DOI:
10.1039/D4NR03011G
(Paper)
Nanoscale, 2024,
16, 19306-19321
Engineering multi-component 3DOM FeVCrOx catalysts with high oxygen mobility for the oxidative dehydrogenation of 1-butene with CO2†
Received
20th July 2024
, Accepted 16th September 2024
First published on 18th September 2024
Abstract
Oxidative dehydrogenation (ODH) of 1-butene with CO2 to 1,3-butadiene (BD) via Fe-based catalysts is a promising strategy, and the mobility of lattice oxygen plays a key role in the catalytic reaction. However, the catalytic activity of Fe-based catalysts is limited by the poor lattice oxygen mobility. To improve the mobility of lattice oxygen and optimize the ODH reaction, a series of 3DOM Fe-based catalysts (FeVAlOx, FeCrAlOx, and FeVCrAlOx) were prepared by PMMA template method. Among these samples, the multi-component FeVCrAlOx samples showed the best catalytic activity, and the 1-butene conversion of the multi-component catalyst could reach 88.3%, the BD yield could reach 27.5%. Further study found that the introduction of multi-component elements (V and Cr) not only promoted the formation of the γ-Fe2O3 phase but also formed more active components (V5+ and Cr6+). More importantly, the lattice oxygen mobility was also significantly improved. In addition, the reaction in the presence of water conditions was studied by activity tests and in situ DRIFTS tests. The results show that CO2 was present in the form of HCO3−. The utilization of CO2 was improved and the reaction path was changed.
1. Introduction
1,3-Butadiene (BD) is a critical building block in the petrochemical industry, and it is used in synthetic rubber, resins, and fine chemicals. Due to the rapid growth of the industry, BD has seen continuous and rapid growth in demand.1–3 Traditionally, it is produced by separating C4 fractions and oxidative dehydrogenation (ODH) procedures.4 Due to the low yield and production of CO2, neither of them is an effective strategy for preparing the BD. Therefore, the development of new processes has become a trend. It is worth noting that CO2-ODH (eqn (1)) is an environmentally friendly and sustainable approach.5 It not only effectively utilizes greenhouse gases, but also produces high-value products like BD. And the weak oxidation of CO2 is helpful in avoiding over-oxidation of the product, reducing coke formation, and improving the stability of the catalyst.6,7 However, there are still other challenges: the breakage energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in CO2 is 750 kJ mol−1, which makes it difficult to utilize CO2.8 The coke formation also reduces the stability of the catalyst.9,10 So, we designed a catalyst and aimed at solving these problems.
O bonds in CO2 is 750 kJ mol−1, which makes it difficult to utilize CO2.8 The coke formation also reduces the stability of the catalyst.9,10 So, we designed a catalyst and aimed at solving these problems.| | 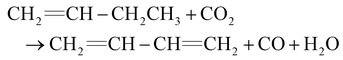 | (1) |
Due to the high catalytic activity, Fe-based catalysts have been widely studied.11–13 In the O2-ODH of butene, Yang et al.14 found that γ-Fe2O3 showed better activity than α-Fe2O3. In Lee's paper,15 a series of MIIFe2O4 catalysts (MII = Zn, Mg, Mn, Ni, Co, Cu) based on iron catalysts were prepared. The result showed that single-element doping changed the surface acidity of the catalysts. Among them, ZnFe2O4 with more surface acidity had a higher BD yield. In the study of Toledo et al.16 Al3+ was introduced into ZnFeO4, and the results showed that the Fe3+ in the octahedral sites was replaced. As a result, the lattice parameters increased, and the symmetry of electron distribution pairs around Fe3+ decreased. Then the charge transfer between Fe3+ and lattice oxygen was promoted. In the chemical looping oxidative dehydrogenation of ethane, Hye Jeong et al. prepared Ce-doped FeTiOx (FeCeTiOx) by one-pot method.17 The introduction of Ce enhanced the lattice oxygen mobility and the catalytic performance of FeCeTiOx was improved. The FeCeTiOx also revealed excellent cyclic stability with a stable C2H4 formation rate and C2H4 selectivity of 84.1%. In the CO2-ODH of propane, Zhang et al. introduced Fe into impregnated V-Al2O3, and the dual-sites catalyst (FeV/Al2O3) including Fe and V was constructed.18 V is the main active site for C3H8 dehydrogenation while Fe is responsible for the CO2 dissociation, replenishing lattice oxygen. And the catalyst achieved a C3H8 conversion at ∼43%, C3H6 selectivity exceeded 80%. It could be found that the introduction of multiple elements could improve the performance of the catalyst. In dehydrogenation reactions, V and Cr are often used as active elements. Moreover, the radius of V, Cr and Fe are similar, and the V and Cr have more valence states. This allows V and Cr to replace iron elements in the lattice, and it will lead to a change in the crystal structure and an increase in lattice defects. Therefore, by introducing V, Cr in Fe-based catalysts, the oxygen mobility and catalytic activity are expected to be improved.19–22
Water is considered to be a frequent influence in the reaction process. Several studies have shown that the introduction of water might lead to the generation of side reactions and the reduction of product selectivity as well as the shortening of catalyst life.23 In the study of the complete oxidation of methane, Zhao et al.24 found that the introduction of water led to the deactivation of the catalyst. The reasons were suggested: on the one hand, the competitive adsorption between water and CH4 at the surface-active sites caused the reversible deactivation. On the other hand, water vapor caused Pd agglomeration on the surface of the catalyst, which led to irreversible deactivation. However, the results of the presence of water were opposite in other studies.25 In the study of non-oxidative propane dehydrogenation, Long et al.26 found that water pretreatment promoted the reduction rearrangement of Co catalytic sites. Compared with the untreated catalyst, the reaction activity was increased by 7 times. The role of water in the O2-ODH of 1-butene was studied by Zeng et al.11 Both experimental and theoretical studies showed that in the presence of water, the surface-unstable O was converted into a more thermally stable hydroxyl group. The hydroxyl group was considered to be the active site of C–H bond cleavage, which is beneficial to the reaction. It could be found that water plays different roles in different reactions. Moreover, the influence of water in the 1-butene CO2-ODH reaction is still open, and the reaction mechanism involving water remains elusive. In this paper, the influence on the catalytic activity under different volume fractions was studied, and the in situ DRIFTS was used to study the reaction path in the presence of water, the possible pathway was suggested.
The paper aimed to investigate the relationship between the introduction of elements and the lattice oxygen mobility. The effect of water on the reactivity was also investigated and the possible reaction pathway was suggested. Hence, a series of multi-component catalysts were prepared for activity testing. Detailed characterizations were also carried out to study the influence of elemental introduction on the catalyst properties. The XPS was used to analyze the changes in lattice oxygen and the in situ DRIFTS tests demonstrated the role of water in the reaction. In addition, TPR, TPD, TPO, SEM, TEM, XRD, Raman, and BET were used to investigate the effect of the element introduction on the performance and morphology structure. The results showed that the multi-component (V, Cr) introduction promoted lattice oxygen mobility, and water facilitated the whole reaction by changing the reaction path. Meanwhile, it was found that the introduction of V played an important role in reducing the coke formation, the reduction ability of the catalyst was improved by the introduction of Cr. In this work, the lattice oxygen mobility was improved by the introduction of multi-component, and the CO2-ODH was optimized by the positive assistance of water. We hope this study could provide new ideas for efficient CO2-ODH.
2. Experimental
2.1. Samples preparation
2.1.1. Preparation of highly ordered polymethyl methacrylate (PMMA) microspheres.
PMMA was prepared in a nitrogen-protected environment.27,28 First, 240 ml of deionized water was added to a three-necked flask and heated to 80 °C. Then 120 ml of MMA was added and mixed thoroughly for 20 minutes. After that, the preheated 80 °C initiator solution (6 g of potassium persulfate dissolved in 40 ml of deionized water) was added to the three-necked flask. Following that, the mixed solution was reacted at 80 °C for 2 h. The solution was filtered through a microfiltration membrane and the filtrate was collected. Finally, the filtrate was centrifuged at 3000 rpm for 10 hours and dried at 40 °C for 12 hours to obtain a three-dimensionally ordered PMMA microsphere template.
2.1.2. Preparation of three-dimensional ordered macro–mesoporous Al2O3 samples.
The soft and hard templates were used to create a three-dimensional ordered macro–mesoporous structure. First, a solution of mesoporous Al precursor was prepared. 20 ml of ethanol was mixed with 1 g of P123, 2.04 g of aluminum isopropoxide, and 1.6 ml of HNO3. After that, the solution was stirred for 5 hours to completely dissolve the solute. Then PMMA was completely impregnated in the precursor solution. Following that, the impregnated samples were dried at 40 °C for 24 hours. Finally, the dried samples were calcined in a tube furnace for 3 hours under the air atmosphere. The heating rate was 1 °C min−1 from room temperature to 650 °C. Finally, a three-dimensional ordered macro–mesoporous structure was obtained.
2.1.3. Preparation of three-dimensional ordered macro–mesoporous Fe-based samples.
The preparation of the FeVCrAlOx catalyst is similar to the preparation method of the three-dimensional ordered macro–mesoporous Al2O3. By adding Fe, V, and Cr to the Al mesoporous precursor solution while keeping other conditions fixed, the FeVCrOx was obtained. Where the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr molar ratio is 10
Cr molar ratio is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Keeping the ratio of Fe and Al constant and changing the proportion of V and Cr a series of Fe-based catalysts FeVaCrbAlOx (a, b = 0, 1, 2, 3) were prepared. Finally, a series of three-dimensional ordered macro–mesoporous Fe-based catalysts were obtained. Full details of the contents could be found in the Table S2.†
1. Keeping the ratio of Fe and Al constant and changing the proportion of V and Cr a series of Fe-based catalysts FeVaCrbAlOx (a, b = 0, 1, 2, 3) were prepared. Finally, a series of three-dimensional ordered macro–mesoporous Fe-based catalysts were obtained. Full details of the contents could be found in the Table S2.†
2.2. Activity measurement
The oxidative dehydrogenation of 1-butene with CO2 was evaluated by a fixed-bed flow reactor operated in steady-state mode. A mixture of 0.2 g catalyst (40–60 mesh) and 0.4 g quartz sand (40–60 mesh) was placed in the reactor. Then the temperature was raised to 600 °C under the protection of nitrogen (60 ml min−1). Subsequently, 60 ml min−1 of feed gas containing 1-butene/CO2 (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was fed. The weight hourly space velocity (WHSV) was 4.5 g (gcat h)−1. Finally, catalyst activity was tested in a stainless-steel reactor at 600 °C. During the reaction, the feed gas was controlled by a flow meter and the water was controlled by the Syringe Pump (TYD01-01). The products were analyzed by gas chromatograph equipped with two different detectors (TCD and FID), and the conversion and selectivity were determined by the external standard method. Calculation details were presented in ESI.†
9) was fed. The weight hourly space velocity (WHSV) was 4.5 g (gcat h)−1. Finally, catalyst activity was tested in a stainless-steel reactor at 600 °C. During the reaction, the feed gas was controlled by a flow meter and the water was controlled by the Syringe Pump (TYD01-01). The products were analyzed by gas chromatograph equipped with two different detectors (TCD and FID), and the conversion and selectivity were determined by the external standard method. Calculation details were presented in ESI.†
3. Results and discussion
3.1. The synthesis mechanism of catalyst
As shown in Scheme 1, the three-dimensional ordered macro–mesoporous structure FeVCrAlOx, FeVAlOx, and FeCrAlOx catalysts were prepared by the soft and hard templates method. First, a solution of Al precursor with ordered mesopores was prepared. In order to improve the catalyst activity, the Fe element was confined into the Al2O3 framework. V and Cr were introduced to modify the crystal phase structure and release reactive oxygen species. The pore structure of the macro–mesoporous structure is aimed to provide a larger specific surface area and facilitate the diffusion of butene. The samples were used to study the synergistic effect of the introduction of multi-component on the catalytic performance.
 |
| | Scheme 1 Schematic showing the synthetic procedures. | |
3.2. Catalytic performance analysis
At 600 °C, the activity of the FeVAlOx, FeCrAlOx, and FeVCrAlOx catalysts was studied. Fig. 1 showed the stability for CO2-ODH over samples. It could be found that the multi-component catalyst FeVCrAlOx exhibited a higher activity compared with the FeVAlOx and FeCrAlOx samples (Fig. 1a). The results showed that the conversion of 1-butene reached 88.3%, the BD yield reached 27.5%, the BD selectivity reached 31.2% and the CO2 conversion reached 15.7%. However, the 1-butene conversion of FeVAlOx and FeCrAlOx was less than 60%, the BD yield was less than 17%, the BD selectivity was less than 30%, and the CO2 conversion was less than 10%. This indicated that the introduction of V and Cr is beneficial to the improvement of activity and it was also further validated by the graph of FeAlOx catalyst activity as presented in Fig. S1.† Fig. S2† shows the product distribution of FeVAlOx FeCrAlOx FeVCrAlOx samples over time. It could be noticed that the FeCrAlOx yield decreased sharply, while the yield of the V-doped samples was relatively stable. This suggests that the introduction of V might be beneficial to improve the BD selectivity. In order to understand the role of V and Cr in catalytic activity, the activity of catalysts with different Cr and V contents was tested. As shown in Fig. 2, with the introduction of Cr, the activity of the catalysts increased first and then decreased. Finally, a maximum value appeared. It was found that the maximum CO2 conversion of FeVCr2AlOx reached 16.7% (Fig. 2d), it was higher than FeVCrAlOx (15.7%). This might indicate that the conversion of CO2 was promoted by Cr. The introduction of V also showed the same trend (Fig. 3). The BD selectivity of FeV2CrAlOx reached 35% (Fig. 3d), which was also higher than FeVCrAlOx (31.2%). It might indicate that the introduction of V contributed to the selectivity of the BD. Therefore, the introduction of Cr was beneficial for the improvement of CO2 conversion, while the introduction of V was beneficial for the improvement of selectivity. Based on the 1-butene conversion and BD yields, the catalysts exhibited the best activity at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of V and Cr (Fig. 1). Although FeVCrAlOx showed the best activity under the synergistic effect of V and Cr, it was quickly deactivated. Then, the conversion rate of the FeVCrAlOx catalyst at different temperatures was analyzed. As shown in Fig. S3,† no reaction occurred at 200 °C. When the temperature was increased to 300 °C, isomerization took place, but 1,3-butadiene was not produced. At 400 °C, dehydrogenation occurred, leading to the formation of 1,3-butadiene. With a further temperature increase to 500–600 °C, isomerization decreased, dehydrogenation increased, coking became significant, and cracking reactions occurred. This suggested that 1-butene first underwent isomerization to form 2-butene, which then converted to 1,3-butadiene. Coke formation likely occurred at higher temperatures, possibly because the products could not be desorbed in time. This will lead to the cracking and even coke formation. This ultimately caused rapid catalyst deactivation. The used catalyst was regenerated, and the results showed that the regeneration process partially recovered its activity but could not fully restore the catalyst to its initial performance levels (Fig. S4†). Subsequently, structural and morphological analyses were conducted to further investigate the issue of high activity but rapid deactivation of the catalyst.
1 ratio of V and Cr (Fig. 1). Although FeVCrAlOx showed the best activity under the synergistic effect of V and Cr, it was quickly deactivated. Then, the conversion rate of the FeVCrAlOx catalyst at different temperatures was analyzed. As shown in Fig. S3,† no reaction occurred at 200 °C. When the temperature was increased to 300 °C, isomerization took place, but 1,3-butadiene was not produced. At 400 °C, dehydrogenation occurred, leading to the formation of 1,3-butadiene. With a further temperature increase to 500–600 °C, isomerization decreased, dehydrogenation increased, coking became significant, and cracking reactions occurred. This suggested that 1-butene first underwent isomerization to form 2-butene, which then converted to 1,3-butadiene. Coke formation likely occurred at higher temperatures, possibly because the products could not be desorbed in time. This will lead to the cracking and even coke formation. This ultimately caused rapid catalyst deactivation. The used catalyst was regenerated, and the results showed that the regeneration process partially recovered its activity but could not fully restore the catalyst to its initial performance levels (Fig. S4†). Subsequently, structural and morphological analyses were conducted to further investigate the issue of high activity but rapid deactivation of the catalyst.
 |
| | Fig. 1 The catalytic performance of FeVAlOx, FeCrAlOx, and FeVCrAlOx samples at 600 °C (a) 1-butene conversion, (b) BD yield, (c) BD selectivity, and (d) CO2 conversion. | |
 |
| | Fig. 2 Catalytic performance of samples with different Cr contents at 600 °C. (a) 1-Butene conversion, (b) BD yield, (c) BD selectivity, and (d) CO2 conversion. | |
 |
| | Fig. 3 Catalytic performance of samples with different V contents at 600 °C. (a) 1-Butene conversion, (b) BD yield, (c) BD selectivity, and (d) CO2 conversion. | |
3.3. Structural and morphological analysis
The surface morphology of the samples was studied by SEM (Fig. 4). It could be found that FeCrAlOx, FeVAlOx, and FeVCrAlOx samples contained abundant ordered macroporous structures. When V and Cr were introduced, the unique macroporous structure of all samples was still maintained. The microstructure of the samples was studied by TEM (Fig. 5). Firstly, it could be found that all the samples maintained an ordered macroporous structure (Fig. 5a–h), which was consistent with the results of SEM. In addition, mesoporous structures were observed in FeCrAlOx, FeVAlOx, and FeVCrAlOx samples (Fig. 5i–l). Without the introduction of elements, Al2O3 had a highly ordered mesoporous structure. When Fe and Cr were introduced, the FeCrAlOx still maintained the ordered mesoporous (Fig. 5f and j). This indicated that the introduction of Cr would not destroy the three-dimensional ordered macro–mesoporous structure. However, after the introduction of V, it was found that the ordered mesoporous structure of FeVAlOx was destroyed (Fig. 5k). And a similar morphology was observed in FeVCrAlOx (Fig. 5l). This might be due to the generation of new crystal phases caused by the introduction of elements. And the lattice distortion might be caused by the formation of new crystal phases. Then the pore structure would deform or reorganize. The transition from ordered structure to disordered structure might indicate the successful introduction of V and Cr.
 |
| | Fig. 4 SEM images of FeCrAlOx (a, d), FeVAlOx (b, e), and FeVCrAlOx (c, f). | |
 |
| | Fig. 5 TEM images of Al2O3 (a, e, i), FeCrAlOx (b, f, j), FeVAlOx (c, g, k), and FeVCrAlOx (d, h, l). | |
The change in crystal structure after the introduction of elements was studied by XRD. The results were shown in Fig. 6 and Fig. S5.† The FeAlO3 (PDF#30-0024) was found in the XRD curves of FeCrAlOx, FeVAlOx, and FeVCrAlOx samples. The XRD curves of FeVAlOx and FeCrAlOx (Fig. 6a and b) showed that the V element exists as VOx when V was introduced into the Fe-based catalysts. This might indicate that the Fe in the lattice could not be replaced when only the V element was introduced. However, when Cr was introduced into iron-based catalysts, Cr1.3Fe0.7O3 (PDF#35-1112) appears in the XRD curve of FeCrAlOx (Fig. 6b). This indicated that Fe in the lattice could be replaced by Cr. Finally, when V and Cr were introduced into the Fe-based catalyst together, the 2θ peaks at 33.44°, 35.79°, 41.17°, 49.72°, 54.35°, 62.90°, were considered as (104), (110), (113), (024), (116), (214) lattice planes of Cr1.3Fe0.7O3 (PDF#35-1112). And the 2θ peaks at 18.96°, 31.01°, 36.67°, 38.59°, 44.68°, and 65.16°, were considered as (111), (220), (311), (222), (400), and (531) lattice planes of Fe2V3O4 (PDF#15-0122). This indicated that V and Cr elements were successfully introduced into the Fe phase, and Cr might play an assistant role in the introduction of V. It's worth noting that, the appearance of 2θ peaks at 23.66°, 25.99°, 37.17°, 62.83°, 64.97°, were considered as (210), (211), (222), (440), (530) lattice planes of γ-Fe2O3 (PDF#39-1346). This indicated that the highly active γ-Fe2O3 phase was formed after the introduction of V and Cr. It could be found that the introduction of V and Cr changed the crystal structure of the catalyst and promoted the formation of γ-Fe2O3. This was beneficial to improving the mobility of lattice oxygen and the catalytic activity. This also elucidated the reason for the good catalytic activity of the catalyst. It should be emphasized that there is a clear difference between the catalyst before and after the reaction. As shown in Fig. S6,† the surface of the used catalyst was coated with a dark-colored substance, which potentially corresponds to catalyst deactivation. According to carbon balance (Fig. S7†), it could be found that the amount of carbon decreased after the reaction. This might be because carbon species. To better understand the catalyst changes before and after the reaction. XRD was performed on the samples. As shown in Fig. 6d, the peaks at 35.78°, 43.98°, 59.01°, and 63.25°, were considered as (010), (111), (104), (212) lattice planes of FeC (PDF#03-0400), and 18.78°, 31.01°, 36.51°, 44.39°, 64.62°, were considered as (111), (220), (311), (400), (440) lattice planes of Fe2Al2O4 (PDF#34-0192). This indicated that the crystalline phase changed after the reaction and the FeC phase was generated. And FeC might be due to deep dehydrogenation of olefins. Besides, Fig. S8† showed the XRD and the surface of the regenerated catalyst, and there was no obvious coke on the surface of catalyst (Fig. S8b†). However, compared to the fresh sample (Fig. S8a†), the regenerated catalyst had a significant decrease in the intensity of the peaks and the shape of the peaks was changed. This suggested the structure of the catalyst might change. Consequently, it can be inferred that the change in crystalline phase and coke formation were the main factors for catalyst deactivation and this make it difficult to restore its initial activity.
 |
| | Fig. 6 XRD patterns of the FeVAlOx (a), FeCrAlOx (b), FeVCrAlOx (c), and used-FeVCrAlOx samples (d). | |
3.4. Study of coke formation
To visually show the type of carbon deposition, the used catalysts were analyzed by Raman spectroscopy. The D and G bands in the Raman spectra of the used catalysts indicated the presence of amorphous carbon deposition and graphitic carbon deposition, respectively.29,30 According to the Tuinstra–Koenig law, the graphite crystallinity of the carbon deposition is inversely related to the integrated ratio of the D and G bands (ID/IG).31,32 In Fig. 7, compared with FeCrAlOx (ID/IG = 0.92), the graphite crystallinity of the V introduced catalyst FeVAlOx (ID/IG = 0.88) was lower. Moreover, compared with FeCrAlOx, the degree of graphitization of the catalyst FeVCrAlOx (ID/IG = 0.91) was also reduced. This suggested that the introduction of V was beneficial to inhibit the graphitizing of carbon deposits. And the lower degree of graphitization means that coke might be eliminated easily, this was consistent with O2-TPO results of the used catalysts (Fig. S9†).33,34 Compared with the FeCrAlOx catalysts, the FeVAlOx catalysts had smaller oxidation peaks and required less time for complete oxidation. This suggested that the coke on the FeVAlOx catalyst was less and it was easier to remove. When V was introduced, the catalyst FeVCrAlOx showed the similar results. This indicated that the catalysts with the introduction of V had better coking resistance.
 |
| | Fig. 7 Raman spectra of the used FeVAlOx, FeCrAlOx, and FeVCrAlOx samples. | |
The surface area, pore volume, and pore size of the FeVAlOx, FeCrAlOx, and FeVCrAlOx catalysts were tested by BET and the results were shown in Fig. 8 and Table 1. All the catalysts showed type IV isotherms with an H1 hysteresis loop, which indicated that the samples had abundant mesoporous structures.35–37 This is consistent with the results of TEM. Table 1 showed the specific surface area in the descending order: FeCrAlOx > FeVCrAlOx > FeVAlOx. Compared with FeCrAlOx, FeVCrAlOx, and FeVAlOx, it was found that the introduction of V resulted in a smaller specific surface area of the catalyst. According to the TEM analysis, the introduction of V destroyed the ordered mesoporous structure, which might be the reason for the reduction of specific surface area. In addition, it could be found that the pore volumes were in the following order: FeVCrAlOx > FeCrAlOx > FeVAlOx. The large pore volume was beneficial in reducing the accumulation of reactants on the catalyst surface, which reduced the possibility of carbon deposition and coking. Owing to the large pore volume of FeVCrAlOx (0.182 cm3 g−1), the catalyst exhibited good catalytic activity with the low coke formation.38,39 Pore size followed the order: FeVAlOx > FeVCrAlOx > FeCrAlOx. The comparison among FeVAlOx, FeCrAlOx, and FeVCrAlOx showed that the catalyst had the smallest pore size after the introduction of Cr (6.78 nm), the pore size became larger after the introduction of V (7.80 nm), and FeVCrAlOx had moderate pore sizes (7.01 nm) when both V and Cr were introduced. This might indicate that the pore size of the catalyst could be adjusted by the introduction of V. And the large pore size would facilitate the flow and diffusion of molecules, thereby reducing the coke formation.40 This is consistent with the Raman results.
 |
| | Fig. 8 Nitrogen adsorption–desorption isotherms (a) and pore size distribution (b) of FeVAlOx, FeCrAlOx, and FeVCrAlOx samples. | |
Table 1 BET result of synthesized FeVAlOx, FeCrAlOx, and FeVCrAlOx catalysts
| Samples |
Surface area (m2 g−1) |
Pore size (nm) |
Pore volume (cm3 g−1) |
| FeVAlOx |
64.04 |
7.80 |
0.166 |
| FeCrAlOx |
92.32 |
6.78 |
0.178 |
| FeVCrAlOx |
84.91 |
7.01 |
0.182 |
3.5. Surface chemical environment and oxygen species analysis
The XPS test was used to study the valence of elements and their relative contents in samples. The results are shown in Fig. 9 and Table 2. As shown in Fig. 9a, the Fe 2p3/2 peaks at 711.2 and 712.8 eV, could be regarded as Fe2+ and Fe3+ species.41–43 The Fe3+ is considered as the active component in the oxidative dehydrogenation process and the oxidative dehydrogenation reaction was carried out through the Fe3+/Fe2+ redox cycle. According to the data in Table 2, the percentage of Fe3+ was 50–60% when only V or Cr was introduced (FeVAlOx or FeCrAlOx). However, when both components were added, the percentage of Fe3+ increased to 73.23% for FeVCrAlOx. According to XRD analysis, the Fe2O3 phase appeared after the introduction of multi-component. This might be the reason for the increase in the percentage of Fe3+. This suggested that multi-component catalysts have a higher oxidative dehydrogenation activity. In addition, the used catalysts were also analyzed to study the percentage of Fe3+. The results showed that there was a decrease in the percentage of Fe3+ from 73.23% to 46.27% after the reaction. This indicated that Fe3+ played a key role in the reaction, its main function was to act as an efficient active component and facilitate the reaction process.
 |
| | Fig. 9 XPS spectra of the (a) Fe 2p, (b) O 1s, (c) V 2p, (d) Cr 2p, on FeVAlOx, FeCrAlOx, FeVCrAlOx, and used-FeVCrAlOx. | |
Table 2 XPS data of FeVAlOx, FeCrAlOx, FeVCrAlOx, and used-FeVCrAlOx catalysts
| Samples |
Fe3+/Fen+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
Cr6+/Crn+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
V5+/Vn+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
Oα/On![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
|
The percentage of surface elements valences (Fen+ = Fe3+ + Fe2+), (Crn+ = Cr6+ + Cr3+), (Vn+ = V5+ + V4+ + V3+), and (On = Oα + Oβ + Oγ) were determined by XPS fitting analysis.
|
| FeVAlOx |
54.20 |
|
47.77 |
26.97 |
| FeCrAlOx |
50.46 |
48.72 |
|
25.67 |
| FeVCrAlOx |
73.23 |
56.85 |
55.23 |
36.10 |
| Used-FeVCrAlOx |
46.27 |
27.11 |
18.43 |
24.94 |
Then, the lattice oxygen content of different catalysts was analyzed. As shown in Fig. 9b, the O 1s peaks at about 530.4, 531.5, and 533 eV, were considered as the lattice oxygen (Oα), the surface adsorbed oxygen (Oβ), and adsorbed water or hydroxyl molecules (Oγ) respectively.44,45 It was noteworthy that the lattice oxygen binding energy of the sample (FeVCrAlOx) was shifted towards lower values. This transition might be attributed to the inherent radius and charge density differences of vanadium (V), chromium (Cr), and iron (Fe). After the introduction of V and Cr, the lattice structure was modified. This led to the change in the local environment around the lattice oxygen, as a result, the strength of the metal–oxygen bond was weakened. The latter would enhance lattice oxygen mobility.46 Moreover, the high proportion of Oα in the catalyst (FeVCrAlOx), confirming the synergistic effect of V and Cr improved the mobility of lattice oxygen. The O2-TPD was used to study the surface oxygen species (Fig. 10 and Table 3). Peaks below 400 °C were attributed to the adsorbed oxygen species. Peaks between 400 and 600 °C were considered as the surface lattice oxygen species. And peaks above 600 °C were regarded as the lattice oxygen species.47–49 As shown in Fig. 10a, FeVCrAlOx showed the highest percentage of surface lattice oxygen species (54.75%). Then, by adjusting the amount of V and Cr introduced, the effect of element introduction on oxygen species was studied. With the introduction of Cr, the lattice oxygen percentage decreased and the surface lattice oxygen percentage increased. While with the introduction of V, the adsorbed oxygen percentage decreased and the surface lattice oxygen percentage increased Under the synergistic effect of V and Cr, the FeVCrAlOx sample had more surface lattice oxygen. This might indicate that V, Cr destroyed the original lattice structure. After the introduction of V and Cr, more lattice oxygen was activated and oxygen mobility was increased.
 |
| | Fig. 10 O2-TPD curves of the FeVAlOx, FeCrAlOx, FeVCrAlOx (a), and the curves of catalysts with different Cr (a) and V (b) contents. | |
Table 3 The percentage of oxygen species by O2-TPD
| Samples |
Adsorbed oxygen (%) |
Surface lattice oxygen (%) |
Lattice oxygen (%) |
| FeVAlOx |
38.66 |
51.24 |
10.10 |
| FeVCrAlOx |
33.26 |
54.75 |
11.99 |
| FeVCr2AlOx |
42.82 |
53.82 |
3.36 |
| FeVCr3AlOx |
43.72 |
52.90 |
3.38 |
| FeCrAlOx |
62.56 |
22.19 |
15.26 |
| FeVCrAlOx |
33.26 |
54.75 |
11.99 |
| FeV2CrAlOx |
42.75 |
42.73 |
14.53 |
| FeV3CrAlOx |
37.63 |
53.57 |
8.80 |
Besides, the V 2p3/2 peaks at 516.5, 517.3, and 517.9 eV in Fig. 9c were considered as V3+, V4+, and V5+ species, respectively.50,51 From the Table 2, it could be found that compared with FeVAlOx (47.77%), FeVCrAlOx (55.23%) contained more V5+ species. This suggested that the introduction of multi-components brought more V5+ species. The analysis of the Used-FeVCrAlOx showed that the percentage of V5+ species was reduced to 18.43%. It demonstrated that V5+ might act as an active phase and participate in the reaction. Cr 2p spectrum was also studied. As shown in Fig. 9d, the peaks at 577 and 579 eV in Cr 2p3/2 were considered as Cr3+ and Cr6+ species.52–55 It could be found that the binding energy of Cr is shifted to low binding energy after the introduction of the V. This might be due to the change in crystal structure after the introduction of V. This led to a change in the electron density around Cr and a decrease in binding energy. Similar to the V species, the Cr6+ species percentage increased from 48.72% to 56.85% when multi-components were introduced. And the percentage of Cr6+ species decreased to 27.11% after the reaction. The result indicated that Cr6+ species were also potential active phases in the reaction. Overall, the introduction of V and Cr not only increased the percentage of Fe3+ but also improved the lattice oxygen mobility. Besides, V and Cr were active components in the reaction. Under the synergistic effect, the multi-component catalyst activity was significantly enhanced.
3.6. Reduction ability and acid/base properties
H2-TPR was used to study the effect of elemental introduction on catalyst activity (Fig. S10 and Table S3†). In Fig. S10,† the peaks between 400–500 °C were attributed to the transition from Fe2O3 to Fe3O4, while the peaks between 600–700 °C were attributed to the transition from Fe3O4 to FeO.56,57 Compared with the peak position of the samples (FeVAlOx, FeCrAlOx, and FeVCrAlOx), the result showed that the addition of Cr led to a shift of the peak towards lower temperatures (from 467 to 464 °C). In order to clarify the influence of Cr and V on the reducibility. The relationship between the different contents of the elements and the reducibility of the catalyst was studied. the results were shown in Fig. S10b and c.† It could be found that the introduction of Cr led to a shift of the peaks toward lower temperatures (from 467 to 445 °C). This demonstrated that doping with Cr was beneficial for improving the reducibility of the catalyst. With the introduction of V, the peak shifted to the high temperatures (from 415 to 558 °C), this indicated that with the introduction of V, the catalyst became difficult to reduce. It is possible that the introduction of V changed the crystal structure. The vanadium species on the crystal surface changed from oligomeric vanadium oxide species to polymeric vanadium oxide species. While there are differences in the reducibility of different vanadium species, this leads to an increase in the reduction temperature.58
CO2-TPD was used to study the basicity of the samples (Fig. S11†) and the results of the test by CO2-TPD were shown in Table 4. As shown in Fig. S11a,† Peaks below 200 °C were considered as the weak basic sites, peaks between 200 and 350 °C were considered as the medium-strong basic sites, and peaks between 350 and 800 °C were considered as the strong basic sites.59,60 Compared with FeCrAlOx, FeVAlOx, and FeVCrAlOx samples (Fig. S11b and c†), it was discovered that FeVCrAlOx had the greatest amount of strong basic sites (86.06%), followed by FeVAlOx (76.91%) and FeCrAlOx (63.39%). This suggested that the introduction of multi-component could effectively improve the strong basic sites. Besides, with the introduction of Cr, the strong basic sites decreased from 86.06% to 76.55% and then to 82.13%. When V was introduced, the percentage of strong basic sites decreased from 86.06% to 69.31%. This indicated that the multi-component had more strong base sites, and the introduction of excessive V or Cr would lead to a decrease in the strong base sites. This would be beneficial for the CO2 utilization.61
Table 4 The percentage of base sites by CO2-TPD
| Samples |
Weak basicity (%) |
Medium basicity (%) |
Strong basicity (%) |
| FeVAlOx |
7.81 |
15.28 |
76.91 |
| FeVCrAlOx |
6.98 |
6.97 |
86.06 |
| FeVCr2AlOx |
9.02 |
14.43 |
76.55 |
| FeVCr3AlOx |
5.13 |
12.73 |
82.13 |
| FeCrAlOx |
16.17 |
20.44 |
63.39 |
| FeVCrAlOx |
6.98 |
6.97 |
86.06 |
| FeV2CrAlOx |
13.41 |
17.22 |
69.37 |
| FeV3CrAlOx |
10.62 |
20.07 |
69.31 |
In oxidative dehydrogenation reactions, acidic sites were found to play a crucial role by facilitating the breaking of C–C, C–H, and C–O bonds.62 NH3-TPD was used to study the acidity of the samples (Fig. S12†). Peaks below 300 °C were attributed to weak acid sites and peaks between 300 and 500 °C were considered as medium-strong acid sites.63–65 Typically, weakly acidic sites provided a suitable environment for adsorption and activation. Strongly acidic sites promoted the reaction rate, while overly acidic sites resulted in reduced selectivity for the target product.66–69 The respective effects of V and Cr were analyzed, and the results showed that the addition of Cr modified the weak and medium strong acidic sites (Fig. S12b†). While the introduction of V decreased the weak acid sites and increased the medium-strong acid sites (Fig. S12c†). Compared with the FeVAlOx, FeCrAlOx, and FeVCrAlOx samples (Fig. S12a†), it could be found that the multi-component catalyst FeVCrAlOx contained more medium strong acid sites. This suggested that the introduction of multicomponent catalysts could increase the medium-strong acid sites. And the suitable ratio of weak and strong acid sites promoted the dehydrogenation reaction.70
3.7. Catalytic performance in the presence of water
In order to study the effect of water on catalytic activity, the performance of the FeVCrAlOx samples was studied under different volume fractions of water (Fig. 11). The volume fraction was recorded as 0 when water was absent in the reaction. It was discovered that when the volume fraction of water rose, the BD yield increased first and then decreased (Fig. 11a). Compared with the yield in the absence of water (27.5%), the yield reached 33.1%, when the water volume fraction was 2.25% (Fig. 11b). Besides, the selectivity of BD increased from 31.2% to 38.9% (Fig. 11c), and the conversion of CO2 increased from 15.7% to 19.3% (Fig. 11d). This demonstrated that the BD yield, BD selectivity, and the CO2 conversion could be improved by a proper volume fraction of water. However, the conversion of 1-butene decreased after the addition of water. This might be due to the competitive adsorption of water with 1-butene on the surface of the catalyst, therefore the conversion of 1-butene was reduced.71,72
 |
| | Fig. 11 Catalytic activity of FeVCrAlOx samples with different water volume fractions at 600 °C. (a) 1-Butene conversion. (b) BD yield, (c) BD selectivity, and (d) CO2 conversion. | |
The catalytic activity of FeVAlOx and FeCrAlOx was studied at different water volume fractions (Fig. S13 and S14†). Fig. S15† showed the reaction orders of FeVAlOx, FeCrAlOx, and FeVCrAlOx, for 1-butene consumption in the presence of water. The data Revealed the sensitivity to the presence of water differed among the different catalysts. The FeCrAlOx catalyst had a reaction order of 0.12, this indicated a relatively low sensitivity to water. The FeVAlOx catalyst had a reaction order of 0.17, suggesting it was more sensitive to the presence of water than the FeCrAlOx. On the other hand, the multicomponent catalyst (FeVCrAlOx) had a reactivity order of only 0.03. This indicated much lower sensitivity to changes in water concentration. This might be due to the strong adsorption capacity of its porous structure, so the adsorption or activation of water molecules is not the rate-determining step.73 In order to study how water promoted the CO2-ODH catalytic process. In situ DRIFTS was used to study the reaction in the absence/presence of water.
3.8. Reaction mechanism study
The in situ DRIFTS spectra of 1-butene adsorption at 100 °C were shown in Fig. 12. The peaks at 3088 cm−1 and 1651 cm−1 were regarded as the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and the C
CH2 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of 1-butene.74 This indicated that butene was effectively adsorbed on the catalyst surface over time. Fig. 13 showed the heating process at 110–350 °C with the introduction of CO2. The peak at 2973 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group75 (Fig. 13c). The peaks at 1654 cm−1 and 1640 cm−1 were regarded as the C
C stretching vibration of 1-butene.74 This indicated that butene was effectively adsorbed on the catalyst surface over time. Fig. 13 showed the heating process at 110–350 °C with the introduction of CO2. The peak at 2973 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group75 (Fig. 13c). The peaks at 1654 cm−1 and 1640 cm−1 were regarded as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of 1-butene and 1,3-butadiene, respectively (Fig. 13b). As the temperature rose, the peak at 2973 cm−1 (–CH3) decreased and then disappeared (the temperature is about 320 °C), which indicated that the terminal methyl group began to be consumed. And the peak at 1654 cm−1 gradually disappeared and the peak at 1640 cm−1 gradually appeared. This indicated that 1,3-butadiene was formed. The shift from 1654 to 1640 cm−1 might be due to the conjugation effect of 1,3-butadiene, then the C
C stretching vibration of 1-butene and 1,3-butadiene, respectively (Fig. 13b). As the temperature rose, the peak at 2973 cm−1 (–CH3) decreased and then disappeared (the temperature is about 320 °C), which indicated that the terminal methyl group began to be consumed. And the peak at 1654 cm−1 gradually disappeared and the peak at 1640 cm−1 gradually appeared. This indicated that 1,3-butadiene was formed. The shift from 1654 to 1640 cm−1 might be due to the conjugation effect of 1,3-butadiene, then the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C force constant was decreased and thus the C
C force constant was decreased and thus the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrational phase was shifted towards the low-wave direction. This eventually leads to a redshift. Similarly, the peaks at 3088 cm−1 and 3072 cm−1 (Fig. 13c) were regarded as the
C stretching vibrational phase was shifted towards the low-wave direction. This eventually leads to a redshift. Similarly, the peaks at 3088 cm−1 and 3072 cm−1 (Fig. 13c) were regarded as the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 stretching vibrations of 1-butene and 1,3-butadiene76,77 respectively. It could be found that as the temperature increased, the peaks at 3088 cm−1 gradually weakened and then disappeared, after that the peaks at 3072 cm−1 appeared and gradually became stronger. This might indicate that the
CH2 stretching vibrations of 1-butene and 1,3-butadiene76,77 respectively. It could be found that as the temperature increased, the peaks at 3088 cm−1 gradually weakened and then disappeared, after that the peaks at 3072 cm−1 appeared and gradually became stronger. This might indicate that the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 of butene is consumed first and then disappeared during the conversion of 1-butene to 1,3-butadiene. Finally, the characteristic peak of 1,3-butadiene appeared. This might be that 1-butene was first converted into 2-butene and then the 2-butene was converted into 1,3-butadiene.78
CH2 of butene is consumed first and then disappeared during the conversion of 1-butene to 1,3-butadiene. Finally, the characteristic peak of 1,3-butadiene appeared. This might be that 1-butene was first converted into 2-butene and then the 2-butene was converted into 1,3-butadiene.78
 |
| | Fig. 12
In situ DRIFTS spectra of 1-butene adsorption at 100 °C over the FeVCrAlOx samples. | |
 |
| | Fig. 13
In situ DRIFTS Spectra of FeVCrAlOx samples exposed to CO2 and 1-butene steams. | |
The reaction in the presence of water was also analyzed by in situ DRIFTS and the adsorption of butene at 100 °C was shown in Fig. S16.† The characteristic peaks at 3088 cm−1 and 1654 cm−1 were regarded as the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and the C
CH2 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of 1-butene, and this indicated that 1-butene was effectively adsorbed on the catalyst surface over time. Fig. 14 showed the variation of peaks from 110–380 °C in the presence of H2O and CO2. The peaks at 3088 cm−1 and 3050 cm−1 could be regarded as the
C stretching vibration of 1-butene, and this indicated that 1-butene was effectively adsorbed on the catalyst surface over time. Fig. 14 showed the variation of peaks from 110–380 °C in the presence of H2O and CO2. The peaks at 3088 cm−1 and 3050 cm−1 could be regarded as the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 stretching vibrations of 1-butene and 1,3-butadiene, respectively. The peak at 2971 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group (Fig. 14c). After the addition of water and CO2, it could be found that the terminal methyl group decreased rapidly and then disappeared (the temperature is about 290 °C), the peaks at 3088 cm−1 gradually weakened and then disappeared, and after that, the peaks at 3050 cm−1 gradually increased (Fig. 14c). This was similar to the change of peak in the absence of water. This indicated that 1,3-butadiene was produced. It is worth noting that when water was added, the peak of OH− (3644 cm−1), HCO3− (1393 cm−1), and HCOO− (1602 cm−1) appeared (Fig. 14b and d), and the hydroxyl peaks on the catalyst surface were enhanced (3400 cm−1).79–82 The reason might be that water was first cleaved to OH− or surface hydroxyl groups by the catalyst, and the OH− reacted with CO2, then the HCO3− was formed. As a result, the peak of surface hydroxyl groups and HCO3− increased. After that HCO3− + C4H8
CH2 stretching vibrations of 1-butene and 1,3-butadiene, respectively. The peak at 2971 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group (Fig. 14c). After the addition of water and CO2, it could be found that the terminal methyl group decreased rapidly and then disappeared (the temperature is about 290 °C), the peaks at 3088 cm−1 gradually weakened and then disappeared, and after that, the peaks at 3050 cm−1 gradually increased (Fig. 14c). This was similar to the change of peak in the absence of water. This indicated that 1,3-butadiene was produced. It is worth noting that when water was added, the peak of OH− (3644 cm−1), HCO3− (1393 cm−1), and HCOO− (1602 cm−1) appeared (Fig. 14b and d), and the hydroxyl peaks on the catalyst surface were enhanced (3400 cm−1).79–82 The reason might be that water was first cleaved to OH− or surface hydroxyl groups by the catalyst, and the OH− reacted with CO2, then the HCO3− was formed. As a result, the peak of surface hydroxyl groups and HCO3− increased. After that HCO3− + C4H8![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4H6 + HCOO− + H2O might happen. This resulted in the peak appearing at 1602 cm−1 (HCOO−). The characteristic peak of
C4H6 + HCOO− + H2O might happen. This resulted in the peak appearing at 1602 cm−1 (HCOO−). The characteristic peak of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (BD) was located at 3050 cm−1, rather than 3072 cm−1, which might be due to the redshift caused by the HCOO−. Finally, through the comparison of methyl peaks with and without water, it could be found that the methyl peaks decreased rapidly in the presence of water. It might be due to the improvement of CO2 utilization in the presence of water. Thus, the reaction rate was accelerated, and the methyl peaks were rapidly consumed. In general, it could be found that the introduction of water not only improved the utilization of CO2 but also changed the reaction path. This might be the reason for the increase in activity.
CH2 (BD) was located at 3050 cm−1, rather than 3072 cm−1, which might be due to the redshift caused by the HCOO−. Finally, through the comparison of methyl peaks with and without water, it could be found that the methyl peaks decreased rapidly in the presence of water. It might be due to the improvement of CO2 utilization in the presence of water. Thus, the reaction rate was accelerated, and the methyl peaks were rapidly consumed. In general, it could be found that the introduction of water not only improved the utilization of CO2 but also changed the reaction path. This might be the reason for the increase in activity.
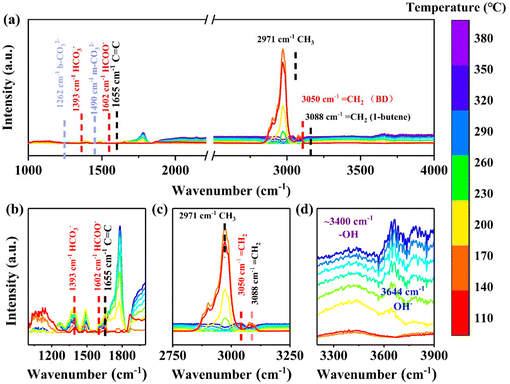 |
| | Fig. 14
In situ DRIFTS Spectra of FeVCrAlOx samples exposed to CO2, 1-butene, and H2O steams. | |
4. Conclusions
To study the synergetic effect of the introduction of multiple elements on the catalytic performance, a series of 3DOM Fe-based catalysts were prepared by the soft and hard templates method. The three-dimensionally ordered macro–mesoporous structures were studied by SEM and TEM images. The FeVCrAlOx catalyst showed a better catalytic performance. The 1-butene conversion of 88.3% and the BD yield of 27.5%, were reached. The synergetic effects of the V and Cr on the catalytic performances were studied respectively. The results showed that the introduction of Cr was beneficial for the conversion of CO2, and the introduction of V was beneficial for the selectivity of BD. Moreover, when V and Cr were introduced together, the formation of γ-Fe2O3 was one of the reasons for the enhancement of catalytic activity. XPS and O2-TPD studies showed that the introduction of multi-components improved the mobility of lattice oxygen. Compared with the FeVAlOx and FeCrAlOx samples, the FeVCrAlOx had surface lattice oxygen, and the proportion reached 36.10%. And the active components were also enriched (the proportion of Fe3+, Cr6+, and V5+ reached 73.23%, 56.85%, and 55.23%, respectively). This might be another reason for the improvement of catalytic activity. The XRD and Raman studies showed that the rapid deactivation of the catalyst was due to the coke formation, and the introduction of V was beneficial for the reduction of coke formation. In addition, by introducing water into the reaction process, the role of water in the catalytic process was clarified. The in situ DRIFTS tests showed that the addition of H2O might enhance the utilization of CO2 and change the reaction route. In the reaction process, water was cleaved to OH− or surface hydroxyl groups. The OH− reacted with CO2. As a result of this, the HCO3− was formed. In this way, CO2 participated in the reaction in the form of HCO3−. The catalytic activity was enhanced by the introduction of water. Finally, this study not only provides useful insights for the efficient utilization of CO2 but also generates new ideas for the design of efficient dehydrogenation catalysts.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Major Science and Technology Project of Gansu (23ZDFA016), Key Research and Development Program of Gansu Province, State Key Laboratory Program of the Lanzhou Institute of Chemical Physics, CAS (No. CHGZ-202213), LICP Cooperation Foundation for Young Scholars (HZJJ23-3) and West Light Foundation of Chinese Academy of Sciences (xbzg-zdsys-202318).
References
- P. Tamizhdurai, V. L. Mangesh, A. A. A. Bahajjaj, U. Rajai, M. Govindasamy, R. Vasanthi, R. Kumaran and T. Augustine, Ionic liquid supported on mesoporous Pd/SBA-15 during the manufacture of 1,3-butadiene using ethanol at lower temperature reaction, Fuel, 2023, 345, 128235 CrossRef CAS.
- L. Qi, Y. Zhang, M. Babucci, C. Chen, P. Lu, J. Li, C. Dun, A. S. Hoffman, J. J. Urban, M. Tsapatsis, S. R. Bare, Y. Han, B. C. Gates and A. T. Bell, Dehydrogenation of Propane and n-Butane Catalyzed by Isolated PtZn4 Sites Supported on Self-Pillared Zeolite Pentasil Nanosheets, ACS Catal., 2022, 12, 11177–11189 CrossRef CAS.
- P. del Campo, M. T. Navarro, S. K. Shaikh, M. D. Khokhar, F. Aljumah, C. Martínez and A. Corma, Propene Production by Butene Cracking. Descriptors for Zeolite Catalysts, ACS Catal., 2020, 10, 11878–11891 CrossRef CAS.
- W. C. White, Butadiene production process overview, Chem.-Biol. Interact., 2007, 166, 10–14 CrossRef CAS PubMed.
- B. Yan, L. Wang, B. Wang, F. Alam, Z. Xiao, J. Li and T. Jiang, Constructing a high-efficiency iron-based catalyst for carbon dioxide oxidative dehydrogenation of 1-butene: The role of oxygen mobility and proposed reaction mechanism, Appl. Catal., A, 2019, 572, 71–79 CrossRef CAS.
- K. Yang, J. Li, C. Wei, Z. Zhao and Z. Liu, Coupling Conversion of CO2 and n-Butane Over Modified ZSM-5: Incorporation of the Carbon from CO2 into Hydrocarbon Products, ACS Catal., 2023, 13, 10405–10417 CrossRef CAS.
- S. T. Rahman, J.-R. Choi, J.-H. Lee and S.-J. Park, The Role of CO2 as a Mild Oxidant in Oxidation and Dehydrogenation over Catalysts: A Review, Catalysts, 2020, 10, 1075 CrossRef CAS.
- X. Li, S. Wang, L. Li, X. Zu, Y. Sun and Y. Xie, Opportunity of Atomically Thin Two-Dimensional Catalysts for Promoting CO2 Electroreduction, Acc. Chem. Res., 2020, 53, 2964–2974 CrossRef CAS PubMed.
- Y. Dai, Y. Wu, H. Dai, X. Gao, S. Tian, J. Gu, X. Yi, A. Zheng and Y. Yang, Effect of coking and propylene adsorption on enhanced stability for Co2+-catalyzed propane dehydrogenation, J. Catal., 2021, 395, 105–116 CrossRef CAS.
- X. Chen, M. Ge, Y. Li, Y. Liu, J. Wang and L. Zhang, Fabrication of highly dispersed Pt-based catalysts on γ-Al2O3 supported perovskite Nano islands: High durability and tolerance to coke deposition in propane dehydrogenation, Appl. Surf. Sci., 2019, 490, 611–621 CrossRef CAS.
- T. Zeng, G. Sun, C. Miao, G. Yan, Y. Ye, W. Yang and P. Sautet, Stabilizing Oxidative Dehydrogenation Active Sites at High Temperature with Steam: ZnFe2O4-Catalyzed Oxidative Dehydrogenation of 1-Butene to 1,3-Butadiene, ACS Catal., 2020, 10, 12888–12897 CrossRef CAS.
- X. Li, B. Yan, S. Yao, S. Kattel, J. G. Chen and T. Wang, Oxidative dehydrogenation and dry reforming of n-butane with CO2 over NiFe bimetallic catalysts, Appl. Catal., B, 2018, 231, 213–223 CrossRef CAS.
- X. Zhang, J. Li, W. Liu, Y. Zheng, J. An, W. Xin, Z. Liu, L. Xu, X. Li and X. Zhu, Synergistic Effect of Zinc and Iron in the CO2-Assisted Oxidative Dehydrogenation of Propane, ACS Catal., 2023, 13, 14864–14873 CrossRef CAS.
- B. L. Yang, M. C. Kung and H. H. Kung, Reasons for the different selectivities in the selective oxidation of butene on α- and γ-Fe2O3, J. Catal., 1984, 89, 172–176 CrossRef CAS.
- H. Lee, J. C. Jung, H. Kim, Y.-M. Chung, T. J. Kim, S. J. Lee, S.-H. Oh, Y. S. Kim and I. K. Song, Effect of Divalent Metal Component (MeII) on the Catalytic Performance of MeIIFe2O4 Catalysts in the Oxidative Dehydrogenation of n-Butene to 1,3-Butadiene, Catal. Lett., 2008, 124, 364–368 CrossRef CAS.
- J. A. Toledo, P. Bosch, M. A. Valenzuela, A. Montoya and N. Nava, Oxidative dehydrogenation of 1-butene over Zn-Al ferrites, J. Mol. Catal. A: Chem., 1997, 125, 53–62 CrossRef CAS.
- M. Hye Jeong, D. Hyung Lee, J. Won Moon, J. Sun, J. Soon Choi, D. Sik Hong, C.-H. Chung and J. Wook Bae, Oxidative dehydrogenation of ethane and subsequent CO2 activation on Ce-incorporated FeTiOx metal oxides, Chem. Eng. J., 2022, 433, 134621 CrossRef CAS.
- S. Zhang, C. Zhou, S. Wang, Z. Qin, G. Shu, C. Wang, L. Song, L. Zheng, X. Wei, K. Ma and H. Yue, Facilitating CO2 dissociation via Fe doping on supported vanadium oxides for intensified oxidative dehydrogenation of propane, Chem. Eng. J., 2024, 481, 148231 CrossRef CAS.
- X. Zheng, B. Li, L. Shen, Y. Cao, Y. Zhan, S. Zheng, S. Wang and L. Jiang, Oxygen vacancies engineering of Fe doped LaCoO3 perovskite catalysts for efficient H2S selective oxidation, Appl. Catal., B, 2023, 329, 122526 CrossRef CAS.
- N. Gao, Y. Zhou, M. Fan, H. Xu, Y. Chen and S. Shen, Promoting effect and role of alkaline earth metal added to ZrO2-TiO2-supported CeO2 for dichloromethane oxidation, Chem. Eng. J., 2020, 396, 125193 CrossRef CAS.
- S. Yang, Z. Qi, Y. Wen, X. Wang, S. Zhang, W. Li and S. Li, Generation of abundant oxygen vacancies in Fe doped δ-MnO2 by a facile interfacial synthesis strategy for highly efficient catalysis of VOCs oxidation, Chem. Eng. J., 2023, 452, 139657 CrossRef CAS.
- L. Song, H. Zhang, Z. Nie, J. Tian, Y. Liu, C. Ma, P. Liu, Q. Ren, J. Xiong, H. Huang, W. Yang, D. Cao, M. Fu and D. Ye, Ni Doping Promotes C–H Bond Activation and Conversion of Key Intermediates for Total Oxidation of Methane over Co3O4 Catalysts, ACS Catal., 2023, 13, 15779–15793 CrossRef CAS.
- Y.-L. Shan, Y.-A. Zhu, Z.-J. Sui, D. Chen and X.-G. Zhou, Insights into the effects of steam on propane dehydrogenation over a Pt/Al2O3 catalyst, Catal. Sci. Technol., 2015, 5, 3991–4000 RSC.
- X. Zhao, T. Chen, Y. Wang, K. Li, R. Zhan and H. Lin, Effect of electric field and water on Pd-based catalyst for complete oxidation of methane: Study on promotion and deactivation, Chem. Eng. J., 2023, 457, 141126 CrossRef CAS.
- H. Tian, W. Li, L. He, Y. Zhong, S. Xu, H. Xiao and B. Xu, Rationalizing kinetic behaviors of isolated boron sites catalyzed oxidative dehydrogenation of propane, Nat. Commun., 2023, 14, 6520 CrossRef CAS PubMed.
- J. Long, S. Tian, S. Wei, H. Lin, G. Shi, X. Zong, Y. Yang, D. Yang, Y. Tang and Y. Dai, Direct dehydrogenation of propane over Co@silicalite-1 zeolite: Steaming-induced restructuring of Co2+ active sites, Appl. Surf. Sci., 2023, 614, 156238 CrossRef CAS.
- Z. Shi, F. Dong, Z. Tang and X. Dong, Design Sr, Mn-doped 3DOM LaCoO3 perovskite catalysts with excellent SO2 resistance for benzene catalytic combustion, Chem.
Eng. J., 2023, 473, 145476 CrossRef CAS.
- W. Han, S. Wu, F. Dong, W. Han, Y. Chu, L. Su and Z. Tang, A confined growth strategy to construct 3DOM SiO2 nanoreactor in situ embedded Co3O4 nanoparticles catalyst for the catalytic combustion of VOCs: Superior H2O and SO2 resistance, Nano Res., 2024, 17, 207–220 CrossRef CAS.
- S. Dong, P. Alvarez, N. Paterson, D. R. Dugwell and R. Kandiyoti, Study on the Effect of Heat Treatment and Gasification on the Carbon Structure of Coal Chars and Metallurgical Cokes using Fourier Transform Raman Spectroscopy, Energy Fuels, 2009, 23, 1651–1661 CrossRef CAS.
- S. Weber, D. Batey, S. Cipiccia, M. Stehle, K. L. Abel, R. Glaser and T. L. Sheppard, Hard X-Ray Nanotomography for 3D Analysis of Coking in Nickel-Based Catalysts, Angew. Chem., Int. Ed., 2021, 60, 21772–21777 CrossRef CAS PubMed.
- J. Chen, Y. Liu, Z. Chen, J. Yue, Y. Tian, C. Zheng and J. Zhang, Highly Efficient Transformation of Tar Model Compounds into Hydrogen by a Ni–Co Alloy Nanocatalyst During Tar Steam Reforming, Environ. Sci. Technol., 2024, 58, 3540–3551 CAS.
- S. A. Theofanidis, V. V. Galvita, H. Poelman, R. Batchu, L. C. Buelens, C. Detavernier and G. B. Marin, Mechanism of carbon deposits removal from supported Ni catalysts, Appl. Catal., B, 2018, 239, 502–512 CrossRef CAS.
- Y. Liu, Y. Chen, Z. Gao, X. Zhang, L. Zhang, M. Wang, B. Chen, Y. Diao, Y. Li, D. Xiao, X. Wang, D. Ma and C. Shi, Embedding high loading and uniform Ni nanoparticles into silicalite-1 zeolite for dry reforming of methane, Appl. Catal., B, 2022, 307, 121202 CrossRef CAS.
- K. Cao, M. Gong, J. Yang, J. Cai, S. Chu, Z. Chen, B. Shan and R. Chen, Nickel catalyst with atomically-thin meshed cobalt coating for improved durability in dry reforming of methane, J. Catal., 2019, 373, 351–360 CrossRef CAS.
- P. A. Monson, Understanding adsorption/desorption hysteresis for fluids in mesoporous materials using simple molecular models and classical density functional theory, Microporous Mesoporous Mater., 2012, 160, 47–66 CrossRef CAS.
- B. Liu, J. Xiao, L. Xu, Y. Yao, B. F. O. Costa, V. F. Domingos, E. S. Ribeiro, F.-N. Shi, K. Zhou, J. Su, H. Wu, K. Zhong, J. A. Paixão and J. M. Gil, Gelatin-assisted sol–gel derived TiO2 microspheres for hydrogen storage, Int. J. Hydrogen Energy, 2015, 40, 4945–4950 CrossRef CAS.
- D. Tuncel and A. N. Ökte, Efficient photoactivity of TiO2-hybrid-porous nanocomposite: Effect of humidity, Appl. Surf. Sci., 2018, 458, 546–554 CrossRef CAS.
- M. Ghazimoradi, S. Soltanali, N. Safari and H. Ghassabzadeh, Synthesis of fluorinated ZSM-5 catalysts: fluoride effect on structure properties and coke resistance in n-hexane catalytic cracking, J. Mater. Sci., 2023, 58, 11551–11567 CrossRef CAS.
- P. Sirous Rezaei, H. Shafaghat and W. M. A. W. Daud, Suppression of coke formation and enhancement of aromatic hydrocarbon production in catalytic fast pyrolysis of cellulose over different zeolites: effects of pore structure and acidity, RSC Adv., 2015, 5, 65408–65414 RSC.
- M.-H. Sun, J. Zhou, Z.-Y. Hu, L.-H. Chen, L.-Y. Li, Y.-D. Wang, Z.-K. Xie, S. Turner, G. Van Tendeloo, T. Hasan and B.-L. Su, Hierarchical Zeolite Single-Crystal Reactor for Excellent Catalytic Efficiency, Matter, 2020, 3, 1226–1245 CrossRef.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide
materials, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- A. Rodriguez-Gomez, S. Ould-Chikh, J. Castells-Gil, A. Aguilar-Tapia, P. Bordet, M. A. Alrushaid, C. Marti-Gastaldo and J. Gascon, Fe-MOF Materials as Precursors for the Catalytic Dehydrogenation of Isobutane, ACS Catal., 2022, 12, 3832–3844 CrossRef CAS.
- Y.-n. Sun, L. Tao, T. You, C. Li and H. Shan, Effect of sulfation on the performance of Fe2O3/Al2O3 catalyst in catalytic dehydrogenation of propane to propylene, Chem. Eng. J., 2014, 244, 145–151 CrossRef CAS.
- Z. Wen, J. Ke, J. Xu, S. Guo, Y. Zhang and R. Chen, One-step facile hydrothermal synthesis of flowerlike Ce/Fe bimetallic oxides for efficient As(V) and Cr(VI) remediation: Performance and mechanism, Chem. Eng. J., 2018, 343, 416–426 CrossRef CAS.
- B. Yang, L. Liu, G. Zou, X. Luo, H. Zhu and S. Xu, The roles of ZnFe2O4 and α-Fe2O3 in the biphasic catalyst for the oxidative dehydrogenation of n-butene, J. Catal., 2020, 381, 70–77 CrossRef CAS.
- Y. Xu, J. Dhainaut, J.-P. Dacquin, A.-S. Mamede, M. Marinova, J.-F. Lamonier, H. Vezin, H. Zhang and S. Royer, La1−x(Sr, Na, K)xMnO3 perovskites for HCHO oxidation: The role of oxygen species on the catalytic mechanism, Appl. Catal., B, 2021, 287, 119955 CrossRef CAS.
- T. Zheng, W. Li, C. Li, S. An, Y. Jiang and L. Yuan, Single-step methane to methanol over lanthanum cerium oxide: The crucial role of surface reactive oxygen and carbonate species, Mol. Catal., 2023, 551, 113623 CrossRef CAS.
- S. Wu, H. Liu, Z. Huang, H. Xu and W. Shen, O-vacancy-rich porous MnO2 nanosheets as highly efficient catalysts for propane catalytic oxidation, Appl. Catal., B, 2022, 312, 121387 CrossRef CAS.
- W. Liu, S. Yang, H. Yu, S. Liu, H. Li, Z. Shen, Z. Song, X. Chen and X. Zhang, Boosting the total oxidation of toluene by regulating the reactivity of lattice oxygen species and the concentration of surface adsorbed oxygen, Sep. Purif. Technol., 2023, 325, 124597 CrossRef CAS.
- K. P. Thaba, M. M. Mphahlele-Makgwane, P. I. Kyesmen, M. Diale, P. G. L. Baker and P. R. Makgwane, Composition-dependent structure evolution of FeVO4 nano-oxide and its visible-light photocatalytic activity for degradation of methylene blue, Colloids Surf., A, 2022, 633, 127856 CrossRef CAS.
- T. Boningari, D. K. Pappas and P. G. Smirniotis, Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen, J. Catal., 2018, 365, 320–333 CrossRef CAS.
- S.-H. Zhang, M.-F. Wu, T.-T. Tang, Q.-J. Xing, C.-Q. Peng, F. Li, H. Liu, X.-B. Luo, J.-P. Zou, X.-B. Min and J.-M. Luo, Mechanism investigation of anoxic Cr(VI) removal by nano zero-valent iron based on XPS analysis in time scale, Chem. Eng. J., 2018, 335, 945–953 CrossRef CAS.
- Y. Qi, M. Jiang, Y.-L. Cui, L. Zhao and S. Liu, Novel reduction of Cr(VI) from wastewater using a naturally derived microcapsule loaded with rutin–Cr(III) complex, J. Hazard. Mater., 2015, 285, 336–345 CrossRef CAS PubMed.
- H. Nguyen Tran, Comment on “simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons”, Chem. Eng. J., 2019, 359, 810–812 CrossRef CAS.
- H.-P. Chao, Y.-C. Wang and H. N. Tran, Removal of hexavalent chromium from groundwater by Mg/Al-layered double hydroxides using characteristics of in situ synthesis, Environ. Pollut., 2018, 243, 620–629 CrossRef CAS.
- M. N. Abu Tahari, F. Salleh, T. S. Tengku Saharuddin, N. Dzakaria, A. Samsuri, M. W. Mohamed Hisham and M. A. Yarmo, Influence of hydrogen and various carbon monoxide concentrations on reduction behavior of iron oxide at low temperature, Int. J. Hydrogen Energy, 2019, 44, 20751–20759 CrossRef CAS.
- M. N. Abu Tahari, F. Salleh, T. S. Tengku Saharuddin, A. Samsuri, S. Samidin and M. A. Yarmo, Influence of hydrogen and carbon monoxide on reduction behavior of iron oxide at high temperature: Effect on reduction gas concentrations, Int. J. Hydrogen Energy, 2021, 46, 24791–24805 CrossRef CAS.
- Y.-P. Tian, P. Bai, S.-M. Liu, X.-M. Liu and Z.-F. Yan, VOx–K2O/γ-Al2O3 catalyst for nonoxidative dehydrogenation of isobutane, Fuel Process. Technol., 2016, 151, 31–39 CrossRef CAS.
- R. Cao, X. Wang, P. Ning, Y. Xie, L. Wang, Y. Ma, X. Li, H. Zhang and J. Liu, Advantageous Role of N-doping on K@Al in COS/CS2 Hydrolysis: Diminished Oxygen Mobility and Rich basic sites, Fuel, 2023, 337, 126882 CrossRef CAS.
- S. B. Alreshaidan, A. A. Ibrahim, A. H. Fakeeha, A. M. Almutlaq, F. A. Ali and A. S. Al-Fatesh, Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane, Catalysts, 2022, 12, 1066 CrossRef CAS.
- Z. Zhang, X. Hu, Y. Wang, S. Hu, J. Xiang, C. Li, G. Chen, Q. Liu, T. Wei and D. Dong, Regulation the reaction intermediates in methanation reactions via modification of nickel catalysts with strong base, Fuel, 2019, 237, 566–579 CrossRef CAS.
- A. T. Al-Qathmi, G. Tanimu, H. S. Alasiri, Z. S. Qureshi, M. M. Hossain and Z. O. Malaibari, Influence of Zn and Fe promoters on Ni-Bi/γ-Al2O3 catalyst for oxidative dehydrogenation of n-butane to butadiene, Mol. Catal., 2023, 540, 113067 CrossRef CAS.
- R. Zong, H. Li, W. Ding and H. Huang, Highly Dispersed Pd on Zeolite/Carbon Nanocomposites for Selective Hydrodeoxygenation of Biomass-Derived Molecules under Mild Conditions, ACS Sustainable Chem. Eng., 2021, 9, 9891–9902 CrossRef CAS.
- M. Wang, N. Shang, W. Gao, X. Cheng, S. Gao and C. Wang, Anchoring Co on CeO2 nanoflower as an efficient catalyst for hydrogenolysis of 5-hydroxymethylfurfural, Fuel, 2023, 354, 129433 CrossRef CAS.
- S. Wu, S. Wu, F. Dong, Y. Xi, P. Wang, Y. Chu, Z. Tang and J. Zhang, Elucidating the nature role of acid etching on the CoMnOx catalyst with outstanding performance for the catalytic combustion of o-dichlorobenzene, Appl. Catal., B, 2024, 342, 123390 CrossRef CAS.
- Y. Chen, Y. Wang, Q. Ma, X. Gao and T.-S. Zhao, Cu modified VOx/Silicalite-1 catalysts for propane dehydrogenation in CO2 atmosphere, Fuel, 2024, 363, 130819 CrossRef CAS.
- S. Rimaz, L. Chen, A. Monzón, S. Kawi and A. Borgna, Enhanced selectivity and stability of Pt-Ge/Al2O3 catalysts by Ca promotion in propane dehydrogenation, Chem. Eng. J., 2021, 405, 126656 CrossRef CAS.
- B. Liu, H. Zhao, J. Yang, J. Zhao, L. Yan, H. Song and L. Chou, Fabrication of hierarchically porous MgFe2O4/N-doped carbon composites for oxidative dehydrogenation of isobutane, Appl. Surf. Sci., 2020, 531, 147219 CrossRef CAS.
- C. Yu, H. Xu, Q. Ge and W. Li, Properties of the metallic phase of zinc-doped platinum catalysts for propane dehydrogenation, J. Mol. Catal. A: Chem., 2007, 266, 80–87 CrossRef CAS.
- S. Lawson, K. Baamran, K. Newport, T. Alghamadi, G. Jacobs, F. Rezaei and A. A. Rownaghi, Integrated direct air capture and oxidative dehydrogenation of propane with CO2 at isothermal conditions, Appl. Catal., B, 2022, 303, 120907 CrossRef CAS.
- G. Liu, H. Sun, H. Wang and Z. Qu, Rational tuning towards B-sites (B = Mn, Co, Al) on CoB2O4 binary oxide for efficient selective catalytic oxidation of ammonia, Chem. Eng. J., 2023, 453, 139941 CrossRef CAS.
- R. Gholami, M. Alyani and K. J. Smith, Deactivation of Pd Catalysts by Water during Low Temperature Methane Oxidation Relevant to Natural Gas Vehicle Converters, Catalysts, 2015, 5, 561–594 CrossRef CAS.
- X. Zhang, J. Li, Y. Zheng, W. Xin, J. An, X. Zhu and X. Li, Structural reconstruction of iron oxide induces stable catalytic performance in the oxidative dehydrogenation of n-butane to 1,3-butadiene, Chem. Eng. J., 2023, 473, 145370 CrossRef CAS.
- Q. Zhu, G. Wang, H. Zhang, X. Zhu and C. Li, n-Butane dehydrogenation over Ni-Sn/SiO2: Adsorption modes and reaction paths of n-butane and 1-butene, Appl. Catal., A, 2018, 566, 113–120 CrossRef CAS.
- C. Hu, J. Sun, D. Kang, Q. Zhu and Y. Yang, Mechanistic insights into complete hydrogenation of 1,3-butadiene over Pt/SiO2: effect of Pt dispersion and kinetic analysis, Catal. Sci. Technol., 2017, 7, 2717–2728 RSC.
- E. Castillejos-López, G. Agostini, M. Di Michel, A. Iglesias-Juez and B. Bachiller-Baeza, Synergy of Contact between ZnO Surface Planes and PdZn Nanostructures: Morphology and Chemical Property Effects in the Intermetallic Sites for Selective 1,3-Butadiene Hydrogenation, ACS Catal., 2016, 7, 796–811 CrossRef.
- C. Hu, J. Sun, Y. Yang, Q. Zhu and B. Yu, Reaction pathway for partial hydrogenation of 1,3-butadiene over Pt/SiO2, Catal. Sci. Technol., 2017, 7, 5932–5943 RSC.
- W. Han, F. Dong, W. Han, X. Huang and Z. Tang, Constructing mesoporous FeAlOx based catalysts through framework-confined strategy with superior performance for the oxidative dehydrogenation of 1-butene with CO2, Chem. Eng. J., 2023, 475, 146432 CrossRef CAS.
- Z.-W. Wang, Q. Wan, Y.-Z. Shi, H. Wang, Y.-Y. Kang, S.-Y. Zhu, S. Lin and L. Wu, Selective photocatalytic reduction CO2 to CH4 on ultrathin TiO2 nanosheet via coordination activation, Appl. Catal., B, 2021, 288, 120000 CrossRef CAS.
- N. Liu, J. Wei, J. Xu, Y. Yu, J. Yu, Y. Han, K. Wang, J. I. Orege, Q. Ge and J. Sun, Elucidating the structural evolution of highly efficient Co–Fe bimetallic catalysts for the hydrogenation of CO2 into olefins, Appl. Catal., B, 2023, 328, 122476 CrossRef CAS.
- X. Li, H. Jiang, C. Ma, Z. Zhu, X. Song, X. Li, H. Wang, P. Huo and X. Chen, Construction of a multi-interfacial-electron transfer scheme for efficient CO2 photoreduction: a case study using CdIn2S4 micro-flower spheres modified with Au nanoparticles and reduced graphene oxide, J. Mater. Chem. A, 2020, 8, 18707–18714 RSC.
- W. Yan, Y. Zhang and Y. Bi, Subnanometric Bismuth Clusters Confined in Pyrochlore-Bi2Sn2O7 Enable Remarkable CO2 Photoreduction, Angew. Chem., Int. Ed., 2023, 63, e202316459 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b,
Xiaosheng
Huang
b,
Xiaosheng
Huang
 b,
Zhicheng
Tang
b,
Zhicheng
Tang
 *b and
Qiuye
Li
*b and
Qiuye
Li
 *a
*a
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in CO2 is 750 kJ mol−1, which makes it difficult to utilize CO2.8 The coke formation also reduces the stability of the catalyst.9,10 So, we designed a catalyst and aimed at solving these problems.
O bonds in CO2 is 750 kJ mol−1, which makes it difficult to utilize CO2.8 The coke formation also reduces the stability of the catalyst.9,10 So, we designed a catalyst and aimed at solving these problems.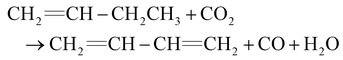
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr molar ratio is 10
Cr molar ratio is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Keeping the ratio of Fe and Al constant and changing the proportion of V and Cr a series of Fe-based catalysts FeVaCrbAlOx (a, b = 0, 1, 2, 3) were prepared. Finally, a series of three-dimensional ordered macro–mesoporous Fe-based catalysts were obtained. Full details of the contents could be found in the Table S2.†
1. Keeping the ratio of Fe and Al constant and changing the proportion of V and Cr a series of Fe-based catalysts FeVaCrbAlOx (a, b = 0, 1, 2, 3) were prepared. Finally, a series of three-dimensional ordered macro–mesoporous Fe-based catalysts were obtained. Full details of the contents could be found in the Table S2.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was fed. The weight hourly space velocity (WHSV) was 4.5 g (gcat h)−1. Finally, catalyst activity was tested in a stainless-steel reactor at 600 °C. During the reaction, the feed gas was controlled by a flow meter and the water was controlled by the Syringe Pump (TYD01-01). The products were analyzed by gas chromatograph equipped with two different detectors (TCD and FID), and the conversion and selectivity were determined by the external standard method. Calculation details were presented in ESI.†
9) was fed. The weight hourly space velocity (WHSV) was 4.5 g (gcat h)−1. Finally, catalyst activity was tested in a stainless-steel reactor at 600 °C. During the reaction, the feed gas was controlled by a flow meter and the water was controlled by the Syringe Pump (TYD01-01). The products were analyzed by gas chromatograph equipped with two different detectors (TCD and FID), and the conversion and selectivity were determined by the external standard method. Calculation details were presented in ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of V and Cr (Fig. 1). Although FeVCrAlOx showed the best activity under the synergistic effect of V and Cr, it was quickly deactivated. Then, the conversion rate of the FeVCrAlOx catalyst at different temperatures was analyzed. As shown in Fig. S3,† no reaction occurred at 200 °C. When the temperature was increased to 300 °C, isomerization took place, but 1,3-butadiene was not produced. At 400 °C, dehydrogenation occurred, leading to the formation of 1,3-butadiene. With a further temperature increase to 500–600 °C, isomerization decreased, dehydrogenation increased, coking became significant, and cracking reactions occurred. This suggested that 1-butene first underwent isomerization to form 2-butene, which then converted to 1,3-butadiene. Coke formation likely occurred at higher temperatures, possibly because the products could not be desorbed in time. This will lead to the cracking and even coke formation. This ultimately caused rapid catalyst deactivation. The used catalyst was regenerated, and the results showed that the regeneration process partially recovered its activity but could not fully restore the catalyst to its initial performance levels (Fig. S4†). Subsequently, structural and morphological analyses were conducted to further investigate the issue of high activity but rapid deactivation of the catalyst.
1 ratio of V and Cr (Fig. 1). Although FeVCrAlOx showed the best activity under the synergistic effect of V and Cr, it was quickly deactivated. Then, the conversion rate of the FeVCrAlOx catalyst at different temperatures was analyzed. As shown in Fig. S3,† no reaction occurred at 200 °C. When the temperature was increased to 300 °C, isomerization took place, but 1,3-butadiene was not produced. At 400 °C, dehydrogenation occurred, leading to the formation of 1,3-butadiene. With a further temperature increase to 500–600 °C, isomerization decreased, dehydrogenation increased, coking became significant, and cracking reactions occurred. This suggested that 1-butene first underwent isomerization to form 2-butene, which then converted to 1,3-butadiene. Coke formation likely occurred at higher temperatures, possibly because the products could not be desorbed in time. This will lead to the cracking and even coke formation. This ultimately caused rapid catalyst deactivation. The used catalyst was regenerated, and the results showed that the regeneration process partially recovered its activity but could not fully restore the catalyst to its initial performance levels (Fig. S4†). Subsequently, structural and morphological analyses were conducted to further investigate the issue of high activity but rapid deactivation of the catalyst.







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%)
a (%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%)
a (%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%)
a (%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%)
a (%)

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and the C
CH2 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of 1-butene.74 This indicated that butene was effectively adsorbed on the catalyst surface over time. Fig. 13 showed the heating process at 110–350 °C with the introduction of CO2. The peak at 2973 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group75 (Fig. 13c). The peaks at 1654 cm−1 and 1640 cm−1 were regarded as the C
C stretching vibration of 1-butene.74 This indicated that butene was effectively adsorbed on the catalyst surface over time. Fig. 13 showed the heating process at 110–350 °C with the introduction of CO2. The peak at 2973 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group75 (Fig. 13c). The peaks at 1654 cm−1 and 1640 cm−1 were regarded as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of 1-butene and 1,3-butadiene, respectively (Fig. 13b). As the temperature rose, the peak at 2973 cm−1 (–CH3) decreased and then disappeared (the temperature is about 320 °C), which indicated that the terminal methyl group began to be consumed. And the peak at 1654 cm−1 gradually disappeared and the peak at 1640 cm−1 gradually appeared. This indicated that 1,3-butadiene was formed. The shift from 1654 to 1640 cm−1 might be due to the conjugation effect of 1,3-butadiene, then the C
C stretching vibration of 1-butene and 1,3-butadiene, respectively (Fig. 13b). As the temperature rose, the peak at 2973 cm−1 (–CH3) decreased and then disappeared (the temperature is about 320 °C), which indicated that the terminal methyl group began to be consumed. And the peak at 1654 cm−1 gradually disappeared and the peak at 1640 cm−1 gradually appeared. This indicated that 1,3-butadiene was formed. The shift from 1654 to 1640 cm−1 might be due to the conjugation effect of 1,3-butadiene, then the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C force constant was decreased and thus the C
C force constant was decreased and thus the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrational phase was shifted towards the low-wave direction. This eventually leads to a redshift. Similarly, the peaks at 3088 cm−1 and 3072 cm−1 (Fig. 13c) were regarded as the
C stretching vibrational phase was shifted towards the low-wave direction. This eventually leads to a redshift. Similarly, the peaks at 3088 cm−1 and 3072 cm−1 (Fig. 13c) were regarded as the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 stretching vibrations of 1-butene and 1,3-butadiene76,77 respectively. It could be found that as the temperature increased, the peaks at 3088 cm−1 gradually weakened and then disappeared, after that the peaks at 3072 cm−1 appeared and gradually became stronger. This might indicate that the
CH2 stretching vibrations of 1-butene and 1,3-butadiene76,77 respectively. It could be found that as the temperature increased, the peaks at 3088 cm−1 gradually weakened and then disappeared, after that the peaks at 3072 cm−1 appeared and gradually became stronger. This might indicate that the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 of butene is consumed first and then disappeared during the conversion of 1-butene to 1,3-butadiene. Finally, the characteristic peak of 1,3-butadiene appeared. This might be that 1-butene was first converted into 2-butene and then the 2-butene was converted into 1,3-butadiene.78
CH2 of butene is consumed first and then disappeared during the conversion of 1-butene to 1,3-butadiene. Finally, the characteristic peak of 1,3-butadiene appeared. This might be that 1-butene was first converted into 2-butene and then the 2-butene was converted into 1,3-butadiene.78
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and the C
CH2 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of 1-butene, and this indicated that 1-butene was effectively adsorbed on the catalyst surface over time. Fig. 14 showed the variation of peaks from 110–380 °C in the presence of H2O and CO2. The peaks at 3088 cm−1 and 3050 cm−1 could be regarded as the
C stretching vibration of 1-butene, and this indicated that 1-butene was effectively adsorbed on the catalyst surface over time. Fig. 14 showed the variation of peaks from 110–380 °C in the presence of H2O and CO2. The peaks at 3088 cm−1 and 3050 cm−1 could be regarded as the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 stretching vibrations of 1-butene and 1,3-butadiene, respectively. The peak at 2971 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group (Fig. 14c). After the addition of water and CO2, it could be found that the terminal methyl group decreased rapidly and then disappeared (the temperature is about 290 °C), the peaks at 3088 cm−1 gradually weakened and then disappeared, and after that, the peaks at 3050 cm−1 gradually increased (Fig. 14c). This was similar to the change of peak in the absence of water. This indicated that 1,3-butadiene was produced. It is worth noting that when water was added, the peak of OH− (3644 cm−1), HCO3− (1393 cm−1), and HCOO− (1602 cm−1) appeared (Fig. 14b and d), and the hydroxyl peaks on the catalyst surface were enhanced (3400 cm−1).79–82 The reason might be that water was first cleaved to OH− or surface hydroxyl groups by the catalyst, and the OH− reacted with CO2, then the HCO3− was formed. As a result, the peak of surface hydroxyl groups and HCO3− increased. After that HCO3− + C4H8
CH2 stretching vibrations of 1-butene and 1,3-butadiene, respectively. The peak at 2971 cm−1 could be regarded as the stretching vibration of the C–H bond of the CH3 group (Fig. 14c). After the addition of water and CO2, it could be found that the terminal methyl group decreased rapidly and then disappeared (the temperature is about 290 °C), the peaks at 3088 cm−1 gradually weakened and then disappeared, and after that, the peaks at 3050 cm−1 gradually increased (Fig. 14c). This was similar to the change of peak in the absence of water. This indicated that 1,3-butadiene was produced. It is worth noting that when water was added, the peak of OH− (3644 cm−1), HCO3− (1393 cm−1), and HCOO− (1602 cm−1) appeared (Fig. 14b and d), and the hydroxyl peaks on the catalyst surface were enhanced (3400 cm−1).79–82 The reason might be that water was first cleaved to OH− or surface hydroxyl groups by the catalyst, and the OH− reacted with CO2, then the HCO3− was formed. As a result, the peak of surface hydroxyl groups and HCO3− increased. After that HCO3− + C4H8![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4H6 + HCOO− + H2O might happen. This resulted in the peak appearing at 1602 cm−1 (HCOO−). The characteristic peak of
C4H6 + HCOO− + H2O might happen. This resulted in the peak appearing at 1602 cm−1 (HCOO−). The characteristic peak of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (BD) was located at 3050 cm−1, rather than 3072 cm−1, which might be due to the redshift caused by the HCOO−. Finally, through the comparison of methyl peaks with and without water, it could be found that the methyl peaks decreased rapidly in the presence of water. It might be due to the improvement of CO2 utilization in the presence of water. Thus, the reaction rate was accelerated, and the methyl peaks were rapidly consumed. In general, it could be found that the introduction of water not only improved the utilization of CO2 but also changed the reaction path. This might be the reason for the increase in activity.
CH2 (BD) was located at 3050 cm−1, rather than 3072 cm−1, which might be due to the redshift caused by the HCOO−. Finally, through the comparison of methyl peaks with and without water, it could be found that the methyl peaks decreased rapidly in the presence of water. It might be due to the improvement of CO2 utilization in the presence of water. Thus, the reaction rate was accelerated, and the methyl peaks were rapidly consumed. In general, it could be found that the introduction of water not only improved the utilization of CO2 but also changed the reaction path. This might be the reason for the increase in activity.





