Graphene oxide-based materials as proton-conducting membranes for electrochemical applications
Itthipon
Moonnee
 ab,
Muhammad Sohail
Ahmad
ab,
Muhammad Sohail
Ahmad
 cd,
Yusuke
Inomata
b,
Worapon
Kiatkittipong
cd,
Yusuke
Inomata
b,
Worapon
Kiatkittipong
 *ad and
Tetsuya
Kida
*ad and
Tetsuya
Kida
 *bcd
*bcd
aDepartment of Chemical Engineering, Faculty of Engineering and Industrial Technology, Silpakorn University, Nakhon Pathom 73000, Thailand. E-mail: kiatkittipong_w@su.ac.th
bGraduate School of Science and Technology, Department of Applied Chemistry and Biochemistry, Faculty of Advanced Science and Technology, Kumamoto University, Kumamoto 860-8655, Japan
cInstitute of Industrial Nanomaterials (IINa), Kumamoto University, Kumamoto 860-8655, Japan
dInternational Research Organization for Advanced Science and Technology, Kumamoto University, Kumamoto 860-8655, Japan. E-mail: tetsuya@kumamoto-u.ac.jp
First published on 3rd October 2024
Abstract
The rapid advancements of graphene oxide (GO)-based membranes necessitate the understanding of their properties and application potential. Generally, proton (H+)-conducting membranes, including GO-based ones, are crucial components in various energy-relevant devices, significantly determining the transport process, selectivity, and overall efficiency of these devices. Particularly, GO-based membranes exhibit great potential in electrochemical applications owing to their remarkable conductivity and ease of undergoing further modifications. This review is aimed at highlighting recent functionalization strategies for GO with diverse substrates. It is also aimed at emphasizing how these modifications can enhance the electrochemical performances of GO-based membranes. Notably, key aspects, such as the enhanced H+-transfer kinetics, improved conductivity, functionalities, and optimization, of these membranes for specific applications are discussed. Additionally, the existing challenges and future directions for the field of functionalized GO are addressed to achieve precise control of the functionalities of these membranes as well as advance next-generation electrochemical devices.
1. Introduction
Graphene-based materials have attracted considerable attention attributed to their remarkable properties, such as large surface areas (SAs), exceptional mechanical strengths, high intrinsic mobilities, and excellent electrical and thermal conductivities.1–14 These outstanding properties make them highly suitable for various applications, specifically in electrochemical energy storage and conversion (as electrodes,15–19 gas sensors,20–23 fuel cells (FCs),24–31 energy storage,32–34etc.). Graphene and its derivatives (e.g., graphene oxide (GO), reduced graphene oxide (rGO), fluorographene, and functionalized graphene)35–37 are considered nobel carbon-based materials that were originally discovered by Geim and Novoselov.38 GO is typically synthesized through the chemical oxidation of graphite, followed by exfoliation.39–41 The GO structure comprises oxygen-rich functional groups, such as epoxy, carboxyl, carbonyl, and quinone, on the basal plane, along with additional functional groups on the edges, making it a fascinating material for several applications.7,42–44 These functional groups can also enhance the mechanical and electrical properties of graphene-based materials.13,45 Further, these functionalities on the graphene surface make GOs excellent candidates for proton (H+)-conduction applications.46The presence of oxygenated functional groups in GO significantly increases the spacing between its nanolayers. These hydrophilic groups also facilitate easy water passage through hydrated GO nanosheets.47 Through π–π interactions, these functional groups play a critical role in modifying the two-dimensional (2D) structure of GO with different substrates,48,49 increasing electrical capacity when deployed as thin film electrodes. These electrodes can be fabricated via techniques, such as casting or coating.50,51 Beyond energy storage, GO is also deployed as an anion-exchange membrane and electrolyte in energy-conversion devices because of the presence of integrated covalent and noncovalent substances within the GO matrix.29,48 These covalent and noncovalent substances demonstrate how surface engineering can be used to fine-tune the physicochemical and electrochemical characteristics of GOs, strengthening their chemical and mechanical properties for efficient electrochemical reactions. Moreover, GO can be deployed in several biological applications, including nanomedicine, drug delivery, biomedical devices, and biosensors.52–55
The last decade witnessed significant electrochemical studies focusing on GO-based materials. Several of these studies have highlighted the importance of GO as an exfoliated graphene material for energy applications.56 GO has attracted attention owing to its facile synthesis, excellent solubility in various solvents, and capacity to store electroactive species on its surface.57–59 Furthermore, its cost-effectiveness makes it a valuable material for applications in currently expensive electrochemical devices. The GO structure features hydrophilic functional groups and a hydrophobic carbon network, imparts GO with amphiphilic properties,60 which facilitate its self-assembling into controlled microstructures exhibiting specific directional properties and make it suitable for different applications, such as catalytic support, conductors, and electrochemical devices.60,61 Owing to the structural diversity and adaptable characteristics of GO, significant efforts have been invested to achieve its application in electrochemical energy storage and conversion devices (Fig. 1).
The oxygen-rich functional groups in GOs bear a negative charge, which facilitates H+ transport, particularly the in-plane transport, in GO nanolayers via the hopping method.62 This characteristic makes GO suitable for application in electrochemical devices for energy conversion, e.g., FCs and electrolyzers. Additionally, recent studies have revealed that GO can capture water vapor from the atmosphere via its hydrophilic functional groups.63 These oxygen-containing groups in GO nanosheets exhibit excellent H+-transfer properties, particularly at high relative humidity. H+ transport in GO nanosheets proceeds through two primary mechanisms: the vehicle mechanism, in which H+s diffuse with the water molecules, and the Grotthuss mechanism involving H+ migration between H+-hopping sites within the nanolayers.64 To generate multilayer GO-composite membranes, which contribute to enhanced thickness and H+ conductivity, a monolayer GO-nanosheet was typically fabricated and assembled through several fabrication techniques. Additionally, researchers have investigated the enhancement of H+ conductivity as well as the mechanical stability of GO-based membranes by integrating GOs with various functional substances.27,65–67
Moreover, GO nanosheets have become a recent research focus. Thus, this review is aimed at elucidating the available H+ conductivities, transport phenomena, performances, and applications of GO nanosheets in electrochemical energy devices.68 Furthermore, this review highlights the protonic-transporting properties of various GO-based membranes, including their synthesis and fabrication processes, electrochemistry analyses, and application potentials. Additionally, the perspectives and current challenges are also discussed. Overall, this review offers a useful guide for researchers focusing on GOs to expand their application scope for next-generation electrochemical devices.
2. Scope of the review
This review provides a detailed and in-depth analysis of the extensive studies on graphene-based materials, which have captivated significant attention in electrochemical applications, specifically focusing on the utilization of these materials as proton-exchange membranes (PEMs). Over the years, researchers have investigated the ionic-conducting properties of graphene-based materials via structural modification using various preparation techniques and/or incorporating additives; this is because several reviews have emphasized GO synthesis, as well as the application of its derivative in catalysis, and batteries.66,68,69 Further, to offer a comprehensive overview of the study on GO-based membranes for electrochemical applications, this review captures the literature (up to March 2024) on GO modification using various additives, specifically focusing on conductivity properties. Further, the review elucidates the synthesis and functionalization of graphene-based materials, briefly highlighting their characterization techniques and discussing their applications and synthesis mechanisms. The concluding part of the review describes the present state-of-the-art graphene-based materials and provides our future perspective.3. Overview of carbon materials in electrochemistry
3.1. Graphene
Graphene has emerged as a beneficial carbon-based material mainly used in electrochemistry owing to its intrinsic physicochemical properties. Graphene, a single atomic layer of carbon atoms comprising an extensively connected sp2-hybridized carbon network strongly bonded by covalent interactions, imparts graphene with exceptional in-plane electrical conductivity as well as thermal conductivity.70–72 Further, the large SA of graphene (theoretical value: 2630 m2 g−1) makes it a very valuable material in electronic and energy storage devices.73 Additionally, graphene is among the strongest existing materials, and this property is attributable to its hexagonal-pattern structure, allowing it to display outstanding flexibility and mechanical strength that ensure its durability and long-term performance in electrochemical devices.74,75 Most evidently, this 2D material is believed to exhibit greater potential than other carbon allotropes.3.2. Porous carbon spheres
Porous carbon spheres (PCSs) are spherical particles comprising developed porous structures; they are typically prepared from carbon-rich precursors. Their controllable mesoporous/microporous structures offer rapid ion and electrolyte accessibility as well as excellent electron conductivity. Their significantly large specific SAs (SSAs) ensure good charge storage and unique surface chemistry, which contribute to improving the energy densities of batteries. The interconnected pore structure of PCS facilitates the volume change during cycling, thereby maintaining stability.76 These advantages make PCSs suitable for energy storage devices, including batteries and supercapacitors77,783.3. Activated carbon fibers
Active carbon fibers (ACFs) are among the ideal candidates for flexible supercapacitors. They exhibit several advantages, including large SSAs as well as good electrical conductivity and mechanical strength.79 Moreover, the porous structure of carbon fibers allows for fast electrolyte penetration and ion diffusion, which are essential for superior electrochemical performances. Additionally, ACFs can be modified via various treatments, such as doping, activation, and functionalization, to tailor their properties for specific applications.80 However, ACFs are susceptible to oxidation and degradation, which decreases their SAs and electrochemical performances.3.4. Graphdiyne
Graphdiyne, a 2D carbon allotrope comprises sp- and sp2-hybridized carbons via a unique acetylenic linkage. This feature imparts it with a high SA, excellent electrical conductivity, and a unique electronic structure that can enhance catalytic activity. Graphdiyne has displayed promise in various applications, including energy storage, electrocatalysis, and sensors.81These carbon-based materials offer unique electrochemical properties and are suitable for various applications.82 However, each material exhibits its unique strengths and weaknesses, and the optimal choice depends on the application-specific requirements.
4. Graphene-oxide-based membranes
4.1. Synthesis of graphene oxide
GO is synthesized using two methods: the top–down and bottom–up methods (Fig. 2).83 In the top–down technique, graphene or GO nanosheets are generally produced from graphite via mechanical exfoliation, chemical exfoliation, thermal reduction, etc. Conversely, in the bottom–up technique, graphene or graphene derivatives are typically generated from small carbon precursors; the method comprises thermal pyrolysis, chemical vapor deposition (CVD), epitaxial growth, etc. The top–down techniques are typically more straightforward, producing higher GO amounts.84 Nevertheless, they may introduce defects and impurities, thus affecting the properties of the final GO product. To achieve the desired functionalization and defect levels, the oxidation conditions must be carefully controlled. Conversely, the bottom–up methods comprising CVD or solution-phase synthesis offer greater control over the GO structure and properties, although it is more complex and expensive than the top–down method.85 These methods are often preferred for applications requiring high-quality, defect-free GOs with specific functionalities. Ultimately, the optimal preparation method depends on the desired GO characteristics, production scale and expensiveness, and specific applications. The literature abounds with reports on the synthesis methods for GO;39,86–88 however, GO synthesis will be synthesized in the following section.Although several methods have been proposed for graphene and GO preparation, this review mainly focuses on the chemical exfoliation methods for synthesizing GO. The most conventional methods involve wet-chemical exfoliation, such as the Brodie,89 Staudenmaier,90 Hummers,91 and Tours methods.51 In these methods, graphite powder is used as the carbon precursor, which is strongly oxidized using an oxidizing agent in an acidic environment, followed by washing and ultrasonication to exfoliate GO in a few layers or monolayers. The Hummers and Tours methods are the most conventional and effective methods owing to their cost-effectiveness, scalability, and environmental friendliness.92
The Hummers’ method, which is a well-known conventional route for GO synthesis, was introduced by Hummers and Offeman (1958).91 They used potassium permanganate as an oxidizing agent and used a mixture of sulfuric acid (H2SO4) and sodium nitrate (NaNO3) as the acid (Fig. 3a). However, this method exhibits disadvantages, such as the generation of NOx substances and the consumption of significant reaction times.39 In 2010, Tour et al. reported a new synthesis technique for GO.39 They used phosphoric acid as the acidic additive instead of NaNO3, thus producing undesired NOxvia the oxidation reaction of graphite. Fig. 4b illustrates the preparation process of GO using the Tours method. The material characteristics of the GO prepared by the Tours method indicated a significant increase in the interlayer distance of the GO nanosheets, as well as an increase in the number of oxygen-containing functional groups; these were examined by X-ray diffraction (XRD) and elemental analyses, respectively.
 | ||
| Fig. 3 Graphical representations of the preparation process of graphene via (a) Hummer's and (b) Tour's methods. Adapted under the terms of the CC-BY license from ref. 91. Copyright 2019. Adapted with permission from ref. 37. Copyright 2020 The Royal Society of Chemistry. | ||
 | ||
| Fig. 4 Functionalization of GO-based materials for electrochemical devices. Adapted with permission from ref. 7 and 6. Copyright 2012 American Chemical Society and Copyright 2019 Wiley. | ||
Furthermore, the morphological analyses of GO via scanning electron microscopy (SEM) and atomic force microscopy (AFM) revealed that GO exhibits a relatively high SA attributed to the layered and wrinkled structures of the GO nanosheets46,93 The characteristic properties of GO significantly vary with the deployed synthesis methods, particularly the Hummers and Tours methods (Table 1). Despite their seemingly rough features, the GOs synthesized by the Hummers’ method often exhibit higher SA than those synthesized by the Tours method. Additionally, the C/O ratio represents another crucial parameter, as it reveals the oxidation degree and significantly impacts various properties, such as the dispersibility, conductivity, and thermal stability of the GO. The strong oxidation facilitated by the Hummers’ method allows their GO to exhibit higher C/O ratios. Conversely, the GO obtained by the Tours method exhibits a slightly lower C/O ratio, indicating a greater proportion of sp2 hybridized carbon network.
Surprisingly, the utilization of expanded graphite powder (∼10 μm) can enhance the structural properties of the synthesized GO, such as its SSA, interlayer spacing, and oxidation ratio, compared with those of the GO prepared using pristine graphite powder (75–500 μm). Similarly, the membrane performance of GOs synthesized by modified methods is significantly enhanced.39,94 Most notably, the GO synthesized by the Tour method exhibits a more regular structure as well as higher amounts of hydrophilic oxidized graphene than those synthesized by the Hummers’ method, and these are crucial to the conductivities of GO materials. Consequently, the GO synthesized by the Tour method is preferred for the preparation of graphene-based membranes for electrochemical applications. More importantly, the Tour method for synthesizing GOs is safer and more environmentally friendly.
4.2. Porous graphene-oxide membranes
Abundant defects/pores are generally present in 2D GO nanosheets. They exhibit different sizes, ranging from several angstroms to nanometers in the graphitic basal plane, which is influenced by several factors during the preparation process, such as oxidation-agent-induced chemical etching, sonication-induced defects, and high-temperature-induced degradation.6 although these defects/pores promote the charge transport in graphene-based materials, especially the through-plane transport, the transport is still insignificant compared with the in-plane transport that is strongly influenced by various oxygenated functional groups. Therefore, the introduction of pores into graphene-based materials represents a promising and necessary strategy for enhancing through-plane H+ transport, improving capacitance activity, and ensuring long-term stability by tailoring the graphene surface layer and morphology. Recently, numerous strategies have been deployed to synthesize porous graphene-based materials, e.g., chemical etching, plasma treatment, and the introduction of templating agents. Studies have revealed that the introduction of pores enhances the performances of GO-based materials in versatile energy-relevant devices.106–108 for instance, Sy et al.109 introduced pores on GO nanosheets via the sonication-assisted Fenton reaction using hydrogen peroxide as a chemical reagent. Consequently, the anisotropic degree of the porous GO membrane decreased significantly owing to the weakening of the π–π stacking interaction between the porous GO nanosheets due to the decrease in the sp2-hybridized carbon and increase in the number of pores on the nanosheets. Moreover, the improved through-plane H+ conductivity was examined under all environmental conditions. Zhang et al.,110 reported the activation of porosity in a graphene-based material via a potassium hydroxide–activation process. The resulting activated rGO films exhibited a significantly enlarged sa, which enhanced its electrochemical performance. The increased porosity increased the charge storage and accelerated ion transport within the supercapacitor, thereby substantially boosting the power density and overall efficiency. Kim et al.111 explored the enhancement effect of modifying the hierarchical pore structure of GO on supercapacitor performance. By applying dual thermal-activation processes, they synthesized a multi-scale pore structure within GO, combining micropores, mesopores, and macropores. This hierarchical porosity significantly improved the SA of the material and facilitated efficient ion transport and charge storage. Consequently, the modified GO material delivered superior electrochemical performance in supercapacitors, including increased capacitance and power density.Additionally, Akada et al.112 explored the surface modification of GO using radio frequency plasma in a nitrogen-rich environment, which facilitated the controlled pore distribution and incorporation of nitrogen into the GO structure. This approach offered a pathway for optimizing GO to enhance conductivity based on the surface modification of the plasma. Surwade et al.113 performed oxygen plasma etching to generate and fine-tune the nanopores in a single-layer graphene. After the treatment, the pore density and size of the membrane were optimized, allowing water molecules to pass through as well as enabling molecular selectivity.
However, the careful controls of the pore sizes, distributions, and morphologies of such membranes still limit the adoption of this approach, namely the introduction of porous structures in GO. As noted, achieving a uniform and precisely controlled porous structure in GO-based materials can be challenging, as the variations among doping agents and preparation methods often result in inconsistent pore characteristics. Additionally, the meticulous management of the pore morphology is essential to maintaining the structural integrity and desired properties of GO-based materials. Thus, rigorous optimization during preparation is key to enhancing the performances of GO-based materials in advanced energy storage and conversion devices114,115
4.3. Functionalized graphene-oxide membranes
Although graphene exhibits exceptional electronic conductivity, which makes it a widely employed material in electronic devices, suppressing this intrinsic characteristic (electronic conductivity) is very necessary for developing graphene-based H+-conducting membranes, as H+ selectivity can be greatly suppressed by high electronic conductivity.1 Undesired electronic conductivity of GO-based membranes can present significant challenges, particularly in applications requiring the precise control of H+ transport. Moreover, excessive conductivity may cause issues, such as current leakage or interference between conductive pathways, which can undermine the efficiency of H+-exchange processes. These issues often arise from the incomplete reduction of GO or the uncontrolled formation of conductive networks that disrupt the intended H+-conducting functions. Thus, the careful management and optimization of the electrical conductivity of GO-based membranes are crucial to ensuring their effectiveness in specific applications.An effective strategy for addressing these challenges comprises the functionalization of GO materials. The adsorbed functional groups, electron-donating or electron-withdrawing groups, enable the modification of the surface chemistry, conductivity, and stability of GO-based materials, thus influencing their electrochemical performances. Additionally, moderating their electronic properties and generating active sites for reactions can significantly enhance their electrochemical activity. However, these modifications can also disrupt the π–π conjugation in graphene, reducing its electronic conductivity, although this may be offset by increasing its ionic conductivity, particularly for applications in PEMs. Additionally, the functional groups influence the interaction of the materials with the electrolyte, affecting their adsorption, diffusion, and overall reaction kinetics.66 This, by tailoring the type and density of these functional groups, the electrochemical properties of GO-based materials can be optimized for specific applications, such as FCs, supercapacitors, or sensors. As previously mentioned, GO contains many oxygen-containing functional groups on the surface of its graphitic plane, and these functional groups facilitate hydration in GO structures.74,116 The interactions between intermolecular hydrogen bonds in GO membranes make them H+-conductive materials, and the GO structure significantly facilitates in-plane H+ transport.46 Moreover, these active functional groups allow for the chemical modification of the GO nanostructure. Numerous studies have revealed that the H+ conductivity in GO-based membranes can be further increased via functionalization using either covalent or noncovalent chemical substances.7,69 Generally, the covalent functionalization in graphene proceeds via two main pathways: (a) the formation of covalent bonds between free radicals and sp2-hybridized carbon and (b) the formation of covalent bonds between organic functional groups and the oxygen-containing groups in GO. In the case of noncovalent functionalization, the organic functional groups and graphene exhibit π-interactions.69
Functionalized GO-based membranes have advanced remarkably, exhibiting various application potential, particularly in electrochemistry. Their remarkable H+ conductivity, chemical and mechanical stability, and multifunctional structures make them highly promising. Therefore, functionalized GOs obtained with intercalated substances have been considered valuable organic fillers in the membrane matrix to promote conductivity and enhance membrane stability. To date, functionalized GO-based membranes can be generally categorized into various types of chemical substrates, including organic compounds (HNO3, H2SO4, and deoxyribonucleic acid (DNA)), metal ions (Al3+, Fe3+, and Ce4+), and polymers (Nafion, chitosan, polyvinyl alcohol (PVA)). Surprisingly, they demonstrated the molecular interaction between GO and covalent and noncovalent reactions. For ease of comparison, this review captures the membrane preparations, H+ conductivities, and electrochemical performances of various functionalized GO membranes.
Another approach comprises composite formation in which graphene is combined with other conductive materials, such as carbon nanotubes (CNTs), conductive polymers, or MOFs. This integration leverages the high conductivity of graphene and incorporates the advantageous properties of the additional materials. For instance, studies have revealed that MOFs can significantly improve the mechanical strength and enhance the performance of graphene-based membranes (Fig. 5).117–119 Nevertheless, this is not covered in this review, as our focus is on the effects of modification using organic chemical, metal, and polymer substrates. The controlled reduction of GO is also a crucial technique for enhancing electronic conductivity. This process involves reducing GO into rGO, thereby restoring much of its inherent conductivity.120 The careful control of the reduction process allows researchers to balance the recovery of electronic conductivity with the retention of essential oxygen-functional groups necessary for H+ conduction. This method ensures that the obtained GO-based membranes achieve optimal conductivity while maintaining effective H+ transport.121
4.4. Fabrication of graphene-oxide-based membranes
Compared with typical three-dimensional (3D) nanomaterials, GO, which is a graphene derivative bearing various oxygen-functionalized groups, is a 2D nanomaterial exhibiting considerable advantages, particularly in electrochemistry. Most evidently, the characteristic dimensions of 2D nanomaterials ease the transportation of molecular components under complex pathways.122 The thickness of the GO layers can be easily adjusted to modify the transportation properties of the membrane. Therefore, the following section discusses the preparation of graphene-based membranes for electrochemical applications.From a practical viewpoint, the large-scale synthesis of single-layer porous graphene membranes with negligible flaws continues still presents a technical challenge. Consequently, researchers have explored the fabrication of multilayer graphene, which can be fabricated as laminates, following the principle of single-layer graphene.2 The assembling of single-layer graphene into laminates can generate complex transport channels among different molecular species by leveraging the advantages of the 2D structure of graphene nanosheets. This can be achieved by adjusting the flake size of the graphene sheet as well as the interlayer spacing between the graphene nanosheets. Accordingly, as the requirements of multilayer graphene are less challenging than those of single-layer graphene, their large-scale, less-expensive synthesis is achievable.
Typically, the primary approach for generating multilayer graphene laminates involves a flow-guided assembly. Multilayer GO nanosheets are conventionally fabricated using porous substrate support. GO laminates are synthesized with the application of appropriate driving force, which facilitates the flow of water molecules within a limited space and generates van der Waals and electrostatic forces between the GO nanosheets.123
Pressure-involving techniques are conventional strategies for preparing GO laminates. The structure of the GO layer obtained using these techniques can be highly organized or loose or very random (Fig. 6a). During the preparation of free-standing GO membranes, many factors, including the deployed GO-preparation technique, GO-suspension concentration, and filtration duration, greatly influence the properties of the resulting GO-laminate membrane, such as its thicknesses and dimensions. Particularly, the time required for the fabrication of complete GO-based membranes through vacuum-assisted filtration varies from several hours to several days. This is because GO significantly restricts the movement of any molecules except for water, thus blocking the generation of porous substrates from GO-nanosheet deposition. Consequently, the slow filtration process causes the inevitable evaporation of water molecules. This phenomenon accounts for the less dense arrangement of the top layer of GO compared with its middle area, resulting in a random assembly of folded, wrinkled, and crumpled forms attributed to insufficient water flow from above to the support.
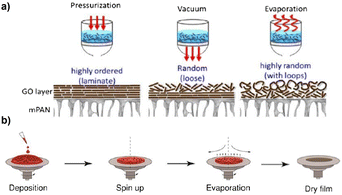 | ||
| Fig. 6 (a) Comparison of the GO nanolayers obtained by pressure-assisted, vacuum-assisted, and evaporation-assisted filtrations. Reprinted with permission from ref. 9, copyright 2015 Elsevier. (b) Assembling of ultrathin GO membranes via the spin coating-method. Reprinted with permission from ref. 36, copyright 2016 American Chemical Society. | ||
Additionally, GO laminates can be generated via coatings or casting approaches, such as dip coating, spray coating, spin coating, and drop casting. These approaches offer simple and effective strategies for assembling GO membranes; they have been deployed for the synthesis of high-quality multilayer GOs. For instance, Chi et al.36 fabricated ultrathin GO membranes through spin coating, which involves high-speed spinning after depositing tiny droplets of GO suspensions onto the substrate (Fig. 6b). In the process, centrifugal force is used to keep the GO film securely connected to the membrane support, allowing for the rapid drainage of excess aqueous solvents.
The drop-casting technique is another method for simply and rapidly generating thin GO membranes while depositing a GO-suspension droplet onto a substrate material (Fig. 7c).124 The desired thickness of the GO membrane can be easily tailored, yielding a self-standing, supported membrane. Moreover, the characteristic stacked GO membranes can be achieved after the evaporation of the GO-suspension by a heating source or at room temperature. This method ensures ease of use as well as control of the membrane thickness; however, it may result in non-uniformity.
 | ||
| Fig. 7 (a) Cross-linked LBL assembling for preparing GO/PEI composite membranes. Reprinted with permission form ref. 8, copyright 2020 Elsevier. (b) Cross-linked GO-laminate membrane fabricated by the doctor-blade technique. Reprinted with permission from ref. 35, copyright 2019 Elsevier. (c) Schematic for preparing GO membranes via drop casting on anodic aluminum oxide substrates. Adapted with permission from ref. 124, copyright 2023 Elsevier. (d) Schematic of the electrophoresis deposition (ED) process and the corresponding cross-sectional SEM image of ED-GO film. Adapted with permission from ref. 126, copyright 2010 American Chemical Society. | ||
Layer-by-layer (LBL) assembling is a technique for fabricating GO-laminate membranes with precisely controlled structure and properties. The LBL technique involves reacting the stacked GO nanosheets with the surface of other materials on the porous substrate to form a multilayered membrane through molecular interactions comprising covalent and hydrogen bonding and hydrophobic and electrostatic interactions. Moreover, the thickness of GO membranes can be simply controlled by repeating the LBL process, although the time consumption of the process represents a major disadvantage.125 Halakoo et al.8 demonstrated LBL assembling by depositing GO and polyethyleneimine (PEI) onto thin-film-composite polyamide support exhibiting a negatively charged surface (Fig. 7a). Thus, the cross-linked PEI/GO membrane with adjustable bilayers was fabricated through electrostatic interaction and hydrogen bonding.
The doctor-blade technique is among the most conventional techniques for preparing GO membranes. It involves coating or casting a GO thin layer on a flat surface, where the blade moves at a constant speed and height, followed by allowing the thin GO layer to dry. For example, Yang et al.35 fabricated large-area cross-linked GO membranes via the doctor-blade technique (Fig. 7b). The obtained laminate membranes displayed a wide thickness range, from ultrathin to a few microns. This technique facilitated the simple and rapid preparation of GO membranes and exhibits great scalability.125
ED is a direct technique for fabricating GO membranes from charged colloidal suspensions based on electrostatic-force interaction. ED allows for the control of membrane thickness and dimension by adjusting the deposition parameters. Compared with drop casting, ED offers better uniformity potential, especially for large-area membranes. The GO sheets are deposited onto the electrode surface by generating an electric field, thereby fabricating a thin GO membrane. Ruoff et al.126 prepared an organized GO membrane via ED and obtained an ED-GO film with considerably lower oxygen content owing to the electrophoresis effect (Fig. 7d).
5. Characterizations of graphene-oxide membranes
Generally, the physical and chemical properties of GO membranes vary with the employed synthesis and modification methods. Thus, to gain insight into the characteristic properties of GO, namely their structural properties (size, shape, and defect) and chemical properties (C/O ratio, chemical composition, and functionalities), various characterization techniques were investigated.127,128XRD analysis is the most extensively deployed technique for characterizing the crystal structure of the material. For example, Üregen et al.41 employed XRD for the characterization of GO membranes synthesized by the modified Hummers’ method. Generally, the XRD pattern of pristine GO displays a diffraction peak at 2θ = 11.5, corresponding to the (002) interlayer spacing of 0.78 nm. Nevertheless, the characteristic graphite-diffraction peak (2θ = 26.5) was not observed in the pristine GO sample owing to the introduction of oxygen-containing groups on the graphitic plane. The surface morphology of the GO nanosheets can also be analyzed by SEM and transmission electron spectroscopy (TEM). SEM provides information regarding the surface topography of the GO membrane, including its roughness, defects, and porosity, whereas TEM can offer a much higher resolution of its internal structure at the nanoscale (can be seen in Fig. 8a and b).
 | ||
| Fig. 8 (a) and (b) SEM images of surface and cross-section of a GO membrane, adapted with permission from ref. 127. Copyright 2014 Elsevier. (c) Characteristic diffraction peak of GO, adapted with permission from ref. 41. Copyright 2017 Elsevier. (d) Fourier-transform infrared (FTIR) spectra of the GO and ionic liquid–GO composite, adapted with permission from ref. 130. Copyright 2018 Elsevier. (e) X-ray photoelectron (XPS) C 1s spectra of the GO membranes. (f) Thermogravometric analysis (TGA) curve of the GO membranes, adapted with permission from ref. 131. Copyright 2020 Springer. | ||
XPS can be used for the elemental and chemical analysis of GO membranes. XPS reveals the specific oxygen-containing groups present in the membrane (e.g., epoxy, hydroxyl, and carbonyl) and their relative abundance; this is crucial, as these groups significantly influence the surface chemistry of the membrane (Fig. 8e). XPS can be used to analyze factors, such as the hydrophilicities, surface charges, and selectivities of the membranes, and these factors are key to the optimization of their performances in various applications. The XPS C 1s spectrum of the GO membrane displayed peak positions corresponding to oxygen-containing groups (C–O–C, –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –COOH) in the carbon structure.129 Further, FTIR is an effective tool for analyzing the chemical composition as well as functional groups in GO membranes; it achieves this by measuring the infrared (IR) light absorbed by the sample. Different functional groups absorb IR light at specific frequencies, and this allows for their identification. Gahlot et al.130 identified peaks corresponding to various functional groups, including O–H, C
O, and –COOH) in the carbon structure.129 Further, FTIR is an effective tool for analyzing the chemical composition as well as functional groups in GO membranes; it achieves this by measuring the infrared (IR) light absorbed by the sample. Different functional groups absorb IR light at specific frequencies, and this allows for their identification. Gahlot et al.130 identified peaks corresponding to various functional groups, including O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C
O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations at 3428, 1726, and 1574 cm−1, respectively. Additionally, the stretching modes of the sp2 skeletal network were observed at 1192 and 1023 cm−1, corresponding to C–O and C–OH, respectively.
C vibrations at 3428, 1726, and 1574 cm−1, respectively. Additionally, the stretching modes of the sp2 skeletal network were observed at 1192 and 1023 cm−1, corresponding to C–O and C–OH, respectively.
TGA provides valuable insights into the thermal behaviors of GO membranes. It clarifies the stability of the membrane by measuring its weight loss with increasing temperature. Zhao et al.131 revealed that a TGA curve of GO displayed several key weight-loss stages of weight loss. At approximately 150 °C, an initial weight loss was observed; it was attributed to the evaporation of water. Thereafter, a steady weight loss was observed up to 300 °C, corresponding to the breakdown of the oxygen-containing groups. Finally, a significant weight loss was observed at higher temperatures (300 °C), corresponding to the decomposition of the GO structure.
Similarly, additional characterization techniques, including AFM,46,93 dynamic light scattering,36 and Raman spectroscopy,132 can be used to examine GO characteristics from different aspects. Ultimately, researchers can comprehensively clarify the characteristics and structural properties of the material by combining these analytical techniques, and this is crucial to the further development and utilization of GO-based membranes.
6. Performance of GO-based membranes
6.1. Theoretical investigations of GO-based membranes
The theoretical investigations of H+ conduction in GO-based membranes offer valuable insights into the fundamental mechanisms governing their performances in electrochemical applications. GO membranes are generally attractive because of their applications in PEM FCs (PEMFCs) and other energy-conversion systems, as they exhibit high SAs and can facilitate H+ transport. Theoretical studies often employ molecular dynamics (MD) simulations and density functional theory (DFT) to model the behaviors of H+ within the GO structure. These simulations elucidate the effects of the interlayer spacing, oxygen-functional groups, and water molecules on H+ mobility and conductivity.133 These theoretical investigations mainly explore the roles of the functional groups, such as –OH and carboxyl groups, in GO membranes, which can significantly impact H+ conduction. The presence of these groups generates hydrophilic sites that attract and retain water molecules, which are crucial for efficient H+ transport. Theoretical models have been developed to explore the effects of the variations in the density and distribution of these functional groups on the overall H+ conductivity. Additionally, studies have investigated the effects of interlayer spacing in GO membranes on the formation of H+-conduction pathways as well as the interaction of H+s with the surface of the membrane.Another notable aspect of theoretical studies covers the impact of membrane hydration on H+ conductivity. Theoretical models have been developed to simulate the interaction between water molecules and GO membranes to determine the effect of hydration on H+ mobility. These models often revealed that optimal hydration levels are key to maintaining high H+ conductivity, as an extremely small amount of water can hinder H+ transport, whereas excessive water may disrupt membrane stability. Thus, the theoretical investigations offer critical insights into balancing the hydration levels to maximize the performance of GO membranes in practical applications. For instance, MD is a valuable technique for simulating the dynamic behavior of a system; it facilitates the investigation of H+ transport between two GO nanosheets.134 Wu and Jiang groups.139 performed reactive MD simulations to demonstrate the rapid and selective H+-hopping mechanism through the –OH groups on the GO membrane in an anhydrous environment. They observed that the calculated H+ conductivities were exceptionally high, indicating the potential of these membranes in H+-based applications. Additionally, Bagusetty et al. studied a specific arrangement of –OH groups and predicted the H+-conduction pathways across the graphene basal plane using a DFT computational model.135
Overall, the theoretical investigations of H+ conduction in GO membranes are key to designing and optimizing materials for H+-exchange applications. By providing a detailed understanding of the underlying mechanisms as well as identifying the key factors influencing H+ transport, these studies can contribute to the development of more efficient and durable GO-based membranes for advanced energy-conversion technologies.136,137
6.2. Electrochemical performances of GO-based membranes
The ionic or protonic conductions in GO-based membranes demonstrate high directional dependences. Generally, this can be observed from their in-plane transport, which occurs at the functional groups (diffusion/hopping), and through-plane transport, which occurs in the defects/pores of the membrane. Moreover, the well-connected network of oxygen-containing functionalized groups ensures that the in-plane H+ conductivity is typically higher than the through-plane one13 Consequently, the in-plane H+ conductivity of GO-based membranes has been deeply investigated even though the through-plane H+ conduction has delivered several favorable results.64 Typically, the conductivity can be calculated using the electrical resistance of the membrane, which is measured by electrochemical impedance spectroscopy (EIS).EIS is the most dependable and effective technique for investigating the electrochemical characteristics of various systems.138 EIS is conventionally performed to determine the H+ conductivity of the GO membranes. The Nyquist plots are generally represented in a complex impedance plane, which comprises the real impedance (Z′) on the X-axis and the imaginary impedance (Z′′) on the Y-axis. EIS can determine the dependence of H+ conductivities in GO membranes at different relative humidities and room temperature. The H+ conductivity of GO membranes can be calculated from the measured current resistance (R) using σ = d/RA, where σ is the H+ conductivity (S cm−1); L and R are the membrane thickness (cm) and cross-sectional area of electrodes (cm−2) respectively; and Rs is the membrane resistance (Ω).
For ease of comparison, the H+ conductivities of the various functionalized GO-based membranes have been summarized in Table 2, including the fabrication methods and experimental conditions of the membranes. For instance, Gao et al.10 enhanced GO membranes via an ozonated reaction. The treatment of GO with ozone significantly increased the H+ conductivity of the membranes by increasing their oxygen contents, generating more H+-transport pathways and enhancing hydrophilicity, and facilitating water uptake and H+ mobility in the GO structure (Fig. 9). Additionally, Hatakeyama et al.12 examined the conducting properties of sGO by filling SO3H. The XRD patterns revealed the increasing interlayer spacing between two opposite GO walls, indicating the presence of bulky sulfate ions in sGO (Fig. 10b). Additionally, the sGO samples displayed several excellent H+-conductivity trends attributed to their high water content and enhanced flexibility.
 | ||
| Fig. 9 (a) H+-transport pathways on the ozonated GO plane. ADF images of (b) GO and (c) OGO on surface of disordered regions that are associated with oxygen functionality. (d) The H+ conductivity on different relative humidity (RH). Adapted with permission from ref. 10. Copyright 2014 Wiley. | ||
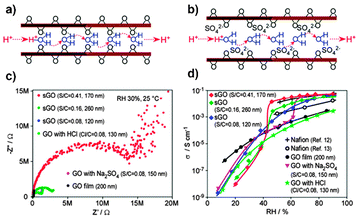 | ||
| Fig. 10 H+-conduction pathways of (a) GO and (b) sulfonated GO (sGO) through nanochannels. (c) Nyquist plots for various sGO samples and (d) the dependence of H+ conductivities on relative humidity. Adapted with permission from ref. 12. Copyright 2014 The Royal Society of Chemistry. | ||
| Category | Materials | Preparation methods | Conductivities (S cm−1) | Experimental conditions | Ref. |
|---|---|---|---|---|---|
| Organic-substance-modified GO membranes | 3D-GO | Pressure-assisted filtration | 3.5 × 10−2 | 70 °C, 100% RH | 148 |
| GO/H2SO4 | Drop casting | 4.2 × 10−3 | 90% RH | 12 and 45 | |
| GO/SO3H | Pressure-assisted filtration | 4.97 × 10−2 | — | 149 | |
| Ozonated GO | Vacuum filtration | 0.23 | 100% RH | 10 | |
| GO/HNO3 | Drop casting | 2.1 × 10−3 | 90% RH | 150 | |
| GO/IM | Evaporation | 7.9 × 10−2 | 100 °C | 151 | |
| GO/naphthalene sulfonate | Vacuum filtration | 1.71 | 80 °C, 95% RH | 139 | |
| GO/sulfonated lignin | Vacuum filtration | 0.346 | 80 °C, 100% RH | 152 | |
| GO/DNA | Vacuum filtration | 0.565 | 80 °C, 98% RH | 153 | |
| Metal-cation-modified GO membranes | GO/Fe3+ | Chemical reduction | 3.2 × 10−2 | 25 °C, 50% RH | 154 |
| GO/Ce4+ | Vacuum filtration | 2.8 × 10−3 | 80% RH | 142 | |
| GO/La3+ | Vacuum filtration | 0.7 × 10−3 | 20 °C, 70% RH | 93 | |
| GO/SO3K | Pressure-assisted filtration | 0.119 | — | 149 | |
| GO/H6Bi12O16 | Vacuum filtration | 0.564 | 80 °C, 70% RH | 143 | |
| Polymer-modified GO membranes | GO/Nafion | Pressure-assisted filtration | 0.58 | 80 °C, 95% RH | 144 |
| GO/chitosan | Spin coating | 1.36 × 10−3 | 25 °C, 50% RH | 155 | |
| GO/PBI | Solution casting | 0.170 | 180 °C, 0% RH | 41 and 156 | |
| GO/QPPO | Solution casting | 0.123 | 80 °C, 100% RH | 147 | |
| GO/PVA | Solution casting | 8.22 × 10−2 | 70 °C | 157 and 158 | |
| GO/PEO | Solution casting | 0.134 | 60 °C, 100% RH | 159 | |
| GO/SPEEK | Solution casting | 0.219 | 90 °C, 50% RH | 160 | |
Moreover, Rahman et al.45 explored the H+ conductivities of 3D-sGO membranes by cross-linking sGO nanosheets with a hyperbranched sulfonated poly(arylene ether sulfonate) polymer. The 3D-sGO membranes exhibited significant out-of-plane and in-plane H+ conductivities of 0.74 and 3.19 S cm−1, respectively, which are relatively higher than those of pristine GO membranes. These higher conductivities were attributed to the interconnected 3D network and abundant sulfonic-acid groups, with enhanced H+-transport pathways and good water uptake. Consequently, the resultant membrane exhibited exceptional performances in PEMFCs, with an excellent power density of 112.62 mW cm−2 at 100% RH and 30 °C. Liu et al.139 introduced SL into GO as a multifunctional intercalator to prepare highly conducting membranes (Fig. 11a). The ether bonds and H2SO4 groups in SL significantly increased the interlayer spacing as well as generated additional H+-hopping sites and increased the H+ mobility in the nanolayers. These imparted the SL/GO membrane with exceptional H+ conductivity at elevated temperatures. Furthermore, the maximum power density of 169.2 mW cm−2 was accomplished from the SL/GO membrane in the single-FC application.
 | ||
| Fig. 11 (a) Illustration of GO/SL membrane preparation process and the digital picture and inter-structure of the membrane. Reprinted with permission from ref. 139. Copyright 2022 Elsevier. (b) Cross-linking preparation of 3D-SGO membranes. Reprinted with permission from ref. 45. Copyright 2023 American Chemical Society. (c) Schematic of proton transport pathway and proton conductivities of DNA@GO membranes. Adapted with permission from ref. 153. Copyright 2020 Elsevier. (d) The increasing in d-spacing of diamine monomers composite membrane. Adapted with permission from ref. 56. Copyright 2014 American Chemical Society. | ||
Functionalizing metal cations onto GO-based membranes is an alternative and promising strategy for improving membrane stability as well as H+ conductivity. Thus far, numerous studies have considered the impact of monovalent (K+ and Na+), divalent (Cu2+ and Mn2+), and trivalent (Fe3+, Al3+, and La3+) cations on interlayer spacing and water transportation in GO nanostructures.14 The mechanisms of cross-linking and incorporating metal cation into GO nanosheets have been proposed through cation–graphite surface interactions (cation–π interaction) and metal–carboxylate chelate formation140,141 (Fig. 12a). For instance, Hamidah et al.142 reported the modification of metal cations by incorporating cerium (IV) sulfate ions onto the surfaces of GO nanosheets, which was prepared via the modified Tour method. The utilization of prepared Ce/GO membranes in the water vapor electrolysis has also been reported. The Ce ions effectively enhanced the H+ conductivity and stability of the modified membranes (Fig. 12c). Liu et al.143 immobilized nanosized bismuth-oxide clusters onto the GO support (H6Bi12O16/GO) and investigated their H+ conductivity along with methanol permeability. The GO-composited membrane exhibited excellent H+ conductivities of 0.564 S cm−1 (in-plane) and 0.1 S cm−1 (out-of-plane), which were better than those of Nafion in an aqueous solution.
 | ||
| Fig. 12 (a) Proposed model for the interactions between the metal cations and GO nanosheets through cation–π interaction. Reprinted with permission from ref. 14. Copyright 2020 Elsevier. (b) Structure model of the H6Bi12O16 cations dispersed on the GO surface. Reprinted with permission from ref. 143. Copyright 2019 The Royal Society of Chemistry (c) H+-transport mechanism in Ce-intercalated GO membranes. Adapted with permission from ref. 142. Copyright 2020 American Chemical Society. | ||
Polymer materials have also attracted considerable attraction over the years. Most evidently, GO is very compatible with Nafion owing to its strong interfacial attraction and has demonstrated potential advantages in membrane modification for PEMFCs.63 The Nafion-modified GO membranes demonstrated promising results in various applications owing to their enhanced H+ conductivity, improved mechanical properties, and tunable functionalities. However, their fabrication challenges, cost considerations, and long-term stability are still critical to their widespread implementation. Most importantly, GO can enhance the side chains and backbone of Nafion, imparting it with increased mechanical and thermal properties as well as improved H+ conductivities for Nafion/GO-composite membranes, which are higher compared with those of pristine Nafion membranes.144,145
Xu et al.146 functionalized polybenzimidazole (PBI) with sGO for PEMFC applications. The XRD patterns revealed that the d-spacing diffraction (001) was shifted, indicating the intercalation of the PBI molecules into the sGO nanostructure. The PBI/GO-composite membrane exhibited significantly enhanced tensile strength and ionic conductivity (2.7 × 10−2 S cm−1), resulting in an incredible performance in PEMFCs. Additionally, Zhang et al.147 reported that the functionalized GO membranes using 2,6-dimethyl-1,4-phenylene oxide (QPPO) displayed an outstanding H+ conductivity of 0.123 S cm−1. In addition to these well-known polymers, GO has been extensively modified using other polymers, revealing its significant contribution and functionalization flexibility. For instance, Zakaria et al.158 explored the enhancement effect of incorporating QPVA with GO on the ionic conductivity of membranes for direct methanol FCs (DMFCs). The ionic conductivity of the QPVA/GO membrane significantly improved at elevated temperatures owing to the incorporation of quaternary-ammonium groups, which enhanced the conducting properties of the membrane by providing additional ionic-transport pathways in the QPVA/GO nanocomposite (Fig. 13c). Feng et al.155 prepared GO/chitosan composite electrolyte films for fabricating electric double-layer transistor devices with multiple lateral-gate electrodes. The highly conductive composite films were obtained (H+ conductivity = ∼13.6 × 10−4 S cm−1 at room temperature; high specific capacitance of ∼3.2 μF cm−2 at 1.0 Hz), demonstrating the capability of the GO/chitosan composite film in new-concept electrochemical-device application (Fig. 13d–f).
 | ||
| Fig. 13 (a) Field-emission SEM images of the surface and cross-section of GO/quaternized PVA (QPVA) membrane. (b) Ionic conductivities of GO/QPVA at different temperatures. (c) Illustration of the cross-linked GO/QPVA nanocomposite membrane. Reprinted with permission from ref. 158. Copyright 2019 Wiley Periodicals. (d and e) Preparation and Nyquist plots of GO/chitosan composite films. (f) Specific capacitance of the GO/chitosan films plotted against the frequency. Adapted with permission from ref. 155. | ||
Ultimately, the incorporation/grafting of polymers into GO membranes offers a valuable strategy for improving their conductivity. The combination of water-filled channels and hydrogen-bonding networks paves the way for efficient H+ transport within functionalized GO-based membranes. Additionally, polymers with specific functionalities can be incorporated to fine-tune the selectivity of the membrane for desired ions or molecules, making them valuable for various applications. However, some polymers can block or suppress the GO nanolayers. Therefore, the polymer must be carefully selected and tailored.
7. Mechanism of proton conduction in graphene-oxide membranes
The recent emergence of GOs as promising materials for 2D H+-conducting materials is particularly attributed to their exceptional properties, including their nano-dimensional structures, amphiphilic structures, and chemical inertness.2,3,161 Furthermore, the H+ transport in graphene-based materials can be conveniently enhanced by modifying the chemical functionality and nanostructure architecture. The GO structure comprises various oxygenated functionalities on its 2D basal planes and edge sites. This exceptional property facilitates the formation of nano-fluidic ion-transport pathways by stacking or assembling the GO nanosheets into multilayered GO membranes.56Generally, two conduction mechanisms have been theoretically and experimentally proposed for the H+-transport characteristics in GO membrane, especially in-plane transport.162,163 H+s can be transferred along the H+-carrying sites via the hydrogen-bonded network, and this is known as the Grotthuss mechanism (Fig. 14). Alternatively, H+s can diffuse along the stream of water in the form of hydronium, and this is known as the vehicle mechanism.134 The schematics of H+ conduction in GO-based membranes through the vehicle and Grotthuss mechanisms are illustrated in Fig. 15b. Studies have revealed that the H+ transport of Nafion, a well-known H+-exchange membrane, is typically dominated by the vehicle and Grotthuss mechanisms at low and high water contents, respectively. Conversely, H+ transport in GO membranes is mainly governed by the vehicle mechanism at a high water content, whereas the Grotthuss mechanism is affected at a low water content.164 This indicates that GO-based membranes can exhibit efficient H+ conduction in all environments, as the Grotthuss mechanism-driven H+ transport is facilitated by the abundant oxygen-containing functional groups on the surface of the GO nanosheets.
 | ||
| Fig. 15 Schematic of H+ conduction: (a) H+ transport through nanopores in multilayered GO, adapted with permission from ref. 13. Copyright 2014 Wiley. (b) H+-conduction mechanisms in GO nanosheets with the stream of water, adapted with permission from ref. 127. Copyright 2016 American Chemical Society. (c) H+ conduction in GO membranes with high water contents, reprinted with permission from ref. 134. Copyright 2022 American Chemical Society. | ||
Regarding the presence of the oxygen-functional groups in the basal plane and edges of GO nanosheets, the membranes prepared from GO-based materials have displayed extraordinary H+-transporting properties and results compared with those prepared from other carbon-based nanomaterials, such as one-dimensional cylindrical CNTs and 2D planar graphene. Moreover, the H+-transport properties of GO can be further investigated using higher water contents, which apparently promote the in-plane H+ conductivity by facilitating H+ movement based on the vehicle-mechanism principle. Both mechanisms have been thoroughly investigated via simulations and experiments, considering the characteristics of the microscopic state of GO-based materials.162,164
8. Optimization of graphene-oxide-based membranes for electrochemical applications
In the realm of electrochemical technologies, GO-based H+-conducting membranes have emerged as versatile materials in numerous energy storage and conversion devices owing to their unique structural and chemical characteristics. The feasibility of tailoring their properties makes them suitable for diverse applications, ranging from energy storage to sensing technologies. However, the performance of GO-based membranes varies significantly with the specific application. This section comprehensively explores GO-based membranes in FC, electrolyzer, battery, supercapacitor, and gas-sensing applications (Table 3).| Electrochemical applications | Material properties |
|---|---|
| FCs | - High H+ conductivity is essential to ensuring efficient H+ transport, as it enhances the overall performance. |
| - Chemical and mechanical stabilities help prevent FC degradation under acidic or alkaline environments. | |
| - Selective permeability is crucial to preventing methanol crossover in DMFCs, thereby enhancing efficient FC durability. | |
| Electrolyzers | - High H+ conductivity facilitates rapid H+ transport for hydrogen production, resulting in excellent conversion efficiency and performance. |
| - Mechanical stability helps the device to withstand harsh environments (high temperature and corrosion) in water-splitting processes. | |
| - High selectivity ensures efficient water splitting while preventing the passage of other unwanted species. | |
| - Water uptake is critical to maintaining efficient operation during water splitting. | |
| Batteries | - High ionic conductivity ensures efficient ion transport (Li+, Na+) across the electrolyte, thus directly affecting the battery performance and energy storage. |
| - High selectivity can reduce the shuttle effect in lithium–sulfur batteries, thereby preventing degradation and enhancing battery cyclability. | |
| - Mechanical strength and stability are key to withstanding physical stress and expansion during operation. | |
| Supercapacitors | - High ionic conductivity is critical to rapid charge and discharge cycles, thus improving efficiency and performance. |
| - Chemical stability can maintain the device performance over time and under various operating conditions. | |
| - Mechanical flexibility is key to designing versatile and flexible supercapacitors. | |
| Gas sensors | - High selectivity allows for the differentiation of analytes or signals. |
| - Rapid sensitivity and response time are essential for detecting the target gas, thus affecting the sensor performance. | |
| - Mechanical stability and flexibility allow the membrane to accommodate different movements and maintain its durability. |
8.1. Proton-exchange membrane in FCs and electrolyzers
H+ exchange or polymer-electrolyte membrane FCs (PEMFCs) are electrolyte materials that are typically utilized as H+ conductors. FCs rely on efficient H+ conductivity to convert chemical energy into electrical energy when supplied with a fuel, e.g., hydrogen, methanol, or natural gas. They exhibit great potential attributed to their high efficiency and clean energy conversion.32 Therefore, GO is an excellent H+-conducting-membrane candidate for superior FC applications. In addition to their remarkable H+ conductivity, GO-based membranes boast effective water retention and robust mechanical stability and flexibility, which can enhance overall performance under high-temperature operation. In addition to the well-known PEMFCs, GO-based membranes also demonstrate outstanding conversion efficiency and performance in other FCs, e.g., DMFCs. Functionalized GO membranes significantly enhance the tolerance of the membrane to methanol, minimizing methanol crossover and improving selectivity in FC devices.165 Interestingly, a potential advantage of GO-based membranes is the possibility of deploying them as filters against impurities, such as carbon monoxide, from hydrogen fuel owing to the efficient architectural structure of GO, which prevents other gaseous species but allows hydrogen to pass through.166The high H+ conductivity in GO-based membranes, achieved through various functionalizations and hybridizations, allows for more effective ion transport between the electrodes, thus facilitating the electrolysis reaction and efficient hydrogen production.167 Surprisingly, GO membranes can prevent hydrogen and oxygen gas crossover in electrolysis devices owing to the structural design of GO. The enhanced mechanical and chemical stabilities of these membranes are essential to withstanding the harsh operation environments; they ensure that the membranes maintain their performance over time, resulting in the prolonged operational life of devices.168,169 Notably, the optimization of high-conductivity GO-based membranes for high current densities and reduced resistive losses is a critical advantage, as these factors significantly influence the energy efficiency of electrolyzers.170
8.2. Batteries
Batteries, particularly lithium-ion and sodium-ion batteries, are crucial energy storage devices that typically rely on ion conduction through their electrolyte membranes. Thus, GO-based membranes are generally employed as conductors and separators in battery applications, allowing the passage of lithium ions while hindering that of negatively charged species. Moreover, the exceptionally high SA of GO extensively increases the battery capacity.171,172 The enhancements in conductivity and interfacial properties can enhance the overall battery performance. For lithium–sulfur batteries, the utilization of GO-based membranes with highly selective permeability could effectively reduce the shuttle effect of polysulfide, which slows down the electrochemical kinetics and induces capacity degradation. Through the interaction between the oxygen-containing groups in GO and negative charges (S2−), optimizing GO membranes can ensure efficient battery performance with increasing cyclability under various operational conditions.688.3. Supercapacitors
Generally, supercapacitors require high H+ conductivities and large SAs for effective charge storage. GO-based membranes can play a significant role in enhancing the performance of supercapacitors owing to their variety of functional groups, offering high power density with rapid charge/discharge capabilities as well as degradation resistance over many charge/discharge cycles.173 GO can be integrated into electrode materials to induce the interaction of the oxygenated functional groups between the electrolyte and electrodes and produce lower mismatch at the interface, thus increasing the specific capacitance and improving the electrochemical performance. Additionally, GO-based membranes can be deployed in electrolytes to facilitate the charge transfer in a supercapacitor or other energy storage devices, thus enhancing ionic conductivity and overall supercapacitor efficiency.174,1758.4. Gas sensors
GO-based membranes can be deployed as solid electrolytes in electrochemical gas sensors for detecting gaseous species, such as H2, CO2, NO2, and NH3. GO-based sensors generally exhibit exceptional electrocatalytic activity attributed to the abundant functional groups at the edge and basal graphitic plane.176,177 These functionalities on the surface of GO nanosheets can be considered interaction sites, making GO-based materials highly sensitive when exposed to gaseous molecules. Furthermore, the high SAs of GO-based sensors allow for advanced interactions between the GO surface and target gas molecules, promoting the gas sensitivity and response time of the devices. By optimizing the functionalization of GO materials, the thermal and chemical stability of GO-based sensors can be significantly improved, making them versatile and practical at elevated operating temperatures. Most importantly, GO-based membranes offer rapid response times and stability, which are crucial for real-time sensing applications.Understanding and addressing the specific requirements of these applications are crucial to advancing the development and deployment of GO-based H+-conducting membranes in various high-performance electrochemical systems.178 Optimizing GO-based H+-conducting membranes for specific electrochemical applications requires a proficient approach tailored to the unique requirements of each application. By focusing on key characteristics, researchers can enhance the performance and applicability of these materials across various fields. Future advancements in membrane fabrication and material science will further refine these optimization strategies, paving the way for more efficient and effective utilization of GO-based membranes in next-generation energy and sensing technologies.
9. Conclusions and perspectives
The physical structure of GO allows for the fabrication of laminate membranes with adjustable nanochannels owing to the presence of oxygen-functional groups. Additionally, the hydrophilic and interlayer distance can be modified by introducing chemical functionalities into the GO structure, thereby enhancing the H+-transport properties. Accordingly, GO has attracted significant interest presently as a promising conducting-membrane material. GO-based membranes exhibit advanced extensive applications in the field of electrochemistry. Multilayered GO membranes can be easily fabricated based on the recent developments in membrane-fabrication techniques, as reviewed in section 4.4.Generally, the GO structure facilitates significant in-plane H+ conductivity, whereas its out-of-plane conductivity is suppressed by the introduction of specific functional groups, such as H2SO4, HNO3, or imidazole groups. These groups act as mobile H+ carriers and minimize the barriers to H+ transport between GO sheets. Nevertheless, the blocking conduction pathways of functional groups must be carefully optimized. The combination of metal cations and polymers offers fascinating possibilities. Metal cations can enhance conductivity and exert synergistic effects, whereas polymers can improve the mechanical strength and advance the processability of composite membranes. However, challenges, such as aggregation, cost, and compatibility, are crucial to the practical implementation of GO-based membranes.
Therefore, a thorough understanding of the architecture and performance mechanism of the functionalized GO-based membrane is greatly desired. These advancements are pushing the boundaries of electrochemical-technology capabilities. However, the future holds even brighter possibilities. Thus, the scalable and cost-effective synthesis of high-conductivity GO-based membranes must be explored to achieve their broader adoption. Additionally, the breakthroughs in the optimization of through-plane conductivity could unlock a new era of high-performance, omnidirectional electrochemical devices. Regarding the existing limitations and functionalities of GO-based materials, researchers can unlock the full potential of these membranes in fabricating a new generation of efficient, sustainable, and high-efficiency electrochemical devices that address key global challenges.
Finally, predicting and optimizing the properties of graphene-based membranes using advanced computational techniques are crucial. Computational modeling directs the experimental design for enhanced performance and aids the understanding of the fundamentals of H+-transport mechanisms inside these materials.
Data availability
The data supporting this review article’s findings are derived from publicly available literature, as referenced throughout the manuscript.All data and materials used in this review are cited and can be accessed through the respective publishers and databases.
Specific data points, tables, and figures are extracted from the cited sources and available within those publication’s context.
No new datasets were generated or analyzed during the current study.
Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research B (24K01300), Researcher Grant-in-Aid for Early-Career Scientists (24K17588), International Research Organization for Advanced Sciences and Technology (IROAST) and Institute of Industrial Nanomaterials (IINa) Kumamoto University.References
- U. R. Farooqui, A. L. Ahmad and N. A. Hamid, Renewable Sustainable Energy Rev., 2018, 82, 714–733 CrossRef.
- L. Nie, C. Y. Chuah, T.-H. Bae and J.-M. Lee, Adv. Funct. Mater., 2021, 31, 2006949 CrossRef.
- V. Hrubý, D. Zaoralová, M. Medveď, A. Bakandritsos, R. Zbořil and M. Otyepka, Nanoscale, 2022, 14, 13490–13499 RSC.
- P. Tripathi, A. K. Verma, A. Vishwakarma, K. Mitra, B. Ray, A. S. K. Sinha and S. Singh, Int. J. Hydrogen Energy, 2022, 47, 36381–36396 CrossRef.
- N. Badi, A. M. Theodore, S. A. Alghamdi, H. A. Al-Aoh, A. Lakhouit, A. S. Roy, A. S. Alatawi and A. Ignatiev, Polymers, 2022, 14, 3837 CrossRef PubMed.
- Y. Zhang, Q. Wan and N. Yang, Small, 2019, 15, 1903780 CrossRef PubMed.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef PubMed.
- E. Halakoo and X. Feng, Chem. Eng. Sci., 2020, 216, 115488 CrossRef.
- C.-H. Tsou, Q.-F. An, S.-C. Lo, M. De Guzman, W.-S. Hung, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Membr. Sci., 2015, 477, 93–100 CrossRef.
- W. Gao, G. Wu, M. T. Janicke, D. A. Cullen, R. Mukundan, J. K. Baldwin, E. L. Brosha, C. Galande, P. M. Ajayan, K. L. More, A. M. Dattelbaum and P. Zelenay, Angew. Chem., Int. Ed., 2014, 53, 3588–3593 CrossRef PubMed.
- T. Syrový, S. Maronová, P. Kuberský, N. V. Ehman, M. E. Vallejos, S. Pretl, F. E. Felissia, M. C. Area and G. Chinga-Carrasco, J. Appl. Polym. Sci., 2019, 136, 47920 CrossRef.
- K. Hatakeyama, M. R. Karim, C. Ogata, H. Tateishi, T. Taniguchi, M. Koinuma, S. Hayami and Y. Matsumoto, Chem. Commun., 2014, 50, 14527–14530 RSC.
- K. Hatakeyama, M. R. Karim, C. Ogata, H. Tateishi, A. Funatsu, T. Taniguchi, M. Koinuma, S. Hayami and Y. Matsumoto, Angew. Chem., Int. Ed., 2014, 53, 6997–7000 CrossRef PubMed.
- K. Ching, B. Lian, G. Leslie, X. Chen and C. Zhao, Carbon, 2020, 170, 646–657 CrossRef.
- T. Kida, Y. Kuwaki, A. Miyamoto, N. L. Hamidah, K. Hatakeyama, A. T. Quitain, M. Sasaki and A. Urakawa, ACS Sustainable Chem. Eng., 2018, 6, 11753–11758 CrossRef.
- J. Hutfles, C. Lumley, X. Chen, Z. J. Ren and J. Pellegrino, Chem. Eng. Sci., 2019, 209, 115221 CrossRef.
- Z. Fakharan, L. Naji and K. Madanipour, J. Colloid Interface Sci., 2019, 540, 272–284 CrossRef PubMed.
- P. Thangavel, M. Ha, S. Kumaraguru, A. Meena, A. N. Singh, A. M. Harzandi and K. S. Kim, Energy Environ. Sci., 2020, 13, 3447–3458 RSC.
- M. R. Islam, S. S. Gupta, S. K. Jana, P. Srikrishnarka, B. Mondal, S. Chennu, T. Ahuja, A. Chakraborty and T. Pradeep, Adv. Mater. Interfaces, 2021, 8, 2001998 CrossRef.
- G. Zardalidis, M. K. Daletou and F. Farmakis, IEEE Sens. J., 2021, 21, 27290–27297 Search PubMed.
- G. Jiang, M. Goledzinowski, F. J. E. Comeau, H. Zarrin, G. Lui, J. Lenos, A. Veileux, G. Liu, J. Zhang, S. Hemmati, J. Qiao and Z. Chen, Adv. Funct. Mater., 2016, 26, 1729–1736 CrossRef.
- J. Zhang, G. Jiang, M. Goledzinowski, F. J. E. Comeau, K. Li, T. Cumberland, J. Lenos, P. Xu, M. Li, A. Yu and Z. Chen, Small Methods, 2017, 1, 1700237 CrossRef.
- A. F. P. Biajoli, C. S. Schwalm, J. Limberger, T. S. Claudino and A. L. Monteiro, J. Braz. Chem. Soc., 2014, 25, 2186–2214 Search PubMed.
- Y. Yin, H. Wang, L. Cao, Z. Li, Z. Li, M. Gang, C. Wang, H. Wu, Z. Jiang and P. Zhang, Electrochim. Acta, 2016, 203, 178–188 CrossRef.
- C. Wang, B. Lin, G. Qiao, L. Wang, L. Zhu, F. Chu, T. Feng, N. Yuan and J. Ding, Mater. Lett., 2016, 173, 219–222 CrossRef.
- A. Marinoiu, M. Raceanu, E. Carcadea, D. Marinescu, C. Teodorescu, A. Mellichio, M. Varlam and I. Stefanescu, React. Kinet., Mech. Catal., 2016, 118, 281–296 CrossRef.
- R. P. Pandey, G. Shukla, M. Manohar and V. K. Shahi, Adv. Colloid Interface Sci., 2017, 240, 15–30 CrossRef PubMed.
- P. Bunlengsuwan, N. Paradee and A. Sirivat, Polym.-Plast. Technol. Eng., 2017, 56, 1695–1703 CrossRef.
- R. Thimmappa, M. Gautam, M. C. Devendrachari, A. R. Kottaichamy, Z. M. Bhat, A. Umar and M. O. Thotiyl, ACS Sustainable Chem. Eng., 2019, 7, 14189–14194 CrossRef.
- M. Ahn, R. Liu, C. Lee and W. Lee, J. Nanomater., 2019, 2019, 6464713 Search PubMed.
- C. Long, C. Lu, Y. Li, Z. Wang and H. Zhu, Int. J. Hydrogen Energy, 2020, 45, 19778–19790 CrossRef.
- R. Kumar, E. Joanni, R. K. Singh, D. P. Singh and S. A. Moshkalev, Prog. Energy Combust. Sci., 2018, 67, 115–157 CrossRef.
- V. P. Vasiliev and V. A. Smirnov, Chem. Phys. Lett., 2019, 726, 99–103 CrossRef.
- P. Krishnan and V. Biju, Phys. E, 2021, 126, 114452 CrossRef.
- E. Yang, H. E. Karahan, K. Goh, C. Y. Chuah, R. Wang and T. H. Bae, Carbon, 2019, 155, 129–137 CrossRef.
- C. Chi, X. Wang, Y. Peng, Y. Qian, Z. Hu, J. Dong and D. Zhao, Chem. Mater., 2016, 28, 2921–2927 CrossRef.
- K. Phasuksom, W. Prissanaroon-Ouajai and A. Sirivat, RSC Adv., 2020, 10, 15206–15220 RSC.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef PubMed.
- L. Sun, Chin. J. Chem. Eng., 2019, 27, 2251–2260 CrossRef.
- N. Üregen, K. Pehlivanoğlu, Y. Özdemir and Y. Devrim, Int. J. Hydrogen Energy, 2017, 4, 2636–2647 CrossRef.
- D. S. Shin, H. G. Kim, H. S. Ahn, H. Y. Jeong, Y.-J. Kim, D. Odkhuu, N. Tsogbadrakh, H.-B.-R. Lee and B. H. Kim, RSC Adv., 2017, 7, 13979–13984 RSC.
- A. T. Dideikin and A. Y. Vul’, Front. Phys., 2019, 6(149) Search PubMed.
- M. Gautam, S. Kanade and B. B. Kale, Energy Fuels, 2023, 37, 17134–17160 CrossRef.
- M. A. Rahman, J. Yagyu, Md. S. Islam, M. Fukuda, S. Wakamatsu, R. Tagawa, Z. Feng, Y. Sekine, J. Ohyama and S. Hayami, ACS Appl. Nano Mater., 2023, 6, 1707–1713 CrossRef.
- K. Hatakeyama, H. Tateishi, T. Taniguchi, M. Koinuma, T. Kida, S. Hayami, H. Yokoi and Y. Matsumoto, Chem. Mater., 2014, 26, 5598–5604 CrossRef.
- L. Wang, K. Lee, Y.-Y. Sun, M. Lucking, Z. Chen, J. J. Zhao and S. B. Zhang, ACS Nano, 2009, 3, 2995–3000 CrossRef PubMed.
- P. Sun, F. Zheng, M. Zhu, Z. Song, K. Wang, M. Zhong, D. Wu, R. B. Little, Z. Xu and H. Zhu, ACS Nano, 2014, 8, 850–859 CrossRef PubMed.
- S. Pandit and M. De, J. Phys. Chem. C, 2017, 121, 600–608 Search PubMed.
- S. S. Suranshe, A. Patil, T. Deshmukh and J. Chavhan, Electrochim. Acta, 2023, 450, 142277 CrossRef.
- S. Sainio, D. Nordlund, M. A. Caro, R. Gandhiraman, J. Koehne, N. Wester, J. Koskinen, M. Meyyappan and T. Laurila, J. Phys. Chem. C, 2016, 120, 8298–8304 CrossRef.
- L. Newman, A. F. Rodrigues, D. A. Jasim, I. A. Vacchi, C. Ménard-Moyon, A. Bianco, C. Bussy and K. Kostarelos, Cell Rep. Phys. Sci., 2020, 1(9), 100176 CrossRef.
- J. Yi, G. Choe, J. Park and J. Y. Lee, Polym. J., 2020, 52, 823–837 CrossRef CAS.
- D. P. Singh, C. E. Herrera, B. Singh, S. Singh, R. K. Singh and R. Kumar, Mater. Sci. Eng., C, 2018, 86, 173–197 CrossRef CAS PubMed.
- J. Lee, J. Kim, S. Kim and D.-H. Min, Adv. Drug Delivery Rev., 2016, 105, 275–287 CrossRef CAS PubMed.
- W.-S. Hung, C.-H. Tsou, M. De Guzman, Q.-F. An, Y.-L. Liu, Y.-M. Zhang, C.-C. Hu, K.-R. Lee and J.-Y. Lai, Chem. Mater., 2014, 26, 2983–2990 CrossRef CAS.
- P. Johari and V. B. Shenoy, ACS Nano, 2011, 5, 7640–7647 CrossRef CAS PubMed.
- P. Ranjan, S. Agrawal, A. Sinha, T. R. Rao, J. Balakrishnan and A. D. Thakur, Sci. Rep., 2018, 8, 12007 CrossRef PubMed.
- H. Ahmadi, E. Hosseini, W. Cha-Umpong, M. Abdollahzadeh, A. H. Korayem, A. Razmjou, V. Chen and M. Asadnia, Adv. Mater. Technol., 2021, 6, 2000665 CrossRef CAS.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS PubMed.
- Y. Tian, Z. Yu, L. Cao, X. L. Zhang, C. Sun and D.-W. Wang, J. Energy Chem., 2021, 55, 323–344 CrossRef CAS.
- B. Liu, Y. Jiang, H. Wang, J. Ge and H. Shi, Energy Fuels, 2020, 34, 2452–2461 CrossRef CAS.
- R. Thimmappa, M. Gautam, Z. M. Bhat, A. R. A. Thodika, M. C. Devendrachari, S. Mukhopadhyay, N. C. Dargily and M. O. Thotiyl, Cell Rep. Phys. Sci., 2021, 2(11), 100627 CrossRef CAS.
- D. W. Chang and J.-B. Baek, Nano Today, 2017, 17, 38–53 CrossRef CAS.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS PubMed.
- M. S. K. Chowdury, Y. J. Cho, S. B. Park and Y. Park, J. Electrochem. Soc., 2023, 170, 033503 CrossRef CAS.
- Y. Yang, C. Han, B. Jiang, J. Iocozzia, C. He, D. Shi, T. Jiang and Z. Lin, Mater. Sci. Eng., R, 2016, 102, 1–72 CrossRef.
- A. Eftekhari, Y. M. Shulga, S. A. Baskakov and G. L. Gutsev, Int. J. Hydrogen Energy, 2018, 43, 2307–2326 CrossRef.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef PubMed.
- A. R. Urade, I. Lahiri and K. S. Suresh, JOM, 2023, 75, 614–630 CrossRef PubMed.
- M. Zhao, H. Li, W. Yuan and C. M. Li, ACS Appl. Energy Mater., 2020, 3, 3966–3977 CrossRef.
- X. Fan, M. Zhao, T. Li, L. Y. Zhang, M. Jing, W. Yuan and C. M. Li, Nanoscale, 2021, 13, 18332–18339 RSC.
- M. Pumera, Chem. Rec., 2009, 9, 211–223 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef PubMed.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef PubMed.
- X. Wu, G. He and Y. Ding, ChemSusChem, 2020, 13, 3376–3390 CrossRef PubMed.
- J. Wang, L. Shen, B. Ding, P. Nie, H. Deng, H. Dou and X. Zhang, RSC Adv., 2014, 4, 7538–7544 RSC.
- X. Cheng, C. Tang, C. Yan, J. Du, A. Chen, X. Liu, L. Jewell and Q. Zhang, Mater. Today Nano, 2023, 22, 100321 CrossRef.
- Y. Xie, Fibers Polym., 2022, 23, 10–17 CrossRef.
- C. Ruan and Y. Xie, RSC Adv., 2020, 10, 37631–37643 RSC.
- Z. Jia, Y. Li, Z. Zuo, H. Liu, C. Huang and Y. Li, Acc. Chem. Res., 2017, 50, 2470–2478 CrossRef CAS PubMed.
- Y. Fang, Y. Liu, L. Qi, Y. Xue and Y. Li, Chem. Soc. Rev., 2022, 51, 2681–2709 RSC.
- M. Ameen and H. S. Shin, Chem. Eng. J. , 2012, 195–196, 307–313 CrossRef.
- N. Kumar, R. Salehiyan, V. Chauke, O. Joseph Botlhoko, K. Setshedi, M. Scriba, M. Masukume and S. Sinha Ray, FlatChem, 2021, 27, 100224 CrossRef.
- K. Sasaki, Y. Uchida and N. Nishiyama, ChemPlusChem, 2023, 88, e202300255 CrossRef PubMed.
- M. S. Ahmad and Y. Nishina, Nanoscale, 2020, 12, 12210–12227 RSC.
- Y. Nishina and S. Eigler, Nanoscale, 2020, 12, 12731–12740 RSC.
- N. Morimoto, H. Suzuki, Y. Takeuchi, S. Kawaguchi, M. Kunisu, C. W. Bielawski and Y. Nishina, Chem. Mater., 2017, 29, 2150–2156 CrossRef.
- B. C. Brodie, Philos. Trans. R. Soc. London, 1997, 149, 249–259 Search PubMed.
- L. Staudenmaier, Ber. Deut. Chem. Ges., 1898, 31, 1481–1487 CrossRef CAS.
- W. S. Hummers Jr. and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef.
- A. Jiříčková, O. Jankovský, Z. Sofer and D. Sedmidubský, Materials, 2022, 15, 920 CrossRef PubMed.
- N. L. Hamidah, M. Shintani, A. S. Ahmad Fauzi, E. G. Mission, K. Hatakeyama, A. T. Quitain and T. Kida, SN Appl. Sci., 2019, 1, 630 CrossRef CAS.
- H. Yang, H. Li, J. Zhai, L. Sun and H. Yu, Ind. Eng. Chem. Res., 2014, 53, 17878–17883 CrossRef CAS.
- P. Feicht, J. Biskupek, T. E. Gorelik, J. Renner, C. E. Halbig, M. Maranska, F. Puchtler, U. Kaiser and S. Eigler, Chem. – Eur. J., 2019, 25, 8955–8959 CrossRef CAS PubMed.
- T. Tsugawa, K. Hatakeyama, J. Matsuda, M. Koinuma and S. Ida, Bull. Chem. Soc. Jpn., 2021, 94, 2195–2201 CrossRef CAS.
- H.-L. Ma, H.-B. Zhang, Q.-H. Hu, W.-J. Li, Z.-G. Jiang, Z.-Z. Yu and A. Dasari, ACS Appl. Mater. Interfaces, 2012, 4(4), 1948–1953 CrossRef CAS PubMed.
- H. L. Poh, F. Šaněk, A. Ambrosi, G. Zhao, Z. Sofer and M. Pumera, Nanoscale, 2012, 4, 3515–3522 RSC.
- M. Nováček, O. Jankovský, J. Luxa, D. Sedmidubský, M. Pumera, V. Fila, M. Lhotka, K. Klímová, S. Matějková and Z. Sofer, J. Mater. Chem. A, 2017, 5, 2739–2748 RSC.
- M. C. F. Costa, V. S. Marangoni, P. R. Ng, H. T. L. Nguyen, A. Carvalho and A. H. Castro Neto, Nanomaterials, 2021, 11, 551 CrossRef CAS PubMed.
- S. You, B. Sundqvist and A. V. Talyzin, ACS Nano, 2013, 7, 1395–1399 CrossRef CAS PubMed.
- H. Bukovska, F. García-Perez, N. Brea Núñez, L. J. Bonales, A. Velasco, M. Á. Clavero, J. Martínez, A. J. Quejido, I. Rucandio and M. B. Gómez-Mancebo, C — Journal of Carbon Research, 2023, 9, 65 CrossRef CAS.
- Z. Benzait, P. Chen and L. Trabzon, Nanoscale Adv., 2021, 3, 223–230 RSC.
- J. Shen, Y. Hu, M. Shi, X. Lu, C. Qin, C. Li and M. Ye, Chem. Mater., 2009, 21, 3514–3520 CrossRef CAS.
- M. R. Karim, K. Hatakeyama, T. Matsui, H. Takehira, T. Taniguchi, M. Koinuma, Y. Matsumoto, T. Akutagawa, T. Nakamura, S. Noro, T. Yamada, H. Kitagawa and S. Hayami, J. Am. Chem. Soc., 2013, 135, 8097–8100 CrossRef CAS PubMed.
- P. Zhang, P. He, Y. Zhao, S. Yang, Q. Yu, X. Xie and G. Ding, Adv. Funct. Mater., 2022, 32, 2202697 CrossRef CAS.
- D. D. Nguyen, Y. T. Megra, T. Lim and J. W. Suk, ACS Appl. Mater. Interfaces, 2023, 15, 7627–7634 CrossRef CAS PubMed.
- H. Eguchi, H. Hayashi and K. Nagata, Polym. J., 2024, 56, 185–192 CrossRef CAS.
- S. Sy, G. Jiang, J. Zhang, H. Zarrin, T. Cumberland, S. Abureden, E. Bell, J. Gostick, A. Yu and Z. Chen, ACS Nano, 2020, 14, 14947–14959 CrossRef CAS PubMed.
- L. L. Zhang, X. Zhao, M. D. Stoller, Y. Zhu, H. Ji, S. Murali, Y. Wu, S. Perales, B. Clevenger and R. S. Ruoff, Nano Lett., 2012, 12, 1806–1812 CrossRef CAS PubMed.
- J. Kim, J.-H. Eum, J. Kang, O. Kwon, H. Kim and D. W. Kim, Sci. Rep., 2021, 11, 2063 CrossRef PubMed.
- K. Akada, S. Obata and K. Saiki, Carbon, 2022, 189, 571–578 CrossRef.
- S. P. Surwade, S. N. Smirnov, I. V. Vlassiouk, R. R. Unocic, G. M. Veith, S. Dai and S. M. Mahurin, Nat. Nanotechnol., 2015, 10, 459–464 CrossRef PubMed.
- A. Devendran and A. Nagai, Mater. Adv., 2023, 4, 2524–2543 RSC.
- H. Huang, H. Shi, P. Das, J. Qin, Y. Li, X. Wang, F. Su, P. Wen, S. Li, P. Lu, F. Liu, Y. Li, Y. Zhang, Y. Wang, Z.-S. Wu and H.-M. Cheng, Adv. Funct. Mater., 2020, 30, 1909035 CrossRef.
- M. R. Karim, M. S. Islam, K. Hatakeyama, M. Nakamura, R. Ohtani, M. Koinuma and S. Hayami, J. Phys. Chem. C, 2016, 120, 21976–21982 CrossRef.
- G. Lu, G. Meng, Q. Liu, L. Feng, J. Luo, X. Liu, Y. Luo and P. K. Chu, Adv. Powder Mater., 2024, 3, 100154 CrossRef.
- E. Bakangura, L. Wu, L. Ge, Z. Yang and T. Xu, Prog. Polym. Sci., 2016, 57, 103–152 CrossRef CAS.
- Y. Y. Cai, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Colloid Interface Sci., 2021, 594, 593–603 CrossRef CAS PubMed.
- R. Singh, S. Ullah, N. Rao, M. Singh, I. Patra, D. A. Darko, C. P. J. Issac, K. Esmaeilzadeh-Salestani, R. Kanaoujiya and V. Vijayan, J. Nanomater., 2022, 8731429 CrossRef CAS.
- V. S. Nanjundappa, T. Ramakrishnappa, K. Sureshkumar, H. R. Prakash and B. M. Praveen, Appl. Surf. Sci. Adv., 2023, 14, 100386 CrossRef.
- B. L. Dasari, J. M. Nouri, D. Brabazon and S. Naher, Energy, 2017, 140, 766–778 CrossRef CAS.
- D. G. Trikkaliotis, A. K. Christoforidis, A. C. Mitropoulos and G. Z. Kyzas, ChemEngineering, 2021, 5, 64 CrossRef CAS.
- T. Kim, H. Cho, S. T. Choi, W. Nam, S. Lee, H. Liang and S. Kim, J. Alloys Compd., 2023, 958, 170464 CrossRef CAS.
- A. Pedico, L. Baudino, A. Aixalà-Perelló and A. Lamberti, Membranes, 2023, 13, 429 CrossRef CAS PubMed.
- S. J. An, Y. Zhu, S. H. Lee, M. D. Stoller, T. Emilsson, S. Park, A. Velamakanni, J. An and R. S. Ruoff, J. Phys. Chem. Lett., 2010, 1, 1259–1263 CrossRef CAS.
- T. Bayer, S. R. Bishop, M. Nishihara, K. Sasaki and S. M. Lyth, J. Power Sources, 2014, 272, 239–247 CrossRef CAS.
- M. Sohail Ahmad, Y. Inomata and T. Kida, Chem. Rec., 2024, 24, e202300163 CrossRef CAS PubMed.
- M. S. K. Chowdury, S. B. Park and Y. Park, Int. J. Precis. Eng. Manuf.–Green Tech., 2020, 7, 669–681 CrossRef.
- S. Gahlot, P. P. Sharma and V. Kulshrestha, Colloids Surf., A, 2018, 538, 622–627 CrossRef CAS.
- X. M. Huang, L. Z. Liu, S. Zhou and J. J. Zhao, Front. Phys., 2020, 15, 33301 CrossRef.
- Y. Wu, P. Jia, L. Xu, Z. Chen, L. Xiao, J. Sun, J. Zhang, Y. Huang, C. W. Bielawski and J. Geng, ACS Appl. Mater. Interfaces, 2017, 9, 4998–5005 CrossRef CAS.
- P. Liu, Y. Liu, K. Wang, S. Shi, M. Jin, J. Liu, T. Qin, Q. Liu, X. Liu and J. He, Nano Res., 2024, 17, 7957–7966 CrossRef CAS.
- L. Zhang, Z. Liu, C. Yang, V. García Sakai, M. Tyagi and L. Hong, ACS Nano, 2022, 16, 13771–13782 CrossRef CAS PubMed.
- A. Bagusetty, P. Choudhury, W. A. Saidi, B. Derksen, E. Gatto and J. K. Johnson, Phys. Rev. Lett., 2017, 118, 186101 CrossRef PubMed.
- Y. Lu, L. Huang, Y. Guo and X. Yang, Carbon, 2021, 183, 355–361 CrossRef CAS.
- Z. F. Wu, P. Z. Sun, O. J. Wahab, Y. T. Tan, D. Barry, D. Periyanagounder, P. B. Pillai, Q. Dai, W. Q. Xiong, L. F. Vega, K. Lulla, S. J. Yuan, R. R. Nair, E. Daviddi, P. R. Unwin, A. K. Geim and M. Lozada-Hidalgo, Nat. Commun., 2023, 14, 7756 CrossRef.
- M. Ates, Prog. Org. Coat., 2011, 71, 1–10 CrossRef.
- Y. Liu, X. Mao, H. Wu, X. Wang, B. Shi, C. Fan, Y. Kong and Z. Jiang, J. Membr. Sci., 2022, 644, 120126 CrossRef.
- W. Yu, T. (Yet) Yu and N. Graham, 2D Mater., 2017, 4, 045006 CrossRef.
- Y. Long, K. Wang, G. Xiang, K. Song, G. Zhou and X. Wang, Adv. Mater., 2017, 29, 1606093 CrossRef.
- N. L. Hamidah, M. Shintani, A. S. Ahmad Fauzi, G. K. Putri, S. Kitamura, K. Hatakeyama, M. Sasaki, A. T. Quitain and T. Kida, ACS Appl. Nano Mater., 2020, 3, 4292–4304 CrossRef.
- B. Liu, D. Cheng, H. Zhu, J. Du, K. Li, H.-Y. Zang, H. Tan, Y. Wang, W. Xing and Y. Li, Chem. Sci., 2019, 10, 556–563 RSC.
- W. Jia, B. Tang and P. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 28955–28963 CrossRef PubMed.
- R. Kumar, C. Xu and K. Scott, RSC Adv., 2012, 2, 8777–8782 RSC.
- C. Xu, Y. Cao, R. Kumar, X. Wu, X. Wang and K. Scott, J. Mater. Chem., 2011, 21, 11359–11364 RSC.
- D. Zhang, S. Xu, R. Wan, Y. Yang and R. He, J. Power Sources, 2022, 517, 230720 CrossRef.
- J. Yagyu, M. S. Islam, Y. Shudo, M. Fukuda, H. Ushijima, J. Ohyama, S. Ida, L. F. Lindoy and S. Hayami, ACS Appl. Energy Mater., 2021, 4, 6296–6301 CrossRef.
- T. M. Subrahmanya, Y. Jo Chi, S. Nayak, S. Habetamu Abebe, W.-S. Hung, G. T. M. Kadja, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Membr. Sci., 2024, 705, 122903 CrossRef.
- K. Wakata, Md. S. Islam, M. R. Karim, K. Hatakeyama, N. N. Rabin, R. Ohtani, M. Nakamura, M. Koinuma and S. Hayami, RSC Adv., 2017, 7, 21901–21905 RSC.
- A. Ouadah, T. Luo, J. Wang, S. Gao, X. Wang, X. Zhang, Z. Fang, Z. Wu, J. Wang and C. Zhu, Compos. Sci. Technol., 2018, 164, 204–213 CrossRef.
- M. Di Virgilio, A. Basso Peressut, S. Latorrata, M. Mariani and G. Dotelli, Solid State Ionics, 2022, 376, 115858 CrossRef.
- Y. Wu, Z. Guo, H. Wu, K. Zhu, L. Yang, Y. Ren, Y. Liu, X. Wu, R. Zhao, N. A. Khan, N. M. Ahmad, M. Younas and Z. Jiang, J. Membr. Sci., 2020, 609, 118215 CrossRef.
- Y. Ikeda, M. R. Karim, H. Takehira, T. Matsui, K. Hatakeyama, Y. Murashima, T. Taniguchi, M. Koinuma, M. Nakamura, Y. Matsumoto and S. Hayami, Bull. Chem. Soc. Jpn., 2014, 87, 639–641 CrossRef.
- P. Feng, P. Du, C. Wan, Y. Shi and Q. Wan, Sci. Rep., 2016, 6, 34065 CrossRef PubMed.
- R. R. R. Sulaiman, R. Walvekar, W. Y. Wong, M. Khalid and M. M. Pang, Membranes, 2022, 12, 344 CrossRef PubMed.
- H. Beydaghi, M. Javanbakht and E. Kowsari, Ind. Eng. Chem. Res., 2014, 53, 16621–16632 CrossRef.
- Z. Zakaria and S. K. Kamarudin, Int. J. Energy Res., 2020, 44, 8988–9000 CrossRef.
- Y.-C. Cao, C. Xu, X. Wu, X. Wang, L. Xing and K. Scott, J. Power Sources, 2011, 196, 8377–8382 CrossRef.
- S. Gao, H. Xu, Z. Fang, A. Ouadah, H. Chen, X. Chen, L. Shi, B. Ma, C. Jing and C. Zhu, Electrochim. Acta, 2018, 283, 428–437 CrossRef.
- M. Aliofkhazraei, N. Ali, W. I. Milne, C. S. Ozkan, S. Mitura and J. L. Gervasoni, Graphene Science Handbook: Applications and Industrialization, CRC Press, Boca Raton, 1st ed., 2016 Search PubMed.
- M. Zakertabrizi, E. Hosseini, A. Habibnejad Korayem and A. Razmjou, Adv. Mater. Technol., 2021, 6, 2001049 CrossRef.
- T. Miyake and M. Rolandi, J. Phys.: Condens. Matter, 2015, 28, 023001 CrossRef PubMed.
- M. Zakertabrizi, E. Hosseini, A. H. Korayem, A. Razmjou, A. G. Fane and V. Chen, J. Membr. Sci., 2021, 618, 118735 CrossRef.
- Z. Jiang, X. Zhao, Y. Fu and A. Manthiram, J. Mater. Chem., 2012, 22, 24862–24869 RSC.
- J. Zhu, X. Meng, J. Zhao, Y. Jin, N. Yang, S. Zhang, J. Sunarso and S. Liu, J. Membr. Sci., 2017, 535, 143–150 CrossRef.
- N. Carboni, L. Mazzapioda, A. Caprì, I. Gatto, A. Carbone, V. Baglio and M. A. Navarra, Electrochim. Acta, 2024, 486, 144090 CrossRef.
- K. Ayers, N. Danilovic, R. Ouimet, M. Carmo, B. Pivovar and M. Bornstein, Annu. Rev. Chem. Biomol. Eng., 2019, 10, 219–239 CrossRef PubMed.
- S. Sun, Z. Shao, H. Yu, G. Li and B. Yi, J. Power Sources, 2014, 267, 515–520 CrossRef.
- C. Li and J.-B. Baek, Nano Energy, 2021, 87, 106162 CrossRef.
- H. Ahmadi, M. Zakertabrizi, E. Hosseini, W. Cha-Umpong, M. Abdollahzadeh, A. H. Korayem, V. Chen, H. K. Shon, M. Asadnia and A. Razmjou, Desalination, 2022, 538, 1–12 CrossRef.
- M. Fouladvand, L. Naji, M. Javanbakht and A. Rahmanian, J. Membr. Sci., 2021, 636, 119563 CrossRef.
- H. Zarrin, S. Sy, J. Fu, G. Jiang, K. Kang, Y.-S. Jun, A. Yu, M. Fowler and Z. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 25428–25437 CrossRef PubMed.
- Y. M. Shulga, S. A. Baskakov, Y. V. Baskakova, Y. M. Volfkovich, N. Y. Shulga, E. A. Skryleva, Y. N. Parkhomenko, K. G. Belay, G. L. Gutsev, A. Y. Rychagov, V. E. Sosenkin and I. D. Kovalev, J. Power Sources, 2015, 279, 722–730 CrossRef.
- Y. M. Shulga, S. A. Baskakov, V. A. Smirnov, N. Y. Shulga, K. G. Belay and G. L. Gutsev, J. Power Sources, 2014, 245, 33–36 CrossRef.
- A. S. Ahmad Fauzi, N. L. Hamidah, S. Sato, M. Shintani, G. K. Putri, S. Kitamura, K. Hatakeyama, A. T. Quitain and T. Kida, Sens. Actuators, B, 2020, 323, 128678 CrossRef.
- A. Miyamoto, Y. Kuwaki, T. Sano, K. Hatakeyama, A. Quitain, M. Sasaki and T. Kida, ACS Omega, 2017, 2, 2994–3001 CrossRef PubMed.
- F. Li, X. Jiang, J. Zhao and S. Zhang, Nano Energy, 2015, 16, 488–515 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |




