Effects of bromine-containing counterion salts in directing the structures of medium-sized silver nanoclusters†
Haoqi
Li
,
Xiao
Wei
,
Xi
Kang
 * and
Manzhou
Zhu
* and
Manzhou
Zhu

Department of Chemistry and Centre for Atomic Engineering of Advanced Materials, Key Laboratory of Structure and Functional Regulation of Hybrid Materials of Ministry of Education, Institutes of Physical Science and Information Technology and Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei, Anhui 230601, China. E-mail: kangxi_chem@ahu.edu.cn
First published on 20th December 2023
Abstract
The preparation and structural determination of silver nanoclusters (especially the medium-sized Ag clusters) remain more challenging relative to those of their gold counterparts because of the comparative instability of the former. In this work, three medium-sized Ag clusters were controllably synthesized and structurally determined, namely, [Ag52(S-Adm)30Br4H20]2− (Ag52 for short), Ag54(S-Adm)30Br4H20 (Ag54 for short), and [Ag58(S-Adm)30Br4(NO3)2H22]2+ (Ag58 for short) nanoclusters. Specifically, the introduction of PPh4Br gave rise to the generation of Ag52 and Ag54 nanoclusters with homologous compositions and configurations, while the TOABr salt selected Ag58 as the sole cluster product, whose geometric structure was completely different from those of Ag52 and Ag54 nanoclusters. In addition, the optical absorptions and emissions of the three medium-sized silver nanoclusters were compared. The findings in this work not only provide three uniquely medium-sized nanoclusters to enrich the silver cluster family but also point out a new approach (i.e., changing the counterion salt) for the preparation of new nanoclusters with novel structures.
Introduction
As a special kind of metal nanomaterial, atomically precise metal nanoclusters, whose sizes are between those of metallic complexes and those of bulk metals, have attracted widespread attention in the past few decades.1–3 Owing to the strong quantum size effect and discrete electronic energy levels, metal nanoclusters or cluster-based nanomaterials exhibit structure-dependent performances, such as optical,4–9 electrical,10–13 magnetic, etc.14–17 Besides, the atomic precision characterization of metal nanoclusters makes them ideal nano-platforms for investigating the structural evolution mechanisms of metal-based nanostructures and the related structure–property correlations, which could, in turn, act as guidelines for the preparation of metal clusters with customized structures and tailored properties. In this context, how to control the size/structure of nanoclusters and regulate their chemical environment to obtain functional nanoclusters has aroused in-depth research interests of nanocluster scientists.18–23Compared with the rich family of gold nanoclusters, there are relatively few structures on the atomically precise silver counterparts. Indeed, the comparative instability of silver nanoclusters relative to gold ones makes their preparation and isolation more challenging. To date, several silver nanoclusters with different core sizes have been prepared and structurally determined by means of one-pot synthesis, ligand engineering, intercluster assembly, etc.24–35 Several of these reported silver nanoclusters comprise small-sized metallic kernels (e.g., Ag14–16,36–38 Ag20–23,39–41 Ag25,42 Ag29,43etc.) or large-sized metallic kernels (e.g., Ag100,44 Ag136,45 Ag141,46 Ag146,47 Ag152,48 Ag206,49 Ag210–211,50 Ag307,51 Ag374,52etc.). In comparison, there are fewer reports about structurally determined silver nanoclusters with medium-sized metallic kernels (i.e., 50–100-atom silver kernels), which partly restrict the formation of a rich family of silver nanoclusters. Besides, ligand engineering has been developed as a versatile approach for the synthesis of silver nanoclusters with novel structures and functionalities, and the ligand effect has been evaluated in such preparations. However, most of these works focused on the thiol or phosphine ligand effects in directing the structures of metal nanoclusters. As the halide-containing salts have been widely exploited for the preparation of silver nanoclusters via forming Ag–halide interactions, more efforts are needed to evaluate the effect of halide-containing counterion salts in the corresponding preparations and structure formations.
In the current work, the effect of bromine-containing salts in directing the structures of silver nanoclusters was investigated, and three clusters with medium-sized metallic kernels were controllably synthesized and structurally determined. Specifically, the introduction of PPh4Br gave rise to the generation of [Ag52(S-Adm)30Br4H20]2− (Ag52 for short) and Ag54(S-Adm)30Br4H20 (Ag54 for short) nanoclusters, while TOABr selected the [Ag58(S-Adm)30Br4(NO3)2H22]2+ (Ag58 for short) nanocluster, where S-Adm is 1-adamantane thiol and TOABr is tetraoctylammonium bromide. The atomically precise structures of the three silver nanoclusters were determined by single-crystal X-ray diffraction (SC-XRD) and further confirmed by electrospray ionization mass spectrometry (ESI-MS) and nuclear magnetic resonance (NMR) measurements. The Ag52 and Ag54 nanoclusters were structurally homologous in terms of the kernel structures and the overall configurations, while the Ag58 nanocluster exhibited a completely different geometric structure. In addition, the optical performances, including optical absorptions and emissions, of the three silver nanoclusters were compared.
Results and discussion
The three medium-sized Ag nanoclusters (i.e., Ag52, Ag54, and Ag58) were controllably synthesized via a one-pot mild-reduction method (see the ESI for more details†). The borane-tert-butylamine complex was exploited as the reducing agent in this work to accomplish the mild reduction of the nanoclusters. Specifically, the structurally homologous Ag52 and Ag54 nanoclusters were prepared simultaneously and distinguished in the SC-XRD process, while the Ag58 nanocluster was prepared separately. The critical points for directing different nanoclusters (Ag52 and Ag54, or Ag58) was the introduction of different bromine-containing counterion salts—PPh4Br or TOABr (Fig. 1). The different cluster products might be attributed to the following two aspects: (i) the alkane chain in TOABr was flexible, while the benzene ring in PPh4Br was rigid. The different steric hindrances of two counterion salts resulted in distinct restriction effects in the structural rearrangement among the cluster formation process; (ii) the different interactions between counter-cations (PPh4+ or TOA+) and cluster molecules would remarkably affect the final cluster formation.The structures of Ag52 and Ag54 clusters were distinguished via SC-XRD (Fig. S1†). As depicted in Fig. 2, the Ag52 and Ag54 nanoclusters were highly structurally homologous in terms of their overall geometric structures. Both Ag52 and Ag54 clusters contained an Ag12 kernel consisting of three octahedral Ag6 units assembled via an Ag3-face-fusing pattern (Fig. 2A and B). The Ag12 kernel was further stabilized by four bromine ligands and seven Ag5(SR)4 motifs via Ag–Br and Ag–S interactions, respectively (Fig. 2C and D). The obtained A46(SR)28Br4 structure presented the C2 symmetry (Fig. 2D). The only difference between the geometric structures between Ag52 and Ag54 nanoclusters was their bridging motifs on two ends: two Ag linkers and two Ag2(SR)1 motifs in the Ag52 nanocluster, or two Ag linkers and two Ag3(SR)1 motifs in the Ag52 nanocluster (Fig. 2E and F). In this context, the Ag52 and Ag54 nanoclusters were highly structurally homologous with subtle differences in their terminal structures. Such similar frameworks might be the root cause for the co-generation of the two nanoclusters and the high similarity of their crystals; accordingly, the Ag52 and Ag54 clusters could only be distinguished and separated via SC-XRD. However, the cocrystallization of the two structure-correlated nanoclusters was not formed, which was probably due to their distinct crystal lattice energies that forced them to be crystallized into their individual crystals.53,54 Indeed, the two nanoclusters followed different crystalline systems and space groups.
Due to their different terminal structures, Ag52 and Ag54 nanoclusters exhibited distinct bond lengths despite their homologous frameworks. Of note, the difference in peripheral structures of nanoclusters would also lead to corresponding changes in their kernel structures. For example, the octahedral Ag6 nuclei underwent elongation with the increasing cluster size. Specifically, the average bond length between Ag atoms in Ag6 nuclei of Ag52 was 2.825 Å, which increased to 2.908 Å for Ag54 (Fig. S2A and S3A†). Besides, the average lengths of the Ag(core)–Ag(motif) and Ag(core)–Br bonds were shortened from 2.992 and 2.762 Å for Ag52 to 2.946 and 2.715 Å for Ag54, respectively. In addition, both average lengths of Ag(motif)–Ag(motif) and Ag(motif)–S(motif) bonds were also reduced with the cluster size growth (Fig. S2 and S3†). The most significant structural difference between Ag52 and Ag54 was their terminal motif structures: Ag2(SR)1 in Ag52 and Ag3(SR)1 in Ag54. Compared to the average Ag–S bond length of 2.603 Å in the Ag2(SR)1 motif of the Ag52 cluster, the corresponding bonds in Ag54 were remarkably shortened to 2.548 Å (Fig. S4†). In this context, the surface structure of Ag54 becomes more densely packed relative to Ag52 due to the incorporation of two more Ag atoms. At the same time, the kernel structure is expanded with the cluster size growth, which might result from the pulling effect of the peripheral motif frameworks of nanoclusters on their innermost kernel structures.
The introduction of the TOABr counterion that contained flexible carbon tails gave rise to the generation of the Ag58 nanocluster. The overall structure of Ag58 contained an Ag6 core with Ag(core)–Ag(core) bond lengths ranging from 2.743 to 2.785 Å (on average, 2.770 Å). At both ends of the Ag6 core, two polar Ag atoms (marked in blue) were symmetrically anchored onto the corresponding Ag3 face, forming two Ag4 tetrahedra and constituting an Ag8 kernel (Fig. 3A and S5–S7†). The Ag8 kernel was fixed by a ring-shaped Ag8Br4 motif, and four Br atoms were connected to four Ag atoms at adjacent positions on the Ag6 core, constituting an Ag16Br4 structure (Fig. 3B). In contrast to the exposed Br atoms in Ag52 and Ag54, the Br atoms in the Ag58 nanocluster were shielded. The average bond length of the Ag(core)–Br bonds in Ag58 was 2.609 Å, indicating a more compact kernel structure relative to those in Ag52 and Ag54 (Fig. S2–S4†). Furthermore, three Ag5(SR)4 motifs further stabilized the Ag16Br4 structure, forming an Ag46(SR)24Br4 framework (Fig. 3C). Each three adjacent Ag5(SR)4 subunits were inter-connected via Ag–S interactions, forming the Ag15(SR)12 structure in a “hand in hand” manner (Fig. S6B†). Besides, each Ag5(SR)4 motif structure corresponded to the three exposed Ag3 faces (blue faces in Fig. 3C), wherein the middle Ag atom in Ag5(SR)4 connected the polar Ag atoms at the ends of the Ag8 kernel. Finally, two Ag6(SR)3(NO3) motifs were attached at both ends of the Ag46(SR)24Br4 structure, giving rise to the overall Ag58(SR)30Br4(NO3)2 structure (Fig. 3D). The average length of the Ag(core)–Ag(core) bonds in the kernel of Ag58 was measured at 2.770 Å, remarkably shorter than those in the Ag52 and Ag54 nanoclusters (Fig. S5A†), while the average Ag(core)–Ag(motif) bond length of Ag58 was much longer than those of Ag52 and Ag54 (Fig. S5B;† 3.191 Å versus 2.992 and 2.946 Å). In this context, the Ag58 nanocluster exhibited a more compact kernel structure compared to a more scattered kernel–motif shell interaction relative to the Ag52 and Ag54 nanoclusters.
Structurally, all three silver nanoclusters contained 30 thiol ligands, and the coordination modes of S (i.e., the interaction modes between S and Ag) in the three nanoclusters were compared. The 30 thiol ligands in Ag52 and Ag54 exhibited three types of bonding patterns, μ2-S, μ3-S, and μ4-S, by analyzing the number of Ag atoms connecting the S atom, while only μ2-S and μ3-S were observed in the Ag58 nanocluster. As the cluster size increased from Ag52 to Ag54, the surface structure of the nanocluster became more complex with a transformation of some μ2-S into μ3-S (Fig. S8†). The average S–Ag coordination numbers of Ag52 and Ag54 nanoclusters were determined to be 2.324 and 2.378, respectively, demonstrating that more S–Ag interactions were generated on the Ag54 nanocluster surface. Moreover, a comparison of crystalline arrangement patterns was conducted (Fig. S9–S12†). The lattice symmetry of Ag52 (orthogonal system), Ag58 (monoclinic system), and Ag54 (triclinic system) was ordered from high to low. Accordingly, the different molecular structures of these nanoclusters could remarkably affect their supramolecular assemblies. Indeed, the supramolecular crystalline density was increased in direct proportion to the size of the nanocluster (Fig. S12†). In addition, the X-ray photoelectron spectroscopy (XPS) results of these nanoclusters demonstrated that there was no change for the Ag 3d, Br 3d, and S 2p binding energies (Fig. S13–15†), further confirming their homologous structural characterization.
The ESI-MS measurement was performed to confirm the compositions and determine the valence states of these silver nanoclusters. The mass peaks at 5484.72 and 5808.24 Da confirmed the compositions of the Ag52 and Ag58 nanoclusters and demonstrated their valence states as [Ag52(S-Adm)30Br4H20]2− and [Ag58(S-Adm)30Br4H22]2+, respectively (Fig. S16†). It should be noted that the two NO3 ligands were dissociated in the ESI-MS measurement. According to the valence states of these nanoclusters, their nominal electron counts were determined as 52(Ag) − 30(SR) − 4(Br) −20(H) + 2(charge) = 0e for [Ag52(SR)30Br4H20]2− and 58(Ag) − 30(SR) − 4(Br) − 22(H) − 2(charge) = 0e for [Ag58(SR)30Br4H22]2+, demonstrating the absence of the free electron in such cluster frameworks. However, no mass signal was detected for the Ag54 cluster (Fig. S17†). Considering that the Ag52 and Ag54 nanoclusters shared a homologous structure, we proposed that the molecular formula for Ag54 should be Ag54(SR)30Br4H20. In this context, the Ag54 nanocluster should be electrically neutral and was difficult to ionize in the mass detection. All three silver clusters contained a large number of hydride ligands, which was rare in previously reported metal nanoclusters. However, the precise locations of such hydrides were hard to determine using an SC-XRD diffractometer due to their low electron densities. To address this issue, we attempted to replace the “H” ligands with “D” to further verify their numbers in nanoclusters by ESI-MS. However, no deuterated borane-tert-butylamine complex (the reducing agent in this work) was commercially available. We have tried several times to replace the reducing agent with NaBH4, for which deuterated NaBD4 was available; however, no cluster product was obtained since all products were decomposed after the preparation. In this context, we just employed 1H NMR spectroscopy to confirm the presence of hydrides in the nanocluster. For the Ag52 nanocluster, three prominent NMR peaks at 4.67, 5.29, and 8.07 ppm were detected for the nanocluster with an area ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while no corresponding signal was observed for the HS-Adm ligand. Consequently, it is reasonable that there are three types of hydrides within the framework of the Ag52 nanocluster.
1, while no corresponding signal was observed for the HS-Adm ligand. Consequently, it is reasonable that there are three types of hydrides within the framework of the Ag52 nanocluster.
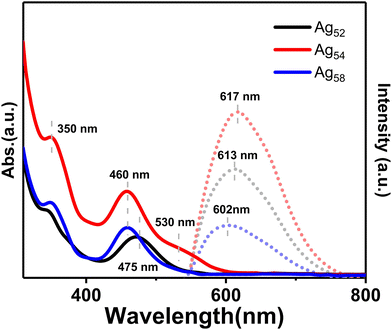
The structure of clusters is the decisive factor of their physical–chemical properties. The differences in molecular structures of the three nanoclusters resulted in their distinct optical absorptions and emissions (Fig. 4). Specifically, the Ag52 nanocluster exhibited two distinctive absorptions at 350 and 475 nm, while Ag54 displayed two absorptions at 350 and 460 nm, and a shoulder band at 530 nm. In comparison, the Ag58 nanocluster presented two absorptions at 350 and 460 nm. Besides, the emission wavelengths of the Ag52, Ag54, and Ag58 nanoclusters were 613, 617, and 602 nm, respectively. In addition, compared to the Ag54 and Ag52 nanoclusters with homologous structures, the Ag58 nanocluster exhibited a remarkably reduced photoluminescence intensity. Besides, we found that the Ag54 nanocluster exhibited an enhanced PL intensity relative to its structure-related Ag52 nanocluster. Such an enhancement was rational by considering that the Ag54 nanocluster showed a more compact molecular structure, which decreased the non-radiative transition and enhanced the radiative transition (i.e., the PL).55,56
Conclusion
In summary, three medium-sized silver nanoclusters, namely, Ag52, Ag54, and Ag58, were controllably synthesized by adjusting the bromine-containing counterion salts in the synthetic process. Accordingly, the effects of Br-based counterion salts in directing the structures of silver nanoclusters were evaluated. Among the three nanoclusters, Ag52 and Ag54 were structurally homologous in terms of their kernel structures and overall configurations, while the configuration of the Ag58 nanocluster was completely different. The structure-dependent optical properties (i.e., optical absorption and emission) of the three nanoclusters were evaluated. The findings in this work not only expand the structural diversity of the silver nanocluster family but also demonstrate the counterion effect in the preparation of new nanoclusters with novel structures.Data availability
CCDC 2301180, 2301182 and 2301183 contain the supplementary crystallographic data for this paper.†Author contributions
H. L. and X. W. performed the experiments. H. L., X. W. and X. K. wrote the manuscript. X. K. and M. Z. supervised the project. All authors commented on and agreed on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the NSFC (21631001, 21871001, 22371003, and 22101001) and the Ministry of Education, and the University Synergy Innovation Program of Anhui Province (GXXT-2020-053).References
- E. Roduner, Chem. Soc. Rev., 2006, 35, 583 RSC.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346 CrossRef CAS PubMed.
- Y. Li, M. Zhou and R. Jin, Adv. Mater., 2021, 33, 2006591 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422 RSC.
- Y. Jin, C. Zhang, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Soc. Rev., 2021, 50, 2297 RSC.
- W. Ishii, Y. Okayasu, Y. Kobayashi, R. Tanaka, S. Katao, Y. Nishikawa, T. Kawai and T. Nakashima, J. Am. Chem. Soc., 2023, 145, 11236 CrossRef CAS PubMed.
- Q. Li, C. J. Zeman IV, G. C. Schatz and X. W. Gu, ACS Nano, 2021, 15, 16095 CrossRef CAS PubMed.
- L. Fang, W. Fan, G. Bian, R. Wang, Q. You, W. Gu, N. Xia, L. Liao, J. Li, H. Deng, N. Yan and Z. Wu, Angew. Chem., Int. Ed., 2023, 62, e202305604 CrossRef CAS PubMed.
- Y. Chen, M. Zhou, Q. Li, H. Gronlund and R. Jin, Chem. Sci., 2020, 11, 8176 RSC.
- K. Kwak and D. Lee, Acc. Chem. Res., 2019, 52, 12 CrossRef CAS PubMed.
- X. Ouyang, Y. Wu, L. Guo, L. Li, M. Zhou, X. Li, T. Liu, Y. Ding, H. Bu, G. Xie, J. Shen, C. Fan and L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202300893 CrossRef CAS PubMed.
- Y. Feng, N. Wang and H. Ju, Sci. China: Chem., 2022, 65, 2417 CrossRef CAS.
- D. Wang, X. Gao, J. Jia, B. Zhang and G. Zou, ACS Nano, 2023, 17, 355 CrossRef CAS PubMed.
- J. Foxley and K. L. Knappenberger, Annu. Rev. Phys. Chem., 2023, 74, 53 CrossRef CAS PubMed.
- Y. Liu, W. Luo, Q. Fan, H. Ma, Y. Yin, Y. Long and J. Guan, Adv. Funct. Mater., 2023, 2303470 CrossRef CAS.
- O. López-Estrada, B. Zuniga-Gutierrez and E. Selenius, Nat. Commun., 2021, 12, 2477 CrossRef PubMed.
- Y. Li, S. Biswas, T. Luo, R. Juarez-Mosqueda, M. G. Taylor, G. Mpourmpakis, N. L. Rosi, M. P. Hendrich and R. Jin, Chem. Mater., 2020, 32, 9238 CrossRef CAS.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Chem. Rev., 2021, 121, 567 CrossRef CAS PubMed.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526 CrossRef CAS PubMed.
- Z. Wang, M. Du, P. Braunstein and J. Lang, J. Am. Chem. Soc., 2023, 145, 9982 CrossRef CAS PubMed.
- J.S. Kim, H. Chang, S. Kang, S. Cha, H. Cho, S.J. Kwak, N. Park, Y. Kim, D. Kang, C.K. Song, J. Kwag, J. Hahn, W.B. Lee, T. Hyeon and J. Park, Nat. Commun., 2023, 14, 3201 CrossRef CAS PubMed.
- W. Suzuki, R. Takahata, Y. Chiga, S. Kikkawa, S. Yamazoe, Y. Mizuhata, N. Tokitoh and T. Teranishi, J. Am. Chem. Soc., 2022, 144, 12310 CrossRef CAS PubMed.
- Y. Wang, B. Nieto-Ortega and T. Burgi, Nat. Commun., 2020, 11, 4562 CrossRef CAS PubMed.
- Y.-P. Xie, Y.-L. Shen, G.-X. Duan, J. Han, L.-P. Zhang and X. Lu, Mater. Chem. Front., 2020, 4, 2205 RSC.
- S. Takano and T. Tsukuda, J. Am. Chem. Soc., 2021, 143, 1683 CrossRef CAS PubMed.
- Y. Cao, S. Malola, M. F. Matus, T. Chen, Q. Yao, R. Shi, H. Häkkinen and J. Xie, Chem., 2021, 7, 2227 CAS.
- S. Takano and T. Tsukuda, J. Am. Chem. Soc., 2021, 143, 1683 CrossRef CAS PubMed.
- Y. Cao, S. Malola, M. F. Matus, T. Chen, Q. Yao, R. Shi, H. Häkkinen and J. Xie, Chem., 2021, 7, 2227 CAS.
- Y. Cao, J. Guo, R. Shi, G. I. N. Waterhouse, J. Pan, Z. Du, Q. Yao, L.-Z. Wu, C.-H. Tung, J. Xie and T. Zhang, Nat. Commun., 2018, 9, 2379 CrossRef PubMed.
- Y. Wang and T. Burgi, Nanoscale Adv., 2021, 3, 2710 RSC.
- C.-Y. Liu, S.-F. Yuan, S. Wang, Z.-J. Guan, D.-E. Jiang and Q.-M. Wang, Nat. Commun., 2022, 13, 2082 CrossRef CAS PubMed.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Acc. Chem. Res., 2018, 51, 3114 CrossRef CAS PubMed.
- Q. Yao, X. Yuan, T. Chen, D. T. Leong and J. Xie, Adv. Mater., 2018, 30, 1802751 CrossRef PubMed.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376 CrossRef CAS PubMed.
- T. Higaki, Q. Li, M. Zhou, S. Zhao, Y. Li, S. Li and R. Jin, Acc. Chem. Res., 2018, 51, 2764 CrossRef CAS PubMed.
- H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Chem. Commun., 2013, 49, 300 RSC.
- X. Shen, X. Ma, Q. Ni, M. Ma, L. Gui, C. Hou, R. Hou and X. Wang, Nanoscale, 2018, 10, 515 RSC.
- H. Yang, Y. Wang and N. Zheng, Nanoscale, 2013, 5, 2674 RSC.
- W. Chang, P. Lee, J. Liao, K. K. Chakrahari, S. Kahlal, Y. Liu, M. Chiang, J. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2017, 56, 10178 CrossRef CAS PubMed.
- R. S. Dhayal, J. Liao, Y. Liu, M. Chiang, S. Kahlal, J. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 3702 CrossRef CAS PubMed.
- C. Liu, T. Li, H. Abroshan, Z. Li, C. Zhang, H. J. Kim, G. Li and R. Jin, Nat. Commun., 2018, 9, 744 CrossRef PubMed.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. D. Gobbo, R. G. AbdulHalim, M. Eddaoudi, D.-E. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970 CrossRef CAS PubMed.
- X. Ma, Y. Bai, Y. Song, Q. Li, Y. Lv, H. Zhang, H. Yu and M. Zhu, Angew. Chem., Int. Ed., 2020, 59, 17234 CrossRef CAS PubMed.
- H. Yang, Y. Wang, X. Chen, X. Zhao, L. Gu, H. Huang, J. Yan, C. Xu, G. Li, J. Wu, A. J. Edwards, B. Dittrich, Z. Tang, D. Wang, L. Lehtovaara, H. Häkkinen and N. Zheng, Nat. Commun., 2016, 7, 12809 CrossRef CAS PubMed.
- L. Ren, P. Yuan, H. Su, S. Malola, S. Lin, Z. Tang, B. K. Teo, H. Häkkinen, L. Zheng and N. Zheng, J. Am. Chem. Soc., 2017, 139, 13288 CrossRef CAS PubMed.
- Y. Song, K. Lambright, M. Zhou, K. Kirschbaum, J. Xiang, A. Xia, M. Zhu and R. Jin, ACS Nano, 2018, 12, 9318 CrossRef CAS PubMed.
- I. Chakraborty, A. Govindarajan, J. Erusappan, A. Ghosh, T. Pradeep, B. Yoon, R. L. Whetten and U. Landman, Nano Lett., 2012, 12, 5861 CrossRef CAS PubMed.
- L. Ren, P. Yuan, H. Su, S. Malola, S. Lin, Z. Tang, B. K. Teo, H. Häkkinen, L. Zheng and N. Zheng, J. Am. Chem. Soc., 2017, 139, 13288 CrossRef CAS PubMed.
- J. Liu, F. Alkan, Z. Wang, Z. Zhang, M. Kurmoo, Z. Yan, Q. Zhao, C. M. Aikens, C. Tung and D. Sun, Angew. Chem., Int. Ed., 2019, 58, 195 CrossRef CAS PubMed.
- M. Ma, X. Ma, G. Liang, X. Shen, Q. Ni, L. Gui, X. Wang, S. Huang and S. Li, J. Am. Chem. Soc., 2021, 143, 13731 CrossRef CAS PubMed.
- H. Yang, Y. Wang, X. Chen, X. Zhao, L. Gu, H. Huang, J. Yan, C. Xu, G. Li, J. Wu, A. J. Edwards, B. Dittrich, Z. Tang, D. Wang, L. Lehtovaara, H. Häkkinen and N. Zheng, Nat. Commun., 2016, 7, 1280 Search PubMed.
- X. Kang and M. Zhu, ACS Mater. Lett., 2020, 2, 1303 CrossRef CAS.
- H. Shen, X. Wei, C. Xu, S. Jin, S. Wang, X. Kang and M. Zhu, Nanoscale, 2021, 13, 7694 RSC.
- J. Xin, J. Xu, C. Zhu, Y. Tian, Q. Zhang, X. Kang and M. Zhu, Chem. Sci., 2023, 14, 8474 RSC.
- X. Kang, S. Wang and M. Zhu, Chem. Sci., 2018, 9, 3062 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S18 and Tables S1–S3 for the structure comparison, and ESI-MS, XPS, and NMR results of nanoclusters. CCDC 2301180, 2301182 and 2301183. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3nr05464k |
| This journal is © The Royal Society of Chemistry 2024 |