Open Access Article
Open Access ArticleSelenium reduction pathways in the colloidal synthesis of CdSe nanoplatelets†
Alessio
Di Giacomo
a,
Alina
Myslovska
a,
Vic
De Roo
 b,
Jan
Goeman
b,
José C.
Martins
b,
Jan
Goeman
b,
José C.
Martins
 b and
Iwan
Moreels
b and
Iwan
Moreels
 *a
*a
aDepartment of Chemistry, Ghent University, 9000-Gent, Belgium. E-mail: iwan.moreels@ugent.be
bDepartment of Organic and Macromolecular Chemistry, Ghent University, 9000-Gent, Belgium
First published on 22nd February 2024
Abstract
Several established procedures are now available to prepare zinc blende CdSe nanoplatelets. While these protocols allow for detailed control over both thickness and lateral dimensions, the chemistry behind their formation is yet to be unraveled. In this work, we discuss the influence of the solvent on the synthesis of nanoplatelets. We confirmed that the presence of double bonds, as is the case for 1-octadecene, plays a key role in the evolution of nanoplatelets, through the isomerization of the alkene, as confirmed by nuclear magnetic resonance spectroscopy and mass spectrometry. Consequently, 1-octadecene can be replaced as a solvent (or solvent mixture), however, only by one that also contains α protons to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. We confirm this via synthesis of nanoplatelets in hexadecane spiked with a small amount of 1-octadecene, and in the aromatic solvent 1,2,3,4-tetrahydronaphthalene (tetralin). At the same time, the chemical reaction leading to the formation of nanoplatelets occurs to some extent in saturated solvents. A closer examination revealed that an alternative formation pathway is possible, through interaction of carboxylic acids, such as octanoic acid, with selenium. Next to shedding more light on the synthesis of CdSe nanoplatelets, fundamental understanding of the precursor chemistry paves the way to use optimized solvent admixtures as an additional handle to control the nanoplatelet synthesis, as well as to reduce potential self-polymerization hurdles observed with 1-octadecene.
C double bonds. We confirm this via synthesis of nanoplatelets in hexadecane spiked with a small amount of 1-octadecene, and in the aromatic solvent 1,2,3,4-tetrahydronaphthalene (tetralin). At the same time, the chemical reaction leading to the formation of nanoplatelets occurs to some extent in saturated solvents. A closer examination revealed that an alternative formation pathway is possible, through interaction of carboxylic acids, such as octanoic acid, with selenium. Next to shedding more light on the synthesis of CdSe nanoplatelets, fundamental understanding of the precursor chemistry paves the way to use optimized solvent admixtures as an additional handle to control the nanoplatelet synthesis, as well as to reduce potential self-polymerization hurdles observed with 1-octadecene.
Introduction
Starting with the pioneering work of Murray et al. in 1993,1 colloidal semiconductors with nanometer dimensions gained progressively more attention, nowadays reaching marketable technological applications such as displays, photodetectors or energy harvesters.2,3 The progress in synthetic procedures over the last three decades has allowed continuous improvement of control over size, size dispersion, and shape, yielding tailored optoelectronic properties and, at the same time, a detailed understanding of the associated nucleation and growth mechanisms.A first, and key step in the synthesis process is given by the formation of monomers from suitable metal and pnictogen,4 chalcogen,5,6 or halogen7 precursors. The monomer formation rate determines the monomer supersaturation in solution, and consequently the critical size of nuclei as well as the final nanocrystal size, size dispersion and concentration.8 Focusing, in the context of this work, on metal chalcogenide nanocrystals, initial studies targeted the mechanistic understanding of the nanocrystal synthesis using phosphine chalcogenides,5,9 yet, in an effort to avoid phosphine-based solvents and precursors, attention rapidly shifted to 1-octadecene (ODE).
The earlier literature often referred to it as a noncoordinating solvent.10–12 On the other hand, the reactivity of chalcogens with carbon–carbon double bonds is well known and also industrially exploited, with vulcanization processes (production of hard crosslinked rubbers) being the best example.13 Also for the CdSe quantum dot synthesis, it was rapidly realized that ODE can yield a source of reactive selenium.14–16 Upon further investigation of the nature of the chalcogen precursor in ODE, Deng et al.17 demonstrated that dehydrogenation of saturated alkane molecules at elevated temperature, of 300 °C, can lead to the formation of H2Se. Others, studying either formation of H2S15 or H2Se16 confirmed that this mechanism is also present in ODE, albeit with a reduced yield compared to a saturated alkane.17 These results align well with the notion that selenium can be used as a dehydrogenating agent in the determination of natural products’ structures, as well as in the aromatization step of complex molecules.18,19 In addition to the formation of H2Se, Bullen et al.20 observed that a different source of reactive Se is produced through isomerization of the double bond in ODE, leading to reactive ODEx-Sey species. In conclusion, the key observation is that all these reactions reduce metallic Se0 to Se2−, a prerequisite to obtain the CdSe monomers.
Among the various nanocrystal shapes that can be produced at present, nanoplatelets (NPLs) have recently emerged as an exciting class of materials. The quantum confinement along their thickness direction is defined by the number of N monolayers (MLs, where the N Se crystalline planes are bound to N + 1 planes of Cd), and is tunable with atomic precision.21 The resulting NPLs possess peculiar optoelectronical properties compared to 0D quantum dots, such as a faster photoluminescence (PL) decay,22 narrower emission line widths, and higher optical gain coefficients,23,24 making them potential candidates for efficient light-emitting diodes or lasers.24–28
Established procedures to synthesize NPLs are mostly based on metal carboxylates as precursors and Se dissolved in ODE, and yield highly luminescent zinc blende CdSe NPLs with precise control over their thickness and lateral dimensions.29–35 The approach can be generalized by the following chemical reaction:
 | (1) |
ΔT refers to the high temperatures, typically exceeding 200 °C, required in these syntheses. In addition, short-chained cadmium carboxylates36 or carboxylic acids34 are added after nucleation of small CdSe seeds to promote lateral growth of 2D nanocrystals. Several synthetic protocols allow for control over the structural and optoelectronic properties of the resulting NPLs,21 but the underlying chemistry remains somewhat ill-defined. In eqn (1), one can notice that the metallic Se0 is employed, which should again be reduced to Se2−. As the metal center retains its oxidation state, the likely redox partner is again ODE.
Considering its central role in the CdSe NPL synthesis, and that recently, practical concerns about the usage of ODE were reported, in particular its ability to form polymers of sizes comparable to the nanocrystals, which can hamper proper sample purification,37,38 we have investigated the precursor chemistry behind the synthesis of CdSe NPLs. Focusing on the behavior of ODE in the presence of Se, we confirm the active participation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in our synthesis, however, here not leading to H2Se, but yielding (ODE–Se)2. We also demonstrate that an alternative pathway exists via a reaction with carboxylic acids, leading to Sex anhydride complexes, further enriching the reaction pathways. We finally demonstrate that we can perform the synthesis in alternative solvents, such as saturated alkanes (hexadecane) spiked with small amounts of ODE, or in aromatic solvents (tetralin), both providing a source of C
C double bond in our synthesis, however, here not leading to H2Se, but yielding (ODE–Se)2. We also demonstrate that an alternative pathway exists via a reaction with carboxylic acids, leading to Sex anhydride complexes, further enriching the reaction pathways. We finally demonstrate that we can perform the synthesis in alternative solvents, such as saturated alkanes (hexadecane) spiked with small amounts of ODE, or in aromatic solvents (tetralin), both providing a source of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds without requiring ODE as main solvent.
C double bonds without requiring ODE as main solvent.
Experimental
Materials
Cadmium oxide (≥99.99%), sodium myristate (Na(myr), ≥99%), octanoic acid (≥98.0%), 1,2,3,4-tetrahydronaphthalene (tetralin, synthesis grade) and chloroform-d (CDCl3, d, 99.8%) were purchased from Merck/Sigma-Aldrich. n-Hexadecane (HD, 95%) was purchased from Fiers. 1-Octadecene (ODE, 90%), oleic acid (OA, 90%) were purchased by Alfa Aesar. Cadmium nitrate tetrahydrate (99+%), propionic acid (99.5+%), toluene (>99%), methanol (99.8+%) and 2-propanol (IPA, 99+%) were purchased from Chem Lab. Acetonitrile (ACN, 99.9%) was purchased from Carl-Roth. Selenium powder (≥99.99%) and cadmium acetate dihydrate 98% were purchased by Acros Organics. All chemicals were used without further purification. A vacuum/dry nitrogen gas Schlenk line was used for nanocrystal syntheses. The purifications were conducted in air unless specified otherwise.Cadmium octanoate preparation (Cd(oct)2)
The cadmium precursor was prepared according to a reported procedure.34 Briefly, in a three-neck flask, 2.0 g (15.7 mmol) of CdO was added to 6.5 mL (41 mmol) of octanoic acid. While stirring, the system was heated under N2 atmosphere to 180 °C, until a colorless solution is obtained. The reaction was kept at 180 °C for 20 minutes and then cooled. During cooling, between 110 °C and 80 °C, the mixture was connected to a vacuum line to remove the water produced in the condensation reaction. The colorless solution was rapidly transferred to centrifugation tubes, acetone was added and a white solid was precipitated after centrifugation. The solid fraction was centrifuged with fresh acetone three additional times to purify the reaction product, then dried overnight under vacuum.Cadmium myristate preparation (Cd(myr)2)
6 g of cadmium nitrate tetrahydrate were mixed with 400 mL of methanol using a stirring bar. Separately, 10 g of Na(myr)2 were mixed with 300 mL of methanol and sonicated for an hour. The Cd solution was then added dropwise to the myristate precursor over a period of approximately 4 hours, while stirring. The mixture was then filtered using a Buchner funnel in a Buchner flask connected to the Schlenk line. The final product was dried overnight under vacuum.Cadmium oleate preparation (Cd(OA)2 in ODE)
In a three-neck round bottom flask CdO (15 mmol) was added to OA (45 mmol) and ODE (15 mL). The mixture was degassed under vacuum at 80 °C for 1 hour. Under nitrogen, the mixture was heated to 200 °C for 15 minutes, until full dissolution of the solid. After removing the thermomantle the mixture was allowed to cool down and finally transferred into vials for storage.Synthesis of ODE–Se reagent
The selenium precursor was prepared according to a reported procedure.20 In a 25 mL three-neck flask 1.02 mmol of selenium powder and 10 mL of ODE were added. The system was placed under N2 and then heated to 200 °C for 2 h. A yellow solution was formed. The reaction mixture was transferred in glovebox and passed through a 450 μm pore size syringe filter to remove eventual unreacted selenium mesh. The reagent was stored in glovebox.Synthesis of OctAc–Se reagent
In a 25 mL round bottom flask, 1.25 mmol of selenium was added to 10 mL of octanoic acid. The suspension was degassed for 1 h at 100 °C, followed by heating, under N2, to 160 °C for 60 minutes. The temperature was then set to 220 °C for 30 minutes, followed by 230 °C for 30 minutes, until everything dissolved. An orange solution was obtained, which upon cooling down turned grey. The reaction mixture was transferred to a glovebox and passed through a 450 μm pore size syringe filter to remove unreacted selenium mesh. A pale yellow solution was obtained.Synthesis of 3.5 ML CdSe NPLs with Cd(oct)2 in different solvents
The NPLs were prepared with slight modifications to a procedure reported elsewhere.34 Briefly, in a 25 mL three-neck flask 0.50 mmol of Cd(oct)2 and 0.26 mmol of Se powder were mixed with ca. 12 mL of the solvent of choice. Four different trials were conducted: HD (12 mL, 40.8 mmol), ODE (12 mL, 37.5 mmol), HD + ODE (two different runs: 40.8 mmol HD + 0.06 mmol ODE and 40.8 mol HD + 0.5 mmol ODE, respectively) and tetralin (12 mL, 88.0 mmol). The suspension was degassed for 1 h at 100 °C, followed by heating the mixture, under N2, to 160 °C for 10 minutes. The temperature was then set to 220 °C and, at 200 °C, 100 μL (1.33 mmol) of propionic acid dispersed in 1 mL of HD, was swiftly injected. The reaction proceeded for 20–185 minutes at 220 °C, then heating was removed to cool the system. When the temperature reached 160 °C, 0.80 g of Cd(OA)2 in ODE was injected, the suspension was cooled to 80 °C and the mixture transferred into vials to proceed with purification.Synthesis of CdSe NPLs with Cd(oct)2 and ODE–Se reagent
The synthesis of CdSe was conducted following the same procedure described previously in HD (12 mL, 40.8 mmol), but replacing selenium mesh with the equimolar amount of ODE–Se solution (2.5 mL, 0.25 mmol Se). The purification was conducted according to the protocol described below.Synthesis of CdSe NPLs with Cd(oct)2 and OctAc–Se reagent
The synthesis followed the same procedure as for ODE–Se, but with 199.4 mg Cd(oct)2, 2.6 mL of OctAc–Se and 9.4 mL of HD. The growth time was set at 60 minutes.General purification protocol for CdSe NPLs
The crude synthesis product was mixed with 15 mL toluene and 10 mL of a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) solution of IPA
1 (v/v) solution of IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN was added. The suspension was centrifuged for 10 minutes at 4500 rpm. The supernatant was discarded, the solid was redispersed in toluene and the precipitation was repeated twice. The final solid was dispersed in 5 mL hexane and centrifuged at 4200 rpm to remove unreacted carboxylates. The solid was discarded and the supernatant, containing CdSe NPLs, was collected and stored in hexane.
ACN was added. The suspension was centrifuged for 10 minutes at 4500 rpm. The supernatant was discarded, the solid was redispersed in toluene and the precipitation was repeated twice. The final solid was dispersed in 5 mL hexane and centrifuged at 4200 rpm to remove unreacted carboxylates. The solid was discarded and the supernatant, containing CdSe NPLs, was collected and stored in hexane.
Specific purification of CdSe NPLs synthesized in tetralin
We applied the general procedure above, however, in the last step, we collected the precipitate containing CdSe NPLs, and stored it in hexane.Synthesis and purification of 2.5 ML CdSe NPLs
In a 25 mL three-neck flask, 0.50 mmol of Cd(oct)2 and 0.26 mmol of Se powder were mixed with 12 mL of HD. The suspension was degassed for 1.5 h at 100 °C, followed by heating the mixture, under N2, to 160 °C for 10 minutes. The temperature was then set to 190 °C and, at 170 °C, 100 μL of propionic acid dispersed in 1 mL of HD, was swiftly injected. After 30 minutes, heating was removed to cool the system, and 1 mL of a 0.5 M Cd(OA)2 solution in ODE was injected, the suspension was cooled to 80 °C and the mixture transferred into vials to proceed with purification.The crude synthesis product was first centrifuged at 2500 rpm for 10 minutes. The supernatant was retained, 1.2 mL of IPA and 6 mL of EtOH were added, the mixture was centrifuged for 5 minutes at 5000 rpm and the resulting precipitate was redispersed in hexane.
Synthesis and purification of 5.5 ML CdSe NPLs
In a 25 mL three-neck flask, 0.30 mmol of Cd(myr)2 was dissolved in 12 mL of hexadecane. The solution was degassed for 1.5 h at 100 °C, followed by heating, under N2, to 250 °C. At that temperature, 24.5 mg of Se-mesh (0.31 mmol) in a mixture of 800 μL of HD and 192 μL of ODE (0.6 mmol) were injected. After one minute, 144 mg of Cd(Ac)2·2H2O was inserted. The reaction proceeded for an additional 440 seconds, then the heating was removed, 300 μL of 0.5 M Cd(OA)2 in ODE was injected, and the reaction mixture was cooled with in a water bath.To purify the NPLs, 10 mL of the crude synthesis product was mixed with 20 mL hexane, 200 μL of oleic acid, 10 mL of IPA and 5 mL of EtOH. The suspension was centrifuged at 4800 rpm for 5 minutes. The precipitate was redispersed in 10 mL of hexane and centrifuged at 3000 rpm for 10 minutes. Finally, the precipitate was discarded, and the supernatant was stored.
Heating up of HD, ODE, and octanoic acid with selenium
In a 25 mL round bottom flask 1.25 mmol of Se was added to 10 mL of the solvent. The suspension was degassed for 1 h at 100 °C, followed by heating the mixture, under N2, to 160 °C for 10 minutes. The temperature was then set to 220 °C for 30 minutes. While hexadecane remained colorless, deep orange suspensions were obtained for ODE and octanoic acid. The solvents turned orange (ODE) and pale yellow (octanoic acid) after returning to room temperature. The reaction mixtures were transferred in a glovebox and passed through a 450 μm pore size syringe filter to remove eventual unreacted selenium mesh. The final products were stored in a glovebox.Transmission electron microscopy
Samples were prepared by drop-casting dilute n-hexane dispersions onto carbon-coated copper grids. Bright field TEM images were acquired on a JEOL JEM-1011 microscope (W filament) operating at an accelerating voltage of 100 kV.Optical characterization
Absorbance spectra were taken with a PerkinElmer Lambda 950 spectrometer. Photoluminescence measurements were performed on an Edinburgh Instruments FLSP920 UV–Vis–NIR spectrofluorometer, using a 450 W xenon lamp as the excitation source.NMR characterization
NMR measurements were recorded on a Bruker Avance III spectrometer operating at a 1H frequency of 500.13 MHz and equipped with a BBI-Z probe. Samples for nuclear magnetic resonance were dispersed in CDCl3. The sample temperature was set to 298 K. Standard pulse sequences as present in the Bruker library were used throughout. 1H and 13C chemical shift scales were calibrated by using the residual solvent signal as a secondary reference against TMS.Liquid and gas chromatography, coupled with mass spectrometry
LC-MS analysis was performed on an Agilent (Santa Clara, CA, U.S.A) 1100 Series HPLC coupled to an Agilent G1946C MS detector equipped with an electrospray ionization source, using a Kinetex column (C18, 150 × 4.60 mm, 5 μm particle size). The flow rate was 1.5 mL min−1 using a gradient consisting of 5 mM NH4OAc in H2O and CH3CN. Optimized gradients were used for each CLiP. GC-MS analysis was performed on an Agilent G1800B GC-MS system equipped with an electron ionization source, using a SSL inlet and helium as carrier gas.Results and discussion
Our study conveys a description of the ODE reactivity in the synthesis of CdSe NPLs. In this study we have worked under reaction conditions used for the synthesis of 3.5 monolayer (ML) thick CdSe NPLs, recently reported.34 We refer to our earlier work for the characterization of the reference NPLs,34 and summarize the procedure in eqn (2): | (2) |
Step (1) consists of raising the precursor mixture to a temperature of 200 °C, inducing a nucleation of CdSe seeds, and subsequently adding propionic acid to promote lateral growth. The NPLs are further grown in step (2), at 220 °C for 20 minutes. Cadmium octanoate (Cd(oct)2) was used as a metal precursor, as the chemical yield in NPLs is expected to be higher compared to other carboxylates.34 The investigation aimed to pinpoint the role of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond moiety in the reaction. If the alkene behaves simply as a noncoordinating solvent, one can rationalize that a solvent with comparable polarity and viscosity would not heavily impact the synthesis. Additionally, the potential self-polymerization issue in ODE37 could be completely avoided using a fully saturated alkane. We selected n-hexadecane (HD) as an alternative to ODE, as this solvent was recently demonstrated to be a viable medium for different nanocrystal syntheses.37,39
C double bond moiety in the reaction. If the alkene behaves simply as a noncoordinating solvent, one can rationalize that a solvent with comparable polarity and viscosity would not heavily impact the synthesis. Additionally, the potential self-polymerization issue in ODE37 could be completely avoided using a fully saturated alkane. We selected n-hexadecane (HD) as an alternative to ODE, as this solvent was recently demonstrated to be a viable medium for different nanocrystal syntheses.37,39
Working in identical conditions as in eqn (2), but changing the solvent composition to HD, Fig. 1 demonstrates that the synthesis progression in the two media is noticeably different. In particular, while in ODE the expected 3.5 ML NPLs with a band gap around 460 nm are readily formed after 120 seconds40 (Fig. 1a), in HD mainly 2.5 ML NPLs with a band gap around 390 nm (ref. 40) are formed (Fig. 1b), in addition to a small fraction of 3.5 ML NPLs and a broad background presumably due to scattering from large sheets.34,41,42 We infer that the different reactivity of the selenium precursor in the presence of ODE can be the explanation for the observed behavior. To strengthen this hypothesis, we have registered control experiments in which we have added known amounts of ODE (0.06 mmol and 0.5 mmol, respectively) to the HD solvent (12 mL, 40.8 mmol). The results are reported in Fig. 1c and d. Even with the small ODE amounts used, the spectra show a reduced background absorption and a relative intensity increase of the signals related to 3.5 ML NPLs, compared to 2.5 ML NPLs. This already suggests that the presence of the carbon–carbon double bond moiety causes ODE to partake in the NPL reaction, and that higher ODE concentrations increase the overall reaction rate, including the transformation rate from 2.5 ML to 3.5 ML NPLs via Ostwald ripening.43 ODE being a reagent has a double importance: firstly, it adds another layer of complexity to the system, as its concentration influences the evolution of the reaction. This information can be used for the optimization of the synthesis conditions e.g., to stabilize different NPL populations at given reaction temperatures. On the other hand, reducing the amount of ODE could reduce the potential self-polymerization issue that was discussed earlier by Dhaene et al.37
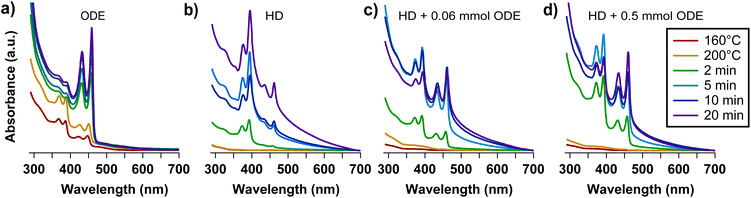 | ||
| Fig. 1 Temporal evolution the absorption spectrum of aliquots collected during NPL synthesis performed under conditions as in eqn (2), using different solvents. (a) ODE (12 mL, 37.5 mmol, reproduced from ref. 34), (b) n-hexadecane (HD, 12 mL, 40.8 mmol), and (c) and (d) HD with known amounts of ODE added (HD + 0.06 mmol ODE and HD + 0.5 mmol ODE, respectively). | ||
As for the reactivity, it was suggested that the naturally occurring polynuclear selenium can form complexes of nuclearity Sex (x = 2–3) when treated at high temperatures (T ≥ 200 °C) with species containing alkene protons, and radical mechanisms were suggested to take place in these conditions.18,19,44,45 To address the solvent-chalcogen reactivity, we heated mixtures of selenium and ODE or HD under the same conditions as in eqn (2), and we sampled the reaction mixture via nuclear magnetic resonance (NMR) spectroscopy. The 1H spectrum of the heated HD-Se mixture proved to be identical to the pristine HD (ESI, Fig. S1†), indicating that HD remains unaltered during heating. In agreement with this, no noticeable color change was observed after the heating (ESI, Fig. S2†). It has been reported in CdX (X = S, Se) syntheses that, starting from elemental chalcogens in saturated hydrocarbon solvents, reactive H2X evolved accompanied by the dehydrogenation of the alkane to alkene.15,17,46 The absence of signals in the characteristic alkene chemical shift region of 5.0–6.0 ppm discards this possibility under the synthesis conditions used in our work. Additionally, a test with Pb(OAc)2 doped paper did not show any precipitate (PbSe). Hence, we did not observe any evidence of selenium reduction from the HD solvent in our experiments.
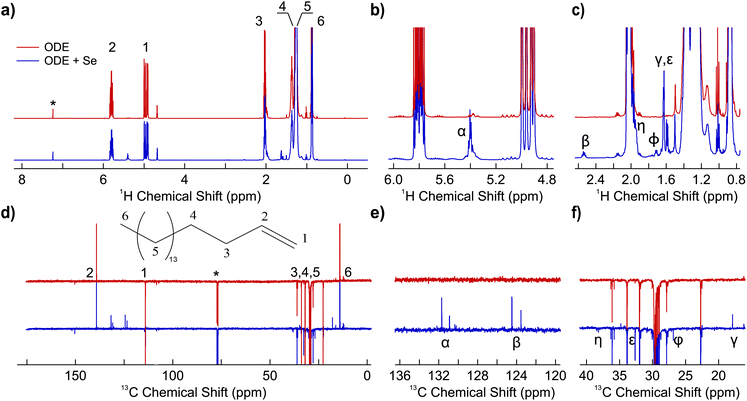
On the other hand, ODE displays a different behavior. After mixing selenium with ODE, the mixture evolves from colorless to dark orange at high temperatures, turning dark yellow after returning to room temperature (ESI, Fig. S2†). Fig. 2 shows the 1H and 13C NMR spectra of the heated mixture of Se and ODE. In the 1H spectra, new resonances are now visible. First, the emerging multiplet appearing around 5.4 ppm (α in Fig. 2b) can be attributed to the presence of non-terminal alkene protons.20 This is due to the formation of additional double bonds, e.g., octadec-1,3-diene, or to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C isomers, e.g., 2-ODE, 3-ODE and related organoselenium complexes. Both options are reported in literature when double bonds with available allylic protons react with selenium at high temperature.18,20,44 The new signals appearing in the region 1.55 ppm–1.75 ppm (ε, ϕ, γ of Fig. 2c) are compatible with allylic CH3 or CH2 in β position to a double bond. Furthermore, the additional resonance appearing around 1.95 ppm (η in Fig. 2c) is compatible with the presence of a CH2 in allylic position to the isomerized double bond, e.g., CH3CH
C isomers, e.g., 2-ODE, 3-ODE and related organoselenium complexes. Both options are reported in literature when double bonds with available allylic protons react with selenium at high temperature.18,20,44 The new signals appearing in the region 1.55 ppm–1.75 ppm (ε, ϕ, γ of Fig. 2c) are compatible with allylic CH3 or CH2 in β position to a double bond. Furthermore, the additional resonance appearing around 1.95 ppm (η in Fig. 2c) is compatible with the presence of a CH2 in allylic position to the isomerized double bond, e.g., CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-CH2R in 2-ODE, as well as to a related organoselenium complex (vide infra). Finally, the multiplet centered around 2.5 ppm (β in Fig. 2c) can be assigned to a CH2 in α position to a double bond of an organo-chalcogen species. Note that this peak also appears in the work of Bullen et al., however, it was not further investigated.20 In conclusion, the analysis of the 1H spectra points toward the isomerization of 1-ODE and the formation of related organo-selenium complexes. It has been proposed that the chalcogen behaves as a hydrogen transfer agent.18 This means that its interaction with C
CH-CH2R in 2-ODE, as well as to a related organoselenium complex (vide infra). Finally, the multiplet centered around 2.5 ppm (β in Fig. 2c) can be assigned to a CH2 in α position to a double bond of an organo-chalcogen species. Note that this peak also appears in the work of Bullen et al., however, it was not further investigated.20 In conclusion, the analysis of the 1H spectra points toward the isomerization of 1-ODE and the formation of related organo-selenium complexes. It has been proposed that the chalcogen behaves as a hydrogen transfer agent.18 This means that its interaction with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds is expected to be similar to the one of dioxygen, with the isomerization of the double bond proceeding via a radical mechanism.18,20,44
C double bonds is expected to be similar to the one of dioxygen, with the isomerization of the double bond proceeding via a radical mechanism.18,20,44
A similar conclusion can be drawn from the analysis of the 13C attached proton test (APT) NMR spectra of the same species, as shown in Fig. 2d–f. 13C APT is routinely used in organic synthesis as it allows to encode the multiplicity n of the different –CHn units in a compound into a positive (for CH/CH3) or negative (for CH2/Cq) signal phase, adding relevant structural information compared to a regular 1D 13C.47 The presence of new positive signals in the region 123.6 ppm–131.7 ppm (α, β, Fig. 2e) is compatible with the formation of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH moieties, as the two groups of signals (123.6–124.5 ppm and 130.9–131.7 ppm) can be related to the migration of the double bond along the aliphatic chain, as a consequence of the proposed radical mechanism.18,20,44 In addition, the two negative signals at 32.6 and 26.9 ppm (ε, ϕ in Fig. 2f) can be attributed to CH2 in α and β position to a double bond, respectively. Finally, the positive signal around 18.2 ppm (γ in Fig. 2f) can be assigned to a CH3 in α position to a double bond. To sum up, chemical-shift based analysis of the 1D NMR experiments support that, in our synthesis conditions, selenium acts as a hydrogen transfer agent, promoting the isomerization of the alkene and the formation of activated organoselenium complexes. The chemical shifts of the emerging species are summarized in Table 1.
CH moieties, as the two groups of signals (123.6–124.5 ppm and 130.9–131.7 ppm) can be related to the migration of the double bond along the aliphatic chain, as a consequence of the proposed radical mechanism.18,20,44 In addition, the two negative signals at 32.6 and 26.9 ppm (ε, ϕ in Fig. 2f) can be attributed to CH2 in α and β position to a double bond, respectively. Finally, the positive signal around 18.2 ppm (γ in Fig. 2f) can be assigned to a CH3 in α position to a double bond. To sum up, chemical-shift based analysis of the 1D NMR experiments support that, in our synthesis conditions, selenium acts as a hydrogen transfer agent, promoting the isomerization of the alkene and the formation of activated organoselenium complexes. The chemical shifts of the emerging species are summarized in Table 1.
| Nucleus | α | β | η | ε | ϕ | γ |
|---|---|---|---|---|---|---|
| 1H (ppm) | 5.40 | 2.54 | 1.96 | 1.59 | 1.71 | 1.63 |
| 13C (ppm) | 132.2 | 125.0 | 36.5 | 33.1 | 27.3 | 18.3 |
To confirm this analysis and reveal the underlying structure of the newly formed molecules, we have also registered 2D NMR experiments. The heteronuclear single quantum correlation (HSQC) spectra of the heated mixture of ODE and Se is shown in ESI, Fig. S3.† Protons with signals around 5.4 ppm (β in Fig. 2) are bound to the emerging ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH with 13C signals in the region 123.6 ppm–131.7 ppm (α, β in Fig. 2e). This is in agreement with the double-bond isomerization (e.g., 2-ODE, 3-ODE) and related organoselenium complexes. The coupling between the new Hγ doublet and the Cγ (Fig. 2) suggests the formation of a CH3 in α position to a double bond (e.g., 2-ODE) and associated organoselenium complex. This is also in agreement with the COSY spectrum (ESI, Fig. S4†), where the new Hα signal is adjacent to the Hγ and Hη (Fig. 2c) as it would be for 2-ODE.
CH with 13C signals in the region 123.6 ppm–131.7 ppm (α, β in Fig. 2e). This is in agreement with the double-bond isomerization (e.g., 2-ODE, 3-ODE) and related organoselenium complexes. The coupling between the new Hγ doublet and the Cγ (Fig. 2) suggests the formation of a CH3 in α position to a double bond (e.g., 2-ODE) and associated organoselenium complex. This is also in agreement with the COSY spectrum (ESI, Fig. S4†), where the new Hα signal is adjacent to the Hγ and Hη (Fig. 2c) as it would be for 2-ODE.
To ensure that the new NMR signals were arising from the ODE-chalcogen interaction, we performed another control reaction. Working in the same conditions as in eqn (2), we heated ODE alone at 220 °C. The heating did not result in an evident colour change (ESI, Fig. S2†) and the proton and carbon NMR spectra did not show significant differences from the pristine ODE (ESI, Fig. S5†). This conveys that the newly formed signals are due to the interaction between the alkene and selenium.
We summarize our experimental results and propose the possible reactive ODE–Se precursor for our CdSe NPL synthesis in Fig. 3. It agrees well with the assignment of Bullen et al.,20 who observed the same species in the CdSe quantum dot synthesis. We confirmed this structure with liquid and gas chromatography coupled to mass spectrometry (LC-MS and GC-MS, respectively). The LC-MS profile (ESI, Fig. S6†) shows a peak at m/z = 663, which can be assigned to the [M + H]+ ion of a species containing 2 Se + 2 ODE fragments (Fig. S6†).20 When we look at the GC-MS profile and its related fractions (ESI, Fig. S7 and S8†), a second species possesses a peak at m/z = 252 (ESI, Fig. S7†), which is attributable to 1-ODE and its isomers (e.g. 2-ODE, 3-ODE etc.). Additional components can be discerned with m/z = 250 (ESI, Fig. S8†), probably due to the formation of conjugated diolefins deriving from subsequent ODE dehydrogenation (e.g., octadec-di-enes). From both NMR and MS, we can thus conclude that, for the CdSe NPL synthesis, the presence of allylic positions to a double bond is an important reactive framework for selenium. Data reveal that the main components formed are 2-ODE, 3-ODE, and ODE–Sex complexes as depicted in Fig. 3.
Since ODE-chalcogen mixtures were already used for colloidal nanocrystal synthesis, we have also prepared and used a ODE–Se suspension.20 We again monitored the gas evolution during the precursor preparation, and a lead acetate paper test did not yield any evidence of H2Se formation during the heating. Next, we performed a synthesis in identical conditions as in eqn (2), but using ODE–Se as chalcogen precursor and hexadecane as solvent. The absorption spectra (Fig. 4a) clearly support the formation of CdSe NPLs, which was confirmed by TEM (Fig. 4b). Also during the synthesis, lead acetate tests did not evidence any evolution of H2Se. This shows that we prepared a similar Se precursor as in typical NPL synthesis in ODE (eqn (2) and Fig. 1a), but now using an ex situ method. In this case, HD can replace ODE as solvent.
 | ||
| Fig. 4 (a) Temporal evolution of the absorption spectra of aliquots collected during a modified version of procedure in eqn (2), using n-hexadecane (HD) as solvent and the ODE–Se reagent as chalcogen source and (b) TEM image of the final particles obtained. (c) Absorption spectrum of NPLs obtained by a modified version of procedure (2), using tetralin (molecular structure in inset) as solvent. (d) TEM of the final particles obtained (scale bar equals 50 nm in both TEM images). | ||
The NMR results demonstrate that an alkene possessing allylic protons behaves as hydrogen transfer agent,18,19 enabling the formation of reactive selenium. With this in mind, we used an aromatic solvent, 1,2,3,4-tetrahydronaphthalene (commonly known as tetralin), as solvent for an NPL synthesis. The solvent possesses a relatively high boiling point (b.p. = 207 °C), has a lower tendency to polymerize when compared to ODE and it has been used as an additive to reduce the average molecular weight of polymers.48 These features are potentially beneficial for the subsequent nanocrystals purification. To test the reactivity of tetralin, we have conducted a synthesis following the procedure in eqn (2) using this solvent. The absorption spectrum and TEM image of NPLs grown for 30 minutes are shown in Fig. 4c and d. Also in this case, the absorption spectra of the aliquots can be attributed to 3.5 ML NPLs, and the TEM analysis confirms NPL formation. Once again, a radical reaction pathway can be expected, based upon literature results.18,19,44,45 Given the lower self-polymerization tendency, tetralin is a promising solvent for NPLs that do not require temperatures above 207 °C for their synthesis. We can conclude that both linear alkene and aromatic solvents are suitable for the synthesis of CdSe NPLs, the only prerequisite being the presence of extractable protons in α to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.
C double bond.
While we have shown that the synthesis of CdSe NPLs is strongly impacted by the choice of the solvent, some NPL formation was still observed in a saturated alkane such as HD. In this respect, Riedinger et al. recently showed that CdSe NPLs can be obtained in a melt of cadmium carboxylate and selenium, hypothesizing that selenium acylates (or acylselenides, (ROC)2Se, see Fig. 6a) are the reactive chalcogen species.49,50 Additionally, they prepared (ROC)2Se with different organic backbones R and used them in an NPL synthesis49 with ODE as a solvent, and in the synthesis of CdSe clusters with HD as a solvent.39,51
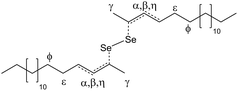
To explore this reaction pathway, we heated up a mixture of selenium and octanoic acid (OctAc, b.p. = 237 °C) at 220 °C and investigated the products formed. As an excess of octanoic acid, and even propionic acid,34 are used in our synthesis, one can assume that either (or both) may interact with selenium. During heating, we observed a color transition from grey to deep orange (ESI, Fig. S9†). This is already similar to what was observed in the heated ODE–Se mixture. The suspension turns pale yellow after cooling to room temperature, together with the separation of a red solid, probably red selenium.18,19,45,521H and 13CNMR spectra were collected from the reaction supernatant, and are shown in Fig. 5 together with the spectrum of pristine octanoic acid. In the 1H spectrum, a new resonance appears around 2.42 ppm (α in Fig. 5b), with an intensity comparable to the 13C satellites of the main octanoic acid α CH2 peak, located at 2.32 ppm. The 13C NMR spectrum is shown in Fig. 5d–f Also here, after heating, two new signals, around 35.3 and 24.2 ppm (α and β, respectively) appear. These can be assigned to new carbons in α and β position to a new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety. A new signal emerging around 169 ppm (γ in Fig. 5) can be assigned to a quaternary carbon. The new signals are summarized in Table 2. We also ran a control reaction, heating octanoic acid alone at 220 °C. The final product was again analyzed via NMR spectroscopy. New signals appear in the 1H and 13C spectra of the heated mixture, at similar chemical shift, yet with reduced intensity (ESI, Fig. S10†).
O moiety. A new signal emerging around 169 ppm (γ in Fig. 5) can be assigned to a quaternary carbon. The new signals are summarized in Table 2. We also ran a control reaction, heating octanoic acid alone at 220 °C. The final product was again analyzed via NMR spectroscopy. New signals appear in the 1H and 13C spectra of the heated mixture, at similar chemical shift, yet with reduced intensity (ESI, Fig. S10†).
| Nucleus | α | β | γ |
|---|---|---|---|
| 1H (ppm) | 2.42 | — | — |
| 13C (ppm) | 35.3 | 24.2 | 169.0 |
Interestingly, comparing our results with the chemical shifts reported by Riedinger et al.49 for acylselenide, we observe key differences. In our case, the chemical shifts of the OctAc–Se mixture and the heated octanoic acid sample can be assigned to octanoic anhydride (spectral database for organic compounds (SDBS),53 no.: 7738). While it is clear from Riedinger et al.49 that acylselenides are a viable Se precursor, we do not find evidence for its formation when heating octanoic acid with selenium, which instead may be activated via formation of a complex with the anhydride. In Fig. 6b we show the possible structure of the selenium complex, taking the formation of anhydrides into account. To demonstrate that it can also lead to the formation of NPLs, we ran a control reaction, using the heated OctAc–Se as precursor. We observed that 3.5 NPLs are indeed formed, together with a sizeable fraction of 0D quantum dots (ESI, Fig. S11†), hence, it is clear that, at high temperatures, selenium forms a reactive complex with organic acids such as octanoic acid, further enriching the Se precursors present during NPL synthesis.
 | ||
| Fig. 6 Proposed selenium reactive species in NPLs syntheses. (a) Acylselenides (as proposed in ref. 49). (b) Selenium complexation by anhydrides formed in situ (x = 1, 2, …, refers to the possibility of formation of multinuclear Se–Se structures). | ||
Finally, adaptation of the synthesis conditions (see Methods section) can also yield NPLs with different thickness. For instance, we already observed that a synthesis in HD yields mostly 2.5 ML NPLs (Fig. 1b). When performing the reaction in HD, using elemental Se, and lowering the growth temperature to 190 °C, we obtained 2.5 ML NPLs (Fig. 7a and b). Interestingly, in contrast with our previous results, where we obtained extended nanosheets by growth at 160 °C,34 here we obtained substantially smaller NPLs, with average width and length of 9 nm by 27 nm (ESI, Fig. S12† for the associated histograms). Additionally, Fig. 1d shows that a reaction performed in HD with 0.5 mmol of ODE, using elemental Se, already yields a sizeable fraction of 3.5 ML NPLs after 20 minutes. Considering that this fraction increases by Ostwald ripening and digesting of the 2.5 ML NPLs,43 by simply extending the growth time, here to 185 minutes, we could obtain nanoribbon-like 11 nm by ca. 150 nm 3.5 ML NPLs (Fig. 7a, c and ESI, Fig. S12†). Finally, with a slight modification of the precursor concentrations with respect to the synthesis of Singh et al.,54 we could obtain 5 nm by 24 nm 5.5 ML NPLs, grown in a solvent mixture of HD with 0.6 mmol of ODE, using elemental Se (Fig. 7a, d and ESI, Fig. S12†). Note that further optimization may still be possible, as the crude product was polydisperse (ESI, Fig. S13†), however, careful purification largely removed the undesired fractions.
Conclusion
In this work, we have explored the reactivity of the different components that lead to the synthesis of CdSe NPLs. Starting from the role of the dispersive medium and from literature reports, we have shown that the role of the typical solvent, ODE, is often overlooked. By combining NMR and MS analyses we confirmed that the carbon–carbon double bond moiety reacts with selenium, allowing for the reduction (and thus activation) of the chalcogen. As we have found no evidence of reaction between selenium and textitn-hexadecane (HD), we have used this solvent to exploit the alkene reactivity in different syntheses, either spiking HD with known amounts of ODE, or using a ODE–Se reagent prepared beforehand. We show that is it possible to reduce the amount of ODE injected in the colloidal synthesis. No clear evidence of H2Se formation was observed, and via NMR and MS studies, we infer that the improved reactivity is due to organo-selenium complexes arising from chalcogen-alkene interaction and the isomerization of the carbon–carbon double bond provided by available allylic protons. This concept was exploited to obtain NPLs using tetralin as solvent, with results similar to ODE. We believe that the dispersive medium should be considered as one of the reagents in the synthesis, unless proven otherwise. Given that NPLs could still be obtained in HD, we have then shifted our attention to the reaction between carboxylic acids and selenium. Via NMR spectroscopy we show that the two species can interact, likely leading to selenium-anhydride complexes. Our results were adapted to grow a variety of CdSe thicknesses, and can lead to rationalization of the chemistry underlying the colloidal synthesis of 2D nanocrystals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 714876 PHOCONA) and the Research Foundation – Flanders (grant no. G037221N). TEM measurements were performed at the UGent TEM Core Facility. We thank the NMR expertise centre (Ghent University) for providing support and access to its NMR infrastructure. The 500 MHz equipment used in this work has been funded by the Hercules Foundation and UGent (grant code AUGE09/006).References
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- J. M. Pietryga, Y.-S. Park, J. Lim, A. F. Fidler, W. K. Bae, S. Brovelli and V. I. Klimov, Chem. Rev., 2016, 116, 10513–10622 CrossRef CAS PubMed.
- F. P. García de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, E. H. Bayer and M. Sargent, Science, 2021, 373, eaaz8541 CrossRef PubMed.
- M. D. Tessier, K. De Nolf, D. Dupont, D. Sinnaeve, J. De Roo and Z. Hens, J. Am. Chem. Soc., 2016, 138, 5923–5929 CrossRef CAS PubMed.
- H. Liu, J. S. Owen and A. P. Alivisatos, J. Am. Chem. Soc., 2007, 129, 305–312 CrossRef CAS PubMed.
- M. P. Hendricks, M. P. Campos, G. T. Cleveland, I. J.-L. Plante and J. S. Owen, Science, 2015, 348, 1226–1230 CrossRef CAS PubMed.
- W. Yin, H. Li, A. S. R. Chesman, B. Tadgell, A. D. Scully, M. Wang, W. Huang, C. R. McNeill, W. W. H. Wong, N. V. Medhekar, P. Mulvaney and J. J. Jasieniak, ACS Nano, 2021, 15, 1454–1464 CrossRef CAS PubMed.
- Z. Hens and R. K. Čapek, Coord. Chem. Rev., 2014, 263–264, 217–228 CrossRef CAS.
- J. S. Owen, E. M. Chan, H. Liu and A. P. Alivisatos, J. Am. Chem. Soc., 2010, 132, 18206–18213 CrossRef CAS PubMed.
- W. W. Yu and X. G. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368–2371 CrossRef CAS PubMed.
- C. R. Bullen and P. Mulvaney, Nano Lett., 2004, 4, 2303–2307 CrossRef CAS.
- J. Park, K. H. Lee, J. F. Galloway and P. C. Searson, J. Phys. Chem. C, 2008, 112, 17849–17854 CrossRef CAS.
- J. E. Mark, B. Erman and M. Roland, The science and technology of rubber, Academic Press, 3rd edn, 2013 Search PubMed.
- J. Jasieniak, C. Bullen, J. van Embden and P. Mulvaney, J. Phys. Chem. B, 2005, 109, 20665–20668 CrossRef CAS PubMed.
- G. G. Yordanov, H. Yoshimura and C. D. Dushkin, Colloid Polym. Sci., 2008, 286, 813–817 CrossRef CAS.
- C. Pu, J. Zhou, R. Lai, Y. Niu, W. Nan and X. Peng, Nano Res., 2013, 6, 652–670 CrossRef CAS.
- Z. Deng, L. Cao, F. Tang and B. Zou, J. Phys. Chem. B, 2005, 109, 16671–16675 CrossRef CAS PubMed.
- W. T. House and M. Orchin, J. Am. Chem. Soc., 1960, 82, 639–642 CrossRef CAS.
- H. A. Silverwood and M. Orchin, J. Org. Chem., 1962, 27, 3401–3404 CrossRef CAS.
- C. Bullen, J. van Embden, J. Jasieniak, J. E. Cosgriff, R. J. Mulder, E. Rizzardo, M. Gu and C. L. Raston, Chem. Mater., 2010, 22, 4135–4143 CrossRef CAS.
- M. Nasilowski, B. Mahler, E. Lhuillier, S. Ithurria and B. Dubertret, Chem. Rev., 2016, 116, 10934–10982 CrossRef CAS PubMed.
- M. D. Tessier, C. Javaux, I. Maksimovic, V. Loriette and B. Dubertret, ACS Nano, 2012, 6, 6751–6758 CrossRef CAS PubMed.
- J. Q. Grim, S. Christodoulou, F. Di Stasio, R. Krahne, R. Cingolani, L. Manna and I. Moreels, Nat. Nanotechnol., 2014, 9, 891–895 CrossRef CAS PubMed.
- B. T. Diroll, D. V. Talapin and R. D. Schaller, ACS Photonics, 2017, 4, 576–583 CrossRef CAS.
- C. She, I. Fedin, D. S. Dolzhnikov, A. Demortiere, R. D. Schaller, M. Pelton and D. V. Talapin, Nano Lett., 2014, 14, 2772–2777 CrossRef CAS PubMed.
- Y. Kelestemur, Y. Shynkarenko, M. Anni, S. Yakunin, M. L. De Giorgi and M. V. Kovalenko, ACS Nano, 2019, 13, 13899–13909 CrossRef CAS PubMed.
- U. Giovanella, M. Pasini, M. Lorenzon, F. Galeotti, C. Lucchi, F. Meinardi, S. Luzzati, B. Dubertret and S. Brovelli, Nano Lett., 2018, 18, 3441–3448 CrossRef CAS PubMed.
- M. İzmir, A. Sharma, S. Shendre, E. G. Durmusoglu, V. K. Sharma, F. Shabani, H. D. Baruj, S. Delikanli, M. Sharma and H. V. Demir, ACS Appl. Nano Mater., 2022, 5, 1367–1376 CrossRef.
- G. H. Bertrand, A. Polovitsyn, S. Christodoulou, A. H. Khan and I. Moreels, Chem. Commun., 2016, 52, 11975–11978 RSC.
- Z. Li and X. Peng, J. Am. Chem. Soc., 2011, 133, 6578–6586 CrossRef CAS PubMed.
- S. Christodoulou, J. I. Climente, J. Planelles, R. Brescia, M. Prato, B. Martin-Garcia, A. H. Khan and I. Moreels, Nano Lett., 2018, 18, 6248–6254 CrossRef CAS PubMed.
- N. Moghaddam, C. Dabard, M. Dufour, H. Po, X. Xu, T. Pons, E. Lhuillier and S. Ithurria, J. Am. Chem. Soc., 2021, 143, 1863–1872 CrossRef CAS PubMed.
- W. Cho, S. Kim, I. Coropceanu, V. Srivastava, B. T. Diroll, A. Hazarika, I. Fedin, G. Galli, R. D. Schaller and D. V. Talapin, Chem. Mater., 2018, 30, 6957–6960 CrossRef CAS.
- A. Di Giacomo, C. Rodà, A. H. Khan and I. Moreels, Chem. Mater., 2020, 32, 9260–9267 CrossRef CAS PubMed.
- D. E. Yoon, J. Lee, H. Yeo, J. Ryou, Y. K. Lee, Y. H. Kim and D. C. Lee, Chem. Mater., 2021, 33, 4813–4820 CrossRef CAS.
- S. Ithurria, M. D. Tessier, B. Mahler, R. P. Lobo, B. Dubertret and A. L. Efros, Nat. Mater., 2011, 10, 936–941 CrossRef CAS PubMed.
- E. Dhaene, J. Billet, E. Bennett, I. Van Driessche and J. De Roo, Nano Lett., 2019, 19, 7411–7417 CrossRef CAS PubMed.
- M. Calcabrini, D. Van den Eynden, S. Sánchez Ribot, R. Pokratath, J. Llorca, J. De Roo and M. Ibánez, JACS Au, 2021, 1, 1898–1903 CrossRef CAS PubMed.
- A. B. Pun, A. S. Mule, J. T. Held and D. J. Norris, Nano Lett., 2021, 21, 7651–7658 CrossRef CAS PubMed.
- S. Ithurria, G. Bousquet and B. Dubertret, J. Am. Chem. Soc., 2011, 133, 3070–3077 CrossRef CAS PubMed.
- D. A. Kurtina, A. V. Garshev, I. S. Vasil'eva, V. V. Shubin, A. M. Gaskov and R. B. Vasiliev, Chem. Mater., 2019, 9652–9663 CrossRef CAS.
- S. Delikanli, G. Yu, A. Yeltik, S. Bose, T. Erdem, J. Yu, O. Erdem, M. Sharma, V. K. Sharma, U. Quliyeva, S. Shendre, C. Dang, D. H. Zhang, T. C. Sum, W. Fan and H. V. Demir, Adv. Funct. Mater., 2019, 1901028–1901037 CrossRef.
- P. N. Knüsel, A. Riedinger, A. A. Rossinelli, F. D. Ott, A. S. Mule and D. J. Norris, Chem. Mater., 2020, 32, 3312–3319 CrossRef.
- B. Hou, D. Benito-Alifonso, R. Webster, D. Cherns, M. C. Galan and D. J. Fermín, J. Mater. Chem. A, 2014, 2, 6879–6886 RSC.
- J. D. Fitzpatrick and M. Orchin, J. Am. Chem. Soc., 1957, 79, 4765–4771 CrossRef CAS.
- G. G. Yordanov, C. D. Dushkin, G. D. Gicheva, B. H. Bochev and E. Adachi, Colloid Polym. Sci., 2005, 284, 229–232 CrossRef CAS.
- H. Günther, NMR spectroscopy: basic principles, concepts and applications in chemistry, John Wiley & Sons, 2013 Search PubMed.
- G. Madras, J. M. Smith and B. J. McCoy, Ind. Eng. Chem. Res., 2002, 34, 4222–4228 CrossRef.
- A. Riedinger, A. S. Mule, P. N. Knusel, F. D. Ott, A. A. Rossinelli and D. J. Norris, Chem. Commun., 2018, 54, 11789–11792 RSC.
- M. Koketsu, F. Nada, S. Hiramatsu and H. Ishihara, J. Chem. Soc., Perkin Trans. 1, 2002, 737–740 RSC.
- A. B. Pun, S. Mazzotti, A. S. Mule and D. J. Norris, Acc. Chem. Res., 2021, 54, 1545–1554 CrossRef CAS PubMed.
- O. Foss and V. Janickis, J. Chem. Soc., Dalton Trans., 1980, 624–627 RSC.
- National Institute of Advanced Industrial Science and Technology, January 2024, https://sdbs.db.aist.go.jp.
- S. Singh, R. Tomar, S. Ten Brinck, J. De Roo, P. Geiregat, J. C. Martins, I. Infante and Z. Hens, J. Am. Chem. Soc., 2018, 140, 13292–13300 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional TEM images, 1H, 1H–1H COSY and 1H–13C HSQC NMR spectra of precursors and solvents, mass spectra of precursors, photographs of precursor solutions. See DOI: https://doi.org/10.1039/d3nr05157a |
| This journal is © The Royal Society of Chemistry 2024 |