Open Access Article
Open Access ArticleOptical grade transformation of monolayer transition metal dichalcogenides via encapsulation annealing†
Huije
Ryu‡
a,
Seong Chul
Hong‡
a,
Kangwon
Kim
b,
Yeonjoon
Jung
a,
Yangjin
Lee
cd,
Kihyun
Lee
cd,
Youngbum
Kim
e,
Hyunjun
Kim
a,
Kenji
Watanabe
 f,
Takashi
Taniguchi
g,
Jeongyong
Kim
f,
Takashi
Taniguchi
g,
Jeongyong
Kim
 e,
Kwanpyo
Kim
cd,
Hyeonsik
Cheong
e,
Kwanpyo
Kim
cd,
Hyeonsik
Cheong
 b and
Gwan-Hyoung
Lee
b and
Gwan-Hyoung
Lee
 *a
*a
aDepartment of Materials Science and Engineering, Seoul National University, Seoul 08826, Republic of Korea. E-mail: gwanlee@snu.ac.kr
bDepartment of Physics, Sogang University, Seoul 04107, Republic of Korea
cDepartment of Physics, Yonsei University, Seoul 03722, Republic of Korea
dCenter for Nanomedicine, Institute for Basic Science, Seoul 03722, Republic of Korea
eDepartment of Energy Science, Sungkyunkwan University, Suwon 16419, Republic of Korea
fResearch Center for Electronic and Optical Materials, National Institute for Materials Science, 1-1 Namiki, Tsukuba 305-0044, Japan
gResearch Center for Materials Nanoarchitectonics, National Institute for Materials Science, 1-1 Namiki, Tsukuba 305-0044, Japan
First published on 26th February 2024
Abstract
Monolayer transition metal dichalcogenides (TMDs) have emerged as highly promising candidates for optoelectronic applications due to their direct band gap and strong light–matter interactions. However, exfoliated TMDs have demonstrated optical characteristics that fall short of expectations, primarily because of significant defects and associated doping in the synthesized TMD crystals. Here, we report the improvement of optical properties in monolayer TMDs of MoS2, MoSe2, WS2, and WSe2, by hBN-encapsulation annealing. Monolayer WSe2 showed 2000% enhanced photoluminescence quantum yield (PLQY) and 1000% increased lifetime after encapsulation annealing at 1000 °C, which are attributed to dominant radiative recombination of excitons through dedoping of monolayer TMDs. Furthermore, after encapsulation annealing, the transport characteristics of monolayer WS2 changed from n-type to ambipolar, along with an enhanced hole transport, which also support dedoping of annealed TMDs. This work provides an innovative approach to elevate the optical grade of monolayer TMDs, enabling the fabrication of high-performance optoelectronic devices.
Introduction
Semiconducting TMDs have significant potential in optical applications owing to their large exciton binding energy, indirect-to-direct band transition, and strong light–matter interaction.1–3 However, exfoliated or synthesized TMDs have shown different optical properties because they are strongly affected by several factors, such as doping, strain, chemical reaction, dielectric environment, and defects, that are also correlated.4–12 For example, chalcogen vacancies cause n-type doping and provide non-radiative recombination pathways in the TMDs.11 Numerous studies have demonstrated improvements in the PL of monolayer TMDs through various approaches, including decreasing chalcogen vacancies, electrostatically doping, or applying mechanical strain.4,7,13,14 However, the critical factor responsible for the enhancement of PL remains elusive. This poses challenges in the fabrication of optical grade TMDs with optical properties approaching the theoretical upper limit. Here, we report a significant enhancement of optical properties in monolayer TMDs, including MoS2, MoSe2, WS2, and WSe2, by hBN-encapsulation annealing. Despite the 400% increase in defect density resulting from annealing hBN-encapsulated 1L-WSe2 at 1000 °C, it astonishingly led to a remarkable 2000% increase in PL quantum yield (QY) and a 1000% extension in PL lifetime. Additionally, transport characteristics of the 1L-WS2 transformed from n-type to ambipolar behavior along with an enhanced hole transport after encapsulation annealing. Our observation suggests that modulation of doping by hBN-encapsulation annealing plays a critical role in the optical grade of monolayer TMDs.Results and discussion
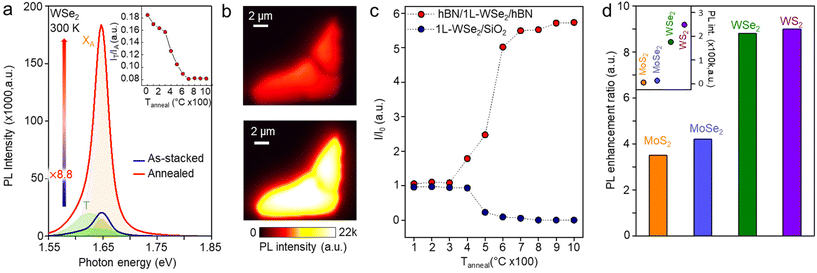
For encapsulation annealing of monolayer TMDs, we fabricated stacks of hBN/TMDs/hBN using a pick-up technique.15Fig. 1a shows an optical microscopic image and atomic force microscopy (AFM) topography image of the as-stacked hBN/1L-WSe2/hBN. After being annealed at 1000 °C in vacuum for 1 hour, no significant change was observed as shown in Fig. 1b. Furthermore, as indicated by thickness profiles (white dashed lines) in Fig. 1a and b, there was no change in the thickness of the 1L-WSe2 after encapsulation annealing. In contrast, the as-exfoliated 1L-WSe2 on a SiO2 substrate was thermally degraded and finally removed at 1000 °C during annealing (Fig. S1†), which is consistent with previous studies.16,17 To examine the structural changes and doping effect due to encapsulation annealing, we measured the Raman spectra of hBN-encapsulated 1L-WSe2 before and after annealing (Fig. 1c). After encapsulation annealing, no noticeable change was observed. To clearly identify the effect of strain and doping, we used 1L-MoS2 as an indicator used in previous studies.18–20Fig. 1d shows the Raman spectra of hBN-encapsulated 1L-MoS2 before and after annealing. The full width at half maximum (FWHM) of both A1g and E12g peaks decreased. Moreover, the encapsulation annealing process leads to a decrease in the intensity of the defect-related LA mode (∼227 cm−1) and an increase in the intensities of A1g and E12g peaks. These results are summarized in Fig. 1e: the FWHM values of A1g and E12g peaks and ratios of I(LA)/I(A1g) and I(LA)/I(E12g) decreased after encapsulation annealing. The position shifts of A1g and E12g peaks in Fig. S2 and S3† show that the 1L-MoS2 becomes dedoped after encapsulation annealing.To study the effect of encapsulation annealing on the optical properties of monolayer TMDs, we measured the PL spectra of 1L-WSe2 before and after encapsulation annealing (Fig. 2a). After encapsulation annealing, the PL intensity of 1L-WSe2 exhibited a significant increase by a factor of 8.8. The PL intensity map in Fig. 2b verifies that the PL was uniformly enhanced over whole area of 1L-WSe2. To explore how annealing temperature influences the PL intensity, we assessed the PL intensity of the annealed hBN/1L-WSe2/hBN and 1L-WSe2/SiO2 structures, as depicted in Fig. 2d. The annealing process was conducted in increments of 100 °C, spanning a range from Tanneal = 100 to 1000 °C. Up to 300 °C, the PL intensity for the hBN/1L-WSe2/hBN remained almost unchanged. It was only after annealing at 400 °C that a noticeable increase in PL intensity became evident. Between 400 °C and 600 °C, there was a sharp increase in PL intensity, aligning with the temperature range where the trion/exciton intensity ratio and FWHM of A exciton peak rapidly decreases, as indicated in the inset of Fig. 2a and S4† respectively. Given that trions primarily follow a non-radiative pathway, the diminished trion/exciton ratio suggests that the radiative recombination of excitons dominates in our samples.4 However, the PL intensity showed no further increase at higher temperature than 700 °C. In contrast, the 1L-WSe2/SiO2 showed a marked decline in PL intensity at 500 °C and no PL at higher temperature due to thermal degradation of WSe2 (see Fig. S5† for PL spectra of 1L-WSe2/SiO2 before and after annealing at 1000 °C). Additionally, we annealed monolayers of MoS2, MoSe2, and WS2 1000 °C for 1 h, all encapsulated with hBN. As shown in Fig. 2d, it was verified that the enhancement in PL is not exclusive to WSe2. The PL intensity of MoS2, MoSe2, and WS2 increased by a factor of 3.5, 4.2, and 9, respectively. The PL intensities of these four monolayer TMDs after encapsulation annealing are displayed in the inset of Fig. 2d.
In Fig. 3, we investigated the optical properties of 1L-WSe2 before and after encapsulation annealing. Fig. 3a and b show the PL contour plots of the as-stacked and annealed 1L-WSe2 as a function of temperature, respectively. Below 100 K, there is no significant difference between PL intensity and peak positions of two samples. However, the A exciton (XA) PL peak of annealed 1L-WSe2 is higher than that of the as-stacked one above 100 K. Fig. 3c shows the temperature-dependent A exciton PL intensities of the two samples. As the temperature decreases, the PL intensity of both samples increases, then decreases around 100 and 120 K for as-stacked and annealed 1L-WSe2, respectively. This is in contrast to the previous report that the PL intensity continuously is reduced as the temperature decreases due to the dominant population of dark excitons of WSe2, which is shown in Fig. 3c.21 The high quality WSe2 synthesized by a flux method in ref. 21 shows the similar trend in the temperature-dependent measurement of PL because of the interplay between defect- and phonon-scattering and the dark exciton state. It is noteworthy that, even though hBN-encapsulation enhances the PL of WSe2, additionally annealed WSe2 shows higher PL intensity in the temperature range above 100 K. Fig. 3d shows the PL spectra of as-stacked and annealed 1L-WSe2 measured at 10 K. The individual PL peaks correspond to the A exciton (P1), biexciton (P2), intervalley trion (P3), intravalley trion (P4), negatively charged biexciton (P5), and localized excitons (P6–P10), respectively as indicated in Fig. 3d.22–24 At 10 K, spin-forbidden dark excitons are prevalent and A exciton PL peaks of both samples show no significant difference. However, after encapsulation annealing, the negatively charged biexciton (P5) is suppressed, while the biexciton (P2) is enhanced. This phenomenon can also be attributed to a dedoping effect as a higher concentration of biexcitons is expected in neutralized WSe2 compared to negatively charged biexcitons. In the case of hBN-encapsulation annealed 1L-MoS2 with bright excitons (Fig. S6†),25 the PL intensity increases with decreasing temperature and A exciton PL peak becomes much higher after encapsulation annealing due to the dedoping effect.
Next, we measured the PL QY of as-stacked and annealed 1L-WSe2 in Fig. 3e. The annealed 1L-WSe2 exhibited higher PL QY of 0.18% at a generation rate of 1017 cm−2 s−1 by 20 times than that of the as-stacked 1L-WSe2 (0.009%). The significant improvement in PL QY can be ascribed to the prevalent formation of neutral excitons, which predominantly follow a radiative recombination path. We also verified that the PL QY of annealed 1L-MoS2 increases by a factor of 5 (Fig. S7a†). To analyze the exciton recombination dynamics of annealed WSe2, time-resolved photoluminescence (TRPL) measurements were performed in Fig. 3f. The PL decay curves were fitted with a single exponential decay function,  , where IRF is a Gaussian instrument response function.26 The annealed WSe2 exhibits a relatively prolonged lifetime of 1.3 ns, surpassing that of the as-stacked one by an order of magnitude. The recombination lifetime (τob) is determined by combination of the radiative (τr) and nonradiative (τnr) lifetimes as per the following equation:
, where IRF is a Gaussian instrument response function.26 The annealed WSe2 exhibits a relatively prolonged lifetime of 1.3 ns, surpassing that of the as-stacked one by an order of magnitude. The recombination lifetime (τob) is determined by combination of the radiative (τr) and nonradiative (τnr) lifetimes as per the following equation:
Because the radiative lifetime is longer than nonradiative lifetime by an order of magnitude, the prolonged lifetime of the annealed WSe2 indicates that the radiative recombination of neutral excitons is dominant and the formation of trions with nonradiative recombination is suppressed due to the dedoping effect.26,27 The annealed 1L-MoS2 also showed an increased lifetime as shown in Fig. S7b.†
The effects of encapsulation annealing on the electrical properties of monolayer TMDs are investigated in Fig. 4. We fabricated an hBN-encapsulated 1L-WS2 device with graphene electrodes as shown in the schematic of Fig. 4a. Fig. 4b shows an optical microscopic image of a representative 1L-WS2 device. As shown in transport curves of Fig. 4c and d, the 1L-WS2 device exhibited enhanced p-type transport after encapsulation annealing. This result also supports that the WS2 channel is dedoped as confirmed by the Raman spectroscopic measurements and PL analyses in Fig. S3† and Fig. 3. Despite dedoping induced by encapsulation annealing, the field effect mobility for electrons increased from 4.94 to 5.14 cm2 V−1 s−1. The n-type transport properties of the 1L-WS2 remained consistent, whereas the p-type transport exhibited enhancement after encapsulation annealing, as shown in the output curves of Fig. 4e and f. These findings indicate that the dedoping induced by encapsulation annealing results in alterations in the electrical properties, while still preserving high electrical performance.
To probe the structural changes of 1L-WSe2 during encapsulation annealing, we employed scanning transmission electron microscopy (STEM) in Fig. 5a–c. For the STEM characterization, 1L-graphene was used as an encapsulation layer as the graphene has a small background signal (see Methods for details of the TEM sample preparation).28–30 The high-angle annular dark field (HAADF) STEM images of Fig. 5a and b show the atomic structures of as-stacked and annealed 1L-WSe2. We calculated the defect densities of two samples using a deep learning process with 1000 simulated STEM images (1024 × 1024 pixels) as we previously reported.31 The defect density increased from 0.33% to 1.38% (see the ESI† for details of the defect density calculation). This is contrary to previous reports that defect density and PL intensity have an inverse correlation.32 In order to differentiate the types of defects in as-stacked and annealed WSe2, we plot the defect density, i.e. counts, as a function of contrast intensity of STEM images as shown in Fig. 5c. It is clear that the encapsulation annealed WSe2 has more defects with relatively high contrast intensity as shown in the inset of Fig. 5c. The vacant sites in defects with higher contrast intensity might be substituted by impurities, such as oxygen, because it has been reported that chalcogen vacancies are substituted by oxygen during post treatments, such as thermal annealing and laser exposure, leading to reduction of vacancy-induced in-gap states below the conduction band.27,33–40
To examine the compositions of as-stacked and annealed WSe2, X-ray photoelectron spectroscopy (XPS) was used. Note that we used 2L-WSe2 for XPS measurements as the monolayer generates low signals. Fig. 5d and e show the XPS spectra of W 4f and Se 3d core levels before and after encapsulation annealing, respectively. The three distinct features of the W 4f7/2, W 4f5/2, and W 5p3/2 are observed at 32.3, 34.4, and 37.6 eV, respectively. The doublets observed at 54.6 and 55.5 eV correspond to the Se 3d5/2 and Se 3d3/2.41 After encapsulation annealing, the binding energies of W 4f and Se 3d core-levels blue-shifted, possibly indicating improved crystallinity. Based on our observed results, the origin of the dedoping effect induced by encapsulation annealing can be ascribed to the substitution of vacancies with oxygen and the enhanced crystallinity in defect-free areas.
Conclusions
In conclusion, we demonstrate a hBN-encapsulation annealing method to achieve the optical grade of monolayer TMDs. After annealing at 1000 °C for 1 h, we observed enhancement of PL intensity, QY, and lifetime in hBN-encapsulated 1L-TMD. The hBN-encapsulated 1L-WS2 exhibited a transition in the transport characteristics from n-type to ambipolar behavior, which can be attributed to the dedoping of WS2 after encapsulation annealing. STEM measurements indicated an increase of defect density after encapsulation annealing. Our observation that the n-type TMD undergoes dedoping despite increased defect density is an exceptional case. Our deep learning analysis implies that the majority of the newly introduced defects might be impurities rather than selenium vacancies. Our work shows a novel way to transform monolayer TMDs into optical grade materials by the hBN-encapsulation annealing and has great potential for various optoelectronic applications.Methods
Chemical vapor deposition growth
1L-MoS2 crystals were grown on silicon dioxide/silicon (SiO2/Si) substrates with SiO2 thickness of 285 nm by the CVD method within a 2-inch quartz tube, conducted under atmospheric pressure. To synthesize 1L-MoS2, a quartz boat containing MoO3 powder (99.97%, Sigma-Aldrich) was positioned in the center of a furnace, and a SiO2/Si substrate was suspended face-down on top of the boat. Another quartz boat loaded with sulfur powder (Sigma-Aldrich) was located upstream within the quartz tube, approximately 21.5 centimeters away from the center of the furnace. The furnace temperature was gradually increased to 750 °C at a rate of 50 °C per minute and maintained for 15 minutes, and then the furnace was naturally cooled to room temperature. Ar (300 sccm) was flowed for the entire growth process.Sample preparation
2D flakes utilized in the fabrication process were mechanically exfoliated onto SiO2/Si substrates. The thickness of each material was individually verified using a combination of Atomic Force Microscopy (AFM) and optical contrast. To encapsulate monolayer TMD between hBN, the pick-up transfer technique was used with a polypropylene carbonate (bisphenol A carbonate, Sigma Aldrich) (PC)-coated poly(dimethyl siloxane) (PDMS) lens mounted on a slide glass to pick-up and release.15 The PDMS/PC/slide glass assembly was manipulated using a 3-axis micromanipulator to control the position of the contact area. By solely regulating the stage temperature within the range of 80–130 °C, the top hBN was picked up completely by the PC, ensuring there were no cracks or folds. Once the hBN had been separated from the substrates, monolayer TMD and the bottom hBN were picked-up by van der Waals forces from the top hBN to form hBN/1L-TMD/hBN ultrathin 2D heterostructures. It's important to note that monolayer TMD had to be fully encapsulated by hBN without any bubbles; otherwise, the monolayer TMD would degrade during the annealing process. After stacking, the heterostructure was transferred onto a clean SiO2/Si substrate by releasing the PC film from the PDMS lens at a temperature exceeding 180 °C. Finally, to remove the PC film, the samples were immersed in chloroform for 30 minutes. Similar to hBN-encapsulated monolayer TMDs, graphene-encapsulated monolayer TMDs for STEM imaging were also prepared using the dry-transfer method.29,30 To prevent degradation of heterostructures during annealing, bi- or trilayer graphene was used. Nonetheless, removing graphene from the SiO2/Si substrate proves challenging due to the stronger adhesion of Gr/SiOx in comparison to hBN/SiOx.42 Therefore, hBN was employed as the upper layer to facilitate the easy detachment of the graphene. Thus, hBN/Gr/TMDs/Gr heterostructures were fabricated initially. Next, XeF2 gas, which can selectively etch only hBN remaining on the graphene, was treated on the heterostructures to remove the top hBN.28 For the encapsulation annealing of monolayer TMD, the sandwiched 1L-TMD was annealed in a vacuum of 10−4 Torr. The furnace temperature was ramped up to the target annealing temperature for 3 h and maintained for 1 h. Then, the furnace was naturally cooled to room temperature.Raman and PL spectroscopy
Room-temperature Raman and PL intensity map images and spectra were obtained using a Raman spectroscopy instrument equipped with a 532 nm laser and a spot size of ∼1 μm (JASCO). To minimize any potential damage to the sample caused by laser exposure, a power of <5 mW was used for an acquisition time of 60 s. Given that the laser spot size was approximately 1 μm, the mapping area was scanned with a point-to-point distance of 1 μm. Low-temperature micro-photoluminescence (micro-PL) measurements were carried out using a diode-pumped solid-state laser with wavelength of 532 nm (2.33 eV) and power of ∼5 μW. We used a 40× objective lens (numerical aperture 0.6) to focus the laser on the samples with a spot of diameter ∼1 μm and to collect PL signals from samples. A substrate with hBN-encapsulated 1L-TMD samples was loaded into an optical cryostat (Oxford MicrostatHe2) and cooled to 10 K with liquid helium. PL signals emitted by the samples were spectrally resolved using a Horiba TRIAX 320 spectrometer (300 grooves per mm) and were detected with a thermoelectrically cooled charge-coupled device (CCD) camera. Raman and PL spectra were obtained at the same position before and after annealing.Atomic force microscopy
AFM (Park Systems, NX10) in the contact mode was used to observe the surface morphology, and thickness of hBN-encapsulated 1L-WSe2.Quantum yield measurement
To estimate the QY of 1L-WSe2 and MoS2, we encapsulated a mechanically exfoliated 1L-WSe2 flake and CVD-grown 1L-MoS2 with hBN and transferred it onto a SiO2 substrate. As a reference sample, we utilized a thin poly(methyl methacrylate) (PMMA) film that contained dispersed rhodamine 6G (R6G).43–45 We acquired photoluminescence (PL) spectra from a 300 nm-thick PMMA film with a concentration of 10−4 M of R6G and from a bare quartz substrate. These spectra were measured utilizing an integrating sphere under a 514 nm laser excitation. We estimated the QY of the reference sample from the emission and absorption of the R6G. Next, we conducted photoluminescence (PL) intensity and absorption measurements on the hBN-encapsulated 1L-WSe2 and MoS2 using a confocal microscope under identical measurement conditions. By comparing the PL intensity and absorption of 1L-WSe2 and MoS2 with those of the reference sample, we were able to estimate the quantum yield (QY) of 1L-WSe2 and MoS2.Time resolved-PL measurement
For TRPL measurements, we employed the same microscope, which was equipped with a pulsed excitation at 488 nm (BDL-488, Becker & Hickl GmbH), with a pulse width of 70 ps and a repetition rate of 80 MHz, along with a high-speed hybrid detector (HPM-100-40, Becker & Hickl GmbH) and a time-correlated single-photon counting module (TCSPC, Becker & Hickl GmbH). All measurements were conducted at room temperature.Device fabrication and electrical measurement
Electron beam lithography was utilized to develop metal pad patterns and establish via contacts for graphene electrodes encapsulated by hBN. The van der Waals heterostructure was etched by exposing the pre-patterned structure to XeF2.28 The top hBN was etched away, and the embedded graphene electrodes stopped the etching process. Metals of Cr/Pd/Au (1 nm/30 nm/40 nm) were then deposited on the exposed graphene electrodes using an e-beam evaporator. Subsequently, a lift-off process was carried out by immersing the samples in acetone. Electrical measurements of the devices were then conducted at room temperature under ambient conditions, employing a parameter analyzer, specifically the Keithley 2400.Transmission electron microscopy
Transmission electron microscopy samples were prepared using a poly(methyl methacrylate)-based wet-transfer method. Samples on poly(methyl methacrylate) film were transferred on Si3N4 nanofilm TEM grids (TEM Windows, SN100-A10Q33B). The PMMA film was removed by placing samples in acetone for 12 h. A double-Cs-aberration-corrected JEOL ARM-200F was used for HAADF-STEM images at an operating voltage of 80 kV with a 23 mrad convergence angle and collection semi angles from 68 to 280 mrad.Model building and training
We generated a ResUNet deep learning model with 5 residual blocks and gradual increased filters from 32 to 128 in the convolutional layers to evaluate the number and type of defects in WSe2. We used 1000 simulated STEM images (1024 × 1024 pixels) of WSe2 as training and validation data. The loss function (categorical cross entropy) decreased under 0.03.31 Pixels with defects were extracted by the model, and we averaged the pixel's intensity out to compare annealed and as-stacked sample. We confirmed that the local intensity of the annealed Se vacancies was higher than that of untreated Se vacancies, which may represent oxygen adsorbed on Se vacancy sites during annealing.X-ray photoelectron spectroscopy
XPS samples were prepared with a top hBN layer approximately 3 nm thick to ensure a distinct signal. The XPS spectra of W and Se are obtained with Al Kα radiation using a 10 μm beam spot size (Versaprobe III).Author contributions
H. R. and G.-H. L. designed and conceived the project. K. W. K. and H. C. performed PL measurements at low temperature. Y. J. synthesized TMDs by CVD. Y. L. and K. P. K. performed HAADF-STEM imaging. K. L. and K. P. K. performed STEM image analysis based on deep learning. Y. K. and J. K. performed QY and TRPL measurements. H. K. performed XPS measurements. K. W. and T. T. supplied boron nitride crystals. H. R., S. C. H., and G.-H. L. collectively analyzed the data and wrote the paper. All authors read and contributed to the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2021R1A2C301431613, NRF-2019R1A2C3006189, and NRF-2017R1A5A1014862 (SRC program: vdWMRC center)) and the Creative-Pioneering Researchers Program through Seoul National University (SNU). K. W. and T. T. acknowledge support from the JSPS KAKENHI (Grant Numbers 21H05233 and 23H02052) and World Premier International Research Center Initiative (WPI), MEXT, Japan. G.-H. L. acknowledges the support from the Research Institute of Advanced Materials (RIAM), Institute of Engineering Research (IER), Institute of Applied Physics (IAP), and Inter-University Semiconductor Research Center (ISRC) at the Seoul National University.References
- A. Raja, A. Chaves, J. Yu, G. Arefe, H. M. Hill, A. F. Rigosi, T. C. Berkelbach, P. Nagler, C. Schuller, T. Korn, C. Nuckolls, J. Hone, L. E. Brus, T. F. Heinz, D. R. Reichman and A. Chernikov, Nat. Commun., 2017, 8, 15251 CrossRef PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- X. Liu, T. Galfsky, Z. Sun, F. Xia, E.-c. Lin, Y.-H. Lee, S. Kéna-Cohen and V. M. Menon, Nat. Photonics, 2015, 9, 30–34 CrossRef CAS.
- D.-H. Lien, S. Z. Uddin, M. Yeh, M. Amani, H. Kim, J. W. Ager, E. Yablonovitch and A. Javey, Science, 2019, 364, 468–471 CrossRef CAS PubMed.
- E. Ji, K. Yang, J. C. Shin, Y. Kim, J. W. Park, J. Kim and G.-H. Lee, Nanoscale, 2022, 14, 14106–14112 RSC.
- S. Kang, Y. S. Kim, J. H. Jeong, J. Kwon, J. H. Kim, Y. Jung, J. C. Kim, B. Kim, S. H. Bae, P. Y. Huang, J. C. Hone, H. Y. Jeong, J. W. Park, C. H. Lee and G.-H. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 1245–1252 CrossRef CAS PubMed.
- H. Kim, S. Z. Uddin, N. Higashitarumizu, E. Rabani and A. Javey, Science, 2021, 373, 448–452 CrossRef CAS PubMed.
- M. Amani, D.-H. Lien, D. Kiriya, J. Xiao, A. Azcatl, J. Noh, S. R. Madhvapathy, R. Addou, S. Kc, M. Dubey, K. Cho, R. M. Wallace, S.-C. Lee, J.-H. He, J. W. Ager, X. Zhang, E. Yablonovitch and A. Javey, Science, 2015, 350, 1065–1068 CrossRef CAS PubMed.
- F. Cadiz, E. Courtade, C. Robert, G. Wang, Y. Shen, H. Cai, T. Taniguchi, K. Watanabe, H. Carrere, D. Lagarde, M. Manca, T. Amand, P. Renucci, S. Tongay, X. Marie and B. Urbaszek, Phys. Rev. X, 2017, 7, 021026 Search PubMed.
- Y. Lin, X. Ling, L. Yu, S. Huang, A. L. Hsu, Y.-H. Lee, J. Kong, M. S. Dresselhaus and T. Palacios, Nano Lett., 2014, 14, 5569–5576 CrossRef CAS PubMed.
- L. Li and E. A. Carter, J. Am. Chem. Soc., 2019, 141, 10451–10461 CrossRef CAS PubMed.
- H. Y. Jeong, Y. Jin, S. J. Yun, J. Zhao, J. Baik, D. H. Keum, H. S. Lee and Y. H. Lee, Adv. Mater., 2017, 29, 1605043 CrossRef PubMed.
- X. Zhang, Q. Liao, S. Liu, Z. Kang, Z. Zhang, J. Du, F. Li, S. Zhang, J. Xiao, B. Liu, Y. Ou, X. Liu, L. Gu and Y. Zhang, Nat. Commun., 2017, 8, 15881 CrossRef CAS PubMed.
- A. O. A. Tanoh, J. Alexander-Webber, Y. Fan, N. Gauriot, J. Xiao, R. Pandya, Z. Li, S. Hofmann and A. Rao, Nanoscale Adv., 2021, 3, 4216–4225 RSC.
- L. Wang, I. Meric, P. Y. Huang, Q. Gao, Y. Gao, H. Tran, T. Taniguchi, K. Watanabe, L. M. Campos, D. A. Muller, J. Guo, P. Kim, J. Hone, K. L. Shepard and C. R. Dean, Science, 2013, 342, 614–617 CrossRef CAS PubMed.
- W. H. Blades, N. J. Frady, P. M. Litwin, S. J. McDonnell and P. Reinke, J. Phys. Chem. C, 2020, 124, 15337–15346 CrossRef CAS.
- P. Chen, W. Xu, Y. Gao, P. Holdway, J. H. Warner and M. R. Castell, J. Phys. Chem. C, 2019, 123, 3876–3885 CrossRef CAS.
- R. Rao, A. E. Islam, S. Singh, R. Berry, R. K. Kawakami, B. Maruyama and J. Katoch, Phys. Rev. B, 2019, 99, 195401 CrossRef CAS.
- M. Velicky, A. Rodriguez, M. Bousa, A. V. Krayev, M. Vondracek, J. Honolka, M. Ahmadi, G. E. Donnelly, F. Huang, H. D. Abruna, K. S. Novoselov and O. Frank, J. Phys. Chem. Lett., 2020, 11, 6112–6118 CrossRef CAS PubMed.
- S. Mignuzzi, A. J. Pollard, N. Bonini, B. Brennan, I. S. Gilmore, M. A. Pimenta, D. Richards and D. Roy, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 195411 CrossRef.
- D. Edelberg, D. Rhodes, A. Kerelsky, B. Kim, J. Wang, A. Zangiabadi, C. Kim, A. Abhinandan, J. Ardelean, M. Scully, D. Scullion, L. Embon, R. Zu, E. J. G. Santos, L. Balicas, C. Marianetti, K. Barmak, X. Zhu, J. Hone and A. N. Pasupathy, Nano Lett., 2019, 19, 4371–4379 CrossRef CAS PubMed.
- M. Barbone, A. R. Montblanch, D. M. Kara, C. Palacios-Berraquero, A. R. Cadore, D. De Fazio, B. Pingault, E. Mostaani, H. Li, B. Chen, K. Watanabe, T. Taniguchi, S. Tongay, G. Wang, A. C. Ferrari and M. Atature, Nat. Commun., 2018, 9, 3721 CrossRef PubMed.
- T. P. Lyons, S. Dufferwiel, M. Brooks, F. Withers, T. Taniguchi, K. Watanabe, K. S. Novoselov, G. Burkard and A. I. Tartakovskii, Nat. Commun., 2019, 10, 2330 CrossRef CAS PubMed.
- J. Jadczak, J. Kutrowska-Girzycka, P. Kapuscinski, Y. S. Huang, A. Wojs and L. Bryja, Nanotechnology, 2017, 28, 395702 CrossRef CAS PubMed.
- M. Koperski, M. R. Molas, A. Arora, K. Nogajewski, A. O. Slobodeniuk, C. Faugeras and M. Potemski, Nanophotonics, 2017, 6, 1289–1308 CAS.
- L. Yuan and L. Huang, Nanoscale, 2015, 7, 7402–7408 RSC.
- Z. Luo, W. Zheng, N. Luo, B. Liu, B. Zheng, X. Yang, D. Liang, J. Qu, H. Liu, Y. Chen, Y. Jiang, S. Chen, X. Zou and A. Pan, Nano Lett., 2022, 22, 2112–2119 CrossRef CAS PubMed.
- J. Son, J. Kwon, S. Kim, Y. Lv, J. Yu, J. Y. Lee, H. Ryu, K. Watanabe, T. Taniguchi, R. Garrido-Menacho, N. Mason, E. Ertekin, P. Y. Huang, G.-H. Lee and A. M. van der Zande, Nat. Commun., 2018, 9, 3988 CrossRef PubMed.
- H. Ryu, Y. Lee, H. J. Kim, S. H. Kang, Y. Kang, K. Kim, J. Kim, B. E. Janicek, K. Watanabe, T. Taniguchi, P. Y. Huang, H. Cheong, I. H. Jung, K. Kim, Y. W. Son and G.-H. Lee, Adv. Funct. Mater., 2021, 31, 2107376 CrossRef CAS.
- H. Ryu, Y. Lee, J. H. Jeong, Y. Lee, Y. Cheon, K. Watanabe, T. Taniguchi, K. Kim, H. Cheong, C. H. Lee and G.-H. Lee, Small, 2023, 19, 2205224 CrossRef CAS PubMed.
- K. Lee, J. Park, S. Choi, Y. Lee, S. Lee, J. Jung, J. Y. Lee, F. Ullah, Z. Tahir, Y. S. Kim, G. H. Lee and K. Kim, Nano Lett., 2022, 22, 4677–4685 CrossRef CAS PubMed.
- M. R. Rosenberger, H.-J. Chuang, K. M. McCreary, C. H. Li and B. T. Jonker, ACS Nano, 2018, 12, 1793–1800 CrossRef CAS PubMed.
- X. Wei, Z. Yu, F. Hu, Y. Cheng, L. Yu, X. Wang, M. Xiao, J. Wang, X. Wang and Y. Shi, AIP Adv., 2014, 4, 123004 CrossRef.
- C. Hou, J. Deng, J. Guan, Q. Yang, Z. Yu, Y. Lu, Z. Xu, Z. Yao and J. Zheng, Phys. Chem. Chem. Phys., 2021, 23, 24579–24588 RSC.
- H. Ardekani, R. Younts, Y. Yu, L. Cao and K. Gundogdu, ACS Appl. Mater. Interfaces, 2019, 11, 38240–38246 CrossRef CAS PubMed.
- S. V. Sivaram, A. T. Hanbicki, M. R. Rosenberger, G. G. Jernigan, H. J. Chuang, K. M. McCreary and B. T. Jonker, ACS Appl. Mater. Interfaces, 2019, 11, 16147–16155 CrossRef CAS PubMed.
- H. Nan, Z. Wang, W. Wang, Z. Liang, Y. Lu, Q. Chen, D. He, P. Tan, F. Miao, X. Wang, J. Wang and Z. Ni, ACS Nano, 2014, 8, 5738–5745 CrossRef CAS PubMed.
- P.-C. Shen, Y. Lin, C. Su, C. McGahan, A.-Y. Lu, X. Ji, X. Wang, H. Wang, N. Mao, Y. Guo, J.-H. Park, Y. Wang, W. Tisdale, J. Li, X. Ling, K. E. Aidala, T. Palacios and J. Kong, Nat. Electron., 2022, 5, 28–36 CrossRef CAS.
- C. Schwermann, T. Stiehm, P. Tonndorf, R. Schneider, R. Schmidt, J. Kern, S. Michaelis de Vasconcellos, R. Bratschitsch and N. L. Doltsinis, Phys. Chem. Chem. Phys., 2018, 20, 16918–16923 RSC.
- Z. Hu, J. Avila, X. Wang, J. F. Leong, Q. Zhang, Y. Liu, M. C. Asensio, J. Lu, A. Carvalho, C. H. Sow and A. H. Castro Neto, Nano Lett., 2019, 19, 4641–4650 CrossRef CAS PubMed.
- F. Bozheyev, K. Harbauer, C. Zahn, D. Friedrich and K. Ellmer, Sci. Rep., 2017, 7, 16003 CrossRef PubMed.
- H. Rokni and W. Lu, Nat. Commun., 2020, 11, 5607 CrossRef CAS PubMed.
- S. Roy, A. S. Sharbirin, Y. Lee, W. B. Kim, T. S. Kim, K. Cho, K. Kang, H. S. Jung and J. Kim, Nanomaterials, 2020, 10, 1032 CrossRef CAS PubMed.
- D. Pan, Y. Fu, N. Spitha, Y. Zhao, C. R. Roy, D. J. Morrow, D. D. Kohler, J. C. Wright and S. Jin, Nat. Nanotechnol., 2021, 16, 159–165 CrossRef CAS PubMed.
- K. Chen, S. Deng, E. Chen, S. Wen, T. Ouyang, X. Wang, R. Zhan, J. Cai, X. Wan and H. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 44814–44823 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr06641j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |