Peng-Fei Huang, Jia-Le Fu, Jia-Jing Huang, Bi-Quan Xiong, Ke-Wen Tang and Yu Liu
Org. Biomol. Chem., 2024,22, 513-520
DOI:
10.1039/D3OB01915B,
Paper
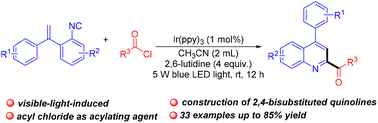
We herein report an efficient photoredox radical cyclization reaction of o-vinylaryl isocyanides with acyl chlorides to access a wide range of 2,4-disubstituted quinolines. Preliminary mechanism experiment results suggested that this reaction was initiated by an acyl radical generated from acyl chlorides through a single-electron-transfer (SET) process. This transformation showed good substrate suitability and functional group compatibility at room temperature.