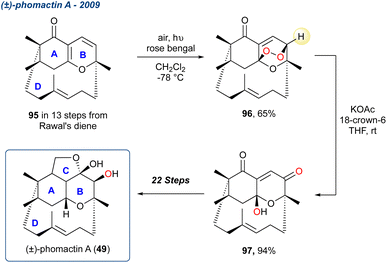
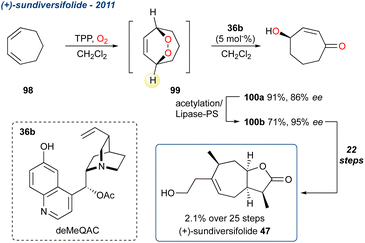
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The Kornblum DeLaMare rearrangement in natural product synthesis: 25 years of innovation
Marc C.
Kimber
 *a and
Darren S.
Lee
*a and
Darren S.
Lee
 *b
*b
aDepartment of Chemistry, School of Science, Loughborough University, Loughborough, LE11 3TU, UK. E-mail: M.C.Kimber@lboro.ac.uk
bCentre for Green Chemistry and Green Engineering at Yale, Yale University, New Haven, CT 06511, USA. E-mail: Darren.lee@yale.edu
First published on 31st January 2024
Abstract
Covering: 1998 up to the end of 2023
Since its initial disclosure in 1951, the Kornblum DeLaMare rearrangement has proved an important synthetic transformation and has been widely adopted as a biomimetic step in natural product synthesis. Utilising the base catalysed decomposition of alkyl peroxides to yield a ketone and alcohol has found use in many syntheses as well as a key strategic step, including the unmasking of furans, as a biomimetic synthetic tool, and the use of the rearrangement to install oxygen enantioselectively. Since ca. 1998, its impact as a synthetic transformation has grown significantly, especially given the frequency of use in natural product syntheses, therefore this 25 year time period will be the focus of the review.
1 Introduction
The base catalysed decomposition of an alkyl-peroxide to give a ketone and alcohol was first reported by Nathan Kornblum and Harold DeLaMare in 1952.1 Unlike previous work by Milas and Surgenor,2 who had demonstrated the stability of t-butyl hydroperoxide to base, Kornblum and DeLaMare exposed 1-phenylethyl-t-butyl peroxide 1 to piperidine giving acetophenone (3) and t-butyl alcohol (5) in 79% and 25%, respectively (Scheme 1a). This reaction was also found to be consistent with potassium hydroxide and sodium ethoxide as the base. Significantly, the authors identified the importance of an α-hydrogen relative to the peroxide, and accordingly they proposed an elimination mechanism as outlined in Scheme 1a. This mechanism was found to have similarities with the base mediated decomposition of nitrate esters3 and has subsequently become the bedrock of what is now known as the Kornblum DeLaMare rearrangement of peroxides, which we will now refer to as the KDM rearrangement within this review.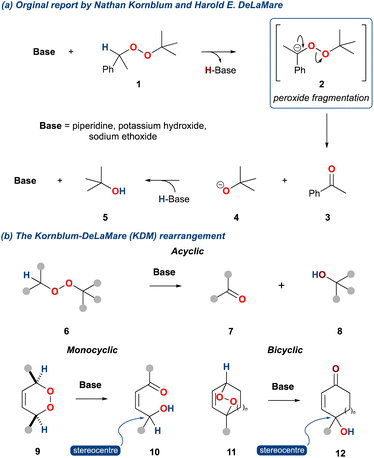 | ||
| Scheme 1 (a) The original report of Nathan Kornblum and Harold DeLaMare; (b) product outcome of the Kornblum DeLaMare rearrangement for acyclic (6), monocyclic (7), and bicyclic peroxides (10). | ||
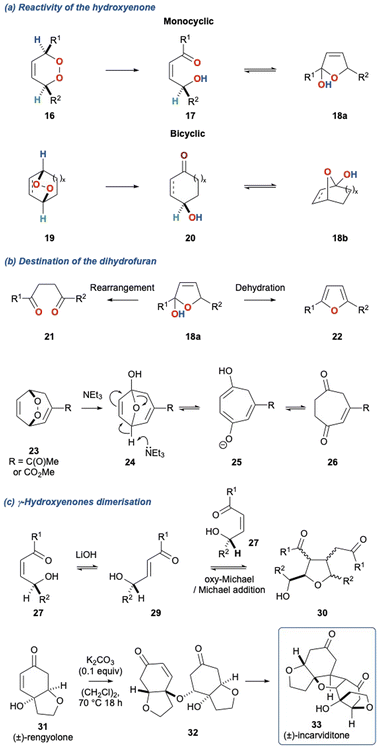
In the KDM rearrangement, acyclic alkyl peroxides (6) upon treatment with base give rise to a ketone and an alcohol (Scheme 1b, 7 and 8, respectively), while cyclic peroxides such as 9 give rise to γ-hydroxyenone 10, and bicyclic peroxides (11) give rise γ-hydroxyenone such as 12. The former variant can be used to access carbonyl products, while the later variant provides a very useful functional group possessing a stereocentre.4 Since the disclosure of the KDM rearrangement considerable focus has been on this later variant, as the functional group, a γ-hydroxyenone, is present in several natural product classes. Furthermore, this functionality is a valuable synthon which can be harnessed in subsequent synthetic transformations. Notable examples of the use of the KDM rearrangement over the past 70 years are its application in tropone and cycloheptatriene chemistry,5–11 its appearance in prostaglandin biosynthesis,12,13 and more recently, its employment as a tool in biomimetic natural product synthesis.
The scope of this review is to show-case the increasing use of the KDM rearrangement in natural product synthesis over the past 25 years. A recent general review on peroxide rearrangements,14,15 of which the KDM rearrangement sits, and the use of singlet oxygen in target synthesis16 provides some excellent context, but the aim of this treatise is to equip the reader with the necessary tools to successfully incorporate the KDM rearrangement in their own natural product syntheses.
2 Mechanism
We will begin with a discussion of the underpinning mechanism of the KDM rearrangement. This is essential when considering the selectivity of the initial base catalysed deprotonation event and is informative when designing and implementing enantioselective variants of the KDM rearrangement.The mechanism is centred on the bond dissociation energy of the oxygen–oxygen σ-bond in the peroxide; the bond energy of organic peroxides is ca. 45 kcal mol−1.17 The KDM rearrangement is initiated by treatment of an endoperoxide with a base. Non-nucleophilic bases promote an initial deprotonation of an α-hydrogen, leading to breakage of the weak oxygen–oxygen σ-bond; an example is shown for the bicyclic endoperoxide 13 (Scheme 2a).18,19 Conversely, when an endoperoxide is treated with a nucleophilic base a competing process can occur. For example, treatment of the bicyclic endoperoxide 13 with an organolithium leads to direct reaction onto one of the oxygen centres and subsequent peroxide cleavage providing the diol 15 (Scheme 2a).
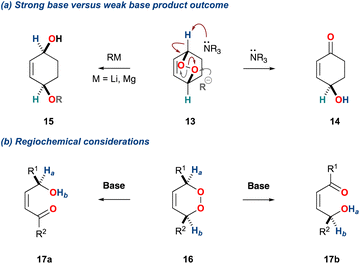 | ||
| Scheme 2 (a) Product outcome of bicyclic peroxides with MgR/LiR versus tertiary amines; (b) regiochemical considerations with monocyclic peroxides. | ||
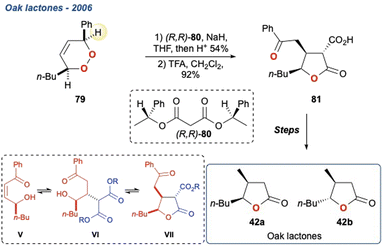
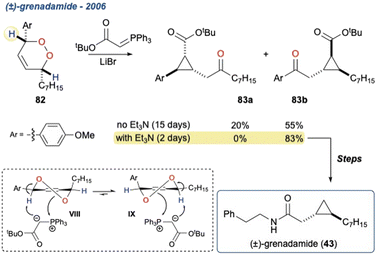
Additionally, the initial α-hydrogen deprotonation becomes crucial when considering the regiochemical outcome. For example, monocyclic peroxides of the type 16, provide two potential α-hydrogens (Scheme 2b, 16 Ha and Hb) which can then deliver to isomeric γ-hydroxyenones 17a and 17b, respectively. This regiochemical complexity in the KDM rearrangement was observed by Taylor and co-workers in their first total synthesis of grenadamide 43, a cyclopropyl amide from the marine cyanobacterium, Lyngbya majuscula;20 this will be discussed later within this review.
Tertiary amines have been the predominant bases employed in the KDM rearrangement, and include Et3N,19,21,22 DIPEA,23 DABCO,24,25 DBU26–28 and piperidine.29 Other bases to be used in the KDM rearrangement include, LiOH,30,31 phosphorus ylides,30 stabilised phosphates,32 malonates,33 SiO2,34 and more recently bases derived from haloforms.35 Each of these bases gives the key product of the KDM rearrangement for cyclic peroxides, the γ-hydroxyenone, whose fate is often defined by the base used in the initial rearrangement. Tertiary amines commonly provide the γ-hydroxyenone which then cyclises to give the dihydrofuran 20 (Scheme 3a).
This also occurs with monocyclic peroxides (16 to 17 to 18a) and bicyclic peroxides (19 to 20 to 18b); note that in each case the γ-hydroxyenone and dihydrofuran are in equilibrium. In the case of dihydrofuran 18 derived from monocyclic peroxides, this can be readily aromatised via dehydration to provide the furan 22; alternatively, depending on the nature of the substituents, this dihydrofuran can rearrange to give a 1,4-diketone 21. Bicyclic peroxides can also undergo this alternate rearrangement, for example peroxide 23 can undergo a NEt3 mediated KDM rearrangement to provide dihydrofuran 24 which then undergoes a subsequent fragmentation to ultimately provide diketone 26. Under basic conditions monocyclic and bicyclic γ-hydroxyenones can also undergo conjugate addition. This is particularly pertinent for γ-hydroxyenones such as 27 which can readily dimerise in the presence of LiOH to provide tetrasubstituted tetrahydrofurans such as 30.31 This transformation is reminiscent of the proposed biomimetic synthesis of (±)-incarviditone (33) through the oxa-Michael/Michael dimerization of (±)-rengyolone (31) described by Sherburn and co-workers (Scheme 3c).23,36 There are sparce examples of alternate bases such as acetates37,38 as well as weak acids such as thiourea.6,39 Bach and co-workers40 described an acid mediated KDM rearrangement via protonation of the peroxide bridge and this report highlights an alternate mechanistic pathway for the KDM rearrangement.
The use of tertiary amine bases in the KDM rearrangement has led to it becoming an increasingly important tool in probing plausible biomimetic pathways in natural product synthesis. This stems from the work of Brame, Morrow and co-workers41,42 who investigated the KDM reaction in the isoprostane pathway, followed by work by Kelly and co-workers19 who identified the potential of an enantioselective isomerisation of a prochiral endoperoxide, and was subsequently realised in 2006 by Staben and Toste (Scheme 4).43 This was achieved by desymmetrisation of meso-endoperoxides using cinchona-alkaloid catalysed enantio-determining deprotonation (Scheme 4b). The model proposed for achieving enantioselective induction is shown in Scheme 4b, where the cinchona alkaloid acts as a bifunctional catalyst providing the γ-hydroxyenone through an E2-elimination. The arrangement of the tertiary amine and the hydroxyl at the 6-position of the quinoline were key for this reaction, facilitating the heterolytic oxygen–oxygen σ-bond cleavage as well as providing a chiral pocket for enantio-discrimination. To achieve high enantio-induction, the prochiral endoperoxide must possess a size differential between the two bridges flanking the oxygen–oxygen bond; this was evidenced by the poor enantio-induction when forming 4-hydroxycyclohex-2-ene-1-one 35f. Additionally, this protocol was shown to give poor enantio-induction for monocyclic endoperoxides.44 It must be noted that the desymmetrisation of prochiral endoperoxides can also be achieved through a homolytic cleavage of the weak oxygen–oxygen σ-bond.45 This also provides a route to enantioenriched γ-hydroxyenones, however, this is not technically a KDM rearrangement and will not be covered within this review.
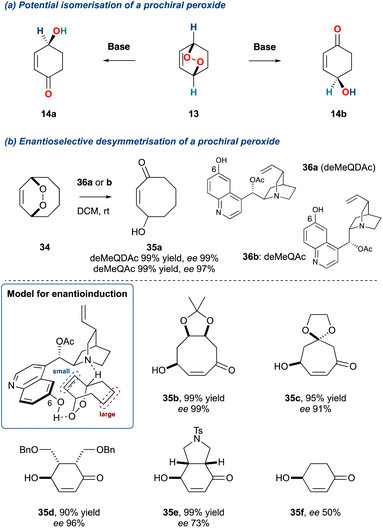 | ||
| Scheme 4 (a) Potential desymmetrisation of a prochiral bicyclic peroxides; (b) Toste and co-workers desymmetrisation protocol. | ||
This important disclosure by Stanben and Toste provides a general approach to the synthesis γ-hydroxyenones, crucial chiral building blocks, and significantly, this method has been harnessed by several groups to synthesise natural products, examples of which are discussed further within this review.
Recently, Greatrex and co-workers divulged an innovative thiourea/amine organocatalytic system to desymmetrise meso-endoperoxide of the type shown in Scheme 5.46 Key to the success of this transformation is the steric requirement of the catalyst, particularly size of the dialkylamine at C1 (cat. 38). The authors observed that if the steric bulk of the amine at C1 increased this was detrimental to the er of the product γ-hydroxyenones. Additionally, for unsymmetrical peroxides such as 39, a kinetic resolution could be realised. The regiochemistry of the KDM rearrangement in this kinetic resolution is confined due to the benzylic proton, which are far more acidic. Therefore, to achieve a satisfactory er in the product γ-hydroxyenones (40a–c) the progress of each reaction required monitoring, and this is highlighted in the percentage completions. However, while this methodology is very recent, it should find applicability within future natural product syntheses.
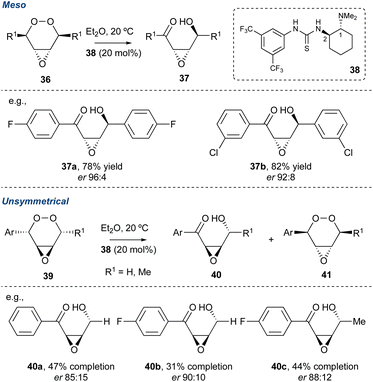 | ||
| Scheme 5 Greatrex and co-workers use of an organocatalysis in the desymmetrisation of endoperoxides. | ||
3 Endoperoxides: the KDM rearrangement precursors
The precursors for a KDM rearrangement are endoperoxides, and their synthesis is achieved by treatment of a 1,3-diene with singlet oxygen, with the latter being generated from oxygen in the presence of a photosensitiser and a visible light source (there are examples of generating singlet oxygen through ‘dark’ processes this has yet to be employed in the synthesis of natural products).47–52 This transformation is well established and there are several reviews that describe the underlining mechanism, reaction scope and limitations.15,16 Over recent decades there have been significant improvements in the practicality of this transformation, which has led to improved yields and more importantly improved safety, given the reputation of endoperoxides for their instability, and the challenges of using molecular oxygen at scale.53,54 This includes the use of continuous flow methodology16,53–55 and reactor design.56–64 Often in natural product syntheses, the formation of the endoperoxide is undertaken in batch conditions. However, improvements in yield should be possible given the increasing availability of commercial continuous flow equipment and accessibility of simple homemade flow reactors.4 The KDM rearrangement and biomimetic relationship
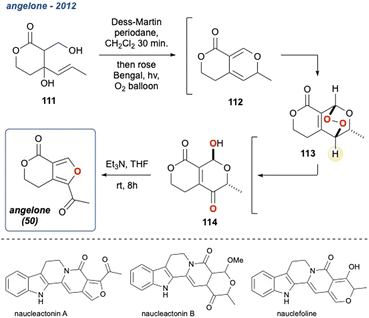
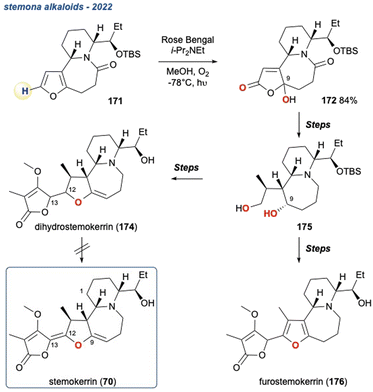
The KDM rearrangement is often described as a biomimetic reaction and as such features in several bioinspired routes to different natural products. The incorporation of the KDM in such a route undoubtedly must proceed through an endo-peroxide intermediate and have an accessible, acidic hydrogen atom that can be removed, see Section 2 for more details on the mechanism. For example, in the synthesis of angelone I, Tan et al. identified that several natural products isolated from Nauclea feature an “oxygenation–deoxygenation relationship” that could be attributed to the reaction of singlet oxygen with diene II and its subsequent KDM rearrangement and O-methylation into III (Scheme 6a).65 The presence of IV was postulated as a ring contraction step going through a 6π-electrocyclic ring opening. With these key steps identified, they targeted I as their synthetic goal and designed their biomimetic retrosynthetic route based around the installation of a peroxide bridge, its subsequent KDM rearrangement and then the ring contraction step (Scheme 6b). They also hypothesised that should the forward route indeed mimic the postulated biomimetic conditions, then the reaction steps could be truncated into a facile process. Indeed, they completed the synthesis of I in a 3-pot procedure with 6 overall steps (Scheme 6c), and a full discussion of this natural product synthesis can be found in Section 5 of this review. Crucially, this example nicely highlights the relevance of the KDM rearrangement as a biomimetic tool and showcases the importance of recognising where it could occur in a biosynthetic pathway, exemplified in the above case between the isolated diene II and the γ-hydroxyenone derivative III.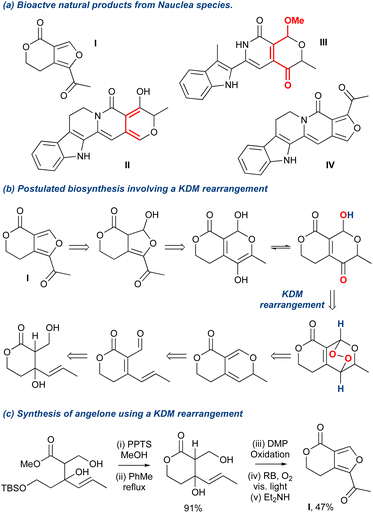 | ||
| Scheme 6 An example of a KDM rearrangement used as a key biomimetic step in the total synthesis of angelone. | ||
5 Natural products
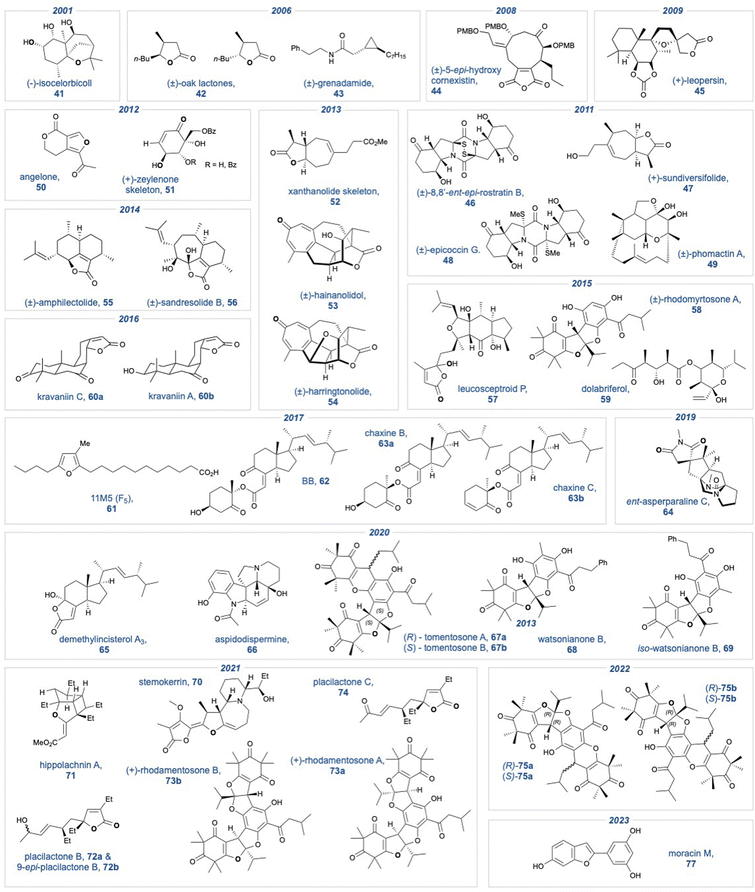
A summary of all natural product targets that have employed a KDM rearrangement over the past 25 years is shown in Fig. 1.We have divided the analysis into two parts. The first section will describe the use of the KDM rearrangement as a key step. We have examined each natural product that uses a KDM rearrangement in chronological order. Where necessary we have provided clarification on the regiochemistry of the products that result from the KDM rearrangement, whether the key KDM rearrangement was enantioselective, and if the KDM rearrangement was employed as a biomimetic tool. The second section of this review describes natural products that specifically contain a butenolide. This structural motif, common to diterpene natural products, can be installed using a late stage KDM rearrangement when performed on a 3-substituted furan.
5.1 KDM rearrangement as a key step
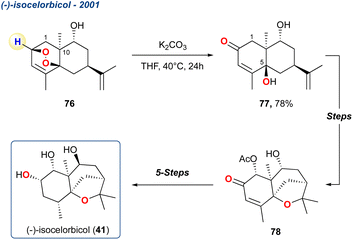 | ||
| Scheme 7 Zhou et al. use of the KDM rearrangement to initially give precursor 77 which was subsequently used to give (−)-isocelorbicol 41. | ||
In their initial report, Zhou et al. prepared peroxide 76 in 8-steps from (−)-carvone and upon treatment with K2CO3 in THF produced diol 77 in high yield, which was subsequently transformed to enone 78 which they suggested was an enantioenriched precursor for polyhydroxylated agarofurans.66 The regiochemistry of the KDM rearrangement is uncomplicated in this example given there is only one available hydrogen. Additionally, the stereochemistry of the OH at C-5 is defined by the initial endoperoxide formation which occurs anti to the methyl group at C-10. In a follow-up report, the group took enone 78 forward and completed the synthesis of 41, which was realised in 5 linear steps.67
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
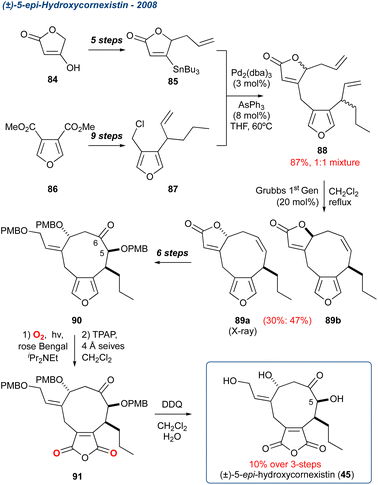
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of isomers, which subsequently underwent an RCM to provide diastereoisomers 89a and 89b in 30% and 47% yield, respectively. Diastereoisomer 89a was then taken forward in 6-steps, introducing oxidation at C5 and C6, as well as opening of the butenolide. The PMB protected precursor 90 was then exposed to KDM rearrangement conditions followed by TPAP oxidation, and PMB global deprotection with DDQ, to give 5-epi-hydroxycornexistin 45 in 10% over these final three steps. While the sequence was low yielding, this synthesis proves the validity of masking the anhydride as its furan.
1 mixture of isomers, which subsequently underwent an RCM to provide diastereoisomers 89a and 89b in 30% and 47% yield, respectively. Diastereoisomer 89a was then taken forward in 6-steps, introducing oxidation at C5 and C6, as well as opening of the butenolide. The PMB protected precursor 90 was then exposed to KDM rearrangement conditions followed by TPAP oxidation, and PMB global deprotection with DDQ, to give 5-epi-hydroxycornexistin 45 in 10% over these final three steps. While the sequence was low yielding, this synthesis proves the validity of masking the anhydride as its furan.
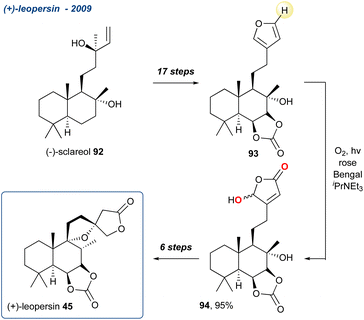
Starting their synthesis from (−)-sclareol (92) they accessed furan intermediate 93 in 17-steps and treated it with singlet oxygen in the presence of DIPEA yielding the γ-hydroxybutenolide 94 in 95% yield. The unmasking of a butenolide using a KDM rearrangement is discussed in more detail in Section 5.2. The synthesis was completed in a further 6-steps with 45 isolated with its C-13 epimer in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
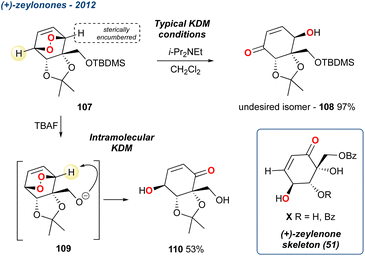 | ||
| Scheme 15 An intramolecular KDM rearrangement, through TBAF deprotection, reported by Palframan et al. in their synthesis of the zeylenone skeleton (51). | ||
When treated with tetrabutylammonium fluoride (TBAF), the resultant alkoxide triggered an intramolecular KDM rearrangement giving the desired isomer in 53% yield, with oxy-anion 109 serving as the base; notably none of the undesired regioisomer 108 was observed. However, the KDM rearrangement product 110 was not taken forward in the synthesis due to its instability and instead the group used additional steps from the diol obtained from the reduced endoperoxide. Nevertheless, this methodology is a useful addition, demonstrating that the regioselectivity of the reaction can be tuned in some circumstances.
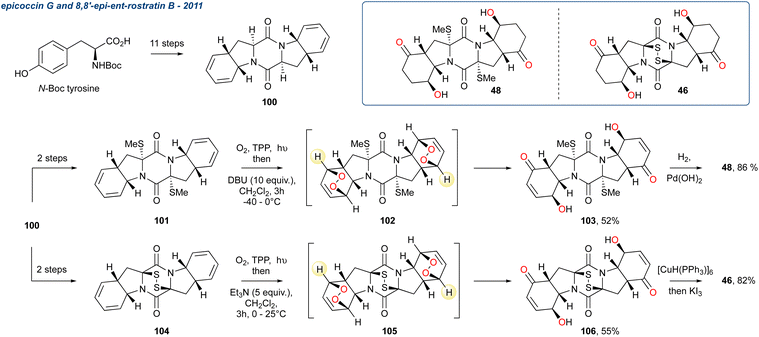
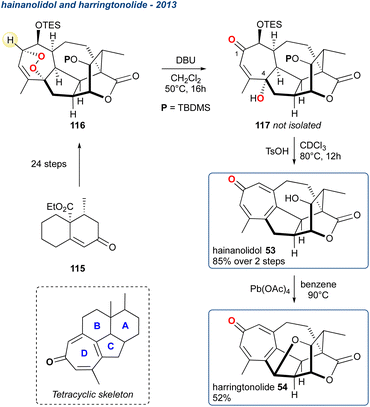
A year later, 54 was isolated again but from C. hainanensis and this alongside the related compound 53 (hainanolidol, formerly named hainanolide) which was later proposed as the precursor to harringtonolide. Whilst 54 shows antineoplastic and antiviral activity the proposed precursor 53 is inactive, suggesting the tetrahydrofuran ring is important for their bioactivity. These two related natural products were synthesised in the laboratory by Zhang et al. starting from enone 115 producing peroxide 116 in 24-steps, which was then subjected to KDM conditions using DBU as the base furnishing the γ-hydroxyenone 117.93 The stereochemistry of the hydroxy group at C4 is a consequence of the [4 + 2]-cycloaddition in the preceding endoperoxide forming reaction, although this is inconsequential given it is subsequently eliminated. Additionally, 117 is used without further purification, being treated immediately with TsOH that gave deprotection and elimination forming the key tropone D-ring (see tetracyclic skeleton) and completing the synthesis of hainanolidol 53, which was isolated in 85% over the 2-steps. Treatment of 53 with Pb(OAc)4 produced the tetrahydrofuran ring, thereby forming harringtonolide 54 in 52%.
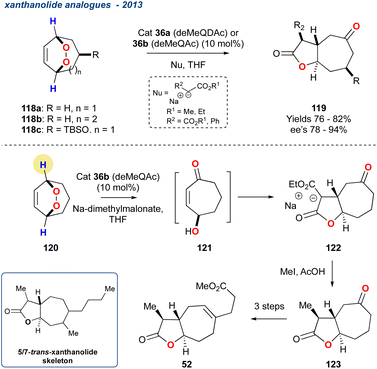 | ||
| Scheme 18 Priest and co-workers use of the desymmetrisation of a prochiral endoperoxides to provide a rapid synthesis of the trans-xanthanolide skeleton. | ||
Catalysts 36a or 36b (see Scheme 4) were used to promote the asymmetric reaction leading to the desired γ-hydroxyenones, that were then treated in situ with various nucleophiles followed by spontaneous lactonization to yield the desired butyrolactones (119) in high yields and ee's of up to 94%. The chemistry was then applied to the synthesis of a trans-xanthanolide analogue 52 with the installation of the methyl group with MeI followed by decarboxylation in AcOH to yield the desired butyrolactones 123 in 79% yield and good diastereoselectivity. An alternative strategy using α-methyldiethylsodiomalonate yielded a similar overall yield (69%). The trans-xanthanolide analogue 52 was realised in 51% yield over an additional 3-steps.
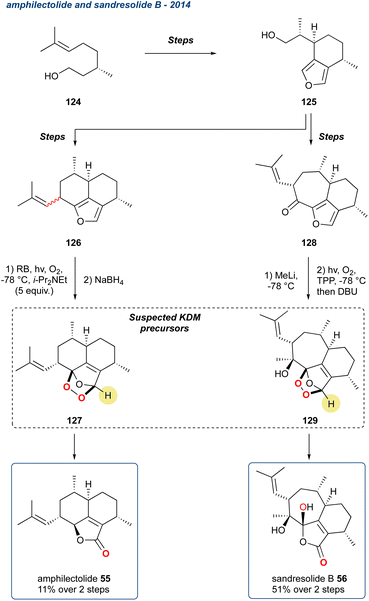 | ||
| Scheme 19 The synthesis of amphilectolide (55) and sandresolide B (56) via a suspected KDM rearrangement from endoperoxide precursors 127 and 129, respectively. | ||
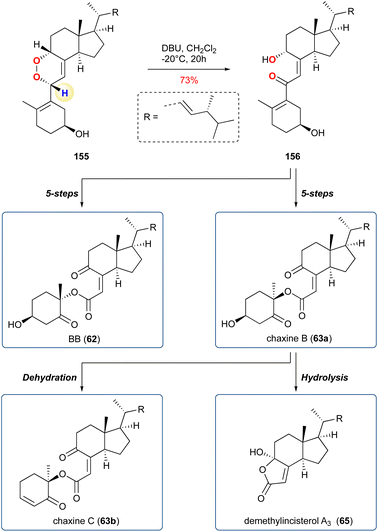
Belonging to a large family of marine metabolites, originally isolated from Pseudopterogorgia elisabethae, a Caribbean coral, natural products of this type demonstrate a broad range of biological activity, i.e., inflammation, tuberculosis, anti-cancer, and antiplasmodial.95 To complete their synthesis of amphilectolide (55) the researchers treated the diastereomeric mixture of 126 with singlet oxygen in the presence of Hünigs base (i-Pr2NEt), and then immediately reduced with NaBH4 yielding 55 in 11%, from a complex mixture of products, over the 2-steps (Scheme 19). It is unclear from the report whether the reaction proceeded by a KDM rearrangement, but given the conditions, the presence of a carbonyl at the 5-membered ring, it is highly likely the KDM precursors was 127. The other target, sandresolide B (56), was obtained in 51% yield over 2-steps, where methylated 128 was subjected to, after some optimization, photo-oxidation with tetraphenylporphyrin (TPP) followed by treatment with DBU to trigger the regioselective KDM rearrangement. During optimisation, the authors found that DBU was the only base to give efficient transformation of the peroxide intermediate.
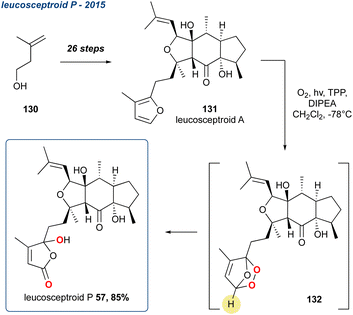 | ||
Scheme 20 Hugelshofer et al. use of the KDM rearrangement in the synthesis of leucosceptroid P. Singlet oxidation of the furan is unselective, therefore leucosceptroid is obtained as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture. 1 mixture. | ||
They are a family of terpenes isolated from Leucosceptrum canum that exhibit antifeedant activities. Building upon their related synthesis of norleucosceptroids A–H, the researchers synthesised leucosceptroid A (131) in 26-steps from alcohol 130.98 Oxidation with singlet oxygen yielded the corresponding endoperoxide (132) which was then converted in situ by Hünigs base forming the γ-hydroxyenone regioselectively in 85% yield. Due to a lack of substrate control, the oxidation of the furan ring by singlet oxygen provides a mixture of endoperoxides. This was predicted by the authors given their previous disclosure on the biomimetic studies on this family of natural products.97 The KDM rearrangement therefore gives a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers in the furan ring, endoperoxide was formed racemically, therefore leading to leucosceptroid P (57) to be obtained as a 1
1 mixture of diastereoisomers in the furan ring, endoperoxide was formed racemically, therefore leading to leucosceptroid P (57) to be obtained as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers at the carbon containing the newly formed hydroxyl group. This is inconsequential as the natural product is originally obtained as a 1
1 mixture of diastereoisomers at the carbon containing the newly formed hydroxyl group. This is inconsequential as the natural product is originally obtained as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture.
1 mixture.
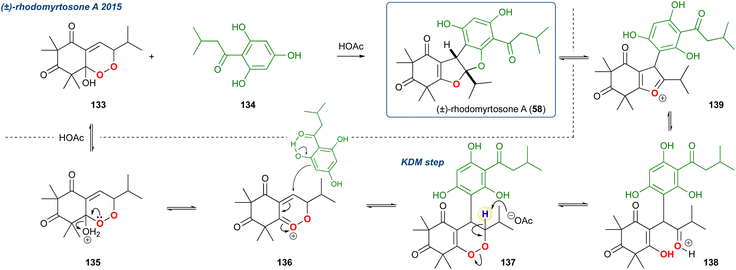 | ||
| Scheme 21 Gervias et al. approach to (±)-rhodomyrtosone A. A unique KDM rearrangement occurs due to an initial conjugate addition to the peroxide oxycation 136. | ||
Following the initial report of this synthesis by Gervais et al. there have been several reports utilizing the same approach, occasionally with minor modification to the conditions, to realise a variety of related natural products isolated from Rhodomyrtus tomentosa or other related plants in the same genus. Watsonianone B (68) and its isomer (69) were synthesised by Zhang et al. in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
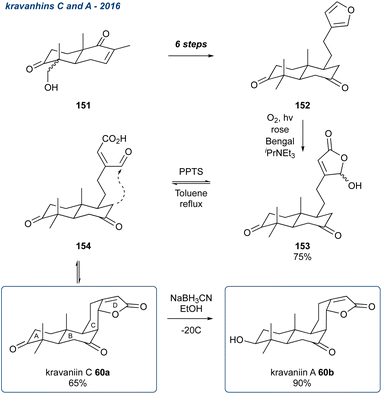
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and 27% isolated yield for the desired 68 (Fig. 2).100 Tomentosones A (67a) and B (67b) were also reported by the same group and synthesised in a similar manner, only this time using pyridinium p-toluenesulfonate (PPTS) in toluene which yielded a 1
1 ratio and 27% isolated yield for the desired 68 (Fig. 2).100 Tomentosones A (67a) and B (67b) were also reported by the same group and synthesised in a similar manner, only this time using pyridinium p-toluenesulfonate (PPTS) in toluene which yielded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixture of 67a and 67b in 73% yield.101 They also reported that the two isomers could be interconverted in the presence of p-toluenesulfonic acid. The following year Deng et al. reported the asymmetric synthesis of rhodomentosones A (73a) and B (73b), a pair of phloroglucinol trimers that contain an unusual 6/5/5/6/5/5/6 fused ring system.102 In this report the group described an asymmetric approach to the domino reaction first reported by Gervais et al. by employing a catalytic amount of a chiral phosphoric acid in the presence of AlF3 as a Lewis acid they could access the desired bis-furan in high ee. After separation of the isomers, the second bis-furan unit was installed giving rhodomentosone A (73a) and B (73b) in a ratio of 1
10 mixture of 67a and 67b in 73% yield.101 They also reported that the two isomers could be interconverted in the presence of p-toluenesulfonic acid. The following year Deng et al. reported the asymmetric synthesis of rhodomentosones A (73a) and B (73b), a pair of phloroglucinol trimers that contain an unusual 6/5/5/6/5/5/6 fused ring system.102 In this report the group described an asymmetric approach to the domino reaction first reported by Gervais et al. by employing a catalytic amount of a chiral phosphoric acid in the presence of AlF3 as a Lewis acid they could access the desired bis-furan in high ee. After separation of the isomers, the second bis-furan unit was installed giving rhodomentosone A (73a) and B (73b) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with 90% ee and a combined yield of 45%. This asymmetric approach was applied again by Deng et al. in the synthesis of four unnamed phloroglucinol trimers (75a and 75b) isolated again from Rhodomyrtus tomentosa.103
2 with 90% ee and a combined yield of 45%. This asymmetric approach was applied again by Deng et al. in the synthesis of four unnamed phloroglucinol trimers (75a and 75b) isolated again from Rhodomyrtus tomentosa.103
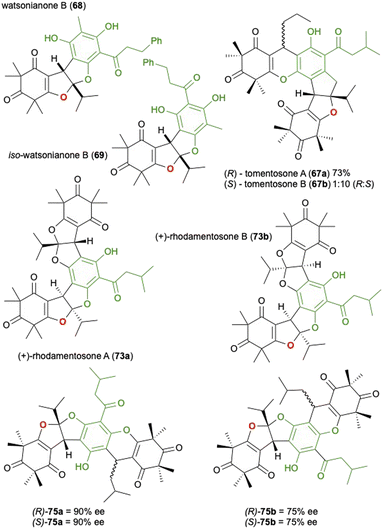 | ||
| Fig. 2 The synthesis of related natural products from Rhodomyrtus tomentosa or related plants in the same genus using the cascade approach described in Scheme 20. | ||
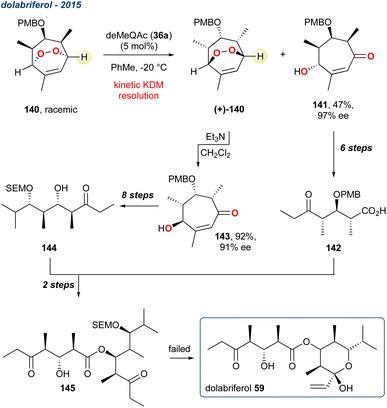 | ||
| Scheme 22 The use of an organocatalyst in a KDM rearrangement and kinetic resolution in the attempted total synthesis of dolabriferol 59. | ||
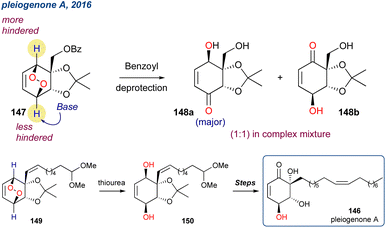
The two enantioenriched fragments of the target were constructed from a common racemic peroxide 140. The authors used the established organocatalysts deMeQAc (36a), but unlike the centrosymmetric desymmetrisation described by Stanben et al.,43 the authors used a racemic endoperoxide to achieve a kinetic resolution. The regioselectivity of the KDM rearrangement is central to this approach, given the highlighted proton in 140 is less sterically encumbered, and therefore more likely to be deprotonated in the key KDM rearrangement step. After some optimisation, they found that in toluene and when 140 was treated with deMeQAc (36a) a selective deprotonation occurred providing enone 141 as a single enantiomer in 47% yield and 97% ee. The remaining enantioenriched peroxide (+)-140 could then be cleanly converted using a subsequent KDM rearrangement but using Et3N, providing enantioenriched enone 143 in 92% yield and 91% ee. Each fragment was then taken forward in several steps and recombined, but unfortunately the synthesis of 59 failed on the last step.
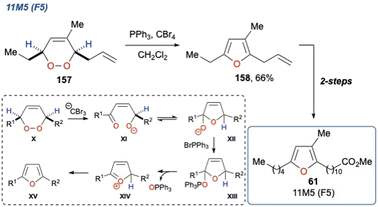 | ||
| Scheme 26 Lee et al. use of the Appel reagent in a KDM rearrangement, and its application to the synthesis of 11M5. | ||
In their synthesis, they employed Appel reaction conditions (PPh3, CBr4) on endoperoxide 157 (which was synthesised in 4-steps) to perform a dehydrative cyclisation to yield furan 158 in 66% yield. In this case the in situ generated CBr3− was used as a base to initiate the KDM rearrangement step yielding enone XI, and upon cyclisation to XII and subsequent dehydration with PPh3Br+ the desired furan XV could be realised. It is not known which proton is deprotonated in the initial KDM step (X to XI); however, this is immaterial given that final product destination is the fully dehydrated furan. The synthesis of 61 was completed with a cross metathesis and hydrogenation to install the ester functionality. The researchers also demonstrated the scope and versatility of the reaction on a range of substrates, yielding a variety of multi-substituted furans in 50–98% yield. The authors framed this as a biosynthetically inspired route to these important natural products, given the biosynthesis of furan fatty acids in marine bacteria.113
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
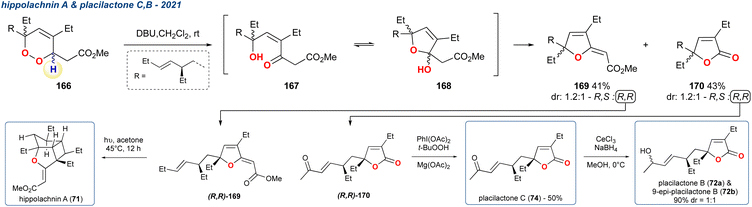
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers, of which the R,R-isomer (R,R-169) underwent UV-induced [2 + 2]-cyclisation to complete the formal synthesis of (+)-hippolachnin A (71). The group also demonstrated that the R,R-isomer of the undesired product (R,R-170) obtained from the KDM rearrangement step could be taken forward to access another series of natural products plakilactone C (74) and B (72) in 1- and 2-steps, respectively.
1 mixture of diastereoisomers, of which the R,R-isomer (R,R-169) underwent UV-induced [2 + 2]-cyclisation to complete the formal synthesis of (+)-hippolachnin A (71). The group also demonstrated that the R,R-isomer of the undesired product (R,R-170) obtained from the KDM rearrangement step could be taken forward to access another series of natural products plakilactone C (74) and B (72) in 1- and 2-steps, respectively.
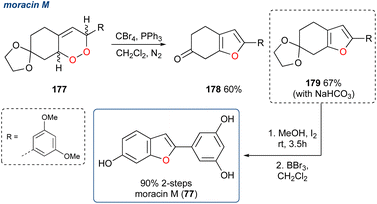 | ||
| Scheme 31 Synthesis of the benzofuran, moracin M (77) using an Appel reagent mediated KDM rearrangement. | ||
Using their reported route to 1,3-dienes122,123 they synthesised endoperoxide 177 and subjected it to Appel conditions, which unexpectedly yielded ketone 178 in 60% yield. Here the furan was successfully constructed but due to the build-up of HBr during the reaction the acetal deprotection occurred and attempts to aromatise 178 failed. A minor modification to the procedure saw the inclusion of NaHCO3 to quench the HBr in situ and prevent deprotection and under these conditions acetal 179 was obtained in 67% yield. The synthesis of moracin M (77) was completed in 90% yield over two deprotection steps.
5.2 Unmasking 3-substituted furans to reveal 4-hydroxybutenolides using the KDM rearrangement
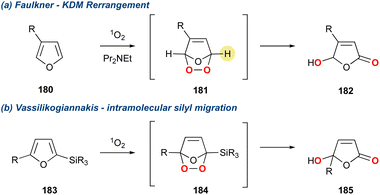
The butenolide motif is found over several natural product families whose synthesis has been recently reviewed.124,125 In 1988 Faulkner and co-workers110 reported a convenient method for the direct conversion of 3-alkyl furans to 4-hydroxybutenolides through the action of singlet oxygen and a hindered base (Scheme 32a).126 This method makes use of a hindered base to direct the deprotonation, thereby proving 182. This methodology was further modified by Vassilikogiannakis and co-workers127 who utilised 2-trialkylsilylfurans which could be directly converted to the corresponding butanolide using singlet oxygen, but in the absence of base (Scheme 32b). This latter method goes via an intramolecular silyl migration as described by Adam and Rodriguez.128 Both methods have found application in the total synthesis of natural products over the past 25 years. However, this section will only centre on those syntheses that convert 3-alkylfurans instead of 2-trialkylsilylfurans, as the former proceeds solely by a base mediated KDM rearrangement.We have summarised all the natural products that have been synthesised using this approach in Fig. 3. Fig. 3A demonstrates natural products giving a γ-hydroxybutenolide in the last step, while Fig. 3B, gives natural products that possess a butenolide, which is installed through a subsequent reduction. Given the uniform approach across all these natural products in unmasking of the 3-substituted furan in the last step using the Faulkner method, detailed analysis has not been undertaken; however, a summary is given below.
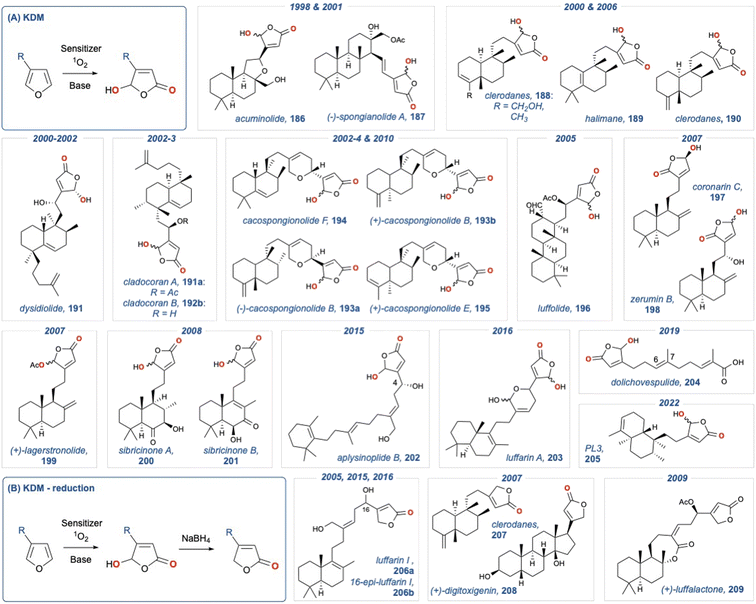 | ||
| Fig. 3 Natural products that have been synthesised using (A) unmasking of a 3-substituted furan in the final step; (b) unmasking of a 3-substituted furan in the final step and then reduction. | ||
Dubay and co-workers synthesised acuminolide (186) in 1998 (and its 17-O-acetyl analogue, 187) using a semisynthetic approach.129 Acuminolide (186) is a labdane diterpene with cytotoxic activity, and their syntheses was achieved from commercially available (+)-sclareolide, with the key butenolide being revealed using a KDM rearrangement. Soetjipto and co-workers disclosed a synthesis of acuminolide (186) in 2001,130 and again, the key butenolide was revealed using a last step KDM rearrangement. The clerodanes and halimanes (188–190) were synthesised by Costa and co-workers. The authors were able to assign the absolute configuration through a successful semi-synthesis from methyl (+)-hardwickiate; again, they utilised the KDM rearrangement to reveal the key butenolide.131,132 Dysiodiolide (191) is a natural product isolated form the marine sponge Dysidera etheris and is a potent inhibitor of the human cdc25A protein phosphatase.133 This natural product has received considerable attention since is identification in 1996. Consequently, several total syntheses134–139 have been reported, with the key butenolide being revealed in the final step. Cladocoran A and B (191a,b), isolated form the coral Cladocora cespitosa140 show structural homology with dysidiolide (191), consequently several groups use a final KDM rearrangement to reveal the butenolide. Marcos et al.141,142 used a semisynthetic approach from ent-halimic acid to give a synthesis of their purported structures. Miyaoka et al.143 then used a de novo synthesis and established clardocoran B (192b) to be an olefinic isomer of dysodiolide (191), with clardocoran A (191a) being its acetate. The cacospongionolides (193–195) are sesterterpenes isolated from sponges of the Thorectidae family and are inhibitors of phospholipase A2 (PLA2) and therefore show potential in treating inflammation. First to be synthesised was (−)- and (+)-cacospongionolides B (193a,b) by Chueng and Snapper,144 who used a KDM rearrangement in the final step to reveal the butenolide, this was followed by a synthesis of (−)-cacospongionolides F (194) by Demeke and Forsyth,145 and a follow up synthesis of cacospongionolides E (195) again by Snapper,146 as well as latter synthesis of (−)-cacospongionolides B (193a) by Oshida et al.147 Luffoilide (196) is a metabolite from the sponge Luffariella, and its semi-synthesis148 was completed using a KDM rearrangement in the final step from methyl isoanticopalate, itself obtained from sclareol. The same group then reported a synthesis of (+)-laffalactone (209);149 however, the key γ-hydroxybutenolide from the final KDM rearrangement was subsequently reduced to provide the desired natural product. Laffarin I (206a) was first synthesised by Urosa and co-workers from commercially available sclareol.150 Luffarin A (203), a related metabolite, was synthesised using a similar last step KDM rearrangement approach, along with luffarin I (206a) and 16-epi-luffarin I (206b); the last two natural products were obtained by a subsequent reduction of the γ-hydroxybutenolide.151 The labdane diterpenes, coronarin C (197) and zerumin B (198) underwent oxidation using rose Bengal or TPP as the photosensitiser. Oxidation using rose Bengal was undertaken in the absence of base, giving the required regioisomer of the butenolide; whereas, in the presence of base the undesired product was isolated.152 Basabe and co-workers completed a semi-synthesis of the diterpene (+)-lagerstronolide (199) from (+)-sclareol.153 (+)-Lagerstronolide (199) is a metabolite isolated from the Lagerstreomia lancasteria shrub in south-eastern Australia.154 The key butenolide was introduced via a KDM rearrangement in the penultimate step, with the resultant γ-hydroxybutenolide being acetylated to give the desired natural product. A de novo synthesis of (+)-digitoxigenin (208) was reported by Homma and Nakata in 2007.155 (+)-Digitoxigenin (208) has clinical value as a treatment for congestive heart failure and as well as displaying anticancer activity. The butenoilide was introduced by a two-step sequence involving a KDM rearrangement with subsequent reduction of the γ-hydroxybutenolide. Marcos and co-workers completed syntheses of both sibiricinone A and B (200 and 201), again from (+)-sclareol.156 Kutsumura and co-workers completed a total synthesis of aplysinoplide B (202),157 a cytotoxic sesterterpeniod isolated from the marine sponge Aplysinopsis digitata.158 The purpose of the synthesis was the promising activity of these natural products against P388 mouse leukemia cells, as well as confirming the C4 hydroxyl group. Again, the butenolide was introduced in the last step via a KDM rearrangement. Dolichovespulide (204) is a sesquiterpene isolated from the body surface extracts of hornet queens (Dolichovespula maculata). This pheromone is a new member of relatively small class of terpenoids isolated from the Vespidae insect family. The key butenolide was unveiled using a KDM rearrangement, and the total synthesis confirmed the C6–C7 double bond geometry.159 Technically, revealing of butenolide was the penultimate step in the synthesis, as the free acid needed to be revealed in the last step. In 2022, Zhu and co-workers provided a modular synthetic approach, using a metal mediated tail-to-head cyclisation strategy, to synthesise the central diterpene ring system of several trans-clerodane analogues. In their report they provided an effective synthesis of annonene, which was oxidised using a KDM rearrangement condition to provide the butenolide PL3 (205).160
6 Conclusions
This review provides valuable insight into the versatility of the KDM rearrangement in natural product synthesis. It is clear from the number of reports that this transformation is increasingly recognised as a key biomimetic step. There are several reports that utilise the enantioselective KDM, but here the methodology is limited to larger rings (≥6-membered) that contain some pre-existing steric bias. New developments in this area would open-up a raft of possibilities for installing chiral alcohols on a wider variety of substrates. Presently, there are limited reports of the reaction taking place under acidic conditions, apart from the report by Bach and co-workers.40 This may provide scope to improve on the existing enantioselective desymmetrisation of prochiral endoperoxides described by Toste.43 This method uses a chiral base to affect the KDM, has substrate limitations. The successful development of an enantioselective acid catalysed process could have significant impact and improve upon the already impressive range of natural products that can be synthesised using the KDM rearrangement.7 Author contributions
MCK and DSL contributed equally to the conceptualisation and investigation of this research. They both contributed equally to the writing and preparation of the manuscript.8 Conflicts of interest
There are no conflicts to declare.9 Acknowledgements
MCK would like to thank Loughborough University. DSL would like to thank the Center for Green Chemistry and Green Engineering for their support and the JA team for their advice and fruitful discussions.10 Notes and references
- N. Kornblum and H. E. DeLaMare, J. Am. Chem. Soc., 1951, 73, 880–881 CrossRef CAS.
- N. A. Milas and D. M. Surgenor, J. Am. Chem. Soc., 1946, 68, 205–208 CrossRef CAS.
- W. Baker and D. M. Easty, Nature, 1950, 166, 156 CrossRef PubMed.
- M. C. Kimber and D. K. Taylor, Trends Org. Chem., 2001, 9, 53 CAS.
- M. Oda and Y. Kitahara, Tetrahedron Lett., 1969, 10, 3295–3296 CrossRef.
- A. Daştan and M. Balci, Tetrahedron, 2006, 62, 4003–4010 CrossRef.
- F. Koc, E. Cadirci, A. Albayrak, Z. Halici, A. Hacimuftuoglu and H. Suleyman, Med. Chem. Res., 2010, 19, 84–93 CrossRef CAS.
- Y. Morita, E. Matsumura, T. Okabe, M. Shibata, M. Sugiura, T. Ohe, H. Tsujibo, N. Ishida and Y. Inamori, Biol. Pharm. Bull., 2003, 26, 1487–1490 CrossRef CAS PubMed.
- M. Balci and B. Atasoy, Tetrahedron Lett., 1984, 25, 4033–4036 CrossRef CAS.
- M. E. Sengül, Z. Ceylan and M. Balci, Tetrahedron, 1997, 53, 10401–10408 CrossRef.
- M. Güney, Z. Ç. Ceylan, A. Daştan and M. Balci, Can. J. Chem., 2005, 83, 227–235 CrossRef.
- M. G. Zagorski and R. G. Salomon, J. Am. Chem. Soc., 1980, 102, 2501–2503 CrossRef CAS.
- M. G. Zagorski and R. G. Salomon, J. Am. Chem. Soc., 1984, 106, 1750–1759 CrossRef CAS.
- I. A. Yaremenko, V. A. Vil', D. V Demchuk and A. O. Terent'ev, Beilstein J. Org. Chem., 2016, 12, 1647–1748 CrossRef CAS PubMed.
- M. Balci, Chem. Rev., 1981, 81, 91 CrossRef CAS.
- A. A. Ghogare and A. Greer, Chem. Rev., 2016, 116, 9994–10034 CrossRef CAS PubMed.
- R. D. Bach and H. B. Schlegel, J. Phys. Chem. A, 2020, 124, 4742–4751 CrossRef CAS PubMed.
- E. Mete, R. Altundas, H. Secen and M. Balci, Turk. J. Chem., 2003, 27, 145 CAS.
- D. R. Kelly, H. Bansal and J. J. G. Morgan, Tetrahedron Lett., 2002, 43, 9331–9333 CrossRef CAS.
- T. D. Avery, J. A. Culbert and D. K. Taylor, Org. Biomol. Chem., 2006, 4, 323–330 RSC.
- N. Akbulut and M. Balci, J. Org. Chem., 1988, 53, 3338–3342 CrossRef CAS.
- T. Fujishima, F. Kitoh, T. Yano and R. Irie, Synlett, 2010, 2010, 2279–2282 CrossRef.
- V. L. Paddock, R. J. Phipps, A. Conde-Angulo, A. Blanco-Martin, C. Giró-Mañas, L. J. Martin, A. J. P. White and A. C. Spivey, J. Org. Chem., 2011, 76, 1483–1486 CrossRef CAS PubMed.
- C. Liu, E. Shi, F. Xu, Q. Luo, H. Wang, J. Chen and X. Wan, Chem. Commun., 2015, 51, 1214–1217 RSC.
- J. Jiang, J. Liu, L. Yang, Y. Shao, J. Cheng, X. Bao and X. Wan, Chem. Commun., 2015, 51, 14728–14731 RSC.
- I. Erden, N. Öcal, J. Song, C. Gleason and C. Gärtner, Tetrahedron, 2006, 62, 10676–10682 CrossRef CAS PubMed.
- X. Zheng, S. Lu and Z. Li, Org. Lett., 2013, 15, 5432–5435 CrossRef CAS PubMed.
- F. Zhang, P. Du, J. Chen, H. Wang, Q. Luo and X. Wan, Org. Lett., 2014, 16, 1932–1935 CrossRef CAS PubMed.
- R. A. Kumar, C. U. Maheswari, S. Ghantasala, C. Jyothi and K. R. Reddy, Adv. Synth. Catal., 2011, 353, 401–410 CrossRef CAS.
- T. D. Avery, D. K. Taylor and E. R. T. Tiekink, J. Org. Chem., 2000, 65, 5531–5546 CrossRef CAS PubMed.
- B. W. Greatrex, M. C. Kimber, D. K. Taylor and E. R. T. Tiekink, J. Org. Chem., 2003, 68, 4239–4246 CrossRef CAS PubMed.
- M. C. Kimber and D. K. Taylor, J. Org. Chem., 2002, 67, 3142–3144 CrossRef CAS PubMed.
- B. W. Greatrex, M. C. Kimber, D. K. Taylor, G. Fallon and E. R. T. Tiekink, J. Org. Chem., 2002, 67, 5307–5314 CrossRef CAS PubMed.
- X. Zhang, S. I. Khan and C. S. Foote, J. Org. Chem., 1993, 58, 7839–7847 CrossRef CAS.
- R. J. Lee, M. R. Lindley, G. J. Pritchard and M. C. Kimber, Chem. Commun., 2017, 53, 6327–6330 RSC.
- P. D. Brown, A. C. Willis, M. S. Sherburn and A. L. Lawrence, Org. Lett., 2012, 14, 4537–4539 CrossRef CAS PubMed.
- M. Rössle, T. Werner, A. Baro, W. Frey and J. Christoffers, Angew. Chem., Int. Ed., 2004, 43, 6547–6549 CrossRef PubMed.
- G. S. Buchanan, K. P. Cole, Y. Tang and R. P. Hsung, J. Org. Chem., 2011, 76, 7027–7039 CrossRef CAS PubMed.
- M. J. Palframan, G. Kociok-Köhn and S. E. Lewis, Chem.–Eur. J., 2012, 18, 4766–4774 CrossRef CAS PubMed.
- C. Wiegand, E. Herdtweck and T. Bach, Chem. Commun., 2012, 48, 10195 RSC.
- L. J. Roberts, R. G. Salomon, J. D. Morrow and C. J. Brame, FASEB J., 1999, 13, 1157–1168 CrossRef CAS PubMed.
- R. G. Salomon, Acc. Chem. Res., 1985, 18, 294–301 CrossRef CAS.
- S. T. Staben, X. Linghu and F. D. Toste, J. Am. Chem. Soc., 2006, 128, 12658–12659 CrossRef CAS PubMed.
- J. Priest, M. R. Longland, M. R. J. Elsegood and M. C. Kimber, J. Org. Chem., 2013, 78, 3476–3481 CrossRef CAS PubMed.
- T. D. Avery, N. F. Jenkins, M. C. Kimber, D. W. Lupton and D. K. Taylor, Chem. Commun., 2002, 28–29 RSC.
- S. V. A.-M. Legendre, C. J. Sumby, A. Karton and B. W. Greatrex, J. Org. Chem., 2023, 88, 11444–11449 CrossRef CAS PubMed.
- J. M. Aubry, J. Am. Chem. Soc., 1985, 107, 5844–5849 CrossRef CAS.
- J. M. Aubry and B. Cazin, Inorg. Chem., 1988, 27, 2013–2014 CrossRef CAS.
- M. Elsherbini, R. K. Allemann and T. Wirth, Chem.–Eur. J., 2019, 25, 12486–12490 CrossRef CAS PubMed.
- D. F. Evans and M. W. Upton, J. Chem. Soc., Dalton Trans., 1985, 1151 RSC.
- W. Adam, D. V. Kazakov and V. P. Kazakov, Chem. Rev., 2005, 105, 3371–3387 CrossRef CAS PubMed.
- M. C. Carreño, M. González-López and A. Urbano, Angew. Chem., Int. Ed., 2006, 45, 2737–2741 CrossRef PubMed.
- C. A. Hone, D. M. Roberge and C. O. Kappe, ChemSusChem, 2017, 10, 32–41 CrossRef CAS PubMed.
- A. Burgard, T. Gieshoff, A. Peschl, D. Hörstermann, C. Keleschovsky, R. Villa, S. Michelis and M. P. Feth, Chem. Eng. J., 2016, 294, 83–96 CrossRef CAS.
- H. F. Grantham, R. J. Lee, G. M. Wardas, J.-R. Mistry, M. R. J. Elsegood, I. A. Wright, G. J. Pritchard and M. C. Kimber, J. Org. Chem., 2024, 89, 484–497 CrossRef CAS PubMed.
- F. Lévesque and P. H. Seeberger, Org. Lett., 2011, 13, 5008–5011 CrossRef PubMed.
- K. N. Loponov, J. Lopes, M. Barlog, E. V. Astrova, A. V. Malkov and A. A. Lapkin, Org. Process Res. Dev., 2014, 18, 1443–1454 CrossRef CAS.
- L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Chem. Rev., 2022, 122, 2752–2906 CrossRef CAS PubMed.
- G. I. Ioannou, T. Montagnon, D. Kalaitzakis, S. A. Pergantis and G. Vassilikogiannakis, ChemPhotoChem, 2017, 1, 173–177 CrossRef CAS.
- C. A. Clark, D. S. Lee, S. J. Pickering, M. Poliakoff and M. W. George, Org. Process Res. Dev., 2016, 20, 1792–1798 CrossRef CAS.
- D. S. Lee, Z. Amara, C. A. Clark, Z. Xu, B. Kakimpa, H. P. Morvan, S. J. Pickering, M. Poliakoff and M. W. George, Org. Process Res. Dev., 2017, 21, 1042–1050 CrossRef CAS PubMed.
- D. S. Lee, M. Sharabi, R. Jefferson-Loveday, S. J. Pickering, M. Poliakoff and M. W. George, Org. Process Res. Dev., 2020, 24, 201–206 CrossRef CAS.
- R. A. Bourne, X. Han, M. Poliakoff and M. W. George, Angew. Chem., Int. Ed., 2009, 48, 5322–5325 CrossRef CAS PubMed.
- A. Chaudhuri, S. D. A. Zondag, J. H. A. Schuurmans, J. van der Schaaf and T. Noël, Org. Process Res. Dev., 2022, 26, 1279–1288 CrossRef CAS PubMed.
- H. Tan, X. Chen, Z. Liu and D. Z. Wang, Tetrahedron, 2012, 68, 3952–3955 CrossRef CAS.
- G. Zhou, X. Gao, W. Z. Li and Y. Li, Tetrahedron Lett., 2001, 42, 3101–3103 CrossRef CAS.
- W.-D. Z. Li, G. Zhou, X. Gao and Y. Li, Tetrahedron Lett., 2001, 42, 4649–4651 CrossRef CAS.
- R. C. Brown, D. K. Taylor and G. M. Elsey, Org. Lett., 2006, 8, 463–466 CrossRef CAS PubMed.
- N. Sitachitta and W. H. Gerwick, J. Nat. Prod., 1998, 61, 681–684 CrossRef CAS PubMed.
- T. D. Avery, B. W. Greatrex, D. K. Taylor and E. R. T. Tiekink, J. Chem. Soc., Perkin Trans. 1, 2000, 1319–1321 RSC.
- T. D. Avery, G. Fallon, B. W. Greatrex, S. M. Pyke, D. K. Taylor and E. R. T. Tiekink, J. Org. Chem., 2001, 66, 7955–7966 CrossRef CAS PubMed.
- J. S. Clark, J. M. Northall, F. Marlin, B. Nay, C. Wilson, A. J. Blake and M. J. Waring, Org. Biomol. Chem., 2008, 6, 4012 RSC.
- S. C. Fields, L. Mireles-Lo and B. C. Gerwick, J. Nat. Prod., 1996, 59, 698–700 CrossRef CAS.
- T. Amagasa, R. N. Paul, J. J. Heitholt and S. O. Duke, Pestic. Biochem. Physiol., 1994, 49, 37–52 CrossRef CAS.
- I. S. Marcos, L. Castañeda, P. Basabe, D. Díez and J. G. Urones, Tetrahedron, 2009, 65, 9256–9263 CrossRef CAS.
- Y. Tang, K. P. Cole, G. S. Buchanan, G. Li and R. P. Hsung, Org. Lett., 2009, 11, 1591–1594 CrossRef CAS PubMed.
- M. Sugano, A. Sato, Y. Iijima, T. Oshima, K. Furuya, H. Kuwano, T. Hata and H. Hanzawa, J. Am. Chem. Soc., 1991, 113, 5463–5464 CrossRef CAS.
- K. P. Cole and R. P. Hsung, Org. Lett., 2003, 5, 4843–4846 CrossRef CAS PubMed.
- K. P. Cole and R. P. Hsung, Chem. Commun., 2005, 5784 RSC.
- M. Kawasumi, N. Kanoh and Y. Iwabuchi, Org. Lett., 2011, 13, 3620–3623 CrossRef CAS PubMed.
- S. Ohno, K. Tomita-Yokotani, S. Kosemura, M. Node, T. Suzuki, M. Amano, K. Yasui, T. Goto, S. Yamamura and K. Hasegawa, Phytochemistry, 2001, 56, 577–581 CrossRef CAS PubMed.
- H. Yokoe, H. Sasaki, T. Yoshimura, M. Shindo, M. Yoshida and K. Shishido, Org. Lett., 2007, 9, 969–971 CrossRef CAS PubMed.
- K. C. Nicolaou, S. Totokotsopoulos, D. Giguère, Y.-P. Sun and D. Sarlah, J. Am. Chem. Soc., 2011, 133, 8150–8153 CrossRef CAS PubMed.
- K. C. Nicolaou, M. Lu, S. Totokotsopoulos, P. Heretsch, D. Giguère, Y.-P. Sun, D. Sarlah, T. H. Nguyen, I. C. Wolf, D. F. Smee, C. W. Day, S. Bopp and E. A. Winzeler, J. Am. Chem. Soc., 2012, 134, 17320–17332 CrossRef CAS PubMed.
- G. R. Schulte and B. Ganem, Tetrahedron Lett., 1982, 23, 4299–4302 CrossRef CAS.
- S. D. Jolad, J. J. Hoffmann, K. H. Schram, J. R. Cole, M. S. Tempesta and R. B. Bates, J. Org. Chem., 1981, 46, 4267–4272 CrossRef CAS.
- C.-R. Zhang, S.-P. Yang, S.-G. Liao, Y. Wu and J.-M. Yue, Helv. Chim. Acta, 2006, 89, 1408–1416 CrossRef CAS.
- Y. Takeuchi, Q. Cheng, Q.-W. Shi, T. Sugiyama and T. Oritani, Biosci., Biotechnol., Biochem., 2001, 65, 1395–1398 CrossRef CAS PubMed.
- D. A. Mulholland, A. Langlois, M. Randrianarivelojosia, E. Derat and J.-M. Nuzillard, Phytochem. Anal., 2006, 17, 87–90 CrossRef CAS PubMed.
- W.-D. Xuan, H.-S. Chen, J.-L. Du, S. Liang, T.-Z. Li and D.-G. Cai, J. Asian Nat. Prod. Res., 2006, 8, 719–722 CrossRef CAS PubMed.
- L. Mao, L. Xin and Y. Dequan, Planta Med., 1984, 50, 459–461 CrossRef CAS PubMed.
- J. G. Buta, J. L. Flippen and W. R. Lusby, J. Org. Chem., 1978, 43, 1002–1003 CrossRef CAS.
- M. Zhang, N. Liu and W. Tang, J. Am. Chem. Soc., 2013, 135, 12434–12438 CrossRef CAS PubMed.
- I. T. Chen, I. Baitinger, L. Schreyer and D. Trauner, Org. Lett., 2014, 16, 166–169 CrossRef CAS PubMed.
- T. J. Heckrodt and J. Mulzer, in Natural Products Synthesis II, ed. J. Mulzer, Spinger, Berlin, Heidelber, 2005, pp. 1–41 Search PubMed.
- C. L. Hugelshofer and T. Magauer, J. Am. Chem. Soc., 2015, 137, 3807–3810 CrossRef CAS PubMed.
- S.-H. Luo, C. L. Hugelshofer, J. Hua, S.-X. Jing, C.-H. Li, Y. Liu, X.-N. Li, X. Zhao, T. Magauer and S.-H. Li, Org. Lett., 2014, 16, 6416–6419 CrossRef CAS PubMed.
- C. L. Hugelshofer and T. Magauer, Angew. Chem., Int. Ed., 2014, 53, 11351–11355 CrossRef CAS PubMed.
- A. Gervais, K. E. Lazarski and J. A. Porco, J. Org. Chem., 2015, 80, 9584–9591 CrossRef CAS PubMed.
- X. Zhang, G. Wu, L. Huo, X. Guo, S. Qiu, H. Liu, H. Tan and Y. Hu, J. Nat. Prod., 2020, 83, 3–7 CrossRef CAS PubMed.
- X. Zhang, C. Dong, G. Wu, L. Huo, Y. Yuan, Y. Hu, H. Liu and H. Tan, Org. Lett., 2020, 22, 8007–8011 CrossRef CAS PubMed.
- L.-M. Deng, L.-J. Hu, Y.-T.-Z. Bai, J. Wang, G.-Q. Qin, Q.-Y. Song, J.-C. Su, X.-J. Huang, R.-W. Jiang, W. Tang, Y.-L. Li, C.-C. Li, W.-C. Ye and Y. Wang, Org. Lett., 2021, 23, 4499–4504 CrossRef CAS PubMed.
- L.-M. Deng, W. Tang, S.-Q. Wang, J.-G. Song, X.-J. Huang, H.-Y. Zhu, Y.-L. Li, W.-C. Ye, L.-J. Hu and Y. Wang, J. Org. Chem., 2022, 87, 4788–4800 CrossRef CAS PubMed.
- M. L. Ciavatta, M. Gavagnin, R. Puliti, G. Cimino, E. Martinez, J. Ortea and C. A. Mattia, Tetrahedron, 1996, 52, 12831–12838 CrossRef CAS.
- M. R. Gesinski, W. E. Brenzovich, S. T. Staben, D. J. Srinilta and F. D. Toste, Tetrahedron Lett., 2015, 56, 3643–3646 CrossRef CAS.
- J. Froese, C. Overbeeke and T. Hudlicky, Chem.–Eur. J., 2016, 22, 6180–6184 CrossRef CAS PubMed.
- M. J. Palframan, G. Kociok-Köhn and S. E. Lewis, Chem.–Eur. J., 2012, 18, 4766–4774 CrossRef CAS PubMed.
- Z. Zhong, G. Zhao, D. Xu, B. Dong, D. Song, X. Xie and X. She, Chem.–Asian J., 2016, 11, 1542–1547 CrossRef CAS PubMed.
- H. Yin, J.-G. Luo and L.-Y. Kong, J. Nat. Prod., 2013, 76, 237–242 CrossRef CAS PubMed.
- M. R. Kernan and D. J. Faulkner, J. Org. Chem., 1988, 53, 2773–2776 CrossRef CAS.
- Y. Hirata, A. Nakazaki, H. Kawagishi and T. Nishikawa, Org. Lett., 2017, 19, 560–563 CrossRef CAS PubMed.
- M. Niki, Y. Hirata, A. Nakazaki, J. Wu, H. Kawagishi and T. Nishikawa, J. Org. Chem., 2020, 85, 4848–4860 CrossRef CAS PubMed.
- N. Shirasaka, K. Nishi and S. Shimizu, Biochim. Biophys. Acta, Lipids Lipid Metab., 1997, 1346, 253–260 CrossRef CAS PubMed.
- I. Dokli, R. Pohl, B. Klepetářová and U. Jahn, Chem. Commun., 2019, 55, 3931–3934 RSC.
- H. Hayahsi, Y. Nishimoto, K. Akiyama and H. Nozaki, Biosci., Biotechnol., Biochem., 2000, 64, 111–115 CrossRef PubMed.
- F. Reuß and P. Heretsch, Org. Lett., 2020, 22, 3956–3959 CrossRef PubMed.
- Q. Li, K. Zhao, A. Peuronen, K. Rissanen, D. Enders and Y. Tang, J. Am. Chem. Soc., 2018, 140, 1937–1944 CrossRef CAS PubMed.
- C. Festa, S. De Marino, M. V. D'Auria, E. Deharo, G. Gonzalez, C. Deyssard, S. Petek, G. Bifulco and A. Zampella, Tetrahedron, 2012, 68, 10157–10163 CrossRef CAS.
- Z. Xue, Q. Li, J. Zhang and Y. Tang, Org. Lett., 2021, 23, 8783–8788 CrossRef CAS PubMed.
- M. Morgenstern, C. Mayer, A. Pöthig and T. Bach, Synthesis, 2023, 55, 1671–1689 CrossRef CAS.
- Y. Al-Jawaheri, M. R. J. Elsegood, J.-R. Mistry and M. C. Kimber, Tetrahedron Lett., 2023, 114, 154273 CrossRef CAS.
- Y. Al-Jawaheri and M. C. Kimber, Org. Lett., 2016, 18, 3502–3505 CrossRef CAS PubMed.
- Y. Al-Jawaheri, M. Turner and M. Kimber, Synthesis, 2018, 50, 2329–2336 CrossRef CAS.
- K. Tadiparthi and S. Venkatesh, J. Heterocycl. Chem., 2022, 59, 1285–1307 CrossRef CAS.
- S. Chatterjee, R. Sahoo and S. Nanda, Org. Biomol. Chem., 2021, 19, 7298–7332 RSC.
- B. L. Feringa, Recl. Trav. Chim. Pays-Bas, 2010, 106, 469–488 CrossRef.
- T. Montagnon, M. Tofi and G. Vassilikogiannakis, Acc. Chem. Res., 2008, 41, 1001–1011 CrossRef CAS PubMed.
- W. Adam and A. Rodriguez, Tetrahedron Lett., 1981, 22, 3505–3508 CrossRef CAS.
- P. A. Zoretic, H. Fang, A. A. Ribeiro and G. Dubay, J. Org. Chem., 1998, 63, 1156–1161 CrossRef CAS.
- N. Furuichi, T. Hata, H. Soetjipto, M. Kato and S. Katsumura, Tetrahedron, 2001, 57, 8425–8442 CrossRef CAS.
- P. M. Imamura and M. Costa, J. Nat. Prod., 2000, 63, 1623–1625 CrossRef CAS PubMed.
- R. M. Ide, M. Costa and P. M. Imamura, J. Braz. Chem. Soc., 2006, 17, 417–420 CrossRef CAS.
- S. P. Gunasekera, P. J. McCarthy, M. Kelly-Borges, E. Lobkovsky and J. Clardy, J. Am. Chem. Soc., 1996, 118, 8759–8760 CrossRef CAS.
- S. R. Magnuson, L. Sepp-Lorenzino, N. Rosen and S. J. Danishefsky, J. Am. Chem. Soc., 1998, 120, 1615–1616 CrossRef CAS.
- D. Demeke and C. J. Forsyth, Tetrahedron, 2002, 58, 6531–6544 CrossRef CAS.
- H. Miyaoka and Y. Yamada, Bull. Chem. Soc. Jpn., 2002, 75, 203–222 CrossRef CAS.
- H. Miyaoka, Y. Kajiwara, Y. Hara and Y. Yamada, J. Org. Chem., 2001, 66, 1429–1435 CrossRef CAS PubMed.
- M. Takahashi, K. Dodo, Y. Hashimoto and R. Shirai, Tetrahedron Lett., 2000, 41, 2111–2114 CrossRef CAS.
- D. Demeke and C. J. Forsyth, Org. Lett., 2000, 2, 3177–3179 CrossRef CAS PubMed.
- A. Fontana, M. L. Ciavatta and G. Cimino, J. Org. Chem., 1998, 63, 2845–2849 CrossRef CAS.
- I. S. Marcos, A. B. Pedrero, M. J. Sexmero, D. Diez, P. Basabe, F. A. Hernández, H. B. Broughton and J. G. Urones, Synlett, 2002, 2002, 0105–0109 CrossRef.
- I. S. Marcos, A. B. Pedrero, M. J. Sexmero, D. Diez, P. Basabe, N. García, R. F. Moro, H. B. Broughton, F. Mollinedo and J. G. Urones, J. Org. Chem., 2003, 68, 7496–7504 CrossRef CAS PubMed.
- H. Miyaoka, M. Yamanishi, Y. Kajiwara and Y. Yamada, J. Org. Chem., 2003, 68, 3476–3479 CrossRef CAS PubMed.
- A. K. Cheung and M. L. Snapper, J. Am. Chem. Soc., 2002, 124, 11584–11585 CrossRef CAS PubMed.
- D. Demeke and C. J. Forsyth, Org. Lett., 2003, 5, 991–994 CrossRef CAS PubMed.
- A. K. Cheung, R. Murelli and M. L. Snapper, J. Org. Chem., 2004, 69, 5712–5719 CrossRef CAS PubMed.
- S. Kobayashi, M. Oshida, M. Ono and A. Nakazaki, Heterocycles, 2010, 80, 313 CrossRef PubMed.
- P. Basabe, S. Delgado, I. S. Marcos, D. Diez, A. Diego, M. De Román and J. G. Urones, J. Org. Chem., 2005, 70, 9480–9485 CrossRef CAS PubMed.
- P. Basabe, O. Bodero, I. S. Marcos, D. Díez, A. Blanco, M. de Román and J. G. Urones, J. Org. Chem., 2009, 74, 7750–7754 CrossRef CAS PubMed.
- A. Urosa, I. Marcos, D. Díez, A. Lithgow, G. Plata, J. Padrón and P. Basabe, Mar. Drugs, 2015, 13, 2407–2423 CrossRef CAS PubMed.
- A. Urosa, I. S. Marcos, D. Díez, G. B. Plata, J. M. Padrón and P. Basabe, Mol. Diversity, 2016, 20, 369–377 CrossRef CAS PubMed.
- J. E. Villamizar, J. Juncosa, J. Pittelaud, M. Hernández, N. Canudas, E. Tropper, F. Salazar and J. Fuentes, J. Chem. Res., 2007, 2007, 342–346 CrossRef.
- P. Basabe, O. Bodero, I. S. Marcos, D. Diez, M. de Román, A. Blanco and J. G. Urones, Tetrahedron, 2007, 63, 11838–11843 CrossRef CAS.
- P. K. Chaudhuri, Phytochemistry, 1987, 26, 3361–3362 CrossRef CAS.
- M. Honma and M. Nakada, Tetrahedron Lett., 2007, 48, 1541–1544 CrossRef CAS.
- I. S. Marcos, L. Castañeda, P. Basabe, D. Díez and J. G. Urones, Tetrahedron, 2008, 64, 10860–10866 CrossRef CAS.
- N. Kutsumura, K. Matsuo and T. Saito, Tetrahedron Lett., 2015, 56, 2602–2604 CrossRef CAS.
- R. Ueoka, Y. Nakao, S. Fujii, R. W. M. van Soest and S. Matsunaga, J. Nat. Prod., 2008, 71, 1089–1091 CrossRef CAS PubMed.
- W. Ren, R. Gries, K. L. Kurita, C. S. McCaughey, S. K. Alamsetti, R. G. Linington, G. Gries and R. Britton, J. Nat. Prod., 2019, 82, 2009–2012 CrossRef CAS PubMed.
- W. Zhu, Q. Yin, Z. Lou and M. Yang, Nat. Commun., 2022, 13, 6633 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |