Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Large area arrays of discrete single-molecule junctions derived from host–guest complexes†
Enrique
Escorihuela
 ab,
Jesús
del Barrio
ac,
Ross J.
Davidson
d,
Andrew
Beeby
d,
Paul J.
Low
ab,
Jesús
del Barrio
ac,
Ross J.
Davidson
d,
Andrew
Beeby
d,
Paul J.
Low
 e,
Francesc
Prez-Murano
e,
Francesc
Prez-Murano
 f,
Pilar
Cea
f,
Pilar
Cea
 *abg and
Santiago
Martin
*abg and
Santiago
Martin
 *abg
*abg
aInstituto de Nanociencia y Materiales de Aragón (INMA), CSIC-Universidad de Zaragoza, 50009, Zaragoza, Spain. E-mail: pilarcea@unizar.es; smartins@unizar.es
bDepartamento de Química Física, Universidad de Zaragoza, 50009, Zaragoza, Spain
cDepartamento de Química Orgánica, Universidad de Zaragoza, 50009, Zaragoza, Spain
dDepartment of Chemistry, Durham University, South Rd, Durham, DH1 3LE, UK
eSchool of Molecular Sciences, University of Western Australia, 35 Stirling Highway, Crawley, 6009, Western Australia, Australia
fInstitute of Microelectronics of Barcelona (IMB-CNM, CSIC), 08193, Bellaterra, Spain
gLaboratorio de Microscopias Avanzadas (LMA), Universidad de Zaragoza, 50018, Zaragoza, Spain
First published on 20th December 2023
Abstract
The desire to continually reduce the lower limits of semiconductor integrated circuit (IC) fabrication methods continues to inspire interest in unimolecular electronics as a platform technology for the realization of future (opto)electronic devices. However, despite successes in developing methods for the construction and measurement of single-molecule and large-area molecular junctions, exercising control over the precise junction geometry remains a significant challenge. Here, host–guest complexes of the wire-like viologen derivative 1,1′-bis(4-(methylthio)-phenyl)-[4,4′-bipyridine]-1,1′-diium chloride ([1][Cl]2) and cucurbit[7]uril (CB[7]) have been self-assembled in a regular pattern over a gold substrate. Subsequently, ligandless gold nanoparticles (AuNPs) synthesized in situ are deposited over the host–guest array. The agreement between the conductance of individual mono-molecular junctions, appropriately chosen as a function of the AuNP diameter, within this array determined by conductive probe atomic force microscope (c-AFM) and true single-molecule measurements for a closely similar host–guest complex within a scanning tunneling microscope break-junction (STM-BJ) indicates the formation of molecular junctions derived from these host–guest complexes without deleterious intermolecular coupling effects.
1. Introduction
The field of molecular electronics is broadly based on the concept that molecules with appropriately designed chemical structures may perform one (or more) of the basic functions of an electronic circuit element.1 In this context, electrode | molecule | electrode junctions have proven to be remarkably versatile research tools, providing an opportunity to directly measure the electrical properties of single molecules connected to two macroscopic electrodes under an applied bias. In turn, such data have informed the establishment of the chemical structure–electrical property relationships that underpin the development of future molecular electronic devices.2–4 However, variations in the electrical response of each individual single-molecule junction that arise from differences and stochastic fluctuations in the molecular conformation, the contact geometry, or the electrode surface geometry within each junction, limit the direct translation of single-molecule junctions from a platform for scientific discovery to a molecular electronic technology.2,5,6‘Large-area’ molecular electronic devices, where molecules are arranged in a parallel fashion within monolayer films, including heterogeneous film structures in which the active molecule is supported within an insulating monolayer provide one solution to the challenges associated with building reproducible and robust junctions.7–9 These film-based approaches are also more likely compatible with fabrication strategies best suited to incorporation of molecular elements into viable devices.10,11 However, whilst film-based large-area junctions may offer advantage in a more uniform molecular geometry, the potential for intermolecular electronic coupling effects and other intermolecular interactions can affect the properties of the individual molecules and hence the electrical properties of any resulting putative device.12–15 Therefore, the fabrication of large area devices where molecules are localized in defined positions on the surface and at a sufficient distance to avoid potentially deleterious intermolecular coupling effects is a key target for research and development of the new generation of molecular electronic devices. Several strategies have been developed in terms to achieve this goal, including: the use of large ‘lily-pad’ anchor groups such as the triazatriangulene platform (TATA) which is functionalised by various wire-like or switching motifs and assembled into regular patterns over a gold surface;16–21 the use of cross-linked metalloporphyrin films to provide a surface template with defined spacing between surface binding sites for the assembly and growth of wire-like structures;22–25 and the use of supramolecular host–guest structures to control molecular structure and insulate molecular components.26–28 Indeed, dynamic processes of supramolecular components arising from external stimuli have been at the heart of some of the most remarkable molecular electronic devices prepared to date, such as Stoddart and Heath's cross-bar molecular memory,10 inspiring the use of supramolecular components in both single-molecule29 and large area junctions.30
We now report the further use of host–guest complexes as components in molecular electronics, whereby the host provides a degree of control over not only the conformation of, and environment around, the guest molecule, but also allows an ordered spatial distribution of the supramolecular structure (Scheme 1). Moreover, by capping the ordered host–guest film with ligandless gold nanoparticles (AuNPs) of varying sizes, an array of single, bi- and multi-molecule junctions can be prepared across the surface. Characterization by conductive AFM measurements reveals the absence of electrical cross-talk between the insulated components under the top-contacting nanoparticles. Therefore, this strategy allows the fabrication of film-based junctions, that preserve the characteristics of monomolecular junctions. In these systems, the individual molecules are arranged in a massively parallel and regular fashion on a surface, while simultaneously avoiding intermolecular electronic coupling effects or other intermolecular interactions that may affect the electrical properties of the large-area junction.
2. Results and discussion
It has been demonstrated elsewhere that the single-molecule conductance of 1,1′-bis(4-(methylthio)-phenyl)-[4,4′-bipyridine]-1,1′-diium chloride ([1][Cl]2) increased upon encapsulation within the hydrophobic cavity of cucurbit[8]uril, CB[8].31 This behavior was interpreted in terms of a two-step (Ulstrup–Kuznetzov) hopping mechanism of charge transport in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex junction, and the reduced outer-sphere reorganization energy associated with electron transfer arising from the pre-organized viologen backbone of [1]2+ within the CB[8] cavity, in line with the expectations of a Marcus-type model. However, analysis of the conductance histograms obtained from STM-BJ measurements also revealed a significant conductance feature associated with ‘bimolecular’ junctions formed from 1
1 complex junction, and the reduced outer-sphere reorganization energy associated with electron transfer arising from the pre-organized viologen backbone of [1]2+ within the CB[8] cavity, in line with the expectations of a Marcus-type model. However, analysis of the conductance histograms obtained from STM-BJ measurements also revealed a significant conductance feature associated with ‘bimolecular’ junctions formed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes, with two viologens incorporated within the same CB[8] cavity. In order to restrict the available space within the cavity and limit the formation of such double-molecule junctions, here attention was turned to the somewhat smaller barrel-shaped host cucurbit[7]uril (CB[7]) (Fig. 1).
2 complexes, with two viologens incorporated within the same CB[8] cavity. In order to restrict the available space within the cavity and limit the formation of such double-molecule junctions, here attention was turned to the somewhat smaller barrel-shaped host cucurbit[7]uril (CB[7]) (Fig. 1).
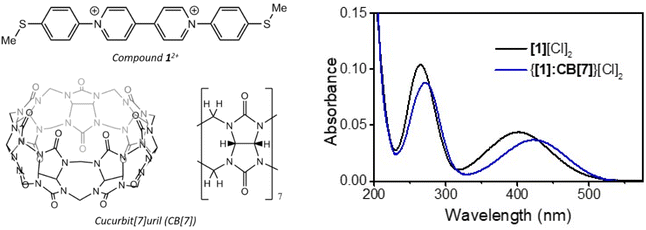
The inclusion of the viologen dication [1]2+ within the CB[7] cavity in aqueous solution was assessed by UV-vis spectroscopy.32 The UV-vis spectrum of [1][Cl]2 displays two intense bands at 264 and 399 nm, which are red-shifted (to 273 and 424 nm, respectively) upon addition of CB[7] (Fig. 1). This red-shift is attributed to the stabilization of the viologen LUMO upon the formation of the binary CB[7]–acceptor complex.33 This complexation process also induced an increase in the intensity of emission from [1][Cl]2 (Fig. S1†), in a similar manner to that reported for other CB[n] inclusion complexes of viologen derivatives.34,35 The formation of a complex of [1][Cl]2 and CB[7] was further verified by isothermal titration calorimetry (ITC) experiments (Fig. S2 and Table S1†).35
Gold-on-glass, gold-on-mica, or gold-on-quartz crystal microbalance (QCM) substrates were incubated in aqueous solutions of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 to promote formation of self-assembled monolayers, taking advantage of both: (i) the intermolecular interactions and close packing of the CB[7] host that promotes good monolayer film characteristics; and (ii) the thiomethyl anchor group of [1]2+ that chemisorbs to the gold substrate surface.9,31,36–42 The formation of the SAMs was monitored by the change in the QCM resonator frequency (Δf) with time immersed in a {[1]
CB[7]}[Cl]2 to promote formation of self-assembled monolayers, taking advantage of both: (i) the intermolecular interactions and close packing of the CB[7] host that promotes good monolayer film characteristics; and (ii) the thiomethyl anchor group of [1]2+ that chemisorbs to the gold substrate surface.9,31,36–42 The formation of the SAMs was monitored by the change in the QCM resonator frequency (Δf) with time immersed in a {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 solution (Fig. S3†). After ca. 30 hours, no further significant changes in resonator frequency were observed, and a surface coverage could be estimated from fitting the QCM data to the Sauerbrey equation (see ESI† for further details). Assuming a 1
CB[7]}[Cl]2 solution (Fig. S3†). After ca. 30 hours, no further significant changes in resonator frequency were observed, and a surface coverage could be estimated from fitting the QCM data to the Sauerbrey equation (see ESI† for further details). Assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex, a surface coverage of 3.7 × 1013 molecules per cm2 (2.7 nm2 per molecule) was obtained. The good agreement between the surface coverage of {[1]
1 host–guest complex, a surface coverage of 3.7 × 1013 molecules per cm2 (2.7 nm2 per molecule) was obtained. The good agreement between the surface coverage of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 and the estimated surface area occupied by a CB[7] molecule given the molecular diameter (ca. 2.0 nm2 per molecule)30 indicates the formation of a homogeneous and well-ordered monolayer film of this host–guest complex. Subsequent AFM imaging of the {[1]
CB[7]}[Cl]2 and the estimated surface area occupied by a CB[7] molecule given the molecular diameter (ca. 2.0 nm2 per molecule)30 indicates the formation of a homogeneous and well-ordered monolayer film of this host–guest complex. Subsequent AFM imaging of the {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 SAM supported by a gold-on-mica substrate revealed a uniform monolayer, largely free of perforations or holes, although a small number of aggregates were also evident (Fig. 2a). A bearing analysis of the AFM image indicates a surface coverage by the {[1]
CB[7]}[Cl]2 SAM supported by a gold-on-mica substrate revealed a uniform monolayer, largely free of perforations or holes, although a small number of aggregates were also evident (Fig. 2a). A bearing analysis of the AFM image indicates a surface coverage by the {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 SAM of 95 ± 1% (Fig. S4†). Additionally, the thickness of the SAM (1.50 ± 0.04) nm, as determined by using the attenuation of the Au 4f signal in the XPS spectra (Fig. S5†), and the S⋯S distance in [1]2+ (ca. 1.95 nm), estimated from Spartan calculations, and the height of CB[7] (ca. 0.9 nm), are also consistent with the formation of a monolayer of {[1]
CB[7]}[Cl]2 SAM of 95 ± 1% (Fig. S4†). Additionally, the thickness of the SAM (1.50 ± 0.04) nm, as determined by using the attenuation of the Au 4f signal in the XPS spectra (Fig. S5†), and the S⋯S distance in [1]2+ (ca. 1.95 nm), estimated from Spartan calculations, and the height of CB[7] (ca. 0.9 nm), are also consistent with the formation of a monolayer of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 on the gold surface.
CB[7]}[Cl]2 on the gold surface.
 | ||
Fig. 2 AFM images of (a) a SAM of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 deposited on a gold-on-mica substrate and (b) the same SAM after incubation for 40 minutes in an aqueous dispersion of ligandless AuNPs. CB[7]}[Cl]2 deposited on a gold-on-mica substrate and (b) the same SAM after incubation for 40 minutes in an aqueous dispersion of ligandless AuNPs. | ||
Having established conditions for the formation of well-ordered and tightly packed SAMs of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 on gold, attention was turned to X-ray photoelectron spectroscopy (XPS) to confirm contact between the thiomethyl sulfur anchor and the gold substrate, as well as further evince the 1
CB[7]}[Cl]2 on gold, attention was turned to X-ray photoelectron spectroscopy (XPS) to confirm contact between the thiomethyl sulfur anchor and the gold substrate, as well as further evince the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the host–guest complex. The XPS data for the S 2p region from a powder sample of [1]Cl2 displays two peaks, as a consequence of spin–orbit splitting, at 163.3 and 164.5 eV; the peak separation of 1.2 eV and ratio of integrated peak areas of 2
1 stoichiometry of the host–guest complex. The XPS data for the S 2p region from a powder sample of [1]Cl2 displays two peaks, as a consequence of spin–orbit splitting, at 163.3 and 164.5 eV; the peak separation of 1.2 eV and ratio of integrated peak areas of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 being as expected.43 In contrast, the XPS data from SAMs of {[1]
1 being as expected.43 In contrast, the XPS data from SAMs of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 or a pristine SAM of uncomplexed [1]Cl2 on a gold substrate (see Experimental section) are more convoluted (Fig. 3). For each monolayer, two sets of S 2p doublet peaks are observed. In each case, a pair of peaks at ca. 163.2 eV and 164.4 eV are observed, at practically the same binding energy as those observed for the powder sample of [1]Cl2, indicating that some of the thioether moieties are free from bonding interactions with the gold substrate. The set of peaks at lower binding energies (161.1 and 162.2 eV) featured in each film are attributed to the thiomethyl sulfur atoms chemisorbed to the gold surfaces.9,44 The XPS data for the N 1s region for powder samples of CB[7] contains a peak at 399.9 eV (Fig. 3, blue line), attributed to the equivalent nitrogen atoms of the glycoluril units. For [1][Cl]2 a peak at 401.7 eV (Fig. 3, red line) is attributed to the positively charged nitrogen atoms of the viologen moiety. Both peaks are observed upon analysis of the SAM of {[1]
CB[7]}[Cl]2 or a pristine SAM of uncomplexed [1]Cl2 on a gold substrate (see Experimental section) are more convoluted (Fig. 3). For each monolayer, two sets of S 2p doublet peaks are observed. In each case, a pair of peaks at ca. 163.2 eV and 164.4 eV are observed, at practically the same binding energy as those observed for the powder sample of [1]Cl2, indicating that some of the thioether moieties are free from bonding interactions with the gold substrate. The set of peaks at lower binding energies (161.1 and 162.2 eV) featured in each film are attributed to the thiomethyl sulfur atoms chemisorbed to the gold surfaces.9,44 The XPS data for the N 1s region for powder samples of CB[7] contains a peak at 399.9 eV (Fig. 3, blue line), attributed to the equivalent nitrogen atoms of the glycoluril units. For [1][Cl]2 a peak at 401.7 eV (Fig. 3, red line) is attributed to the positively charged nitrogen atoms of the viologen moiety. Both peaks are observed upon analysis of the SAM of {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 with relative integrated areas of 1
CB[7]}[Cl]2 with relative integrated areas of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, in perfect agreement with the 1
14, in perfect agreement with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nature of the supramolecular complex observed in solution. The weak peak at 400 eV observed in the [1][Cl]2 powder sample (Fig. 3, grey line) is attributed to adventitious nitrogen in the chamber and does not have any relevant implications in the quantification process.
1 nature of the supramolecular complex observed in solution. The weak peak at 400 eV observed in the [1][Cl]2 powder sample (Fig. 3, grey line) is attributed to adventitious nitrogen in the chamber and does not have any relevant implications in the quantification process.
Together, QCM, AFM and XPS data indicate that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex {[1]
1 host–guest complex {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl] is readily transferred to a gold substrate through simple self-assembly methods. The high packing density and uniform film surface indicates that this simple in situ assembly and deposition approach represents a good strategy to create arrays of parallel unimolecular devices in which the relative positions of the wire-like guests are controlled by the dimensions of the barrel-shaped host.
CB[7]}[Cl] is readily transferred to a gold substrate through simple self-assembly methods. The high packing density and uniform film surface indicates that this simple in situ assembly and deposition approach represents a good strategy to create arrays of parallel unimolecular devices in which the relative positions of the wire-like guests are controlled by the dimensions of the barrel-shaped host.
To complete each of the individual molecular junctions within the film prior to evaluation of their electrical properties, a gold substrate modified by a monolayer of the supramolecular complex, Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2, was prepared and subsequently incubated for a second time, but now in a freshly prepared aqueous dispersion of ligandless gold nanoparticles (AuNPs).45,46 The attachment of the AuNPs on the top surface of the film through the exposed thiomethyl anchors was followed by QCM measurements, with a deposition time of 20 min proving to be optimal (Fig. S5†). Information about the distribution, shape, and size of these top-contacting gold nanoparticles over the Au|{[1]
CB[7]}[Cl]2, was prepared and subsequently incubated for a second time, but now in a freshly prepared aqueous dispersion of ligandless gold nanoparticles (AuNPs).45,46 The attachment of the AuNPs on the top surface of the film through the exposed thiomethyl anchors was followed by QCM measurements, with a deposition time of 20 min proving to be optimal (Fig. S5†). Information about the distribution, shape, and size of these top-contacting gold nanoparticles over the Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 surface was obtained by AFM imaging (Fig. 2b). Analysis of the images reveals that the ligandless AuNPs are distributed homogenously on the surface with a surface coverage by the AuNPs of ca. 45 ± 1% (Fig. S8†). These surface confined particles exhibit an average height of 4.1 ± 2 nm and an average diameter (corrected by the tip convolution47) of 5.5 ± 2 nm (see ESI† for further details of the size distribution of the as prepared AuNPs).
CB[7]}[Cl]2 surface was obtained by AFM imaging (Fig. 2b). Analysis of the images reveals that the ligandless AuNPs are distributed homogenously on the surface with a surface coverage by the AuNPs of ca. 45 ± 1% (Fig. S8†). These surface confined particles exhibit an average height of 4.1 ± 2 nm and an average diameter (corrected by the tip convolution47) of 5.5 ± 2 nm (see ESI† for further details of the size distribution of the as prepared AuNPs).
From the estimates of surface coverage by the supramolecular complex (2.7 nm2 per molecule) and the size distribution and surface coverage of the AuNPs, three broad scenarios can be envisioned: (1) given the incomplete surface coverage by the AuNPs, some supramolecular complexes within the SAM are not capped; (2) single AuNPs less than 3 nm in diameter will contact an individual host–guest complex; (3) single AuNPs larger than 3 nm in diameter will contact 2, 3 or more individual complexes. In contrast, a gold substrate modified by a simple SAM of [1][Cl]2, (denoted Au|1[Cl]2) features a more densely packed array of exposed surface thiomethyl groups and when incubated in the gold nanoparticle solution gave a more complete surface coverage of 92 ± 1% by the metal particles and a more conventional array of multiple molecule junctions contacted by individual AuNPs (Fig. S8†).
To evaluate the electrical properties of these 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes I–V curves, recorded with a conductive atomic force microscope in the peak force tapping mode,45,46,48 were registered for the Au|{[1]
1 host–guest complexes I–V curves, recorded with a conductive atomic force microscope in the peak force tapping mode,45,46,48 were registered for the Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2|AuNP structure but only for those structures formed from AuNPs less than 3 nm in diameter to ensure the measurement of a single-molecule junction. Multi-molecule junctions formed by AuNPs larger than 3 nm in diameter were excluded from the reported I–V curves to prevent convolution of putative cross-talk effects with those of multi-molecule transport.
CB[7]}[Cl]2|AuNP structure but only for those structures formed from AuNPs less than 3 nm in diameter to ensure the measurement of a single-molecule junction. Multi-molecule junctions formed by AuNPs larger than 3 nm in diameter were excluded from the reported I–V curves to prevent convolution of putative cross-talk effects with those of multi-molecule transport.
In the peak force tapping mode, damage of the surface and detrimental lateral forces are limited as the tip makes intermittent contact with the surface at a frequency of 2 kHz and a low maximum force (peak-force). This soft contact makes PF-TUNA a valuable method for conductivity mapping of delicate samples. The most suitable contact force to record the I–V curves demands a compromise between sufficient force to give good electrical contact between the tip and the AuNP without a considerable deformation and yet not a so large force that would result in large deformation of the monolayer underlying the AuNPs and, therefore, in unreasonably high conductance values (Fig. 4a).
A range of set-point forces were explored in order to optimize the tip-particle contact. At set points below 2 nN, no significant current through the junction was detected beyond the background noise. For set-point forces between 5 and 20 nN, good electrical contact is obtained between the tip and the particle without deforming considerably the monolayer, permitting conductance values to be extracted from the slope of a linear fit to the Ohmic region of each curve. Higher set-point forces (beyond 20 nN) result in high conductance values, caused by AuNPs being pushed down into the relatively soft underlying monolayer. As the monolayer deforms the probability of interparticle contacts, and also contacts between the tip and more than one AuNP, increase, both of which would also contribute to the higher currents observed. A log–log plot of junction conductance versus the applied set-point force49 (Fig. 4a inset) shows two distinct power law regimes consistent with this observation: between 5 and 20 nN, the power law exponent is n = 1.75; above 20 nN the exponent n increases to 5.3.
Taking into account these findings, set-point forces above 5 and below 20 nN seem to be optimum to provide an effective electrical contact without significant deformation of the monolayer film. As indicated previously, conductance data was recorded after positioning the PF-TUNA cantilever over an isolated AuNP with a pre-determined 2–3 nm diameter to assure the contact with a unique supramolecular complex. The data sets were collected from multiple examples of such junctions, as well using several independently prepared substrates, using set points of 5 and 10 nN. From these data collected by locating the AFM tip across multiple points on the surface and different junctions, average I–V curves were constructed (Fig. 4b). For completeness, a plot illustrating all the I–V curves (ca. 250 curves) recorded, superposed by the average (red lines) at these two set-point forces are shown in Fig. S9.† A variation of less than half an order of magnitude in the obtained current for all the recorded I–V curves (Fig. S9†) demonstrates the reproducibility, reliability, and low fluctuations of these Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2|AuNP structures. This low variation in measurement compares with typical single-molecule measurements, in which current data spanning one or more orders of magnitude are commonly recorded due to the greater dispersion of molecular geometries and contacts within unconstrained junctions.6,50–57 Importantly, these curves do not show low resistance traces which are characteristic of metallic short circuits but a linear section at relatively low bias voltages and an increase of the curvature at higher bias; the common behavior observed in metal–molecule–metal junctions. From these average curves, conductance values of 2 × 10−5 and 6.5 × 10−5G0 (G0 = 77.5 μS) were obtained for set-point forces of 5 and 10 nN, respectively. This small increase in the junction conductance as the set-point force increases from 5 to 10 nN is attributed to a small deformation of the 1
CB[7]}[Cl]2|AuNP structures. This low variation in measurement compares with typical single-molecule measurements, in which current data spanning one or more orders of magnitude are commonly recorded due to the greater dispersion of molecular geometries and contacts within unconstrained junctions.6,50–57 Importantly, these curves do not show low resistance traces which are characteristic of metallic short circuits but a linear section at relatively low bias voltages and an increase of the curvature at higher bias; the common behavior observed in metal–molecule–metal junctions. From these average curves, conductance values of 2 × 10−5 and 6.5 × 10−5G0 (G0 = 77.5 μS) were obtained for set-point forces of 5 and 10 nN, respectively. This small increase in the junction conductance as the set-point force increases from 5 to 10 nN is attributed to a small deformation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex within the junction and improved tip–AuNP electrical contacts. These values are in good agreement with the single-molecule conductance value determined for the {[1]
1 host–guest complex within the junction and improved tip–AuNP electrical contacts. These values are in good agreement with the single-molecule conductance value determined for the {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8]}[Cl]2 host guest complex using the STM break junction (STM-BJ) method (5.4 × 10−5G0).31 The electrical properties of the parent supramolecular structures within the film were also determined for comparison by positioning the near atomically sharp tip of the c-AFM directly above the film in a region free of AuNPs (Fig. 2b). Set point forces similar to those employed for the determination the electrical properties of the Au| {[1] : CB[7]}[Cl]2|AuNP structures were employed for consistency. With the tip in contact with a single host-guest complex within these nanoparticle free regions of the film, conductance values essentially identical to those determined from the sub 3nm AuNP contacted junctions were obtained (Fig. S12)†. The similarity of these results is consistent with the absence of cross-talk between components within the film and the contact of a single 1 : 1 host–guest complex with the AuNP contacted junctions featuring the smallest diameter particles.
CB[8]}[Cl]2 host guest complex using the STM break junction (STM-BJ) method (5.4 × 10−5G0).31 The electrical properties of the parent supramolecular structures within the film were also determined for comparison by positioning the near atomically sharp tip of the c-AFM directly above the film in a region free of AuNPs (Fig. 2b). Set point forces similar to those employed for the determination the electrical properties of the Au| {[1] : CB[7]}[Cl]2|AuNP structures were employed for consistency. With the tip in contact with a single host-guest complex within these nanoparticle free regions of the film, conductance values essentially identical to those determined from the sub 3nm AuNP contacted junctions were obtained (Fig. S12)†. The similarity of these results is consistent with the absence of cross-talk between components within the film and the contact of a single 1 : 1 host–guest complex with the AuNP contacted junctions featuring the smallest diameter particles.
To demonstrate the role of the supramolecular assembly within the junction on the electrical properties of the devices, the electrical properties of a host-free Au|1[Cl]2|AuNP structure were also determined using the same methodology as indicated above. These more traditional self-assembled molecular ‘large area’ junctions gave higher conductance values than the supramolecular Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2|AuNP junctions when similar set-point forces are applied: 1.8 × 10−4 or 3.9 × 10−4G0 (Au|1[Cl]2|AuNP) vs. 2 × 10−5 or 6.5 × 10−5G0 (Au|{[1]
CB[7]}[Cl]2|AuNP junctions when similar set-point forces are applied: 1.8 × 10−4 or 3.9 × 10−4G0 (Au|1[Cl]2|AuNP) vs. 2 × 10−5 or 6.5 × 10−5G0 (Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2|AuNP) at 5 nN or 8–10 nN, respectively (Fig. 4 and Fig. S10†). Applying a set-point force of 12 nN to the Au|1[Cl]2|AuNP junction provokes a considerable increase of the contact both between neighboring AuNPs and between the AFM tip and these AuNPs as consequence of the high deformation of the monolayer as revealed by the very high conductance value obtained, 2.1 × 10−3G0, where the current recorded saturates at ±120 nA limited by the current amplifier employed (Fig. 4a and Fig. S11†). These results imply that even for the Au|1[Cl]2|AuNP structures formed from small diameter AuNPs, either multiple [1]2+ structures are contacted by individual AuNPs and/or that there is a contact between several AuNPs contacting multiple [1]2+ structures. This result is in contrast to the single-molecule junctions formed from small diameter AuNPs and SAMs of the 1
CB[7]}[Cl]2|AuNP) at 5 nN or 8–10 nN, respectively (Fig. 4 and Fig. S10†). Applying a set-point force of 12 nN to the Au|1[Cl]2|AuNP junction provokes a considerable increase of the contact both between neighboring AuNPs and between the AFM tip and these AuNPs as consequence of the high deformation of the monolayer as revealed by the very high conductance value obtained, 2.1 × 10−3G0, where the current recorded saturates at ±120 nA limited by the current amplifier employed (Fig. 4a and Fig. S11†). These results imply that even for the Au|1[Cl]2|AuNP structures formed from small diameter AuNPs, either multiple [1]2+ structures are contacted by individual AuNPs and/or that there is a contact between several AuNPs contacting multiple [1]2+ structures. This result is in contrast to the single-molecule junctions formed from small diameter AuNPs and SAMs of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex of cucurbit[7]uril and [1]Cl2, and further highlighting the important role played by the insulating CB[7] host on the electrical properties of these assemblies.
1 host–guest complex of cucurbit[7]uril and [1]Cl2, and further highlighting the important role played by the insulating CB[7] host on the electrical properties of these assemblies.
3. Conclusions
In conclusion, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex formed between cucurbit[7]uril and a viologen derivative has been self-assembled on gold substrates to give well-ordered supramolecular arrays with excellent spatial control over a large surface area through close packing of the barrel-like host structures (a challenge for the fabrication of a future generation of electronic devices). Given the molecule spacing, appropriately sized ligandless gold nanoparticles (AuNPs) were deposited in situ on top of this host–guest arrangement as the top-contact electrode. Given the distribution in size of the deposited AuNPs, only AuNPs less than 3 nm in diameter were used to determine the electrical properties of single-molecule junctions located across the SAM. A variation of less than half an order of magnitude in the current, obtained from the slope of a linear fit to the Ohmic region for all I–V curves, demonstrates the reproducibility, reliability, and low fluctuations of the Au|{[1]
1 host–guest complex formed between cucurbit[7]uril and a viologen derivative has been self-assembled on gold substrates to give well-ordered supramolecular arrays with excellent spatial control over a large surface area through close packing of the barrel-like host structures (a challenge for the fabrication of a future generation of electronic devices). Given the molecule spacing, appropriately sized ligandless gold nanoparticles (AuNPs) were deposited in situ on top of this host–guest arrangement as the top-contact electrode. Given the distribution in size of the deposited AuNPs, only AuNPs less than 3 nm in diameter were used to determine the electrical properties of single-molecule junctions located across the SAM. A variation of less than half an order of magnitude in the current, obtained from the slope of a linear fit to the Ohmic region for all I–V curves, demonstrates the reproducibility, reliability, and low fluctuations of the Au|{[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2|AuNP structure. Additionally, the electrical properties of these unimolecular devices are in agreement to the single molecule conductance determined for this kind of host guest complexes. Future work will be devoted to assessing the suitability of this methodology to other molecular components exhibiting a wider range of electrical functions as well as to other hosts that allow to modulate both the single conductance behavior and shape dependent molecular effects and local volume for electromechanically induce configurational modifications in the guest molecule.
CB[7]}[Cl]2|AuNP structure. Additionally, the electrical properties of these unimolecular devices are in agreement to the single molecule conductance determined for this kind of host guest complexes. Future work will be devoted to assessing the suitability of this methodology to other molecular components exhibiting a wider range of electrical functions as well as to other hosts that allow to modulate both the single conductance behavior and shape dependent molecular effects and local volume for electromechanically induce configurational modifications in the guest molecule.
4. Experimental section
General information
Water was purified on a Milli-Q system (resistivity 18.2 MΩ cm). The compounds 1,1′-bis(4-(methylthio)-phenyl)-[4,4′-bipyridine]-1,1′-diium chloride ([1][Cl]2)31 and cucurbit[7]uril (CB[7])58,59 were synthesized according to previously published procedures, other reagents were purchased and used as received. All processes and manipulations were carried out under ambient atmosphere and conditions unless otherwise indicated. Gold-on-glass substrates (purchased from Arrandee, Germany) were flame-annealed at approximately 800–1000 °C with a Bunsen burner to generate Au(111) atomically flat terraces;60 whilst gold-on-mica substrates (Georg Albert PVD Beschichtungen, Germany) were used as received.Solution preparation of the host–guest complex, film fabrication and characterization techniques
An aqueous (Milli-Q) solution of the host–guest complex {[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[7]}[Cl]2 (10−6 M) was prepared by mixing aqueous solutions (2 × 10−6 M) of both components in a 1
CB[7]}[Cl]2 (10−6 M) was prepared by mixing aqueous solutions (2 × 10−6 M) of both components in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. Self-assembled monolayers (SAMs) of the host–guest complex were subsequently prepared by immersing a gold-on-glass or gold-on-mica substrate in this solution and left undisturbed for 30 hours. After this time, the substrates were carefully removed, rinsed with water and then dried under a N2 flow. The host-free SAMs of [1][Cl]2 were prepared by immersing a gold-on-glass or gold-on-mica substrate in an aqueous 10−6 M solution of this compound for 24 hours.
1 volume ratio. Self-assembled monolayers (SAMs) of the host–guest complex were subsequently prepared by immersing a gold-on-glass or gold-on-mica substrate in this solution and left undisturbed for 30 hours. After this time, the substrates were carefully removed, rinsed with water and then dried under a N2 flow. The host-free SAMs of [1][Cl]2 were prepared by immersing a gold-on-glass or gold-on-mica substrate in an aqueous 10−6 M solution of this compound for 24 hours.
Isothermal titration calorimetry experiments were carried out using a VP-ITC titration calorimetric system (MicroCal LLC, Northampton, MA). Typically, an aqueous solution of [1][Cl]2 (50 μM) in the calorimetric cell was titrated with an aqueous solution of CB[7] (0.90 mM). All solutions were thoroughly degassed and carefully loaded into the cells to avoid bubble formation during stirring. The heat evolved after each injection of ligand was obtained from the integral of the calorimetric signal. The heat associated with the binding reaction was obtained as the difference between the heat of reaction and the corresponding heat of dilution, the latter estimated as a constant heat throughout the experiment and included as an adjustable parameter in the analysis.
UV-visible spectra of solutions were obtained with a Varian Cary 50 Bio UV-vis spectrophotometer in quartz cuvettes with an incident angle of 90°. A Bruker Multimode 8 microscope with a Nanoscope V control unit was used to record the atomic force microscopy (AFM) images under ambient conditions at a scan rate of 1 Hz in the tapping mode using RTESPA-150 AFM probes purchased from Bruker (90–210 kHz resonant frequency, 5 N m−1 spring constant and 8 nm nominal tip radius). X-Ray Photoelectron Spectroscopy (XPS) measurements were obtained with a Kratos AXIS ultra DLD spectrometer with a monochromatic Al Kα X-ray source (1486.6 eV) using a pass energy of 20 eV. The photoelectron take-off angle was 90° with respect to the sample plane. Binding energies of the samples were referenced to the C 1s peak (284.8 eV) to compensate surface charge effects. Quartz Crystal Microbalance (QCM) measurements were carried out on AT-cut a-quartz crystals (resonant frequency of 5 MHz) patterned with circular gold electrodes on both sides using a Stanford Research Systems microbalance model QCM200. The fluorescence spectra were recorded by means of a Varian Cary Eclipse spectrophotometer in quartz cuvettes of 1 cm.
Gold nanoparticle preparation
Ligandless gold nanoparticles (5.5 ± 2 nm diameter) were prepared in situ by adding an aqueous solution of NaBH4 (1.0 × 10−3 M, 0.5 mL) to a vigorously stirred aqueous solution of HAuCl4 (1.0 × 10−5 M, 30 mL) held at 0 °C using an ice-water bath.45 Incubation of the SAM modified gold substrate in the dispersion of AuNPs took place immediately after mixing the reactants with the solution while being stirred at 0 °C.Molecular conductance measurements
A Bruker ICON microscope in the Peak Force Tunneling AFM (PF-TUNA™) mode with a PF-Tapping™ cantilever tip from Bruker (coated with Pt/Ir 20 nm, ca. 25 nm radius, 0.4 N m−1 spring constant and 70 kHz resonance frequency) was used to perform the conductive-AFM (c-AFM) measurements under conditions of closely controlled humidity (ca. 30%), in an N2 atmosphere.Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. E. gratefully acknowledges the award of a DGA fellowship from the Government of Aragon. P. C. and S. M. are grateful for financial assistance in the framework of the projects PID2019-105881RB-I00, TED2021-131318B-I00, and PID2022-141433OB-I00 funded by MCIN/AEI/10.13039/501100011033 and European Union “NextGenerationEU”/PRTR as well as Gobierno de Aragón through the grant E31_23R with European Social Funds (Construyendo Europa desde Aragón). J. D. B acknowledges grant RYC-2015-18471, funded by MCIN/AEI/10.13039/501100011033 and by “European Social Fund Investing in your future”; and the grant CTQ2017-84087-R supported by MCIN/AEI/10.13039/501100011033, and by “European Regional Development Fund a way of making Europe” by the European Union. R. J. D. and A. B. gratefully acknowledge the EPSRC grant EP/M029204/1. P. J. L. gratefully acknowledges support for work in the area of molecular electronics from the Australian Research Council Discovery Program (DP190100073, DP190100075 and DP220100790).References
- T. A. Su, M. Neupane, M. L. Steigerwald, L. Venkataraman and C. Nuckolls, Nat. Rev. Mater., 2016, 1, 16002 CrossRef CAS.
- L. Sun, Y. A. Diaz-Fernandez, T. A. Gschneidtner, F. Westerlund, S. Lara-Avila and K. Moth-Poulsen, Chem. Soc. Rev., 2014, 43, 7378–7411 RSC.
- D. Xiang, X. L. Wang, C. C. Jia, T. Lee and X. F. Guo, Chem. Rev., 2016, 116, 4318–4440 CrossRef CAS PubMed.
- C. W. Fuller, P. S. Padayatti, H. Abderrahim, L. Adamiak, N. Alagar, N. Ananthapadmanabhan, J. Baek, S. Chinni, C. Choi, K. J. Delaney, R. Dubielzig, J. Frkanec, C. Garcia, C. Gardner, D. Gebhardt, T. Geiser, Z. Gutierrez, D. A. Hall, A. P. Hodges, G. Hou, S. Jain, T. Jones, R. Lobaton, Z. Majzik, A. Marte, P. Mohan, P. Mola II, P. Mudondo, J. Mullinix, T. Nguyen, F. Ollinger, S. Orr, V. Ouyang, P. Pan, N. Park, D. Porras, K. Prabhu, C. Reese, T. Ruel, T. Sauerbrey, J. R. Sawyer, P. Sinha, J. Tu, A. G. Venkatesh, V. S. , L. Zheng, S. Jin, J. M. Tour, G. M. Church, P. W. Mola and B. Merriman, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2112812119 CrossRef CAS PubMed.
- H. M. Liu, N. Wang, J. W. Zhao, Y. Guo, X. Yin, F. Y. C. Boey and H. Zhang, ChemPhysChem, 2008, 9, 1416–1424 CrossRef CAS PubMed.
- W. Haiss, S. Martin, E. Leary, H. van Zalinge, S. J. Higgins, L. Bouffier and R. J. Nichols, J. Phys. Chem. C, 2009, 113, 5823–5833 CrossRef CAS.
- S. Casalini, C. A. Bortolotti, F. Leonardi and F. Biscarini, Chem. Soc. Rev., 2017, 46, 40–71 RSC.
- L. Herrer, S. Martin, A. Gonzalez-Orive, D. C. Milan, A. Vezzoli, R. J. Nichols, J. L. Serrano and P. Cea, J. Mater. Chem. C, 2021, 9, 2882–2889 RSC.
- E. Escorihuela, P. Cea, S. Bock, D. C. Milan, S. Naghibi, H. M. Osorio, R. J. Nichols, P. J. Low and S. Martin, J. Mater. Chem. C, 2020, 8, 672–682 RSC.
- J. E. Green, J. W. Choi, A. Boukai, Y. Bunimovich, E. Johnston-Halperin, E. DeIonno, Y. Luo, B. A. Sheriff, K. Xu, Y. S. Shin, H. R. Tseng, J. F. Stoddart and J. R. Heath, Nature, 2007, 445, 414–417 CrossRef CAS PubMed.
- A. J. Bergren, L. Zeer-Wanklyn, M. Semple, N. Pekas, B. Szeto and R. L. McCreery, J. Phys.: Condens.Matter, 2016, 28, 094011 CrossRef PubMed.
- H. Song, H. Lee and T. Lee, J. Am. Chem. Soc., 2007, 129, 3806–3807 CrossRef CAS PubMed.
- Y. Dubi, J. Phys. Chem. C, 2014, 118, 21119–21127 CrossRef CAS.
- K. Slowinski, R. V. Chamberlain, C. J. Miller and M. Majda, J. Am. Chem. Soc., 1997, 119, 11910–11919 CrossRef CAS.
- A. Martin-Barreiro, R. Soto, S. Chiodini, A. Garcia-Serrano, S. Martin, L. Herrer, F. Perez-Murano, P. J. Low, J. L. Serrano, S. Marcos, J. Galban and P. Cea, Adv. Mater. Interfaces, 2021, 8, 2100876 CrossRef CAS.
- H. Jacob, S. Ulrich, U. Jung, S. Lemke, T. Rusch, C. Schutt, F. Petersen, T. Strunskus, O. Magnussen, R. Herges and F. Tuczek, Phys. Chem. Chem. Phys., 2014, 16, 22643–22650 RSC.
- U. Jung, S. Kuhn, U. Cornelissen, F. Tuczek, T. Strunskus, V. Zaporojtchenko, J. Kubitschke, R. Herges and O. Magnussen, Langmuir, 2011, 27, 5899–5908 CrossRef CAS PubMed.
- S. Kuhn, B. Baisch, U. Jung, T. Johannsen, J. Kubitschke, R. Herges and O. Magnussen, Phys. Chem. Chem. Phys., 2010, 12, 4481–4487 RSC.
- F. L. Otte, S. Lemke, C. Schutt, N. R. Krekiehn, U. Jung, O. M. Magnussen and R. Herges, J. Am. Chem. Soc., 2014, 136, 11248–11251 CrossRef CAS PubMed.
- Z. M. Wei, X. T. Wang, A. Borges, M. Santella, T. Li, J. K. Sorensen, M. Vanin, W. P. Hu, Y. Q. Liu, J. Ulstrup, G. C. Solomon, Q. J. Chi, T. Bjornholm, K. Norgaard and B. W. Laursen, Langmuir, 2014, 30, 14868–14876 CrossRef CAS PubMed.
- Q. Wang, L. Ma, Z. H. Liu, X. Zhang, Z. Y. Zhang, Z. C. Shangguan, X. H. Huang, Y. Q. Liu, J. T. Lv, H. M. Zhang, L. F. Chi and T. Li, Sci. China Mater., 2018, 61, 1345–1350 CrossRef CAS.
- Q. Ferreira, L. Alcacer and J. Morgado, Nanotechnology, 2011, 22, 435604 CrossRef PubMed.
- Q. Ferreira, A. M. Braganca, L. Alcacer and J. Morgado, J. Phys. Chem. C, 2014, 118, 7229–7234 CrossRef CAS.
- Z. S. Wang, K. Qian, M. A. Oner, P. S. Deimel, Y. Wang, S. Zhang, X. X. Zhang, V. Gupta, J. Li, H. J. Gao, D. A. Duncan, J. V. Barth, X. Lin, F. Allegretti, S. X. Du and C. A. Palma, ACS Appl. Nano Mater., 2020, 3, 11752–11759 CrossRef CAS.
- E. Escorihuela, A. Concellon, I. Marin, V. J. Kumar, L. Herrer, S. A. Moggach, A. Vezzoli, R. J. Nichols, P. J. Low, P. Cea, J. L. Serrano and S. Martin, Mater. Today Chem., 2022, 26, 101067 CrossRef CAS.
- D. C. Milan, M. Krempe, A. K. Ismael, L. D. Movsisyan, M. Franz, I. Grace, R. J. Brooke, W. Schwarzacher, S. J. Higgins, H. L. Anderson, C. J. Lambert, R. R. Tykwinski and R. J. Nichols, Nanoscale, 2017, 9, 355–361 RSC.
- Y. Ie, Y. Okamoto, T. Inoue, S. Tone, T. Seo, Y. Honda, S. Tanaka, S. K. Lee, T. Ohto, R. Yamada, H. Tada and Y. Aso, J. Phys. Chem. Lett., 2019, 10, 3197–3204 CrossRef CAS PubMed.
- L. Herrer, S. Naghibi, I. Marin, J. S. Ward, J. M. Bonastre, S. J. Higgins, S. Martin, A. Vezzoli, R. J. Nichols, J. L. Serrano and P. Cea, Adv. Mater. Interfaces, 2023, 10, 2300133 CrossRef CAS.
- H. L. Chen and J. F. Stoddart, Nat. Rev. Mater., 2021, 6, 804–828 CrossRef CAS.
- C. Q. Yang and H. L. Chen, ACS Appl. Nano Mater., 2022, 5, 13874–13886 CrossRef CAS.
- W. Zhang, S. Y. Gan, A. Vezzoli, R. J. Davidson, D. C. Milan, K. V. Luzyanin, S. J. Higgins, R. J. Nichols, A. Beeby, P. J. Low, B. Y. Li and L. Niu, ACS Nano, 2016, 10, 5212–5220 CrossRef CAS PubMed.
- H. J. Kim, W. S. Jeon, Y. H. Ko and K. Kim, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5007–5011 CrossRef CAS PubMed.
- F. Biedermann and O. A. Scherman, J. Phys. Chem. B, 2012, 116, 2842–2849 CrossRef CAS PubMed.
- M. Fathalla, N. L. Strutt, J. C. Barnes, C. L. Stern, C. F. Ke and J. F. Stoddart, Eur. J. Org. Chem., 2014, 2873–2877 CrossRef CAS.
- M. Freitag, L. Gundlach, P. Piotrowiak and E. Galoppini, J. Am. Chem. Soc., 2012, 134, 3358–3366 CrossRef CAS PubMed.
- Y. S. Park, A. C. Whalley, M. Kamenetska, M. L. Steigerwald, M. S. Hybertsen, C. Nuckolls and L. Venkataraman, J. Am. Chem. Soc., 2007, 129, 15768–15769 CrossRef CAS PubMed.
- E. Leary, A. La Rosa, M. T. Gonzalez, G. Rubio-Bollinger, N. Agrait and N. Martin, Chem. Soc. Rev., 2015, 44, 920–942 RSC.
- L. Li, J. Z. Low, J. Wilhelm, G. M. Liao, S. Gunasekaran, C. R. Prindle, R. L. Starr, D. Golze, C. Nuckolls, M. L. Steigerwald, F. Evers, L. M. Campos, X. D. Yin and L. Venkataraman, Nat. Chem., 2022, 14, 1061–1067 CrossRef CAS PubMed.
- X. T. Wang, T. L. R. Bennett, A. Ismael, L. A. Wilkinson, J. Hamill, A. J. P. White, I. M. Grace, O. V. Kolosov, T. Albrecht, B. J. Robinson, N. J. Long, L. F. Cohen and C. J. Lambert, J. Am. Chem. Soc., 2020, 142, 8555–8560 CrossRef CAS PubMed.
- A. Ismael, X. T. Wang, T. L. R. Bennett, L. A. Wilkinson, B. J. Robinson, N. J. Long, L. F. Cohen and C. J. Lambert, Chem. Sci., 2020, 11, 6836–6841 RSC.
- L. J. O'Driscoll, X. T. Wang, M. Jay, A. S. Batsanov, H. Sadeghi, C. J. Lambert, B. J. Robinson and M. R. Bryce, Angew. Chem., Int. Ed., 2020, 59, 882–889 CrossRef PubMed.
- T. L. R. Bennett, M. Alshammari, S. Au-Yong, A. Almutlg, X. T. Wang, L. A. Wilkinson, T. Albrecht, S. P. Jarvis, L. F. Cohen, A. Ismael, C. J. Lambert, B. J. Robinson and N. J. Long, Chem. Sci., 2022, 13, 5176–5185 RSC.
- H. R. Tseng, S. A. Vignon and J. F. Stoddart, Angew. Chem., Int. Ed., 2003, 42, 1491–1495 CrossRef CAS PubMed.
- F. Sander, J. P. Hermes, M. Mayor, H. Hamoudi and M. Zharnikov, Phys. Chem. Chem. Phys., 2013, 15, 2836–2846 RSC.
- H. M. Osorio, P. Cea, L. M. Ballesteros, I. Gascon, S. Marques-Gonzalez, R. J. Nichols, F. Perez-Murano, P. J. Low and S. Martin, J. Mater. Chem. C, 2014, 2, 7348–7355 RSC.
- A. Moneo, A. Gonzalez-Orive, S. Bock, M. Fenero, I. L. Herrer, D. C. Milan, M. Lorenzoni, R. J. Nichols, P. Cea, F. Perez-Murano, P. J. Low and S. Martin, Nanoscale, 2018, 10, 14128–14138 RSC.
- J. Canet-Ferrer, E. Coronado, A. Forment-Aliaga and E. Pinilla-Cienfuegos, Nanotechnology, 2014, 25, 395703 CrossRef PubMed.
- L. M. Ballesteros, S. Martin, J. Cortes, S. Marques-Gonzalez, F. Perez-Murano, R. J. Nichols, P. J. Low and P. Cea, Adv. Mater. Interfaces, 2014, 1, 1400128 CrossRef.
- D. J. Wold and C. D. Frisbie, J. Am. Chem. Soc., 2001, 123, 5549–5556 CrossRef CAS PubMed.
- R. J. Nichols, W. Haiss, S. J. Higgins, E. Leary, S. Martin and D. Bethell, Phys. Chem. Chem. Phys., 2010, 12, 2801–2815 RSC.
- S. Martin, W. Haiss, S. J. Higgins and R. J. Nichols, Nano Lett., 2010, 10, 2019–2023 CrossRef CAS PubMed.
- C. S. Wang, A. S. Batsanov, M. R. Bryce, S. Martin, R. J. Nichols, S. J. Higgins, V. M. Garcia-Suarez and C. J. Lambert, J. Am. Chem. Soc., 2009, 131, 15647–15654 CrossRef CAS PubMed.
- X. L. Li, J. He, J. Hihath, B. Q. Xu, S. M. Lindsay and N. J. Tao, J. Am. Chem. Soc., 2006, 128, 2135–2141 CrossRef CAS PubMed.
- F. Chen, X. L. Li, J. Hihath, Z. F. Huang and N. J. Tao, J. Am. Chem. Soc., 2006, 128, 15874–15881 CrossRef CAS PubMed.
- M. Kamenetska, S. Y. Quek, A. C. Whalley, M. L. Steigerwald, H. J. Choi, S. G. Louie, C. Nuckolls, M. S. Hybertsen, J. B. Neaton and L. Venkataraman, J. Am. Chem. Soc., 2010, 132, 6817–6821 CrossRef CAS PubMed.
- S. Y. Quek, M. Kamenetska, M. L. Steigerwald, H. J. Choi, S. G. Louie, M. S. Hybertsen, J. B. Neaton and L. Venkataraman, Nat. Nanotechnol., 2009, 4, 230–234 CrossRef CAS PubMed.
- E. J. Dell, B. Capozzi, K. H. DuBay, T. C. Berkelbach, J. R. Moreno, D. R. Reichman, L. Venkataraman and L. M. Campos, J. Am. Chem. Soc., 2013, 135, 11724–11727 CrossRef CAS PubMed.
- J. Kim, I. S. Jung, S. Y. Kim, E. Lee, J. K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540–541 CrossRef CAS.
- A. Day, A. P. Arnold, R. J. Blanch and B. Snushall, J. Org. Chem., 2001, 66, 8094–8100 CrossRef CAS PubMed.
- W. Haiss, D. Lackey, J. K. Sass and K. H. Besocke, J. Chem. Phys., 1991, 95, 2193–2196 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr05122f |
| This journal is © The Royal Society of Chemistry 2024 |