Open Access Article
Open Access ArticleEstimating the extent of adulteration of the popular herbs black cohosh, echinacea, elder berry, ginkgo, and turmeric – its challenges and limitations
Nilüfer
Orhan
 *,
Stefan
Gafner
*,
Stefan
Gafner
 and
Mark
Blumenthal
and
Mark
Blumenthal
American Botanical Council, 6200 Manor Road, 78723, Austin, TX, USA. E-mail: nympis@gmail.com
First published on 7th August 2024
Abstract
Covering: up to July 2023
Botanical natural medicinal products and dietary supplements are utilized globally for their positive impacts on health and wellness. However, the effectiveness and safety of botanical products can be compromised by unintentional or intentional adulteration. The presence of adulterated botanical ingredients in the global market has been documented in the published literature but a key question, namely what the extent of adulteration is, remains to be answered. This review aims to estimate the prevalence of adulteration in preparations made from black cohosh rhizome, echinacea root or herb, elder berry, ginkgo leaf, and turmeric root/rhizome. According to the information provided in the 78 publications retrieved for this paper, 818 of 2995 samples were reported to be adulterated and/or mislabeled. Ginkgo leaf samples (n = 533) had the highest adulteration rate with 56.7%, followed by black cohosh rhizome (n = 322) samples with 42.2%, echinacea root/herb (n = 200) with 28.5%, elder berry (n = 695) with 17.1%, and turmeric root/rhizome (n = 1247) with 16.5%. Products sold as licensed or registered herbal medicines were found to have a lower risk of adulteration compared to products sold as dietary/food supplements. The data show that the adulteration rate substantially differs from one ingredient to the other. Due to the significant limitations of the available data upon which the estimated extent of adulteration is based, and the rapidly changing botanical dietary supplement market, conclusions from the five herbs examined in this publication cannot be applied to other botanicals traded in the global market. However, the data clearly show that a substantial portion of the botanical dietary supplements do not contain what is claimed on their labels.
1. Introduction
According to the 2022 Global Supplement Business Report of the Nutrition Business Journal, total global herb and botanical sales reached $37.506 billion in 2021, representing a 5.6% growth over 2020.1 Annual retail sales of herbal dietary supplements in the United States alone totalled $12.350 billion in the same year, according to the American Botanical Council's (ABC's) 2021 herb market report. This represents the highest recorded annual spending on these products in the U.S. and a 9.7% increase from the previous year.2 These values are also very conservative as they are limited to sales that are electronically tracked and do not capture sales by multilevel marketing or health professionals.While the supplement industry is growing, as is the botanical sector of this industry, so are the number of documented cases of adulteration of herbal medicine and dietary supplement ingredients and finished products. In fact, the recent industry growth and increased popularity of these products may be placing additional strain on supply chains, exacerbating the adulteration problem.
1.1. Terminology and history of adulteration
Adulteration has multiple definitions. For this paper, adulteration is defined as accidental or intentional deviations in declarations of botanical ingredient identity, strength, composition, geographic origin, or production methods from the named or implied claims made for a drug, dietary/food supplement, spice, or food, or an ingredient to be used in such goods. Failure to meet the declared strength was not considered as adulteration unless the ingredient or product contained the declared botanical only in trace amounts. Economically motivated adulteration (EMA) is defined by the United States Pharmacopeia (USP) as the fraudulent addition of non-authentic substances or removal or replacement of authentic substances without the purchaser's knowledge for the economic gain of the seller. In the vast majority of cases, the adulterants do not represent a safety risk, although there are some notable exceptions, such as the adulteration of skullcap (Scutellaria lateriflora, Lamiaceae) with hepatotoxic germander (Teucrium spp., Lamiaceae),3 or the presence of various undeclared colorants in ingredients such as St. John's wort (Hypericum perforatum, Hypericaceae) or bilberry (Vaccinium myrtillus, Ericaceae).4 A particularly unfortunate practice is the addition of lead chromate to turmeric (Curcuma longa, Zingiberaceae)5,6 or marigold (Tagetes patula, Asteraceae),7 since the presence of elevated blood lead levels is known to increase the risk of damage to the central nervous system, and is known to lead to slower growth, decreased learning ability, and behavior issues in children. Moreover, in 2023 and 2024 the US Food and Drug Administration (FDA) initiated recalls for various dietary supplements marketed for weight loss due to intentional adulteration with yellow oleander (Cascabela thevetia, Apocynaceae) seeds. These supplements, which were labeled to contain either Mexican hawthorn (Crataegus mexicana, Rosaceae) root, candlenut (Aleurites moluccanus, Euphorbiaceae) seeds, or Brazil seeds (Bertholletia excelsa, Lecythidaceae), reportedly caused severe adverse effects including nausea, dizziness, and changes in cardiac rhythm.8 Adulteration is an ancient and ongoing occurrence that has been described since the first centuries by scholars in the history of botany and pharmacy such as Dioscorides (c. 40–90 AD), Pliny the Elder (AD 23/24 – AD 79), Theophrastus (c. 371 – c. 287 BC), and Galen (129–216 AD).9 However, advances in producing herbal medicines and botanical dietary supplements, particularly the use of extracts instead of whole, cut, or powdered medicinal plants, as well as the increased popularity of essential oils from plants, have made the detection of adulteration more challenging.1.2. Types of intentional botanical ingredient adulteration
Intentional botanical ingredient adulteration currently occurs in many ways, such as the mislabeling of a material containing a lower-cost material, the undeclared admixing of lower-cost plant parts of a species to the labeled plant part, adding lower-cost plants or plant extracts with similar chemical profiles to the target plant without indicating them on the label or certificate of analysis, adding undeclared food colorants, or the undeclared “fortifying” an ingredient or product with lower-cost synthetic or nature-identical pure compounds to impart a sense of higher product quality based on the analysis of chemical markers.4 Additionally, some producers substitute or dilute raw plant materials or extracts of plant materials with excessive amounts of bulking agents such as maltodextrin or starches to increase the profit margin.10 Another type of adulteration involves the illegal sale of undeclared pharmaceutical drugs as botanical food or dietary supplements to create a sense of effectiveness, even if it is unrelated to the labeled botanical ingredient. These unethical and illegal practices represent a substantial challenge for the responsible members of the global herbal products, dietary supplement, and natural products marketplace and undermine products' effectiveness, safety, and consumer trust in them.41.3. Studies of the botanical adulterants prevention program
Many studies have been published to identify adulterants in botanical bulk ingredients and commercial finished products using a variety of analytical laboratory methods.11–13 These methods, including macroscopic and microscopic examinations, chemical techniques (including analytical instrumentation methods), and DNA-based diagnostics, have their benefits and specific strengths, and limitations, in the authentication of botanical materials.4 Many of these studies have been reviewed and summarized by the ABC-AHP-NCNPR Botanical Adulterants Prevention Program (BAPP), a research and educational partnership among the nonprofit American Botanical Council (ABC), the American Herbal Pharmacopoeia (AHP), and the University of Mississippi's National Center for Natural Products Research (NCNPR). BAPP aims to raise awareness and provide solutions to adulteration issues to members of the global herb, dietary supplement, and natural products industries. To date (March 2024), BAPP has published 84 peer-reviewed documents to report adulteration of specific ingredients, and/or to evaluate laboratory test methods to authenticate legitimate ingredients, and to detect the potential presence of undeclared adulterants in botanical materials, dietary supplements, and other natural products.14Despite the extensive documentation and confirmation of the presence of adulterated botanical ingredients in the global market, one of the key questions that remains to be answered is the extent of adulteration. Three reviews11–13 attempted to answer this question. Researchers assessed adulteration frequency by reporting on studies that used macroscopic and microscopic examinations, genetic testing or analysis by chromatographic or spectrometric/spectroscopic means. The overall authenticity of the microscopically examined commercial products was 59% (n = 300).13 Of the 5957 commercial herbal products analyzed by genetic methods, 1611 or 27% were reportedly adulterated.11 Similarly, when chromatographic or spectrometric/spectroscopic techniques were used, 652 (27%) of the 2386 commercial herbal products were found to be adulterated.12
1.4. Challenges and limitations of estimating the extent of adulteration
There are many challenges in determining the accurate number of adulterated products for a specific market. Results from comprehensive testing/analyzing of all products sold in a geographic region are not available. In fact, in most papers, the names of the tested products and their respective market shares or importance in the market are not provided. Therefore, it is not clear if the products analyzed represent niche products with a small market share, or if they are among the top-sellers in each market, or both. Even in cases where the brand names are made available, data on their market share are usually not provided by the authors, making an assessment of their impact on the market impractical.In market tests of botanical dietary supplement products, the testing entity may sometimes employ methods that are not fit-for-purpose for a specific product or its unique combination of ingredients. This can make it challenging to detect or identify certain ingredients in the product or lead to erroneous conclusions. Hence, the assessment of adulteration in each of the 78 publications reviewed is author-specific and depends on the analytical methods used. The authors of this paper have not attempted to assess the accuracy of the findings in the publications included in this review. As an example, the study on ginkgo product authenticity published by Little (2014) detected DNA for Ginkgo biloba in 31 of the 40 samples tested.15 Six of the remaining nine samples contained rice DNA, while no DNA was obtained for the other three samples. Since the absence of ginkgo plant DNA in a highly processed ingredient is not necessarily evidence of adulteration, just as the presence of ginkgo DNA does not confirm the authenticity of a product, the adulteration rate could not be determined. However, based on the data, between 0 and nine out of 40 products (0–22%) would be considered adulterated. This is considerably lower than adulteration rates of similar investigations using chromatographic methods of analysis, which show an average of 57% for ginkgo leaf extract (Section 3.4 Ginkgo).
Another factor impacting results is the source of information. Investigation of the authenticity of elder berry by Gafner et al.16 reported an average adulteration rate of 7% when samples were analyzed by contract analytical laboratories, while the average adulteration rate was 40% when samples were analyzed by manufacturers of elder berry dietary supplements. A proposed explanation for this apparent discrepancy is that companies that test ingredients or products through qualified third-party laboratories are likely to be actively engaged in regulatory compliance and have appropriate supplier qualification protocols. This results in higher quality ingredients (i.e., with respect to their authenticity) which are unlikely to fail the tests. Whereas manufacturers that analyze competitors' products may be subject to selection bias by more frequently analyzing products that are suspicious based on low costs or marketing claims and hence at higher prevalence of adulteration. Furthermore, data from contract analytical laboratories often include multiple batches from the same qualified supplier; hence adulteration results can be skewed in one direction if a supplier asks to have a high number of authentic samples tested at a laboratory. Duplicate analysis of the same products is also an issue when publications from different authors are summarized. Several of the publications investigating ginkgo leaf extract adulteration included the extract EGb 761® (W. Schwabe, Karlsruhe, Germany), the clinically-tested ginkgo leaf extract that established the market for ginkgo leaf extracts, as a control for authentic products.
2. Materials and methods
2.1. Selection of botanical ingredients
Although there are numerous challenges in calculating the frequency of adulteration in the global supply chain, this review aims to estimate the extent of adulteration based on the available published literature. To improve the estimate, we limited the analysis to collect adulteration data on botanicals that are common in the international supply chain, and for which a relatively large amount of data on identity are available. As a proxy for common use globally, we examined sales data from the U.S. dietary supplement industry for which a substantial number of products had been tested, and the results made public. According to published literature, five common ingredients that had analytical testing data available in the public literature – black cohosh (Actaea racemosa, Ranunculaceae), echinacea (Echinacea angustifolia, E. pallida, and E. purpurea, Asteraceae), elder berry (Sambucus nigra, Viburnaceae), ginkgo (Ginkgo biloba, Ginkgoaceae), and turmeric (Curcuma longa, Zingiberaceae) – were selected for this study.2.2. Search strategy and study selection
A systematic literature search was conducted on Google Scholar, PubMed, and ScienceDirect to find studies on the investigation of adulteration in commercial bulk ingredients and finished herbal products. The database search was carried out for papers published between January 2000–July 2023. Full-text articles, reports, theses, and posters were examined, and duplicates and studies that did not report data relevant to the authentication of commercial raw materials or herbal products were excluded. Additionally, the publications retrieved were searched for further papers, and when available, data from investigations by researchers from the private sector were included. The data collected are summarized in tables (Tables 1–5) for each plant individually. The extent of adulteration of each of these five ingredients is calculated as the total number of adulterated samples (sum of the numbers of adulterated samples provided by the authors of each paper) in relation to the total number of samples analyzed.| Number of samples | Detection method | Adulteration percentage (#: number of adulterated products/number of total products) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ad. samples% (#) | Ad. bulk S% (#) | Ad. extract S% (#) | Ad. mixed S% (#) | Ad. powdered S% (#) | Ad. Europe S% (#) | Ad. North America S% (#) | |||
| a Sample details such as material type (herb powder, dry extract) or origin of the samples (Europe or North America) are included when available. Ref: reference, S: samples, Ad.: adulterated, Chem: chemical, Gen: genetic, —: no information. b In this study, genetic and chemical methods were used for investigating the authenticity of one bulk and 14 dietary supplements but only six of them could be assessed for authenticity and two of them were found to be adulterated. No data were given about the authenticity of the other eight products. | |||||||||
| 4 | Chem | 25.0 (1/4) | 0 | 0 (0/2) | — | 0 | 0 | 25.0 (1/4) | 17 and 18 |
| 2 | Gen | 0.0 (0/2) | 0.0 (0/1) | — | — | — | 0 | 0.0 (0/2) | 19 |
| 6 | Chem | 16.7 (1/6) | 0 | 0.0 (0/3) | 0.0 (0/1) | 50.0 (1/2) | 0 | 16.7 (1/6) | 20 |
| 11 | Chem | 36.4 (4/11) | 0 | 30.0 (3/10) | 0 | 100.0 (1/1) | 0 | 36.4 (4/11) | 21 |
| 4 | Chem | 100.0 (4/4) | 0 | 100.0 (4/4) | 0 | 0 | 100.0 (4/4) | 0 | 22 |
| 40 | Gen | 22.5 (9/40) | 0 | 0 | 0 | 22.5 (9/40) | — | — | 23 |
| 4 | Gen | 25.0 (1/4) | — | — | — | — | 0 | 25.0 (1/4) | 24 |
| 16 | Chem, Gen | 25.0 (4/16) | 0 | 14.3 (1/7) | 0.0 (0/3) | 50.0 (3/6) | 0 | 26.7 (4/15) | 25 |
| 34 | Chem, Gen | 38.2 (13/34) | 50.0 (8/16) | 33.3 (3/9) | 0.0 (0/5) | 50.0 (2/4) | 29.4 (5/17) | 47.1 (8/17) | 26–28 |
| 30 | Chem, Gen | 40.0 (12/30) | 100.0 (5/5) | 13.3 (2/15) | 60.0 (3/5) | 40.0 (2/5) | — | — | 29 and 30 |
| 5 | Chem | 20.0 (1/5) | 20.0 (1/5) | 0 | 0 | 0 | 25.0 (1/4) | 0 | 31 |
| 15 | Chem, Gen | 13.33 (2/15) | 100.0 (1/1) | — | — | — | 0 | 7.1 (1/14) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| 1 | Gen | 0.0 (0/1) | 0.0 (0/1) | 0 | 0 | 0 | 0 | 0.0 (0/1) | 33 |
| 36 | Chem | 83.3 (30/36) | 0 | 0 | 92.3 (12/13) | 78.3 (18/23) | — | — | 34 |
| 39 | Chem | 51.3 (20/39) | 0 | 57.1 (16/28) | 0 | 45.5 (4/11) | — | — | 35 |
| 13 | Gen | 0.0 (0/13) | 0 | 0.0 (0/6) | 0 | 0.0 (0/7) | 0 | 0.0 (0/13) | 36 |
| 60 | Chem | 55.0 (33/60) | 66.7 (18/27) | 54.5 (6/11) | 0 | 40.9 (9/22) | 0 | 55.0 (33/60) | 37 |
| 320 | 42.2 (135/320) | 58.9 (33/56) | 36.8 (35/95) | 55.6 (15/27) | 40.5 (49/121) | 32.3 (10/31) | 31.9 (53/166) | Total | |
| Number of samples | Detection method | Adulteration percentage (#: number of adulterated products/number of total products) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ad. samples% (#) | Ad. bulk S% (#) | Ad. extract S% (#) | Ad. mixed S% (#) | Ad. powder S% (#) | Ad. Europe S% (#) | Ad. North America S% (#) | |||
| a Sample details such as material type (herb powder, extract) or origin of the samples (Europe or North America) are included when available. S: samples, Ad.: adulterated, Chem: chemical, Gen: genetic, —: no information. | |||||||||
| 55 | Chem | 43.6 (24/55) | — | — | — | — | 0 | 43.6 (24/55) | 38 |
| 9 | Chem | 0.0 (0/9) | 0 | 0.0 (0/9) | 0 | 0 | 0 | 0.0 (0/9) | 39 |
| 3 | Chem | 66.7 (2/3) | 0 | 66.7 (2/3) | 0 | 0 | 0 | 0 | 40 |
| 20 | Chem | 50.0 (10/20) | — | 100 (3/3) | 0.0 (0/1) | 36.4 (4/11) | — | — | 41 |
| 19 | Chem | 36.8 (7/19) | — | — | — | — | 0 | 36.8 (7/19) | 42 |
| 53 | Chem, Gen | 9.4 (5/53) | — | 14.3 (1/7) | — | 5.9 (1/17) | 11.1 (5/45) | 0.0 (0/7) | 43 |
| 18 | Chem | 33.3 (6/18) | 23.1 (3/13) | — | — | 60.0 (3/5) | 0 | 33.3 (6/18) | 44 |
| 23 | Chem | 13.0 (3/23) | 0 | 13.6 (3/19) | (1/3) | 0.0 (0/1) | 13.0 (3/23) | 0 | 37 |
| 200 | 28.5 (57/200) | 23.1 (3/13) | 22.0 (9/41) | 25.0 (1/4) | 23.1 (8/34) | 11.8 (8/68) | 34.3 (37/108) | Total | |
| Number of samples | Detection method | Adulteration percentage (#: number of adulterated products/number of total products) | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| Ad. samples% (#) | Ad. bulk S% (#) | Ad. extract S% (#) | Ad. powder S% (#) | Ad. Europe S% (#) | Ad. North America S% (#) | |||
| a Sample details such as material type (powder, extract) or origin of the samples (Europe or North America) are included when available. S: samples, Ad.: adulterated, Chem: chemical, Gen: genetic, —: no information. b Syrups (10) and liquid (4) commercial products are included in the extract group. c Samples in this study were purchased from Türkiye; despite Türkiye's being a transcontinental country spanning across Europe and Asia, these products were added to Europe in the table. | ||||||||
| 30 | Chem | 30.0 (9/30) | — | — | 100.0 (1/1) | 0 | 30.0 (9/30) | 45 |
| 73 | Chem | 24.7 (18/73) | 14.6 (6/41) | 38.7 (12/31)b | 0.0 (0/1) | — | 37.5 (6/16) | 46 |
| 532 | Chem, Gen | 10.9 (58/532) | — | — | — | 0 | 10.9 (58/532) | 16 |
| 11c | Chem | 81.8 (9/11) | 0 | 81.8 (9/11) | 0 | 81.8 (9/11) | 0 | 47 |
| 31 | Chem | 67.7 (21/31) | — | 77.8 (7/9) | 63.6 (14/22) | 0 | 67.7 (21/31) | 48 |
| 18 | Chem | 22.2 (4/18) | 0 | 44.4 (4/9) | 0 (0/9) | 0 | 22.2 (4/18) | 49 |
| 695 | 17.1 (119/695) | 14.6 (6/41) | 53.3 (32/60) | 45.6 (15/33) | 81.8 (9/11) | 15.6 (98/627) | Total | |
| Number of samples | Detection method | Adulteration percentage (#: number of adulterated products/number of total products) | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad. S% (#) | Ad. bulk S% (#) | Ad. extract S% (#) | Ad. mixed S% (#) | Ad. PD S% (#) | Ad. ANZ S% (#) | Ad. Asia S% (#) | Ad. Eu S% (#) | Ad. NA S% (#) | Ad. SA S% (#) | |||
| a Sample details such as material type (powder, extract) or origin of the samples are included when available. S: samples, Ad.: adulterated, Chem: chemical, Gen: genetic, n: adulterated product number, PD: powdered leaves, Eu: Europe, ANZ: Australia + New Zealand, NA: North America, SA: South America, —: no information. b Samples in this study were purchased from Türkiye and were added to Europe in the table. | ||||||||||||
| 10 | Chem | 20.0 (2/10) | 0 | 20.0 (2/10) | 0 | 0 | — | — | — | — | — | 50 |
| 14 | Chem | 28.6 (4/14) | — | — | — | — | 0 | 0 | 0 | 28.6 (4/14) | 0 | 51 |
| 22 | Chem | 54.6 (12/22) | 0 | 54.6 (12/22) | 0 | 0 | 0 | 0 | 0 | 54.6 (12/22) | 0 | 52 |
| 19 | Chem | 15.8 (3/19) | 0 | 15.8 (3/19) | 0 | 0 | 0 | 15.8 (3/19) | 0 | 0 | 0 | 53 |
| 5 | Chem | 40.0 (2/5) | 0 | 40.0 (2/5) | 0 | 0 | 0 | 0 | 40.0 (2/5) | 0 | 0 | 54 |
| 16 | Chem | 6.3 (1/16) | 0 | 6.3 (1/16) | 0 | 0 | 0 | 0 | 6.3 (1/16) | 0 | 0 | 55 |
| 11 | Chem | 72.7 (8/11) | 0 | 72.7 (8/11) | 0 | 0 | 0 | 0 | 72.7 (8/11) | 0 | 0 | 56 |
| 14 | Chem | 50.00 (7/14) | 0.00 (0/4) | 70.00 (7/10) | 0 | 0 | 0 | 0 | 50.0 (7/14) | 0 | 0 | 57 |
| 8 | Chem | 37.5 (3/8) | 37.5 (3/8) | 0 | 0 | 0 | 0 | 37.5 (3/8) | 0 | 0 | 0 | 58 |
| 18 | Chem | 38.9 (7/18) | 0 | 38.9 (7/18) | 0 | 0 | 0 | 0 | 0 | 38.9 (7/18) | 0 | 59 |
| 22 | Chem | 13.6 (3/22) | 0 | 13.6 (3/22) | 0 | 0 | 0 | 18.8 (3/16) | 0.0 (0/6) | 0 | 0 | 60 |
| 22 | Chem | 72.7 (16/22) | 0 | 72.7 (16/22) | 0 | 0 | 0 | 0 | 0 | 0 | 72.7 (16/22) | 61 |
| 8 | Chem | 25.0 (2/8) | 0 | 25.0 (2/8) | 0 | 0 | 0 | 0 | 25.0 (2/8) | 0 | 0 | 62 |
| 13b | Chem | 38.5 (5/13) | 0 | 38.5 (5/13) | 0 | 0 | 0 | 0 | 38.5 (5/13) | 0 | 0 | 63 |
| 8 | Chem | 37.5 (3/8) | 0 | 37.5 (3/8) | 0 | 0 | 50.0 (3/6) | 0 | 0.0 (0/2) | 0 | 0 | 64 |
| 25 | Chem | 80.0 (20/25) | 0 | 80.0 (20/25) | 0 | 0 | 0 | 0 | 0 | 80.0 (20/25) | 0 | 65 |
| 35 | Chem | 94.3 (33/35) | 0 | 94.0 (29/31) | 100.0 (2/2) | 100.0 (2/2) | 0 | 0 | 94.3 (33/35) | 0 | 0 | 66 |
| 29 | Chem | 51.7 (15/29) | 26.7 (4/15) | 78.6 (11/14) | 0 | 0 | — | — | — | 18.18 (4/22) | — | 67 |
| 20 | Chem, Gen | 85.0 (17/20) | 0 | 93.8 (15/16) | 66.7 (2/3) | 0.0 (0/1) | 0 | 0 | 0 | 85.0 (17/20) | 0 | 68 |
| 11 | Chem | 72.7 (8/11) | 0 | 77.8 (7/9) | 0 | 50.0 (1/2) | 0 | 0 | 72.7 (8/11) | 0 | 0 | 69 |
| 22 | Chem | 72.7 (16/22) | 0 | 72.7 (16/22) | 0 | 0 | 28.6 (2/7) | 0 | 93.3 (14/15) | 0 | 0 | 70 |
| 36 | Gen | 25.0 (9/36) | 0 | 32.1 (9/28) | 0 | 0.0 (0/8) | — | — | — | — | — | 71 |
| 10 | Chem | 20.0 (2/10) | 0 | 20.0 (2/10) | 0 | 0 | 0 | 0 | 20.0 (2/10) | 0 | 0 | 72 |
| 59 | Chem | 81.4 (48/59) | 0 | 78.8 (41/52) | 100 (2/2) | 100 (5/5) | 100.0 (1/1) | 0 | 80.9 (38/47) | 57.1 (4/7) | 100.0 (4/4) | 73 |
| 7 | Chem | 42.9 (3/7) | 0 | 60.0 (3/5) | 0 | 0.0 (0/2) | 0 | 0 | 42.9 (3/7) | 0 | 0 | 74 |
| 22 | Chem | 68.2 (15/22) | 0 | 68.2 (15/22) | 0 | 0 | 0 | 0 | 68.2 (15/22) | 0 | 0 | 75 |
| 24 | Chem | 75.0 (18/24) | 90.0 (18/20) | 0.0 (0/4) | 0 | 0 | 0 | 0 | 75.0 (18/24) | 0 | 76 | |
| 23 | Chem | 82.6 (19/23) | 0 | 82.6 (19/23) | 0 | 0 | 0 | 0 | 82.6 (19/23) | 0 | 0 | 77 |
| 533 | 56.5 (301/533) | 53.2 (21/47) | 58.0 (258/445) | 85.7 (6/7) | 40.0 (8/20) | 42.9 (6/14) | 20.9 (9/43) | 64.1 (157/245) | 56.6 (86/152) | 76.9 (20/26) | Total | |
| Number of samples | Detection method | Adulteration percentage (#: number of adulterated products/number of total products) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad. samples% (#) | Ad. bulk S% (#) | Ad. extract S% (#) | Ad. mixed S% (#) | Ad. powder S% (#) | Ad. Asia S% (#) | Ad. Europe S% (#) | Ad. NA S% (#) | |||
| a Sample details such as material type (powder, extract) or origin of the samples are included when available. S: samples, Ad.: adulterated, Chem: chemical, Gen: genetic, NA: North America, —: no information. | ||||||||||
| 3 | Gen | 100.0 (3/3) | 0 | 0 | 0 | 100.0 (3/3) | 100.0 (3/3) | 0 | 0 | 78 |
| 712 | Chem | 14.8 (105/712) | 0 | 0 | 0 | 14.8 (105/712) | 14.8 (105/712) | 0 | 0 | 79 |
| 6 | Gen | 66.7 (4/6) | 0 | 0 | 0 | 66.7 (4/6) | 66.7 (4/6) | 0 | 0 | 80 |
| 6 | Chem | 0.0 (0/6) | — | — | — | — | 0 | 0 | 0.0 (0/6) | 81 |
| 22 | Chem | 54.6 (12/22) | 40.0 (2/5) | 100 (7/7) | 66.7 (2/3) | 14.3 (1/7) | 33.3 (4/12) | 100.0 (5/5) | 60.0 (3/5) | 82 |
| 12 | Chem | 33.3 (4/12) | 0.0 (0/2) | 44.4 (4/9) | 0 | 0.0 (0/1) | 0 | 0 | 33.3 (4/12) | 83 |
| 35 | Chem | 14.3 (5/35) | 0 | 15.8 (3/19) | 14.3(2/14) | 0.0 (0/2) | 0 | 0 | 14.3 (5/35) | 84 |
| 45 | Chem | 24.4 (11/45) | 0 | 33.3 (4/12) | 41.2 (7/17) | 0.0 (0/16) | — | — | — | 85 |
| 16 | Chem | 25.0 (4/16) | 0 | 25.0 (4/16) | 0 | 0 | 25.0 (4/16) | 0 | 0 | 86 |
| 18 | Chem | 38.9 (7/18) | 0 | 31.3 (5/16) | 100.0 (1/1) | 100.0 (1/1) | 0 | 38.9 (7/18) | 0 | 87 |
| 2 | Chem | 100.0 (2/2) | 0 | 100.0 (2/2) | 0 | 0 | 0 | 100.0 (2/2) | 0 | 88 |
| 316 | Chem, Gen | 10.8 (34/316) | 0 | 0 | 0 | 0 | 0 | 10.8 (34/316) | 0 | 89 |
| 14 | Chem | 35.7 (5/14) | 0 | 50.0 (1/2) | 40.0 (4/10) | 0.0 (0/2) | 0 | 0 | 35.7 (5/14) | 90 |
| 36 | Chem | 61.1 (9/36) | 50.0 (1/2) | 27.3 (6/22) | 0.0 (0/1) | 18.2 (2/11) | 83.3 (5/6) | 13.0 (3/23) | 0.0 (0/7) | 91 |
| 4 | Chem | 25.0 (1/4) | 0 | 25.0 (1/4) | 0 | 0 | 0 | 25.0 (1/4) | 0 | 92 |
| 1247 | 16.5 (206/1247) | 33.3 (3/9) | 42.2 (46/109) | 34.8 (16/46) | 15.2 (116/761) | 16.8 (125/746) | 14.1 (52/368) | 21.5 (17/79) | Total | |
2.3. Additional information on the products and analytical methods included
The term “bulk material” is used for unfinished commercial products sold in large quantities prior to manufacturing. Preparations in tea bag form are counted as powdered herbs and added to the tables as such. Products containing both powdered plants and extracts are listed as mixed products. The number of adulterated samples was based on the study authors' assessment and, in rare cases, by the authors of this review. The investigated commercial products were purchased from pharmacies, grocery stores, mass market retailers, health food stores, markets, online retailers, or in some cases directly from the producers in various countries. When information was provided, herbal products were grouped as “bulk,” “powdered plant,” “extract”, and “mixed products”.The methods used for the investigation of adulteration were also provided and grouped under genetic and chemical titles. Chemical methods of analysis included chromatographic (TLC, HPTLC, HPLC, UHPLC) methods with various detector systems, spectrometric (MS), and spectroscopic (IR, NIR, NMR) methods. The data collected on the five selected plants are provided in adulteration report summary tables (Tables 1–5). Studies that used the same datasets by the same/different research groups are given in the same row in Table 1.
3. Results of the adulteration studies on selected botanical ingredients
3.1. Black cohosh (Actaea racemosa L., syn. Cimicifuga racemosa (L.) Nutt.)
The dried rhizome and root of black cohosh are used as an aid in the management of premenstrual discomfort, dysmenorrhea, and climacteric symptoms such as hot flashes, profuse sweating, sleeping disorders, and nervous irritability.93 The roots and rhizomes are sold in whole, chopped, or powdered forms, and their liquid or dried extracts are available mainly as tinctures, tablets, or capsules.94 Between 2011 and 2017, black cohosh was consistently one of the 10 top-selling herbs in the mainstream market and has ranked among the 30 top-selling herbs in the natural foods sector in the U.S.95–104 According to data from the market research company SPINS, at least 76 black cohosh dietary supplements (this number includes stock keeping units [SKUs] from the same brand sold under different sizes or into special markets if the SKU number is different) have reported sales in the 52 weeks prior to October 18, 2022 in the combined US natural and multi-outlet channels (personal e-mail from Haleigh Resetar, October 18, 2022, SPINS).To determine the frequency of adulteration in black cohosh bulk ingredients and dietary supplements containing them, investigations into their authenticity published between 2000 and 2023 were reviewed, including 21 studies with data on ingredient and product authenticity. Several studies17,18,26–30 used the same set of black cohosh samples for their research. The results of the studies that used the common sample sets are given in the same row in Table 1.
Chromatographic methods were used in 10 studies,17,18,20–22,27,31,34,35,37 while genetic methods to detect adulteration were reported in six publications including a total of 68 samples.19,23,24,26,33,36 Five studies with 155 samples used both chemical and genetic methods to determine the authenticity of products.25,28–30,32 DNA could not be found in 14 samples out of all the materials submitted to genetic testing. These 14 samples were not classified as adulterated, as it is well-known that highly processed herbal materials often do not yield extractable DNA. The main adulterants of black cohosh were determined as Actaea species originating from Asia: A. asiatica, A. brachycarpa, A. cimicifuga, A. dahurica, A. heracleifolia, and A. simplex. In the case of black cohosh, the primary constituents of interest are the 9,19-cycloartenol type triterpene glycosides (actein [1], 23-epi-26-deoxyactein [2], cimiracemoside A [3], etc.) along with phenolic acids, tannins, fatty acids, and nitrogen-containing compounds such as alkaloids.94 The adulterating Asian Actaea species reportedly also contain 9,19-cycloartenol type triterpene glycosides and some of the same phenolic acids as black cohosh.105,106
However, these Asian species reportedly lack 1 and 2, or have them present at substantially lower concentrations compared to authentic North American black cohosh. In addition, the presence of dihydrofurochromones, such as cimifugin (4) and cimifugin-3-O-glycoside (5), is indicative of adulteration.105,106
According to the data summarized in Table 1, 320 products labeled as containing “black cohosh” were investigated in total; these included 56 bulk root and rhizome samples, 95 dietary supplement extracts, and 121 products containing powdered root and rhizome. Twenty-seven of the black cohosh dietary supplements were labeled as containing black cohosh extract with root powder. These products were listed in a different column under the mixed samples title. Eight of the investigated products were licensed as an herbal medicinal product (herbal drug, a regulatory designation in the EU and/or elsewhere) and 175 commercial samples were sold as a dietary supplement. Information about the regulatory category (herbal drugs or dietary supplements) for the remaining products was not provided by the authors.
Adulterated or mislabeled products were found to represent 42% (i.e., 135 of 320 samples). The percentage of adulterated ingredients sold in bulk (59%) was higher than of finished products sold as mixed products (56%), extracts (37%), or powdered root and rhizome (41%). In almost half of the samples tested, the authors did not specify the origin of the bulk ingredients or finished products. Of the samples for which the purchasing country was indicated (n = 205), 166 were from North America (USA, Canada), 31 were from Europe (Germany, UK, Bulgaria), 6 were from Asia (Japan, China), and 2 were from Australia and New Zealand. Except for North America (32% of 166 samples adulterated), the sample numbers were too low to calculate a meaningful adulteration percentage. One study from 2022 included data on 60 black cohosh dietary supplements sold on the U.S. market, of which 33 products were deemed to be adulterated. Considering that SPINS recorded 57 black cohosh SKUs sold in the US, the 60 samples analyzed by Frommenwiler et al.37 represent a substantial part of the U.S. black cohosh market and hence provide a good estimate of black cohosh product authenticity in this country.
All the tested products sold as licensed or registered herbal medicines (n = 8) were found to be authentic, while for dietary supplements, the frequency of adulteration was calculated as 21% (36 out of 175). This is an expected result since herbal medicines are licensed as herbal medicinal products in the European Union and they are produced according to stricter rules, including premarket approvals (for authenticity, claims, etc.) with quality control and quality assurance documentation, than those that are required for food supplements in the EU.
3.2. Echinacea (Echinacea spp.)
Preparations containing aerial parts and/or roots of Echinacea purpurea (Asteraceae) and roots of E. angustifolia or E. pallida are commonly used by consumers to prevent or manage symptoms of colds and infections of the upper respiratory tract (and, in some cases, lower urinary tract) due to their documented immunomodulatory effects.107,108 They are sold as dry herb (whole, cut and sift, or powdered), liquid or dry extracts, tinctures, and dried or fresh-pressed juice in various pharmaceutical forms such as liquid preparations, pastilles, lozenges, tablets, or capsules.107,108 Echinacea is one of the most popular herbs in the U.S. and recently experienced one of the strongest sales growth (36.8% in 2020 over 2019 sales) in the mainstream retail channel.95–101,103,104,109 SPINS data show that 457 echinacea dietary supplement SKUs were sold over the 52 weeks period (from October 15, 2021 to October 15, 2022) in the combined natural and multi-outlet channels in the U.S. (personal e-mail from Haleigh Resetar, October 18, 2022, SPINS).The echinacea adulteration report summary is shown in Table 2. The investigations conducted on echinacea bulk ingredients, dietary supplements, or licensed or registered herbal medicines published between 2000 and 2023 were reviewed, and eight studies37–44 were qualified for inclusion in this review. To detect adulteration, various chemical methods such as thin-layer chromatography (TLC) or high-performance thin-layer chromatography (HPTLC), high-performance liquid chromatography (HPLC) with charged aerosol detection (CAD), ultraviolet spectroscopy (UV-vis) detection, or mass spectrometric (MS) detection, hyperspectral imaging, or flow-injection mass spectrometry (FIMS) were used in eight studies along with a genetic method in one study.
Data summarized in Table 2 show that 200 echinacea products, collected from different continents (Europe = 68, North America = 108, Australia and New Zealand = 3, Asia = 1) were investigated. No commercial products were purchased from South America and Africa. Most of the commercial finished products (n = 138) were collected from pharmacies and health food shops and only one product was purchased from online retailers. Eleven of the products were licensed as traditional herbal medicinal products and 93 of them were dietary supplements. Although roots of Parthenium integrifolium (Asteraceae), Lespedeza capitata (Fabaceae), Eryngium aquaticum (Apiaceae), Rudbeckia nitida (Asteraceae), Helianthus annuus (Asteraceae), Liatris aspera (Asteraceae) and Cistanche species (Orobanchaceae) have been documented as adulterants of “Radix Echinacea”, none of these adulterants were detected in the studies summarized here.4,9
The main problems observed were the sale of products containing little or no echinacea material, partial or entire substitution with echinacea species other than the declared species, and blending of large amount of the aerial parts with roots (and vice versa) without declaration of both plant parts.
It is not clear if echinacea adulteration is done on purpose or accidental, as cases of inaccurate seed labeling or species confusion are known from this genus. Admixture of the wrong plant part or species can be detected using the fingerprints of the alkylamides (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) alkamides) or phenolic constituents.110
alkamides) or phenolic constituents.110
Larger concentrations of chichoric acid (6) and caftaric acid (7) are characteristic for E. purpurea, while high amounts of echinacoside (8) are indicative of E. angustifolia or E. pallida. Cynarin (9) is reportedly present in E. angustifolia, but not in the roots of the other two species in commerce.111,112 Adulteration with echinacoside-containing Cistanche species, possibly C. deserticola, has been reported in 2023.4Cistanche deserticola contains substantial amounts of verbascoside (10), which can help in the detection of adulteration.
More than 20 alkylamides have been reported from the roots of echinacea species; the highest concentration is in E. angustifolia, followed by E. purpurea, with E. pallida having the lowest amounts. They are also found, albeit less abundantly, in the aerial parts of E. purpurea. According to most authors, the main alkylamides consist of a mixture of isomeric dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides (11a/b).39,43,44,108 Binns et al. reported dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide (12) as the most abundant alkylamide in wild harvested E. purpurea.112 Additionally, undeca-2E-ene-8,10-diynoic acid isobutylamide (13) is described as a marker compound for E. angustifolia roots.113,114
In total, 57 of the bulk ingredients and finished products were found to be adulterated and/or mislabeled (29%). The results of a study combining DNA metabarcoding with HPTLC showed the presence of Echinacea spp. in 34 out of 38 sequenced samples, while the number of investigated samples was 53 in total.43 Even though echinacea is one of the best-known and most highly consumed herbs, the number of samples analyzed represents the smallest sample size among the five selected herbs in this review. Additionally, in some of the publications mentioned in Table 2, the product label information of the adulterated samples was not given in detail and therefore not included in Table 3 (marked with “–”). Hence, the extract and powdered dietary/food supplement adulteration percentages probably do not reflect the actual market situation. The extent of echinacea adulteration in North America (34%, 37 of 108 samples) appears to be higher than in Europe (12%, 8 of 68 samples). However, compared to the total number of SKUs sold in the U.S. (n = 457), the number of echinacea dietary supplement samples tested from the U.S. and Canadian markets is relatively low (n = 108) and therefore results may not be representative of the overall quality of products marketed in North America.
3.3. Elder berry (Sambucus nigra L.)
Sambucus nigra (Viburnaceae), also known as black elder or European elder,115 is distributed throughout most of Europe and has been introduced to the Americas and Oceania. Elder berries are mostly used in the form of capsules, gummies, and tablets, or as fruit juice or syrup to prevent or treat symptoms of the common cold and flu, for general immune health, or for respiratory support.16In 2020, elder berry was the top-selling herbal dietary supplement ingredient in mainstream retail outlets in the United States. Elder berry sales in this channel more than doubled each year from 2018 to 2020, and the increase in popularity moved the herb from the 25th top-selling ingredient in 2015 to the top spot in 2020.95–101,103,104,109
For elder berry retail sales over the 52 weeks ending on October 18, 2022, in the combined natural and multi-outlet channel in the US, 152 unique commercial products containing elder berry were sold according to SPINS (personal e-mail from Haleigh Resetar, October 18, 2022, SPINS).
Based on the search of publications between 2000 and 2023, six analytical studies on elder berry commercial samples were found. The summary of investigations into the authenticity of commercial elder berry bulk ingredients and finished dietary and herbal medicinal products is given in Table 3. Samples (n = 695) were procured mainly from North America (n = 627), with a small number also from Europe (n = 11) and the remainder of unknown provenance, and the exact region of harvest or manufacturing was not detailed in most of them. Authenticity was assessed using predominantly chromatographic (HPTLC, TLC, HPLC-UV/Vis, and HPLC-MS) methods, which are listed as chemical methods in Table 3. Only two bulk ingredients were evaluated by a genetic method and they were both found to be authentic.16 Sixty of the investigated commercial products were labeled to contain elder berry extracts while the number of powdered elder berry commercial products was 33 and of bulk samples was 41. Ninety of the products were classified as dietary supplements. Since the European Medicines Agency's assessment report on elder berry concluded that the available information was insufficient to use elder berry as a registered herbal medicine or under the traditional herbal medicinal use regulation, there are no registered herbal medicinal elder berry products available in Europe;116 they are mainly sold as food supplements, medical devices, or as food or beverage products. Eleven products were collected from pharmacies and health shops, and 49 products were purchased from online sources.
Elder berries are rich in anthocyanins, flavonoids, organic acids, and caffeoylquinic acids. The anthocyanins in European elder berry are dominated by cyanidin-3-O-glucoside (14) and cyanidin-3-O-sambubioside (15). Total anthocyanins, measured by UV/Vis spectrophotometry, is often the sole chemical specification for marketed extracts.117 Hence elder berry adulterants generally consist of ingredients that have compounds absorbing at the same wavelengths in the visible range, such as extracts from lower-cost anthocyanin-rich plants, like black rice (Oryza sativa, Poaceae). Black rice extract adulteration can be detected by the presence of peonidin-3-O-glucoside (16).117,118 Other reports have evidenced adulteration with purple carrot (Daucus carota, Apiaceae) extract or rutin-rich extracts, and flowers of elder berry. In rare cases, berries of other Sambucus spp. (S. ebulus and S. canadensis) have also been detected. European elder berry can be distinguished by American elder berry by the absence of acylated anthocyanins, particularly cyanidin-3-O-[6-O-(E)-p-coumaroyl]sambubioside-5-O-glucoside (17), which is the main anthocyanin in American elder berry.117,118
The total number of adulterated elder berry products was calculated as 119 (17%), and 32 of 60 (53%) commercial products labeled to contain elder berry extract were found to contain undisclosed other ingredients. The main adulterant of European elder berry was determined as black rice. Additional adulterants detected included purple carrot, other Sambucus spp. (S. ebulus and S. canadensis), and undeclared synthetic colorants such as Brilliant Blue FCF and Amaranth.117
The only data on elder berry food supplement quality in Europe come from a publication by Turkish researchers, which determined nine of 11 products (82%) did not meet the ingredient label claims.47 In the U.S., the adulteration rate was calculated as 16% based on 627 samples tested. None of the samples listed under “North America” in Table 4 were from Canada or Mexico.
The lowest adulteration percentage was found in the study with the highest number of products analyzed.16 A possible explanation for the low adulteration rate is that the products were tested by a third-party analytical lab; these were submitted by lab clients and not necessarily randomly selected from the market. In this study, 470 of 532 samples were analyzed by contract analytical laboratories. The samples included multiple batches from the same vendor, and tested ingredients and products are more likely from a sector of the dietary supplement industry that is actively in compliance with regulations. Hence, the likelihood of adulteration was expected to be low. Omitting these 470 samples, of which 34 were listed as adulterated, would raise the overall adulteration rate (85 samples out of 225) to 38%. Regarding the results from the U.S. market of products tested in this study, a total of 82 elder berry dietary supplements were evaluated, and of these, 38 (46%) were reportedly adulterated. Compared to the 152 SKUs sold on the U.S. market in 2022, the data in Table 4 may represent about half of the products marketed in the U.S., considering that some samples of the same manufacturer may have been analyzed in more than one study.
3.4. Ginkgo (Ginkgo biloba L.)
Ginkgo biloba (Ginkgoaceae) leaf standardized extracts are used for the symptomatic treatment of cerebrovascular insufficiency, dementia, difficulties in concentration, tinnitus, headache, vertigo, and to improve pain-free walking distance in people with peripheral arterial occlusive disease.119In addition to the standardized leaf extracts, upon which most of the clinical evidence is based, whole, cut, and powdered dry leaf, liquid leaf extracts are used to produce coated tablets, capsules, and liquids for oral administration.119
In the United States, ginkgo has ranked among the top 25 best-selling dietary supplements from 2011–2020 in the mainstream multi-outlet channel.95–101,103,104,109 However, SPINS data show that over 245 items containing ginkgo have been sold over the 52 weeks before October 18, 2022 in the U.S. (personal e-mail from Haleigh Resetar, October 18, 2022, SPINS).
Ginkgo is one of the most popular medicinal herbs worldwide and adulteration of ginkgo products is well documented in the literature. In 28 studies published between 2000–2023,50–77 26 of them used chemical methods (HPLC, HPTLC, LC-MS/MS, FTIR, and NMR), one study used a genetic method and one study examined the adulteration by using both chemical and genetic methods and a total of 533 ginkgo commercial products were examined for their authenticity. Most of the products were collected from European countries (n = 245), while the number of samples from North America was 152, followed by Asia (n = 43), South America (n = 26), and Australia and New Zealand (combined, n = 14). According to the declared sample specifications, 445 of the samples contained leaf extracts. The number of bulk products tested was 47 and the number of products containing powdered ginkgo leaves was 20. Seven of the investigated products were classified under mixed products that contain ginkgo extracts with powdered leaves. Of the products where the regulatory category was indicated, most were labeled as dietary supplements (n = 160), while 30 were sold as licensed or registered herbal medicinal products.
Ginkgo leaf extracts are standardized to flavonol glycosides and terpene lactones, and ginkgo products are mainly adulterated by the undeclared addition of pure flavonoids (e.g., rutin [18], quercetin [19], kaempferol [20]) or extracts from other botanicals which are rich in flavonol glycosides such as flower and fruits of Japanese sophora (Styphnolobium japonicum, syn: Sophora japonica, Fabaceae) and possibly buckwheat (Fagopyrum esculentum, Polygonaceae).120 The isoflavone genistein (21) is proposed as a marker compound for the detection of ginkgo leaf extract adulteration with Japanese sophora. None of the reported adulterants of ginkgo are considered harmful.
According to data obtained from the studies summarized in Table 4, the overall frequency of adulteration was calculated as 57% (301 of 533 samples). Among 36 ginkgo samples, 34 were found to have ginkgo DNA sequences, and 9 were found to have Sophora japonica DNA sequences using the recombinase polymerase amplification assay.71 The extent of adulteration based on the available data was similar in Europe (64%, 157 of 245 samples) and North America (57%, 86 of 152 samples). The number of samples from other geographic areas was too small for an accurate adulteration frequency determination. All the tested products licensed as registered herbal medicinal products were found to be authentic, while the adulteration rate for dietary supplements was 73% (n = 116). Of note, products containing the extract EGb 761® were used as a control in several of the publications investigating ginkgo leaf extract adulteration; therefore, this extract was tested and counted multiple times. Since a substantial number (n = 152) of ginkgo products sold in the North American markets (USA and Canada) were evaluated, the numbers presented here may be indicative of the authenticity of ginkgo leaf dietary supplements sold in the U.S. and Canada.
3.5. Turmeric (Curcuma longa L.)
The deep orange-yellow powder known as turmeric is prepared from peeled, boiled, and dried rhizomes of Curcuma longa (Zingiberaceae). Turmeric-based products have seen a steady increase in popularity and turmeric has been the top-selling herbal dietary supplement ingredient in natural retail stores in the United States between 2013 and 2018.95–101,103,104,109 According to SPINS data, over 263 SKUs containing turmeric have reported sales over the 52 weeks ending on October 18, 2022 in the combined natural and multi-outlet channel (personal e-mail from Haleigh Resetar, October 18, 2022, SPINS).The rhizomes of turmeric are used as a fresh root, as powder, as herbal tea, or after extraction, as oleoresin, dry extract, or tincture. The rhizome of turmeric is used as a spice, a natural coloring agent, and as an ingredient in herbal medicines and dietary supplements. The beneficial effect of turmeric on the treatment of excessive gastric acid production, flatulence, or dyspepsia is supported by human clinical data. Additionally, it is used in the treatment of peptic ulcers, pain, and inflammation due to rheumatoid arthritis, amenorrhea, dysmenorrhea, diarrhea, epilepsy, skin diseases, and as a cholagogue in traditional medicine systems.121
For turmeric extracts, adulteration schemes vary depending on the region. In South Asian countries such as India and Bangladesh, undeclared synthetic colorants such as Sudan I, metanil yellow, lead chromate, or tartrazine may be added to improve the visual aspects of the root or rhizome. Sudan I, metanil yellow, and lead chromate are known to be toxic to humans and their use to color turmeric rhizomes represents a potentially serious public health safety issue.79,89,122 In Europe and North America, synthetic curcuminoids, predominantly curcumin (22), are the primary adulterants detected in commercial turmeric products.122
Fifteen publications78–92 were found reporting evaluations of the authenticity of commercial turmeric products sold as either a spice or a dietary supplement (Table 5). Twelve of these studies used chromatographic, spectrometric, or spectroscopic techniques, such as HPLC, HPTLC, UPLC/MS, and NMR, to detect adulteration and two studies used only genetic methods78,80 while one study89 combined both chemical and genetic methods. According to selected studies published between 2000–2023, 1247 commercial samples collected from Asia (n = 746), Europe (n = 368), and North America (n = 79) were investigated. Most of the tested products were in powdered rhizome form (n = 761) while the number of products containing extracts was 109 and mixed products was 46. Only a small number of test samples (n = 9) were sold in bulk. One hundred twenty-two samples were sold as dietary supplements, while one of the tested products was licensed as an herbal medicinal product. Most of the commercial products (n = 1113) were purchased from pharmacies and health stores, and 43 products were bought from online retailers.
Turmeric root, powdered root, and turmeric extracts are reportedly adulterated with yellow colorants such as lead chromate and metanil yellow, and synthetic curcumin. In rare instances, adulteration with other species of the genus Curcuma, in particular with zedoary (Curcuma zedoaria, Zingiberaceae), has been described. Additionally, previously extracted (spent) underground parts of turmeric may be sold instead of genuine turmeric root and rhizome without proper declaration. According to data from these publications, the frequency of adulteration was calculated as 17% with 206 adulterated or mislabeled products. Publications using genetic methods reported the presence of C. zedoaria and C. malabarica in seven of nine samples.78,80 According to Maquet et al.,89 DNA of non-declared plant material, mostly paprika (Capsicum spp., Solanaceae), and starch-containing species was detected in 24 of 316 samples. The highest adulteration rate was 42% (46 of 109 samples) in the turmeric extract group followed by mixed products (35%, 16 out of 46), bulk samples (33%, 3 of 9 samples), and powdered turmeric root/rhizome (15%, 116 of 761). The small sample size for bulk ingredients, however, does not allow for a robust evaluation of the authenticity of turmeric products sold in bulk. The adulterated sample percentage was highest in the North American group (21%, 17 of 79) while it was 16% for the Asia group (125 of 746) and 14% for Europe (52 of 368). Compared to the 263 turmeric dietary supplement SKUs sold in the U.S. market, the small sample set (n = 79) from North America evaluated to date is insufficient to generalize about the extent of product adulteration in this region.
4. Discussion
Adulteration of plant-based medicinal products and dietary supplements is a global problem that is well documented. This review aims to estimate the frequency of adulteration based on the analysis of public data on five commonly sold herbs. The available data on botanical adulteration is limited and includes heterogeneous studies that use different sample collection and different analytical methods, which restricts the ability to generalize these results to the global botanical supply chain.The analysis of adulteration data on common botanicals demonstrates that adulteration of botanical products that are regulated as registered or licensed as medicinal products are considerably less adulterated than products regulated as food or dietary supplements.
4.1. Discussion on the findings
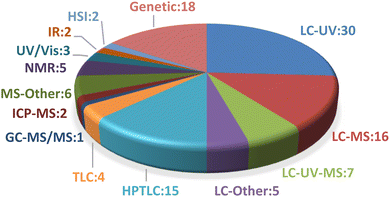
In the present review, 78 studies published between 2000 and 2023 were reviewed and adulteration summary reports of five selected botanical ingredients were presented. Among these, 47 studies employed a single method for analyzing adulterants, while 31 utilized multiple methods. Genetic methods (including qualitative and digital PCR, DNA barcoding, DNA metabarcoding, whole genome sequencing, recombinase polymerase amplification (RPA) assay, randomly amplified polymorphic DNA, and sequence characterized amplified region marker analysis) were featured in 18 studies. Liquid chromatography (LC), i.e., high-performance or ultrahigh-performance liquid chromatography, was used in 58 studies in conjunction with different ultraviolet (UV) detectors including fixed wavelength or photodiode array detectors (LC-UV) in 30 studies. Mass spectrometry (MS) served as the detector in liquid chromatography (LC-MS) in 16 studies. Seven studies combined UV and MS with LC (LC-UV-MS), while five studies employed different detectors with the LC system (LC-Other), such as charged aerosol detection, fluorescence detector, evaporative light scattering detector, or pulsed amperometric detection. Planar chromatography was used in 19 studies with 15 implementing HPTLC and four utilizing TLC. In one study, gas chromatography coupled with tandem mass spectrometry (GC-MS/MS) was utilized, while two studies featured inductively coupled plasma mass spectrometry (ICP-MS). Additionally, mass spectrometry alone or combined with other techniques (MS-Other) such as accelerator, easy ambient sonic-spray ionization (EASI-MS), electrospray ionization (ESI-MS), flow injection, was used in six studies. Other methods included nuclear magnetic resonance (NMR, n = 5), ultraviolet-visible spectrophotometry (UV/Vis, n = 3), infrared spectroscopy (IR, n = 2), and hyperspectral imaging (HSI, n = 2). Fig. 1 illustrates the distribution of methods used in the reviewed studies.The advantages and disadvantages of each analytical method have been discussed elsewhere.105,120,122,123 For most botanicals, a number of orthogonal methods may be necessary to detect all types of adulteration, with each of them having its strengths and its limitations. This can be demonstrated using turmeric adulteration as an example. Genetic identification methods may help with the detection of other Curcuma species or the presence of starches as bulking agents in turmeric spice samples if DNA of sufficient quality is present. However, genetic tests will not provide information about adulteration with colorants or synthetic curcuminoids. For metanil yellow, a spot test using water yields a bright yellow color immediately. It can also be detected with HPTLC or HPLC/UHPLC methods specifically targeting food dyes. Detection of lead chromate adulteration is more challenging due to the poor solubility in water and organic solvents.122 However, this lack of solubility enables its detection by microscopy, allowing to see the lead chromate crystals. Microscopy is also helpful to detect the undeclared addition of starches to bulk turmeric powder. Alternatively, ICP-MS is a suitable method for lead chromate detection. In addition, Forsyth et al. reported the use of handheld X-ray fluorescent detectors by inspectors of the Bangladeshi Food Safety Authority for finding turmeric samples adulterated with lead chromate.124 Liquid chromatography methods are helpful to detect adulteration with other Curcuma species or the presence of spent turmeric, i.e., turmeric from which the therapeutically important curcuminoids have been removed. The detection of spiking with synthetic curcumin is quite challenging, although absence of demethoxycurcumin and bisdemethoxycurcumin, or unusually high relative curcumin amounts in turmeric extracts are a good indication of synthetic curcumin being used.90 Girme et al. proposed (1E,4Z)-5-hydroxy-1-(3-hydroxy-4-methoxyphenyl) hexa-1,4-dien-3-one as a marker compound for synthetic curcumin, although this marker may not be present in all adulterated samples.86 This byproduct of the curcumin synthesis can be detected by HPLC-UV/Vis or HPTLC. Direct detection of the presence of non-biobased curcuminoids can be achieved with 14C-isotope mass spectrometry.
The highest total sample number was 1247 for turmeric while echinacea is found at the other end of the scale with 200 products evaluated. The most frequently investigated plant was ginkgo with 28 analytical publications whereas there were only six studies on the quality control and ingredient authentication of elder berry products. The results of this review showed estimated adulteration percentages for black cohosh, echinacea, elder berry, ginkgo, and turmeric-based commercial products of 42%, 29%, 17%, 57%, and 17%, respectively. The total number of adulterated or mislabeled commercial products was 818 out of 2995 resulting in an average frequency of adulteration of 27% for these five botanicals.
As outlined in the introduction, it is impossible to make an accurate projection of the frequency of adulteration from the limited number of studies reporting adulteration of botanicals since the investigated products may not represent the reality of the market in a specific geographic location and of adulteration worldwide. Additionally, the focus of this review has been on top-selling botanicals in the US market; hence the number of investigated samples in studies is often larger in North America and Europe since this is where these ingredients are popular. In this review, the highest number of commercial products collected was from North America (n = 1132). The number of Asian products was 796, followed by European products (n = 723), 26 from South America, and 19 from Australia and New Zealand (Table 6). There were no products from Africa. The origin of a total of 299 investigated products is unknown. These samples are not included in Table 6, leaving 2696 samples for the assessment. The calculated adulteration percentages are also shown by bar graphs with standard errors in Fig. 2.
| Plant name | Number of adulterated products/number of total products | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asia | Europe | N America | S America | ANZ | Bulk | Extract | Mixed | Powder | Ad/Total | |
| a ANZ: Australia + New Zealand, N: North, S: South, —: no data, Ad: adulterated. | ||||||||||
| Black cohosh | 1/6 | 10/31 | 53/166 | — | 0/2 | 33/56 | 35/95 | 15/27 | 49/121 | 135/320 |
| Echinacea | 0/1 | 8/68 | 37/108 | — | 2/3 | 3/13 | 9/41 | 1/4 | 8/34 | 57/200 |
| Elder berry | — | 9/11 | 98/627 | — | — | 6/41 | 32/60 | — | 9/33 | 119/695 |
| Ginkgo | 9/43 | 157/245 | 86/152 | 20/26 | 6/14 | 21/47 | 258/445 | 6/7 | 8/20 | 301/533 |
| Turmeric | 125/746 | 52/368 | 17/79 | — | — | 3/9 | 46/109 | 16/46 | 116/761 | 206/1247 |
| Total | 135/796 | 236/723 | 291/1132 | 20/26 | 8/19 | 66/166 | 380/750 | 38/84 | 190/969 | 818/2995 |
| Adulteration% | 17.0 | 32.6 | 25.7 | 76.9 | 42.1 | 39.8 | 50.7 | 45.2 | 19.6 | 27.3 |
 | ||
| Fig. 2 Adulteration percentages with standard error bars according to location, product type, and ingredient of the products. | ||
The data gathered in this review allow for comparing the effect of product type on the frequency of adulteration. According to Table 6, the highest adulteration percentage was calculated for finished products containing botanical extracts (51%) or mixed products (45%), followed by bulk ingredients (40%). Finished products containing the plant in powdered form had the lowest rate of adulteration (20%). The sample sizes of these four groups were not homogeneous, so it is difficult to determine the effect of product type on adulteration.
However, powdered plants may be less likely to be adulterated since the ingredient costs are lower, decreasing the financial incentive to cheat, and adulteration in some cases may be more easily detected using relatively low-cost analytical tests such as microscopic evaluation.
Another question often raised is if products available only from online sellers are more prone to adulteration than the products sold in retail pharmacies, grocery stores, and health food shops (many of which are also available online). Since the retail outlet of the products was mentioned only in a few studies and the results were not discussed with the market channel in mind, the available information does not permit the authors of this article to make any conclusions regarding this question.
The impact of the regulatory category on adulteration is another factor to consider, but the lack of sufficient data hinders making definitive conclusions on this matter. Nevertheless, a few studies have examined products registered as herbal medicinal products in the European Union to assess their authenticity, and all these products were confirmed to be authentic. Given that herbal products are subject to more stringent regulations compared to food and dietary supplements, necessitating the submission of robust dossiers that validate the quality, safety, and efficacy of a product, it's reasonable to assume that manufacturers are motivated to ensure the consistent quality and authenticity of their products. This, in turn, should result in a lower frequency of adulteration for herbal medicinal products compared to food and dietary supplements.
4.2. Comparison of the findings with the previous reviews
Previous assessments of the extent of adulteration in the botanical dietary supplement and herbal medicine trade are rare, but three reviews related to the detection of adulteration in botanical materials and dietary supplements may be useful as they contain additional information regarding the extent of adulteration in the global market.11–13 However, all of these reviews are subject to the same limitations as this present study.Ichim et al.13 reviewed 28 publications reporting the use of hand lenses, light, stereo, and scanning electron microscopes for the microscopic authentication of 508 commercial herbal products from South America (n = 167), Asia (n = 164), North America (n = 128), and Europe (n = 49), while the number of products from Africa and Australia was zero. In 20 papers, at least one additional technique (DNA or/and chemistry-based methods) was used to test product authenticity. Overall, 59% of the commercial products (n = 300) were found to be authentic, while the rest (41%, n = 208) were deemed adulterated. Almost half (49%) of the total products microscopically authenticated in Asia were reported to be adulterated, with lower adulteration rates in South America (40%), Europe (39%), and North America (33%). In the U.S. (n = 128) a substantial part of the samples (>30%) did not comply with the labeled ingredient declaration.13
A review of 206 publications that used DNA-based methods to detect adulteration was reported by Ichim.11 Commercial herbal products (n = 5957) from six continents were investigated in this review. A substantial number (27%) of the herbal products reviewed was found to be adulterated when their content was tested to verify the authenticity of the labeled ingredient(s). The percentages of adulterated products varied significantly among continents, being highest in Australia (79%) and South America (67%), lower in Europe (47%), North America (33%), Africa (27%), and lowest in Asia (23%). However, the number of products investigated per continent was low in some cases, allowing only a very limited assessment of these markets. For nine countries (Brazil, Taiwan, India, the U.S., Malaysia, Japan, South Korea, Thailand, and China), more than 100 products were authenticated successfully by DNA-based methods. From these countries, the highest percentage of adulterated products was reported for Brazil (68%, 104 out of 154), and the adulteration percentage for the U.S. was found to be 29% (134 out of 465).11 Although DNA-based methods for ingredient identification in botanicals is a highly dependable and promising tool under specific conditions, they also have limitations: They cannot detect adulteration with other (usually lower-cost) plant parts of the same species, undeclared food colorants and dyes, or pure chemical compounds. In addition, genetic testing of some botanical extracts can be inadequate to determine proper identity due to the occasional or frequent degradation of the DNA in the sample resulting from processing, e.g., extraction steps that include heat and/or some specific solvents. These limitations have been reviewed by several authors.4,11,43,125,126
Ichim and Booker12 reviewed the studies on different chemical techniques used for the authentication of 2386 commercial herbal products. More than a quarter of the analyzed products were found to be adulterated (27%). Again, the number of products and the adulteration percentages varied very widely according to the country. Yet, the adulteration reported for four countries, from which more than 100 commercial products were investigated, was 37% (United Kingdom), 31% (Italy), 27% (U.S), and 21% (China).12
5. Conclusion
The extent of adulteration was found to be ingredient-dependent, with some botanicals being less prone to adulteration than others. Furthermore, the estimated percentages should be viewed in the context of the specific products tested in the referenced studies. There may be a greater risk of intentional economic adulteration in expensive or high-demand products compared to relatively niche products sold at low cost. This trend is particularly evident in the spice and seasoning industry, where the most expensive spices are often more susceptible to adulteration for economic gain. The extent of adulteration determined as a result of this review of five popular botanicals is in line with previous studies using chemical and genetic methods of authentication conducted by Ichim and Ichim and Booker, both of whom reported adulteration rates of 27%.11,12 Contrary to products regulated as food or dietary supplements labeled to contain black cohosh root/rhizome, echinacea root or herb, elder berry, ginkgo leaf, or turmeric root/rhizome, products regulated as registered or licensed herbal medicinal products had no incidence of adulteration. This highlights the importance of regulatory frameworks that mandate quality control and proper identity analysis to ensure the effectiveness and safety of botanical products (although safety concerns have not been documented in most of the published literature with respect to these five botanicals). Quality control methods used by suppliers, manufacturers, researchers, and regulators need to be sufficiently specific to detect possible adulterants in botanical ingredients and produce high-quality authentic products. There are numerous examples where some analytical methods used in industry quality control or government regulatory laboratories have been shown to be inadequate to detect adulteration and properly authenticate the botanical material(s). For this reason, BAPP has published a series of extensively peer-reviewed Laboratory Guidance Documents that evaluate the fitness for purpose of various analytical methods employed for some botanicals that BAPP has determined are subject to adulteration.4,16,94,105,120,127 There were notable constraints in establishing an accurate estimate of botanical adulteration within the global supply chain. For a more precise evaluation of adulteration, forthcoming large-scale trials should adopt uniform fit-for-purpose methods and encompass products from all continents. Furthermore, these studies should define consistent specifications to discern when a product is considered adulterated. For instance, deviations in the chemical fingerprint might stem from processing anomalies rather than adulteration. Additionally, future research needs to define what constitutes an acceptable level of unlabeled excipients in botanical raw materials.6. Author contributions
Conceptualization: NO, SG, MB, data curation: SG, MB, formal Analysis: NO, SG, funding acquisition: MB, investigation: NO, SG, methodology: NO, SG, MB, project administration: SG, MB, resources: NO, SG, MB, supervision: SG, MB, visualization: NO, SG, writing – original draft: NO, SG, writing – review & editing: NO, SG, MB.7. Conflicts of interest
The authors declare that they have no conflict of interest.8. Acknowledgments
This study was financed by the ABC-AHP-NCNPR Botanical Adulterants Prevention Program (BAPP). ABC is the American Botanical Council, a nonprofit, tax-exempt research and education organization in Austin, Texas USA; AHP is the American Herbal Pharmacopoeia, a nonprofit, tax-exempt organization in Scotts Valley, California, USA; NCNPR is the National Center for Natural Products Research at the University of Mississippi in Oxford, Mississippi, USA. BAPP is financially supported by members of the herb, medicinal plant, dietary supplement, tea, and natural product industries in the United States and internationally. BAPP also receives financial support from nonprofit research and standard-setting organizations. Funders and supporters of BAPP are not responsible for its publications. The authors would like to express our sincere gratitude to Onur Toka, PhD from the Department of Statistics at Hacettepe University (Ankara, Türkiye) for his help with the statistical analysis conducted in this manuscript, and to Haleigh Resetar from SPINS (Chicago, Illinois, USA) for providing the number of dietary supplement SKUs sold in the U.S. of the five botanicals assessed in this review.9. References
- Herbs and botanicals meet blended demands of traditional wellness and validated efficacy – analysis, https://www.naturalproductsinsider.com/herbs-botanicals/herbs-and-botanicals-meet-blended-demands-traditional-wellness-and-validated, accessed 03/20/2024, 2022.
- T. Smith, H. Resetar and C. Morton, HerbalGram, 2022, 136, 42–69 Search PubMed.
- S. Foster, HerbalGram, 2012, 93, 34–41 Search PubMed.
- S. Gafner, M. Blumenthal, S. Foster, J. H. Cardellina, II, I. A. Khan and R. Upton, J. Nat. Prod., 2023, 86, 460–472 CrossRef CAS PubMed.
- W. K. Forsyth JE, K. Maher, M. Saiful Islam, R. Raqib, M. Rahman, S. Fendorf and S. P. Luby, Environ. Sci. Technol., 2019, 53, 11429–11436 CrossRef PubMed.
- N. S. Forsyth JE, I. S. Sahiful, M. Baker, D. Yeasmin, M. Saiful Islam, M. Rahman, S. Fendorf, N. M. Ardoin, P. J. Winch and S. P. Luby, Environ. Res., 2019, 179, 108722 CrossRef PubMed.
- A. K. Forsyth JE, M. Jintcharadze, E. Nash, P. Sharov, A. Temnikova and C. Elmera, Environ. Res., 2024, 250, 118504 CrossRef PubMed.
- S. Gafner, HerbalEGram, 2024, vol. 21, https://www.herbalgram.org/resources/herbalegram/volumes/volume-21/issue-5-may/news-and-features-2/yellow-oleander-adulteration/, accessed 08/02/2024.
- S. Foster, HerbalGram, 2011, 92, 42–57 Search PubMed.
- S. Gafner, L. Loffredo, J. Kababick, S. M. Wise and R. Upton, HerbalGram, 2024, 140, 44–51 Search PubMed.
- M. C. Ichim, Front. Pharmacol, 2019, 10, 1227 CrossRef CAS PubMed.
- M. C. Ichim and A. Booker, Front. Pharmacol, 2021, 12, 666850 CrossRef CAS PubMed.
- M. C. Ichim, A. Häser and P. Nick, Front. Pharmacol, 2020, 11, 876 CrossRef CAS.
- American Botanical Council, ABC-AHP-NCNPR Botanical Adulterants Prevention Program, https://www.herbalgram.org/resources/botanical-adulterants-prevention-program/, accessed 03/20/2024.
- D. P. Little, Genome, 2014, 57, 513–516 CrossRef CAS PubMed.
- S. Gafner, T. Borchardt, M. Bush, S. Sudberg, N. G. Feuillère, M. Y. R. Tenon, J. H. Jolibois, P. J. N. Bellenger, H. You, R. E. Adams, J. Stewart, I. Dagan, T. Murray and D. L. Erickson, HerbalGram, 2021, 130, 24–31 Search PubMed.
- K. He, B. Zheng, C. H. Kim, L. Rogers and Q. Zheng, Planta Med., 2000, 66, 635–640 CrossRef CAS PubMed.
- K. He, G. F. Pauli, B. Zheng, H. Wang, N. Bai, T. Peng, M. Roller and Q. Zheng, J. Chromatogr. A, 2006, 1112, 241–254 CrossRef CAS PubMed.
- N. J. Zerega, S. Mori, C. Lindqvist, Q. Zheng and T. J. Motley, Econ. Bot., 2002, 56, 154–164 CrossRef CAS.
- H.-K. Wang, N. Sakurai, C. Y. Shih and K.-H. Lee, J. Agric. Food Chem., 2005, 53, 1379–1386 CrossRef CAS PubMed.
- B. Jiang, F. Kronenberg, P. Nuntanakorn, M.-H. Qiu and E. J. Kennelly, J. Agric. Food Chem., 2006, 54, 3242–3253 CrossRef CAS.
- R. Marles, S. Omar, S. Jordan, M. Murty, S. Perwaiz, R. Bertrand and P. Lacroix, presented in part at the Developments in Botanical Dietary Supplements Research from 1994 to Today, University of Illinois at Chicago, 2010 Search PubMed.
- D. A. Baker, D. W. Stevenson and D. P. Little, J. AOAC Int., 2012, 95, 1023–1034 CrossRef CAS PubMed.
- L. J. Wallace, S. M. Boilard, S. H. Eagle, J. L. Spall, S. Shokralla and M. Hajibabaei, Food Res. Int., 2012, 49, 446–452 CrossRef CAS.
- S. Masada-Atsumi, M. Onuma, E. Suenaga, T. Maruyama, A. Hishida, F. Kiuchi, S. Kobayashi, Y. Goda and T. Hakamatsuka, Jpn. J. Food Chem. Saf., 2013, 20, 178–189 Search PubMed.
- S. Williams, C. Howard, J. Dixon, R. Middleton, A. Slater and E. Koch, Planta Med., 2014, 80, LP69 Search PubMed.
- M. H. M. Sharaf, J. Yuk, K. Yu, M. Wrona and G. Isaac, Planta Med., 2016, 82, SL36 Search PubMed.
- S. Williams, PhD, Degree of Doctor of Philosophy, Faculty of Health and Life Science, De Montford University, Leicester, 2017.
- S. Masada-Atsumi, Y. Kumeta, Y. Takahashi, T. Hakamatsuka and Y. Goda, Biol. Pharm. Bull., 2014, 37, 454–460 CrossRef CAS PubMed.
- S. Masada, J. Nat. Med., 2016, 70, 361–375 CrossRef PubMed.
- M. Bittner, R. Schenk and M. F. Melzig, Phytochem. Lett., 2016, 18, 220–225 CrossRef.
- J. Harnly, P. Chen, J. Sun, H. Huang, K. L. Colson, J. Yuk, J.-A. H. McCoy, D. T. H. Reynaud, P. B. Harrington and E. J. Fletcher, Planta Med., 2016, 82, 250–262 CAS.
- C.-I. Michel, R. S. Meyer, Y. Taveras and J. Molina, J. Appl. Res. Med. Aromat. Plants, 2016, 3, 94–100 Search PubMed.
- S. Tankeu, I. Vermaak, W. Chen, M. Sandasi, G. Kamatou and A. Viljoen, Planta Med., 2018, 84, 407–419 CrossRef CAS.
- P. Geng, P. Chen, J. Sun, J.-A. H. McCoy and J. M. Harnly, Anal. Bioanal. Chem., 2019, 411, 7147–7156 CrossRef CAS.
- J. Shanmughanandhan, D. Shanmughanandhan, S. Ragupathy, T. A. Henry and S. G. Newmaster, J. AOAC Int., 2021, 104, 836–846 CrossRef.
- D. A. Frommenwiler, E. Reich, M. H. Sharaf, S. Cañigueral and C. J. Etheridge, Front. Pharmacol, 2022, 13, 925298 CrossRef CAS PubMed.
- C. M. Gilroy, J. F. Steiner, T. Byers, H. Shapiro and W. Georgian, Arch. Intern. Med., 2003, 163, 699–704 CrossRef PubMed.
- E. Mudge, D. Lopes-Lutz, P. Brown and A. Schieber, J. Agric. Food Chem., 2011, 59, 8086–8094 CrossRef CAS PubMed.
- S. Agatonovic-Kustrin, C. M. Loescher and R. Singh, Phytochem. Anal., 2013, 24, 303–308 CrossRef CAS PubMed.
- M. Sandasi, I. Vermaak, W. Chen and A. M. Viljoen, Molecules, 2014, 19, 13104–13121 CrossRef PubMed.
- J. Harnly, Y. Lu, J. Sun and P. Chen, J. Food Compos. Anal., 2017, 64, 85–92 CrossRef CAS.
- A. C. Raclariu, C. E. Ţebrencu, M. C. Ichim, O. T. Ciuperca, A. K. Brysting and H. de Boer, Phytomedicine, 2018, 44, 32–38 CrossRef CAS.
- S. Waidyanatha, J. Pierfelice, T. Cristy, E. Mutlu, B. Burback, C. V. Rider and K. Ryan, Food Chem. Toxicol., 2020, 137, 111125 CrossRef CAS PubMed.
- J. A. Galetti, Degree of Doctor of Philosophy, Food and Nutrition Sciences, The University of Maine, 2016.
- D. T. Hänni M and E. Reich, Evaluation of elderberry fruits and extracts to create a basis for identification, species discrimination and detection of adulteration, Muttenz, Switzerland, Camag AG, 2021 Search PubMed.
- E. Güzelmeriç, C. Çelik, N. B. Şen, M. A. Oçkun and E. Yeşilada, J. Res. Pharm., 2021, 25, 238–248 Search PubMed.
- B. Avula, K. Katragunta, Y.-H. Wang, Z. Ali, R. Srivedavyasasri, S. Gafner, R. Slimestad and I. A. Khan, J. Food Compos. Anal., 2022, 110, 104584 CrossRef CAS.
- C. Crawford, B. Avula, A. T. Lindsey, A. Walter, K. Katragunta, I. A. Khan and P. A. Deuster, JAMA Netw. Open, 2022, 5, e2226040 CrossRef.
- B. D. Sloley, S. R. Tawfik, K. A. Scherban and Y. K. Tam, J. Food Drug Anal., 2003, 11(2), 11 Search PubMed.
- C. Liu, R. Mandal and X.-F. Li, Analyst, 2005, 130, 325–329 RSC.
- C. Bewicke, Ginkgo biloba extract:Brabd QC analytical report, Ethical Naturals Inc., San Anselmo, CA, 2006 Search PubMed.
- P. Xie, S. Chen, Y.-z. Liang, X. Wang, R. Tian and R. Upton, J. Chromatogr. A, 2006, 1112, 171–180 CrossRef CAS PubMed.
- S. Ding, E. Dudley, S. Plummer, J. Tang, R. Newton and A. Brenton, Phytochemistry, 2008, 69, 1555–1564 CrossRef CAS PubMed.
- S. Agnolet, J. W. Jaroszewski, R. Verpoorte and D. Staerk, Metabolomics, 2010, 6, 292–302 CrossRef CAS PubMed.
- A. Gawron-Gzella, P. Marek, J. Chanaj and I. Matlawska, Acta Pol. Pharm., 2010, 67, 335–343 CAS.
- M. Tawab, M. Krzywon and M. Schubert-Zsilavecz, Pharm. Ztg., 2010, 20, 62–67 Search PubMed.
- A. Chandra, Y. Li, J. Rana, K. Persons, C. Hyun, S. Shen and T. Mulder, J. Funct. Foods, 2011, 3, 107–114 CrossRef CAS.
- J. M. Harnly, D. Luthria and P. Chen, J. AOAC Int., 2012, 95, 1579–1587 CrossRef CAS PubMed.
- Y. Kakigi, T. Hakamatsuka, T. Icho, Y. Goda and N. Mochizuki, Biosci., Biotechnol., Biochem., 2012, 76, 1003–1007 CrossRef CAS.
- E. M. Borges, D. A. Volmer and M. N. Eberlin, Can. J. Chem., 2013, 91, 671–678 CrossRef CAS.
- J. Ronowicz, B. Kupcewicz and E. Budzisz, Open Life Sci., 2013, 8, 374–385 CAS.
- L. Ö. Demirezer, A. Büyükkaya, E. Uçaktürk, A. Kuruüzüm-Uz, Z. Güvenalp and E. Palaska, Rec. Nat. Prod., 2014 Search PubMed.
- H. Wohlmuth, K. Savage, A. Dowell and P. Mouatt, Phytomedicine, 2014, 21, 912–918 CrossRef CAS PubMed.
- B. Avula, S. Sagi, S. Gafner, R. Upton, Y.-H. Wang, M. Wang and I. A. Khan, Anal. Bioanal. Chem., 2015, 407, 7733–7746 CrossRef CAS PubMed.
- A. Booker, D. Frommenwiler, E. Reich, S. Horsfield and M. Heinrich, J. Herb. Med., 2016, 6, 79–87 CrossRef.
- Y.-C. Ma, A. Mani, Y. Cai, J. Thomson, J. Ma, F. Peudru, S. Chen, M. Luo, J. Zhang and R. G. Chapman, Phytomedicine, 2016, 23, 377–387 CrossRef CAS PubMed.
- R. S. Pawar, S. M. Handy, R. Cheng, N. Shyong and E. Grundel, Planta Med., 2017, 83, 921–936 CrossRef CAS PubMed.
- S. Czigle, J. Tóth, N. Jedlinszki, E. Háznagy-Radnai, D. Csupor and D. Tekeľová, Planta Med., 2018, 84, 475–482 CrossRef CAS PubMed.
- S. Govindaraghavan, Fitoterapia, 2018, 131, 146–159 CrossRef CAS PubMed.
- Y. Liu, X.-y. Wang, X.-m. Wei, Z.-t. Gao and J.-p. Han, Sci. Rep., 2018, 8, 1–8 Search PubMed.
- M. Budeč, J. Bošnir, A. Racz, D. Lasić, D. Brkić, A. Mosović Ćuić, Ž. Kuharić, G. Jurak and L. Barušić, Acta Clin. Croat., 2019, 58, 672–691 Search PubMed.
- D. A. Frommenwiler, A. Booker, R. Vila, M. Heinrich, E. Reich and S. Cañigueral, J. Ethnopharmacol., 2019, 243, 112084 CrossRef CAS PubMed.
- M. Subhi Sammani, S. Clavijo, A. González and V. Cerdà, Anal. Lett., 2019, 52, 1298–1314 CrossRef CAS.
- A. Walkowiak, Ł. Ledziński, M. Zapadka and B. Kupcewicz, Spectrochim. Acta, Part A, 2019, 208, 222–228 CrossRef CAS PubMed.
- B. J. Collins, S. P. Kerns, K. Aillon, G. Mueller, C. V. Rider, E. F. DeRose, R. E. London, J. M. Harnly and S. Waidyanatha, Anal. Bioanal. Chem., 2020, 412, 6789–6809 CrossRef CAS PubMed.
- A. Walkowiak, K. Wnuk, M. Cyrankiewicz and B. Kupcewicz, Molecules, 2022, 27, 433 CrossRef CAS PubMed.
- B. Sasikumar, S. Syamkumar, R. Remya and T. John Zachariah, Food Biotechnol., 2004, 18, 299–306 CrossRef CAS.
- S. Dixit, S. K. Purshottam, S. K. Khanna and M. Das, Food Addit. Contam., 2009, 26, 1227–1231 CrossRef CAS.
- K. Dhanya, S. Syamkumar, S. Siju and B. Sasikumar, Food Res. Int., 2011, 44, 2889–2895 CrossRef CAS.
- B. Avula, Y.-H. Wang and I. A. Khan, J. Chromatogr. Sep. Tech., 2012, 3, 1000120 Search PubMed.
- A. Booker, D. Frommenwiler, D. Johnston, C. Umealajekwu, E. Reich and M. Heinrich, J. Ethnopharmacol., 2014, 152, 292–301 CrossRef CAS PubMed.
- E. Mudge, M. Chan, S. Venkataraman and P. N. Brown, Food Anal. Methods, 2016, 9, 1428–1435 CrossRef.
- M. B. Skiba, P. B. Luis, C. Alfafara, D. Billheimer, C. Schneider and J. L. Funk, Mol. Nutr. Food Res., 2018, 62, 1800143 CrossRef PubMed.
- L. Chatzinasiou, A. Booker, E. MacLennan, M. Mackonochie and M. Heinrich, J. Herb. Med., 2019, 17, 100281 CrossRef.
- A. Girme, G. Saste, A. K. Balasubramaniam, S. Pawar, C. Ghule and L. Hingorani, J. Pharm. Biomed. Anal., 2020, 191, 113603 CrossRef CAS PubMed.
- F. Menniti-Ippolito, I. Ippoliti, A. A. Pastorelli, I. Altieri, F. Scalise, B. De Santis, F. Debegnach, C. Brera, R. Pacifici and S. Pichini, Ann. Ist. Super. Sanita, 2020, 56, 462–469 CAS.
- S. B. Kim, J. Bisson, J. B. Friesen, L. Bucchini, S. Gafner, D. C. Lankin, S.-N. Chen, G. F. Pauli and J. B. McAlpine, J. Nat. Prod., 2021, 84, 846–856 CrossRef CAS PubMed.
- A. Maquet, A. Lievens, V. Paracchini, G. Kaklamanos, B. de la Calle, L. Garlant, S. Papoci, D. Pietretti, T. Zdiniakova, A. Breidbach, J. Omar Onaindia, A. Boix Sanfeliu, T. Dimitrova and F. Ulberth, Results of an EU wide coordinated control plan to establish the prevalence of fraudulent practices in the marketing of herbs and spices, Publications Office of the European Union, Luxembourg, 2021 Search PubMed.
- H. You, H. Gershon, F. Goren, F. Xue, T. Kantowski and L. Monheit, Food Chem., 2022, 370, 131007 CrossRef CAS PubMed.
- E. Brusač, M.-L. Jeličić, B. Nigović, D. Amidžić Klarić and A. Mornar, Food Technol. Biotechnol., 2022, 60, 434–448 Search PubMed.
- P. Siudem, Ł. Szeleszczuk, A. Zielińska and K. Paradowska, Molecules, 2023, 28, 3442 CrossRef CAS PubMed.
- World Health Organization, in WHO Monographs on Selected Medicinal Plants, World Health Organization, 1999, vol. 2, p. 58 Search PubMed.
- S. Gafner, Adulteration of Actaea racemosa, Botanical Adulterants Prevention Bulletin, ABC-AHP-NCNPR Botanical Adulterants Prevention Program, 2016 Search PubMed.
- M. Blumenthal, A. Lindstrom, C. Ooyen and M. E. Lynch, HerbalGram, 2012, 95, 60–64 Search PubMed.
- A. Lindstrom, C. Ooyen, M. Lynch and M. Blumenthal, HerbalGram, 2013, 99, 60–65 Search PubMed.
- A. Lindstrom, C. Ooyen, M. E. Lynch, M. Blumenthal and K. Kawa, HerbalGram, 2014, 103, 52–56 Search PubMed.
- T. Smith, M. E. Lynch, J. Johnson, K. Kawa, H. Bauman and M. Blumenthal, HerbalGram, 2015, 107, 52–59 Search PubMed.
- T. Smith, K. Kawa, V. Eckl and J. Johnson, HerbalGram, 2016, 111, 67–73 Search PubMed.
- T. Smith, K. Kawa, V. Eck, C. Morton and R. Stredney, HerbalGram, 2017, 115, 56–65 Search PubMed.
- T. Smith, K. Kawa, V. Eckl, C. Morton and R. Stredney, HerbalGram, 2018, 119, 62–71 Search PubMed.
- T. Smith, M. Gillespie, V. Eckl, J. Knepper and C. M. Reynolds, HerbalGram, 2019, 123, 62–73 Search PubMed.
- T. Smith, G. May, V. Eckl and C. M. Reynolds, HerbalGram, 2020, 127, 54–69 Search PubMed.
- T. Smith, F. Majid, V. Eckl and C. M. Reynolds, HerbalGram, 2021, 131, 52–65 Search PubMed.
- S. Gafner, Black cohosh laboratory guidance document, ABC-AHP-NCNPR Botanical Adulterants Prevention Program, 2015 Search PubMed.
- A. Imai, D. C. Lankin, T. Gödecke, S. N. Chen and G. F. Pauli, Fitoterapia, 2020, 146, 104686 CrossRef CAS PubMed.
- World Health Organization, in WHO Monographs on Selected Medicinal Plants, World Health Organization, 1999, vol. 1, pp. 136–144 Search PubMed.
- World Health Organization, in WHO Monographs on Selected Medicinal Plants, 1999, vol. 1, pp. 125–135 Search PubMed.
- T. Smith, H. Bauman, H. Resetar and E. Craft, HerbalGram, 2024, 139, 52–68 Search PubMed.
- M. Williams, A. Jenks and J. Brinckmann, HerbalGram, 2023, 138, 12–21 Search PubMed.
- J. Barnes, L. A. Anderson, S. Gibbons and J. D. Phillipson, J. Pharm. Pharmacol., 2005, 57, 929–954 CrossRef CAS PubMed.
- S. E. Binns, J. F. Livesey, J. T. Arnason and B. R. Baum, J. Agric. Food Chem., 2002, 50, 3673–3687 CrossRef CAS PubMed.
- N. B. Cech, V. Kandhi, J. M. Davis, A. Hamilton, D. Eads and S. M. Laster, Int. Immunopharmacol., 2010, 10, 1268–1278 CrossRef CAS PubMed.
- S. E. Binns, J. T. Arnason and B. R. Baum, Biochem. Syst. Ecol., 2002, 30, 837–854 CrossRef CAS.
- M. Zimmermann, H. E. Johnson, M. McGuffin and W. Applequist, The American Herbal Products Association's Herbs of Commerce, Silver Spring, MD: American Herbal Products Association, 3rd edn, 2023 Search PubMed.
- European Medicine Agency Committee on Herbal Medicinal Products (HMPC), Assessment report on Sambucus nigra L., fructus, EMA/HMPC/44208/2012, 2014.
- S. Gafner and J. Brinckmann, Adulteration of European elder (Sambucus nigra) berries and berry extracts, Botanical Adulterants Prevention Bulletin, ABC-AHP-NCNPR Botanical Adulterants Prevention Program, 2023 Search PubMed.
- J. Lee and C. E. Finn, J. Sci. Food Agric., 2007, 87, 2665–2675 CrossRef CAS PubMed.
- World Health Organization, in WHO Monographs on Selected Medicinal Plants, WHO Monographs on Selected Medicinal Plants, 1999, vol. 154–167 Search PubMed.
- S. Gafner, Ginkgo leaf extract laboratory guidance document, ABC-AHP-NCNPR Botanical Adulterants Prevention Program, 2022 Search PubMed.
- World Health Organization, in WHO Monographs on Selected Medicinal Plants, World Health Organization, 1999, vol. 115–124 Search PubMed.
- J. H. Cardellina II, Turmeric raw material and products laboratory guidance document, ABC-AHP-NCNPR Botanical Adulterants Prevention Program, 2020 Search PubMed.
- R. Upton, B. David, S. Gafner and S. Glasl, Phytochem. Rev., 2020, 19, 1157–1177 CrossRef CAS.
- J. E. Forsyth, M. Baker, S. Nurunnahar, S. Islam, M. S. Islam, T. Islam, E. Plambeck, P. J. Winch, D. Mistree, S. P. Luby and M. Rahman, Environ. Res., 2023, 232, 116328 CrossRef CAS PubMed.
- I. Parveen, S. Gafner, N. Techen, S. J. Murch and I. A. Khan, Planta Med., 2016, 82, 1225–1235 CrossRef CAS PubMed.
- A. C. Raclariu, M. Heinrich, M. C. Ichim and H. de Boer, Phytochem. Anal., 2018, 29, 123–128 CrossRef CAS PubMed.
- S. Gafner, R. Upton, I. Khan, J. Cardellina, S. Foster and M. Blumenthal, Planta Med., 2017, 4, S01 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |