: substrate 1a (1.0 mmol), 2a–2u (1.0 mmol), Cat-V (5 mol%, 25 mg), Cat-III (5 mol%, 32 mg), toluene (15 mL), refluxed overnight; yield: isolated yield; N.D. not detected. |
|---|
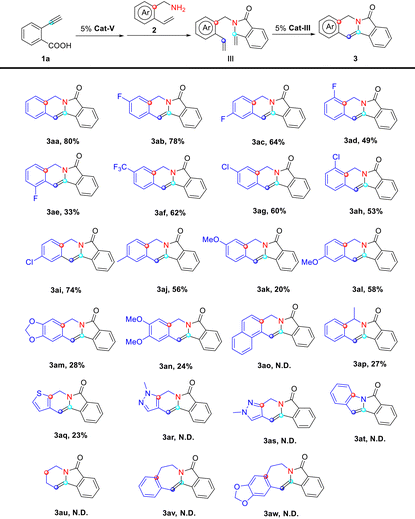
|
To further extend the substrate scope of this multicatalytic reaction, a number of diverse alkynoic acids (1) were tested (Table 5). To our delight, the substitution on benzoic acid is well-tolerated. Both alkynoic acids bearing electron-donating and electron-withdrawing groups gave indolizine 3 in moderate to good yields (51–83%). The highest yield was observed in the case of fluorine-substituted alkynoic acid 1h (83%), whereas methoxyl-substituted alkynoic acid 1e and bromo-substituted alkynoic acid 1f also gave the corresponding indolizine 3 in a satisfactory yield of over 80%. Even heterocyclic alkynoic acid (1i) and aliphatic alkynoic acid (1j) reacted smoothly with 2a to give the expected product in reasonably good yields (71% and 72% yields, respectively). The only exception is alkynoic acid 1c, whose enamide intermediate formed compound 3ca′ instead of the expected indolizine via intermolecular cross metathesis, indicating that an increase of steric hindrance might still be a challenge for ring-closing metathesis in this protocol.
| a Conditions: substrate 1 (1.0 mmol), 2a (1.0 mmol), Cat-V (5 mol%, 25 mg), Cat-III (5 mol%, 32 mg), toluene (15 mL), refluxed overnight; yield: isolated yield; N.D. not detected. b DCE (10 mL) was used as solvent in the hydrocarboxylation step, removed after the formation of intermediate II and switched to toluene (10 mL) as solvent afterwards. |
|---|
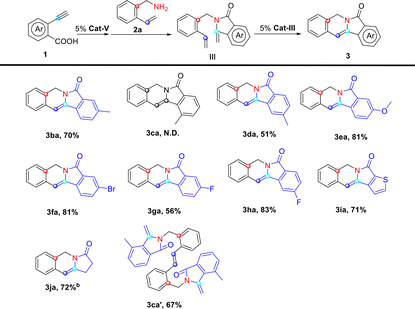
|
Conclusions
In summary, this study presents a simple, practical and environmentally benign protocol involving Au(I)-catalyzed hydrocarboxylation, aminolysis, cyclization and ruthenium-catalyzed ring-closing metathesis. This unified approach provided indolizin-3-ones in moderate to good yields with satisfying substrate scope and functional group tolerance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21472024 and 21242008), the Shanghai Municipal Health Commission (No. 20490740500) and the Science and Technology Commission of Shanghai Municipality (No. 202040114) for the financial support. We are grateful to Prof. Yingxia Li and Prof. Ran Hong for their helpful discussion.References
- (a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (b) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed; (c) N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed; (d) M. M. Heravi and V. Zadsirjan, RSC Adv., 2020, 10, 44247–44311 RSC.
- (a) K. Kong, N. J. Rahier, B. M. Eisenhauer, R. Gao, S. J. Thomas and S. M. Hecht, J. Am. Chem. Soc., 2005, 127, 838–839 CrossRef PubMed; (b) G. Schultz, Chem. Rev., 1973, 73, 385–405 CrossRef PubMed; (c) F. Grillet, B. Baumlová, G. Prévost, J.-F. Constant, S. Chaumeron, D. C. H. Bigg, A. E. Greene and A. Kanazawa, Bioorg. Med. Chem. Lett., 2008, 18, 2143–2146 CrossRef CAS PubMed; (d) R. Boulahjar, A. Ouach, C. Matteo, S. Bourg, M. Ravache, R. le Guével, S. Marionneau, T. Oullier, O. Lozach, L. Meijer, C. Guguen-Guillouzo, S. Lazar, M. Akssira, Y. Troin, G. Guillaumet and S. Routier, J. Med. Chem., 2012, 55, 9589–9606 CrossRef CAS PubMed; (e) J.-H. Lee, J.-M. Lee, J.-K. Kim, S.-K. Ahn, S.-J. Lee, M.-Y. Kim, S.-S. Jew, J.-G. Park and G. I. Hong, Arch. Pharmacal Res., 1998, 21, 581–590 CrossRef CAS PubMed.
- (a) N. Kise, S. Isemoto and T. Sakurai, J. Org. Chem., 2011, 76, 9856–9860 CrossRef CAS PubMed; (b) G. Lahm, A. Stoye and T. Opatz, J. Org. Chem., 2012, 77, 6620–6623 CrossRef CAS PubMed; (c) Y. A. Amador-Sánchez, A. Aguilar-Granda, R. Flores-Cruz, D. González-Calderón, C. Orta, B. Rodríhnez-Molina, A. Jiménez-Sánchez and L. D. Miranda, J. Org. Chem., 2020, 85, 633–649 CrossRef PubMed; (d) X.-M. Xu, L. Zhao, J. Zhu and M.-X. Wang, Angew. Chem., Int. Ed., 2016, 55, 3799–3803 CrossRef CAS PubMed; (e) P. Šafář, Š. Marchalín, M. Šoral, J. Moncal and A. Daȉch, Org. Lett., 2017, 19, 4742–4745 CrossRef PubMed; (f) N. Kise, T. Manto and T. Sakurai, Tetrahedron, 2020, 76, 131725 CrossRef CAS; (g) N. J. Owen and M. G. McLaughlin, Org. Biomol. Chem., 2022, 20, 8209–9211 RSC; (h) C. V. Gärtner and C. Schneider, Org. Lett., 2022, 24, 3560–3564 CrossRef PubMed; (i) K. S. Mandrekar and S. G. Tilve, RSC Adv., 2022, 12, 17701–17705 RSC.
- (a) P. T. Anastas and J. C. Warrner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed; (b) B. W. Cue, in Green Techniques for Organic Synthesis and Medicinal Chemistry, ed. W. Zhang and B. W. Cue, Wiley, Chichester (UK), 2012, pp. 553–569 Search PubMed; (c) S. G. Akakios, M. L. Bode and R. A. Sheldon, Green Chem., 2021, 23, 3334–3347 RSC.
- S. Martínez, L. Veth, B. Lainer and P. Dydio, ACS Catal., 2021, 11, 3891–3915 CrossRef.
- F. Tang, C. Chen, Y. Zhou, C. Lina and J. Zhang, RSC Adv., 2014, 4, 51298–51301 RSC.
- B.-J. Lee, G.-P. Hong and G. Kim, Tetrahedron Lett., 2016, 57, 5348–5350 CrossRef CAS.
- H. K. Saini, S. Dhiman, N. K. Nandwana, R. Krishnan and A. Kumar, Org. Biomol. Chem., 2019, 17, 4281–4290 RSC.
- C. R. Reddy and K. Mallesh, Org. Lett., 2018, 20, 150–153 CrossRef PubMed.
- (a) U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S. G. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed; (b) M. M. Lorion, K. Maindan, A. R. Kapdi and L. Ackermann, Chem. Soc. Rev., 2017, 46, 7399–7420 RSC; (c) L. Wei and C.-J. Wang, Chem. Catal., 2023, 3, 100455 CrossRef CAS; (d) L. K. G. Ackerman-Biegasiewicz, S. K. Kariofillis and D. J. Weix, J. Am. Chem. Soc., 2023, 145, 6596–6614 CrossRef CAS PubMed; (e) C.-G. Zhao, S. Xia, C. Wang, W. Wang and J. Xie, Chem. Catal., 2022, 2, 458–467 CrossRef CAS; (f) X. Huo, G. Li, X. Wang and W. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202210086 CrossRef CAS PubMed; (g) J. Fu, X. Huo, B. Li and W. Zhang, Org. Biomol. Chem., 2017, 15, 9747–9759 RSC; (h) T. Zhong, C. Gu, Y. Li, J. Huang, J. Han, C. Zhu, J. Han and J. Xie, Angew. Chem., Int. Ed., 2023, 62, e202310762 CrossRef CAS PubMed.
- (a) Q. Zhang, S. Wang, J. Yin, T. Xiong and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202202713 CrossRef CAS PubMed; (b) X. Chang, X. Cheng, X. T. Liu, C. Fu, W. Y. Wang and C.-J. Wang, Angew. Chem., Int. Ed., 2022, 61, e202206517 CrossRef CAS PubMed; (c) Y. Yuan, F. P. Wu, J. X. Xu and X.-F. Wu, Angew. Chem., Int. Ed., 2020, 59, 17055–17061 CrossRef CAS PubMed; (d) S. A. Green, T. R. Huffman, R. O. McCourt, V. van der Puyl and R. A. Shenvi, J. Am. Chem. Soc., 2019, 141, 7709–7714 CrossRef CAS PubMed; (e) P. Peng, Y. Zhong, C. Zhou, Y. Tao, D. Li and Q. Lu, ACS Cent. Sci., 2023, 9, 756–762 CrossRef CAS PubMed.
- T. Yang, L. Campbell and D. J. Dixon, J. Am. Chem. Soc., 2007, 129, 12070–12071 CrossRef CAS PubMed.
- M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796–10797 CrossRef CAS PubMed.
- N. T. Patil, V. S. Shinde and B. Sridhar, Angew. Chem., Int. Ed., 2013, 52, 2251–2255 CrossRef CAS PubMed.
- N. T. Patil, P. G. V. V. Lakshmi, B. Sridhar, S. Patra, M. P. Bhadra and C. R. Patra, Eur. J. Org. Chem., 2012, 1790–1799 CrossRef CAS.
- N. T. Patil, A. K. Mutyala, P. G. V. V. Lakshmi, B. Gajula, B. Sridhar, G. R. Pottireddygari and T. P. Rao, J. Org. Chem., 2010, 75, 5963–5975 CrossRef CAS PubMed.
- J. Qiao, X. Jia, P. Li, X. Liu, J. Zhao, Y. Zhou, J. Wang, H. Liu and F. Zhao, Adv. Synth. Catal., 2019, 361, 1419–1440 CrossRef CAS.
- E. Feng, Y. Zhou, D. Zhang, L. Zhang, H. Sun, H. Jiang and H. Liu, J. Org. Chem., 2010, 75, 3274–3282 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhai, X. Ji, G. Liu, E. Feng, D. Ye, L. Zhao, H. Jiang and H. Liu, Adv. Synth. Catal., 2010, 352, 373–378 CrossRef CAS.
- Y. Zhou, X. Ji, G. Liu, D. Zhang, L. Zhao, H. Jiang and H. Liu, Adv. Synth. Catal., 2010, 352, 1711–1717 CrossRef CAS.
- Y. Zhou, J. Li, X. Ji, W. Zhou, X. Zhang, W. Qian, H. Jiang and H. Liu, J. Org. Chem., 2011, 76, 1239–1249 CrossRef CAS PubMed.
- E. Feng, Y. Zhou, F. Zhao, X. Chen, L. Zhang, H. Jiang and H. Liu, Green Chem., 2012, 14, 1888–1895 RSC.
- X. Ji, Y. Zhou, J. Wang, L. Zhao, H. Jiang and H. Liu, J. Org. Chem., 2013, 78, 4312–4318 CrossRef CAS PubMed.
- Z. Li, J. Li, N. Yang, Y. Chen, Y. Zhou, X. Ji, L. Zhang, J. Wang, X. Xie and H. Liu, J. Org. Chem., 2013, 78, 10802–10811 CrossRef CAS PubMed.
- S. Naidu and S. R. Reddy, RSC Adv., 2016, 6, 62742–62746 RSC.
- Y. Zheng, J. Liu and X. Lei, Org. Chem. Front., 2020, 7, 660–665 RSC.
- A. H. Hoveyda and A. R. Zhugralin, Nature, 2007, 450, 243–251 CrossRef CAS PubMed.
- K. Melis, T. Opstal and F. Verpoort, Eur. J. Org. Chem., 2022, 3779–3784 Search PubMed.
- A. Padwa, P. Rashatasakhon and M. Rose, J. Org. Chem., 2003, 68, 5139–5146 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2073382. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00067f |
| ‡ Co-first authors. |
| This journal is © The Royal Society of Chemistry 2024 |