Diastereoselective synthesis of trans-2,3-dihydroindoles via formal [4 + 1] annulation reactions of a sulfonium ylide†
Received
2nd November 2023
, Accepted 15th December 2023
First published on 18th December 2023
Abstract
We have established an in situ generated sulfonium-ylide mediated annulation to construct 2,3-disubstituted-2,3-dihydroindoles. The [4 + 1] annulation approach relied on Michael addition/substitution reactions. These reactions were carried out at ambient temperature to deliver dihydroindoles with excellent yields and diastereoselectivities. Moreover, the versatility of this approach allows for the introduction of various functional groups, enabling further diversification of the dihydroindoles. Also, the cascade approach was broadened to synthesize dihydrobenzofurans.
Introduction
[4 + 1] Annulation is an effective technique that enables the construction of diversely substituted five-membered rings in a one-pot operation.1–4 It is successfully mediated by a number of combinations of A4- and A1-synthons (Scheme 1), which produce a variety of five-membered heterocyclic compounds, including fused and spirocyclic frameworks.1–4 Various Michael acceptors, including conjugated carbonyl compounds, conjugated imines, azoalkenes, and many more, often serve as A4-synthons in [4 + 1] annulation reactions.5,6 Besides a variety of A1-synthons,7–9 ylides are one of the most powerful and versatile synthons in synthetic organic chemistry, offering a wide range of reactivity patterns and functional group compatibility.10,11 Consequently, they hold great potential for synthesizing complex natural and biologically active compounds.12 They have both electrophilic and nucleophilic centers localized on the same carbon atom. Among the manifold applications of ylides, their participation in formal [4 + 1] annulation reactions stands out as the most prominent and promising area of research. Several cascade approaches have been established utilizing ylide chemistry to access N-heterocycles with remarkable efficiency and selectivity.10,11,13–18
 |
| | Scheme 1 General strategy of the [4 + 1] annulation. | |
N-heterocycles have significant importance in medicinal chemistry, drug discovery, and the synthesis of natural products.19–21 In particular, 2,3-disubstituted dihydroindoles (DHIs) play key roles in the development of new pharmacological drugs. DHIs are prevalent in various bioactive compounds and alkaloids, e.g., vindoline,22 aspidospermidine,23 strychnine,24 flustramine B,25 physostigmine,26 and WAY-163909![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (Fig. 1).
27 (Fig. 1).
 |
| | Fig. 1 Selected examples of bioactive indolines. | |
The majorly reported strategy for the synthesis of DHIs is reduction of indoles via catalytic hydrogenation or dearomative annulation.28,29 In the last decade, synthetic techniques for dihydroindoles have expanded to other organo- as well as metal-catalysed approaches.30–33 Recently, our group has demonstrated a supported pyridinium ylide-mediated cascade synthesis of trans-2,3-dihydroindoles34 and trans-2,3-dihydrobenzofurans.35 In continuation of our study, we hypothesized that a sulfonium salt would participate in the in situ generation of a sulfonium ylide. This sulfonium ylide might then undergo a 1,4-conjugate addition with an ortho-aminochalcone followed by subsequent cyclization through N-substitution, yielding the corresponding DHIs.
Results and discussion
At the outset, an experiment was conducted wherein a reaction was performed using phenacyl sulfonium salt 1a and ortho-aminochalcone 2aa in the presence of triethylamine as a base at ambient temperature in toluene. Pleasingly, the cascade reaction led to 2,3-disubstituted dihydroindole 3aaa in a viable yield (Table 1, entry 1). The structure of product 3aaa as a trans-isomer was confirmed by single crystal X-ray analysis.
Table 1 Optimization of the cascade reaction conditions with different bases
Next, we conducted a systematic investigation of several organic and inorganic bases and solvents to determine the optimal reaction conditions for this cascade approach. Almost a similar outcome was observed with N-ethyl diisopropylamine as the base (Table 1, entry 2). When the reaction was carried out with stronger bases, namely 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), significant reduction in the reaction time was observed (Table 1, entries 3 and 4). However, the yield of the desired product was unaltered. A slight increment in the yield was noted with N-methyl pyrrolidine (NMP) and N-methyl piperidine (NMPPR) (Table 1, entries 5 and 6). The yield of product 3aaa was drastically decreased with the inorganic base sodium carbonate (Table 1, entry 7). Notably, cesium carbonate was identified as an appropriate base in terms of yield and time. Subsequently, several solvents were evaluated (Table 2). Eventually, toluene emerged as the most favourable solvent for this cascade approach. To further check the reactivity and selectivity of the reaction, we explored the cyclic sulfonium salt of phenacyl bromide (Table 2, entries 7 and 8). Remarkably, the cascade reaction proceeded smoothly, albeit with a slight reduction in the yield. After thorough evaluation, the dimethyl sulfonium salt 1a was identified as the optimal A1-synthon for this cascade approach, offering maximum efficiency.
Table 2 Effect of solvents
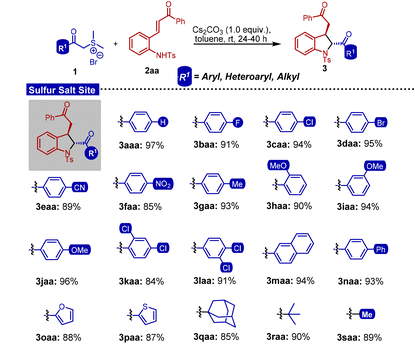
Having established the optimal reaction conditions, we explored the substrate generality of this [4 + 1] annulation reaction (Tables 3 and 4). At first, we examined the effect of the substitution pattern on the phenacyl sulfonium salt (Table 3). Specifically, we investigated the effects of introducing halogens (1b–d) (fluoro, chloro, and bromo), electron-withdrawing groups (1e and 1f) (cyano and nitro), and the methyl group 1g at the para-position of the aryl ring of compound 1. Remarkably, all of these led to the corresponding DHIs (3baa–gaa) in excellent yields.
Table 3 Substrate scope of sulfonium salts
Reaction conditions: 1 (0.3 mmol), 2aa (0.2 mmol), Cs2CO3 (0.2 mmol), and toluene (2 mL), unless specified. Diastereoisomeric ratios for all entries (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were determined by 1H-NMR analysis. 1) were determined by 1H-NMR analysis. |
|
Table 4 Substrate scope of ortho-aminochalcones
Reaction conditions: 1a (0.3 mmol), 2 (0.2 mmol), Cs2CO3 (0.2 mmol), and toluene (2 mL), unless specified. Diastereoisomeric ratios for all entries (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were determined by 1H-NMR analysis. 1) were determined by 1H-NMR analysis. |

|
The introduction of an electron-donating group, specifically methoxy (1h–j), at the ortho-, meta- and para-positions of the aryl ring was observed to be well tolerated under the optimized conditions. Excellent yields were observed when dichloro-substitution was introduced at both the 2,5- and 3,4-positions (1k and 1l) of the aryl ring in sulfonium salt 1. Importantly, 2-naphthyl (1m) and biphenyl (1n) substituted sulfonium salts also resulted in the formation of the corresponding DHIs (3maa and 3naa) in excellent yields. Additionally, we discovered that sterically and electronically diverse R1 groups, including heterocycles (1o and 1p) i.e., 2-furyl and 2-thiophenyl, and aliphatic groups (1q–s) i.e., adamantyl, tert-butyl, and methyl, were also well suited for the reaction and successfully transformed into the desired dihydroindoles. Next, our investigation focused on elucidating the effect of substitution on ortho-aminochalcones 2 (Table 4). To begin with, the influence of halogens on the 5-position of the aryl ring was investigated and fluoro, chloro, and bromo substituted DHIs were constructed (3aba–3ada). To further expand the scope of the [4 + 1] annulation reaction, we varied the R2 group of ortho-aminochalcone 2. For instance, halogen, cyano, and methyl were strategically introduced at the para-positions of the aryl ring of compound 2. Remarkably, all of these substitutions were well tolerated. Amenable outcomes were obtained when the R2 group was represented by differently substituted methoxy groups on the aryl ring. Additionally, a favourable outcome was observed with R2 groups as 2-naphthyl, biphenyl, heteroaryl, and alkyl. Notably, it was seen that all the dihydroindoles were obtained exclusively as a single trans-isomer.
Furthermore, we explored various sulfonium salt derivatives (1t–1v) including secondary sulfonium salt 1w (Table 5). Among them, ester-derived sulfonium salt 1t was found to be efficient, leading to dihydroindole 3taa. However, the cascade reaction with other salts (1u–1w) did not yield the anticipated products. Next, the reaction with the electronically distinct ortho-aminochalcone bearing ester functionality 2ap provided a trace amount of the corresponding DHI at elevated temperatures.
Table 5 Substrate scope
| Reaction conditions: 1 (0.3 mmol), 2 (0.2 mmol), Cs2CO3 (0.2 mmol), and toluene (2 mL), unless specified. The reaction was conducted at 80 °C and the formation of a trace amount of the product was confirmed by HRMS analysis and 1H NMR of the crude reaction mixture. |

|
Drawing upon insights from the previous reports,34,35 a plausible mechanism for this [4 + 1] annulation reaction was proposed, as shown in Fig. 2. The cascade reaction is initiated with base promoted in situ generation of a sulfonium ylide, which functioned as the active A1 synthon. The in situ generated sulfonium ylide then reacts with aminochalcone 2 in a conjugate addition fashion, forming intermediate A. Notably, the sulfonium ylide can also be prepared and later used in the cascade reaction with or without any base to provide the same products (Table 6). This suggests that the base has a minimal role in the subsequent steps. A proton is transferred from –NHTs to the enolate/carbanion leading to B. HRMS data analysis of the crude reaction mixture showed the presence of a protonated form of intermediate A (or B). Finally, an intramolecular nucleophilic substitution by an amine led to the formation of trans-DHIs 3 with the removal of dimethyl sulfide.
 |
| | Fig. 2 Plausible mechanism of the [4 + 1] annulation approach. | |
Table 6 Study of the protecting amino group of 2
| Reaction conditions: 1 (0.3 mmol), 2 (0.2 mmol), and toluene (2 mL), unless specified. The reaction was conducted with 10 mol% of Cs2CO3 and the reaction was completed in 24 h. |

|
To demonstrate the practicality and scalability of the developed methodology, the [4 + 1] annulation reaction was carried out at the gram-scale, resulting in the formation of product 3aaa in excellent yield and dr (Scheme 2).
 |
| | Scheme 2 Cascade synthesis at the gram-scale. | |
Furthermore, the [4 + 1] annulation reaction was also evaluated using the preformed sulfonium ylide 1a′. Although, the reaction took a longer time to achieve completion in the absence of any base, the desired DHI 3aaa was formed in a comparable yield (Table 6). With a catalytic amount of Cs2CO3 (10 mol%), the reaction was relatively faster. Next, we tested a variety of protecting groups including Bn and Boc on the nitrogen of ortho-aminochalcone 2. However, the corresponding DHIs did not form with the Bn- and Boc-groups. Surprisingly, the Boc-group protected amine yielded a three-membered product (6aaa′) in a moderate yield.
Next, product 3aaa was transformed into tricyclic diazepino-indole derivative 4aaa. Interestingly, during this transformation, an intriguing observation on the removal of the tosyl group was made (Scheme 3).
 |
| | Scheme 3 Synthetic transformations. | |
To access enantioenriched DHIs, reactions were carried out with sulfonium salt 1a and ortho-aminochalcone 2aa in the presence of chiral cyclohexane-diamine derived H-bond donor catalysts C1–C3 (Scheme 4). Pleasingly, the cascade reaction proceeded smoothly at room temperature to afford the desired product 3aaa in a good yield. However, no enantiofacial selectivity was achieved. On the other hand, a similar outcome was observed in the reaction of the preformed sulfur ylide 1a′ and ortho-aminochalcone 2aa in the presence of Cinchona-derived bifunctional catalysts C4–C6 (Scheme 4).
 |
| | Scheme 4 Asymmetric variant of the cascade [4 + 1] annulation approach. | |
Furthermore, the scope of the reaction was expanded by replacing the aminochalcone with an ortho-hydroxychalcone. To our delight, the corresponding DHBs 8 were formed in excellent yields and diastereomeric ratios (Table 7).
Table 7 Synthesis of DHBs
Reaction conditions: 1 (0.3 mmol), 7 (0.2 mmol), Cs2CO3 (0.2 mmol), and toluene (2 mL), unless specified. Diastereoisomeric ratios for all entries (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were determined by 1H-NMR analysis. 1) were determined by 1H-NMR analysis. |

|
Conclusions
We have developed a [4 + 1] annulation reaction of in situ generated sulfonium ylides with ortho-aminochalcones for the synthesis of trans-dihydroindoles at room temperature. This cascade approach exhibited tolerance towards a wide variety of substrate groups, demonstrating its ability and versatility to accommodate diverse chemical functionalities. Additionally, successful gram-scale synthesis demonstrates its feasibility and scalability in large-scale manufacturing processes, highlighting its potential for future applications. Importantly, this cascade [4 + 1] annulation reaction was found to be efficient at room temperature under mild reaction conditions. Unlike ammonium ylides which are relatively unstable and cannot be isolated, sulfur ylides can easily be prepared and the same can later be employed in the reactions. All the synthesized dihydroindole derivatives might have significant potential as good candidates for drug discovery and synthetic manipulations. Furthermore, the developed methodology was extended to construct dihydrobenzofurans.
Experimental section
General information
Unless otherwise noted, all reactions were carried out in a closed vial. 1H NMR spectra were recorded on a 500 MHz spectrometer (125 MHz for 13C{1H} NMR). The following abbreviations were used to designate chemical shift multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, and m = multiplet. An oil bath on top of a hot plate was used for heating wherever required. TLC was performed using silica gel GF254 precoated on aluminium plates and spots were visualized with UV. Flash column chromatography was performed on silica gel with the use of CombiFlash. IR spectra were recorded on a FT-IR spectrometer and only major peaks were reported in cm−1. High-resolution mass spectra were obtained by the ESI-TOF method (Agilent LC/Q-TOF 6546). Sulfonium salt 1, ortho-aminochalcones 2 and ortho-hydroxychalcones 7 were prepared according to the reported methods.36 All the other reagents were purchased from commercial sources and used as received, unless specified.
General procedure for the synthesis of product 3
The pre-formed sulfonium salt 1 (0.3 mmol) and ortho-aminochalcone 2 (0.2 mmol) were added to toluene (2 mL). After stirring the mixture for 5 min, Cs2CO3 (65.2 mg, 0.2 mmol) was added and stirring was continued at rt. The progress of the reaction was monitored by TLC. After the completion of the reaction, the crude product 3 was purified by flash column chromatography on a silica support (hexane/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Synthetic procedure for the asymmetric synthesis of product 3aaa.
The pre-formed sulfonium salt 1a (0.3 mmol) and ortho-aminochalcone 2aa (0.2 mmol) were added to toluene (2 mL). The catalyst (C1–C6) (0.02 mmol, 10 mol%) was then added. After stirring the mixture for 5 min, K2CO3 (0.2 mmol) was added and stirring was continued at rt. The progress of the reaction was monitored by TLC. After the completion of the reaction, the crude product 3aaa was purified using flash chromatography with hexane/ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluents and silica gel (100–200 mesh) as the stationary solid phase.
1) as eluents and silica gel (100–200 mesh) as the stationary solid phase.
Synthetic procedure for the scale-up synthesis of product 3aaa.
The pre-formed sulfonium salt 1a (1.04 g, 3.98 mmol) and ortho-aminochalcone 2aa (1.0 g, 2.65 mmol) were added to toluene (25 mL). After stirring the mixture for 5 min, Cs2CO3 (0.86 g, 2.65 mmol) was added and stirring was continued at rt. The progress of the reaction was monitored by TLC. After the completion of the reaction, the crude product 3aaa was purified by flash column chromatography on a silica support (hexane/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-phenylethan-1-one (3aaa).
White solid, yield = 97% (96.1 mg); 1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 7.6 Hz, 2H), 7.64–7.61 (m, 5H), 7.56–7.46 (m, 2H), 7.43–7.40 (m, 2H), 7.37–7.34 (m, 2H), 7.21 (t, J = 7.9 Hz, 1H), 7.11 (d, J = 7.8 Hz, 2H), 7.03 (d, J = 7.3 Hz, 1H), 6.96 (t, J = 7.4 Hz, 1H), 5.32 (d, J = 2.7 Hz, 1H), 3.82 (s, 1H), 2.89 (dd, J = 17.8, 6.0 Hz, 1H), 2.50 (dd, J = 17.8, 7.7 Hz, 1H), 2.27 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.01, 195.58, 144.47, 141.61, 136.24, 135.18, 134.79, 133.74, 133.71, 133.70, 129.98, 129.32, 128.94, 128.81, 128.76, 128.10, 127.50, 125.11, 124.76, 116.13, 69.80, 44.87, 41.09, 21.70; IR (ATR): ν 3187, 3103, 2980, 2888, 1795, 1772, 1738, 1716, 1695, 1683, 1651, 1634, 1557, 1520, 1506, 1474, 1420, 1362, 1275 cm−1; HRMS (ESI+) calc. for C30H26NO4S [M + H]+: 496.1577, found: 496.1585.
Enantioenriched 3aaa was isolated as a white solid. Enantiomeric excess of the product was determined by chiral stationary phase HPLC analysis using a ChiralPak IA column (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 hexane/i-PrOH at 1 mL min−1, λ = 254 nm); major enantiomer: tR = 15.522 min, minor enantiomer: tR = 28.131 min.
30 hexane/i-PrOH at 1 mL min−1, λ = 254 nm); major enantiomer: tR = 15.522 min, minor enantiomer: tR = 28.131 min.
2-(2-(4-Fluorobenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3baa).
White solid, yield = 91% (93.5 mg); 1H NMR (500 MHz, CDCl3) δ 8.19–8.05 (m, 2H), 7.68 (dd, J = 15.5, 7.7 Hz, 5H), 7.58 (t, J = 7.3 Hz, 1H), 7.44 (t, J = 7.7 Hz, 2H), 7.29 (t, J = 7.6 Hz, 1H), 7.21–7.12 (m, 4H), 7.10 (d, J = 7.2 Hz, 1H), 7.05 (t, J = 7.4 Hz, 1H), 5.30 (d, J = 3.3 Hz, 1H), 3.92–3.79 (m, 1H), 3.01 (dd, J = 18.0, 5.4 Hz, 1H), 2.50 (dd, J = 18.0, 8.5 Hz, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.16, 194.38, 166.23 (J(C–F) = 253.75 Hz), 144.57, 141.55, 136.16, 134.97, 133.80, 133.77, 132.2 (J(C–F) = 10.00 Hz), 131.25 (J(C–F) = 2.50 Hz), 130.01, 129.00, 128.81, 128.11, 127.49, 124.95, 124.90, 116.17 (J(C–F) = 23.75 Hz), 115.90, 69.58, 44.80, 41.10, 21.71; IR (ATR): ν 3168, 3140, 3138, 3006, 2990, 1796, 1771, 1736, 1716, 1699, 1684, 1652, 1595, 1560, 1541, 1516, 1474, 1415, 1275, 1264 cm−1; HRMS (ESI+) calc. for C30H25FNO4S [M + H]+: 514.1483, found: 514.1472.
2-(2-(4-Chlorobenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3caa).
White solid, yield = 94% (99.6 mg); 1H NMR (500 MHz, CDCl3) δ 8.01 (d, J = 8.6 Hz, 2H), 7.74–7.62 (m, 5H), 7.58 (t, J = 7.4 Hz, 1H), 7.49–7.39 (m, 4H), 7.34–7.27 (m, 1H), 7.16 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 7.3 Hz, 1H), 7.08–7.02 (m, 1H), 5.29 (d, J = 3.4 Hz, 1H), 3.92–3.77 (m, 1H), 3.01 (dd, J = 18.0, 5.4 Hz, 1H), 2.49 (dd, J = 18.0, 8.6 Hz, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.12, 194.85, 144.59, 141.50, 140.22, 136.12, 134.89, 133.80, 133.69, 133.25, 130.84, 130.01, 129.13, 129.01, 128.95, 128.80, 128.10, 127.47, 124.93, 116.27, 69.60, 44.77, 41.05, 21.70; IR (ATR): ν 3158, 3122, 3009, 2988, 1790, 1776, 1735, 1718, 1695, 1652, 1636, 1617, 1560, 1474, 1457, 1419, 1362, 1276, 1263 cm−1; HRMS (ESI+) calc. for C30H24ClNNaO4S [M + Na]+: 552.1007, found: 552.1005.
2-(2-(4-Bromobenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3daa).
White solid, yield = 95% (109.2 mg); 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.6 Hz, 2H), 7.75–7.60 (m, 7H), 7.61–7.54 (m, 1H), 7.43 (t, J = 7.8 Hz, 2H), 7.33–7.27 (m, 1H), 7.16 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 7.3 Hz, 1H), 7.05 (td, J = 7.4, 0.8 Hz, 1H), 5.28 (d, J = 3.5 Hz, 1H), 3.90–3.77 (m, 1H), 3.01 (dd, J = 18.0, 5.4 Hz, 1H), 2.48 (dd, J = 18.1, 8.6 Hz, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.11, 195.06, 144.60, 141.47, 136.09, 134.86, 133.80, 133.67, 133.66, 132.10, 130.90, 130.01, 129.02, 129.00, 128.79, 128.09, 127.45, 124.94, 124.93, 116.26, 69.57, 44.75, 41.03, 21.69; IR (ATR): ν 3150, 3101, 3066, 3006, 2990, 2834, 1795, 1772, 1734, 1717, 1699, 1636, 1559, 1542, 1507, 1474, 1276, 1252 cm−1; HRMS (ESI+) calc. for C30H25BrNO4S [M + H]+: 574.0682, found: 574.0676.
4-(3-(2-Oxo-2-phenylethyl)-1-tosylindoline-2-carbonyl)benzonitrile (3eaa).
White solid, yield = 89% (92.7 mg); 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 8.3 Hz, 2H), 7.79 (d, J = 8.3 Hz, 2H), 7.69 (dd, J = 7.7, 2.3 Hz, 3H), 7.61 (dd, J = 16.6, 7.8 Hz, 3H), 7.45 (t, J = 7.7 Hz, 2H), 7.37–7.28 (m, 1H), 7.18–7.04 (m, 4H), 5.25 (d, J = 3.6 Hz, 1H), 3.87 (dt, J = 8.7, 4.2 Hz, 1H), 3.07 (dd, J = 18.2, 4.8 Hz, 1H), 2.39 (dd, J = 18.2, 9.3 Hz, 1H), 2.32 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.18, 195.35, 144.78, 141.30, 138.51, 135.96, 134.51, 133.94, 133.47, 132.52, 130.09, 129.80, 129.15, 128.85, 128.09, 127.46, 125.18, 124.85, 118.18, 116.67, 116.49, 69.73, 44.64, 40.86, 21.70; IR (ATR): ν 3141, 3115, 3079, 3005, 2990, 1792, 1774, 1734, 1717, 1699, 1635, 1617, 1541, 1505, 1489, 1474, 1419, 1396, 1362, 1274 cm−1; HRMS (ESI+) calc. for C31H25N2O4S [M + H]+: 521.1530, found: 521.1534.
2-(2-(4-Nitrobenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3faa).
White solid, yield = 85% (91.9 mg); 1H NMR (500 MHz, CDCl3) δ 8.33 (d, J = 8.7 Hz, 2H), 8.22 (d, J = 8.7 Hz, 2H), 7.77–7.66 (m, 3H), 7.61 (dd, J = 17.7, 7.8 Hz, 3H), 7.44 (t, J = 7.8 Hz, 2H), 7.38–7.28 (m, 1H), 7.21–7.05 (m, 4H), 5.26 (d, J = 3.6 Hz, 1H), 3.95–3.83 (m, 1H), 3.08 (dd, J = 18.3, 4.7 Hz, 1H), 2.38 (dd, J = 18.3, 9.4 Hz, 1H), 2.32 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.19, 195.35, 150.51, 144.83, 141.27, 140.16, 135.93, 134.44, 133.96, 133.44, 130.42, 130.10, 129.19, 128.85, 128.09, 127.46, 125.22, 124.85, 123.85, 116.52, 69.92, 44.65, 40.85, 21.70; IR (ATR): ν 3130, 3115, 3005, 2989, 2879, 1790, 1771, 1750, 1733, 1716, 1634, 1684, 1663, 1617, 1559, 1522, 1489, 1474, 1434, 1397, 1275, 1262 cm−1; HRMS (ESI+) calc. for C30H25N2O6S [M + H]+: 541.1428, found: 541.1420.
2-(2-(4-Methylbenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3gaa).
White solid, yield = 93% (94.8 mg); 1H NMR (500 MHz, CDCl3) δ 7.94 (d, J = 8.0 Hz, 2H), 7.77–7.61 (m, 5H), 7.56 (t, J = 6.8 Hz, 1H), 7.42 (t, J = 7.1 Hz, 2H), 7.33–7.24 (m, 3H), 7.18 (d, J = 7.8 Hz, 2H), 7.08 (d, J = 7.4 Hz, 1H), 7.01 (t, J = 7.4 Hz, 1H), 5.35 (d, J = 3.3 Hz, 1H), 3.87 (s, 1H), 2.95 (dd, J = 17.8, 6.2 Hz, 1H), 2.60 (dd, J = 17.8, 7.6 Hz, 1H), 2.42 (s, 3H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.04, 195.12, 144.67, 144.41, 141.67, 136.29, 135.26, 133.83, 133.67, 132.18, 129.96, 129.56, 129.47, 128.90, 128.76, 128.12, 127.51, 125.10, 124.69, 116.07, 69.72, 44.89, 41.17, 21.90, 21.71; IR (ATR): ν 3123, 3099, 3006, 2989, 1792, 1771, 1735, 1718, 1698, 1685, 1640, 1620, 1542, 1517, 1474, 1419, 1263 cm−1; HRMS (ESI+) calc. for C31H27NNaO4S [M + Na]+: 532.1553, found: 532.1550.
2-(2-(2-Methoxybenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3haa).
White solid, yield = 90% (94.6 mg); 1H NMR (500 MHz, CDCl3) δ 7.76–7.67 (m, 3H), 7.67–7.60 (m, 3H), 7.55 (t, J = 7.4 Hz, 1H), 7.53–7.47 (m, 1H), 7.41 (t, J = 7.7 Hz, 2H), 7.26–7.20 (m, 3H), 7.10–7.01 (m, 2H), 6.98–6.91 (m, 2H), 5.50 (d, J = 2.8 Hz, 1H), 4.01–3.93 (m, 1H), 3.70 (s, 3H), 2.74 (qd, J = 17.4, 6.8 Hz, 2H), 2.40 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.05, 196.81, 158.40, 144.22, 141.87, 136.53, 135.84, 134.19, 134.17, 133.51, 131.77, 129.85, 128.75, 128.69, 128.04, 127.55, 125.80, 125.43, 124.48, 121.33, 115.97, 111.49, 73.69, 55.59, 45.74, 40.38, 21.76; IR (ATR): ν 3110, 3079, 3055, 3006, 2990, 2888, 1792, 1774, 1750, 1717, 1699, 1679, 1653, 1617, 1521, 1489, 1473, 1419, 1263 cm−1; HRMS (ESI+) calc. for C31H28NO5S [M + H]+: 526.1683, found: 526.1691.
2-(2-(3-Methoxybenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3iaa).
White solid, yield = 94% (98.8 mg); 1H NMR (500 MHz, CDCl3) δ 7.69 (dd, J = 13.1, 4.7 Hz, 5H), 7.62 (d, J = 7.7 Hz, 1H), 7.60–7.53 (m, 2H), 7.40 (dt, J = 19.4, 7.9 Hz, 3H), 7.30–7.26 (m, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.14 (ddd, J = 8.2, 2.6, 0.6 Hz, 1H), 7.09 (d, J = 7.5 Hz, 1H), 7.02 (td, J = 7.5, 0.8 Hz, 1H), 5.35 (d, J = 3.3 Hz, 1H), 3.89 (td, J = 7.0, 3.3 Hz, 1H), 3.83 (s, 3H), 2.95 (dd, J = 17.8, 6.3 Hz, 1H), 2.60 (dd, J = 17.8, 7.7 Hz, 1H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.97, 195.33, 159.94, 144.46, 141.62, 136.25, 136.03, 135.24, 133.71, 133.69, 129.98, 129.77, 128.95, 128.76, 128.10, 127.49, 125.15, 124.74, 121.84, 120.59, 116.09, 113.34, 70.07, 55.52, 44.89, 41.16, 21.70; IR (ATR): ν 3115, 3098, 3006, 2989, 1792, 1770, 1734, 1717, 1699, 1684, 1636, 1559, 1541, 1507, 1489, 1457, 1369, 1275 cm−1; HRMS (ESI+) calc. for C31H28NO5S [M + H]+: 526.1683, found: 526.1684.
2-(2-(4-Methoxybenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3jaa).
White solid, yield = 96% (100.9 mg); 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J = 8.8 Hz, 2H), 7.69 (dt, J = 13.1, 5.9 Hz, 5H), 7.57 (t, J = 7.4 Hz, 1H), 7.43 (t, J = 7.7 Hz, 2H), 7.28 (d, J = 7.6 Hz, 1H), 7.18 (d, J = 8.1 Hz, 2H), 7.09 (d, J = 7.4 Hz, 1H), 7.02 (t, J = 7.5 Hz, 1H), 6.97 (d, J = 8.9 Hz, 2H), 5.34 (d, J = 3.3 Hz, 1H), 3.88 (s, 4H), 2.98 (dd, J = 17.9, 6.0 Hz, 1H), 2.58 (dd, J = 17.9, 7.9 Hz, 1H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.15, 194.02, 164.12, 144.39, 141.72, 136.30, 135.24, 133.97, 133.68, 131.79, 129.95, 128.87, 128.77, 128.13, 127.59, 127.52, 125.04, 124.69, 116.08, 114.11, 69.47, 55.64, 44.88, 41.29, 21.71; IR (ATR): ν 3135, 3109, 3081, 3066, 3006, 2990, 1792, 1772, 1734, 1699, 1684, 1636, 1559, 1541, 1507, 1473, 1457, 1276, 1261 cm−1; HRMS (ESI+) calc. for C31H28NO5S [M + H]+: 526.1683, found: 526.1676.
2-(2-(2,4-Dichlorobenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3kaa).
White solid, yield = 84% (94.8 mg); 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 8.1 Hz, 1H), 7.63–7.54 (m, 5H), 7.49 (d, J = 8.3 Hz, 1H), 7.46–7.38 (m, 3H), 7.33 (dd, J = 8.3, 1.9 Hz, 1H), 7.31–7.26 (m, 1H), 7.13 (d, J = 7.4 Hz, 1H), 7.12–7.04 (m, 3H), 5.22 (d, J = 2.2 Hz, 1H), 4.04–3.94 (m, 1H), 2.81 (dd, J = 17.9, 5.3 Hz, 1H), 2.29 (s, 3H), 2.06 (dd, J = 17.9, 9.4 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 197.24, 196.79, 144.65, 140.97, 137.35, 136.01, 135.81, 134.83, 134.13, 133.68, 132.44, 130.33, 130.20, 130.03, 129.02, 128.73, 127.99, 127.40, 127.13, 125.43, 125.05, 117.24, 72.02, 44.82, 39.33, 21.69; IR (ATR): ν 3121, 3103, 3081, 3006, 2991, 1790, 1772, 1749, 1734, 1717, 1670, 1653, 1617, 1565, 1542, 1490, 1457, 1420, 1400, 1261 cm−1; HRMS (ESI+) calc. for C30H24Cl2NO4S [M + H]+: 564.0798, found: 564.0793.
2-(2-(3,4-Dichlorobenzoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3laa).
White solid, yield = 91% (102.7 mg); 1H NMR (500 MHz, CDCl3) δ 8.14 (d, J = 2.0 Hz, 1H), 7.92 (dd, J = 8.4, 2.0 Hz, 1H), 7.70 (dd, J = 9.7, 8.3 Hz, 3H), 7.64 (d, J = 8.3 Hz, 2H), 7.59 (dd, J = 14.3, 7.9 Hz, 2H), 7.44 (t, J = 7.8 Hz, 2H), 7.34–7.27 (m, 1H), 7.15 (d, J = 8.1 Hz, 2H), 7.08 (dt, J = 14.6, 7.0 Hz, 2H), 5.23 (d, J = 3.5 Hz, 1H), 3.85 (dt, J = 8.7, 4.3 Hz, 1H), 3.05 (dd, J = 18.1, 5.0 Hz, 1H), 2.45 (dd, J = 18.1, 9.2 Hz, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.19, 194.13, 144.70, 141.41, 138.29, 136.05, 134.76, 134.63, 133.89, 133.52, 133.50, 131.43, 130.83, 130.05, 129.10, 128.84, 128.48, 128.13, 127.48, 125.04, 124.87, 116.36, 69.58, 44.70, 41.03, 21.71; IR (ATR): ν 3102, 3080, 3006, 2990, 2926, 1790, 1775, 1734, 1717, 1698, 1684, 1641, 1617, 1560, 1510, 1473, 1419, 1260 cm−1; HRMS (ESI+) calc. for C30H24Cl2NO4S [M + H]+: 564.0798, found: 564.0795.
2-(2-(2-Naphthoyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3maa).
White solid, yield = 94% (102.6 mg); 1H NMR (500 MHz, CDCl3) δ 8.66 (s, 1H), 8.03 (dd, J = 8.6, 1.7 Hz, 1H), 7.97 (d, J = 8.1 Hz, 1H), 7.89 (dd, J = 11.0, 8.6 Hz, 2H), 7.77–7.65 (m, 5H), 7.65–7.59 (m, 1H), 7.57–7.50 (m, 2H), 7.41 (t, J = 7.8 Hz, 2H), 7.30 (t, J = 7.8 Hz, 1H), 7.18 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 7.5 Hz, 1H), 7.04 (td, J = 7.5, 0.9 Hz, 1H), 5.56 (d, J = 3.2 Hz, 1H), 3.98–3.91 (m, 1H), 3.04 (dd, J = 17.8, 5.8 Hz, 1H), 2.68 (dd, J = 17.8, 8.2 Hz, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.11, 195.52, 144.45, 141.71, 136.24, 135.96, 135.37, 133.78, 133.70, 132.61, 132.05, 131.44, 130.09, 129.97, 128.97, 128.85, 128.77, 128.66, 128.13, 127.89, 127.50, 126.84, 125.06, 124.74, 124.71, 116.04, 69.98, 44.93, 41.39, 21.69; IR (ATR): ν 3135, 3102, 3067, 3006, 2990, 1795, 1772, 1749, 1734, 1717, 1698, 1684, 1636, 1559, 1541, 1457, 1257 cm−1; HRMS (ESI+) calc. for C34H28NO4S [M + H]+: 546.1734, found: 546.1730.
2-(2-([1,1′-Biphenyl]-4-carbonyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3naa).
White solid, yield = 93% (106.3 mg); 1H NMR (500 MHz, CDCl3) δ 8.14 (d, J = 8.3 Hz, 2H), 7.78–7.67 (m, 7H), 7.67–7.61 (m, 2H), 7.57 (t, J = 7.4 Hz, 1H), 7.48 (t, J = 7.5 Hz, 2H), 7.42 (dd, J = 15.9, 7.9 Hz, 3H), 7.30 (t, J = 7.8 Hz, 1H), 7.19 (d, J = 8.1 Hz, 2H), 7.11 (d, J = 7.4 Hz, 1H), 7.04 (t, J = 7.2 Hz, 1H), 5.40 (d, J = 3.4 Hz, 1H), 3.97–3.89 (m, 1H), 3.01 (dd, J = 17.9, 6.0 Hz, 1H), 2.62 (dd, J = 17.9, 7.9 Hz, 1H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.08, 195.21, 146.34, 144.48, 141.64, 140.04, 136.23, 135.13, 133.79, 133.71, 133.43, 129.98, 129.96, 129.07, 128.94, 128.77, 128.38, 128.11, 127.51, 127.47, 127.45, 125.08, 124.77, 116.12, 69.78, 44.87, 41.19, 21.70; IR (ATR): ν 3152, 3113, 3006, 2989, 1792, 1773, 1734, 1717, 1699, 1653, 1603, 1559, 1541, 1507, 1489, 1419, 1397, 1262 cm−1; HRMS (ESI+) calc. for C36H30NO4S [M + H]+: 572.1890, found: 572.1877.
2-(2-(Furan-2-carbonyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3oaa).
White solid, yield = 88% (85.5 mg); 1H NMR (500 MHz, CDCl3) δ 7.76–7.63 (m, 5H), 7.60–7.56 (m, 2H), 7.48 (dd, J = 3.6, 0.5 Hz, 1H), 7.43 (t, J = 7.8 Hz, 2H), 7.31–7.26 (m, 1H), 7.21 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 7.5 Hz, 1H), 7.02 (td, J = 7.5, 0.9 Hz, 1H), 6.58 (dd, J = 3.6, 1.7 Hz, 1H), 5.09 (d, J = 3.7 Hz, 1H), 3.95 (td, J = 6.9, 3.7 Hz, 1H), 2.89 (dd, J = 17.7, 6.7 Hz, 1H), 2.67 (dd, J = 17.8, 7.2 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.89, 184.10, 150.54, 147.45, 144.56, 141.59, 136.34, 134.88, 133.70, 133.69, 129.99, 128.94, 128.79, 128.10, 127.63, 125.18, 124.86, 120.20, 116.19, 112.87, 70.41, 45.08, 41.18, 21.74; IR (ATR): ν 3019, 3006, 2990, 2925, 2856, 1793, 1770, 1749, 1734, 1684, 1653, 1559, 1507, 1458, 1276, 1259 cm−1; HRMS (ESI+) calc. for C28H24NO5S [M + H]+: 486.1370, found: 486.1384.
1-Phenyl-2-(2-(thiophene-2-carbonyl)-1-tosylindolin-3-yl)ethan-1-one (3paa).
White solid, yield = 87% (87.3 mg); 1H NMR (500 MHz, CDCl3) δ 8.05 (dd, J = 3.8, 1.0 Hz, 1H), 7.74–7.68 (m, 6H), 7.56 (dd, J = 10.5, 4.3 Hz, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.29 (t, J = 7.7 Hz, 1H), 7.25–7.13 (m, 3H), 7.09 (d, J = 7.4 Hz, 1H), 7.04 (dd, J = 10.8, 4.1 Hz, 1H), 5.06 (d, J = 3.8 Hz, 1H), 3.95 (dd, J = 10.4, 6.8 Hz, 1H), 2.94 (dd, J = 18.0, 6.3 Hz, 1H), 2.57 (dd, J = 18.0, 7.6 Hz, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.94, 188.84, 144.65, 141.48, 140.74, 136.17, 135.34, 134.45, 134.33, 133.79, 133.66, 129.97, 128.88, 128.73, 128.47, 128.03, 127.52, 125.05, 124.97, 116.20, 71.19, 44.71, 41.50, 21.66; IR (ATR): ν 3130, 3095, 3005, 2988, 1791, 1772, 1735, 1716, 1699, 1683, 1652, 1616, 1560, 1541, 1505, 1489, 1456, 1360, 1263 cm−1; HRMS (ESI+) calc. for C28H24NO4S2 [M + H]+: 502.1141, found: 502.1138.
2-(2-((1S,3S)-Adamantane-1-carbonyl)-1-tosylindolin-3-yl)-1-phenylethan-1-one (3qaa).
White solid, yield = 85% (94.1 mg); 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 8.2 Hz, 2H), 7.63 (dd, J = 12.7, 4.7 Hz, 3H), 7.57 (t, J = 7.4 Hz, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.26–7.19 (m, 3H), 7.05 (d, J = 7.4 Hz, 1H), 6.94 (t, J = 7.5 Hz, 1H), 5.00 (d, J = 1.8 Hz, 1H), 3.62 (t, J = 6.3 Hz, 1H), 2.60 (dd, J = 17.5, 8.1 Hz, 1H), 2.54–2.42 (m, 1H), 2.36 (d, J = 16.2 Hz, 3H), 2.04 (dd, J = 24.6, 7.3 Hz, 6H), 1.93 (d, J = 12.2 Hz, 3H), 1.80–1.72 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 208.55, 196.84, 144.31, 141.86, 136.34, 135.76, 134.26, 133.68, 129.96, 128.81, 128.74, 128.07, 127.31, 125.45, 124.66, 116.23, 67.50, 46.31, 45.33, 40.64, 38.27, 36.51, 27.99, 21.71; IR (ATR): ν 3069, 3005, 2990, 2910, 2851, 1792, 1770, 1724, 1717, 1700, 1683, 1653, 1559, 1541, 1507, 1455, 1277 cm−1; HRMS (ESI+) calc. for C34H36NO4S [M + H]+: 554.2360, found: 554.2370.
2,2-Dimethyl-1-(3-(2-oxo-2-phenylethyl)-1-tosylindolin-2-yl)propan-1-one (3raa).
White solid, yield = 90% (85.6 mg); 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 12.0, 8.2 Hz, 3H), 7.63–7.59 (m, 2H), 7.57 (t, J = 7.4 Hz, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.22 (dd, J = 14.0, 8.1 Hz, 3H), 7.05 (d, J = 7.5 Hz, 1H), 6.97 (t, J = 7.5 Hz, 1H), 4.97 (d, J = 1.8 Hz, 1H), 3.64 (t, J = 6.4 Hz, 1H), 2.60 (dd, J = 17.7, 7.6 Hz, 1H), 2.45–2.28 (m, 4H), 1.31 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 209.87, 196.83, 144.37, 141.91, 136.33, 135.65, 134.51, 133.70, 129.98, 128.87, 128.77, 128.06, 127.36, 125.31, 124.86, 116.59, 68.03, 45.40, 44.14, 41.04, 26.93, 21.73; IR (ATR): ν 3066, 3005, 2965, 2921, 2851, 1772, 1716, 1681, 1653, 1634, 1597, 1579, 1559, 1540, 1530, 1507, 1477, 1459, 1398, 1355, 1275, 1257 cm−1; HRMS (ESI+) calc. for C28H30NO4S [M + H]+: 476.1890, found: 476.1875.
2-(2-Acetyl-1-tosylindolin-3-yl)-1-phenylethan-1-one (3saa).
White solid, yield = 89% (77.2 mg); 1H NMR (500 MHz, CDCl3) δ 8.05–7.95 (m, 2H), 7.72 (d, J = 8.3 Hz, 2H), 7.60 (t, J = 8.5 Hz, 2H), 7.48 (t, J = 7.7 Hz, 2H), 7.26 (dd, J = 12.9, 5.2 Hz, 3H), 7.07–6.97 (m, 2H), 5.23 (d, J = 4.0 Hz, 1H), 3.78–3.53 (m, 1H), 2.48 (dd, J = 17.9, 6.5 Hz, 1H), 2.40 (s, 3H), 2.28 (dd, J = 17.9, 7.4 Hz, 1H), 1.97 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 205.70, 195.55, 144.56, 141.51, 135.10, 134.68, 133.73, 133.06, 129.90, 129.23, 128.93, 128.82, 127.61, 124.95, 124.54, 115.55, 70.12, 49.25, 40.77, 30.18, 21.68; IR (ATR): ν 3065, 3005, 2991, 1790, 1764, 1733, 1685, 1617, 1594, 1540, 1474, 1420, 1362, 1262 cm−1; HRMS (ESI) m/z: [M + H]+ calcd for C25H24NO4S: 434.1421; found: 434.1430.
Ethyl 3-(2-oxo-2-phenylethyl)-1-tosylindoline-2-carboxylate (3taa).
White solid, yield = 87% (80.7 mg); 1H NMR (500 MHz, CDCl3) δ 7.69–7.65 (m, 5H), 7.57 (t, J = 7.4 Hz, 1H), 7.43 (t, J = 7.8 Hz, 2H), 7.25 (dd, J = 9.6, 5.4 Hz, 1H), 7.16 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 7.5 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H), 4.48 (d, J = 3.4 Hz, 1H), 4.27–4.23 (m, 2H), 4.03–3.88 (m, 1H), 2.83 (dd, J = 17.8, 6.2 Hz, 1H), 2.41 (dd, J = 17.8, 8.0 Hz, 1H), 2.33 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 196.71, 170.33, 144.46, 141.19, 136.28, 135.00, 133.62, 133.57, 129.93, 128.83, 128.74, 128.05, 127.46, 124.94, 124.78, 116.30, 77.41, 77.16, 76.91, 67.99, 62.02, 44.96, 41.03, 21.67, 14.19; IR (ATR): ν 3168, 3112, 3068, 3010, 2995, 1842, 1801, 1778, 1760, 1735, 1716, 1684, 1541, 1475, 1332 cm−1; HRMS (ESI+) calc. for C26H26NO5S+ [M + H]+: 464.1526, found: 464.1530.
2-(2-Benzoyl-5-fluoro-1-tosylindolin-3-yl)-1-phenylethan-1-one (3aba).
White solid, yield = 90% (92.4 mg); 1H NMR (500 MHz, CDCl3) δ 8.03 (dd, J = 8.2, 0.9 Hz, 2H), 7.76–7.54 (m, 7H), 7.47 (dt, J = 32.5, 7.8 Hz, 4H), 7.19 (d, J = 8.1 Hz, 2H), 6.98 (td, J = 8.8, 2.6 Hz, 1H), 6.83 (dd, J = 8.1, 2.5 Hz, 1H), 5.39 (d, J = 3.0 Hz, 1H), 3.94–3.75 (m, 1H), 2.88 (dd, J = 18.0, 6.3 Hz, 1H), 2.48 (dd, J = 18.0, 7.8 Hz, 1H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.67, 195.38, 160.37 (J(C–F) = 242.50 Hz), 144.68, 137.66, 136.22 (J(C–F) = 8.75 Hz), 136.05, 134.79 (J(C–F) = 26.25 Hz), 133.88, 133.84, 130.09, 129.33, 128.88, 128.81, 128.09, 127.52, 117.62 (J(C–F) = 7.5 Hz), 115.60 (J(C–F) = 22.50 Hz), 112.69, 112.49, 69.66, 44.53, 40.89, 21.73; IR (ATR): ν 3136, 3006, 2990, 1792, 1770, 1717, 1698, 1652, 1597, 1558, 1540, 1517, 1474, 1415, 1263 cm−1; HRMS (ESI+) calc. for C30H25FNO4S [M + H]+: 514.1483, found: 514.1482.
2-(2-Benzoyl-5-chloro-1-tosylindolin-3-yl)-1-phenylethan-1-one (3aca).
White solid, yield = 90% (95.4 mg); 1H NMR (500 MHz, CDCl3) δ 8.07–8.00 (m, 2H), 7.73–7.64 (m, 4H), 7.62–7.59 (m, 3H), 7.50 (t, J = 7.8 Hz, 2H), 7.44 (t, J = 7.8 Hz, 2H), 7.26–7.23 (m, 1H), 7.19 (d, J = 8.1 Hz, 2H), 7.09 (d, J = 1.7 Hz, 1H), 5.40 (d, J = 3.1 Hz, 1H), 3.88–3.72 (m, 1H), 2.92 (dd, J = 18.0, 6.1 Hz, 1H), 2.52 (dd, J = 18.0, 7.9 Hz, 1H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.61, 195.27, 144.76, 140.42, 136.02, 135.89, 134.95, 134.62, 133.91, 133.86, 130.12, 130.04, 129.33, 129.01, 128.89, 128.82, 128.10, 127.46, 125.44, 117.24, 69.60, 44.60, 40.87, 21.73; IR (ATR): ν 3120, 3005, 2989, 1792, 1771, 1733, 1716, 1699, 1636, 1617, 1559, 1475, 1457, 1418, 1362, 1264 cm−1; HRMS (ESI+) calc. for C30H25ClNO4S [M + H]+: 530.1187, found: 530.1163.
2-(2-Benzoyl-5-bromo-1-tosylindolin-3-yl)-1-phenylethan-1-one (3ada).
White solid, yield = 94% (108.0 mg); 1H NMR (500 MHz, CDCl3) δ 8.07–7.99 (m, 2H), 7.71–7.65 (m, 4H), 7.65–7.54 (m, 3H), 7.50 (t, J = 7.8 Hz, 2H), 7.46–7.37 (m, 3H), 7.23 (d, J = 1.5 Hz, 1H), 7.19 (d, J = 8.1 Hz, 2H), 5.40 (d, J = 3.1 Hz, 1H), 3.89–3.78 (m, 1H), 2.93 (dd, J = 18.0, 6.0 Hz, 1H), 2.52 (dd, J = 18.0, 8.0 Hz, 1H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.60, 195.23, 144.77, 140.94, 136.23, 135.98, 134.91, 134.58, 133.90, 133.85, 131.88, 130.12, 129.31, 128.87, 128.81, 128.29, 128.08, 127.41, 117.62, 117.50, 69.50, 44.62, 40.82, 21.71; IR (ATR): ν 3101, 3005, 2990, 2835, 1795, 1771, 1734, 1717, 1698, 1636, 1559, 1542, 1507, 1473, 1276, 1234 cm−1; HRMS (ESI+) calc. for C30H25BrNO4S [M + H]+: 574.0682, found: 574.0688.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(4-fluorophenyl)ethan-1-one (3aab).
White solid, yield = 95% (97.6 mg); 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.1 Hz, 2H), 7.79–7.68 (m, 4H), 7.65 (d, J = 8.1 Hz, 1H), 7.63–7.57 (m, 1H), 7.55–7.45 (m, 2H), 7.34–7.27 (m, 1H), 7.20 (d, J = 8.0 Hz, 2H), 7.09 (t, J = 7.7 Hz, 3H), 7.02 (t, J = 7.5 Hz, 1H), 5.36 (d, J = 3.5 Hz, 1H), 3.89 (dd, J = 9.4, 6.9 Hz, 1H), 2.97 (dd, J = 17.7, 6.1 Hz, 1H), 2.62 (dd, J = 17.7, 7.7 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.57, 195.43, 166.09 (J(C–F) = 255.00 Hz), 144.45, 141.62, 135.24, 134.78, 133.75, 133.52, 132.53 (J(C–F) = 3.75 Hz), 130.82 (J(C–F) = 8.75 Hz), 129.96, 129.32, 129.02, 128.83, 127.59, 124.01 (J(C–F) = 43.75 Hz), 116.03, 116.01, 115.83, 69.91, 44.71, 41.10, 21.73; IR (ATR): ν 3005, 2990, 2980, 1790, 1735, 1716, 1684, 1652, 1636, 1559, 1542, 1457, 1276, 1263 cm−1; HRMS (ESI+) calc. for C30H25FNO4S [M + H]+: 514.1483, found: 514.1484.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(4-chlorophenyl)ethan-1-one (3aac).
White solid, yield = 93% (98.6 mg); 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 7.3 Hz, 2H), 7.70 (d, J = 8.2 Hz, 2H), 7.68–7.56 (m, 4H), 7.49 (t, J = 7.8 Hz, 2H), 7.39 (d, J = 8.5 Hz, 2H), 7.28 (t, J = 7.8 Hz, 1H), 7.19 (d, J = 8.1 Hz, 2H), 7.08 (d, J = 7.4 Hz, 1H), 7.02 (t, J = 7.4 Hz, 1H), 5.35 (d, J = 3.5 Hz, 1H), 3.88 (td, J = 7.2, 3.6 Hz, 1H), 2.96 (dd, J = 17.8, 6.1 Hz, 1H), 2.61 (dd, J = 17.8, 7.8 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.85, 195.54, 144.47, 141.62, 140.25, 135.22, 134.77, 134.56, 133.76, 133.46, 129.97, 129.53, 129.32, 129.10, 129.04, 128.84, 127.57, 125.06, 124.75, 116.06, 69.87, 44.75, 41.05, 21.74; IR (ATR): ν 3064, 3006, 2990, 2956, 2923, 2852, 1791, 1778, 1751, 1735, 1698, 1685, 1652, 1636, 1558, 1541, 1458, 1276, 1255 cm−1; HRMS (ESI+) calc. for C30H25ClNO4S [M + H]+: 530.1187, found: 530.1160.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(4-bromophenyl)ethan-1-one (3aad).
White solid, yield = 97% (111.5 mg); 1H NMR (500 MHz, CDCl3) δ 8.07–8.00 (m, 2H), 7.70 (d, J = 8.3 Hz, 2H), 7.66 (d, J = 8.1 Hz, 1H), 7.63–7.58 (m, 1H), 7.58–7.53 (m, 4H), 7.48 (dd, J = 10.7, 4.8 Hz, 2H), 7.33–7.26 (m, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 7.4 Hz, 1H), 7.02 (td, J = 7.5, 0.9 Hz, 1H), 5.35 (d, J = 3.5 Hz, 1H), 3.88 (td, J = 7.3, 3.5 Hz, 1H), 2.96 (dd, J = 17.8, 6.1 Hz, 1H), 2.60 (dd, J = 17.9, 7.8 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.06, 195.54, 144.47, 141.60, 135.20, 134.95, 134.76, 133.76, 133.44, 132.09, 129.96, 129.61, 129.31, 129.04, 128.98, 128.83, 127.56, 125.05, 124.75, 116.05, 69.84, 44.72, 41.03, 21.73; IR (ATR): ν 3105, 3057, 3004, 2988, 1789, 1776, 1750, 1730, 1717, 1636, 1607, 1522, 1489, 1437, 1361, 1220 cm−1; HRMS (ESI+) calc. for C30H25BrNO4S [M + H]+: 574.0682, found: 574.0686.
4-(2-(2-Benzoyl-1-tosylindolin-3-yl)acetyl)benzonitrile (3aae).
White solid, yield = 90% (93.7 mg); 1H NMR (500 MHz, CDCl3) δ 8.02 (d, J = 7.6 Hz, 2H), 7.79 (d, J = 8.2 Hz, 2H), 7.75–7.66 (m, 4H), 7.66–7.57 (m, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.33–7.26 (m, 1H), 7.20 (d, J = 7.9 Hz, 2H), 7.07 (d, J = 7.5 Hz, 1H), 7.02 (t, J = 7.4 Hz, 1H), 5.35 (d, J = 1.5 Hz, 1H), 3.90 (dd, J = 9.9, 6.7 Hz, 1H), 3.04 (dd, J = 18.0, 6.1 Hz, 1H), 2.73 (dd, J = 18.0, 7.5 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.84, 195.44, 144.51, 141.60, 139.01, 135.19, 134.71, 133.83, 133.05, 132.61, 129.95, 129.27, 129.17, 128.86, 128.54, 127.63, 125.03, 124.74, 117.79, 116.99, 115.96, 69.93, 44.94, 40.89, 21.73; IR (ATR): ν 3110, 3066, 3004, 2990, 2891, 1792, 1771, 1750, 1718, 1683, 1634, 1617, 1571, 1507, 1472, 1430, 1390, 1278 cm−1; HRMS (ESI+) calc. for C31H25N2O4S [M + H]+: 521.1530, found: 521.1535.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(p-tolyl)ethan-1-one (3aaf).
White solid, yield = 92% (93.8 mg); 1H NMR (500 MHz, CDCl3) δ 8.17–7.94 (m, 2H), 7.69 (dd, J = 12.8, 8.2 Hz, 3H), 7.59 (t, J = 8.8 Hz, 3H), 7.48 (t, J = 7.7 Hz, 2H), 7.28 (d, J = 7.5 Hz, 1H), 7.20 (dd, J = 10.0, 8.5 Hz, 4H), 7.09 (d, J = 7.4 Hz, 1H), 7.02 (dd, J = 7.5, 6.9 Hz, 1H), 5.37 (d, J = 3.4 Hz, 1H), 3.88 (td, J = 7.4, 3.4 Hz, 1H), 2.94 (dd, J = 17.7, 6.2 Hz, 1H), 2.57 (dd, J = 17.7, 7.8 Hz, 1H), 2.41 (s, 3H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.63, 195.61, 144.62, 144.43, 141.61, 135.21, 134.80, 133.85, 133.81, 133.69, 129.97, 129.43, 129.33, 128.90, 128.80, 128.25, 127.51, 125.12, 124.71, 116.06, 69.87, 44.76, 41.17, 21.82, 21.72; IR (ATR): ν 3115, 3006, 2988, 1793, 1734, 1716, 1699, 1653, 1636, 1606, 1522, 1507, 1489, 1474, 1438, 1397, 1274 cm−1; HRMS (ESI+) calc. for C31H28NO4S [M + H]+: 510.1734, found: 510.1709.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(2-methoxyphenyl)ethan-1-one (3aag).
White solid, yield = 90% (94.6 mg); 1H NMR (500 MHz, CDCl3) δ 8.08–7.97 (m, 2H), 7.73 (d, J = 8.3 Hz, 2H), 7.61–7.57 (m, 3H), 7.52–7.42 (m, 3H), 7.27–7.22 (m, 1H), 7.17 (d, J = 8.1 Hz, 2H), 7.08 (d, J = 7.4 Hz, 1H), 7.01–6.97 (m, 2H), 6.91 (d, J = 8.3 Hz, 1H), 5.41 (d, J = 4.0 Hz, 1H), 3.97–3.82 (m, 1H), 3.77 (s, 3H), 3.17 (dd, J = 17.4, 5.9 Hz, 1H), 2.81 (dd, J = 17.4, 8.0 Hz, 1H), 2.32 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 199.07, 196.04, 158.75, 144.28, 141.64, 135.22, 134.89, 134.27, 133.59, 133.57, 130.61, 129.80, 129.29, 128.75, 128.68, 127.57, 127.37, 125.01, 124.28, 120.91, 115.18, 111.65, 70.10, 55.54, 49.92, 41.66, 21.67; IR (ATR): ν 3080, 3064, 3005, 2989, 1794, 1765, 1734, 1716, 1683, 1616, 1560, 1521, 1490, 1458, 1398, 1261 cm−1; HRMS (ESI+) calc. for C31H28NO5S [M + H]+: 526.1683, found: 526.1696.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(3-methoxyphenyl)ethan-1-one (3aah).
White solid, yield = 93% (97.8 mg); 1H NMR (500 MHz, CDCl3) δ 8.08–7.97 (m, 2H), 7.69 (t, J = 8.3 Hz, 3H), 7.60 (t, J = 7.4 Hz, 1H), 7.49 (t, J = 7.7 Hz, 2H), 7.36–7.26 (m, 3H), 7.20 (d, J = 8.0 Hz, 3H), 7.10 (dd, J = 13.5, 5.0 Hz, 2H), 7.03 (t, J = 7.5 Hz, 1H), 5.36 (d, J = 3.4 Hz, 1H), 3.92–3.72 (m, 4H), 2.95 (dd, J = 17.8, 6.1 Hz, 1H), 2.58 (dd, J = 17.8, 7.8 Hz, 1H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.87, 195.61, 160.02, 144.57, 141.63, 137.62, 135.16, 134.82, 133.74, 133.73, 130.01, 129.72, 129.34, 128.97, 128.83, 127.51, 125.11, 124.77, 120.66, 119.86, 116.16, 112.73, 69.84, 55.63, 45.02, 41.14, 21.67; IR (ATR): ν 3120, 3101, 3080, 3004, 2989, 1794, 1770, 1730, 1716, 1698, 1684, 1635, 1505, 1457, 1275, 1259 cm−1; HRMS (ESI+) calc. for C31H28NO5S [M + H]+: 526.1683, found: 526.1689.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(4-methoxyphenyl)ethan-1-one (3aai).
White solid, yield = 95% (99.9 mg); 1H NMR (500 MHz, CDCl3) δ 8.07–7.96 (m, 2H), 7.71 (d, J = 8.3 Hz, 2H), 7.69–7.64 (m, 3H), 7.59 (t, J = 7.4 Hz, 1H), 7.48 (t, J = 7.7 Hz, 2H), 7.31–7.26 (m, 1H), 7.20 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 7.5 Hz, 1H), 7.04–6.98 (m, 1H), 6.91–6.85 (m, 2H), 5.38 (d, J = 3.4 Hz, 1H), 3.91–3.85 (m, 4H), 2.93 (dd, J = 17.5, 6.2 Hz, 1H), 2.57 (dd, J = 17.5, 7.8 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.62, 195.47, 163.94, 144.40, 141.61, 135.25, 134.80, 133.82, 133.68, 130.45, 129.96, 129.41, 129.33, 128.88, 128.80, 127.54, 125.14, 124.67, 115.98, 113.89, 69.92, 55.68, 44.51, 41.29, 21.74; IR (ATR): ν 3115, 3055, 3005, 2988, 2895, 1792, 1734, 1716, 1684, 1636, 1601, 1576, 1473, 1456, 1320, 1261 cm−1; HRMS (ESI+) calc. for C31H28NO5S [M + H]+: 526.1683, found: 526.1690.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(naphthalen-2-yl)ethan-1-one (3aaj).
White solid, yield = 94% (102.6 mg); 1H NMR (500 MHz, CDCl3) δ 8.20 (s, 1H), 8.14–8.00 (m, 2H), 7.89 (dd, J = 15.0, 8.3 Hz, 3H), 7.81 (dd, J = 8.6, 1.6 Hz, 1H), 7.72 (d, J = 8.3 Hz, 2H), 7.68 (d, J = 8.1 Hz, 1H), 7.66–7.54 (m, 3H), 7.50 (t, J = 7.7 Hz, 2H), 7.29 (t, J = 7.8 Hz, 1H), 7.20–7.10 (m, 3H), 7.08–6.98 (m, 1H), 5.45 (d, J = 3.5 Hz, 1H), 4.01–3.87 (m, 1H), 3.17 (dd, J = 17.7, 6.0 Hz, 1H), 2.77 (dd, J = 17.7, 8.0 Hz, 1H), 2.26 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.03, 195.70, 144.50, 141.67, 135.86, 135.25, 134.85, 133.74, 133.71, 133.62, 132.51, 129.95, 129.66, 129.40, 128.99, 128.97, 128.84, 128.70, 128.62, 127.99, 127.57, 127.19, 125.12, 124.70, 123.66, 116.00, 69.90, 44.95, 41.29, 21.68; IR (ATR): ν 3095, 3052, 3005, 2988, 1790, 1770, 1750, 1735, 1686, 1652, 1575, 1540, 1507, 1485, 1457, 1419, 1362, 1262 cm−1; HRMS (ESI+) calc. for C34H28NO4S [M + H]+: 546.1735, found: 546.1731.
1-([1,1′-Biphenyl]-4-yl)-2-(2-benzoyl-1-tosylindolin-3-yl)ethan-1-one (3aak).
White solid, yield = 96% (109.8 mg); 1H NMR (500 MHz, CDCl3) δ 8.06 (d, J = 7.3 Hz, 2H), 7.77 (d, J = 8.4 Hz, 2H), 7.71 (dd, J = 14.9, 8.2 Hz, 3H), 7.68–7.58 (m, 5H), 7.49 (td, J = 7.9, 1.7 Hz, 4H), 7.42 (t, J = 7.3 Hz, 1H), 7.29 (t, J = 7.8 Hz, 1H), 7.21 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 7.4 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 5.41 (d, J = 3.4 Hz, 1H), 3.99–3.87 (m, 1H), 3.01 (dd, J = 17.7, 6.1 Hz, 1H), 2.63 (dd, J = 17.7, 7.8 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.59, 195.61, 146.39, 144.45, 141.62, 139.70, 135.20, 134.92, 134.80, 133.73, 133.71, 129.99, 129.33, 129.17, 128.94, 128.82, 128.72, 128.60, 127.52, 127.36, 127.35, 125.12, 124.75, 116.09, 69.83, 44.89, 41.16, 21.74; IR (ATR): ν 3145, 3095, 3005, 2991, 1791, 1772, 1731, 1719, 1617, 1545, 1501, 1473, 1420, 1360, 1261 cm−1; HRMS (ESI+) calc. for C36H30NO4S [M + H]+: 572.1890, found: 572.1896.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(furan-2-yl)ethan-1-one (3aal).
White solid, yield = 89% (86.4 mg); 1H NMR (500 MHz, CDCl3) δ 8.04–7.98 (m, 2H), 7.74 (d, J = 8.3 Hz, 2H), 7.64 (d, J = 8.1 Hz, 1H), 7.61–7.56 (m, 1H), 7.52 (d, J = 1.0 Hz, 1H), 7.46 (t, J = 7.8 Hz, 2H), 7.27 (dd, J = 11.9, 3.7 Hz, 1H), 7.23 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 7.5 Hz, 1H), 7.04–6.97 (m, 2H), 6.50 (dd, J = 3.6, 1.7 Hz, 1H), 5.45 (d, J = 3.5 Hz, 1H), 3.82 (td, J = 7.2, 3.4 Hz, 1H), 2.87 (dd, J = 17.1, 6.4 Hz, 1H), 2.54 (dd, J = 17.1, 7.9 Hz, 1H), 2.36 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.36, 186.09, 152.28, 146.81, 144.48, 141.53, 135.25, 134.61, 133.71, 133.14, 129.92, 129.21, 128.97, 128.82, 127.52, 125.11, 124.55, 117.73, 115.71, 112.58, 69.78, 44.39, 40.97, 21.69; IR (ATR): ν 3100, 3078, 2989, 1790, 1771, 1732, 1701, 1686, 1673, 1634, 1616, 1607, 1540, 1498, 1472, 1419, 1395, 1262 cm−1; HRMS (ESI+) calc. for C28H24NO5S [M + H]+: 486.1370, found: 486.1356.
2-(2-Benzoyl-1-tosylindolin-3-yl)-1-(thiophen-2-yl)ethan-1-one (3aam).
White solid, yield = 87% (87.3 mg); 1H NMR (500 MHz, CDCl3) δ 8.04–7.97 (m, 2H), 7.74 (d, J = 8.3 Hz, 2H), 7.68–7.63 (m, 2H), 7.61–7.55 (m, 1H), 7.47 (t, J = 7.7 Hz, 2H), 7.37 (dd, J = 3.8, 1.0 Hz, 1H), 7.27 (s, 1H), 7.23 (d, J = 8.4 Hz, 2H), 7.11–7.06 (m, 2H), 7.01 (dd, J = 7.9, 7.1 Hz, 1H), 5.44 (d, J = 3.3 Hz, 1H), 3.85 (td, J = 7.1, 3.4 Hz, 1H), 2.90 (dd, J = 17.0, 6.4 Hz, 1H), 2.62 (dd, J = 17.0, 7.7 Hz, 1H), 2.38 (d, J = 8.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 195.33, 189.84, 144.49, 143.58, 141.59, 135.33, 134.60, 134.54, 133.76, 133.23, 132.38, 129.99, 129.26, 129.04, 128.86, 128.25, 127.55, 125.15, 124.64, 115.87, 69.82, 45.23, 41.35, 21.72; IR (ATR): ν 3133, 3096, 3006, 2989, 1792, 1772, 1734, 1717, 1699, 1684, 1653, 1617, 1559, 1541, 1507, 1489, 1457, 1361, 1262 cm−1; HRMS (ESI+) calc. for C28H24NO4S2 [M + H]+: 502.1141, found: 502.1152.
1-(2-Benzoyl-1-tosylindolin-3-yl)-3,3-dimethylbutan-2-one (3aan).
White solid, yield = 89% (84.7 mg); 1H NMR (500 MHz, CDCl3) δ 8.00–7.92 (m, 2H), 7.73 (d, J = 8.2 Hz, 2H), 7.65 (d, J = 8.1 Hz, 1H), 7.59 (t, J = 7.4 Hz, 1H), 7.47 (t, J = 7.7 Hz, 2H), 7.26 (dd, J = 11.5, 5.0 Hz, 3H), 7.03–6.94 (m, 2H), 5.18 (d, J = 3.7 Hz, 1H), 3.76 (td, J = 6.8, 3.8 Hz, 1H), 2.48–2.29 (m, 5H), 0.94 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 212.71, 195.38, 144.53, 141.55, 135.26, 134.70, 133.69, 133.52, 129.96, 129.15, 128.94, 128.85, 127.59, 125.17, 124.56, 115.75, 70.66, 44.10, 43.23, 40.95, 26.07, 21.70; IR (ATR): ν 3065, 3007, 2990, 1798, 1771, 1752, 1731, 1685, 1616, 1595, 1540, 1477, 1415, 1362, 1266 cm−1; HRMS (ESI+) calc. for C28H30NO4S [M + H]+: 476.1890, found: 476.1905.
1-(2-Benzoyl-1-tosylindolin-3-yl)propan-2-one (3aao).
White solid, yield = 87% (75.4 mg); 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 8.2 Hz, 1H), 7.63 (d, J = 8.0 Hz, 2H), 7.57 (t, J = 7.5 Hz, 3H), 7.43 (t, J = 7.8 Hz, 2H), 7.30 (t, J = 7.6 Hz, 1H), 7.12–7.03 (m, 4H), 4.38 (d, J = 2.9 Hz, 1H), 3.99–3.89 (m, 1H), 2.72 (dd, J = 18.2, 5.8 Hz, 1H), 2.50 (s, 3H), 2.29 (s, 3H), 2.06 (dd, J = 18.2, 8.6 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 205.60, 196.98, 144.67, 140.87, 136.19, 134.36, 134.33, 133.63, 130.02, 128.92, 128.73, 128.00, 127.49, 125.42, 125.12, 117.22, 73.70, 44.60, 39.02, 27.09, 21.68; IR (ATR): ν 3065, 3008, 2996, 1793, 1760, 1756, 1733, 1685, 1616, 1595, 1541, 1476, 1420, 1362, 1261 cm−1; HRMS (ESI) m/z: [M + H]+ calcd for C25H24NO4S: 434.1421; found: 434.1430.
Procedure for the synthesis of 1,4-diphenyl-3,10-dihydro-[1,2]diazepino[4,5-b]indole 4aaa.
A solution of DHI 3aaa (99.1 mg, 0.2 mmol) in ethyl acetate (1 ml) was added to 24% aq. solution of hydrazine hydrate (19.2 mg, 0.6 mmol). The biphasic solution was vigorously stirred at 95 °C for 30 h. The reaction was monitored by TLC. After the complete consumption of reactant 3aaa, ethyl acetate was added and the organic phase was separated and dried over Na2SO4. The organic phase was evaporated under reduced pressure and the crude product was purified using flash chromatography with hexane/ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluents and silica gel (100–200 mesh) as the stationary solid phase. The product 4aaa was obtained as an off-white solid (56.2 mg, 79%).
1) as eluents and silica gel (100–200 mesh) as the stationary solid phase. The product 4aaa was obtained as an off-white solid (56.2 mg, 79%).
1,4-Diphenyl-3,10-dihydro-[1,2]diazepino[4,5-b]indole (4aaa).
White solid, yield = 79% (53.0 mg); 1H NMR (500 MHz, CDCl3) δ 8.27 (s, 1H), 8.02–7.96 (m, 2H), 7.95–7.88 (m, 3H), 7.53–7.47 (m, 3H), 7.43–7.34 (m, 5H), 7.35–7.27 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 152.08, 148.97, 138.17, 137.25, 136.40, 130.48, 129.93, 129.29, 129.16, 128.98, 128.81, 127.64, 127.19, 125.24, 124.15, 120.78, 119.30, 119.05, 112.19; IR (ATR): ν 3310, 3188, 3102, 3006, 1684, 1653, 1636, 1559, 1521, 1507, 1474, 1419, 1362, 1276 cm−1; HRMS (ESI) m/z: [M + H]+ calcd for C23H18N3: 336.1495; found: 336.1497.
tert-Butyl (2-(2,3-dibenzoylcyclopropyl)phenyl)carbamate (6aaa′).
White solid, yield = 42% (37.1 mg); 1H NMR (500 MHz, CDCl3) δ 8.09 (d, J = 8.1 Hz, 1H), 8.02–8.00 (m, 4H), 7.58–7.53 (m, 2H), 7.51 (s, 1H), 7.44 (t, J = 7.8 Hz, 4H), 7.34–7.28 (m, 1H), 7.23 (d, J = 7.6 Hz, 1H), 7.04 (td, J = 7.5, 1.0 Hz, 1H), 3.37–3.31 (m, 3H), 1.46 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 194.67, 153.12, 138.65, 136.77, 133.48, 128.71, 128.48, 128.36, 126.67, 125.78, 122.67, 119.79, 80.36, 77.28, 77.03, 76.77, 34.94, 28.23, 27.30; HRMS (ESI+) calc. for C28H28NO4+ [M + H]+: 442.2013, found: 442.2010.
General procedure for the synthesis of product 8.
The pre-formed sulfonium salt 1 (0.3 mmol) and ortho-hydroxychalcone 7 (0.2 mmol) were added to toluene (2 mL). After stirring the mixture for 5 min, Cs2CO3 (65.2 mg, 0.2 mmol) was added and stirring was continued at rt. The progress of the reaction was monitored by TLC. After the completion of the reaction, the crude product 8 was purified by flash column chromatography on a silica support (hexane/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
2-(2-Benzoyl-2,3-dihydrobenzofuran-3-yl)-1-phenylethan-1-one (8aaa).
White solid, yield = 94% (64.4 mg); 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 7.4 Hz, 2H), 7.96 (d, J = 7.4 Hz, 2H), 7.66–7.53 (m, 2H), 7.52–7.44 (m, 4H), 7.23 (d, J = 7.4 Hz, 1H), 7.17 (t, J = 7.7 Hz, 1H), 6.95–6.82 (m, 2H), 5.66 (d, J = 5.5 Hz, 1H), 4.56 (dd, J = 13.5, 5.9 Hz, 1H), 3.64 (dd, J = 17.7, 6.0 Hz, 1H), 3.44 (dd, J = 17.7, 8.1 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 197.89, 194.90, 158.74, 136.56, 135.13, 133.70, 133.65, 129.52, 128.98, 128.86, 128.79, 128.25, 124.93, 121.55, 110.11, 87.78, 77.41, 77.16, 76.91, 44.23, 39.76; IR (ATR): ν 3050, 3003, 2991, 1731, 1686, 1650, 1600, 1580, 1555, 1541, 1506, 1473, 1443, 1280, 1258, 1210 cm−1; HRMS (ESI+) calc. for C23H19O3+ [M + H]+: 343.1329, found: 343.1320.
2-(2-Benzoyl-5,7-di-tert-butyl-2,3-dihydrobenzofuran-3-yl)-1-phenylethan-1-one (8aba).
White solid, yield = 89% (80.9 mg); 1H NMR (500 MHz, CDCl3) δ 8.15–8.07 (m, 2H), 8.02–7.90 (m, 2H), 7.61–7.55 (m, 2H), 7.47 (dd, J = 16.2, 8.1 Hz, 4H), 7.15 (d, J = 1.9 Hz, 1H), 7.13–7.07 (m, 1H), 5.62 (d, J = 5.3 Hz, 1H), 4.54–4.50 (m, 1H), 3.63 (dd, J = 17.5, 5.5 Hz, 1H), 3.45 (dd, J = 17.5, 8.6 Hz, 1H), 1.30 (s, 9H), 1.29 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 198.33, 195.40, 154.17, 144.19, 136.80, 135.26, 133.50, 133.46, 132.57, 129.56, 128.82, 128.71, 128.62, 128.29, 122.85, 119.19, 87.96, 77.41, 77.16, 76.91, 44.44, 39.60, 34.71, 34.37, 31.92, 29.51; IR (ATR): ν 3101, 3064, 2992, 1830, 1790, 1778, 1730, 1715, 1665, 1639, 1620, 1577, 1541, 1503, 1485, 1472, 1421, 1341, 1230 cm−1; HRMS (ESI+) calc. for C31H35O3+ [M + H]+: 455.2581, found: 455.2579.
2-(2-Benzoyl-2,3-dihydrobenzofuran-3-yl)-1-(naphthalen-1-yl)ethan-1-one (8aab).
White solid, yield = 91% (71.4 mg); 1H NMR (500 MHz, CDCl3) δ 8.64 (d, J = 8.5 Hz, 1H), 8.20–8.06 (m, 2H), 8.01 (d, J = 8.2 Hz, 1H), 7.93–7.86 (m, 2H), 7.66–7.45 (m, 6H), 7.27 (d, J = 6.0 Hz, 1H), 7.18 (t, J = 7.7 Hz, 1H), 6.90 (dd, J = 17.2, 7.8 Hz, 2H), 5.74 (d, J = 5.6 Hz, 1H), 4.64 (dd, J = 13.4, 6.2 Hz, 1H), 3.71 (dd, J = 17.5, 6.1 Hz, 1H), 3.56 (dd, J = 17.5, 7.9 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 201.71, 195.07, 158.78, 135.19, 134.99, 134.15, 133.73, 133.53, 130.28, 129.53, 129.01, 128.93, 128.82, 128.63, 128.42, 128.40, 126.74, 125.91, 124.94, 124.44, 121.60, 110.16, 87.81, 77.41, 77.16, 76.91, 47.19, 40.33; IR (ATR): ν 3001, 2878, 2832, 1850, 1750, 1725, 1710, 1684, 1640, 1520, 1475, 1460, 1268 cm−1; HRMS (ESI+) calc. for C27H21O3+ [M + H]+: 393.1485, found: 393.1489.
1-(2-Benzoyl-2,3-dihydrobenzofuran-3-yl)propan-2-one (8aac).
White solid, yield = 87% (48.7 mg); 1H NMR (500 MHz, CDCl3) δ 8.07 (dd, J = 8.3, 1.2 Hz, 2H), 7.64–7.57 (m, 1H), 7.49 (dd, J = 10.8, 4.7 Hz, 2H), 7.19–7.13 (m, 2H), 6.93–6.82 (m, 2H), 5.54 (d, J = 5.8 Hz, 1H), 4.35 (dd, J = 13.5, 6.4 Hz, 1H), 3.08 (dd, J = 17.7, 6.3 Hz, 1H), 2.90 (dd, J = 17.7, 7.8 Hz, 1H), 2.19 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 206.60, 195.04, 158.62, 135.03, 133.76, 129.46, 128.98, 128.80, 128.72, 124.73, 121.55, 110.10, 87.65, 77.41, 77.16, 76.91, 48.76, 39.55, 30.37; IR (ATR): ν 3174, 3140, 3068, 3008, 2984, 2955, 1796, 1769, 1738, 1710, 1670, 1635, 1592, 1541, 1449, 1278 cm−1; HRMS (ESI+) calc. for C18H17O3+ [M + H]+: 281.1172, found: 281.1179.
2-(2-(4-Bromobenzoyl)-2,3-dihydrobenzofuran-3-yl)-1-phenylethan-1-one (8daa).
White solid, yield = 92% (77.5 mg); 1H NMR (500 MHz, CDCl3) δ 8.23–7.80 (m, 4H), 7.58 (q, J = 7.2 Hz, 1H), 7.51–7.39 (m, 4H), 7.23 (t, J = 6.5 Hz, 1H), 7.17 (q, J = 7.1 Hz, 1H), 6.94–6.90 (m, 1H), 6.89–6.80 (m, 1H), 5.59 (d, J = 5.5 Hz, 1H), 4.60–4.49 (m, 1H), 3.64 (dt, J = 17.8, 6.2 Hz, 1H), 3.48–3.36 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 197.94, 193.84, 158.55, 140.20, 136.48, 133.73, 133.51, 131.01, 129.12, 129.04, 128.90, 128.86, 128.24, 124.86, 121.68, 110.13, 87.78, 77.41, 77.16, 76.91, 44.14, 39.61; IR (ATR): ν 3060, 3002, 2991, 1777, 1760, 1734, 1710, 1684, 1598, 1560, 1510, 1479, 1431, 1276, 1228 cm−1; HRMS (ESI+) calc. for C23H17BrO3+ [M]+: 420.0361, found: 420.0357.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
NKR is thankful to the Science and Engineering Research Board (SERB), India (CRG/2020/001940) and the Council of Scientific and Industrial Research (CSIR), India (02(0424)/21/EMR-II). AJ and AK thank the Ministry of Education, India for research fellowships.
Notes and references
- J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Rev., 2015, 115, 5301–5365 CrossRef CAS PubMed.
- T. Kaur, P. Wadhwa, S. Bagchia and A. Sharma, Chem. Commun., 2016, 52, 6958–6976 RSC.
- C. Zhu, Y. Ding and L.-W. Ye, Org. Biomol. Chem., 2015, 13, 2530–2536 RSC.
- A. Kruithof, E. Ruijter and R. V. A. Orru, Chem. – Asian J., 2015, 10, 508–520 CrossRef CAS PubMed.
- S. M. M. Lopes, A. L. Cardoso, A. Lemos and T. M. V. D. Pinho e Melo, Chem. Rev., 2018, 118, 11324–11352 CrossRef CAS.
- A. A. Tabolin, A. Yu. Sukhorukov, S. L. Ioffe and A. D. Dilman, Synthesis, 2017, 3255–3268 CAS.
- H. Zhang and R. Zhou, Eur. J. Org. Chem., 2020, 4098–4107 CrossRef CAS.
- X. Mi, C. Pi, W. Feng and X. Cui, Org. Chem. Front., 2022, 9, 6999–7015 RSC.
- Y. Liu, F. Sun and Z. He, Tetrahedron Lett., 2018, 59, 4136–4148 CrossRef CAS.
- P. Yu. Ushakov, S. L. Ioffe and A. Yu. Sukhorukov, Org. Chem. Front., 2022, 9, 5358–5382 RSC.
- L. Roiser, K. Zielke and M. Waser, Asian J. Org. Chem., 2018, 7, 852–864 CrossRef CAS PubMed.
-
Modern Tools for the Synthesis of Complex Bioactive Molecules, ed. S. Bur and A. Padwa, Wiley, Weinheim, 2012, pp. 433–484 Search PubMed.
- Y. Lv, J. Meng, C. Li, X. Wang, Y. Ye and K. Sun, Adv. Synth. Catal., 2021, 363, 5235–5265 CrossRef CAS.
- Q.-Q. Yang, Q. Wang, J. An, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Chem. – Eur. J., 2013, 19, 8401–8404 CrossRef CAS PubMed.
- M. Yu and S.-G. Kim, Tetrahedron Lett., 2015, 56, 7034–7037 CrossRef CAS.
- A. Kim, C. Kim and S.-G. Kim, Bull. Korean Chem. Soc., 2015, 36, 417–420 CrossRef CAS.
- J. Wang, X. Pan, L. Zhao, L. Zhao, J. Liu, Y. Zhi, A. Wang, K. Zhao and L. Hu, Org. Biomol. Chem., 2019, 17, 10158–10162 RSC.
- K. Kawai, H. Uno, D. Fujimoto and N. Shibata, Helv. Chim. Acta, 2021, 104, e2000217 CrossRef CAS.
- R. R. Gataullin, Helv. Chim. Acta, 2020, 103, e2000137 CrossRef CAS.
- N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed.
- Y. Li, T. Liu and J. Sun, Molecules, 2023, 28, 733 CrossRef CAS PubMed.
- H. Ishikawa, G. I. Elliott, J. Velcicky, Y. Choi and D. L. Boger, J. Am. Chem. Soc., 2006, 128, 10596–10612 CrossRef CAS PubMed.
- R. Iyengar, K. Schildknegt, M. Morton and J. Aubé, J. Org. Chem., 2005, 70, 10645–10652 CrossRef CAS PubMed.
- H. Zhang, J. Boonsombat and A. Padwa, Org. Lett., 2006, 9, 279–282 CrossRef PubMed.
- J. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5482–5487 CrossRef CAS PubMed.
- T. Bui, S. Syed and C. F. Barbas, J. Am. Chem. Soc., 2009, 131, 8758–8759 CrossRef CAS.
- K. L. Marquis, A. L. Sabb, S. F. Logue, J. A. Brennan, M. J. Piesla, T. A. Comery, S. M. Grauer, C. R. Ashby, H. Q. Nguyen, L. A. Dawson, J. E. Barrett, G. Stack, H. Y. Meltzer, B. L. Harrison and S. Rosenzweig-Lipson, J. Pharmacol. Exp. Ther., 2006, 320, 486–496 CrossRef PubMed.
- C. Lu, B. Hao, Y. Han and Y. Liang, Asian J. Org. Chem., 2022, 11, e202200312 CrossRef CAS.
- S. R. Mangaonkar, H. Hayashi, H. Takano, W. Kanna, S. Maeda and T. Mita, ACS Catal., 2023, 13, 2482–2488 CrossRef CAS.
- S. Anas and H. B. Kagan, Tetrahedron: Asymmetry, 2009, 20, 2193–2199 CrossRef CAS.
- D. Liu, G. Zhao and L. Xiang, Eur. J. Org. Chem., 2010, 3975–3984 CrossRef CAS.
- Y. Mo, J. Zhao, W. Chen and Q. Wang, Res. Chem. Intermed., 2014, 41, 5869–5877 CrossRef.
- T. S. Silva, M. T. Rodrigues, H. Santos, L. A. Zeoly, W. P. Almeida, R. C. Barcelos, R. C. Gomes, F. S. Fernandes and F. Coelho, Tetrahedron, 2019, 75, 2063–2097 CrossRef CAS.
- A. Jain, A. Regina, A. Kumari, R. Patra, M. Paranjothy and N. K. Rana, Org. Lett., 2023, 25, 3790–3795 CrossRef CAS PubMed.
- A. Kumari, A. Jain, K. Shukla, R. Patra and N. K. Rana, Org. Biomol. Chem., 2023, 21, 5542–5546 RSC.
- Y.-Q. Gao, Y. Hou, L. Zhu, J. Chen, R. Li, S.-Y. Zhang, Y.-P. He and W. Xie, Chem. Commun., 2020, 56, 6739–6742 RSC.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  ,
Akanksha
Kumari
,
Akanksha
Kumari
 ,
Ramesh K.
Metre
,
Ramesh K.
Metre
 and
Nirmal K.
Rana
and
Nirmal K.
Rana
 *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (Fig. 1).
27 (Fig. 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were determined by 1H-NMR analysis.
1) were determined by 1H-NMR analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were determined by 1H-NMR analysis.a dr = 20
1) were determined by 1H-NMR analysis.a dr = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluents and silica gel (100–200 mesh) as the stationary solid phase.
1) as eluents and silica gel (100–200 mesh) as the stationary solid phase.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 hexane/i-PrOH at 1 mL min−1, λ = 254 nm); major enantiomer: tR = 15.522 min, minor enantiomer: tR = 28.131 min.
30 hexane/i-PrOH at 1 mL min−1, λ = 254 nm); major enantiomer: tR = 15.522 min, minor enantiomer: tR = 28.131 min.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluents and silica gel (100–200 mesh) as the stationary solid phase. The product 4aaa was obtained as an off-white solid (56.2 mg, 79%).
1) as eluents and silica gel (100–200 mesh) as the stationary solid phase. The product 4aaa was obtained as an off-white solid (56.2 mg, 79%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).











