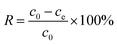Open Access Article
Open Access ArticleIndium-based quantum dots trapped in solid-state matrices: a one-pot synthesis, thermoresponsive properties, and enhanced micropollutant removal†
Nida
Ük
 a,
Sümeyye
Aykut
a,
Sümeyye
Aykut
 b,
Hadi
Jahangiri
c,
Ilgın
Nar
b,
Hadi
Jahangiri
c,
Ilgın
Nar
 d and
Caner
Ünlü
d and
Caner
Ünlü
 *ab
*ab
aIstanbul Technical University, Polymer Science and Technology, Maslak, 34469, Istanbul, Turkey. E-mail: canerunlu@itu.edu.tr
bIstanbul Technical University, Faculty of Science and Letters, Department of Chemistry, 34469, Maslak, Istanbul, Turkey
cKoç University Surface Science and Technology Center (KUYTAM), Koc University, Sariyer 34450, Istanbul, Turkey
dIstanbul Technical University Nanotechnology Research and Application Center (ITUNano), Istanbul, Turkey
First published on 9th May 2024
Abstract
Indium-based quantum dots (QDs), such as copper indium disulfide and zinc copper indium sulfide, have been the center of research for decades due to their low toxicity and unique photophysical properties. In contrast, versatile indium-based materials like In2S3 and ZnIn2S4 have been rarely studied in their QD form because of the challenges in their synthesis and used in solid-state material based applications because of their colloidal nature. In this study, a one-pot single-step method to synthesize In2S3, ZnIn2S4, and Cu-doped ZnIn2S4 QDs trapped in insoluble solid-state oleic acid matrices was developed. The QDs in solid-state matrices exhibited bright orange colored fluorescence with controllable emission properties achieved by altering the chemical composition. Among these QDs, the ZnIn2S4 QDs displayed thermo-responsive properties. As the temperature increased, the fluorescence intensity of ZnIn2S4 QDs decreased. In addition, all QDs demonstrated high removal efficiency for micropollutants in the aqueous medium, especially against cationic organic dyes. This study represents one of the first attempts at the direct development of QDs trapped in insoluble solid-state matrices. The QDs in solid-state matrices hold promise for applications in thermal sensors and studies related to the micropollutant removal.
Introduction
Indium-based quantum dots (QDs) have long fascinated researchers due to their intriguing photophysical properties and low toxicity, making them promising candidates for various applications.1–3 Copper indium disulfide QDs, in particular, have been extensively studied in the literature.1–3 However, the exploration of alternative indium-based materials in their QD form has been relatively limited, with a particular lack of studies focusing on indium sulfide (In2S3) and zinc indium sulfide (ZnIn2S4) QDs, primarily because of their comparatively weak photophysical properties compared to copper indium disulfide QDs.4–7Like most QDs, indium-based QDs have been synthesized in their colloidal form.1–7 In addition to their superior photophysical properties, colloidal QDs have a non-porous structure with an extremely small size (4–7 nm for the indium based ones),1–7 making their utilization impractical for water decontamination studies. This is noteworthy even though indium-based materials have often been employed in water decontamination, particularly in the removal of organic micropollutants from aqueous solutions.8–12
Environmental conditions, including factors like temperature, pressure, and humidity, are critical for sustaining life, with temperature being particularly crucial due to its narrow tolerance range.13,14 Monitoring rapid temperature fluctuations, especially in workplaces like mines, is vital for ensuring safety.13,14 Thermoresponsive materials, specifically thermoresponsive fluorescent compounds, have gained significant attention for their ability to reversibly change fluorescence intensity or emission wavelength in response to temperature variations, making them invaluable in applications such as temperature sensing, biological sensing, and drug delivery.15–17 Their sensitivity to temperature changes enables precise tracking in complex settings, including challenging work environments and biological systems.15–17 Although quantum dots are frequently used as thermosensors,18–20 their colloidal structure limits their utilization in many applications which requires solid-state thermoresponsive materials.
In this study, In2S3, ZnIn2S4, and Cu:ZnIn2S4 QDs trapped in oleic acid based solid-state matrices were developed using a one-pot single-step synthesis technique. These QDs in the size range of 4 nm to 7 nm were dispersed in a solidified network formed by surfactant molecules which was not soluble in any kind of solvent. QDs in solid-state matrices (SSQDs) emitted orange color fluorescence with considerably high fluorescence quantum yields (up to 31%) and by adjusting the composition, the emission characteristics of these SSQDs could be controlled. Notably, the ZnIn2S4 SSQDs exhibited a unique thermo-responsive behavior. When subjected to heat, the fluorescence of ZnIn2S4 SSQDs decreased gradually until 100 °C, and completely quenched at 100 °C. Until 160 °C, the decrease in fluorescence was reversible, allowing the fluorescence of ZnIn2S4 SSQDs to recover at room temperature. Nevertheless, once the temperature exceeded 160 °C, the fluorescence recovery was no longer observable. In addition, the indium-based SSQDs exhibited great potential in water decontamination from organic micropollutants. All the synthesized SSQDs showed significantly high removal efficiency against cationic organic dyes (methylene blue, methyl violet 2B and rhodamine B). In particular, In2S3 SSQDs were able to remove anionic dyes (methyl orange and sodium fluorescein) with considerably high removal efficiency.
Materials and methods
Chemicals
To synthesize SSQDs, copper(I) iodide (CuI, 99.9%, Aldrich), indium(III) acetate (In(C2H3O2)3 99.9%, Aldrich), zinc stearate (C36H70O4, technical grade, Aldrich), thioacetamide (CH3CSNH2, >99.0%, Aldrich), oleic acid (OA, 90%, Aldrich), and 1-octadecene (CH3(CH2)15CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, technical grade, 90%, Aldrich) were used without further purification.
CH2, technical grade, 90%, Aldrich) were used without further purification.
Synthesis of copper doped indium zinc sulfide (Cu:ZnIn2S4) SSQDs
0.0267 g (0.14 mmol) of CuI, 0.0409 g (0.14 mmol) of In(CH3COO)3, 0.0759 g (0.12 mmol) of Zn-(CH3(CH2)16COO)2, 0.056 g (0.745 mmol) of thioacetamide, and 0.23 mL (0.72 mmol) of oleic acid were mixed with 1-octadecene (16 mL) in a 250 mL three-necked round reaction flask under a nitrogen atmosphere and heated. As the solution reached nearly 130 °C, all the materials dissolved. The reaction mixture was kept at 150 °C and refluxed for 40 minutes. At the end of 40 minutes, the reaction was stopped by cooling down the reaction mixture. The crude product, SSQDs, precipitated within minutes and was obtained as a solid residue in the reaction medium. Finally, SSQDs were washed with deionized water and acetone and then dried by using dry air at room temperature.Copper doped indium zinc sulfide SSQDs were also synthesized by using 1-dodecanethiol or 3-mercaptopropionic acid instead of thioacetamide using the same synthesis and purification method.
Synthesis of zinc indium sulfide (ZnIn2S4) SSQDs
0.0409 g (0.14 mmol) of In(CH3COO)3, 0.0759 g (0.12 mmol) of Zn–(CH3(CH2)16COO)2, 0.056 g (0.745 mmol) of thioacetamide, and 0.23 mL (0.72 mmol) of oleic acid were mixed with 1-octadecene (16 mL) in a 250 mL three-necked round bottom reaction flask under a nitrogen atmosphere and heated. As the solution reached nearly 130 °C, all the materials dissolved. The reaction mixture was kept at 150 °C and refluxed for 40 minutes. At the end of 40 minutes, the reaction was stopped by cooling down the reaction mixture. The crude product, SSQDs, precipitated within minutes and was obtained as a solid residue in the reaction medium. Finally, SSQDs were washed with deionized water and acetone and then dried by using dry air at room temperature.Synthesis of indium sulfide (In2S3) SSQDs
In this study, a traditional solvothermal method to synthesize Cu:ZnIn2S4 colloidal SSQDs was modified by changing the sulfur source to thioacetamide instead of dodecanethiol.21 0.0409 g (0.14 mmol) of In(CH3COO)3, 0.056 g (0.745 mmol) of thioacetamide, and 0.23 mL (0.72 mmol) of oleic acid were mixed with 1-octadecene (16 mL) in a 250 mL three-necked round reaction flask under a nitrogen atmosphere and heated. As the solution reached nearly 130 °C, all the materials dissolved. The reaction mixture was kept at 150 °C and refluxed for 40 minutes. At the end of 40 minutes, the reaction was stopped by cooling down the reaction mixture. The crude product, SSQDs, precipitated within minutes and was obtained as a solid residue in the reaction medium. Finally, SSQDs were washed with deionized water and acetone and then dried by using dry air at room temperature.Observation of the thermoresponsive properties of ZnIn2S4 SSQDs
In order to observe the thermoresponsive properties, approximately 25 mg of ZnIn2S4 SSQDs were used for the temperature test of the SSQDs. The sample was placed on a black cardboard and heated on a hot plate, increasing the temperature by 10 degrees each time. Once the desired temperature was reached, it was maintained on the hot plate for 4 minutes. Simultaneously, an equivalent amount of the reference sample was subjected to UV light. Images were captured by bringing the heated sample next to the reference. When the temperature surpassed 50 °C, it was observed that the fluorescence color under UV light initially decreased. However, as the observation continued, it was noticed that within approximately 45 seconds, the fluorescence properties returned to its original state. This phenomenon of fluorescence presence upon cooling (at room temperature) and disappearance upon heating persisted up to 160 °C. After reaching 160 °C, it was observed that upon cooling, the fluorescence properties were lost, indicating structural degradation.Treatment of micropollutants with SSQDs
Methylene blue, methyl violet 2B, and rhodamine B were selected as cationic dyes, while methyl orange and sodium fluorescein were chosen as anionic dyes. Aqueous solutions of methyl orange (MO), sodium fluorescein (NF), methylene blue (MB), methyl violet 2B (MV), and rhodamine B (RB) were prepared at a concentration of 20 μM. Subsequently, 1 mL of solutions containing 5 mg of SSQDs were prepared for each micropollutant. The glass vials were subsequently wrapped with aluminum foil and agitated on a shaker at 400 rpm for 24 hours in the absence of light. After 24 hours, a dilution process was performed to better observe the intensity changes in the solutions. 300 microliters of each solution were extracted and diluted to 3 mL with distilled water. The micropollutants were similarly diluted, and measurements were taken using a UV-vis spectrophotometer to observe the differences in absorption intensities.Structural and optical characterization of SSQDs
The crystal structures of Cu:ZnIn2S4, ZnIn2S4, and In2S3 SSQDs were analyzed using an RIGAKU SmartLab X-ray diffraction spectrometer with Cu kα radiation at a wavelength of 1.5406 Å at 30 kV voltage. The Cu:ZnIn2S4, ZnIn2S4, In2S3 SSQDs were measured in the 2-theta range between 15° and 70° for 4 seconds with a step size of 0.01°.The shape and size of the SSQDs were determined using transmission electron microscopy (TEM). TEM measurements were conducted using a JEOL JEM 1220 instrument with an accelerating voltage of 100 kV.
The elemental composition of each nanocrystal was checked by X-ray photoelectron spectroscopy (XPS). XPS measurements were carried out with a Thermo Scientific K-Alpha with a monochromatic 1486.68 eV Al Kα X-ray line source and a 400 μm beam size.
The surface properties of SSQDs were characterized by attenuated total reflectance FTIR (ATR-FTIR) spectroscopy. The FTIR spectra of the SSQDs were measured in the range of 700–4000 cm−1 by using a PerkinElmer ATR-FTIR spectrophotometer.
The absorption spectrum of SSQDs was recorded using an Agilent double-beam ultraviolet-visible (UV-vis) spectrophotometer. For SSQDs, the QDs were dispersed in dicholoromethane and then dropped on quartz glass to form thin films. Afterwards, the absorption spectrum of SSQDs was collected from these thin films.
Fluorescence analysis of SSQDs was performed using a RENISHAW inVia spectrometer with a 532 nm excitation laser, 1% laser power, 10 s acquisition time, and ×50 objective lens in fluorescence mode at room temperature. The quantum yield (QY) and time resolved fluorescence measurements of SSQDs were conducted with an Edinburgh Instruments FLS1000 spectrometer. For QY measurements of SSQDs, an integrating sphere was used. The time-resolved fluorescence measurements of SSQDs were performed by using 377 nm excitation wavelength and a 585 ± 17 nm band-pass emission filter.
Results and discussion
Trapping QDs in solid-state matrices and formation of SSQDs
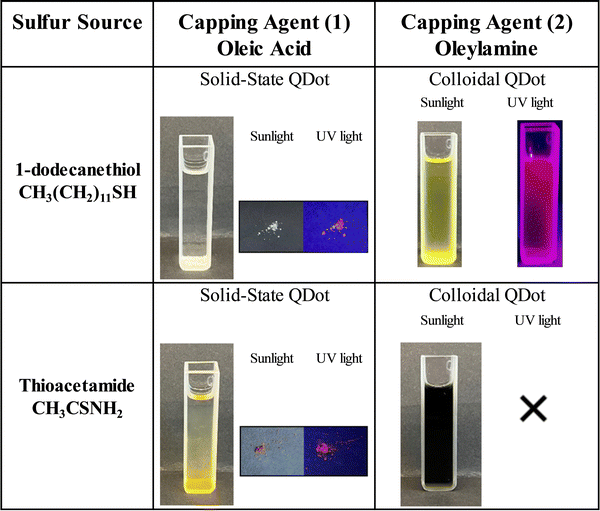
To synthesize In-based SSQDs, first, the traditional synthesis method was altered by substituting the surfactant (oleylamine) with another frequently used surfactant for cadmium-based QDs, oleic acid.22–24 It should be noted that the same procedure to synthesize SSQDs was repeated by keeping oleylamine as the surfactant and colloidal QDs were obtained (see the ESI† for experimental details and Fig. S1 for characterization of the colloidal QDs). As the surfactant was changed, formation of insoluble solid-state materials, in which QDs were dispersed, was observed instead of colloidal QDs. The insoluble nature of solid-state materials arises due to formation of solid networks most likely because of intermolecular interactions between oleic acid molecules, as was observed for different types of QDs25 and oleic acid alone26 in the literature before and discussed in more detail later in the text. To determine whether the solid-state material was soluble in any kind of solvent, efforts were made to dissolve the solid-state material in various solvents: toluene, chloroform, acetone, dimethylsulfoxide, N,N-dimethylformamide, tetrahydrofuran, methanol, ethanol, and water. However, none of these solvents were successful in dissolving the solid-state material at room temperature, confirming its insoluble nature. In addition, the effect of substituting sulfur precursors from 1-dodecanethiol to thiocatamide was checked by keeping oleylamine as the surfactant when QDs were synthesized in the colloidal form. In the literature, it has been demonstrated that the utilization of various sulfur precursors leads to the formation of distinct crystal structures.27 Specifically, the use of thioacetamide or 1-dodecanethiol as sulfur precursors has been shown to result in the formation of completely distinct types of crystals.27 1-Dodecanethiol has a slow reaction rate and possesses a long carbon chain, which generally leads to the formation of wurtzite type crystals and has been widely used as the S precursor in the synthesis of indium-based quantum dots such as copper indium sulfide and zinc copper indium sulfide.21,28 On the flip side, thioacetamide exhibits a significantly faster reaction rate, leading to the formation of an entirely distinct crystal type, such as keskerite.27 It is important to note that despite thioacetamide being frequently used as the S precursor for cadmium chalcogenide and copper indium disulfide quantum dots,21,28,29 opting for thioacetamide as the sulfur precursor to synthesize indium sulfide or zinc indium sulfide nanocrystals resulted in nanocrystals with sizes larger than the Bohr exciton radius.5,30–32 As a consequence, quantum confinement effects and thus fluorescent indium sulfide and zinc indium sulfide nanocrystals cannot be observed.5,30,31 In simpler terms, neither indium sulfide nor zinc indium sulfide colloidal quantum dots have been successfully synthesized using thioacetamide as the S precursor.5,30,31 In this study, substituting the sulfur precursor from 1-dodecathiol to thioacetamide by using oleylamine as the surfactant resulted in the formation of black colored solution without any precipitate, which did not display any sort of emissive properties (Fig. 1). It should be noted that initially all the syntheses were performed at 200 °C because when 1-dodecanethiol was used as the S precursor, the metal sulfide nanocrystals start to form above 200 °C;21 however, high/fast reactivity of thioacetamide might cause over-growth of nanocrystals,5,30,31 thus in order to slow down the crystal growth, the temperature of the synthesis set-up was decreased to 150 °C, yet there was no indication of quantum dot formation. To sum up, only changing the S precursor to thioacetamide resulted in the formation of colloidal particles without any fluorescence properties, indicating that thioacetamide was not an ideal S precursor to synthesize colloidal indium-based quantum dots.Effect of different sulfur precursors on fluorescence of CuInZnS SSQDs
To investigate the effect of different sulfur precursors on SSQDs, CuInZnS SSQDs were synthesized by using three sulfur sources: thioacetamide, 1-dodecanethiol (DDT) and 3-mercaptopropionic acid (3-MPA). Although each of these precursors resulted in the formation of QDs in solid-state matrices, the brightness of the SSQDs synthesized with DDT and 3-MPA was lower compared to the brightness of CuInZnS SSQDs synthesized with thioacetamide (Fig. 2). Additionally, the full width at half maximum (FWHM) of the fluorescence peak collected from SSQDs increased significantly (35 nm for thioacetamide, 105 nm for 3-MPA, and 100 nm for DDT) (Fig. 2). The FWHM of the fluorescence spectrum can be regarded as a significant indicator of the size distribution of quantum dots. Due to the quantum confinement effect, the band gap between the valence and conduction bands of the quantum dots becomes dependent on the size of the quantum dots. As the size increases, the band gap decreases, and vice versa. As the fluorescence of a material is defined as the transition from the lowest vibrational level of the excited state to the ground state, the broadness of the fluorescence spectrum of quantum dots can be considered as evidence of the monodispersity or polydispersity of the size of quantum dots.33 Therefore, the increase in the FWHM suggested the possible formation of SSQDs with polydispersed sizes. As a result, further experiments were conducted using thioacetamide as the sulfur precursor.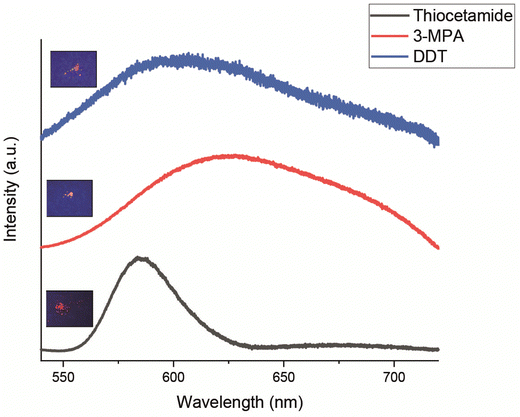 | ||
| Fig. 2 Photoluminescence spectrum of CuInZnS SSQDs in which a sulfur precursor was used as thioacetamide, DDT and 3-MPA. The inset fluorescence pictures were taken under 366 nm UV-light. | ||
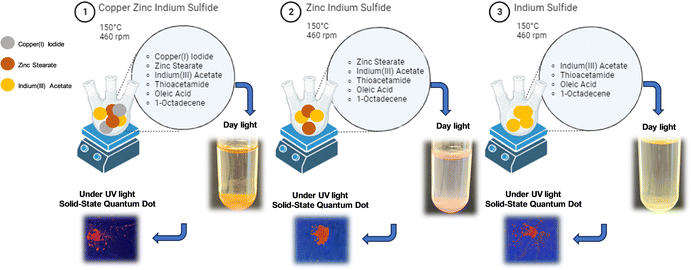
Synthesis of In2S3, ZnIn2S4 and Cu:ZnIn2S4 SSQDs
Although colloidal copper indium disulfide (CIS) and zinc copper indium sulfide (ZCIS) QDs have been synthesized in various ways and extensively explored in the literature, the synthesis of In2S3 and ZnIn2S4 QDs has received relatively little attention, with only a few studies conducted on these particles. However, it is important to note that ZnIn2S4 is a particularly crucial material which is frequently used in various applications such as photocatalytic degradation of dyes and artificial photosynthesis solar cells.6,34 The main reason why ZnIn2S4 and In2S3 QDs haven't garnered much research attention is the superior photophysical properties of CIS and ZCIS QDs, including a wide color gamut, high photoluminescence quantum yield, and high photostability, in contrast to the photophysical properties of In2S3 and ZnIn2S4 QDs.35,36 In our study, In2S3 and ZnIn2S4 SSQDs were successfully synthesized with bright fluorescence intensity, particularly with ZnIn2S4 SSQDs being the brightest among them all (Fig. 3).Structural and elemental characterization of In2S3, ZnIn2S4 and Cu:ZnIn2S4 SSQDs
To obtain more insight into the nature of SSQDs, the crystal structure, elemental composition and size of the quantum dots were determined by using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) and Fourier transform infrared (FTIR) spectroscopy.The size and size distribution of SSQDs were determined by TEM. The TEM analysis of solid-state materials showed that the SSQDs were trapped in solidified oleic acid matrices with a rough surface (Fig. 4). The TEM results of In2S3 SSQDs showed that the QDs were dispersed in the solid structure and had an average size of 7 nm (see Fig. S2 for the size histogram of In2S3 QDs dispersed in solid matrices, ESI†). The size of the In2S3 SSQDs was significantly lower than the Bohr exciton radius of In2S3 (33 nm), which showed that the In2S3 nanocrystals were within the quantum confinement regime.37,38 As was observed for In2S3 SSQDs, the TEM results showed that ZnIn2S4 QDs were also dispersed in a solid structure and had an average size of 6 nm (Fig. 4, see Fig. S2 (ESI†) for the size histogram of In2S3 SSQDs dispersed in solid matrices). It should be noted that even though the exact Bohr exciton radius of ZnIn2S4 has not been exactly determined, the ZnIn2S4 SSQDs show quantum confinement effects below 6 nm radius, and the ZnIn2S4 SSQDs that were produced in this study were within the quantum confinement regime.6 Also, the TEM results showed that Cu:ZnIn2S4 SSQDs were dispersed in the solid structure and had an average size of 6 nm, which showed that the Cu:ZnIn2S4 SSQDs were in the quantum confinement regime (Fig. 4, see Fig. S2 (ESI†) for the size histogram of In2S3 SSQDs dispersed in solid matrices).6
 | ||
| Fig. 4 TEM images of (a) and (b) In2S3, (c) and (d) ZnIn2S4 and (e) and (f) Cu:ZnIn2S4 SSQDs. The scale bar for (a), (c) and (e) is 200 nm. The scale bar for (b), (d) and (f) is 50 nm. | ||
The crystal structure of SSQDs was determined by XRD. The XRD pattern of In2S3 SSQDs matched with the XRD pattern of cubic In2S3 (JCPDS: card no. 05-00731 cubic In2S3)39 but with significantly broadened peaks (Fig. 5). The identified planes for the In2S3 peaks were as follows: (111) at 2θ: 27.3°, (200) at 2θ: 33.15°, (220) at 2θ: 47.9°, and the (311) plane at 2θ: 56.18°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 (Fig. 5). The broadening of the peaks was a result of the nanosize of the In2S3 SSQDs.39 When the Zn precursor was added to the synthesis medium, a dramatic change in the crystal structure was observed, which suggested that a new type of crystal formed instead of Zn doped In2S3 (Fig. 5). The most significant change was the disappearance of the (0012) peak in the XRD pattern of In2S3 (Fig. 5). The new nanocrystal structure matched with hexagonal ZnIn2S4, whose XRD characteristics were identified in the literature before4 (JCPDS: card no. 65-2023 hexagonal ZnIn2S4). The identified planes for the ZnIn2S4 peaks were as follows: (101) at 2θ: 28.85°, (110) at 2θ: 47.5° and (202) at 2θ: 56.1° (Fig. 5). Similar to In2S3, the broadening of the ZnIn2S4 peaks was a consequence of the nanosize of the SSQDs. Adversely, when the Cu precursor was added in addition to the Zn precursor, the XRD pattern of the new nanocrystal did not show a significant difference. The XRD peaks slightly shifted towards lower angles. This effect was expected since the XRD pattern of tetragonal CuZnInS had been observed at lower angles in the literature before40 (JCPDS: 47-1371). These results indicated that the amount of Cu should be lower compared to the rest of the elements (Zn, In, and S) since there was no dramatic difference in the shape and position of the XRD peaks of Cu:ZnIn2S4 (Fig. 5).
39 (Fig. 5). The broadening of the peaks was a result of the nanosize of the In2S3 SSQDs.39 When the Zn precursor was added to the synthesis medium, a dramatic change in the crystal structure was observed, which suggested that a new type of crystal formed instead of Zn doped In2S3 (Fig. 5). The most significant change was the disappearance of the (0012) peak in the XRD pattern of In2S3 (Fig. 5). The new nanocrystal structure matched with hexagonal ZnIn2S4, whose XRD characteristics were identified in the literature before4 (JCPDS: card no. 65-2023 hexagonal ZnIn2S4). The identified planes for the ZnIn2S4 peaks were as follows: (101) at 2θ: 28.85°, (110) at 2θ: 47.5° and (202) at 2θ: 56.1° (Fig. 5). Similar to In2S3, the broadening of the ZnIn2S4 peaks was a consequence of the nanosize of the SSQDs. Adversely, when the Cu precursor was added in addition to the Zn precursor, the XRD pattern of the new nanocrystal did not show a significant difference. The XRD peaks slightly shifted towards lower angles. This effect was expected since the XRD pattern of tetragonal CuZnInS had been observed at lower angles in the literature before40 (JCPDS: 47-1371). These results indicated that the amount of Cu should be lower compared to the rest of the elements (Zn, In, and S) since there was no dramatic difference in the shape and position of the XRD peaks of Cu:ZnIn2S4 (Fig. 5).
 | ||
| Fig. 5 (a) XRD spectra of In2S3, ZnIn2S4 and Cu:ZnIn2S4 SSQDs (InS represents In2S3, ZnInS represents ZnIn2S4 and CuZnInS represents Cu:ZnIn2S4). | ||
The elemental composition of each SSQD was determined by XPS measurements. According to the XPS results, the In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio in In2S3 SSQDs was 2.3
S atomic ratio in In2S3 SSQDs was 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.4, the Zn
3.4, the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In
In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio in ZnIn2S4 SSQDs was 1.1
S atomic ratio in ZnIn2S4 SSQDs was 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7, and the Cu
3.7, and the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In
In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio in Cu
S atomic ratio in Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnIn2S4 SSQDs was 0.2
ZnIn2S4 SSQDs was 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1
2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 (Fig. 6). The atomic ratios of In
3.5 (Fig. 6). The atomic ratios of In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S in In2S3 SSQDs and Zn
S in In2S3 SSQDs and Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In
In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S in ZnIn2S4 SSQDs were consistent with the XRD results (Fig. 6). Additionally, the atomic ratio of Cu in Cu
S in ZnIn2S4 SSQDs were consistent with the XRD results (Fig. 6). Additionally, the atomic ratio of Cu in Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnIn2S4 SSQDs was relatively low, which indicated that Cu was a dopant in Cu
ZnIn2S4 SSQDs was relatively low, which indicated that Cu was a dopant in Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnIn2S4 SSQDs and this result was in line with the XRD pattern of Cu
ZnIn2S4 SSQDs and this result was in line with the XRD pattern of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnIn2S4 QDs (Fig. 5 and 6). The HR-XPS spectra of the common elements in the SSQD structures, In(3d) and S(2p), indicated that the bonding characteristics of In and S were different in each SSQD (Fig. 6). The HR-XPS peak of S(2p) composed of 2 peaks merged into each other for each QD; one peak at a high energy region which corresponds to 2p3/2 (162.6 eV for In2S3 and 163.1 eV for ZnIn2S4 and Cu:ZnIn2S4) accompanied by another peak at a lower energy region which corresponds to 2p1/2 (161.4 eV for In2S3 and 161.9 eV for ZnIn2S4 and Cu:ZnIn2S4). Both of these peaks shifted towards a higher energy region in ZnIn2S4 and Cu:ZnIn2S4 SSQDs compared to In2S3 SSQDs (see the ESI† for the deconvoluted HR-XPS S(2p) spectrum for each SSQD, Fig. S3). This observation was expected due to the higher electronegativity (EN) difference between Zn (EN = 1.6) and S (EN = 2.5) compared to the EN difference between In (EN = 1.7) and S (Fig. 6). Furthermore, the 3d5/2 and 3d3/2 peaks (appeared at 452.5 eV and 445.1 eV, respectively) in the HR-XPS spectrum of In(3d) broadened in ZnIn2S4 and Cu:ZnIn2S4 SSQDs (the FWHM of the 3d5/2 peak: 1.1 eV for In2S3 and 1.4 eV for ZnIn2S4 and Cu:ZnIn2S4 and the FWHM of the 3d3/2 peak: 1.1 eV for In2S3 and 1.6 eV for ZnIn2S4 and Cu:ZnIn2S4) since the presence of Zn affected the bonding characterization of In by creating an extra interaction and led to broadening in the XPS peaks (Fig. 6). As the HR-XPS of C (1s) was investigated, it was observed that the C
ZnIn2S4 QDs (Fig. 5 and 6). The HR-XPS spectra of the common elements in the SSQD structures, In(3d) and S(2p), indicated that the bonding characteristics of In and S were different in each SSQD (Fig. 6). The HR-XPS peak of S(2p) composed of 2 peaks merged into each other for each QD; one peak at a high energy region which corresponds to 2p3/2 (162.6 eV for In2S3 and 163.1 eV for ZnIn2S4 and Cu:ZnIn2S4) accompanied by another peak at a lower energy region which corresponds to 2p1/2 (161.4 eV for In2S3 and 161.9 eV for ZnIn2S4 and Cu:ZnIn2S4). Both of these peaks shifted towards a higher energy region in ZnIn2S4 and Cu:ZnIn2S4 SSQDs compared to In2S3 SSQDs (see the ESI† for the deconvoluted HR-XPS S(2p) spectrum for each SSQD, Fig. S3). This observation was expected due to the higher electronegativity (EN) difference between Zn (EN = 1.6) and S (EN = 2.5) compared to the EN difference between In (EN = 1.7) and S (Fig. 6). Furthermore, the 3d5/2 and 3d3/2 peaks (appeared at 452.5 eV and 445.1 eV, respectively) in the HR-XPS spectrum of In(3d) broadened in ZnIn2S4 and Cu:ZnIn2S4 SSQDs (the FWHM of the 3d5/2 peak: 1.1 eV for In2S3 and 1.4 eV for ZnIn2S4 and Cu:ZnIn2S4 and the FWHM of the 3d3/2 peak: 1.1 eV for In2S3 and 1.6 eV for ZnIn2S4 and Cu:ZnIn2S4) since the presence of Zn affected the bonding characterization of In by creating an extra interaction and led to broadening in the XPS peaks (Fig. 6). As the HR-XPS of C (1s) was investigated, it was observed that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak around 288 eV almost disappeared. The HR-XPS of C (1s) displayed a single-narrow peak at 284.9 eV, which belonged to sp3 hybridized carbons.41 The HR-XPS spectrum of C (1s) revealed that the sp3 hybridized carbons dominated the structure and the presence of sp2 hybridized carbons was almost insignificant (the XPS peak of sp2 hybridized carbons should be around 284 eV). This result indicated that the double bond in oleic acid, an unsaturated fatty acid, was most probably broken and may lead to the formation of a cross-linked structure, which was observed for a different type of quantum dot before in the literature.25
O peak around 288 eV almost disappeared. The HR-XPS of C (1s) displayed a single-narrow peak at 284.9 eV, which belonged to sp3 hybridized carbons.41 The HR-XPS spectrum of C (1s) revealed that the sp3 hybridized carbons dominated the structure and the presence of sp2 hybridized carbons was almost insignificant (the XPS peak of sp2 hybridized carbons should be around 284 eV). This result indicated that the double bond in oleic acid, an unsaturated fatty acid, was most probably broken and may lead to the formation of a cross-linked structure, which was observed for a different type of quantum dot before in the literature.25
The FTIR spectrum of In2S3, ZnIn2S4 SSQDs and Cu:ZnIn2S4 SSQDs displayed similar characteristics, indicating that these quantum dots had comparable surface properties. In order to understand the solid nature of the material, the FTIR spectra of SSQDs (Fig. S4, ESI†) were compared with the FTIR spectrum of colloidal oleic acid capped CdSeS QDs (Fig. S5, ESI†). The disappearance of the hydroxyl (–OH) peak at around 3100–3400 cm−1 indicated that oleic acid surrounded the QDs through the carboxyl group (Fig. S4, ESI†).25,42 Another noteworthy observation was the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak of the carboxylate group at 1700 cm−1, suggesting that carboxyl groups interacted with metals within the quantum dots (Fig. S4, ESI†).25,42 Also, the C
O peak of the carboxylate group at 1700 cm−1, suggesting that carboxyl groups interacted with metals within the quantum dots (Fig. S4, ESI†).25,42 Also, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak, which arose around 3000–3020 cm−1 in the FTIR spectrum of CdSeS QDs (Fig. S4, ESI†), disappeared while an increase in the band around 1000–1100 cm−1 that corresponds to C–C bonds was observed (Fig. S4, ESI†). It should be noted that the band around 1000–1100 cm−1 was not observed in the FTIR spectrum of oleic acid capped CdSeS QDs (Fig. S5, ESI†). These results were in line with the XPS results of C (1s). In short, these observations supported the XPS data and suggested the formation of a cross-linked structure which was observed in the literature before for different types of QDs25 and oleic acid alone,26 which would explain the insoluble nature for In based SSQDs.
C peak, which arose around 3000–3020 cm−1 in the FTIR spectrum of CdSeS QDs (Fig. S4, ESI†), disappeared while an increase in the band around 1000–1100 cm−1 that corresponds to C–C bonds was observed (Fig. S4, ESI†). It should be noted that the band around 1000–1100 cm−1 was not observed in the FTIR spectrum of oleic acid capped CdSeS QDs (Fig. S5, ESI†). These results were in line with the XPS results of C (1s). In short, these observations supported the XPS data and suggested the formation of a cross-linked structure which was observed in the literature before for different types of QDs25 and oleic acid alone,26 which would explain the insoluble nature for In based SSQDs.
Optical characterization of In2S3, ZnIn2S4 and Cu:ZnIn2S4 SSQDs in solid-state oleic acid matrices
The temporal evolution of SSQDs was observed on analysing the absorption spectrum of each quantum dot at different time intervals. The SSQDs displayed typical absorption behaviour of traditional SSQDs; each quantum dot had a broad absorption spectrum with a characteristic shoulder that corresponds to crystal band-edge transition (Fig. 7). The band-edge peak is slightly shifted to a lower energy region as Zn and Cu were incorporated into the SSQD structure; the position of the peak was 574 nm for In2S3, 577 nm for ZnIn2S4 and 580 nm for Cu:ZnIn2S4. In order to observe the growth of SSQDs over time, the shift of the band-edge transition peak with respect to different experiment times was checked (Fig. 7). In theory, the SSQDs should increase with longer synthesis time and as a consequence of the quantum confinement effect, the band–edge transition peak should red-shift with the increase of SSQDs.43 In our study, the band-edge transition peak shifted slightly towards longer wavelengths as the synthesis time increased, as expected (Fig. 7).The SSQDs synthesized in this study exhibited insolubility in both polar and non-polar solvents. They were insoluble in their synthesis solvent, 1-octadecene, as well as other solvents with varying polarities such as toluene, chloroform, acetone, dimethylsulfoxide, N,N-dimethylformamide, tetrahydrofuran, methanol, ethanol, and water. Therefore, the photoluminescence spectra of the SSQDs were obtained from the solid product using Raman microscopy with an excitation wavelength of 532 nm (Fig. 7). The excitation wavelength was decided with respect to the band-edge absorption peak of SSQDs (Fig. 7). Additionally, the emission of SSQDs was visualized under an excitation wavelength of 366 nm, within the typical excitation wavelength range for QDs,22,23,44 resulting in an orange color emission for each SSQD (Fig. 7). Upon 532 nm excitation, the In2S3 SSQDs exhibited a single emission peak around 580 nm. The emission peak of ZnIn2S4 SSQDs slightly shifted towards longer wavelengths, appearing at 583 nm. The addition of Cu caused an additional red-shift in the emission peak of Cu:ZnIn2S4 SSQDs, which appeared at 586 nm. Similar behaviour was observed in the absorption spectrum of SSQDs; integration of Zn caused a slight red-shift in the band-edge transition peak in the absorption spectrum, while integration of Cu to ZnInS caused even further shift towards the lower energy region in the band-edge transition peak in the absorption spectrum. The fluorescence quantum yields of the SSQDs were measured as follows: 31.73% for ZnIn2S4 QDs, 4.74% for Cu:ZnIn2S4 SSQDs and below 1% for In2S3 SSQDs (see Fig. S6, ESI†). As the fluorescence decays of SSQDs were observed, it was found that the ZnIn2S4 SSQDs possessed the longest average fluorescence lifetime (1.34 ns). The average fluorescence lifetimes of Cu:ZnIn2S4 SSQDs and In2S3 SSQDs were 0.704 ns and 0.540 ns, respectively. The fluorescence lifetimes of SSQDs were coherent with the quantum yields of SSQDs; as the quantum yield of the SSQD decreased, the fluorescence lifetime of the SSQD became shorter. The fluorescence decay of ZnIn2S4 SSQDs was monoexponential, indicating that mainly a single radiative process took place. However, the fluorescence decays of Cu:ZnIn2S4 QDs and In2S3 SSQDs were biexponential with a fast-dominant component (for Cu:ZnIn2S4; 0.614 ns with an amplitude of 82% and 1.127 ns with an amplitude of 18%, for In2S3; 0.425 ns with an amplitude of 92% and 1.190 ns with an amplitude of 8%), suggesting that a fast radiative process took place in these QDs resulting in a decrease in the quantum yield.
Thermoresponsive properties of ZnIn2S4 SSQDs
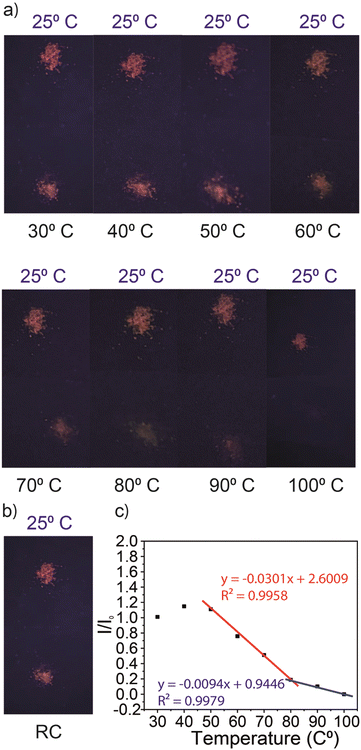
The thermal response of fluorescence of colloidal quantum dots has been widely studied in the literature.18–20 Yet, to date, quantum dots have been rarely tested as a potential temperature sensor in solid-state materials as they display different photophysical properties in colloidal and aggregate forms.18–20 In our study, the ZnInS SSQDs in solid-state matrices displayed thermoresponsive properties while others did not. To investigate the response of ZnIn2S4 SSQDs to different temperatures, a heating experiment was conducted, and the photoluminescence of the SSQDs under UV-light was monitored (Fig. 8). The emission of ZnIn2S4 QDs was examined at various temperatures ranging from 25 °C to 100 °C (Fig. 8). Up to 50 °C, there was no observable change in the intensity and color of the fluorescence of ZnIn2S4 QDs (Fig. 8). However, beyond 50 °C, a gradual decrease in the fluorescence intensity was observed as the temperature increased (Fig. 8). At 100 °C, the fluorescence of ZnIn2S4 QDs was completely quenched (Fig. 8). Remarkably, when the quenched ZnIn2S4 SSQDs were brought back to room temperature which were heated up to 160 °C, their fluorescence recovered within seconds (Fig. 8). Conversely, when the ZnIn2S4 SSQDs were heated beyond 160 °C, the recovery of fluorescence was not observed, and the quenching of fluorescence became permanent.As the quenching of fluorescence with respect to the increase in temperature was checked, it was observed that the fluorescence intensity of ZnIn2S4 SSQDs decreased linearly between 50 °C and 100 °C (Fig. 8). The decrease between 50 °C and 80 °C was steeper compared to the decrease between 80 °C and 100 °C (Fig. 8). This observation showed that the conversion rate between the fluorescence state and quenched state of ZnIn2S4 SSQDs was faster between 50 °C and 80 °C; however, this conversion rate slowed down as the temperature increased and ZnIn2S4 SSQDs were completely converted to the quenched state at 100 °C (Fig. 8). In order to understand the mechanism behind the quenching, several experiments were conducted at 70 °C. The fluorescence recovery of SSQDs at room temperature was within seconds (∼5 seconds) which was a proof of the intact structure of nanocrystals in the SSQD structure (see the Video in the ESI†). The fluorescence of SSQDs increases because of the quantum confinement effect that nanocrystals undergo; therefore the recovery of fluorescence was an indication of the stable structure of nanocrystals. As the quantum yields of the SSQDs were measured, it was observed that the quantum yield of the SSQD was dropped to 3% (Fig. S7, ESI†). A fluorescence lifetime experiment on SSQDs at 70 °C was carried out in order to comprehend the decrease in the quantum yield. At this temperature, there was a noticeable change in the fluorescence decay: instead of being monoexponential, it became biexponential as a result of the formation of a fast decay component (Fig. S8, ESI†). This finding implied that the electrons of SSQDs underwent a transition to a different vibrational energy level during heating, which prevented the formation of fluorescence. Upon cooling, this transition was cancelled and the fluorescence was recovered.
As was mentioned before, this conversion was reversible up to 160 °C. After 160 °C, the conversion was irreversible and QDs became permanently quenched. To investigate the reason behind the permanent quenching of ZnIn2S4 QDs, the X-ray diffraction (XRD) pattern of ZnIn2S4 SSQDs following permanent quenching was examined (Fig. S9, ESI†). The crystallinity of ZnIn2S4 SSQDs treated at 200 °C exhibited significant differences (Fig. S9, ESI†). The intensity of the characteristic ZnIn2S4 XRD peaks decreased considerably upon heating up to 200 °C (Fig. S6, ESI†). These results indicated that as the temperature increased, the ZnIn2S4 SSQD crystal structure started to be defected and consequently, the fluorescence of the SSQDs, which arose from their nanocrystal characteristics due to the quantum confinement effect, quenched.
Micropollutant removal from aqueous medium
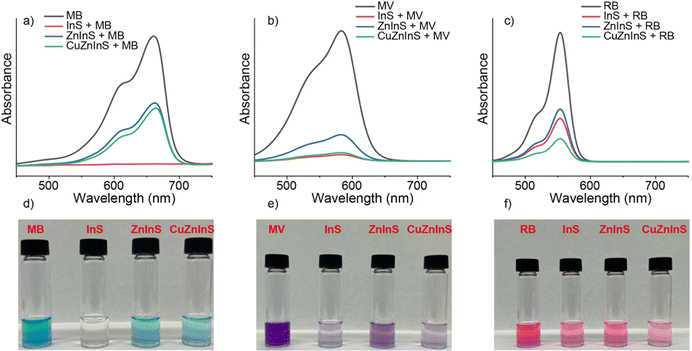
The quantum dots trapped in solid-state matrices were also tested for their potential to remove various micropollutants from aqueous environments. In the literature, indium-incorporated cage structures, such as indium-incorporated MOFs or COFs, have frequently been used in dye adsorption studies, particularly for cationic dyes.8–12 Additionally, specifically modified cross-linked structures have been proposed as potential agents for removing dyes due to their insoluble, solid, and porous nature.45 In our study, In2S3, ZnIn2S4, and Cu:ZnIn2S4 SSQDs were employed as removal agents to eliminate several different cationic and anionic dyes from their aqueous solutions. Methylene blue, methyl violet 2B, and rhodamine B were selected as cationic dyes, while methyl orange and sodium fluorescein were chosen as anionic dyes.When aqueous solutions of cationic dyes were agitated with SSQDs, the color of the solutions faded, especially when In2S3 was used, which almost completely removed methylene blue from its aqueous solution (Fig. 9). Also, SSQDs were quenched upon interaction, which showed that nanocrystals in the solid state matrices were mainly interacting with dyes (Fig. S10, ESI†). To understand the extent of the removal, the removal efficiencies for dyes were calculated (Table 1).
| MB (%) | MV (%) | RB (%) | MO (%) | NF (%) | |
|---|---|---|---|---|---|
| In2S3 SSQDs | 98.9 | 95.0 | 66.5 | 42.1 | 38.4 |
| ZnIn2S4 SSQDs | 56.1 | 79.7 | 59.5 | 8.6 | 8.6 |
| Cu:ZnIn2S4 SSQDs | 51.9 | 93.7 | 82.1 | 7.5 | 12.1 |
The removal efficiency (R) of each SSQD for each dye was calculated by using the following equation:
In terms of cationic dye removal efficiency, In2S3 exhibited the highest performance for methylene blue and methyl violet 2b (Table 1). For rhodamine B, Cu:ZnIn2S4 SSQDs displayed the highest removal efficiency (Table 1). Additionally, ZnIn2S4 SSQDs showed notably high removal efficiencies when removing cationic dyes from aqueous solutions (Table 1).
In contrast, the anionic dyes ZnIn2S4 and Cu:ZnIn2S4 SSQDs did not significantly remove the micropollutants from their aqueous solutions (Fig. 10). On the other hand, In2S3 exhibited considerable efficiency in removing both methyl orange and sodium fluorescein (Table 1). These findings suggest that In2S3 SSQDs hold significant promise as a micropollutant removal agent for water treatment (Table 1). Furthermore, ZnIn2S4 and Cu:ZnIn2S4 SSQDs can be utilized for removing anionic dyes from aqueous solutions.
It should be noted that particularly when it comes to treating dye-related wastewater, indium-based materials have shown great promise for adsorption research.8–12 For example, indium-based metal–organic frameworks (In-MOFs) exhibited remarkable adsorption efficiency for a variety of cationic dyes, including methylene blue, azure A, and others.8,9 Furthermore, materials such as indium-based mesoporous adsorbents exhibited better adsorption capabilities and improved efficiency for dyes like methylene blue and basic yellow-28 due to the structural incorporation of indium atoms.10 Moreover, the removal of rhodamine B from water demonstrates how indium enhances adsorption performance when combined with other materials, such as graphite oxide in the In-MOF@GO composite.11 In comparison to the conventional In-MOF, the addition of graphene oxide to the composite greatly increases adsorption activity, making it a viable wastewater treatment solution.11 To sum up, the remarkable adsorption properties of materials based on indium originate from their structural features, which offer a multitude of active sites for interactions with molecules of dyes. On the other hand, when zinc and copper were incorporated into the structure, additional interactions would occur between SSQDs and dyes which would affect the adsorption capacity of the SSQDs. Other than rhodamine B, all the adsorption capacities decreased upon these interactions.
Conclusions
In conclusion, a one-pot single-step synthesis technique to create solid-state matrices incorporating In2S3, ZnIn2S4, and Cu:ZnIn2S4 SSQDs, which were trapped in oleic acid, was utilized. These SSQDs, ranging in size from 4 nm to 7 nm, were dispersed within a solidified network formed by surfactant molecules, rendering them insoluble in any type of solvent. The SSQDs emitted fluorescence in the wavelength range of 580–586 nm (which could be finely tuned by controlling the chemical composition) with a notably high fluorescence quantum yield, reaching up to 31%. These SSQDs possessed an average fluorescence lifetime between 1.34 ns and 0.540 ns. Remarkably, the ZnIn2S4 SSQDs exhibited a distinctive thermo-responsive behavior. Upon heat exposure, the fluorescence of ZnIn2S4 SSQDs gradually diminished until reaching 100 °C, completely extinguishing at this point. Between 100 °C and 160 °C, the decrease in fluorescence was reversible, allowing for recovery at room temperature. However, beyond 160 °C, the fluorescence recovery was no longer observed. Additionally, the indium-based SSQDs demonstrated significant potential in water decontamination from organic micropollutants. All synthesized SSQDs displayed notably high removal efficiency against cationic organic dyes (such as methylene blue, methyl violet 2B, and rhodamine B). Specifically, In2S3 SSQDs exhibited remarkable efficiency in removing anionic dyes (methyl orange and sodium fluorescein).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Ege University – Central Research Test and Analysis Laboratory Application and Research Center (EGE–MATAL) for the XPS measurements. The fluorescence quantum yield and fluorescence lifetime measurements were conducted at KUYTAM – Koç University Surface Science and Technology Center. This work was supported by the TUBITAK (The Scientific and Technological Research Council of Turkey) under The Scientific and Technological Research Projects Funding Program (programme no. 2244) [grant number: 119C197].References
- P. M. Allen and M. G. Bawendi, J. Am. Chem. Soc., 2008, 130, 9240–9241 CrossRef CAS PubMed.
- Y. H. Won, O. Cho, T. Kim, D. Y. Chung, T. Kim, H. Chung, H. Jang, J. Lee, D. Kim and E. Jang, Nature, 2019, 575, 634–638 CrossRef CAS PubMed.
- L. Li, T. J. Daou, I. Texier, T. T. K. Chi, N. Q. Liem and P. Reiss, Chem. Mater., 2009, 21, 2422–2429 CrossRef CAS.
- J. Wang, Y. Chen, W. Zhou, G. Tian, Y. Xiao, H. Fu and H. Fu, J. Mater. Chem. A, 2017, 5, 8451–8460 RSC.
- X. Wang, J. Damasco, W. Shao, Y. Ke and M. T. Swihart, ChemPhysChem, 2016, 17, 687–691 CrossRef CAS PubMed.
- Y. Pan, X. Yuan, L. Jiang, H. Yu, J. Zhang, H. Wang, R. Guan and G. Zeng, Chem. Eng. J., 2018, 354, 407–431 CrossRef CAS.
- Z. Long, W. Zhang, J. Tian, G. Chen, Y. Liu and R. Liu, Inorg. Chem. Front., 2021, 8, 880–897 RSC.
- K. Maru, S. Kalla, A. K. Ghosh and R. Jangir, Res. Chem. Intermed., 2024, 50, 147–174 CrossRef CAS.
- L. Feng, X. Zhang, Z. Jin, J. Chen, X. Duan, S. Ma and T. Xia, Molecules, 2023, 28, 4980 CrossRef CAS PubMed.
- R. K. Khaled, M. A. Wahba, M. D. Badry, M. F. Zawrah and E. A. Heikal, J. Mater. Sci., 2022, 57, 4504–4527 CrossRef CAS.
- C. Yang, S. Wu, J. Cheng and Y. Chen, J. Alloys Compd., 2016, 687, 804–812 CrossRef CAS.
- P. Huang, C. Chen, M. Wu, F. Jiang and M. Hong, Dalton Trans., 2019, 48, 5527–5533 RSC.
- S. M. Aminossadati, N. M. Mohammed and J. Shemshad, Tunn. Undergr. Sp. Technol., 2010, 25, 220–229 CrossRef.
- D. Chenyu, J. Phys.: Conf. Ser., 2021, 2005, 012113 CrossRef.
- A. C. Benniston, G. Copley, A. Harriman and R. Ryan, J. Mater. Chem., 2011, 21, 2601–2608 RSC.
- P. Yao, W. Qiao, Y. Wang, H. Peng, X. Xie and Z. Li, Chem. – Eur. J., 2022, 28, e202200725 CrossRef CAS PubMed.
- O. Tagit, D. Jańczewski, N. Tomczak, M. Y. Han, J. L. Herek and G. J. Vancso, Eur. Polym. J., 2010, 46, 1397–1403 CrossRef CAS.
- Y. Han, Y. Liu, H. Zhao, A. Vomiero and R. Li, J. Mater. Chem. B, 2021, 9, 4111–4119 RSC.
- P. Haro-González, L. Martínez-Maestro, I. R. Martín, J. García-Solé and D. Jaque, Small, 2012, 8, 2652–2658 CrossRef PubMed.
- C. Wang, H. Lin, Z. Xu, Y. Huang, M. G. Humphrey and C. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 6621–6628 CrossRef CAS PubMed.
- G. Lisensky, R. McFarland-Porter, W. Paquin and K. Liu, J. Chem. Educ., 2020, 97, 806–812 CrossRef CAS.
- C. Ünlü, Opt. Mater., 2019, 89, 361–367 CrossRef.
- S. M. Kestir, S. Şahin Keskin, Ö. Ergüder, N. Ük, Y. Türker, I. Nar, L. Trabzon and C. Ünlü, Dalton Trans., 2023, 52, 5704–5714 RSC.
- M. Erkan, N. Ük, I. Nar and C. Ünlü, Opt. Mater., 2023, 138, 113713 CrossRef CAS.
- C. D. Dieleman, W. Ding, L. Wu, N. Thakur, I. Bespalov, B. Daiber, Y. Ekinci, S. Castellanos and B. Ehrler, Nanoscale, 2020, 12, 11306–11316 RSC.
- R. Bonetti and W. O. Parker, JAOCS, J. Am. Oil Chem. Soc., 2019, 96, 1181–1184 CrossRef CAS.
- Z. Li, A. L. K. Lui, K. H. Lam, L. Xi and Y. M. Lam, Inorg. Chem., 2014, 53, 10874–10880 CrossRef CAS PubMed.
- K. Yu, P. Ng, J. Ouyang, M. B. Zaman, A. Abulrob, T. N. Baral, D. Fatehi, Z. J. Jakubek, D. Kingston, X. Wu, X. Liu, C. Hebert, D. M. Leek and D. M. Whitfield, ACS Appl. Mater. Interfaces, 2013, 5, 2870–2880 CrossRef CAS PubMed.
- S. Wang, J. Yu, P. Zhao, S. Guo and S. Han, ACS Omega, 2021, 6, 7139–7146 CrossRef CAS PubMed.
- S. Shen, L. Zhao and L. Guo, Int. J. Hydrogen Energy, 2010, 35, 10148–10154 CrossRef CAS.
- Z. Chen, D. Li, W. Zhang, C. Chen, W. Li, M. Sun, Y. He and X. Fu, Inorg. Chem., 2008, 47, 9766–9772 CrossRef CAS PubMed.
- O. Cavdar, M. Baluk, A. Malankowska, A. Żak, W. Lisowski, T. Klimczuk and A. Zaleska-Medynska, J. Colloid Interface Sci., 2023, 640, 578–587 CrossRef CAS PubMed.
- D. Mutavdžić, J. Xu, G. Thakur, R. Triulzi, S. Kasas, M. Jeremić, R. Leblanc and K. Radotić, Analyst, 2011, 136, 2391–2396 RSC.
- G. Yadav and M. Ahmaruzzaman, Mater. Sci. Eng. B, 2023, 292 Search PubMed.
- L. Li, A. Pandey, D. J. Werder, B. P. Khanal, J. M. Pietryga and V. I. Klimov, J. Am. Chem. Soc., 2011, 133, 1176–1179 CrossRef CAS PubMed.
- G. Morselli, M. Villa, A. Fermi, K. Critchley and P. Ceroni, Nanoscale Horiz., 2021, 6, 676–695 RSC.
- P. Zhao, T. Huang and K. Huang, J. Phys. Chem. C, 2007, 111, 12890–12897 CrossRef CAS.
- N. Gao, R. Zhang, B. Chen, J. Zhang, X. Zhang and A. L. Rogach, Nano Res., 2021, 14, 2321–2329 CrossRef CAS.
- N. M. Huang, J. Nanomater., 2011, 2011, 815709 Search PubMed.
- D. Cai, X. Yuan, D. Zhu, H. Zhou, H. Li and J. Zhao, Mater. Res. Bull., 2017, 94, 241–246 CrossRef CAS.
- B. Lesiak, L. Kövér, J. Tóth, J. Zemek, P. Jiricek, A. Kromka and N. Rangam, Appl. Surf. Sci., 2018, 452, 223–231 CrossRef CAS.
- J. Ibarra, J. Melendres, M. Almada, M. G. Burboa, P. Taboada, J. Juárez and M. A. Valdez, Mater. Res. Express, 2015, 2, 095010 CrossRef.
- C. Ünlü, G. Ü. Tosun, S. Sevim and S. Özçelik, J. Mater. Chem. C, 2013, 1, 3026–3034 RSC.
- Y. Coşkun, F. Y. Ünlü, T. Yllmaz, Y. Türker, A. Aydogan, M. Kuş and C. Ünlü, ACS Omega, 2022, 7, 18840–18851 CrossRef PubMed.
- F. Y. Ünlü and A. Aydogan, ACS Appl. Polym. Mater., 2023, 5, 7193–7200 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj01219d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |