High-load Mg2Ni nanoparticle-carbon nanofiber composites for hydrogen storage†
Eduardo David
Ruiz-Santacruz
a,
José de Jesús
Vega-Soria
a,
Aura Karina
Cruz-Jiménez
a,
Uriel
Caudillo-Flores
b,
Nidia Libia
Torres-García
a and
Karina
Suárez-Alcántara
 *a
*a
aMorelia Unit of Materials Institute Research, National Autonomous University of Mexico, Antigua Carretera a Pátzcuaro No. 8701, Col. Ex Hacienda de San José de la Huerta, Morelia CP 58190, Mexico. E-mail: karina_suarez@materiales.unam.mx
bCentro de Nanociencias y Nanotecnología, Universidad Nacional Autónoma de México, Ensenada, B.C. CP 22860, Mexico
First published on 10th September 2024
Abstract
Herein, a simple synthesis method for Mg2Ni composites with carbon nanofibers capable of hydrogen storage is presented. Specifically, n-butyl-sec-butyl-magnesium solution in hexane (C8H18Mg, 0.7 M) and bis-cyclopentadienyl nickel(II) (nickelocene or NiCp2) were used as precursors for the Mg2Ni nanoparticles. Subsequently, the nanoparticles were composited with carbon nanofibers (CNF) with high loading of Mg2Ni of 50 wt%, 75 wt%, 90 wt%, and 100 wt%. The physicochemical characterization of the materials indicated that the size of the as-prepared Mg2Ni nanoparticles was less than 5 nm and they were highly agglomerated due to a carbon-based binder. The best hydrogen storage values were determined to be 2.6–2.7 wt%. Among the tested materials, the composite with 75 wt% of Mg2Ni in CNF presented the best hydrogen uptake. The pressure–composition–temperature curves indicated changes in the hydriding equilibrium pressures of the Mg2Ni nanoparticles compared to the material with a similar composition produced using ball-milling and thermodynamic calculations. Thus, the results presented herein indicate the beneficial effect of nanosizing on hydriding reactions.
1. Introduction
Hydrogen storage methods are necessary for the use of hydrogen as a fuel or reagent in different applications. Today, hydrogen storage as a cryogenic liquid and pressurized gas can be considered a mature but inefficient method of storage. Ultimately, hydrogen storage in solid materials is highly desirable. MgH2 has long been studied for hydrogen storage because of its high hydrogen storage capacity, low cost, and low toxicity.1,2 However, the two major problems associated with MgH2, namely its low dehydriding kinetics and high dehydriding temperature, prevent its widespread use. Thus, Ni, Mg2Ni, or Mg2NiH4 has been used as catalysts together with Mg or MgH2 processed using different techniques.3–7 The addition of Ni to Mg or MgH2 frequently leads to the partial or total formation of Mg2Ni or its hydride, Mg2NiH4.8 Alternatively, Mg2Ni/Mg2NiH4 is a possible candidate for hydrogen storage in a solid material.9–11Recently, the formation of Mg-nanoparticles, nanoconfinement, or nanomodification has been proposed as strategies to surpass the kinetic and thermodynamic limitations of bulk Mg.12–15 In theory, reducing the particle size can lower the dehydrogenation temperature and significantly increase the kinetics. The particle size threshold for Mg has been reported to be as low as 1.3 nm.13 Employing the same approach, the formation of Mg2Ni/Mg2NiH4 nanoparticles can be of interest. However, the number of published papers on this subject is limited. Thus, this work presents the synthesis and characterization of Mg2Ni nanoparticles composited with a carbon material. The selected synthesis method is a bottom-to-top method using organometallic precursors, and the results are compared to a “classic” material prepared via the mechanical milling of elements.
M. Fichtner proposed that the reversibility of hydriding/dehydriding reactions in nanoparticles of complex hydrides may be reduced if the nanoparticles are free-standing on a surface and enhanced if they are enclosed.16 Thus, the compositing of materials plays an important role in the fabrication of nanomaterials for hydrogen storage. Hydrogen storage nanoparticles have been mixed with several carbon materials such as activated carbon, graphite, carbon aerogel scaffolds, carbon aerogel microspheres, mesoporous carbon, graphene, carbon nanospheres, a variety of carbon nanotubes, and carbon nanofibers.17–25 Thus, carbon nanofibers (CNF) can be an interesting compositing material, given that they are cheaper than other nanosized carbons and have a high surface area. Herein, we present the use of carbon nanofibers as a compositing material for Mg2Ni nanoparticles. Alternatively, the loading of hydrogen storage nanoparticles in carbon materials is typically low (about 10–20 wt%).26,27 This translates into a reduction in the hydrogen storage capacity due to the weight of the compositing material. Thus, studies on hydrogen storage nanomaterials with a high loading of active materials are necessary. In the present work, we used a variation of the synthetic method of Zlotea et al.26 with high loads of Mg2Ni in carbon nanofibers of 50 wt%, 75, wt.% and 90 wt%; and 100 wt% Mg2Ni nanoparticles (i.e. no addition of CNF) for comparison.
2. Experimental
2.1. Preparation of materials
Carbon nanofibers (CNF) were used as the composite material for Mg2Ni, which were purchased from Sigma-Aldrich with the characteristics of graphitized, iron-free, 100 nm in diameter, and 20–200 μm long. The CNFs were degassed at 120 °C for 4 h under vacuum in a Schlenk line with a liquid-N2 cold-finger trap before use. n-Butyl-sec-butyl-magnesium solution in hexane (C8H18Mg, 0.7 M) and bis-cyclopentadienyl nickel(II) (NiCp2 hereafter) were purchased from Sigma-Aldrich and used without further purification or treatment. Anhydrous THF was added as a co-solvent in a volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of hexane and THF. C8H18Mg (0.7 M in hexane), NiCp2, CNF, and THF were added in stoichiometric quantities to form Mg2Ni in a concentration of 50 wt%, 75 wt%, and 90 wt% in CNF, and 100 wt%. In the first step, NiCp2 was dissolved in THF in a reaction glass flask. The reaction glass flask already contained the appropriate amount of carbon nanofibers. After a few minutes of agitation, C8H18Mg (0.7 M in hexane reagent) was added with a syringe. The C8H18Mg (0.7 M in hexane), NiCp2, CNF, and THF in the glass flask were mixed by magnetic agitation for 24 h. Storage of the precursors, handling, and mixing were performed in a glovebox filled with high-purity argon with less than 5 ppm of oxygen and moisture. After mixing for 24 h, the glass flask was moved out of the glovebox and attached to a Schlenk line. Then, the material was heated to 80 °C for the coarse elimination of the solvents upon forming a sludge. Then, the mixture was heated to 140 °C for complete elimination of the solvent. The process of drying was performed under vacuum in a Schlenk line with a liquid-N2 cold-finger trap for 6 h. The drying eliminated the solvents and started the decomposition of both organometallic precursors to generate Mg2Ni nanoparticles. Then, the dried materials were recovered and stored in a glovebox until further hydriding and characterization.
1 of hexane and THF. C8H18Mg (0.7 M in hexane), NiCp2, CNF, and THF were added in stoichiometric quantities to form Mg2Ni in a concentration of 50 wt%, 75 wt%, and 90 wt% in CNF, and 100 wt%. In the first step, NiCp2 was dissolved in THF in a reaction glass flask. The reaction glass flask already contained the appropriate amount of carbon nanofibers. After a few minutes of agitation, C8H18Mg (0.7 M in hexane reagent) was added with a syringe. The C8H18Mg (0.7 M in hexane), NiCp2, CNF, and THF in the glass flask were mixed by magnetic agitation for 24 h. Storage of the precursors, handling, and mixing were performed in a glovebox filled with high-purity argon with less than 5 ppm of oxygen and moisture. After mixing for 24 h, the glass flask was moved out of the glovebox and attached to a Schlenk line. Then, the material was heated to 80 °C for the coarse elimination of the solvents upon forming a sludge. Then, the mixture was heated to 140 °C for complete elimination of the solvent. The process of drying was performed under vacuum in a Schlenk line with a liquid-N2 cold-finger trap for 6 h. The drying eliminated the solvents and started the decomposition of both organometallic precursors to generate Mg2Ni nanoparticles. Then, the dried materials were recovered and stored in a glovebox until further hydriding and characterization.
A material containing 75 wt% of Mg2Ni and 25 wt% of carbon nanofibers was prepared by ball milling as a reference. Stoichiometric quantities of Mg, Ni (Alfa-Aesar, 325 mesh, 99.86 purity), and CNF powders were loaded in a milling vial together with 6 balls with a diameter of 1 cm. The milling balls were made of yttria-stabilized zirconia and the milling vial with a volume of 50 mL was made of stainless steel. The total milling time was 20 h and the milling batch was 1 g. The milling was performed in a Retsch® mill working in room-temperature mode at an agitation rate of 25 Hz. Then, the ball-milled precursors were annealed at 400 °C for 1 h under high vacuum.9,28 The ball-milling/annealing preparation of Mg2Ni is well-known. However, the formation of Mg2Ni just by ball-milling is rare, while the formation of Mg2Ni is easy by applying heating under vacuum or cycling in the hydrogen of the milled precursors. The milling and annealed material was characterized, tested, and presented as needed for comparison.
2.2. Pressure–composition–temperature curves (PCT)
PCT curves were recorded in a Quantachrome iSorb-100 machine. A sample of about 0.3 g of as-dried materials was transferred to the machine without air contact utilizing a sample holder with an isolation valve. The as-dried materials were heated at 260 °C under dynamic vacuum. These conditions were maintained for 4 h to eliminate the organic fraction of the precursors. After cooling under vacuum, the calibration of void volume was performed with ultrahigh-purity helium. The PCT curves were recorded with a progressive increase (in steps) in the hydrogen pressure from 0.01 to 15 bar, followed by a progressive decrease (in steps) in the hydrogen pressure from 15 to 0.01 bar. PCT curves must be recorded under equilibrium conditions, which means long-term experiments. The equilibrium condition was assumed when the pressure presented a variation smaller than 0.1 × 10−3 bar during 30 min or a maximum time of 240 min for each step. Reaching equilibrium directed the change to the next step. The equilibrium condition criteria directed the total time employed at each experiment, normally between 2 and 4 days per curve. The experiments were performed at 100 °C, 200 °C, and 300 °C. Each experiment was performed with fresh samples of the same batch. The hydrogen used during experiments was of chromatographic purity. All the reported pressures correspond to the absolute pressure scale, with 0.8 bar the average atmospheric pressure in our location.2.3. Characterization of materials
All the as-dried (as-prepared) and PCT-tested (hydrided) materials were characterized using the following techniques.X-ray diffraction (XRD) characterization was performed in a Bruker D2 Phaser diffractometer. Cu Kα (1.540598 Å) wavelength was used. The powders were compacted in a dedicated sample holder; then, they were covered with Kapton® tape for protection against ambient oxygen and moisture. Crystalline phase identification was performed with the help of the Maud software and crystallographic databases including Inorganic Crystal Structure Database Karlsruhe (ICSD) and Crystallography Open Database (COD).
Scanning electron microscopy (SEM) images of selected samples were collected in a JSM-IT300 microscope. The samples were dispersed on carbon tape inside an argon-filled glovebox. Then, they were transferred to the SEM chamber reducing the oxygen contact using a glove bag. However, brief air exposure occurred during the transfer to the vacuum chamber of the SEM apparatus. SEM images were obtained with secondary or backscattered electrons at an acceleration voltage of 10 and 20 kV. Elemental mapping was performed by an SDD X-MaxN EDS detector, Oxford Instruments attached to the microscope with an energy resolution of 127 eV.
Transmission electron microscopy (TEM) images were collected on a JEOL ARM-200F, Schottky-type field emission transmission electron microscope with atomic resolution, equipped with a spherical aberration corrector (Cs) in STEM mode. A few milligrams of each studied material were dispersed in THF by ultrasonic agitation. The samples and THF were confined in small vials and sealed in argon inside a glovebox. Then, the sealed vials were taken out of the glovebox and mixed in an ultrasonic bath for 10 min. Then, the vials were returned to the glovebox, and the slurries were deposited dropwise on dedicated copper grids and left to dry at room temperature. The materials on the grid were transported to the transmission electron microscope in vials sealed in argon. The materials were briefly exposed to air during transfer to the TEM apparatus. TEM images were taken in both dark-field and bright-field modes.
Fourier-transformed infrared spectroscopy (FTIR) was performed on a Thermo Scientific Nicolet iS10 FTIR spectrometer using an attenuated total reflectance (ATR) accessory with a diamond crystal. About 2 mg of materials was mixed with 50 mg of dry KBr in an agate mortar and compacted in a dedicated press. The KBr was dried in a vacuum oven at 120 °C for 2 h, and then quickly transferred to a glovebox. The mixing and compaction of the materials were performed inside the glovebox. However, the materials were briefly exposed to air during data collection. The presented spectra are an average of 32 spectra collected for each sample in the range of 4000–650 cm−1 at a spectral resolution of 1 cm−1.
X-ray photoelectron spectroscopy (XPS) analysis was performed to determine the chemical state of the studied materials. All measurements were performed in a SPECS spectrometer with a PHOIBOS® 150 WAL hemispherical energy analyzer with angular resolution (<0.5°), equipped with an XR 50 Al-X-ray and μ-FOCUS 500 X-ray monochromator (Al excitation line) sources. To this end, the materials were transported to the XPS in sealed argon-filled vials. The samples were degassed at 10−5 mbar in the pretreatment chamber before being transferred to the analysis chamber. The measurement chamber had a residual pressure of less than 5 × 10−9 mbar during the measurements. Data was processed with the CasaXPS software and the background was modeled as Shirley-type.
3. Results and discussion
3.1. Preparation and characterization of as-prepared materials
NiCp2 can easily decompose in the presence of hydrogen.29,30 However, it cannot decompose below 200 °C in the absence of hydrogen.31 C. Peng et al. described a change in the color of the NiCp2 from green to orange due to the formation of intermediates during hydrogenolysis, generating Ni nanoparticles.29 This change was observed after 7–8 h at 70 °C and hydrogen injection.29 In our case, a change in color from green to red and slight heating above room temperature were noticed upon the addition of C8H18Mg 0.7 M/hexane to the NiCp2/CNF/THF solution in an argon atmosphere, i.e. in the absence of hydrogen. This color change was completed within a few minutes. The color change can be associated with the partial decomposition of the precursors. Heating at 80 °C coarsely eliminated the solvents, forming a black sludge, while complete removal of the solvents occurred at 140 °C. However, as presented in the next sections, a carbon-based material remained in the as-prepared materials. Characterization of the as-prepared materials was performed in this stage.Further heating to 260 °C under high vacuum was performed just before the PCT experiments to eliminate the organic part of the precursors. This step was essential for hydrogen storage, given that preliminary experiments without heating under vacuum resulted in poor hydrogen storage. The proposed complete reaction for the formation of Mg2Ni is as follows:
| 2C8H18Mg + NiCp2 → Mg2Ni + nCxHy, | (1) |
Other reports indicate the direct formation of hydrided nanoparticles of Mg, Mg–Ni, or Mg–Fe when the heating of organometallic precursors in a hydrogen atmosphere is performed.26 However, this method is more complicated because of the use of a pressurized gas reacting with a liquid solution.
3.2. Morphology and characterization of as-prepared materials
Fig. 1 presents selected SEM images of the as-prepared Mg2Ni-CNF materials, i.e. after solvent removal. Fig. S1 to S10 in the ESI† present additional SEM images of the as-received CNF, as-prepared (50% to 100% Mg2Ni), and milled materials. In general, the as-prepared Mg2Ni-CNF materials present high agglomeration. Fig. 1(a) presents the as-prepared 50%Mg2Ni-CNF, where the high proportion of CNF is evident by the presence of long fibers in this material. The carbon nanofibers are more visible at the edges of big broken blocks of materials. In comparison with the other materials (Fig. 1(b)–(e) and Fig. S4–S10†), a continuous decrease in the quantity of CNF and agglomeration can be observed. Fig. 1(b) presents the as-prepared 75%Mg2Ni-CNF, where the low-magnification image reveals huge blocks of agglomerated materials, which is consistent with the breaking induced by the drying of a sludgy material. This is a common characteristic of all the materials after solvent removal at 140 °C. Fig. 1(c) presents the image of the as-prepared 90%Mg2Ni-CNF, which confirmed the decrease in the content of carbon nanofibers. Fig. 1(d) presents the image of the as-prepared 100% Mg2Ni, where the size of the agglomerates in this material is smaller than the other materials. According to the SEM images (Fig. 1 and ESI S1–S10†), we can correlate the reduction in the agglomerate size with a reduction in CNF content. The reason for these differences can be attributed to the affinity of carbon nanofibers to hexane, which affected the drying of the materials. In our preliminary experiments, we only used the C8H18Mg 0.7 M in hexane reagent with NiCp2 and CNF, but the drying was almost impossible. When we added THF as a co-solvent, the drying was effective. We also observed that with a lower content of CNF, the drying was easier, and a general deduction of agglomerate size was obtained. Fig. 1(e) and Fig. S9 and S10† present the SEM images of the ball-milled Mg2Ni with CNF after annealing. The morphology of the particles indicates a typical ball-milled material; however, the absence of long carbon nanofibers is notable. We explain the absence of long CNF as the result of cutting during ball milling and integration of the short CNF with the metallic matrix in the composite. Carbon materials usually act as lubricants in ball-milling processes, but the conditions used here during the ball-milling process can be considered highly energetic, resulting in the shortening of the carbon nanofibers. Also, the bright spots (corresponding with Ni) in these images indicate that despite the annealing process, a small segregation of Mg, Ni, and Mg–Ni zones still existed. Finally, SEM-EDS mapping (ESI, Fig. S11 to S15†) demonstrated homogeneous materials, i.e. no segregation of precursor, and strong presence of a carbon-based material.Fig. 2 presents a series of TEM images of the as-prepared samples. Fig. S16 to S26 (ESI†) show the TEM images at different magnifications of as-received CNF, as-prepared materials, and particle size distributions. The image of the as-received CNF (Fig. S16, ESI†) is consistent with the characteristics reported by the supplier, i.e. 100 nm in diameter, 20–200 μm long, and a conical assembly of graphitic platelets. In Fig. S16,† it can be noticed that the center of the as-received CNFs is empty and their ends are closed. Fig. 2(a)–(d) demonstrated that independently of the Mg2Ni load, the as-prepared materials were in the form of nanoparticles. Q. Peng et al. suggested that the hydrogenolysis of NiCp2 produced finer Ni-nanoparticles than other forms of Ni-nanoparticle production.29 After dispersion in THF for TEM sample preparation, the big agglomerates observed in SEM were destroyed and the nanoparticles were observed to be distributed in agglomerates (Fig. S17 and S20†) or on the surface of the CNF (Fig. S21 and S23†). The most frequent nanoparticle diameters are 3.7 nm, 2.3 nm, 4.0 nm, and 3.1 nm for 50%Mg2Ni-CNF, 75%Mg2Ni-CNF, 90%Mg2Ni-CNF, and 100%Mg2Ni, respectively. The size distributions are presented in Fig. S18, S22, S24, and S26 of the ESI,† respectively. We can remark that the synthesis route is simple, without the need for special equipment, and even in the absence of a hydrogen atmosphere during the preparation of the materials, the precursors reacted, and the produced nanoparticles are small and homogenous. Additionally, according to the comparison of the TEM images (Fig. S25†) and the characterization presented in the next sections, we conclude that the nanoparticles are surrounded by an amorphous carbon-based material produced by the partial release and decomposition of the organic part of the precursors. The formation of a carbon layer was also observed by other researchers when using organic precursors and solvents. For example, X. Lu et al. produced Mg2Ni nanoparticles with a size of 300–500 nm by the wet chemical ball milling of the Mg and Ni elements and a mixture of organic agents and solvents.32 Carbon layers may help decrease the unwanted oxidation and sintering of nanoparticles, but also reduce the interaction of hydrogen with the nanoparticles.
 | ||
| Fig. 2 TEM images of (a) as-prepared 50%Mg2Ni-CNF, (b) as-prepared 75%Mg2Ni-CNF, (c) as-prepared 90%Mg2Ni-CNF and (d) as-prepared 100% Mg2Ni. | ||
The XRD patterns of the as-prepared materials are presented in Fig. 3. All diffractograms present the diffraction of the Kapton tape routinely used to protect the samples from unwanted oxidation. Kapton produces three amorphous peaks in the 2θ range of 15° to 25° and a strong background that extends up to 34°. From the bottom to top, the first frame (Fig. 3(a)) presents the X-ray diffraction pattern of the as-received carbon nanofibers. The CNF material presented diffraction peaks consistent with graphite but with changes in the expected peak intensity due to the alignment of the graphitic platelets. The most intense peak is located at the 2θ value of 26.23°, corresponding to (002). The other CNF diffraction peaks are much less intense than expected. The graphite peak positions are shown in Fig. 3 as green lines. The second frame (Fig. 3(b)) presents the pattern of 75%Mg2Ni-CNF-MM produced by ball-milling and annealing. All the diffraction peaks correspond to Mg2Ni. Fig. S27 of the ESI† presents the tracking of the ball-milling time of Mg and Ni. Long milling times resulted in the amorphization of Mg and Ni. However, the formation of Mg2Ni only appeared after annealing,9 indicating the importance of this process for the formation of the intermetallic material. The most intense peak of CNF is very low in the ball-milled material, indicating a decrease in the size of the carbon nanofibers, which is consistent with the SEM images. The rest of the frames (Fig. 3(c)–(f)) present the diffraction of the Mg2Ni nanoparticles from the wet-synthesis with a decrease in the content of CNF. The intensity of the (002) peak progressively decreased. In the case of the 50 wt%, 75 wt%, and 90 wt% content of Mg2Ni from the wet synthesis, no indications of the formation of crystalline micro-sized materials can be observed. Thus, we conclude that all the formed Mg2Ni are nanoparticles for the series of materials with carbon nanofibers. For the Mg2Ni without CNF (100%Mg2Ni), a series of peaks indicate the presence of some unreacted NiCp2 precursor. Additionally, the 100%Mg2Ni material also presents an unknown peak at the 2θ value of 10.94°, which is probably related to the decomposition of an intermediate.
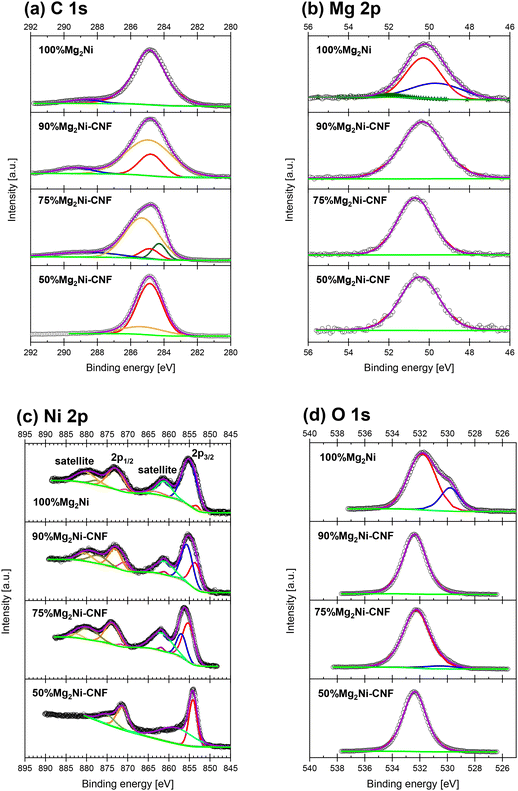
Fig. 4 presents the experimental XPS and deconvoluted data at the C 1s, Mg 2p, Ni 2p, and O 1s edges. Fig. 4(a) presents the C 1s edge, where the four composites present the characteristic graphite (C–C) peak of C at 284.8 eV (red lines in Fig. 4(a)).33 In the case of the 50%Mg2Ni-CNF material, the graphite peak is dominant in the XPS spectrum. However, another component of the materials is located at 285.3 eV (dark yellow peaks), which is attributed to C–H.33 The C–H peak presented a small shift to 285.0 eV in the 75%Mg2Ni-CNF material and 284.9 eV in the 90%Mg2Ni-CNF material. Interestingly, this peak was not present on the surface of the 100%Mg2Ni material. Meanwhile, the 75%Mg2Ni-CNF presented an additional peak (dark green) located at 284.3 eV, which can be related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.33 Finally, the 75%Mg2Ni-CNF, 90%Mg2Ni-CNF, and 100%Mg2Ni materials present a small peak (blue peaks) that can be interpreted as C–O interactions at about 290–288 eV.
C.33 Finally, the 75%Mg2Ni-CNF, 90%Mg2Ni-CNF, and 100%Mg2Ni materials present a small peak (blue peaks) that can be interpreted as C–O interactions at about 290–288 eV.
Mg and its alloys easily oxidize in the presence of even minor quantities of oxygen. The formed oxide can reduce the kinetics and hydrogen uptake. In Mg and its alloys, the presence of oxides is evident by a prominent peak at 51.2 eV.34 The metallic Mg 2p XPS signal is expected at lower energies (49.6 eV (ref. 34 and 35)). At the oxidized surface of Mg, the XPS data was deconvoluted clearly into two or more peaks. Alternatively, the Mg 2p XPS signal of the materials with CNF was deconvoluted in a single peak. However, we did not discard the presence of a small amount of oxide because oxygen is practically omnipresent. In the Mg 2p XPS data of the Mg2Ni-CNF materials, the main peak values are 50.5 eV, 50.7 eV, 50.3 eV, and 50.3 eV for 50%Mg2Ni-CNF, 75%Mg2Ni-CNF, 90%Mg2Ni-CNF, and 100%Mg2Ni, respectively. These values indicate that there is no particular trend with respect to the CNF content. These peaks can be related to the presence of a partially reduced Mg precursor. Accordingly, 100%Mg2Ni was better deconvoluted into three peaks. In addition to the peak at 50.3 eV, the binding energy of the second peak is located at 49.6 eV (blue line), indicating the presence of metallic Mg. The third peak is located at 51.8 eV and is related to the formation of a small quantity of surface oxides of Mg (dark-green peak near the baseline in light green). Together with the presence of unreacted and decomposed intermediates of NiCp2 in the XRD characterization, the XPS data indicate that the Mg-organometallic precursor can be reduced to some degree even if NiCp2 is not.
Fig. 4(c) presents the Ni 2p XPS signal of the studied composites. The Ni 2p edge is composed of Ni 2p3/2 (major intensity), Ni 2p1/2, and their satellite signals. The positions and intensities of the main peaks and satellites depend on the oxidation state and geometry of the Ni compounds.36 The satellites associated with the 2p of transition metal compounds are interpreted as the excitation of a metal 3d electron into an unoccupied metal orbital concurrently with the emitted photoelectron.36,37 The intensity of satellites is dependent on the probability of the material being excited during the photoemission process.38 The Ni 2p3/2 of metallic Ni is expected at 852.6 eV, while NiO and Ni(OH)2 show the main peaks at 853–853.5 eV and 856.5 eV, respectively.35 NiO presents a clear splitting of the Ni 2p peaks,39 while the Ni(OH)2 peak presents a similar shape to the metallic Ni, but is wider.35,40 The XPS spectra in Fig. 4(c) are shifted from the expected metallic value to that for Ni2+. The Ni 2p peaks are located between the values of the NiO and Ni(OH)2.41 However, the peaks evolved with a decrease in the CNF content and presented other characteristics that discard the massive unwanted oxidation or contamination with the formation of NiO and Ni(OH)2. The red deconvoluted peak component of Ni 2p3/2 appeared at 854.1 eV, 855.3 eV, 853.5 eV and 853.2 eV for 50%Mg2Ni-CNF, 75%Mg2Ni-CNF, 90%Mg2Ni-CNF, and 100%Mg2Ni, respectively. Additionally, the peak intensity progressively decreased to a small peak in the 100%Mg2Ni material. Conversely, a dark-blue peak appeared in 75%Mg2Ni-CNF, and also shifted to a lower binding energy in the 90%Mg2Ni-CNF and 100%Mg2Ni materials. This peak appeared at 856.9 eV, 855.6 eV, and 855.0 eV for 75%Mg2Ni-CNF, 90%Mg2Ni-CNF, and 100%Mg2Ni, respectively. Thus, the peak position and intensity evolution can be interpreted as the partial formation of nanoparticles in this stage of the synthesis and the presence of unreacted or partially reacted NiCp2. To confirm this, the O 1s XPS spectra of the materials are presented in Fig. 4(d). This series of O 1s XPS spectra is dominated by a peak at around 531.8 eV to 532.4 eV (red or purple for a single peak deconvolution), corresponding to different carbon–oxygen interactions.42,43 Another peak is located at 529.8–530.5 eV (blue peaks in Fig. 4(d)), which can be associated with the formation of surface magnesium oxides. Despite this peak being prominent in the O 1s (Fig. 4(d)) for 100%Mg2Ni, the corresponding information at Mg 2p (Fig. 4(b)) indicates the formation of a small amount of oxides.
Additionally, the infrared spectroscopy characterization of the as-prepared nanosized materials is presented in Fig. S28 in the ESI.† For comparison, the infrared characterization of the as-received NiCp2 is presented in Fig. S28(a).† NiCp2 presented the characteristic peaks,44 with the most intense peaks observed at 768 cm−1 and 1001 cm−1. The C–H stretching bands of n-butyl-sec-butyl-Mg are expected at 2800–3300 cm−1. In the case of CNF, it presents weak peaks at around 2000–2300 cm−1 due to its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.45–48 The as-prepared nanosized materials (Fig. S28(b) to (e)†) present a progressive increase in the relative intensity of the most intense peaks of NiCp2, which is in agreement with the increase in concentration but somehow deformed. In parallel, Fig. S28(b) to (e)† present a decrease in the intensity of some minor peaks such as the peak at 1422 cm−1, and the progressive change in the position of the peak at 3082 cm−1, which is attributed to the C–H bonds, ending as a complex peak at 2800–2900 cm−1. The described changes are consistent with the partial decomposition of NiCp2 and n-butyl-sec-butyl-Mg during drying at 140 °C. The relative intensity of the remaining peaks indicates the progressive difficulty in the formation of Mg2Ni with an increase in the load of the precursors, NiCp2 and n-butyl-sec-butyl-Mg, and a decrease in the load of carbon nanofibers.
C bonds.45–48 The as-prepared nanosized materials (Fig. S28(b) to (e)†) present a progressive increase in the relative intensity of the most intense peaks of NiCp2, which is in agreement with the increase in concentration but somehow deformed. In parallel, Fig. S28(b) to (e)† present a decrease in the intensity of some minor peaks such as the peak at 1422 cm−1, and the progressive change in the position of the peak at 3082 cm−1, which is attributed to the C–H bonds, ending as a complex peak at 2800–2900 cm−1. The described changes are consistent with the partial decomposition of NiCp2 and n-butyl-sec-butyl-Mg during drying at 140 °C. The relative intensity of the remaining peaks indicates the progressive difficulty in the formation of Mg2Ni with an increase in the load of the precursors, NiCp2 and n-butyl-sec-butyl-Mg, and a decrease in the load of carbon nanofibers.
3.3. Hydriding and dehydriding reactions
As mentioned in the Experimental section, the materials were heated at 260 °C under dynamic vacuum for 4 h to eliminate most of the organic fraction of the precursors before the PCT experiments. This process led to a notable reduction in the weight of the samples, with an average reduction of 30% of the initial weight. The hydrogen uptake values presented below were calculated based on the weight after heating at 260 °C. Fig. 5 shows a comparison of the PCT curves of the Mg2Ni nanoparticles supported in CNF and the ball-milled Mg2Ni-CNF. The expected maximum hydrogen uptake is 1.8 wt%, 2.7 wt%, 3.2 wt%, and 3.6 wt% for 50%Mg2Ni-CNF, 75%Mg2Ni-CNF, 90%Mg2Ni-CNF and 100%Mg2Ni, respectively. The observed hydrogen uptake at 300 °C was 1.7 wt%, 2.6 wt%, 2.1 wt%, and 2.7 wt% for 50%Mg2Ni-CNF, 75%Mg2Ni-CNF, 90%Mg2Ni-CNF and 100%Mg2Ni, respectively. This means a hydriding efficiency of 94.4%, 96.3%, and 65.6% for the materials with CNF, i.e., 50%Mg2Ni-CNF, 75%Mg2Ni-CNF, and 90%Mg2Ni-CNF, respectively. The hydriding efficiency for the material without CNF, 100%Mg2Ni, was 75%. An additional characteristic of the PCT curves is the dependency of the hydrogen uptake with the temperature for the 50%Mg2Ni-CNF and 75%Mg2Ni-CNF materials, as shown in Fig. 5(a) and (b), respectively. These two materials showed a progressive increase in hydrogen uptake with an increase in the test temperature, achieving the above-mentioned hydriding efficiency. In the case of the 90%Mg2Ni-CNF and 100%Mg2Ni materials (Fig. 5(c) and (d), respectively), their hydrogen uptake was notably lower than that expected at all temperatures. We attribute the incomplete hydriding to the following reasons: (i) the presence of carbon materials as the residue of the organic part of the precursors, which contributes extra weight to the samples and (ii) the high aggregation of particles, which prevents a good distribution of hydrogen to all the particles at progressively high loads of Mg2Ni. Regarding the 100%Mg2Ni material, the presence of unreacted NiCp2 can also be a factor reducing its hydrogen storage capacity because of the lack of Mg2Ni. The PCT curves in Fig. 5 indicate that among the tested materials, 75%Mg2Ni-CNF presents the best level of CNF content and hydrogen uptake. The PCT curve of the ball-milled (MM) 75%Mg2Ni-CNF material is presented in Fig. 5(e) for comparison, where it can be seen that the hydrogen uptake of the 75%Mg2Ni-CNF-MM material is lower than that of the nanosized 75%Mg2Ni-CNF. This is an improvement due to formation of Mg2Ni nanoparticles. | ||
| Fig. 5 Pressure–composition isotherms of (a) 50%Mg2Ni-CNF, (b) 75%Mg2Ni-CNF, (c) 90%Mg2Ni-CNF, (d) 100% Mg2Ni, and (e) 75%Mg2Ni-CNF-MM, ball milled and annealed under high vacuum. | ||
The shape of the PCT curves presented in Fig. 5 indicates the ease of hydriding and the difficulty of dehydriding reaction for the nanosized materials. The incomplete dehydriding on the nanosized materials indicates the need for a moderate-high vacuum, which was poorly managed in our Sieverts apparatus. The dehydriding reaction on the nanosized materials could be constrained by the stability of the nanoparticles gained upon hydride formation. Meanwhile, at the ball-milled 75%Mg2Ni-CNF, the equilibrium pressure at 300 °C is well defined for the hydride and dehydriding reactions. M. Fichtner proposed a dependency of the enthalpy of the hydriding reaction, and in turn the dependency of the equilibrium pressure on particle size.16 However, the parameters of the Fichtner equations are not yet defined experimentally.16 Nonetheless, the effect of particle size is relevant to the position of equilibrium pressure for hydriding/dehydriding reactions.
Fig. 5(a)–(c) demonstrate the low equilibrium hydriding pressure of the Mg2Ni nanoparticles. In the case of the 75%Mg2Ni-CNF material, the equilibrium pressure is about 0.09 bar, 0.6 bar, and 5.2 bar at 100 °C, 200 °C and 300 °C, respectively. Meanwhile, the ball-milled 75%Mg2Ni-CNF-MM material presented hydriding equilibrium pressures of 0.44 bar, 0.78 bar, and 2 bar at 100 °C, 200 °C, and 300 °C, respectively. The listed values indicate a notable reduction of the equilibrium pressure in the nanoparticles of Mg2Ni.
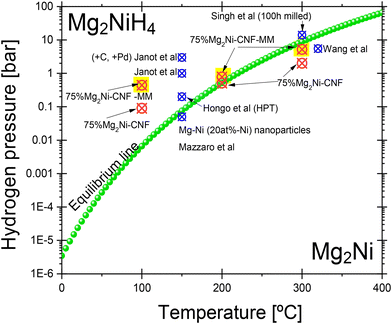
Several experimental conditions for the preparation of materials can affect the hydriding and dehydriding reactions. Thus, it is difficult to compare the “same materials” reported by different research groups. Nevertheless, we include published reports to compare nano- and micro-sized Mg2Ni. Alternatively, the best way to qualify experimental results is to compare the hydriding/dehydriding equilibrium pressures versus thermodynamic data. Fig. 6 presents a comparison of the results of equilibrium pressures of this work with other thermodynamic and experimental values. Thermodynamic equilibrium pressures can be calculated according to,49 as follows:
 | (2) |
 indicates the equilibrium pressure, ΔH0 is the standard formation enthalpy of Mg2NiH4 (64 kJ mol−1 H2−1
indicates the equilibrium pressure, ΔH0 is the standard formation enthalpy of Mg2NiH4 (64 kJ mol−1 H2−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), ΔS0 = 130 J K−1 mol−1 is the change in enthalpy of the gaseous hydrogen entering an ordered system (the solid-state material),49 and R and T have their usual meaning. With these values and the proper unit conversion to bar and °C, the expected equilibrium pressure is 6.6 × 10−3 bar, 0.52 bar, and 8.9 bar at 100 °C, 200 °C, and 300 °C, respectively. The equilibrium temperature for 1 bar hydrogen pressure is calculated as 220 °C. The green line in Fig. 6 indicates the equilibrium line between Mg2Ni and Mg2NiH4 calculated using eqn (2). The red dots indicate the experimental values obtained in this work for the nanosized 75%Mg2Ni-CNF; meanwhile, the red dots with a yellow background indicate the ball-milled 75%Mg2Ni-CNF-MM equilibrium pressures. Deviations in the experimental data of the equilibrium pressure from the expected thermodynamic values are more evident at low temperatures (100 °C).
50), ΔS0 = 130 J K−1 mol−1 is the change in enthalpy of the gaseous hydrogen entering an ordered system (the solid-state material),49 and R and T have their usual meaning. With these values and the proper unit conversion to bar and °C, the expected equilibrium pressure is 6.6 × 10−3 bar, 0.52 bar, and 8.9 bar at 100 °C, 200 °C, and 300 °C, respectively. The equilibrium temperature for 1 bar hydrogen pressure is calculated as 220 °C. The green line in Fig. 6 indicates the equilibrium line between Mg2Ni and Mg2NiH4 calculated using eqn (2). The red dots indicate the experimental values obtained in this work for the nanosized 75%Mg2Ni-CNF; meanwhile, the red dots with a yellow background indicate the ball-milled 75%Mg2Ni-CNF-MM equilibrium pressures. Deviations in the experimental data of the equilibrium pressure from the expected thermodynamic values are more evident at low temperatures (100 °C).
 | ||
| Fig. 6 Thermodynamic equilibrium line (green) for Mg2Ni/Mg2NiH4. Red dots: hydriding equilibrium pressure of the 75%Mg2Ni-CNF and 75%Mg2Ni-CNF-MM materials. Blue dots: hydriding4,11,52,53 or dehydriding51 equilibrium reported data. | ||
Despite the importance of Mg2Ni, relatively few PCT curves have been reported. Together with the available reports, the PCT curves present different equilibrium pressures. The blue dots in Fig. 6 show the reported equilibrium pressures and serve for discussion. A. K. Singh et al. reported the desorption PCT curve of Mg2NiH4 prepared by 100 h of ball milling of the elements and after the fifth cycle;51 the equilibrium pressure was not well defined in that report, but the middle point of the curve was located at about 14 bar, and 2 wt%. Y. Wang et al. reported the PCT curves of pure Mg2Ni ball milled with low quantities of multi-walled carbon nanotubes or graphite (1–5 wt%), whose hydriding equilibrium pressure is located at about 5.5 bar at 320 °C; they achieved 3.5 wt% hydrogen storage.52 No influence of the multi-walled carbon nanotubes or graphite on the PCT curves of Mg2Ni was reported in that work.52 R. Janot et al. demonstrated a hydriding equilibrium pressure of about 3 bar at 150 °C for ball-milled Mg2Ni/10 wt%C + 5 wt%Pd and about 1 bar for the material without the addition of C and Pd.11 They achieved 2.7 and 1.4 wt% for Mg2Ni/10 wt%C + 5 wt%Pd and Mg2Ni, respectively.11 T. Hongo et al.53 presented similar PCT curves to that in Fig. 5(a)–(d), i.e. not achieving full dehydriding of the materials. In that report, Mg2Ni was processed by high-pressure torsion (HPT) and the hydriding equilibrium pressure was located at about 0.2 bar at 150 °C (as read from the plots).53 Also,53 the hydrogen uptake was about 3.25 wt%. Additionally, the desorption branch of the PCT curve was not defined53 even at low pressures as 0.01 bar. In this case, dehydriding was achieved by heating under dynamic vacuum for 20 h at 150 °C.53 R. Mazzaro et al. reported the formation of Mg–Ni (20 at%–Ni) nanoparticles whose hydriding equilibrium pressure is located at about 0.05 bar at 150 °C.4 The reviewed reports4,11,51–53 present a great variety of preparation techniques and experimental measurement conditions; nevertheless, the nano-sized Mg2Ni developed in this work can achieve similar hydrogen uptake levels. The variation in the equilibrium pressures can be a result of the preparation techniques and additives, which produce changes in the structure (superficial or bulk) of the materials.
3.4. Morphology and characterization of hydrided materials
In general, the studied materials did not present a relevant dehydriding reaction during the PCT experiments, and thus the characterization of the hydrided materials was performed immediately after the PCT experiment at 300 °C. The exception was the ball-milled 75%Mg2Ni-CNF-MM material, which was subjected to further hydriding at 300 °C, 14 bar, and 2 h in the same Sieverts apparatus. The hydrided materials are hereafter identified by the addition of “PCT-300” to the original name.Fig. 7 presents the SEM images of the hydrided (PCT) Mg2Ni-CNF materials at 300 °C. In general, the materials presented a breaking-up of the initial agglomerates, confirming the presence of the CNF and the existence of a binder material that is assumed to be composed of carbon material as a residue of the organic part of the precursors. The 50%Mg2Ni-CNF PCT-300 material developed a net structure due to the high amount of CNF. Meanwhile, the 75%Mg2Ni-CNF PCT-300 material developed a partial net structure due to the reduction in the content of CNF. Both materials reached near full hydrogen storage according to the Mg2Ni content; however, a low load of hydrogen storage material is not recommended due to the low hydrogen uptake. In this case, the high-load materials, 90%Mg2Ni-CNF PCT-300 and 100%Mg2Ni-PCT-300, did not present the formation of net structures, which can justify the lower hydrogen uptake than that expected. The high-load materials maintained the presence of blocks of about 10–30 μm. Additional SEM images of the hydrided materials can be found in the ESI, Fig. S29 to S32.†
Fig. 8 presents a series of TEM images of the hydrided (PCT) nanosized materials at similar magnifications. Fig. S33 to S42 of the ESI† present more TEM images at different magnifications as a complement. Fig. 8(a) presents the TEM image of 50%Mg2Ni-CNF, where the nanoparticles are dispersed on the CNF surface. Meanwhile, the TEM image of 75%Mg2Ni-CNF (Fig. 8(b)) presents a dispersion of particles, which can be interpreted as being within the CNF. This is not the only image indicating this characteristic (see Fig. S33, S36, and S37 of the ESI†). Also, the TEM images of 90%Mg2Ni-CNF and 100%Mg2Ni (Fig. 8(c) and (d)) corroborated the presence of a material whose function is a binder of the nanoparticles of Mg2Ni and Mg2NiH4, respectively. It can be concluded that the Mg2NiH4 nanoparticles are distributed on the CNF surface, between the binder material, and a minor proportion inside the CNF (Fig. 8(b)).
The sintering of materials is a common feature of nanoparticles after heating under pressure. Accordingly, in the present materials after hydriding, the nanoparticles showed a change in size. The particle size distributions are presented in Fig. S35, S38 and S40.† In the case of the hydrided 50%Mg2Ni-CNF and 75%Mg2Ni-CNF, their particle size increased to 7.9 nm, and 4.4 nm, respectively (most frequent sizes). These numbers indicate an increase of almost 100%. The 90%Mg2Ni-CNF (Fig. S40†) material presented almost the same most frequent particle size of ∼4 nm. However, the image in Fig. S40† presented a high degree of uncertainty. 100%Mg2Ni (Fig. 8(d)) presented particle sizes of 20–50 nm. In the case of the hydrided 90%Mg2Ni-CNF and 100%Mg2Ni, the determination of particle size became more complex. We attributed the reduction and lack of CNF, respectively, as a factor reducing the ability to keep the nanoparticles fixed during their preparation for TEM imaging. The role played by the CNF can be established as a support, confinement, and spacer of particles. The other roles of carbon additives are hydrogen diffusers and heat-conducting materials, where both effects improve the hydrogen storage capabilities of hydrogen storage materials.17,54
Fig. 9 presents the X-ray diffraction characterization of the hydrided materials. From the bottom to top, Fig. 9(a) presents the ball-milled and hydrided Mg2Ni, which presents a combination of low- and high-temperature phases of Mg2NiH4 (all the peaks labeled as Mg2NiH4). Also, the presence of unreacted Ni was observed. Ni particles were observed in the SEM image in Fig. 1(e); however, only after heating in hydrogen, Ni was sintered and presented diffraction peaks. No indications of crystalline Mg or MgH2 were observed. The XRD pattern of the ball-milled and hydrided 75%Mg2Ni-CNF-MM was consistent with the partial hydriding observed in the PCT experiments. In the case of the nanosized and hydrided materials (Fig. 9(b)–(e)), an increase in the peak located at the 2θ value of 29.37° can be observed. In addition to this peak, the hydrided 100%Mg2Ni presented a series of other minor peaks. All these peaks are labeled “unknown” because they do not correspond to any of the multiple known phases of Mg2NiH4; however, these peaks are close to some of the expected diffraction peaks of the low-temperature phase of Mg2NiH4.55
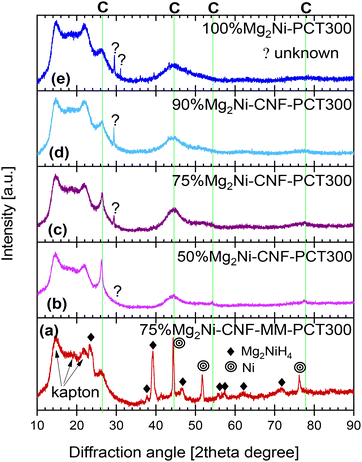 | ||
| Fig. 9 X-ray diffraction characterization of hydrided materials: (a) 75%Mg2Ni-CNF-MM-PCT300, (b) 50%Mg2Ni-CNF-PCT-300, (c) 75%Mg2Ni-CNF-PCT-300, (d) 90%Mg2Ni-CNF-PCT-300, and (e) 100%Mg2Ni-PCT-300. | ||
Fig. 10 presents the XPS signal of the PCT hydrided materials. Fig. 10(a) presents the C 1s edge, where in comparison with the as-prepared materials of Fig. 4(a), the PCT hydrided materials presented more peaks and complex fitting. The XPS of the hydrided materials present the common C–C peaks at 284.7–284.8 eV (red deconvoluted peaks). The additional peaks are as follows: (i) C–H at 285.4–285.7 eV (blue deconvoluted peaks).33 (ii) Small peaks at 281.7–283.1 eV in 75% to 100%Mg2Ni -PCT 300 materials, which can be ascribed to the presence of disordered (amorphous) C.56,57 (iii) The coexistence of small peaks at 290–288 eV, which can be interpreted as C–O interactions.33 Very interestingly, a small peak appeared at 283.9 eV (dark-red deconvoluted peak for the 75%Mg2Ni-CNF PCT-300 material), which can be related to C bonded with Ni in Ni3C.56,57
Fig. 10(b) presents the Mg 2p XPS spectra of the hydrided materials. The main peak (red or purple for accumulative single peak) is located at 50.7, 50.1, 50.4, and 50.2 eV for 50%Mg2Ni-CNF-PCT-300, 75%Mg2Ni-CNF-PCT-300, 90%Mg2Ni-CNF-PCT-300, and 100%Mg2Ni-PCT-300, respectively. Interestingly, the 75%Mg2Ni-CNF-PCT-300 material presented a clear shift to a lower binding energy compared to the as-prepared material (i.e. 50.7 eV) (Fig. 4(b) to 50.1 eV). This small shift can be related to the formation of Mg2NiH4. Also, the appearance of a peak at 49.8 eV is very interesting for the 100%Mg2Ni-PCT-300 material, which can be related to the presence of metallic Mg. The as-prepared material also presented this peak but with a higher relative intensity. The reduction in the relative intensity of the metallic Mg peak in the hydrided material can be explained by the formation of Mg2NiH4 upon heating in hydrogen, which consumed some of the Mg observed in the first stage of preparation of the particles. Additionally, 50%Mg2Ni-CNF-PCT-300 presented a prominent peak at 52.1 eV (orange deconvoluted peak), which shifted to a too high binding energy to be considered MgO. M. Ramachandran et al. reported a similar peak at 52.3 eV as Mg2C3; however, as mentioned by them, no XPS reference (standard) for Mg2C3 exists.58
Fig. 10(c) presents the Ni 2p XPS spectra of the hydrided materials. Similar to the as-prepared materials, the signal is dominated by the 2p3/2 peaks together with the presence of 2p1/2 and satellite peaks. Unlike the as-prepared materials, the hydrided materials stood out due to the presence of a new peak (red deconvoluted peak) located between 852.8 eV and 852.9 eV. The Ni main peak of the Mg2Ni was reported to be located at 852.6 eV.35 The small shift to higher binding energies can be related to the formation of the hydrided form of Mg2Ni. The XPS spectrum of 75%Mg2Ni-CNF-PCT-300 was not suitable for deconvolution but its characteristics indicate that its main peak is consistent with the formation of Mg2NiH4. The other deconvoluted peaks of the main 2p3/2 signal (orange peaks) are located at 856 eV, 855.5 eV, and 855.7 eV for 50%Mg2Ni-CNF-PCT-300, 90%Mg2Ni-CNF-PCT-300, and 100%Mg2Ni-PCT-300, respectively. The origin of these peaks can be related to the presence of unreacted NiCp2 or decomposition of the intermediates. Finally, Fig. 10(d) presents the O 1s peaks of the hydrided materials. All these spectra have in a common peak between 531.5–532.2 eV, corresponding to the usual surface oxygen. An additional peak at 532.5 eV improved the fitting of the 75%Mg2Ni-CNF-PCT-300 material. This peak can also be related to other surface oxygen species rather than the formation of metallic oxides. As a brief general conclusion of the characterization of the as-prepared and hydrided materials by XPS, this technique revealed the complexity of the intermediates and by-products, which are most probably amorphous or present in low concentration and can intervene in the decomposition of the precursors and the hydriding reactions. For example, several metal carbides have been used as catalysts of MgH2 and Mg2NiH4 in the field of hydrogen storage.59,60 Similar effects can occur in the materials reported here.
As a remark on the synthesis and characterization findings, we can mention that the formation of Mg2Ni nanoparticles supported in the CNF can be interesting for hydrogen storage. However, the principal observed limitation is the difficulty of dehydriding. Alternatively, the reversibility observed for the ball-milled 75%Mg2Ni-CNF-MM may be related to the presence of Ni and other species, which can work as a catalyst. Further research must be done to explore the use of catalysts and other supporting materials for improving dehydriding. Developing hydrogen storage materials must be optimized in future to enhance the performance of actual hydrogen storage methods.
4. Conclusions
The present work presented the formation of Mg2Ni nanoparticles mixed with carbon nanofibers, which were proved to be capable of hydrogen storage. The method for the synthesis of Mg2Ni nanoparticles is simple and does not require special equipment. Among the tested materials, 75%Mg2Ni-CNF presented the optimum level of CNF content and hydrogen uptake. Carbon nanofibers played the role as a support, confinement, and spacer of the particles. Also, a high load of nanoparticles in the CNF-supporting material was feasible. The size of Mg2Ni was determined to be about 5 nm, which was highly agglomerated due to the presence of the carbon-base material. This binder material originated from the partial decomposition of the organic part of the precursors. The binder protected the active material from unwanted oxidation but reduced the hydrogen uptake. Ni and metal carbides can act as catalysts for hydriding reactions. The results presented herein indicated the beneficial effect of nano-sizing on hydriding reactions but not in dehydriding. Further research must be done to explore the use of catalysts and other supporting materials.Author contributions
Experimentation: Eduardo David Ruiz-Santacruz, José de Jesús Vega-Soria, and Aura Karina Cruz-Jiménez. XPS data collection: Uriel Caudillo-Flores Supervision: Nidia Libia Torres-García. Conceptualization, methodology, and writing Karina Suárez-Alcántara.Data availability
Data for this article are available at https://osf.io/y2b8r/files/osfstorage.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
This research was funded by UNAM-DGAPA-PAPIIT [IN200122] Nano-confinamiento de materiales de almacenamiento de hidrógeno. All authors thank Dr Orlando Hernandez Cristobal for the SEM images and Dr Josue Romero for the TEM images. NLTG (#CVU: 631953) appreciates the support provided by CONAHCYT within Estancias Posdoctorales por Mexico 2022 (3) academic modality. U.C.-F. was funded by the PAPIIT DGAPA-UNAM projects: IN116424 and IN112922. U.C.-F. want to thank the expert technical assistance of D. Dominguez and P. Casillas.References
- J. Zhang, Z. Li, Y. Wu, X. Guo, J. Ye and B. Yuan, et al., Recent advances on the thermal destabilization of Mg-based hydrogen storage materials, RSC Adv., 2019, 9, 408–428, 10.1039/C8RA05596C.
- Q. Li, Y. Lu, Q. Luo, X. Yang, Y. Yang and J. Tan, et al., Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials, J. Magnesium Alloys, 2021, 9, 1922–1941, DOI:10.1016/j.jma.2021.10.002.
- S. Wang, M. Gao, Z. Yao, K. Xian, M. Wu and Y. Liu, et al., High-loading, ultrafine Ni nanoparticles dispersed on porous hollow carbon nanospheres for fast (de)hydrogenation kinetics of MgH2, J. Magnesium Alloys, 2022, 10, 3354–3366, DOI:10.1016/j.jma.2021.05.004.
- R. Mazzaro and L. Pasquini, Structure and hydrogen sorption properties of Mg-Mg2Ni nanoparticles prepared by gas phase condensation, J. Alloys Compd., 2022, 911, 165014, DOI:10.1016/j.jallcom.2022.165014.
- Y. Fu, Z. Ding, S. Ren, X. Li, S. Zhou and L. Zhang, et al., Effect of in situ formed Mg2Ni/Mg2NiH4 compounds on hydrogen storage performance of MgH2, Int. J. Hydrogen Energy, 2020, 45, 28154–28162, DOI:10.1016/j.ijhydene.2020.03.089.
- F. Guo, T. Zhang, L. Shi and L. Song, Hydrogen absorption/desorption cycling performance of Mg-based alloys with in situ formed Mg2Ni and LaHx (x = 2, 3) nanocrystallines, J. Magnesium Alloys, 2023, 11, 1180–1192, DOI:10.1016/j.jma.2021.06.013.
- L. Ren, W. Zhu, Q. Zhang, C. Lu, F. Sun and X. Lin, et al., MgH2 confinement in MOF-derived N-doped porous carbon nanofibers for enhanced hydrogen storage, Chem. Eng. J., 2022, 434, 134701, DOI:10.1016/j.cej.2022.134701.
- X. Ding, R. Chen, X. Chen, H. Fang, Q. Wang and Y. Su, et al., A novel method towards improving the hydrogen storage properties of hypoeutectic Mg-Ni alloy via ultrasonic treatment, J. Magnesium Alloys, 2023, 11, 903–915, DOI:10.1016/j.jma.2021.06.003.
- F. H. Matheus, G. Zepon, V. B. Oliveira and D. R. Leiva, Highly reactive hydrogen storage Mg2Ni alloy prepared by mechanochemistry and H-cycling, Int. J. Hydrogen Energy, 2024, 51, 320–328, DOI:10.1016/j.ijhydene.2023.04.295.
- J. Lyu, A. Lider and V. Kudiiarov, Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview, Metals, 2019, 9, 768, DOI:10.3390/met9070768.
- R. Janot, L. Aymard, A. Rougier, G. A. Nazri and J. M. Tarascon, Fast hydrogen sorption kinetics for ball-milled Mg2Ni alloys☆, J. Phys. Chem. Solids, 2004, 65, 529–534, DOI:10.1016/j.jpcs.2003.08.038.
- C. Zlotea, Y. Oumellal, S. J. Hwang, C. M. Ghimbeu, P. E. De Jongh and M. Latroche, Ultrasmall MgH2 Nanoparticles Embedded in an Ordered Microporous Carbon Exhibiting Rapid Hydrogen Sorption Kinetics, J. Phys. Chem. C, 2015, 119, 18091–18098, DOI:10.1021/acs.jpcc.5b05754.
- R. W. P. Wagemans, J. H. Van Lenthe, P. E. De Jongh, A. J. Van Dillen and K. P. De Jong, Hydrogen storage in magnesium clusters: Quantum chemical study, J. Am. Chem. Soc., 2005, 127, 16675–16680, DOI:10.1021/ja054569h.
- Y. Luo, Q. Wang, J. Li, F. Xu, L. Sun and Y. Zou, et al., Enhanced hydrogen storage/sensing of metal hydrides by nanomodification, Mater Today Nano, 2020, 9, 100071, DOI:10.1016/j.mtnano.2019.100071.
- S. Cheung, W.-Q. Deng, A. C. T. Van Duin and W. A. Goddard, ReaxFFMgH reactive force field for magnesium hydride systems, J. Phys. Chem. A, 2005, 109, 851–859 CrossRef CAS PubMed.
- M. Fichtner, Properties of nanoscale metal hydrides, Nanotechnology, 2009, 20, 0–4, DOI:10.1088/0957-4484/20/20/204009.
- M. Mohan, V. K. Sharma, E. A. Kumar and V. Gayathri, Hydrogen storage in carbon materials—A review, Energy Storage, 2019, 1, e35, DOI:10.1002/est2.35.
- S. Zhang, A. F. Gross, S. L. Van Atta, M. Lopez, P. Liu and C. C. Ahn, et al., The synthesis and hydrogen storage properties of a MgH2 incorporated carbon aerogel scaffold, Nanotechnology, 2009, 20, 204027, DOI:10.1088/0957-4484/20/20/204027.
- D. Peng, Z. Ding, L. Zhang, Y. Fu, J. Wang and Y. Li, et al., Remarkable hydrogen storage properties and mechanisms of the shell–core MgH2@carbon aerogel microspheres, Int. J. Hydrogen Energy, 2018, 43, 3731–3740, DOI:10.1016/j.ijhydene.2018.01.004.
- H. Cheng, G. Chen, Y. Zhang, Y. Zhu and L. Li, Boosting low-temperature de/re-hydrogenation performances of MgH2 with Pd-Ni bimetallic nanoparticles supported by mesoporous carbon, Int. J. Hydrogen Energy, 2019, 44, 10777–10787, DOI:10.1016/j.ijhydene.2019.02.218.
- Y. Huang, G. Xia, J. Chen, B. Zhang, Q. Li and X. Yu, One-step uniform growth of magnesium hydride nanoparticles on graphene, Prog. Nat. Sci.: Mater. Int., 2017, 27, 81–87, DOI:10.1016/j.pnsc.2016.12.015.
- J. Zhang, Y. Zhu, H. Lin, Y. Liu, Y. Zhang and S. Li, et al., Metal Hydride Nanoparticles with Ultrahigh Structural Stability and Hydrogen Storage Activity Derived from Microencapsulated Nanoconfinement, Adv. Mater., 2017, 29, 1–6, DOI:10.1002/adma.201700760.
- T. K. Nielsen, K. Manickam, M. Hirscher, F. Besenbacher and T. R. Jensen, Confinement of MgH2 Nanoclusters within Nanoporous Aerogel Scaffold Materials, ACS Nano, 2009, 3, 3521–3528, DOI:10.1021/nn901072w.
- B.-J. Kim, Y.-S. Lee and S.-J. Park, A study on the hydrogen storage capacity of Ni-plated porous carbon nanofibers, Int. J. Hydrogen Energy, 2008, 33, 4112–4115, DOI:10.1016/j.ijhydene.2008.05.077.
- J. M. Blackman, J. W. Patrick, A. Arenillas, W. Shi and C. E. Snape, Activation of carbon nanofibres for hydrogen storage, Carbon, 2006, 44, 1376–1385, DOI:10.1016/j.carbon.2005.11.015.
- C. Zlotea, C. Chevalier-César, E. Léonel, E. Leroy, F. Cuevas and P. Dibandjo, et al., Synthesis of small metallic Mg-based nanoparticles confined in porous carbon materials for hydrogen sorption, Faraday Discuss., 2011, 151, 117–131, 10.1039/c0fd00016g.
- R. Bogerd, P. Adelhelm, J. H. Meeldijk, K. P. de Jong and P. E. de Jongh, The structural characterization and H2 sorption properties of carbon-supported Mg1−x Nix nanocrystallites, Nanotechnology, 2009, 20, 204019, DOI:10.1088/0957-4484/20/20/204019.
- A. Ebrahimi-Purkani and S. F. Kashani-Bozorg, Nanocrystalline Mg2Ni-based powders produced by high-energy ball milling and subsequent annealing, J. Alloys Compd., 2008, 456, 211–215, DOI:10.1016/j.jallcom.2007.02.003.
- C. Peng, Y. Li and Q. Zhang, Enhanced hydrogen desorption properties of MgH2 by highly dispersed Ni: The role of in situ hydrogenolysis of nickelocene in ball milling process, J. Alloys Compd., 2022, 900, 163547, DOI:10.1016/j.jallcom.2021.163547.
- S. Yagi, T. Koyanagi, H. Nakanishi, T. Ichitsubo and E. Matsubara, Formation of Nickel Nanoparticles by Electroless Deposition Using NiO and Ni(OH)2 Suspensions, J. Electrochem. Soc., 2008, 155, D583, DOI:10.1149/1.2948380.
- L. Brissonneau, R. Sahnoun, C. Mijoule and C. Vahlas, Investigation of Nickelocene Decomposition during Chemical Vapor Deposition of Nickel, J. Electrochem. Soc., 2000, 147, 1443, DOI:10.1149/1.1393375.
- X. Lu, L. Zhang, J. Zheng and X. Yu, Construction of carbon covered Mg2NiH4 nanocrystalline for hydrogen storage, J. Alloys Compd., 2022, 905, 164169, DOI:10.1016/j.jallcom.2022.164169.
- X. Chen, X. Wang and D. Fang, A review on C1s XPS-spectra for some kinds of carbon materials, Fullerenes, Nanotubes Carbon Nanostruct., 2020, 28, 1048–1058, DOI:10.1080/1536383X.2020.1794851.
- O. Friedrichs, L. Kolodziejczyk, J. C. Sánchez-López, C. López-Cartés and A. Fernández, Synthesis of nanocrystalline MgH2 powder by gas-phase condensation and in situ hydridation: TEM, XPS and XRD study, J. Alloys Compd., 2007, 434–435, 721–724, DOI:10.1016/j.jallcom.2006.08.286.
- L. Schlapbach, D. Shaltiel and P. Oelhafen, Catalytic effect in the hydrogenation of Mg and Mg compounds: Surface analysis of Mg-Mg2Ni and Mg2Ni+, Mater. Res. Bull., 1979, 14, 1235–1246, DOI:10.1016/0025-5408(79)90220-4.
- J. Matienzo, L. I. Yin, S. O. Grim and W. E. Swartz, X-ray photoelectron spectroscopy of nickel compounds, Inorg. Chem., 1973, 12, 2762–2769, DOI:10.1021/ic50130a005.
- I. Pollini, Chemical bond and hybridization in nickel compounds, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 045107, DOI:10.1103/PhysRevB.65.045107.
- D. N. G. Krishna and J. Philip, Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges, Appl. Surf. Sci. Adv., 2022, 12, 100332, DOI:10.1016/j.apsadv.2022.100332.
- A. N. Mansour, Characterization of NiO by XPS, Surf. Sci. Spectra, 1994, 3, 231–238, DOI:10.1116/1.1247751.
- A. N. Mansour, Characterization of β -Ni(OH)2 by XPS, Surf. Sci. Spectra, 1994, 3, 239–246, DOI:10.1116/1.1247752.
- M. C. Biesinger, B. P. Payne, L. W. M. Lau and A. Gerson, Smart RSC, X–ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems, Surf. Interface Anal., 2009, 41, 324–332, DOI:10.1002/sia.3026.
- M. Smith, L. Scudiero, J. Espinal, J.-S. McEwen and M. Garcia-Perez, Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations, Carbon, 2016, 110, 155–171, DOI:10.1016/j.carbon.2016.09.012.
- J.-H. Zhou, Z.-J. Sui, J. Zhu, P. Li, D. Chen and Y.-C. Dai, et al., Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR, Carbon, 2007, 45, 785–796, DOI:10.1016/j.carbon.2006.11.019.
- Y. M. Kimel'fel'd, E. M. Smirnova and V. T. Aleksanyan, The vibrational spectra of molecular crystals of ferrocene, ruthenocene, osmocene and nickelocene, J. Mol. Struct., 1973, 19, 329–346, DOI:10.1016/0022-2860(73)85275-5.
- S. Korkmaz, İ.A Kariper, C. Karaman and O. Karaman, MWCNT/Ruthenium hydroxide aerogel supercapacitor production and investigation of electrochemical performances, Sci. Rep., 2022, 12, 12862, DOI:10.1038/s41598-022-17286-w.
- M. T. Caccamo, G. Mavilia and S. Magazù, Thermal Investigations on Carbon Nanotubes by Spectroscopic Techniques, Appl. Sci., 2020, 10, 8159, DOI:10.3390/app10228159.
- Ü. Çalışır and B. Çiçek, Synthesis of thiol-glycol-functionalized carbon nanotubes and characterization with FTIR, TEM, TGA, and NMR technics, Chem. Pap., 2020, 74, 3293–3302, DOI:10.1007/s11696-020-01158-6.
- M. Hazarika, P. Chinnamuthu and J. P. Borah, Enhanced photocatalytic efficiency of MWCNT/NiFe2O4 nanocomposites, Phys. E, 2022, 139, 115177, DOI:10.1016/j.physe.2022.115177.
- L. Schlapbach and A. Züttel, Hydrogen-storage materials for mobile applications, Nature, 2001, 414, 353–358, DOI:10.1038/35104634.
- J. F. Herbst, On extending Miedema's model to predict hydrogen content in binary and ternary hydrides, J. Alloys Compd., 2002, 337, 99–107, DOI:10.1016/S0925-8388(01)01939-9.
- A. K. Singh, A. K. Singh and O. N. Srivastava, On the synthesis of the Mg2Ni alloy by mechanical alloying, J. Alloys Compd., 1995, 227, 63–68, DOI:10.1016/0925-8388(95)01625-2.
- Y. Wang, X. Liu, Y. Chen, X. Cai and L. Zhou, High energy ball milling composite modification of Mg2Ni hydrogen storage alloy by graphene and MWCNTs, Int. J. Hydrogen Energy, 2024, 50, 1562–1573, DOI:10.1016/j.ijhydene.2023.10.125.
- T. Hongo, K. Edalati, M. Arita, J. Matsuda, E. Akiba and Z. Horita, Significance of grain boundaries and stacking faults on hydrogen storage properties of Mg2Ni intermetallics processed by high-pressure torsion, Acta Mater., 2015, 92, 46–54, DOI:10.1016/j.actamat.2015.03.036.
- H. Pan, CHAPTER 5. Nanotubes for Energy Storage, Nanofabrication and its Application in Renewable Energy, in G. Zhang and N. Manjooran, 2014, pp. 121–198. 10.1039/9781782623380-00121.
- D. Noréus and P.-E. Werner, The structure of the low temperature phase Mg2NiH4(LT), Mater. Res. Bull., 1981, 16, 199–206, DOI:10.1016/0025-5408(81)90082-9.
- E. Grigore, A. A. El Mel, A. Granier and P. Y. Tessier, The influence of Ni content on the characteristics of C–Ni thin films, Surf. Coat. Technol., 2012, 211, 188–191, DOI:10.1016/j.surfcoat.2011.09.049.
- A. Wiltner and C. Linsmeier, Formation of endothermic carbides on iron and nickel, Phys. Status Solidi, 2004, 201, 881–887, DOI:10.1002/pssa.200304362.
- M. Ramachandran and R. G. Reddy, Direct reduction of magnesium oxide to magnesium using thermal plasma technology, Miner. Metall. Process., 2015, 32, 30–37, DOI:10.1007/BF03402355.
- L. Yao, X. Lyu, J. Zhang, Y. Liu, Y. Zhu and H. Lin, et al., Remarkable synergistic effects of Mg2NiH4 and transition metal carbides (TiC, ZrC, WC) on enhancing the hydrogen storage properties of MgH2, Int. J. Hydrogen Energy, 2020, 45, 6765–6779, DOI:10.1016/j.ijhydene.2019.12.139.
- Z. Tian, Z. Wang, P. Yao, C. Xia, T. Yang and Q. Li, Hydrogen storage behaviors of magnesium hydride catalyzed by transition metal carbides, Int. J. Hydrogen Energy, 2021, 46, 40203–40216, DOI:10.1016/j.ijhydene.2021.09.212.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr01725k |
| This journal is © The Royal Society of Chemistry 2024 |