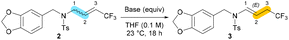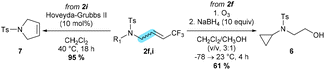Open Access Article
Open Access ArticleMetal free regio – and stereoselective semireduction of CF3-substituted N-allenamides†
Clément
Gommenginger‡
 ,
Maxime
Hourtoule‡
,
Maxime
Hourtoule‡
 ,
Marco
Menghini
,
Marco
Menghini
 and
Laurence
Miesch
and
Laurence
Miesch
 *
*
Equipe Synthèse Organique et Phytochimie, Institut de Chimie, CNRS-UdS, UMR 7177, 4 rue Blaise Pascal, CS 90032, 67081 Strasbourg, France. E-mail: lmiesch@unistra.fr
First published on 30th December 2023
Abstract
We developed a chemoselective metal-free access for the 1,2- and 2,3-semireduction of CF3-N-allenamides. The enamide functionality of CF3-substituted N-allenamides could be efficiently reduced by Et3SiH/BF3·OEt2 in total regioselectivity and good stereoselectivity, whereas DBU promoted the isomerization of the resulting allyl amide leading exclusively to the E-configurated enamide.
Introduction
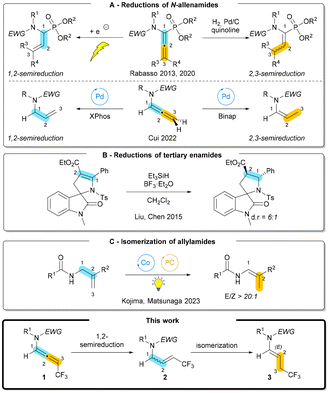
Allenamides represent a privileged subclass of allenes and have become key substrates in myriad transformations,1 including those leading to the construction of nitrogen-containing scaffolds.2 Regioselective semireduction of N-allenamides paves the way for the synthesis of both enamides and allylic amides, two ubiquitous classes of synthetic intermediates critical for the synthesis of heterocyclic compounds. Enamides and allylic amides represent bioactive pharmacophores in drug molecules and have become more popular reagents for the incorporation of nitrogen-based functional groups.3 In particular, unsaturated nitrogen containing fluorinated compounds are of ongoing interest in synthetic methodologies.4 Nonetheless, the semireduction of allenes is confronted with significant challenges such as the control of chemo-, regio-, and stereo-selectivities. Both π-bonds can be reduced to the corresponding alkane, and the differentiation of 1,2- vs. 2,3-semireduction becomes complicated.5 Furthermore, the stereocontrol of the formed alkene adds another challenge to the problematic semireduction of allenes. In the case of N-allenamides, the problems remain and in addition, these points have been less explored for those substrates. Specific semireduction has been examined by classical methods and specific tools including electrochemical reduction. Notably, Rabasso et al. have reported the chemo- and stereoselective reduction of α-amino allenylphosphonates to α-amino vinylphosphonates by using H2 and a poisoned palladium catalyst.6 Recently, these authors developed an electrochemical process to reduce the tertiary enamide moiety of α-amino allenylphosphonates (Scheme 1A).7 More recently, Cui et al. reported a ligand-controlled regiodivergent semireduction. XPhos and BINAP allowed a switch between the 1,2- and 2,3-semireduction reactions with the same palladium precatalyst (Scheme 1A).8Documented examples of either the reduction of tertiary enamides or the isomerization of allylamides are rare. In this regard, spirocyclic oxindoles have been efficiently reduced by a combination of Et3SiH and BF3·OEt2 (Scheme 1B).9 A combination of cobalt and photoredox catalysis was used to promote an isomerization by a hydrogen atom transfer to produce polysubstituted enamides (Scheme 1C).10
Results and discussion
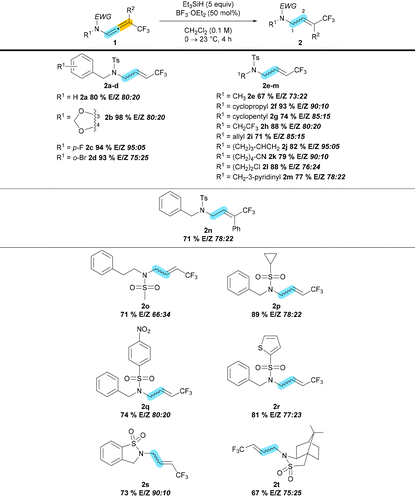
Herein we report a formal metal-free 1,2- and 2,3-semireduction of CF3-substituted N-allenamides. Given the unusual reactivity of N-allenamides and inspired by literature precedents, we first examined the possibility of discriminating one functional group in our substrate i.e., either the enamide-substituted alkene or the trifluoromethyl-functionalized olefin. Importantly, delocalization of the nitrogen lone pair toward the allenic moiety confers a dual reactivity to these entities and dictates the addition of a nucleophile α or γ to the nitrogen atom.11 Trifluoromethylated N-allenamides were obtained by treatment of terminal ynamides with trifluoromethylated diazomethane according to our previously developed strategy.12We anticipated that the enamide moiety would be reduced by using a mixture of a hydride source with a Lewis acid similar to systems used with simple enamides.9,13 Gratifyingly, as shown in Table 1, when CF3-N-allenamide 1 was exposed to a mixture of Et3SiH and BF3·OEt2, N-allyl amide 2 was exclusively obtained as a E/Z mixture (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) with 90% yield (Table 1, entry 1). Next, diverse Lewis acids were investigated (Table 1, entries 2–6).
15) with 90% yield (Table 1, entry 1). Next, diverse Lewis acids were investigated (Table 1, entries 2–6).
| Entrya | Acid (equiv.) | Time (h) | Ratio E/Zb | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: to a solution of 1 (0.15 mmol) in CH2Cl2 (1.5 mL), were added Et3SiH (0.75 mmol) and the acid at 0 °C and then the mixture was stirred at 23 °C. b E/Z ratio was determined by 1H-NMR-spectroscopy. c Isolated yields. d Only degradation of starting material was observed. e The starting material was fully recovered. f Hantzsch ester (5 equiv.) was used instead of Et3SiH, and the reaction mixture was stirred at 40 °C. g 1.2 equiv. of Et3SiH was used instead of 5 equiv. | ||||
| 1 | BF3·OEt2 (5) | 1 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
90 |
| 2 | FeCl3 (0.2) | 1 | —d | —d |
| 3 | InCl3 (0.2) | 6 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
89 |
| 4 | TMSOTf (5) | 1 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
34 |
| 5 | AgOTf (0.2) | 4 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
75 |
| 6 | AgNTf2 (0.2) | 2 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
78 |
| 7 | Tf2O (5) | 1 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 53 |
51 |
| 8 | AcOH (5) | 4 | —e | —e |
| 9f | BF3·OEt2 (5) | 4 | —d | —d |
| 10g | BF3·OEt2 (1.2) | 1 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
45 |
| 11 | BF3·OEt2 (0.5) | 4 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
98 |
| 12 | — | 18 | —e | —e |
Whereas FeCl3 only led to degradation of the starting material, InCl3, TMSOTf, AgNTf2 provided the desired 1,2-semireduction of the CF3-N-allenamide 1, although with lower selectivities except for AgNTf2 (Table 1, entry 6). Notably, the selectivity was inverted by employing AgOTf as Lewis acid (Table 1, entry 5). Even though the desired compound was obtained with triflic anhydride (Tf2O), there was almost no selectivity in this case (Table 1, entry 7). Unfortunately, with AcOH instead of BF3·OEt2 the starting material was fully recovered (Table 1, entry 8). Replacing Et3SiH by Hantzsch ester or lowering the amount of Et3SiH was not effective (Table 1, entries 9 and 10). There was no significant erosion in selectivity when the loading of Lewis acid was lowered to 0.5 mol%, and a good yield (98%) was obtained in this case (Table 1, entry 11). Without any Lewis acid the reduction did not take place (Table 1, entry 12).
Using the determined optimal conditions, the scope of the reaction was examined (Table 2). Aryls (2a–d), hetero-aryls (2m), alkyls (2e), cycloalkyls (2f–g), as well as functionalized side-chains (2h–l) were accommodated in this transformation. The disubstituted N-allenamide 2n underwent the 1,2-semireduction with 71% yield. Not only tosyl derivatives but also mesyl (2o), nosyl (2q), cyclopropyl (2p), thienyl (2r), as well as cyclic sulfonamides (2s–t) participated in this reduction. In this study, carbamates could not be investigated because we failed to obtain the corresponding CF3-substituted N-allenamides.
Next, we wondered whether it would be possible to deprotonate the α-position of the nitrogen of the N-allyl-amide 2. When the latter was treated with t-BuOK, enamide 3 was obtained, albeit with a moderate yield (Table 3, entry 1). The formation of an exclusive E-configured tertiary enamide 3 resulted formally in a 2,3-semireduction of CF3-N-allenamide 1. Whereas cesium carbonate was ineffective (Table 3, entry 2) for this reaction, tertiary ammonium salts showed acceptable yields of the target compound 3 (Table 3, entries 3–6). Although triethylamine (Et3N) and tetramethyguanidine (TMG) were unproductive (Table 3, entries 7 and 8), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) was well-suited for this transformation and provided the best yield for the formation of enamide 3 (Table 3, entry 9). Lowering the amount of DBU caused a decrease in the yield of the target compound (Table 3, entry 10).
| Entrya | Base (equiv.) | Yieldb (%) |
|---|---|---|
| a Reaction conditions: to a solution of 2 (0.10 mmol) in THF (1.0 mL), the base was added, and then the mixture was stirred at 23 °C for 18 h. b Isolated yields. c The starting material was fully recovered. | ||
| 1 | t-BuOK (5) | 30 |
| 2 | Cs2CO3 (5) | —c |
| 3 | TBAF (1M THF) (5) | 48 |
| 4 | TBAF (1M THF) (1.3) | 57 |
| 5 | Triton B (40% wt. CH3OH) (5) | 12 |
| 6 | Triton B (40% wt. H2O) (1.3) | 66 |
| 7 | Et3N (5) | —c |
| 8 | TMG (1.3) | —c |
| 9 | DBU (1.3) | 89 |
| 10 | DBU (50 mol%) | 78 |
We then focused on the generality of this formal 2,3-semireduction. Various benzylamine derivatives were examined to react with DBU. As shown in Scheme 2, neutral (3a), different functional groups on the benzyl ring were tolerated (3b–d). Good yields were obtained as well with the disubstituted compound 3n. X-Ray analyses of 3b confirmed the structure of the E-configured enamide 3b obtained (CCDC 2301994† contains the supplementary crystallographic data for the structure).14 Linear alkyls (3e), cyclic alkyls (3f–g), as well as fluorinated side-chains (3h) on the nitrogen atom were compatible with this transformation. Moreover, the reaction proceeded smoothly in the presence of functionalized side-chains including unsaturated (3i–j), nitrile (3k), halogen (3l), disulfonamide (3u), pyridine (3m), as well as tetralone (3v) substituents. Enamides (3u, 3v) were directly obtained in a sequential two step procedure from the corresponding N-allenamides because of the instability of allylamides 2u and 2v. Good yields were also obtained through modification of the electron-withdrawing group on the nitrogen atom (3o–r). Even the hindered cyclic sulfonamides exhibited good reactivity with the current protocol (3s–t).
To gain further insights into the reaction mechanism, we subjected E- and Z-configured allyl-amides 2t-E and 2t-Z to DBU. E-Enamide 3t was the sole product formed in 83% and 75% yield, resepectively from the two isomeric starting materials (Scheme 3A), establishing that both stereoisomers 2t-E and 2t-Z react and form the same compound 3t. Moreover, when terminal N-allenamide 4 was treated with Et3SiH and BF3·OEt2, the totally reduced amide 5 was isolated in 42% yield, confirming that with unsubstituted N-allenamides 4, a 1,4-addition of hydride takes place first, followed by reduction of the enamide formed (Scheme 3B).11 Based on literature and on the experimental results, we propose the following mechanism (Scheme 3C). Activation of the CF3-N-allenamide with BF3·OEt2 results in the formation of conjugated iminium ion A. Hydride addition occurs exclusively on the iminium ion, affording allylamides 2.13 No addition on the trifluoromethylated alkene has been observed. Accordingly, addition of Et3SiH to CF3-N-allenamide 1 (Table 1, entry 12) did not promote the addition of hydride on the trifluoromethyl-substituted alkene moiety. Deprotonation of 2 by DBU and subsequent delocalization of the carbanion α to the CF3 moiety produces B. Protonation of the latter finally forms the thermodynamically more stable E-configured tertiary enamide 3.
Unfortunately, when we tried to deprotect the nosyl group in 2q, deprotection and isomerization took place simultaneously leading to an unstable enamine. We then carried out some additional transformations with allyl-amides as depicted in Scheme 4. Reductive ozonolysis of 2f took place forming alcohol 6, key building blocks for the construction of heterocyclic compounds in drug design.15 Additionally, the requisite functionalized allyl-amide 2i was transformed into unsaturated tosylsulfonamide 7 with good yields (Scheme 4).
Conclusions
In conclusion, we achieved a 1,2-semireduction of CF3-substituted N-allenamides with Et3SiH and BF3·OEt2. An isomerization of the formed allylamides in the presence of DBU resulted in a 2,3-semireduction of the CF3-N-allenamide. We offer a metal-free facile and practical method for the regioselective access to various allylamides bearing a vinyl-CF3 moiety as well as the formation of corresponding tertiary enamides. A wide variety of side chains could be adapted at the nitrogen atom including functional groups together with unique sulfonyl derivatives.Author contributions
C. G. and M. H. contributed equally. L. M., C. G., and M. H. conceived and designed the experiments. L. M. directed the project. C. G., M. H., M. M. performed the experiments. L. M. wrote the paper. L. M., M. H., and C. G. discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Support for this work was provided by CNRS and Université de Strasbourg. C. G. and M. H. thanks M.R.T. for a research fellowship.References
- For selected recent developments in N-allenamide chemistry: (a) T. R. Pradhan, H. W. Kim and J. K. Park, Angew. Chem., Int. Ed., 2018, 57, 9930–9935 CrossRef CAS PubMed; (b) J. N. Ayres, M. T. J. Williams, G. J. Tizzard, S. J. Coles, K. B. Ling and L. C. Morrill, Org. Lett., 2018, 20, 5282–5285 CrossRef CAS PubMed; (c) J. Hédouin, C. Schneider, I. Gillaizeau and C. Hoarau, Org. Lett., 2018, 20, 6027–6032 CrossRef PubMed; (d) R. Blieck, R. Abed Ali Abdine, M. Taillefer and F. Monnier, Org. Lett., 2018, 20, 2232–2235 CrossRef CAS PubMed; (e) D. C. Marcote, I. Varela, J. Fernández-Casado, J. L. Mascareñas and F. López, J. Am. Chem. Soc., 2018, 140, 16821–16833 CrossRef CAS PubMed; (f) S. Samanta and A. Hajra, J. Org. Chem., 2018, 83, 13157–13165 CrossRef CAS PubMed; (g) J. An, L. Lombardi, S. Grilli and M. Bandini, Org. Lett., 2018, 20, 7380–7383 CrossRef CAS PubMed; (h) L. Chen, J. Yu, S. Tang, Y. Shao and J. Sun, Org. Lett., 2019, 21, 9050–9054 CrossRef CAS PubMed; (i) S. Banerjee, B. Senthilkumar and N. T. Patil, Org. Lett., 2019, 21, 180–184 CrossRef CAS PubMed; (j) P. Pertejo, I. Carreira-Barral, P. Peña-Calleja, R. Quesada and M. García-Valverde, J. Org. Chem., 2020, 85, 2291–2302 CrossRef CAS PubMed; (k) F. Zhong, Q. Xue and L. Yin, Angew. Chem., Int. Ed., 2020, 59, 1562–1566 CrossRef CAS PubMed.
- (a) L. Wei, H. Xiong and R. P. Hsung, Acc. Chem. Res., 2003, 36, 773–782 CrossRef CAS PubMed; (b) T. Lu, Z. Lu, Z.-X. Ma, Y. Zhang and R. P. Hsung, Chem. Rev., 2013, 113, 4862–4904 CrossRef CAS PubMed; (c) G. Broggini, G. Poli, E. M. Beccalli, F. Brusa, S. Gazzola and J. Oble, Adv. Synth. Catal., 2015, 357, 677–682 CrossRef CAS; (d) M. Hourtoule and L. Miesch, Org. Lett., 2023, 25, 1727–1731 CrossRef CAS PubMed; (e) C. Gommenginger, Y. Zheng, D. Maccarone, I. Ciofini and L. Miesch, Org. Chem. Front., 2023, 10, 4055–4060 RSC.
- (a) S. J. Meek, R. V. O'Brien, J. Llaveria, R. R. Schrock and A. H. Hoveyda, Nature, 2011, 471, 461–466 CrossRef CAS PubMed; (b) B. M. Trost, J. J. Cregg and N. Quach, J. Am. Chem. Soc., 2017, 139, 5133–5139 CrossRef CAS PubMed; (c) G. Choi, S. Jo, J. Mun, Y. Jeong, S.-H. Kim and J.-W. Jung, Molecules, 2019, 24, 4495 CrossRef PubMed; (d) F. Beltran and L. Miesch, Synthesis, 2020, 2497–2511 CAS; (e) T. Varlet and G. Masson, Chem. Commun., 2021, 57, 4089–4105 RSC; (f) J. C. Vantourout and S. B. Beil, Trends Chem., 2022, 4, 569–572 CrossRef CAS.
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed; (b) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305–321 CrossRef CAS; (c) D. E. Yerien, S. Barata-Vallejo and A. Postigo, ACS Catal., 2023, 13, 7756–7794 CrossRef CAS.
- (a) W. Bonrath, J. A. Medlock and M. A. Müller, Science of Synthesis, vol. 5, 2017, pp. 195–228 Search PubMed; (b) T. Mandai, Modern Allene Chemistry, 2004, pp. 925–972 Search PubMed.
- P. Adler, F. Gomes, A. Fadel and N. Rabasso, Eur. J. Org. Chem., 2013, 7546–7555 CrossRef CAS.
- P. Adler, P. de Oliveira and N. Rabasso, Eur. J. Org. Chem., 2020, 3918–3925 CrossRef CAS.
- M. Hajiloo Shayegan, Z. Li and X. Cui, Chem. – Eur. J., 2022, 28, e202103402 CrossRef CAS PubMed.
- P.-F. Zheng, Q. Ouyang, S.-L. Niu, L. Shuai, Y. Yuan, K. Jiang, T.-Y. Liu and Y.-C. Chen, J. Am. Chem. Soc., 2015, 137, 9390–9399 CrossRef CAS PubMed.
- Y. Seino, Y. Yamaguchi, A. Suzuki, M. Yamashita, Y. Kamei, F. Kamiyama, T. Yoshino, M. Kojima and S. Matsunaga, Chem. – Eur. J., 2023, 29, e202300804 CrossRef CAS PubMed.
- M. Hourtoule and L. Miesch, Org. Biomol. Chem., 2022, 20, 9069–9084 RSC.
- Y. Zheng, B. Moegle, S. Ghosh, A. Perfetto, D. Luise, I. Ciofini and L. Miesch, Chem. – Eur. J., 2022, 28, e202103598 CrossRef CAS PubMed.
- F. Beltran, L. Andna and L. Miesch, Org. Chem. Front., 2019, 6, 373–376 RSC.
- CCDC 2301994 (3b)† contains the supplementary crystallographic data for this paper.
- B. Lv, D. Cui, Q. Zhang, C. Chai, H. Liang and D. Zhang, (Suzhou Zelgen Biopharmaceuticals Co Ltd, Shanghai Zelgen Pharma Tech Co Ltd), WO2022171018A1, 2022.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2301994. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ob01859h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |