Open Access Article
Open Access ArticleUnlocking the potential of NiCo2O4 nanocomposites: morphology modification based on urea concentration and hydrothermal and calcination temperature
Ataollah
Niyati
 *,
Arianna
Moranda
,
Juan Felipe
Basbus
and
Ombretta
Paladino
*,
Arianna
Moranda
,
Juan Felipe
Basbus
and
Ombretta
Paladino
Department of Civil, Chemical and Environmental Engineering, University of Genoa (UNIGE-DICCA), Via All’Opera Pia 15, 16145 Genoa, Italy. E-mail: ataollah.niyati@edu.unige.it
First published on 22nd May 2024
Abstract
Oxygen evolution reaction (OER) electrocatalysts are critical in minimizing energy loss during the anodic four-electron transfer process that is required for water oxidation. Improving and selecting optimal non-noble OER electrocatalysts are key strategies for elevating their overall performance and efficiency of energy storage and conversion. Eight NiCo2O4 electrocatalysts were synthesized using the hydrothermal method by changing the amount of urea as a nucleation agent and hydrothermal and calcination temperature to achieve an outstanding catalyst in terms of morphology and electrochemical activity. For examining the physicochemical properties of the electrocatalyst, analyses such as XRD, SEM, TEM, and EDS were conducted. Although XRD analysis revealed the formation of pure NiCo2O4 for all eight samples, SEM and TEM analysis unraveled the best electrocatalyst in terms of morphology to be NiCo-S3 (urea: 10 times higher, Thydrothermal: 120 °C, and TCalcination: 350 °C) and NiCo-S4 (urea: 10 times higher, Thydrothermal: 120 °C, and TCalcination: 400 °C) with a mum-flower-like shape and particle dimension between 20 and 45 nm. NiCo-S4 displayed robust electrochemical activity, primarily in the OER, with an overpotential of 327 mV at 10 mA cm−2 in 1.0 M aqueous KOH solution. The OER performance was enhanced as demonstrated by the exceptional durability of >24 hours and a Tafel slope of 79.7 mV dec−1. Electrochemical impedance spectroscopy (EIS) revealed a low resistance of 1.03 Ω and a double-layer capacitance of 2.43 mF cm−2, substantiating the outstanding OER performance of NiCo-S4.
1. Introduction
In recent years, the issue of energy storage has become increasingly important, and to simultaneously protect the environment and promote economic growth, some efforts should be made.1 The growing concerns about global warming and the depletion of fossil fuels have made it clear that we need to prioritize the development of methods by leveraging renewable energy sources.2–4 As a result, there is a growing demand for technologies that can efficiently collect, store, and use energy.5 In view of this, electrochemical conversion and energy storage technologies such as water electrolysis, fuel cells, and redox flow batteries can address the problems by employing renewable energy sources such as solar, wind, and wave energy, thus enabling the storage and utilization of energy for power supply.6–9 Hydrogen, a carbon-neutral form of energy, can be electrocatalytically produced via water electrolysis, stored and used as a fuel source in power generation devices whenever needed.10 With reference to these technologies, the OER on the anode side is a crucial step in producing hydrogen.11 For instance, concerning water electrolysis, identifying a good OER electrocatalyst is of the utmost importance because it plays a fundamental role in minimizing energy loss at the anode due to the four-electron transfer during water oxidation.12 By selecting, studying, and improving OER electrocatalysts, we can significantly enhance the overall performance and efficiency of energy storage and conversion systems.13Many studies have been conducted on transition metal oxides operating as oxygen evolution anodes under alkaline conditions, and they demonstrated appropriate overpotential and stability.14–16 Spinel electrocatalysts, a type of transition metal oxide represented by the AB2O4 formula (A and B are metal ions), have garnered considerable interest for their potential applications as OER electrodes in water electrolyzers owing to their unique chemical makeup, structure, valence, and morphology.17–19 NiCo2O4, more commonly referred to as cobalt–nickel oxide, is an extraordinary spinel catalyst due to its high capacity, excellent redox activity, affordability, and abundant availability in nature, which makes it highly desirable for use in the energy storage sector.20,21 To be more precise, NiCo2O4 is composed of mixed-valence transition metal oxides in which nickel occupies the octahedral sites, whereas cobalt occupies both the octahedral and tetrahedral sites.22 Although NiCo2O4 has strong characteristics, the particle size and morphology are vital, especially in systems with porous conductive substrates as an anode and electrocatalysts as a reactive layer. As a result, the appropriate shape and size of the electrocatalyst should be in a way that does not block the pore sites of the substrate, allowing water and generated oxygen to pass easily without adding additional resistance to the system and, more crucially, improving electrocatalytic activity.23
A variety of techniques to synthesize NiCo2O4 spinel have been developed, including hydroxide decomposition,24 nano casting,25 electrodeposition,26 coprecipitation,27 and hydrothermal synthesis.28 Among these methods, hydrothermal synthesis seems suitable for controlling the shape and producing a catalyst that can be used for deposition onto the surface of different substrates.29–33 The hydrothermal method using a hydrolysis agent such as urea can make the desired morphology of the final electrocatalyst by the production of hydroxide in the solution which can firstly decompose the precursor salts and in the second step bond with Ni2+ and Co2+, producing double-layered hydroxide (LDH) of Ni and Co, which can with thermal treatment produce pure single phase NiCo2O4.34,35
NiCo2O4 has been synthesized in a wide variety of structural forms, spanning nanoparticles,36 nanowires,37 nanoflowers,38 nanosheet arrays,39 and nanoneedle arrays,40 and the results of these studies demonstrate the important role of shape in electrochemical characteristics.41 The essential truth is that most of the manufactured NiCo2O4 electrocatalysts do not have a single morphology, consisting of a combination of rods and sheets, which may be ineffective when sprayed on conductive substrates for OER applications.
To overcome this issue, a hydrothermal method followed by a tempering step was used to construct NiCo2O4 with a finely controlled morphology for its use as an electrocatalyst in the OER. Three important factors were investigated in this work to optimize the material's morphology, i.e. hydrothermal temperature, calcination temperature, and the quantity of urea, which is used as a nucleation agent, facilitating the controlled release of metal ions and contributing to the formation of catalytically active sites. Furthermore, the electrochemical performance of the salient samples was thoroughly studied in order to determine the most promising contender. Several characterization techniques were used to provide a thorough understanding of the morphology and purity of the synthesized materials.
2. Experimental
2.1. Materials
NiCl2·6H2O (99% purity) as a Ni precursor, CoCl2·6H2O (98% purity) as a Co precursor, KOH (99% purity), and urea (99% purity) were acquired from Carlo Erba in order to make the requisite electrocatalysts. Ultra-pure deionized water was used for all cleaning and synthesis methods, which was purchased from Exaxol (Genova, Italy). Ethanol and acetone, which were used after filtration of electrocatalysts to remove any remaining residue, were purchased from Sigma Aldrich. To be more precise, all the reactants were used without further purification.2.2. Preparation of NiCo2O4 nano electrocatalysts
The attainment of an electrocatalyst with a suitable morphology that can be readily deposited onto diverse electrodes without obstructing the desired electrode's pore structure is crucial. In order to address this requirement, three factors, namely, urea amount as factor 1, hydrothermal temperature as factor 2, and calcination temperature as factor 3, were systematically varied and investigated during the synthesis of NiCo2O4. A total of eight samples were synthesized and designated as NiCo-S1 to NiCo-S8, each exhibiting distinct characteristics, as outlined in Table 1. These modifications enable a comprehensive study of the influence of these factors on the morphology and properties of the NiCo2O4 electrocatalyst. Fig. 1 illustrates the synthesis procedure employed for the fabrication of NiCo2O4 using a facile hydrothermal method at a temperature of 120 °C or 180 °C, followed by subsequent calcination at 350 °C or 400 °C. The total amount of final powder obtained for each catalyst was 2 grams of NiCo2O4. The synthesis procedure involved several steps. Initially, 2.015 grams of NiCl2·6H2O was dissolved in 25 mL of deionized water (DI) and gradually added drop by drop to the CoCl2·6H2O solution, which contained 4.0354 grams of Co precursor and 40 mL of DI. In the second step, urea was added in two different quantities, namely, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and a 1
2 and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio, and the solution was vigorously stirred for 30 minutes. Subsequently, the solution was subjected to sonication in a bath for an additional 30 minutes to promote better nucleation and the formation of nanoparticles. The resulting solution was then transferred to a 100 mL Teflon-lined stainless-steel autoclave for the hydrothermal reaction, which was carried out for a duration of 10 hours. After the completion of the reaction, the powder was collected after washing with DI and ethanol. The collected powder was then filtered and dried in a vacuum oven at 60 °C. In the final step, the dried powder was placed in a furnace and subjected to a thermal treatment at either 350 °C or 400 °CC for a period of 3 hours, with a heating ramp of 10 °C min−1. This process ensured the successful synthesis of NiCo2O4 with the desired characteristics for subsequent analysis and application.
10 molar ratio, and the solution was vigorously stirred for 30 minutes. Subsequently, the solution was subjected to sonication in a bath for an additional 30 minutes to promote better nucleation and the formation of nanoparticles. The resulting solution was then transferred to a 100 mL Teflon-lined stainless-steel autoclave for the hydrothermal reaction, which was carried out for a duration of 10 hours. After the completion of the reaction, the powder was collected after washing with DI and ethanol. The collected powder was then filtered and dried in a vacuum oven at 60 °C. In the final step, the dried powder was placed in a furnace and subjected to a thermal treatment at either 350 °C or 400 °CC for a period of 3 hours, with a heating ramp of 10 °C min−1. This process ensured the successful synthesis of NiCo2O4 with the desired characteristics for subsequent analysis and application.
| Name | Catalyst | Urea | T Hydrothermal | T Calcination |
|---|---|---|---|---|
| NiCo-S1 | NiCo2O4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
120 | 350 |
| NiCo-S2 | NiCo2O4 | “ | “ | 400 |
| NiCo-S3 | NiCo2O4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
“ | 350 |
| NiCo-S4 | NiCo2O4 | “ | “ | 400 |
| NiCo-S5 | NiCo2O4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
180 | 350 |
| NiCo-S6 | NiCo2O4 | “ | “ | 400 |
| NiCo-S7 | NiCo2O4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
“ | 350 |
| NiCo-S8 | NiCo2O4 | “ | “ | 400 |
2.3. Characterization of NiCo2O4 nano electrocatalysts
Several analytical techniques were used to analyze the materials' physical and chemical structure. X-ray diffraction (XRD) studies were carried out at room temperature in air using a PANalytical AERIS equipment to investigate the crystal structure and content. With the aid of scanning electron microscopy (SEM), the morphology of the samples was evaluated by using a TESCAN device. Using energy-dispersive spectroscopy (EDS) with a Hitachi SU3500 detector, it was possible to analyze the composition of NiCo2O4 and possible impurities in the synthesized samples. To examine the intricate structure and morphology, transmission electron microscopy (TEM) was carried out utilizing a JEM 2100 Plus by JEOL Ltd (Japan).2.4. Electrochemical characterization
Electrocatalyst measurements were conducted using an IVIUM Vertex.10A potentiostat workstation (Ivium Technologies B.V., Netherlands) in a 1 M KOH electrolyte solution. The working electrode consisted of the NiCo2O4 deposited onto the Ni-felt by just putting a bare Ni felt inside the reaction reactor, while a Hg/HgO electrode served as the reference electrode, and a platinum wire was utilized as the counter electrode. The polarization curves were recorded at a scan rate of 5 mV s−1. To determine the double-layer capacitance (Cdl), cyclic voltammograms (CVs) were obtained by varying the scan rates within a limited potential range. Electrochemical impedance spectroscopy (EIS) was performed over a frequency range spanning from 0.01 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The time-dependent potential response was measured over a period of 24 hours, specifically under a current of 10 mA. All the reported potentials were calibrated with respect to a reversible hydrogen electrode (RHE) and were adjusted for the 80% iR drop using the equation E(RHE) = E(Hg/HgO) + 0.927 V − iRs, where Rs represents the equivalent series resistance derived from fitting calculations.42
000 Hz. The time-dependent potential response was measured over a period of 24 hours, specifically under a current of 10 mA. All the reported potentials were calibrated with respect to a reversible hydrogen electrode (RHE) and were adjusted for the 80% iR drop using the equation E(RHE) = E(Hg/HgO) + 0.927 V − iRs, where Rs represents the equivalent series resistance derived from fitting calculations.42
3. Results and discussion
3.1. Mum flower-like NiCo2O4 nano electrocatalyst characterization
 | ||
| Fig. 3 SEM images of NiCo2O4 synthesised under different conditions: (a) NiCo-S2, (b) NiCo-S3, (c) NiCo-S4, (d) NiCo-S6 and (e) NiCo-S8. | ||
The results of an EDS examination are shown in Fig. 4 for two samples: (a) NiCo-S3 and (b) NiCo-S4. Ni, Co, and O elements have been identified using EDS in three different zones. It is evident that the percentage of Ni, Co, and O is nearer the NiCo2O4 theoretical composition, which confirms the accurate synthesis and dispersion of elements in these electrocatalysts.
The NiCo-S3 and NiCo-S4 are further examined by using TEM, which can show the dispersion of particles, grain size, and morphology in nanometer dimensions. In Fig. 5, Fig. 5a is the TEM image of NiCo-S3, and Fig. 5b is the TEM image of NiCo-S4 on the scale of 50 nm. For both samples, after measuring their dimension distribution, their particle size is between 20 nm and 45 nm. The lattice spacing for NiCo-S3 and NiCo-S4 is 0.249 and 0.246 nm, respectively, which is related to the (311) plane of NiCo2O4. Also, for NiCo-S4 the lattice spacing of 0.203 nm which is related to the (400) plane was observed. These results confirm further what was obtained by SEM and EDS analysis and endorse the purity of the synthesized samples in nanometer dimensions.
 | ||
| Fig. 6 OER property of NiCo-S3 and NiCo-S4 samples: (a) IR-corrected LSV plot, (b) overpotentials, (c) Tafel slopes, and (d) Nyquist plot. | ||
Moreover, by obtaining the Tafel slope based on backward LSV, the catalytic activity can be revealed by showing which electrode needs the lowest energy to be activated. In Fig. 6c, the Tafel slope for NiCo-S3, NiCo-S4, and NiFelt is depicted, which is 117, 79.7, and 130.4 mV dec−1, respectively. The Tafel slope for NiCo-S4 at 79.7 mV dec−1 shows that this electrode has very good kinetics and needs lower energy to be activated in comparison to NiCo-S3 and NiFelt. NiCo-S4 shows a good performance in terms of Tafel slope, too, if compared with similar studies. Kumar et al. computed a Tafel slope of 99 and 95 mV dec−1 for the two modified samples, while it was 104 mV dec−1 for the standard NiCo2O4.43 The Tafel slope obtained by Liu et al. in 1.0 M KOH on NiCo2O4 synthesized by co-electrodeposition on a graphite fiber support was 88 mV dec−1.47 Also, for NiCo2O4/CoNx-NMC (Nitrogen doped mesoporous carbon), NiCo2O4 on NiFoam prepared by the solvothermal method, and metallic NiCo nitride nanoparticle/NiCo2O4 nanoflake/graphite fibers synthesized via co-electrodeposition, the calculated Tafel slope was 99, 53, and 61 mV dec−1, respectively.45,46,48
Furthermore, at the open circuit voltage, electrochemical impedance spectroscopy (EIS) was carried out to investigate the system's intrinsic transport characteristics. To assess the findings, an equivalent circuit was obtained by data analysis. Fig. 6d illustrates that the resistance measured at high frequencies is related to the uncompensated solution resistance (RΩ), which is constant for all examined electrodes, at around 1.17 Ω. In particular, the semicircle diameter of the NiCo-S4 and NiCo-S3 electrodes, which is related to charge transfer resistance (Rct), decreased considerably when compared to bare NiFelt, especially in the mid and low-frequency ranges. The Rct for NiCo-S4, NiCo-S3, and NiFelt is 1.03, 1.16 and 3.516 Ω, respectively. This finding implies that the charge transfer kinetics of the NiCo-S4 and NiCo-S3 electrodes have been significantly improved and supports the results gathered through backward LSV and SEM analysis, which indicate that NiCo-S4 is an electrocatalyst with a lowered potential energy barrier for driving current through the catalyst, hence increasing OER efficiency. NiCo-S3 and NiCo-S4 also confirmed good behavior in this test. The EIS test done by Park et al. on NiCo2O4 obtained by direct growth via the hydrothermal method on graphite fiber gave an Rs of 2.768 Ω and an Rct of 1.814 Ω.49 Kaur et al. reported a NiCo2O4 obtained by co-precipitation, in which the Rs and Rct were 0.684 and 4.64 Ω, respectively.50
In order to have an estimation of the electrochemical active surface area (ECSA), double layer capacitance (Cdl) is calculated based on cyclic voltammetry (CV) in the region where there is no Faraday potential, and the results are given in Fig. 7. For all the electrodes, including NiFelt, the potential range is between 0 and 0.3 V vs. RHE, and different scan rates starting from 100 mV s−1 to 400 mV s−1 were used to extract Cdl by depicting the linear relationship between current density and scan rate. From Fig. 7d, the Cdl for NiCo-S3, NiCo-S4, and NiFelt is 2.43, 2.55 and 0.886 mF cm−2, respectively. These values were obtained by calculating the difference between cathodic and anodic peaks for each scan rate, which are plotted with a first-order model at 0.22 V vs. RHE, and the slope of each electrode corresponds to the Cdl. As the Cdl has a direct relationship with ECSA, an electrode that has higher Cdl will have higher ECSA, too. In this study, NiCo-S4 showed higher Cdl, which means that the electrochemical active surface area is higher when using this electrocatalyst in comparison to bare NiFelt and NiCo-S3. To have a comparison, Shuai et al. computed a Cdl of 2.6 mF cm−2 by performing a CV test in 1 M KOH electrolyte for NiCo2O4/RGO obtained by the precipitation method, which is near the value calculated for NiCo-S4 without using RGO.51
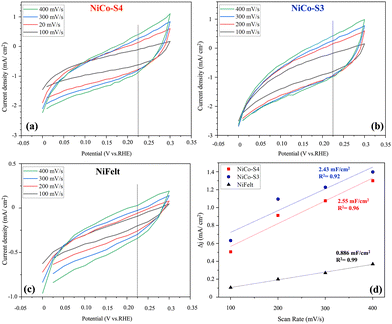 | ||
| Fig. 7 CV curves of electrodes: (a) NiCo-S4, (b) NiCo-S3, and (c) NiFelt; (d) double-layer capacitance. | ||
As an indicator of the intrinsic catalytic activity, turnover frequency (TOF) can be calculated to show the rate of oxygen produced per active site of the material based on the formula TOF = j*A/(4*F*m), in which j is the current density at a specific potential (A cm−2), A is the surface area of the electrode (1 cm−2), F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), and m is the amount of active material (mol cm−2).52,53 TOFOER for NiCo-S3 and NiCo-S4 is given in Table 2. As can be seen, the TOF for NiCo-S3 and NiCo-S4 is 1.69 and 2.51 (10−3 S−1), respectively, with almost 48% higher TOF for NiCo-S4, in line with the results obtained by LSV, Tafel slope and ECSA.
485 C mol−1), and m is the amount of active material (mol cm−2).52,53 TOFOER for NiCo-S3 and NiCo-S4 is given in Table 2. As can be seen, the TOF for NiCo-S3 and NiCo-S4 is 1.69 and 2.51 (10−3 S−1), respectively, with almost 48% higher TOF for NiCo-S4, in line with the results obtained by LSV, Tafel slope and ECSA.
| Name | Catalyst | Potential V. vs. RHE | Current density (mA cm−2) | TOF value (10−3 S−1) |
|---|---|---|---|---|
| NiCo-S3 | NiCo2O4 | 1.6 | 21.71 | 1.69 |
| NiCo-S4 | NiCo2O4 | 1.6 | 32.25 | 2.51 |
Electrocatalytic stability is another critical element in determining the usability and performance of the synthesized catalysts; the results are provided for the selected best OER electrodes in Fig. 8. The stability test was performed at 10 mA cm−2 over 24 h for NiCo-S4, and the result is shown in Fig. 8e. The potential started at around 1.56 V vs. RHE, and at the end, it slightly decreased to about 1.553 V, which means that the electrocatalyst for the OER is stable. By comparing stability results with other works, Gao et al. synthesized a hierarchical NiCo2O4 hollow microcuboid by the hydrothermal method, the durability test was done under galvanostatic conditions at a current density of 10 mA cm−2 for 32 h, and the overpotential registered an increment of 10 mV, which is in accordance with NiCo-S4.45 Liu et al. measured durability in a two electrode set-up by applying a current of 10 mA for 40 hours in 1.0 M KOH electrolyte for NiCo-nitrides/NiCo2O4/GF, and the test displayed a little degradation and an increase of potential while oxygen bubbles arose.48 For two NiCo2O4 samples (modified with grape juice) reported by Kumar et al. the stability test over 40 h by chronoamperometry at 20 mA cm−1 in 1.0 M KOH for the best sample displayed negligible losses.43
Moreover, backward LSV was performed at the end of the 24 h and compared with the initial one. The results are given in Fig. 8a. The backward LSV after the stability test showed higher activity than the initial one. This behavior could be related to the production of gas bubbles at the beginning of the process, which cannot diffuse from the pores of NiFelt to the solution media.
Furthermore, as the NiCo2O4 has super capacitance behavior, some energy is gathered to charge the electrode initially. Accordingly, more investigations were planned to verify the dimension of the bubbles attached to the electrodes as suggested by Huang et al., and also the gas bubble removal method with pressure swing for foam electrodes, which is suggested and evaluated by Bleeker et al. by using fast pressure swing of N2 gas under 1 second of time54,55 Comparing the decay behavior with other relevant work, Kumar et al. performed the LSV after the stability test, which showed that the current density at 1.6 V is almost stable at about 120 mA cm−2 for NiCo2O4 modified with grape juice.43
The Nyquist plot for NiCo-S4 before and after the 24-hour stability test was recorded, and the results are depicted in Fig. 8b. Charge transfer resistance after the stability test increased to 2.15 Ω while for fresh NiCo-S4 it was 1.03 Ω. Comparing the results of EIS and LSV after the stability test shows that as NiCo-S4 is deposited in all volumes of the NiFelt, it is active and produces oxygen at a rapid pace inside the pores, which requires some time to desorb these bubbles to the electrolyte and achieve desired potential. On the other hand, higher Rct after stability shows some detachments of this catalyst but not in a way to reduce the efficiency of electrochemical activity.
Besides the long-term durability of NiCo-S4, multi-potential as well as multi-current measurements were carried out for both NiCo-S4 and NiCo-S-3, and the results are presented in Fig. 8c and d. For chronopotentiometry (Fig. 8c), each step was performed for 100 s and started from 10 mA to 60 mA of current density. As can be seen, NiCo-S4 is more stable and reaches a lower potential, which means that it has lower resistance and higher electrochemical activity. On the other side, chronoamperometry was applied starting from 350 mV to 600 mV, and each step was carried out for 100 s, too. In this analysis, again, NiCo-S4 showed superior characteristics and achieved higher current when compared to NiCo-S3, and hence it was selected as the salient electrode for the OER process.
To have a better idea about the NiCo-S4 after the stability test, physicochemical analysis has been performed and the results are given in Fig. 9. The XRD analysis (Fig. 9a) showed no difference between the used and fresh NiCo-S4 electrocatalysts and aligned with the JCPDS No. 20-0781. Moreover, SEM analysis (Fig. 9b) showed that the mum-flower structure remained stable with small quantities of flower rods detached from this structure after the stability test but the material is NiCO2O4 and can be electrochemically active based on the results of the stability test.
4. Conclusions
In this work, eight samples of NiCo2O4 catalysts were synthesized by the hydrothermal method adopting different amounts of nucleating agent, and hydrothermal, and calcination temperatures. The study drove the selection of the two best samples first, from a morphological point of view: NiCo-S3 and NiCo-S4. The two samples showed a single-phase morphology with an urchin-like shape. TEM analyses displayed the outstanding result of obtained particle dimensions (20–45 nm). From the electrochemical tests, NiCo-S4 showed the most promising results: an overpotential of 327 mV at 10 mA cm−2, a Tafel slope of 79,7 mV dec−1, a low charge transfer resistance (Rct) of 1.03 Ω and a Cdl of 2,43 mF cm−2. The results displayed are meaningful and show the appropriate OER activity of this catalyst. Moreover, the stability test showed a retention of 99% of performances over 24 h. The LSV test after the stability test highlighted how performances slightly improved while the charge transfer resistance computed from EIS after the stability test doubled. From these results, it can be said that the electrode made with foam electrodes and felts needs a period to reach mass transport stability for removing bubbles. The study of the effects of the three key operating variables chosen as discriminating factors drove the selection of the best ones to synthesize the NiCo2O4 with the desired characteristics.Author contributions
Ataollah Nyati: conceptualization, investigation, formal analysis, visualization, writing – original draft, writing – review & editing. Arianna Moranda: validation, formal analysis, writing – review & editing. Juan Felipe Basbus: analysis, investigation. Ombretta Paladino: writing – reviewing and editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank Prof. Antonio Comite (Head of the Elemental lab at DCCI, Department of Chemistry of University of Genoa) and Mrs Laura Negretti who carried out TEM analyses at DCCI. The present work was funded by EU Next Generation - PNRR, M2C213.5, project NEMESI_ID: RSH2B_000002, and by the PhD grant FSE + 2021-2027, ESO 4.6 at the University of Genova, Italy.Notes and references
- T. Xu, Y. Wang, Y. Xue, J. Li and Y. Wang, Chem. Eng. J., 2023, 470, 144247 CrossRef CAS.
- D. Yu, X. Wan and B. Gu, Chemosphere, 2023, 323, 138182 CrossRef CAS PubMed.
- H. A. Miller, K. Bouzek, J. Hnat, S. Loos, C. I. Bernäcker, T. Weißgärber, L. Röntzsch and J. Meier-Haack, Sustainable Energy Fuels, 2020, 4, 2114–2133 RSC.
- D. Henkensmeier, M. Najibah, C. Harms, J. Žitka, J. Hnát and K. Bouzek, J. Electrochem. Energy Convers. Storage, 2021 DOI:10.1115/1.4047963.
- D. Gielen, F. Boshell, D. Saygin, M. D. Bazilian, N. Wagner and R. Gorini, Energy Strategy Rev., 2019, 24, 38–50 CrossRef.
- F. Van Der Linden, E. Pahon, S. Morando and D. Bouquain, J. Power Sources, 2023, 575, 233168 CrossRef CAS.
- S. Huang, Z. Yuan, M. Salla, X. Wang, H. Zhang, S. Huang, D. G. Lek, X. Li and Q. Wang, Energy Environ. Sci., 2023, 16, 438–445 RSC.
- G. Yang, Y. Zhu, Z. Hao, Y. Lu, Q. Zhao, K. Zhang and J. Chen, Adv. Mater., 2023, 35(33) DOI:10.1002/adma.202301898.
- S. Bonizzoni, D. Stucchi, T. Caielli, E. Sediva, M. Mauri and P. Mustarelli, ChemElectroChem, 2023, 10(6) DOI:10.1002/celc.202201077.
- P. Hota, A. Das and D. K. Maiti, Int. J. Hydrogen Energy, 2023, 48, 523–541 CrossRef CAS.
- L. Lu, Y. Zheng, R. Yang, A. Kakimov and X. Li, Mater. Today Chem., 2021, 21, 100488 CrossRef CAS.
- B. Guo, Y. Ding, H. Huo, X. Wen, X. Ren, P. Xu and S. Li, Nanomicro. Lett., 2023, 15, 57 CAS.
- L. Yang, J. Shui, L. Du, Y. Shao, J. Liu, L. Dai and Z. Hu, Adv. Mater., 2019, 31, 1804799 CrossRef PubMed.
- A. Moysiadou and X. Hu, J. Mater. Chem. A, 2019, 7, 25865–25877 RSC.
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet and X. Hu, J. Am. Chem. Soc., 2018, 140, 7748–7759 CrossRef CAS PubMed.
- H. Osgood, S. V. Devaguptapu, H. Xu, J. Cho and G. Wu, Nano Today, 2016, 11, 601–625 CrossRef CAS.
- J. X. Flores-Lasluisa, F. Huerta, D. Cazorla-Amorós and E. Morallón, Environ. Res., 2022, 214, 113731 CrossRef CAS PubMed.
- X.-M. Liu, X. Cui, K. Dastafkan, H.-F. Wang, C. Tang, C. Zhao, A. Chen, C. He, M. Han and Q. Zhang, J. Energy Chem., 2021, 53, 290–302 CrossRef CAS.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed.
- S. Liu, L. Hu, X. Xu, A. A. Al-Ghamdi and X. Fang, Small, 2015, 11, 4267–4283 CrossRef CAS PubMed.
- L. Hu, L. Wu, M. Liao, X. Hu and X. Fang, Adv. Funct. Mater., 2012, 22, 998–1004 CrossRef CAS.
- C. Peng, H. Liu, J. Chen, Y. Zhang, L. Zhu, Q. Wu, W. Zou, J. Wang, Z. Fu and Y. Lu, Appl. Surf. Sci., 2021, 544, 148897 CrossRef CAS.
- W. Sudarsono, S. Y. Tan, W. Y. Wong, F. S. Omar, K. Ramya, S. Mehmood, A. Numan, R. Walvekar and M. Khalid, J. Ind. Eng. Chem., 2023, 122, 1–26 CrossRef CAS.
- Z. Wu, X. Pu, Y. Zhu, M. Jing, Q. Chen, X. Jia and X. Ji, J. Alloys Compd., 2015, 632, 208–217 CrossRef CAS.
- D. Yadav, P. Singh and R. Prasad, Int. J. Hydrogen Energy, 2019, 44, 29057–29065 CrossRef CAS.
- M. Kaur, P. Chand and H. Anand, J. Energy Storage, 2022, 52, 104941 CrossRef.
- M. Kaur, P. Chand and H. Anand, Chem. Phys. Lett., 2022, 786, 139181 CrossRef CAS.
- S. Yan, S. Luo, M. Sun, Q. Wang, Y. Zhang and X. Liu, Int. J. Energy Res., 2021, 45, 20186–20198 CrossRef CAS.
- E. Abbasi, M. Haghighi, M. Shabani, A. Niyati and S. Mahboob, Mater. Today Sustainability, 2023, 24, 100580 CrossRef.
- B. Sriram, S. Kogularasu, S.-F. Wang and J.-K. Sheu, ACS Appl. Nano Mater., 2023, 6, 17593–17602 CrossRef CAS.
- J. I. Orege, J. Wei, Q. Ge and J. Sun, Nano Today, 2023, 51, 101914 CrossRef CAS.
- Y. Zhang, W. Zhang, J. Zhou, X. Li, W. Zhou, Y. Xie, J. Mao and K. Dai, J. Electrochem. Soc., 2023, 170, 090532 CrossRef.
- A. Niyati, M. Haghighi and M. Shabani, Mater. Today Sustainability, 2023, 24, 100478 CrossRef.
- H. Fu, L. Chen, Y. Shi, W. Kong, X. Zhang, J. Hou, H. Li, G. Wang, F. Yu and X. Guo, Electrochim. Acta, 2019, 320, 134581 CrossRef CAS.
- H. Fu, Y. Liu, L. Chen, Y. Shi, W. Kong, J. Hou, F. Yu, T. Wei, H. Wang and X. Guo, Electrochim. Acta, 2019, 296, 719–729 CrossRef CAS.
- M. Chatterjee, S. Saha, S. Das and S. K. Pradhan, J. Alloys Compd., 2020, 821, 153503 CrossRef CAS.
- S. Yadav, A. Sharma Ghrera and A. Devi, Mater. Today Proc., 2023, 74, 281–288 CrossRef CAS.
- R. Packiaraj, P. Devendran, K. S. Venkatesh, K. Mahendraprabhu and N. Nallamuthu, J. Energy Storage, 2021, 34, 102029 CrossRef.
- X. Zhang, F. Yang, H. Chen, K. Wang, J. Chen, Y. Wang and S. Song, Small, 2020, 16(44) DOI:10.1002/smll.202004188.
- G. Yang and S.-J. Park, Electrochim. Acta, 2018, 285, 405–414 CrossRef CAS.
- Z.-Q. Liu, Q.-Z. Xu, J.-Y. Wang, N. Li, S.-H. Guo, Y.-Z. Su, H.-J. Wang, J.-H. Zhang and S. Chen, Int. J. Hydrogen Energy, 2013, 38, 6657–6662 CrossRef CAS.
- W. Zheng, ACS Energy Lett., 2023, 8, 1952–1958 CrossRef CAS.
- S. Kumar, A. Tahira, M. Emo, B. Vigolo, A. Infantes-Molin, A. M. Alotaibi, S. F. Shaikh, A. Nafady and Z. H. Ibupoto, J. Energy Storage, 2023, 68, 107708 CrossRef.
- Z. Liu, H. Tan, D. Liu, X. Liu, J. Xin, J. Xie, M. Zhao, L. Song, L. Dai and H. Liu, Adv. Sci., 2019, 6(5) DOI:10.1002/advs.201801829.
- X. Gao, H. Zhang, Q. Li, X. Yu, Z. Hong, X. Zhang, C. Liang and Z. Lin, Angew. Chem., Int. Ed., 2016, 55, 6290–6294 CrossRef CAS PubMed.
- M.-F. Qiao, Y. Wang, L. Li, G.-Z. Hu, G.-A. Zou, X. Mamat, Y.-M. Dong and X. Hu, Rare Met., 2020, 39, 824–833 CrossRef CAS.
- Z. Liu, H. Tan, D. Liu, X. Liu, J. Xin, J. Xie, M. Zhao, L. Song, L. Dai and H. Liu, Adv. Sci., 2019, 6(5) DOI:10.1002/advs.201801829.
- Z. Liu, H. Tan, D. Liu, X. Liu, J. Xin, J. Xie, M. Zhao, L. Song, L. Dai and H. Liu, Adv. Sci. DOI:10.1002/advs.201801829.
- H. Park, B. H. Park, J. Choi, S. Kim, T. Kim, Y.-S. Youn, N. Son, J. H. Kim and M. Kang, Nanomaterials, 2020, 10, 1727 CrossRef CAS PubMed.
- M. Kaur, P. Chand and H. Anand, Chem. Phys. Lett., 2022, 786, 139181 CrossRef CAS.
- C. Shuai, Z. Mo, X. Niu, X. Yang, G. Liu, J. Wang, N. Liu and R. Guo, J. Mater. Sci., 2020, 55, 1627–1636 CrossRef CAS.
- Y. Cheng, P. Fu, X. Yang, Y. Zhang, S. Jin, H. Liu, Y. Shen, X. Guo and L. Chen, J. Mater. Chem. A, 2023, 11, 24764–24776 RSC.
- Y. Zheng, L. Wang, J. Pang, K. Sun, J. Hou, G. Wang, W. Guo and L. Chen, J. Colloid Interface Sci., 2023, 637, 85–93 CrossRef CAS PubMed.
- C. Huang, Z. Wang, Z. Yao, Y. Ma, F. Guo and L. Chai, Electrochim. Acta, 2024, 477, 143792 CrossRef CAS.
- J. Bleeker, C. van Kasteren, J. R. van Ommen and D. A. Vermaas, Int. J. Hydrogen Energy, 2024, 57, 1398–1407 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |