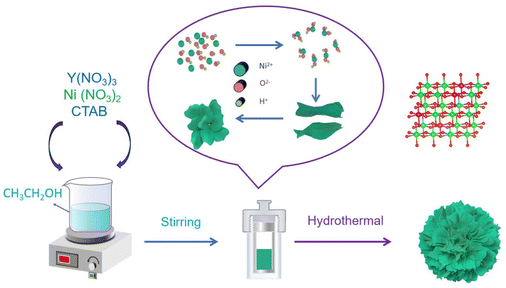
Yttrium doping stabilizes the structure of Ni3(NO3)2(OH)4 cathodes for application in advanced Ni–Zn batteries†
Xinyu
Feng
a,
Siwen
Zhang
a,
Jiazhuo
Li
a,
Yingfang
Hu
a,
Rongyuan
Ge
a,
Yaowen
Shi
a,
Yali
Yao
b,
Bosi
Yin
*a and
Tianyi
Ma
 *c
*c
aInstitute of Clean Energy Chemistry, Key Laboratory for Green Synthesis and Preparative Chemistry of Advanced Materials of Liaoning Province, College of Chemistry, Liaoning University, Shenyang 110036, China. E-mail: yinbosi@lnu.edu.cn
bInstitute for Catalysis and Energy Solutions (ICES), University of South Africa, Roodepoort 1710, South Africa
cCentre for Atomaterials and Nanomanufacturing (CAN), School of Science, RMIT University, Melbourne, VIC 3000, Australia. E-mail: tianyima@swin.edu.au
First published on 8th August 2024
Abstract
Ni3(NO3)2(OH)4 has a high theoretical specific capacitance, low cost, and environmental friendliness, making it a promising electrode material. Specifically, Ni3(NO3)2(OH)4 electrodes have a larger layer spacing (c = 6.898 Å) than Ni(OH)2 electrodes since NO3− has a much larger ionic radius than OH−. The larger layer spacing stores more electrolyte ions, significantly improving the electrochemical activity of the electrodes. Additionally, the interlayer NO3− can enhance the structural stability of Ni3(NO3)2(OH)4. However, since Ni3(NO3)2(OH)4 has a higher molar mass than Ni(OH)2, it has a lower theoretical specific capacity. Consequently, Ni3(NO3)2(OH)4 has not been used in zinc-based alkaline batteries. Studies showed that doping could enhance the electrochemical performance of electrode materials. Therefore, this study used a simple solvothermal reaction to synthesize yttrium-doped Ni3(NO3)2(OH)4 (Y-Ni3(NO3)2(OH)4), assembling a Y-Ni3(NO3)2(OH)4//Zn battery for electrochemical testing. Y-Ni3(NO3)2(OH)4 served as the cathode in the battery. The analysis of Y-Ni3(NO3)2(OH)4 showed that yttrium (Y) doping increased the specific surface area and pore size of Ni3(NO3)2(OH)4 significantly. The increased specific surface area improved the active material utilization, and the abundant mesopores facilitated OH− transport, substantially enhancing the battery's specific capacity and energy density. Ultimately, the specific discharge capacity of the advanced Y-Ni3(NO3)2(OH)4//Zn battery reached 177.97 mA h g−1 at a current density of 4 A g−1, nearly doubling the capacity of the earlier Ni3(NO3)2(OH)4//Zn battery (103.59 mA h g−1).
Introduction
The demand for energy has increased due to rapid economic development and the gradual increase in population. Due to the gradual depletion of non-renewable energy sources, such as fossil fuels, it is imperative to develop safe, low-cost, and high-efficiency renewable energy storage devices.1–7 Lithium-ion batteries have attracted much attention over the past few decades due to their rechargeability and high energy density. In addition to their advantages, lithium-ion batteries have several disadvantages, such as their flammability and high cost, and the limited lithium resources.8–12 Moreover, aqueous rechargeable batteries have gained increasing attention due to their high safety and low cost, the abundant resources, and their environmental friendliness. Among the aqueous batteries, nickel-based batteries are particularly noteworthy for their high energy density, power density, and affordability.13–17 Nickel-based batteries include nickel–cadmium (Ni//Cd),18 nickel–iron (Ni//Fe),19 nickel–zinc (Ni//Zn),20 nickel–hydrogen (Ni//H),21 and nickel–metal hydride (Ni//MH) batteries.22Among many nickel-based batteries, Ni//Zn batteries have attracted a lot of research in recent years due to the abundant reserves of zinc metal and their low price, safety, and environmental friendliness, with a high theoretical capacity (820 mA h g−1).23,24 Moreover, studies have shown that Ni//Zn batteries have a high discharge voltage platform (∼1.7 V),25 which has a high practical application value and is considered to be an alternative to lithium-ion batteries. However, Ni//Zn batteries have poor stability due to the inevitable formation of zinc dendrites and the irreversibility of nickel-based cathodes, which is the main bottleneck for their widespread use. Therefore, considerable efforts have been made to improve the cycling stability of Ni//Zn batteries to overcome this limitation.26 For example, Guo et al. reported a specific capacity of 177.8 mA h g−1 for CoNiO2//Zn at a current density of 0.5 A g−1, with a maximum energy density of 308.14 W h kg−1 at 880.40 kW kg−1 power density.27 Zhu et al. reported a high capacity of 155 mA h g−1 for NiO@carbon nanotubes//Zn sheet at a current density of 1 A g−1, retaining 65% of its capacity after 500 cycles.28 Although the research on Ni//Zn batteries has made some progress, their cycle life and capacity fail to meet the market demands. Therefore, low-cost Ni//Zn batteries with higher energy densities and better rate capabilities are urgently needed.
This study used a simple solvothermal method to synthesize nanoflowers of yttrium-doped Ni3(NO3)2(OH)4 (Y-Ni3(NO3)2(OH)4). NO3− reacted with CH3CH2OH to release OH− according to the reaction: 4CH3CH2OH + NO3− → 4CH3CHO + NH3↑ + OH− + 2H2O.29 Ni2+ and Y3+ reacted with OH− to form Y-Ni3(NO3)2(OH)4. The synthesis and crystal structure of Y-Ni3(NO3)2(OH)4 nanoflowers are shown in Fig. 1. The specific surface area of Ni3(NO3)2(OH)4 increased significantly, and numerous mesopores appeared after yttrium (Y) doping, improving the conductivity and reactivity of Y-Ni3(NO3)2(OH)4. Subsequently, this increased the efficiency of redox reactions in the electrochemical process, improving the electrochemical performance. Furthermore, Y-Ni3(NO3)2(OH)4 exhibited a remarkable electrochemical performance when assembled in a zinc-based alkaline battery. As expected, the Y-Ni3(NO3)2(OH)4 cathode exhibited excellent electrochemical performance, with a discharge capacity of 177.97 mA h g−1 at a current density of 4 A g−1 (almost double compared to that before doping) and an energy density of 379.35 W h kg−1. Therefore, many results indicated that Y doping significantly improved the electrochemical performance of the material, making it promising for future practical applications.
Experimental section
Materials
Ni(NO3)2·6H2O, CTAB, and KOH were purchased from Tianjin Da Mao Chemical Reagent Factory, and absolute ethanol was purchased from Tianjin Yongda Chemical Reagent Co., Ltd. Y(NO3)3·6H2O, acetylene black, N-methyl pyrrolidine (NMP, 98%), and polyvinylidene fluoride (PVDF) were purchased from Aladdin Reagent (Shanghai) Co., Ltd.Material syntheses
The typical procedure for the synthesis of Y-Ni3(NO3)2(OH)4 is as follows: 4.0 mmol of Ni(NO3)2·6H2O, 0.4 mmol Y(NO3)3·6H2O and 1.0 mmol of CTAB are weighed and added to 70 mL of absolute ethanol. The mixture is stirred for 30 minutes to mix completely, then the mixed solution is transferred to a 100 mL stainless steel reactor lined with polytetrafluoroethylene (PTFE). The solution is reacted at 150 °C for 18 hours using a solvothermal method to produce Y-doped Ni3(NO3)2(OH)4 nanoflowers. After the reactor has completely cooled to room temperature, the Y-doped nickel hydroxide is removed and washed with deionized water and absolute ethanol by centrifugation three times each. Finally, it is dried under vacuum at 60 °C for 12 hours.Characterization
X-ray diffraction patterns of the samples were obtained by X-ray diffraction (XRD, Bruker-D8-AXS). The surface valence state and chemical composition of the products were analysed by X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha). The morphological structures of the samples were studied by scanning electron microscopy (SEM, Hitathi SU1080) and transmission electron microscopy (TEM, JEM 2100). Raman spectra were obtained using a laser excitation of 532 nm (Raman, inVia Reflex, Renishaw). The Fourier transform infrared spectra of the samples were obtained using a Fourier transform infrared (FTIR) spectrometer (Bruker Tensor II). The specific surface area and pore size of the sample were tested using the Brunauer–Emmett–Teller (BET) method (Micromeritics ASAP 2460).Electrochemical characterization
The electrochemical properties of the electrode materials were investigated using a VMP3 multi-channel electrochemical workstation and a NEWARE Battery Test System, and the single-electrode properties were tested in a three-electrode system, with the electrolyte being a 3 M KOH solution. Ni3(NO3)2(OH)4 or Y-Ni3(NO3)2(OH)4 (1.2 cm × 1.2 cm) was used as the working electrode, the mass loading of Y-Ni3(NO3)2(OH)4 was 1.70 mg cm−2, a Hg/HgO electrode was used as the reference electrode, a platinum sheet was used as the counter electrode, and the test environment was room temperature.30 The performance of the electrodes was tested and calculated by cyclic voltammetry (CV), galvanostatic cycling with potential limitation (GCPL) and electrochemical impedance spectroscopy (EIS). The battery performance was tested in a two-electrode system with an electrolyte of 3 M KOH solution, Ni3(NO3)2(OH)4 or Y-Ni3(NO3)2(OH)4 as the anode material and zinc flakes as the cathode material, with the test environment being room temperature. The performance of the electrodes was tested and calculated by cyclic voltammetry scanning and constant current charging and discharging.Results and discussion
The crystal structure of the samples was investigated using XRD (Fig. 2a). The diffraction peaks at 2θ = 12.81°, 25.80°, 33.01°, 35.55°, 42.36°, 55.73°, 58.93°, and 60.59° could be categorized as the (001), (002), (100), (101), (102), (103), (110), and (111) crystal faces of Ni3(NO3)2(OH)4 (JCPDS card no. 22-0752, a = b = 3.131 Å and c = 6.898 Å).31,32 The XRD results showed no phase of the doped Y ions, which may be due to their low content. Additionally, Y doping did not destroy the lattice structure of Ni3(NO3)2(OH)4. Compared to Ni3(NO3)2(OH)4, the (001) diffraction peak of Y-Ni3(NO3)2(OH)4 was broader and less intense, and shifted to a lower angle (Fig. S1†). This may be because the difference between the ionic radii of Y3+ (0.90 Å) and Ni2+ (0.69 Å) distorted the Ni3(NO3)2(OH)4 lattice and reduced its crystallinity.33Furthermore, the samples were subjected to FTIR to investigate the changes due to Y doping, as shown in the results in Fig. 2b. The peaks at 653, 1365, and 1618 cm−1 represent the bending vibration of the Ni–O–H plane, the stretching vibration of the N–O bond in NO3−, and the bending vibration of O–H, respectively. A broad, strong absorption peak at 3423 cm−1 represents the stretching vibration of H–O–H. Moreover, this demonstrates the successful synthesis of Ni3(NO3)2(OH)4, confirming the XRD results. After Y doping, the infrared peaks of Y-Ni3(NO3)2(OH)4 shifted slightly to higher wavelengths due to doping-induced lattice defects.
Additionally, Raman spectroscopy was used to confirm the successful Y doping. As shown in the Raman spectra in Fig. 2c, Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 exhibited peaks at 463 and 468 cm−1, respectively, which are attributed to the stretching vibration of Ni–O.34 The Y-doped samples exhibited a greater intensity of peaks than the undoped samples, indicating that the Y-doped samples had more structural defects and a greater disorder, which is consistent with the XRD results.
Furthermore, XPS was used to determine the elemental content and valence state of Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4. As shown in Fig. 2d, elements Ni and O were present in the full spectrum XPS images. The C 1s peak at 284.6 eV for carbon was used as a correction reference for the subsequent elements. In particular, the XPS spectrum of Y-Ni3(NO3)2(OH)4, with a peak at 158.08 eV, demonstrated the successful doping of Y into the sample. As shown in the Ni 2P fine spectrum in Fig. 2e, Ni 2p3/2 and Ni 2p1/2 had characteristic Ni2+ peaks with binding energies of 854.98 and 872.88 eV, respectively, a spin–orbit split of 17.9 eV, and characteristic Ni3+ peaks at 856.58 eV and 874.38 eV.35 Utilizing XPS to analyze the split peak area, it was determined that the relative abundances of Ni3+ ions in Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 were 14.68% and 18.58%, respectively. This observation underscores how the introduction of Y3+ ions through doping serves to elevate the concentration of Ni3+ ions within the compound, whereas an increase in the Ni3+ ion content increases the number of oxygen vacancy defects and improves the charge transfer efficiency and electrical conductivity of the material.36
As shown in the Y 3d fine spectrum in Fig. 2f, the peaks at 159.8 and 157.7 eV corresponded to the Y 3d5/2 and Y 3d3/2 orbitals, respectively, and the peaks at 160.6 and 158.4 eV were possibly due to the formation of carbonate since Y2O3 adsorbed CO2 from the air.37 This differed from the XRD results primarily because the Y2O3 content on the surface was too low to be detected in XRD, whereas XPS had more sensitivity for the surface. The specific surface area and pore structure of the electrode materials significantly influence their electrochemical properties. Therefore, the porous properties of the samples were investigated using the specific surface area (BET) test. Additionally, the specific surface area, pore volume, and corresponding pore size distribution graphs were calculated based on the N2 adsorption–desorption isotherms, as shown in Fig. 2(g–i). Both samples had type IV adsorption isotherms, and their relative pressures (P/P0) were in the 0.4–1.0 range with significant hysteresis loops, indicating that they had mesoporous structures.38 The results show that for pure Ni3(NO3)2(OH)4, the specific surface area was 26.76 m2 g−1, the average pore diameter was 7.36 nm, and the pore volume was 0.0498 cm3 g−1. However, Y doping increased the specific surface area, pore diameter, and pore volume to 226.04 m2 g−1, 7.45 nm, and 0.416 cm3 g−1, respectively. Y doping increased the specific surface area of the material significantly, shortening the ion diffusion path and promoting full contact between the material and the electrolyte to improve the capacity and cycling stability of the material. Additionally, the significant increase in the specific surface area could reduce the polarization of the battery, reducing the energy loss due to polarization.39,40
The morphology and structure of Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 were studied using SEM and TEM, as shown in Fig. 3, S2, and S3.† Both samples had the same morphology and structure.41 Moreover, both of them had a flowery structure of about 4 μm. However, the nanosheets of the samples increased after Y doping, which increased their specific surface area significantly and was consistent with the results of the BET tests. Additionally, energy dispersive X-ray spectroscopy (EDS) could confirm the successful Y doping in Y-Ni3(NO3)2(OH)4. As shown in Fig. 3(e) and (f), the Ni, Y, and O elements were distributed uniformly in the sample, which was consistent with the results of XPS and Raman spectroscopy. The TEM images shown in Fig. 3(g) and (h) indicated that the microspheres of Y-Ni3(NO3)2(OH)4 were made up of nanosheets. The prepared Y-Ni3(NO3)2(OH)4 nanoflowers were assembled from ultrathin (∼10 nm) nanosheets, shortening the ion transfer path and providing more electroactive sites. As shown in Fig. 3(i), the selected area electron diffraction (SAED) pattern of Y-Ni3(NO3)2(OH)4 had diffuse rings, indicating the polycrystalline nature of Y-Ni3(NO3)2(OH)4. As shown in Fig. 3j and S3d,† high-resolution transmission electron microscopy (HRTEM) provides detailed information about the crystal structures of the Y-Ni3(NO3)2(OH)4 and Ni3(NO3)2(OH)4 nanosheets. The 0.19 nm spacing of Ni3(NO3)2(OH)4 corresponded to its (103) crystal plane. In contrast, the 0.249 nm spacing of Y-Ni3(NO3)2(OH)4 corresponded to its (102) crystal plane.42
A series of electrochemical performance tests was conducted using a conventional three-electrode system in a 3 M KOH electrolyte to examine the effect of Y doping on the electrochemical properties of pristine Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 samples. As shown in Fig. 4(a), Y-Ni3(NO3)2(OH)4 had a more prominent redox peak and larger area compared to Ni3(NO3)2(OH)4, which indicated that Y doping improved the electrochemical activity of the active material effectively to increase the battery capacity. The CV curves of Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 electrodes at various scanning rates are shown in Fig. 4(b) and S4.† The samples before and after doping showed different pairs of redox peaks, corresponding to the charging and discharging plateaus of the galvanostatic charge and discharge (GCD) curves. The redox current peaks could be attributed to the reversible surface reactions of Ni2+/Ni3+. The oxidation and reduction peaks shifted in the directions of positive and negative potentials with increased sweep rates, respectively. This peak shift phenomenon was due to the increased sweep rate and enhanced polarization.43
As a nickel-based transition metal hydroxide, Ni3(NO3)2(OH)4 underwent redox reactions on the surface and in the interior during electrochemical testing. Consequently, it exhibited the properties of a battery and pseudo-capacitance. The CV test revealed different peak current values (i in mA) at different voltage scan rates (v in mV s−1). v was correlated with i to distinguish between the diffusive and pseudo-capacitive behavior of a battery during charging and discharging. i varied with the 0.5 power of v if the process was diffusion-controlled to indicate the battery behavior. In contrast, i varied linearly with v if the process was capacitively controlled to indicate the pseudo-capacitive behavior. Furthermore, the value of b for the electrode material could be calculated using eqn (1) and (2) to determine whether there was pseudo-capacitive behavior during charging and discharging.44
| i = aνb | (1) |
log(i) = log(a) + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) log(v) | (2) |
The value of b could be calculated using linear curve fitting. When the b value reached 0.5, the material primarily exhibited battery-type properties. When the b value was equal to 1, the material was determined to be purely capacitor-type.45 A b value between 0.5 and 1 indicated that the material was in the region of transition between capacitor-type and battery-type materials. Generally, the effect of diffusion-controlled processes was more significant with a decreased b value, while the surface capacitance contribution gradually increased with an increased b value.
Based on the calculations shown in Fig. 4(c), the b values of the oxidation and reduction peaks of Ni3(NO3)2(OH)4 were 0.5442 and 0.6049, respectively. This suggested that the Ni3(NO3)2(OH)4 electrode material had more similarities to the battery material, and solid-state diffusion control dominated the electrochemical reaction. Furthermore, to better understand the contribution rate of pseudo-capacitance, it is calculated under a specific scanning rate using eqn (3) and (4).46
| i = k1v + k2v1/2 | (3) |
| i/v1/2 = k1v1/2 + k2 | (4) |
As demonstrated in Fig. 4(d) and S5,† the capacitance contributions of Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 were 67.24% and 60.43% at a scanning rate of 4 mV s−1, respectively.
Furthermore, comparison of the capacitance contributions of Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 illustrated in Fig. 4(e) and (f) revealed an increased pseudo-capacitance contribution. This may be due to the increased specific surface area, which enhanced the actual contact area between the electrolyte and the active substance and increased the number of redox reactions.47
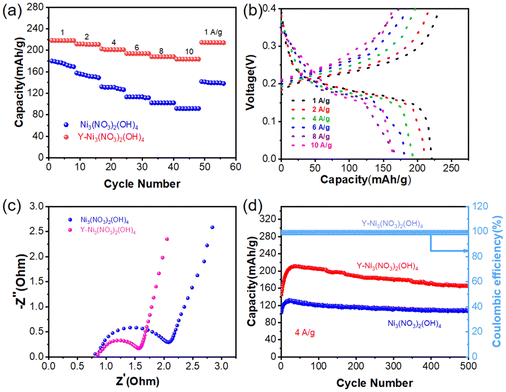
A performance comparison of electrode materials before and after doping is shown in Fig. 5(a). Y-Ni3(NO3)2(OH)4 had a higher specific capacity and better rate performance than Ni3(NO3)2(OH)4. As shown in Fig. 5(b), the battery discharge voltage was around 0.2 V, which was consistent with the position of the reduction peak observed in the CV curve. Furthermore, EIS was used to investigate the reasons for the improved electrochemical performance of the Y-Ni3(NO3)2(OH)4 electrode, as shown in Fig. 5(c). The Nyquist plot consisted of two parts, including the semicircular slope in the high and low-frequency regions, which was related to the diffusion-limited electron transfer process. Compared to the as-prepared Ni3(NO3)2(OH)4, Y-Ni3(NO3)2(OH)4 had a smaller charge transfer resistance (Rct) in the high-frequency region and a larger slope in the low-frequency region. This indicated that Y doping improved the conductivity of Ni3(NO3)2(OH)4 and increased the ion diffusion rate, which was possible because of the numerous mesopores on the electrode surface due to Y doping that benefitted ion diffusion.48 As seen in Fig. 5(d), the initial capacity of the battery before doping was 96 mA h g−1, which gradually increased to 124 mA h g−1 during the first 30 cycles. After doping, the initial capacity of the battery was 143 mA h g−1, which gradually increased to 216 mA h g−1 during the first 30 cycles. The capacity was 163 mA h g−1 even after 500 cycles.
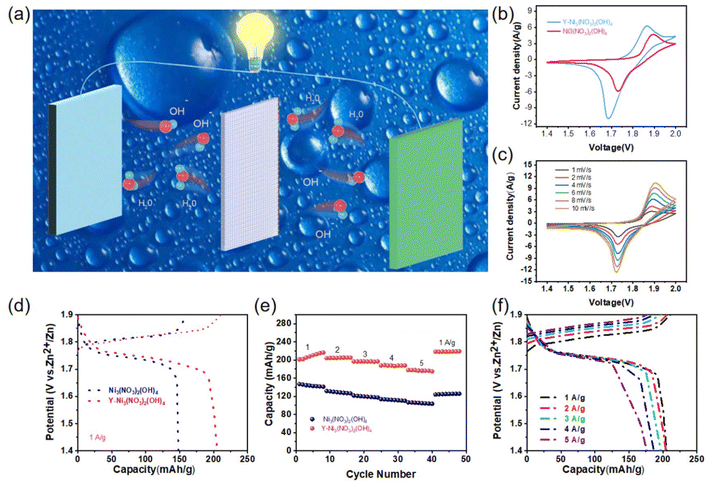
Moreover, a typical Ni//Zn battery was assembled to demonstrate the feasibility of using the Y-Ni3(NO3)2(OH)4 electrode in a water-based rechargeable Ni//Zn battery. The Y-Ni3(NO3)2(OH)4 electrode, commercial zinc foil, and 3 M KOH were used as the cathode, anode, and electrolyte, respectively. A schematic diagram of the internal structure and the electrochemical process of a Y-Ni3(NO3)2(OH)4//Zn battery is presented in Fig. 6(a). During charging and discharging, OH− migrated to the reactive sites through the three-dimensional pores inside the nanoflower. Additionally, an oxidation reaction occurred at the cathode active site, oxidizing Ni2+ to Ni3+. The H2O molecules moved through the three-dimensional pores inside the nanoflower into the solution. Electrons were released and transferred from the cathode nanoflower to the anode. A comparison of the CV curves of Ni3(NO3)2(OH)4 and Y-Ni3(NO3)2(OH)4 at a scan rate of 1 mV s−1 is shown in Fig. 6(b). The CV curve of Y-Ni3(NO3)2(OH)4 exhibited a larger integrated area compared to Ni3(NO3)2(OH)4 since Y doping enhanced the conductivity and specific surface area of the cathode material, increasing the number of active sites. This indicated that the Y-Ni3(NO3)2(OH)4 electrode had a higher specific capacity than the Ni3(NO3)2(OH)4 electrode. The CV curves of the Y-Ni3(NO3)2(OH)4//Zn battery at different scan rates are shown in Fig. 6(c). The curves display a pair of redox peaks at different scan rates, confirming a highly reversible redox reaction in the Y-Ni3(NO3)2(OH)4//Zn battery under alkaline electrolytic conditions. As shown in Fig. 6(d), the discharge voltage plateau of the Y-Ni3(NO3)2(OH)4//Zn battery is approximately 1.75 V, higher than that of the Ni3(NO3)2(OH)4//Zn battery. This indicates that Y doping in the Ni3(NO3)2(OH)4 electrode could lower the electrochemical polarization. The discharge capacities of the Ni3(NO3)2(OH)4//Zn and Y-Ni3(NO3)2(OH)4//Zn batteries were compared at current densities in the range of 1–5 A g−1, as shown in Fig. 6(e) and (f). Y doping significantly enhanced the discharge capacity of the Y-Ni3(NO3)2(OH)4//Zn battery, achieving 216.52, 205.23, 196.82, 187.85, and 176.42 mA h g−1 discharge capacities at current densities of 1, 2, 3, 4, and 5 A g−1, respectively. Restoring the current density to 0.1 A g−1 could recover the discharge capacity to 218.42 mA h g−1. The Y-Ni3(NO3)2(OH)4//Zn battery had almost twice the capacity of the Ni3(NO3)2(OH)4//Zn battery at a current density of 5 A g−1. The improved performance could be attributed to the significant increase in the specific surface area and the emergence of many mesopores in the material after Y doping, which enhanced the conductivity and reactivity of the Y-Ni3(NO3)2(OH)4 material, improved the efficiency of redox reactions during the electrochemical process, and ultimately improved the electrochemical performance.49
Cycle stability is an extremely significant metric in electrochemical testing. As shown in Fig. 7(a), the energy density and power density of the Y-Ni3(NO3)2(OH)4//Zn battery are further compared with those of recently reported aqueous alkaline batteries in the Ragone diagram. The results show that the energy density of the Y-Ni3(NO3)2(OH)4//Zn battery is 379 W h kg−1 at a power density of 1749 W kg−1. Impressively, the electrochemical performance of the Y-Ni3(NO3)2(OH)4//Zn battery is significantly superior to that of the recently reported zinc-based alkaline batteries, such as Co–Ni(OH)2//Zn (148.5 W h kg−1),50 NiO//Zn (228 W h kg−1),51 Fe-Co3O4//Zn (287 W h kg−1),38 D-Co3O4//Zn(202 W h kg−1),52 and CoNiO2//Zn (177 W h kg−1).27 The cycling stability of the batteries before and after doping at 4 A g−1 current density is shown in Fig. 7(b). The Ni3(NO3)2(OH)4//Zn battery had an initial capacity of 90.88 mA h g−1, which gradually increased to 104.62 mA h g−1 over the first 20 cycles and decreased to 49.68 mA h g−1 after 400 cycles (the capacity retention was 47.49%). In contrast, the Y-Ni3(NO3)2(OH)4//Zn battery had an initial capacity of 133.44 mA h g−1, which gradually increased to 196.92 mA h g−1 over the first 50 cycles and maintained a capacity of 162.34 mA h g−1 even after 400 cycles (the capacity retention was 82.44%). Moreover, as shown in Fig. 7(c) and (d), a mini electric fan and a light-emitting diode (LED) could be powered using a Y-Ni3(NO3)2(OH)4//Zn battery. To evaluate the changes in morphology and elemental valence states, we performed SEM and XPS tests on the composite electrodes after long-term charge/discharge experiments. As shown in Fig. S7 and S8,† the Y-Ni3(NO3)2(OH)4 electrode was able to maintain the nanoflower structure after cycling, and the XPS results showed that the elemental valence states did not change significantly. This series of test results demonstrated that the Y-Ni3(NO3)2(OH)4 nanoflower electrode could potentially be used in a high-performance energy storage device in the future.
Conclusions
This study used a simple solvothermal reaction to successfully synthesize Y-Ni3(NO3)2(OH)4//Zn, which exhibited excellent electrochemical performance with potential practical applications. After Y doping, the Y-Ni3(NO3)2(OH)4 material had significantly improved conductivity and dramatically increased specific surface area with many mesopores. The increased specific surface area was beneficial for improving the utilization efficiency of active materials. During the electrochemical reaction, more surface atoms could participate, increasing the capacity and energy density of the battery. The increased number of mesopores benefitted the transport of OH− during the electrochemical process. The mesopores could provide more ion channels, allowing OH− to reach the inside of the electrode material more easily, accelerating the reaction rate and improving the power density of the battery. Finally, during the charge–discharge process, the increased specific surface area and the mesoporous structure could provide a certain buffering space, which mitigated the impact of volume changes on the electrode structure and improved the stability and cycle life of the battery. The results showed that the Y-Ni3(NO3)2(OH)4//Zn battery had a high capacity of 218 mA h g−1 at 1 A g−1 and maintained an 82.44% capacity retention rate after 400 cycles at 4 A g−1.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors thank Shiyanjia Lab (https://www.shiyanjia.com) for supporting the SEM, TEM, XPS and BET tests. This work was supported by the National Natural Science Foundation of China (No. 52071171, 52202248), the Key Research Project of Department of Education of Liaoning Province (LJKZZ20220015), the Australian Research Council (ARC) through the Future Fellowship (FT210100298), the Discovery Project (DP220100603) and Linkage Project (LP210200504, LP220100088) schemes, the Australian Government through the Cooperative Research Centres Projects (CRCPXIII000077), the Australian Renewable Energy Agency (ARENA) as part of ARENA's Transformative Research Accelerating Commercialisation Program (TM021), the Research Fund for the Doctoral Program of Liaoning Province (2022-BS-114), and the Chunhui Program of the Ministry of Education of the People's Republic of China (202201135).References
- J. Yao, H. Wan, C. Chen, J. Ji, N. Wang, Z. Zheng, J. Duan, X. Wang, G. Ma and L. Tao, Nano-Micro Lett., 2021, 13, 1–14 CrossRef.
- H. Chen, Z. Shen, Z. Pan, Z. Kou, X. Liu, H. Zhang, Q. Gu, C. Guan and J. Wang, Adv. Sci., 2019, 6, 1802002 CrossRef.
- H. Zhang, R. Wang, D. Lin, Y. Zeng and X. Lu, ChemNanoMat, 2018, 4, 525–536 CrossRef CAS.
- S. W. Zhang, B. S. Yin, Y. Z. Luo, L. Shen, B. S. Tang, Z. K. Kou, X. X. Liu, D. M. Gu, Z. B. Wang and H. Gong, Nano Energy, 2022, 68, 104314 CrossRef.
- T. Kou, S. Wang, J. L. Hauser, M. Chen, S. R. J. Oliver, Y. Ye, J. Guo and Y. Li, ACS Energy Lett., 2019, 4, 622–628 CrossRef CAS.
- Y. Sun, B. S. Yin, J. Z. Yang, Y. X. Ding, M. D. Li, H. Li, J. Z. Li, B. H. Jia, S. W. Zhang and T. Y. Ma, Energy Environ. Sci., 2023, 16, 5568–5604 RSC.
- X. Cheng, L. Zhou, Y. Lu, W. Xu, P. Zhang and X. Lu, Electrochim. Acta, 2018, 263, 311–317 CrossRef CAS.
- M. D. Li, Y. X. Ding, S. W. Zhang, M. H. Liu, Y. Sun, Y. S. Zhang, B. S. Yin and T. Y. Ma, J. Mater. Chem. A, 2024, 12, 10269–10278 RSC.
- R. Sharma, H. Kumar, G. Kumar, S. Sharma, R. Aneja, A. K. Sharma, R. Kumar and P. Kumar, Chem. Eng. J., 2023, 468, 143706 CrossRef CAS.
- M. Aizudin, W. Fu, R. P. Pottammel, Z. Dai, H. Wang, X. Rui, J. Zhu, C. C. Li, X. L. Wu and E. H. Ang, Small, 2024, 20, 2305217 CrossRef CAS.
- F. Lionetto, N. Arianpouya, B. Bozzini, A. Maffezzoli, M. Nematollahi and C. Mele, J. Energy Storage, 2024, 84, 110849 CrossRef.
- M. D. Li, Y. X. Ding, S. W. Zhang, Y. Sun, M. H. Liu, J. W. Zhao, B. S. Yin and T. Y. Ma, Small Struct., 2024, 2, 2300371 CrossRef.
- H. Wu, J. Hao, Y. Jiang, Y. Jiao, J. Liu, X. Xu, K. Davey, C. Wang and S. Z. Qiao, Nat. Commun., 2024, 15, 575 CrossRef CAS.
- B. Liu, Q. Zhang, L. Zhang, X. Yong, L. Li and C. Wang, Energy Storage Mater., 2024, 66, 103221 CrossRef.
- L. Li, S. Cheng, L. Deng, T. Liu, W. Dong, Y. Liu, L. Huang, H. Yao and X. Ji, ACS Appl. Mater. Interfaces, 2023, 15, 3953–3960 CrossRef CAS.
- F. Zhang, J. Ma, H. Song, L. He, J. Zhang and E. Wang, J. Colloid Interface Sci., 2023, 652, 104–112 CrossRef CAS PubMed.
- W. Xie, K. Zhu, H. Yang, W. Jiang, W. Li, Z. Wang and W. Yang, Angew. Chem., 2023, 135, e202303517 CrossRef.
- Y. Dai, S. Cheng, Q. J. Gan, T. J. Yu, X. Wu and F. L. Bi, J. Cent. South Univ., 2021, 28, 2919–2930 CrossRef CAS.
- X. Wu, H. B. Wu, W. Xiong, Z. Le, F. Sun, F. Liu, J. Chen, Z. Zhu and Y. Lu, Nano Energy, 2016, 30, 217–224 CrossRef CAS.
- W. Zhao, Z. Liu, C. Zhong, Z. Shen, X. Jin and H. Zhang, J. Colloid Interface Sci., 2020, 579, 823–831 CrossRef CAS.
- W. Chen, Y. Jin, J. Zhao, N. Liu and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11694–11699 CrossRef CAS.
- M. M. Li, C. C. Wang and C. C. Yang, J. Power Sources, 2021, 491, 229585 CrossRef CAS.
- Y. Yan, S. Liang, X. Wang, M. Zhang, S. Hao, X. Cui, Z. Li and Z. Lin, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2110036118 CrossRef CAS.
- L. Tang, H. Peng, J. Kang, H. Chen, M. Zhang, Y. Liu, D. H. Kim, Y. Liu and Z. Lin, Chem. Soc. Rev., 2024, 53, 4877–4925 RSC.
- K. Wang, X. Fan, S. Chen, J. Deng, L. Zhang, M. Jing, J. Li, L. Gou, D. Li and Y. Ma, Small, 2023, 19, 2206287 CrossRef CAS.
- N. Wang, H. Wan, J. Duan, X. Wang, L. Tao, J. Zhang and H. Wang, Mater. Today Adv., 2021, 11, 100149 CrossRef CAS.
- J. Ye, X. Zhai, L. Chen, W. Guo, T. Gu, Y. Shi, J. Hou, F. Han, Y. Liu, C. Fan, G. Wang, S. Peng and X. Guo, J. Energy Chem., 2021, 62, 252–261 CrossRef CAS.
- X. Wang, M. Li, Y. Wang, B. Chen, Y. Zhu and Y. Wu, J. Mater. Chem. A, 2015, 3, 8280–8283 RSC.
- Y. Zhang, Y. Zhao, W. An, L. Xing, Y. Gao and J. Liu, J. Mater. Chem. A, 2017, 5, 10039–10047 RSC.
- Y. Zhang, D. Sun, Y. Wang, X. Liu, H. Sun, T. Cai, X. Li, H. Hu, X. Zhang, W. Xing and Z. Yan, Chem. Eng. J., 2023, 453, 139736 CrossRef CAS.
- L. Xie, M. Shi, H. Kimura, M. Cui, K. Wang, B. Cui, W. Du and L. Kang, J. Mater. Sci.: Mater. Electron., 2022, 33, 17284–17294 CrossRef CAS.
- C. Tang, G. Li and L. Li, Chem. Lett., 2008, 37, 1138–1139 CrossRef CAS.
- C. Chen, M. Shi, Y. Zhao, C. Yang, L. Zhao and C. Yan, Chem. Eng. J., 2021, 422, 130375 CrossRef CAS.
- M. Xie, S. Duan, Y. Shen, K. Fang, Y. Wang, M. Lin and X. Guo, ACS Energy Lett., 2016, 1, 814–819 CrossRef CAS.
- K. Tao, P. Li, L. Kang, X. Li, Q. Zhou, L. Dong and W. Liang, J. Power Sources, 2015, 293, 23–32 CrossRef CAS.
- J. Gao, H. Xie, F. Zuo, H. Liu, Y. Zhao and C. Yang, Chem. – Eur. J., 2024, 30, e202400170 CrossRef CAS.
- Y. Jiang, T. Fu, J. Liu, J. Zhao, B. Li and Z. Chen, RSC Adv., 2022, 12, 4805–4812 RSC.
- J. Li, S. Zhang, Y. Ding, Y. Sun, J. Yang, H. Li, T. Ma and B. Yin, Chem. Commun., 2023, 59, 4316–4319 RSC.
- M. Liang, Z. Li, Y. Kang, X. Zhao, X. Zhang, H. Zhang, H. Wang, Z. Miao and C. Fu, J. Mater. Chem. A, 2024, 12, 4623–4634 RSC.
- X. Wang, T. T. Li and Y. Q. Zheng, Int. J. Hydrogen Energy, 2018, 43, 2009–2017 CrossRef CAS.
- L. B. Kong, L. Deng, X. M. Li, M. C. Liu, Y. C. Luo and L. Kang, Mater. Res. Bull., 2012, 47, 1641–1647 CrossRef CAS.
- N. Balke, S. Jesse, A. N. Morozovska, E. Eliseev, D. W. Chung, Y. Kim, L. Adamczyk, R. E. García, N. Dudney and S. V. Kalinin, Nat. Nanotechnol., 2010, 5, 749–754 CrossRef CAS.
- Q. Wei, Q. Li, Y. Jiang, Y. Zhao, S. Tan, J. Dong, L. Mai and D. L. Peng, Nano-Micro Lett., 2021, 13, 1–13 CrossRef CAS.
- J. Come, P. L. Taberna, S. Hamelet, C. Masquelier and P. Simon, J. Electrochem. Soc., 2011, 158, A1090 CrossRef CAS.
- C. Liu, E. I. Gillette, X. Chen, A. J. Pearse, A. C. Kozen, M. A. Schroeder, K. E. Gregorczyk, S. B. Lee and G. W. Rubloff, Nat. Nanotechnol., 2014, 9, 1031–1039 CrossRef CAS.
- Y. Ren, F. Meng, S. Zhang, B. Ping, H. Li, B. Yin and T. Ma, Carbon Energy, 2022, 4, 446–457 CrossRef CAS.
- Y. Xu, Q. Ding, L. Li, Z. Xie and G. Jiang, New J. Chem., 2018, 42, 20069–20073 RSC.
- R. Tatara, P. Karayaylali, Y. Yu, Y. Zhang, L. Giordano, F. Maglia, R. Jung, J. P. Schmidt, I. Lund and Y. Shao-Horn, J. Electrochem. Soc., 2019, 166, A5090–A5098 CrossRef CAS.
- Z. Li, J. Yu, C. Shi, H. Su, L. Bai and L. Yan, Appl. Surf. Sci., 2023, 640, 158320 CrossRef CAS.
- C. Xu, J. Liao, C. Yang, R. Wang, D. Wu, P. Zou, Z. Lin, B. Li, F. Kang and C. Wong, Nano Energy, 2016, 30, 900–908 CrossRef CAS.
- X. Wang, M. Li, Y. Wang, B. Chen, Y. Zhu and Y. Wu, J. Mater. Chem. A, 2015, 3, 8280–8283 RSC.
- J. Li, Y. Ding, S. Zhang, H. Li, B. Yin and T. Ma, J. Power Sources, 2022, 524, 231074 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr02011a |
| This journal is © The Royal Society of Chemistry 2024 |