Open Access Article
Open Access ArticleStructural basis for Cu(II) metallocycle hexamer formation†
Matthew L.
Bracken
 *a,
Manuel A.
Fernandes
*a,
Manuel A.
Fernandes
 a,
Daniel
Wamwangi
a,
Daniel
Wamwangi
 bc and
Orde Q.
Munro
bc and
Orde Q.
Munro
 ad
ad
aMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, PO Wits 2050, Johannesburg, South Africa. E-mail: matthewbracken125@gmail.com
bSchool of Physics, University of the Witwatersrand, Wits 2050, Johannesburg, South Africa
cDSI-NRF Centre of Excellence in Strong Materials, University of the Witwatersrand, Wits 2050, Johannesburg, South Africa
dSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds LS2 9JT, UK
First published on 8th May 2024
Abstract
NNN bis-aryl amide pincer ligands may be designed to meet the structural requirements for Cu(II) metallocycle hexamer formation, giving supramolecular crystals containing solvent-accesible voids.
Introduction
Metal–organic frameworks are known for their applications in nanotechnology1,2 and these supermolecules may be constructed by design. Cu(II) metallocycles have been synthesized from various ligands and we introduce a few examples here to highlight requirements for metallocycle formation. We then find that the NNN bis-aryl amide pincer ligand meets these requirements and may be easily functionalized for crystal engineering. Metallocycle hexamers have been constructed from tripodal benzimidazole ligands3 where the cyclic coordination follows the pattern Cu(II)–N–C–N–C–C–O–Cu(II) and the bridging group involves a (1-H-benzimidazol-1-yl)acetic acid moiety. The flexibility of the tripod reduces steric clashes in the hexamer and the good π-accepting ability of the ligand allows the anionic acetate moiety to serve as a bridge between metal centres. These electronic requirements for metallocycle formation have been highlighted by contrasting pyrene-appended ligands.4,5 The pyrene-appended acylhydrazide ligand provides a single anionic donor while an axially coordinated nitrate provides charge balance and increased electron density at the Cu(II) centre. The acylhydrazide contains a carbonyl that is a good π-acceptor and thus serves as a bridge between copper centres allowing the metallocycle to form. However, the pyrene-appended hydrazone contains neither a carbonyl, nor a suitable σ-donor or π-acceptor moiety to serve as a bridge between metal centres, and hence metallocycle formation does not take place. However, well-balanced electronics are revealed in a pyrazolyl urea ligand,6 which contains two anionic σ-donors and a π-acceptor amide carbonyl that serves as the bridging moiety across the metallocycle hexamer. This cyclic coordination, Cu(II)–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oamide–Cu(II), follows the same pattern as the Cu(II) metallocycles studied in this work, and inspired the use of the previously reported NNN bis-aryl carboxamide pincer ligand7–9 for the construction of metallocycles. Due to facile functionalization of the bis-aryl rings, the carboxamide pincer ligand enables the construction, and engineering, of Cu(II) metallocycle hexamers.
Oamide–Cu(II), follows the same pattern as the Cu(II) metallocycles studied in this work, and inspired the use of the previously reported NNN bis-aryl carboxamide pincer ligand7–9 for the construction of metallocycles. Due to facile functionalization of the bis-aryl rings, the carboxamide pincer ligand enables the construction, and engineering, of Cu(II) metallocycle hexamers.
Results and discussion
The copper pincers presented here, Cu1–Cu3, all crystallized in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a single copper metallocycle in the unit cell and the asymmetric unit described by three monomers of the hexamer. The hexanuclear Cu(II) macrocycle coordination patterns are shown in Fig. 1 and the thermal ellipsoids are shown in Fig. S1.1–S1.3 (ESI†). The coordination follows the sequence Cu(II)–N–C
with a single copper metallocycle in the unit cell and the asymmetric unit described by three monomers of the hexamer. The hexanuclear Cu(II) macrocycle coordination patterns are shown in Fig. 1 and the thermal ellipsoids are shown in Fig. S1.1–S1.3 (ESI†). The coordination follows the sequence Cu(II)–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oamide–Cu(II) where the amide carbonyl of the complex acts a bridging ligand between copper centres, engendering S2 symmetry for each supermolecule. The mean plane of each metallocycle lies in the plane of the six nitrogens that make up the coordination pattern and three alternating Cu(II) centres lie in a plane above, and below, the mean plane. The plane-to-plane distance between the Cu(II) triads averages 1.35 ± 0.25 Å and the metallocycle mean plane bisects this interplanar distance. The inner coordination sphere of the Cu(II) centres adopts square pyramidal geometry where the tridentate ligand chelates Cu(II) through the pyridine nitrogen (Npyr) and two amide nitrogens (Namide) while exogenous ligands coordinate to make up the square plane as well as occupy the axial position. The copper-based atomic 3dx2−y2 orbital lies in the square plane where the Cartesian x-axis is colinear with the Npyr–Cu bond and the y-axis is colinear with the Namide–Cu–Namide bond as shown in Fig. 2a. The bond lengths between Cu(II) and the neutral donor atoms Npyr and Ocarbonyl are 1.91 ± 0.01 and 1.92 ± 0.02 Å, respectively, while the anionic Namide donors have bond lengths to Cu(II) that average 2.04 ± 0.02 Å. The strong interelectronic repulsions between the metal ion and the four donor atoms within the square plane raise the energy of the 3dx2−y2 orbital and promote electronic delocalization. This is reflected in the bond lengths of the amide carbonyls that make up the chelate ring and serve as bridging ligands between metal centres.
Oamide–Cu(II) where the amide carbonyl of the complex acts a bridging ligand between copper centres, engendering S2 symmetry for each supermolecule. The mean plane of each metallocycle lies in the plane of the six nitrogens that make up the coordination pattern and three alternating Cu(II) centres lie in a plane above, and below, the mean plane. The plane-to-plane distance between the Cu(II) triads averages 1.35 ± 0.25 Å and the metallocycle mean plane bisects this interplanar distance. The inner coordination sphere of the Cu(II) centres adopts square pyramidal geometry where the tridentate ligand chelates Cu(II) through the pyridine nitrogen (Npyr) and two amide nitrogens (Namide) while exogenous ligands coordinate to make up the square plane as well as occupy the axial position. The copper-based atomic 3dx2−y2 orbital lies in the square plane where the Cartesian x-axis is colinear with the Npyr–Cu bond and the y-axis is colinear with the Namide–Cu–Namide bond as shown in Fig. 2a. The bond lengths between Cu(II) and the neutral donor atoms Npyr and Ocarbonyl are 1.91 ± 0.01 and 1.92 ± 0.02 Å, respectively, while the anionic Namide donors have bond lengths to Cu(II) that average 2.04 ± 0.02 Å. The strong interelectronic repulsions between the metal ion and the four donor atoms within the square plane raise the energy of the 3dx2−y2 orbital and promote electronic delocalization. This is reflected in the bond lengths of the amide carbonyls that make up the chelate ring and serve as bridging ligands between metal centres.
The amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O that forms part of the chelate ring, but is not a bridging group, has an average bond length of 1.24 ± 0.01 Å. However, when the amide C
O that forms part of the chelate ring, but is not a bridging group, has an average bond length of 1.24 ± 0.01 Å. However, when the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O acts as bridging group and forms a Cu–Ocarbonyl bond, the bond length increases to an average of 1.27 ± 0.01 Å for Cu1–Cu3. This increase in C
O acts as bridging group and forms a Cu–Ocarbonyl bond, the bond length increases to an average of 1.27 ± 0.01 Å for Cu1–Cu3. This increase in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond length is due to back-donation of electron density from the 3dx2−y2 into a vacant σ* molecular orbital on the carbonyl, thereby decreasing Coulombic repulsion within the metal-based orbital. There is additional overlap between the atomic 3dxy orbital and π* molecular orbitals of the carbonyl, resulting in further bond length elongation for the bridging moiety. The orbital analysis has been presented in Fig. 2. The inner coordination sphere of Cu1 is shown in Fig. 2a, and the only symmetry element is a vertical mirror plane, σV. The mirror plane defines the Cartesian z-axis as the principal z-axis is collinear with the highest symmetry element. As a result, the x-axis is perpendicular to the z-axis and collinear with highest number of identical atoms, i.e., the two Namide. That leaves the y-axis to pass through Npyr and Ocarbonyl. The bridging carbonyl interaction is then shown in Fig. 2b. The bonding involves predominately σ-overlap of the metal-based 3dx2−y2 atomic orbital and σ* molecular orbital of the carbonyl. There is additional bonding involving π-overlap of the metal-based 3dxy atomic orbital and the π* molecular orbitals of the carbonyl. This donation of electron density from the metal-based orbitals into antibonding orbitals of the carbonyl causes the bond length to increase for the bridging moiety. This was confirmed with DFT shown in Fig. S2.1–S2.4 (ESI†), as the spin-density plot shows significant 3dx2−y2 character as well as σ-overlap between copper and the carbonyl. The antibonding π-overlap between carbonyl π* molecular orbitals and the atomic 3dxy orbital was observed for the LUMOs of the asymmetric unit for Cu1–Cu3.
O bond length is due to back-donation of electron density from the 3dx2−y2 into a vacant σ* molecular orbital on the carbonyl, thereby decreasing Coulombic repulsion within the metal-based orbital. There is additional overlap between the atomic 3dxy orbital and π* molecular orbitals of the carbonyl, resulting in further bond length elongation for the bridging moiety. The orbital analysis has been presented in Fig. 2. The inner coordination sphere of Cu1 is shown in Fig. 2a, and the only symmetry element is a vertical mirror plane, σV. The mirror plane defines the Cartesian z-axis as the principal z-axis is collinear with the highest symmetry element. As a result, the x-axis is perpendicular to the z-axis and collinear with highest number of identical atoms, i.e., the two Namide. That leaves the y-axis to pass through Npyr and Ocarbonyl. The bridging carbonyl interaction is then shown in Fig. 2b. The bonding involves predominately σ-overlap of the metal-based 3dx2−y2 atomic orbital and σ* molecular orbital of the carbonyl. There is additional bonding involving π-overlap of the metal-based 3dxy atomic orbital and the π* molecular orbitals of the carbonyl. This donation of electron density from the metal-based orbitals into antibonding orbitals of the carbonyl causes the bond length to increase for the bridging moiety. This was confirmed with DFT shown in Fig. S2.1–S2.4 (ESI†), as the spin-density plot shows significant 3dx2−y2 character as well as σ-overlap between copper and the carbonyl. The antibonding π-overlap between carbonyl π* molecular orbitals and the atomic 3dxy orbital was observed for the LUMOs of the asymmetric unit for Cu1–Cu3.
Our DFT calculations show significant 3dx2−y2 character in the square plane of the Cu(II) centre as well as spin density localized on the bridging carbonyl which is crucial to metallocycle formation. The delocalization of the paramagnetic electron density leads to the emergence of exchange interactions over the three bridging atoms between the metal centres. Cu1 shows low temperature antiferromagnetic coupling at T < 9.3 K at a field strength of 0.1 T. Cu2 shows competing antiferromagnetic and paramagnetic phases below the Néel temperature (T < 8 K). Cu3 is diamagnetically ordered under an applied magnetic field at room temperature. At 100 K, Cu3 undergoes a magnetic moment reversal from diamagnetic to paramagnetic with complete paramagnetism at 2 K. These magnetic measurements are presented in Fig. S3.1–S3.6 (ESI†). Exchange interactions and magnetic ordering have been reported to influence coordination-based solid-state architecture.10–12 Particularly, hexanuclear copper-based exchange coupling has been compared to the bonding in benzene.13 The DFT spin-density plot is consistent with magnetic ordering and shows that paramagnetic electron density is delocalized into the aromatic rings of the ligand and reflects the energy lowering of the metal-based orbitals as a result of the nephelauxetic effect. The coordination pattern Cu(II)–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oamide–Cu(II) that drives metallocycle formation is dominated by 3dx2−y2 character as shown in S3 (ESI†). The Cu(II) based atomic 3dx2−y2 orbital is instrumental to metallocycle formation and the resulting coordinating forces impart significant tensional integrity to the cyclic assembly.
Oamide–Cu(II) that drives metallocycle formation is dominated by 3dx2−y2 character as shown in S3 (ESI†). The Cu(II) based atomic 3dx2−y2 orbital is instrumental to metallocycle formation and the resulting coordinating forces impart significant tensional integrity to the cyclic assembly.
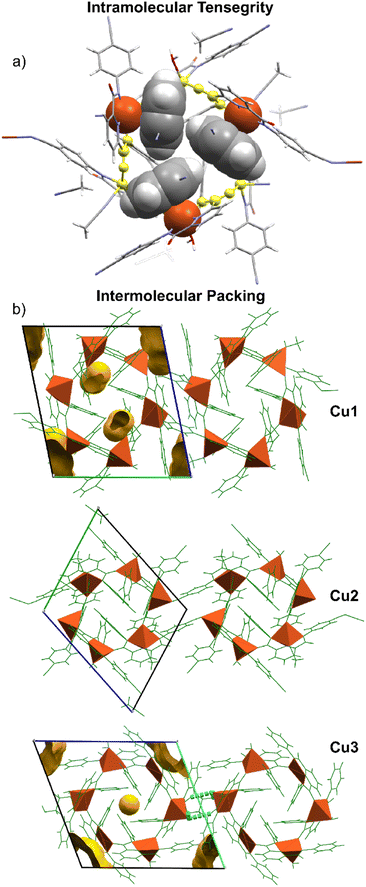
The metallocycle hexamer is held together by a synergistic combination of intramolecular forces that facilitate the tensegrity observed for the supramolecular assembly. The term “tensegrity” was originally coined by the architect, Buckminster Fuller. These forces include coordinative bonding through the cyclic structure, magnetic ordering, van der Waals forces, C–H⋯π14 and d⋯π15 interactions. The tensegrity of the copper metallocycles has been highlighted in Fig. 3 as well as the supramolecular packing. The strongest intramolecular forces holding the cyclic structure together are the coordinating bonds that result in the pattern Cu(II)–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oamide–Cu(II). The short bond lengths Cu–Ocarbonyl indicate that the negative charge from the amidate nitrogen has been delocalized into the carbonyl oxygen making it a better σ-donor. Significant C–H⋯π and d⋯π interactions are observed to stabilize the cyclic assembly and impart remarkable tensegrity. This self-recognition and intramolecular stabilisation have been shown in Fig. S4.1–S4.4 (ESI†). The bonding exists between the Cu(II) triad that lies above the mean plane of the metallocycle, and these C–H⋯π and d⋯π interactions are inverted about the centroid of the hexamer and exist between the Cu(II) triad that lies below the mean plane. The aromatic rings that serve as d⋯π acceptors on one face of the aromatic ring, function as C–H⋯π acceptors on the reflected face. Notably, the atom-to-plane distances for the d⋯π interactions average 2.88 ± 0.09 Å which indicates that although the bonding may be weak, it is significant. Also noteworthy is the fact that d⋯π bond angles vary between about 50° and 60°, suggesting that atomic 3dxz and 3dyz orbitals are principally involved with d–π overlap. Hence, numerous forces stabilize the supramolecular assembly through intramolecular interactions. There are significantly fewer intermolecular interactions that facilitate packing of the cyclic hexamers in the lattice that result in void space (Fig. S5.1–S5.3, ESI†).
Oamide–Cu(II). The short bond lengths Cu–Ocarbonyl indicate that the negative charge from the amidate nitrogen has been delocalized into the carbonyl oxygen making it a better σ-donor. Significant C–H⋯π and d⋯π interactions are observed to stabilize the cyclic assembly and impart remarkable tensegrity. This self-recognition and intramolecular stabilisation have been shown in Fig. S4.1–S4.4 (ESI†). The bonding exists between the Cu(II) triad that lies above the mean plane of the metallocycle, and these C–H⋯π and d⋯π interactions are inverted about the centroid of the hexamer and exist between the Cu(II) triad that lies below the mean plane. The aromatic rings that serve as d⋯π acceptors on one face of the aromatic ring, function as C–H⋯π acceptors on the reflected face. Notably, the atom-to-plane distances for the d⋯π interactions average 2.88 ± 0.09 Å which indicates that although the bonding may be weak, it is significant. Also noteworthy is the fact that d⋯π bond angles vary between about 50° and 60°, suggesting that atomic 3dxz and 3dyz orbitals are principally involved with d–π overlap. Hence, numerous forces stabilize the supramolecular assembly through intramolecular interactions. There are significantly fewer intermolecular interactions that facilitate packing of the cyclic hexamers in the lattice that result in void space (Fig. S5.1–S5.3, ESI†).
The Cu(II) NNN amide pincers presented here all adopt hexameric cyclic coordination in the solid-state. A CSD16 search of the general amide pincer scaffold yielded 78 unique results, and these have been presented in Table S1 (ESI†). The table shows the search query submitted to the CSD, the coordination number at the metal centre, the charge on the copper ion, and the substitution on the aryl rings. The search filtered out structures with R1 > 7.5%. The 78 copper NNN amide pincers contain only 5 structures (6.4%) where the aryl ring has been substituted on the 3-position. All 5 of these structures contain dinucleating ligands. Most structures (90%) contain ligands where the aryl ring has been substituted on the 2- and 6-positions or 2-,4-and 6-positions. None of the structures adopt the metallocycle architecture observed for the Cu(II) NNN amide pincers presented in this work. It is suspected that metallocycle formation requires the aryl ring to be substituted in the 3- or 4-position so that the substituents do not sterically block metallocycle formation. The stereoelectronic requirements for hexamer formation are presented in Fig. 4. The metallocycle follows the bonding pattern Cu(II)–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oamide–Cu(II) and so all 78 CSD structures possess the necessary coordination pattern yet none of them form cyclic supermolecules. It is suggested here that this is because 90% of structures are substituted in the 2-,6-position and the substituents sterically block coordination from one copper pincer to another. When only the 2-position is substituted, the CSD structures do not adopt a hexameric geometry because there is intramolecular self-recognition between the substituent and the Cu(II) centre, or alternatively, the complex is anionic and electrostatic forces dominate the solid-state architecture. The structures that possess no substitution on the aryl rings do not form cyclic supermolecules because they are all anionic. The CSD structures with aryl rings substituted in the 3-position are dinucleating structures and hence the flexibility of these ligands is reduced such that metallocycle formation does not occur.
Oamide–Cu(II) and so all 78 CSD structures possess the necessary coordination pattern yet none of them form cyclic supermolecules. It is suggested here that this is because 90% of structures are substituted in the 2-,6-position and the substituents sterically block coordination from one copper pincer to another. When only the 2-position is substituted, the CSD structures do not adopt a hexameric geometry because there is intramolecular self-recognition between the substituent and the Cu(II) centre, or alternatively, the complex is anionic and electrostatic forces dominate the solid-state architecture. The structures that possess no substitution on the aryl rings do not form cyclic supermolecules because they are all anionic. The CSD structures with aryl rings substituted in the 3-position are dinucleating structures and hence the flexibility of these ligands is reduced such that metallocycle formation does not occur.
The coordination mode of the Cu(II) NNN amide pincer has been presented in Fig. 4 with the general requirements that permit metallocycle hexamer formation. Firstly, the ligand must be suitably functionalized to avoid steric repulsions. The copper complex must also be neutral and have an open face that is available for coordination by a neighbouring monomer. The variable geometry of the copper ion permits supramolecular coordination as bridging groups may adopt favourable conformations around the metal centre. The metallocycles discussed here contain the Cu(II) ion with square pyramidal,6,17 trigonal bipyramidal3 and octahedral4 geometries depending on the ligand's steric requirements and the metal centre's electronic requirements.
The pincers presented here coordinate Cu(II) via anionic amidate donors which increase electron density at the metal centre through σ-donation. The interelectronic repulsion is relieved at the metal centre upon coordination of a σ*- or π*-acceptor that functions as a bridging group. Therefore, electronic requirements of the metal facilitate metallocycle formation as electron density is balanced through a synergistic interplay of σ- and π-effects. Hence, a general coordination pattern for the copper hexamers may be presented as Cu(II)–Xnπ*–Cu(II), where X represents n number of any bridging atoms and the bridging group must be a π-acceptor. This depends on the electronics at the Cu(II) centre, as seen in the structure reported by Fu et al.3 When the ligand coordinates Cu(II) through only one anionic donor, and the ligand is a good π-acceptor, the electron deficient metal centre may coordinate to a bridging σ-donor moiety. Hence, the general coordination pattern may be presented as Cu(II)–Xnσ–Cu(II), where X represents n number of any bridging atoms that contains a good σ-donor. Ultimately, electron density at the metal centre must be appropriately balanced through σ- and π-effects for the general Cu(II)–Xn–Cu(II) cyclic coordination pattern to form. The size of the metallocycle follows the simple arithmetic sequence 6 + 6n, where n is the number of bridging atoms. Hence, an 18-membered metallocycle is formed when two bridging atoms are present, and 24-membered when three bridging atoms are present, and so on.
Experimental
Materials and instrumentation
All chemicals were purchased from Sigma-Aldrich and used without further purification. All solvents were purchased from Merck, and when necessary, freshly distilled. The copper metalation reactions were carried out using inhibitor-free THF. NMR data were acquired on Bruker Avance III 400 spectrometer at a frequency of 400 MHz (1H) and 100 MHz (13C) using a 5 mm BBOZ probe. All proton and carbon chemical shifts are quoted relative to the relevant solvent reference signal. Spectra were analysed with MestReNova (Version 12.0.0-20080). Data were acquired at a temperature of 300 K. Infrared spectra of pure powder or crystalline samples were recorded using a Bruker Alpha FTIR spectrometer using a Bruker Platinum diamond Attenuated Total Reflectance sampling accessory. OPUS software (Version 7.5) was used to analyse spectra. The spectra were acquired over 32 scans at 20 °C with a spectral resolution of 4 cm−1. X-ray intensity data were collected on a Bruker D8 Venture Bio PHOTON III diffractometer with a Mo Kα Iμ DIAMOND source (50 kV, 1.4 mA). The collection method involved ω- and ϕ-scans with 1536 × 1024 bit data frames. The unit cell and full data set were collected using APEX4;18 SAINT was used to integrate the data, and SADABS was used to make empirical absorption corrections and scale the data. Space group assignments were made using XPREP on all compounds. Using Olex2,19 the crystal structures were solved with the ShelXT20 structure solution program using Intrinsic Phasing and refined with the ShelXL21 refinement package using Least Squares minimization. Non-hydrogen atoms were first refined isotropically followed by anisotropic refinement by full-matrix least-squares calculations based on F2.Synthesis and spectroscopy
1H NMR: (400 MHz, DMSO-d6, 300 K) [δ, ppm] 11.27 (s, 2H), 8.48–8.42 (m, 4H), 8.36 (dd, J = 8.6, 6.9 Hz, 1H), 8.24 (dq, J = 7.8, 2.7 Hz, 2H), 7.75–7.65 (m, 4H). 13C NMR: (100 MHz, DMSO-d6, 300 K) [δ, ppm] 162.53, 148.78, 140.80, 139.35, 130.80, 128.40, 126.32, 126.15, 124.12, 119.15, 112.10. FTIR (cm−1): 3288.66 (m, asymmetric N–H stretch), 3086.81 (w, aromatic C–H stretch), 2230.18 (m, symmetric C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch), 1667.75 (s, asymmetric C
N stretch), 1667.75 (s, asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch; N–H wag), 1534.30 (s, asymmetric N–H wag).
O stretch; N–H wag), 1534.30 (s, asymmetric N–H wag).
1H NMR: (400 MHz, DMSO-d6, 300 K) [δ, ppm] 11.26 (s, 2H), 8.41 (d, J = 8.0 Hz, 2H), 8.31 (t, J = 7.9 Hz, 1H), 8.17 (d, J = 8.8 Hz, 4H), 7.91 (d, J = 8.7 Hz, 4H). 13C NMR: (100 MHz, DMSO-d6, 300 K) [δ, ppm] 160.40, 146.58, 140.65, 138.56, 131.59, 124.27, 118.98, 117.32, 116.39. FTIR (cm−1): 3340.51 (m, asymmetric N–H stretch), 3099.14 (w, aromatic C–H stretch), 2217.87 (m, symmetric C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch), 1693.08 (s, asymmetric C
N stretch), 1693.08 (s, asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch; N–H wag), 1581.22 (s, asymmetric N–H wag).
O stretch; N–H wag), 1581.22 (s, asymmetric N–H wag).
1H NMR: (400 MHz, DMSO-d6, 300 K) [δ, ppm] 11.12 (s, 2H), 8.41 (d, J = 8.2 Hz, 2H), 8.31 (t, J = 8.0 Hz, 1H), 7.96 (d, J = 8.0 Hz, 4H), 7.46 (d, J = 7.5 Hz, 4H), 7.20 (t, J = 7.4 Hz, 2H). 13C NMR: (100 MHz, DMSO-d6, 300 K) [δ, ppm] 162.19, 149.40, 140.40, 138.56, 129.22, 125.80, 124.85, 121.64. FTIR (cm−1): 3268.21 (m, asymmetric N–H stretch), 2803.26 (w, aromatic C–H stretch), 1671.83 (s, asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch; N–H wag), 1599.41 (s, asymmetric N–H wag).
O stretch; N–H wag), 1599.41 (s, asymmetric N–H wag).
Cu1: H2L1 (101 mg, 0.275 mmol) was dissolved in 50 mL of tetrahydrofuran (THF) before adding Cu(OAc)2·H2O (186 mg, 0.929 mmol) to the mixture. Instantly, a light-green precipitate began to form. The reaction was refluxed at 50 °C open to the air for 24 hours. The mixture was then allowed to cool to room temperature before filtering under vacuum. The isolated green powder was then washed with 10 mL of THF to yield Cu1·H2O, (59.8 mg, 49%). The copper chelate may be crystalized by evaporation from a mixture of methanol (MeOH) and ethyl acetate (EtOAc) over 3 weeks at ambient temperatures. Similar crystals can be grown by vapour diffusion of diethyl ether (Et2O) into a saturated MeOH solution containing Cu1 over 2–4 weeks at ambient temperature, 4 °C and −20 °C.
FTIR (cm−1): 3626.76 (w, O–H stretch), 3066.06 (w, aromatic C–H stretch), 2226.17 (s, nitrile stretch), 1626.57 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch). Crystal data: C128H78Cu6N30O16, 1.5[CH3OH] (M = 2721.50 g mol−1): triclinic, space group P
O stretch). Crystal data: C128H78Cu6N30O16, 1.5[CH3OH] (M = 2721.50 g mol−1): triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 12.8621(7) Å, b = 14.6778(8) Å, c = 17.1404(9) Å, α = 76.085(2)°, β = 70.053(2)°, γ = 81.249(2)°, V = 2943.8(3) Å3, Z = 1, T = 173.0 K, μ(MoKα) = 1.147 mm−1, Dcalc = 1.535 g cm−3, 343
(no. 2), a = 12.8621(7) Å, b = 14.6778(8) Å, c = 17.1404(9) Å, α = 76.085(2)°, β = 70.053(2)°, γ = 81.249(2)°, V = 2943.8(3) Å3, Z = 1, T = 173.0 K, μ(MoKα) = 1.147 mm−1, Dcalc = 1.535 g cm−3, 343![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382 reflections measured (4.23° ≤ 2Θ ≤ 51.992°), 11
382 reflections measured (4.23° ≤ 2Θ ≤ 51.992°), 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 unique (Rint = 0.1174, Rsigma = 0.0282) which were used in all calculations. The final R1 was 0.0361 (I > 2σ(I)) and wR2 was 0.0873 (all data). CCDC 2340637.†
552 unique (Rint = 0.1174, Rsigma = 0.0282) which were used in all calculations. The final R1 was 0.0361 (I > 2σ(I)) and wR2 was 0.0873 (all data). CCDC 2340637.†
Cu2: H2L2 (113 mg, 0.308 mmol) was dissolved in 50 mL of THF before adding Cu(OAc)2·H2O (154 mg, 0.771 mmol) to the mixture. Instantly, a green precipitate began to form. The reaction was refluxed at 50 °C open to the air for 24 hours. The mixture was then allowed to cool to room temperature before filtering under vacuum. The isolated green powder was then washed with 10 mL of THF to yield Cu2·H2O, (59.1 mg, 45%). The copper chelate was crystalized by vapour diffusion of Et2O into a saturated MeCN solution containing Cu2 over 3 weeks at ambient temperature.
FTIR (cm−1): 3396.60 (w, O–H stretch), 3090.05 (w, aromatic C–H stretch), 2223.05 (s, nitrile stretch), 1625.64 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch). Crystal data: C130H76Cu6N32O14, 4[C2H3N] (M = 2855.68 g mol−1): triclinic, space group P
O stretch). Crystal data: C130H76Cu6N32O14, 4[C2H3N] (M = 2855.68 g mol−1): triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 14.4764(11) Å, b = 15.1477(11) Å, c = 16.4380(10) Å, α = 106.667(2)°, β = 95.920(3)°, γ = 110.424(3)°, V = 3152.1(4) Å3, Z = 1, T = 173.0 K, μ(MoKα) = 1.075 mm−1, Dcalc = 1.504 g cm−3, 162
(no. 2), a = 14.4764(11) Å, b = 15.1477(11) Å, c = 16.4380(10) Å, α = 106.667(2)°, β = 95.920(3)°, γ = 110.424(3)°, V = 3152.1(4) Å3, Z = 1, T = 173.0 K, μ(MoKα) = 1.075 mm−1, Dcalc = 1.504 g cm−3, 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 reflections measured (4.062° ≤ 2Θ ≤ 56.86°), 15
869 reflections measured (4.062° ≤ 2Θ ≤ 56.86°), 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772 unique (Rint = 0.1107, Rsigma = 0.0649) which were used in all calculations. The final R1 was 0.0482 (I > 2σ(I)) and wR2 was 0.1261 (all data). CCDC 2340638.†
772 unique (Rint = 0.1107, Rsigma = 0.0649) which were used in all calculations. The final R1 was 0.0482 (I > 2σ(I)) and wR2 was 0.1261 (all data). CCDC 2340638.†
Cu3: H2L3 (112 mg, 0.353 mmol) was dissolved in 50 mL of THF before adding Cu(OAc)2·H2O (176 mg, 0.882 mmol) to the mixture. Instantly, a green precipitate began to form. The reaction was refluxed at 50 °C open to the air for 24 hours. The mixture was then allowed to cool to room temperature before filtering under vacuum. The isolated green powder was then washed with 10 mL of THF to yield Cu3·H2O, (40.0 mg, 30%). The copper chelate was crystalized by vapour diffusion of Et2O into a saturated MeOH solution containing Cu3 and a drop of ethylene glycol over 2 weeks at ambient temperature.
FTIR (cm−1): 2854.32 (w, aromatic C–H stretch), 1769.46 (w, aqua O–H wag), 1629.90 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch). Crystal data: C114H86Cu6N18O16, C2H6O2, 5[CH3OH] (M = 2567.52 g mol−1): triclinic, space group P
O stretch). Crystal data: C114H86Cu6N18O16, C2H6O2, 5[CH3OH] (M = 2567.52 g mol−1): triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 10.8153(11) Å, b = 17.0091(16) Å, c = 17.4196(16) Å, α = 106.568(5)°, β = 100.629(5)°, γ = 107.750(5)°, V = 2793.0(5) Å3, Z = 1, T = 173.0 K, μ(MoKα) = 1.204 mm−1, Dcalc = 1.526 g cm−3, 94
(no. 2), a = 10.8153(11) Å, b = 17.0091(16) Å, c = 17.4196(16) Å, α = 106.568(5)°, β = 100.629(5)°, γ = 107.750(5)°, V = 2793.0(5) Å3, Z = 1, T = 173.0 K, μ(MoKα) = 1.204 mm−1, Dcalc = 1.526 g cm−3, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 reflections measured (2.554° ≤ 2Θ ≤ 45°), 7314 unique (Rint = 0.3031, Rsigma = 0.1282) which were used in all calculations. The final R1 was 0.0681 (I > 2σ(I)) and wR2 was 0.1446 (all data). CCDC 2340639.†
743 reflections measured (2.554° ≤ 2Θ ≤ 45°), 7314 unique (Rint = 0.3031, Rsigma = 0.1282) which were used in all calculations. The final R1 was 0.0681 (I > 2σ(I)) and wR2 was 0.1446 (all data). CCDC 2340639.†
Conclusions
MOFs that contain void space have industrially and globally relevant applications. We have shown that novel NNN bis-aryl Cu(II) pincer chelates may be used as building blocks for the supramolecular construction of porous MOFs. The structural basis for Cu(II) metallocycle formation has been delineated and the facile functionalization of the carboxamide ligand enables the engineering of these solid-state structures.Author contributions
M. L. B. performed all experimental work, writing, and concept. M. A. F. was crystallographer and editor. D. W. collected and processed magnetic measurements. O. Q. M. conceptualized, directed, and funded the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is based on research supported by the South African Research Chairs Initiative of the Department of Science and Innovation (DSI) and National Research Foundation (NRF) of South Africa (Grant No. 64799, OQM). The authors thank WITS University and the NRF for funding to purchase a dual-wavelength Bruker D8 Venture X-ray diffractometer (Grant No. 129920, OQM). We would like to further thank the NRF for funding through the NEP program (grant number UID: 116181). We also thank the Centre for High Performance Computing (Project CHEM1065, CHPC, Cape Town) for access to supercomputing infrastructure.Notes and references
- J. Yang, H. Wang, J. Liu, M. Ding, X. Xie, X. Yang, Y. Peng, S. Zhou, R. Ouyang and Y. Miao, RSC Adv., 2021, 11, 3241–3263 RSC.
- N. Hanikel, M. S. Prévot and O. M. Yaghi, Nat. Nanotechnol., 2020, 15, 348–355 CrossRef CAS PubMed.
- L. Fu, Y. Liu, M. Pan, X.-J. Kuang, C. Yan, K. Li, S.-C. Wei and C.-Y. Su, J. Mater. Chem. A, 2013, 1, 8575 RSC.
- S. M. Hossain, V. Prakash, P. Mamidi, S. Chattopadhyay and A. K. Singh, RSC Adv., 2020, 10, 3646–3658 RSC.
- S. M. Hossain, G. K. Dam, S. Mishra and A. K. Singh, New J. Chem., 2020, 44, 15186–15194 RSC.
- G. O. Lloyd and J. W. Steed, Chem. Commun., 2014, 50, 1426–1428 RSC.
- M. Ray, D. Ghosh, Z. Shirin and R. Mukherjee, Inorg. Chem., 1997, 36, 3568–3572 CrossRef CAS PubMed.
- A. K. Patra and R. Mukherjee, Inorg. Chem., 1999, 38, 1388–1393 CrossRef CAS.
- A. K. Patra, M. Ray and R. Mukherjee, Inorg. Chem., 2000, 39, 652–657 CrossRef CAS PubMed.
- A. S. Lytvynenko, S. V. Kolotilov, O. Cador, S. Golhen, L. Ouahab and V. V. Pavlishchuk, New J. Chem., 2011, 35, 2179 RSC.
- S. Giri, S. Biswas, M. G. B. Drew, A. Ghosh and S. K. Saha, Inorg. Chim. Acta, 2011, 368, 152–156 CrossRef CAS.
- I.-R. Jeon and R. Clérac, Dalton Trans., 2012, 41, 9569 RSC.
- A. M. Toader, M. C. Buta, F. Cimpoesu, A.-I. Toma, C. M. Zalaru, L. O. Cinteza and M. Ferbinteanu, Chemistry, 2021, 3, 411–439 CrossRef CAS.
- M. Brandl, M. S. Weiss, A. Jabs, J. Sühnel and R. Hilgenfeld, J. Mol. Biol., 2001, 307, 357–377 CrossRef CAS PubMed.
- D. L. Lichtenberger, S. K. Renshaw and R. M. Bullock, J. Am. Chem. Soc., 1993, 115, 3276–3285 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- T. Higa, M. Moriya, Y. Shimazaki, T. Yajima, F. Tani, S. Karasawa, M. Nakano, Y. Naruta and O. Yamauchi, Inorg. Chim. Acta, 2007, 360, 3304–3313 CrossRef CAS.
- APEX4version 2021.4-1 Data Collection Software Which Includes SAINT Version 8.40B, SADABS-2016/2, and XPREP Version 2014/2. Bruker AXS Inc., Madison, Wisconsin, USA Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. J. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, A71, 3–8 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, C71, 3–8 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2340637–2340639. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4nj01330a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |