Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Circularly polarized luminescence activity in the near infrared spectral region of a water-soluble ytterbium(III) complex containing a conjugated chromophoric ligand†
Silvia
Mizzoni
 ab,
Annika
Sickinger
ab,
Annika
Sickinger
 b,
Silvia
Ruggieri
b,
Silvia
Ruggieri
 a,
François
Riobé
a,
François
Riobé
 b,
Laure
Guy
b,
Laure
Guy
 b,
Olivier
Maury
b,
Olivier
Maury
 *b,
Bruno
Baguenard
c,
Amina
Bensalah-Ledoux
c,
Yannick
Guyot
*b,
Bruno
Baguenard
c,
Amina
Bensalah-Ledoux
c,
Yannick
Guyot
 c,
Stéphan
Guy
c,
Stéphan
Guy
 c,
Martina
Sanadar
c,
Martina
Sanadar
 d,
Andrea
Melchior
d,
Andrea
Melchior
 *d and
Fabio
Piccinelli
*d and
Fabio
Piccinelli
 *a
*a
aLuminescent Materials Laboratory, DB, Università di Verona, and INSTM, UdR Verona, Strada Le Grazie 15, 37134 Verona, Italy. E-mail: fabio.piccinelli@univr.it
bUniv Lyon, ENS de Lyon, CNRS UMR 5182, Laboratoire de Chimie, Lyon F-69342, France. E-mail: olivier.maury@ens-lyon.fr
cUniv. Lyon, Institut Lumière Matière, UMR 5306 CNRS–Université Claude Bernard, 10 rue Ada Byron, Lyon 1, F-69622 Villeurbanne Cedex, France
dDipartimento Politecnico di Ingegneria e Architettura, Laboratorio di Tecnologie Chimiche, Università di Udine, via Cotonificio 108, 33100 Udine, Italy. E-mail: andrea.melchior@uniud.it
First published on 9th May 2024
Abstract
Two cationic enantiomeric complexes [(R,R)-[YbL]Cl and (S,S)-[YbL]Cl with L = N,N′-bis(2-pyridylmethyl)-1,2-(R,R or S,S)-cyclohexanediamine functionalized at sp3 N with picolinate antennae] have been synthesized and spectroscopically characterized in polar protic solvents, such as water and methanol. The Yb(III) luminescence at about 980 nm is efficiently sensitized upon excitation of the picolinate antenna in the UV spectral region around 330 nm. The complexes exhibit good CPL activity for the 2F5/2 → 2F7/2 magnetic dipole (MD) allowed transition (glum values of |0.02| and |0.04| at 984 nm and 1021 nm, respectively) and high solubility in aqueous solution. The YbL species is largely predominant at physiological pH (7.4), given its high thermodynamic stability (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βYbL = 20.98). As elucidated by DFT calculations, among the possible isomeric species the one characterized by the cis-O, O–N, N geometry was found to be dominant. Furthermore, one solvent molecule is bound to Ln(III) in water, giving rise to 9-fold coordination at the metal ion. The enantiomeric (R,R)-[YbL]Cl and (S,S)-[YbL]Cl complexes can be considered promising candidates as NIR-to-NIR chiroptical bioprobes also for in vivo experiments.
βYbL = 20.98). As elucidated by DFT calculations, among the possible isomeric species the one characterized by the cis-O, O–N, N geometry was found to be dominant. Furthermore, one solvent molecule is bound to Ln(III) in water, giving rise to 9-fold coordination at the metal ion. The enantiomeric (R,R)-[YbL]Cl and (S,S)-[YbL]Cl complexes can be considered promising candidates as NIR-to-NIR chiroptical bioprobes also for in vivo experiments.
Introduction
Materials chemistry and physics are increasingly pervaded by the possible technological application of circularly polarized luminescence (CPL).1,2 In particular, during the last few years the scientific community working in the field of chiral lanthanide(III)-based complexes has shown interest in the investigation of materials emitting CPL in the near-infrared (NIR) spectral region.3–13The specificity, selectivity and sensitivity which are typical of the chiroptical counterpart of emission spectroscopy (i.e., CPL) stemming from lanthanide(III) ions have proven to be very important in the context of biological applications, such as microscopy and bioassays.14–17 Only recently Stachelek et al. were able to determine, thanks to CPL, the enantioselective localization of the two enantiomeric Eu(III) complexes in two different cell environments (lysosomes and mitochondria).18 This contribution paves the way for the potential and unprecedented use of CPL in in vivo bioimaging experiments. So far, the use of chiroptical probes working in the near-infrared spectral region is still quite limited and the design of new NIR-to-NIR chiroptical probes would have a significant impact on their biological applications. Recently, some of us demonstrated that the Yb(III) luminescence can be efficiently sensitized upon excitation of a conjugated ligand system in different spectral regions: from UV to Vis.19,20 In order to efficiently sensitize the Ln(III) luminescence at NIR wavelengths, one possible strategy is the design of ligands including a two-photon absorbing chromophoric antenna capable of transferring the excitation energy to the Ln(III) ion. Two-photon (2P) excitation is a non-linear optical phenomenon which takes place when an excited state of a chromophore can be populated by the concomitant absorption of two photons, whose wavelength is twice that of the single photon excitation. This technique has been already conveniently employed in Ln(III)-based coordination compounds with potential biological applications.21–23 If the two-photon absorption phenomenon allows shifting of the excitation wavelength from UV (1P excitation) to NIR (2P excitation) the proper choice of the Ln(III) ions enables to produce NIR emission. Indeed, as we previously demonstrated, the luminescence of an Eu(III) complex (at 620 nm) can be sensitized upon NIR excitation (around 720 nm).24 In this context, the analogous Yb(III) complex bears high potential for in cellulo experiments as a NIR-to-NIR optical probe23 that is active in the short wavelength range of the so-called “biological window” (650–1450 nm).
As a logical extension of a recent work in which the Eu(III) and Sm(III) chiral complexes of ligand L [which is N,N′-bis(2-pyridylmethyl)-1,2-(R,R or S,S)-cyclohexanediamine functionalized at sp3 N with the picolinate antennae; Fig. 1] have been in-depth investigated,24 we synthesized and spectroscopically characterized in this contribution both the enantiomers of the chiral Yb(III)-based counterpart [namely (S,S)-[YbL]Cl and (R,R)-[YbL]Cl complexes depicted in Fig. 2]. The presence of conjugated picolinate antennae ensures 2P absorption features and thanks to a ligand-to-metal energy transfer process (antenna effect) the metal-centered luminescence can be triggered, like in the case of Eu(III) and Sm(III) complexes. We also demonstrated that the octadentate ligand system enables the emission of circularly polarized (CP) light by the coordinated Ln(III). All these aspects make this pair of complex enantiomers ideal candidates for emerging chiroptical applications in the field of in vivo bioimaging.
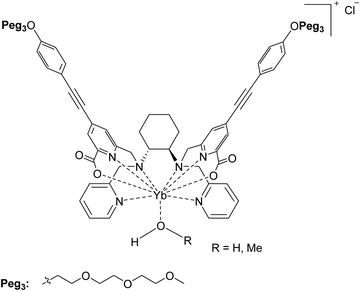 | ||
| Fig. 1 (S,S)-[YbL]Cl complex discussed in this contribution. Also the (R,R) enantiomer is considered in this work. | ||
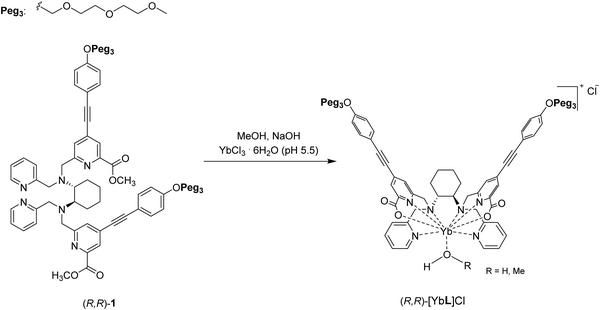 | ||
| Fig. 2 Synthetic pathway for the synthesis of the (S,S) or (R,R)-[YbL]Cl complexes. For the sake of simplicity, only the (R,R) enantiomer is reported. | ||
Experimental section
General
All reactions of air- and/or water-sensitive compounds were carried out under an inert gas atmosphere using standard Schlenk techniques. Solvents were purchased from Fisher Scientific, VWR Chemicals or Carlo Erba Reagents and used without further purification. All the solvents used for the synthesis were stored over 3 Å molecular sieves. Starting materials were purchased from Sigma-Aldrich, TCI, Alfa Aesar or Acros Organics. Column chromatography was performed using silica gel (40–63 μm) from VWR Chemicals. IR Spectra were recorded on a Spectrum 65, 100 and 400 series FT-IR spectrometer. Typical 32 scans were accumulated for each spectrum (resolution of 4 cm−1). High resolution mass spectrometry measurements (HR-MS) were performed at the Centre Commun de Spectrometrie de Masse (Villeurbanne, France) with a MicroOTOFQ II (Bruker) using electrospray ionization (ESI). Both the enantiomers of 1 and L (Fig. 2) have been synthesized, respectively, as reported in the literature.24 YbCl3·6H2O (Aldrich, 98%) has been stored under vacuum for several days at 80 °C and then transferred into a glove box.ESI-HR-MS (positive, CH3CN; m/z) calcd for [C62H68N6O12Yb]+: 1262.4278, found: 1262.4268 (S,S)-[YL]Cl, 1262.4275 (R,R)-[YbL]Cl, [M]+. See Fig. S1 (ESI†).
FT-IR (cm−1, ATR): C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 1634; C
O: 1634; C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C: 2206 (Fig. S2, ESI†).
C: 2206 (Fig. S2, ESI†).
Spectrophotometric titrations
Stock solutions of NaOH and HCl were prepared using Fixanal 0.1 M (Fluka Analytical) standard solution and ultrapure water (>18 MΩ cm) from a MilliQ system (ELGA Purelab UHQ). The ionic strength of all solutions was adjusted to 0.1 M with NaCl (Riedel-de Haën). Stock solutions of Yb(III) (14.6 mM) were prepared by dissolving the chloride hexahydrate salts (SigmaAldrich) and standardized by titration with EDTA and xylenol orange as the indicator in acetate buffer.25 Stock solutions of Zn(II) (15.0 mM) and Ca(II) (17.5 mM) were prepared by dissolving the chloride hexahydrate salts (SigmaAldrich) and standardized by titration with EDTA and eriochrome black T as the indicator in buffer pH 10.25 Complex formation constants were determined by combined potentiometric/UV-Vis titrations in the range pH = 1.96–11.47.26,27 Electromotive force (emf) data were collected by using a computer-controlled potentiometer (Amel Instruments, 338 pH Meter) connected to a combined glass electrode (Metrohm Unitrode 6.0259.100). The electrode was calibrated before each lecture by acid–base titration with standard HCl and NaOH solutions. The content of carbonate in solution, as well as free acid concentrations in stock metal ion solutions, was determined using Gran's method.28 Absorption spectra were collected using a Varian Cary 50 spectrophotometer equipped with an optical fiber probe (1 cm optical path length) which was inserted into the titration cell which contained a solution with the ligand (total concentration = 0.016 mM) and an equimolar quantity of Yb(III)/Zn(II)/Ca(II). In all cases, the titration cells were maintained at a constant temperature (25 °C) with a circulatory bath and under Ar radial flux to avoid carbonate contamination. Absorbance data at multiple (>40) wavelengths in the 250–380 nm range were analyzed using the HypSpec program29 to obtain the complex formation constants using the previously determined ligand pKa (pKa: HL = 9.65, H2L = 8.88, H3L = 3.60, H4L = 2.20).24DFT calculations
All molecular structures of the complexes were obtained by means of DFT calculations run in Gaussian 16 (rev. A.03).30 The ωB97X–D functional31 was used with the 6–31+G(d) basis set for all ligand atoms and MWB59 pseudopotential and valence electron basis set for Yb.32 To reduce the computational cost of the geometry searches, the PEGylated chain of L has been replaced by a methyl ether group (–OCH3). Geometry optimizations were carried including solvent (water) by means of the polarizable continuum model (PCM).33Photophysical measurements
Absorption spectra were recorded using a JASCO V-650 spectrophotometer in dilute solution (ca. 10−5 or 10−6 M), using spectrophotometric grade solvents. Emission spectra were measured using a Horiba–Jobin–Yvon Fluorolog-3 fluorimeter. The steady-state luminescence was excited by unpolarized light from a 450 W xenon continuous wave (CW) lamp and detected at an angle of 90° for measurements of dilute solutions (10 mm quartz cuvette) by using a Hamamatsu R928 photomultiplier tube. Spectra were corrected for both excitation source light–intensity variation and emission spectral responses. For luminescence lifetimes, the sample in an EPR quartz tube was excited using a pulsed Nd:YAG laser (SpectraPhysics), operating at 10 Hz. Light emitted at right angles to the excitation beam was focused onto the slits of a monochromator (PTI120), which was used to select the appropriate wavelength. The growth and decay of luminescence at selected wavelengths were detected using a Ge photodiode (Edinburgh Instruments, EI-P) and recorded using a digital oscilloscope (Tektronix TDS320) before being transferred for analysis. Luminescence lifetimes were obtained by iterative reconvolution of the detector response (obtained by using a scatterer) with exponential components for growth and decay of the metal-centered luminescence.Luminescence decay curves were fitted using the mono-exponential equation:
Chiroptical measurements
Measurements of circular dichroism in the UV/Vis and NIR regions were performed on a JASCO J-710 spectropolarimeter on complex solutions with concentrations around 10−5 (UV/Vis) and 1 M (NIR). CPL spectra were recorded with a bandwidth of 3 nm, on a homemade apparatus at the Institute of Light and Matter, Villeurbanne.34 Solutions (10−4 M) in a quartz cuvette are excited by UV light from a diode (365 nm). The fluorescence is collected with a lens and separated by means of an achromatic λ/4 waveplate (451) and a polarizing beam splitter. With this arrangement, the light is split into two components of either left- or right circularly polarized light. Each arm is further imaged on one side of a fiber bundle. The other extremity of the bundle is focused on the entrance slit of a spectrophotometer. The spectrally separated light is imaged on a CCD camera. The “upper/lower” part of the camera records the left- or right-handed circularly polarized spectra. Because of the brightness of the molecules as well as their high glum, this set-up allows fast recording of the CPL spectra in less than ten seconds.Results and discussion
Synthesis of ytterbium complexes
Both complexes have been obtained in high chemical yield [94% and 92% for (S,S)-[YbL]Cl and (R,R)-[YbL]Cl, respectively] following the synthetic path depicted in Fig. 2. The reaction was completed after 12 hours, when the signal of the ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at about 1725 cm−1 in the FT-IR absorption spectrum disappeared (Fig. S2, ESI†). Furthermore, the presence of the peak related to the [YbL]+ cation in the ESI-MS spectrum (Fig. S1, ESI†) confirmed the successful complexation reaction.
O stretching at about 1725 cm−1 in the FT-IR absorption spectrum disappeared (Fig. S2, ESI†). Furthermore, the presence of the peak related to the [YbL]+ cation in the ESI-MS spectrum (Fig. S1, ESI†) confirmed the successful complexation reaction.
Complex formation equilibria and structure
The complexation constants for ligand L are reported in Table 1, along with those relative to other ligands previously studied by some of us17 or others35–37 (Fig. 3) for comparison.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) for ligand L with Yb(III), Ca(II) and Zn(II) obtained at 25 °C in NaCl 0.1 M. Additional complex formation constants data with other ligands in Fig. 3 are also reported as a comparison. Charges omitted for clarity
β) for ligand L with Yb(III), Ca(II) and Zn(II) obtained at 25 °C in NaCl 0.1 M. Additional complex formation constants data with other ligands in Fig. 3 are also reported as a comparison. Charges omitted for clarity
| Equilibrium | L | bpcd 17 | CHXoctapa 36 | octapa | EDTA 35 | CDTA 35 | DTPA 35 |
|---|---|---|---|---|---|---|---|
L + Yb ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) YbL YbL |
20.98 ± 0.05 | — | 19.65 | 20.1037 | 19.6 | 21.2 | 22.7 |
YbL + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) YbLH YbLH |
4.29 ± 0.05 | — | 1.89 | 3.1437 | — | — | — |
YbLH + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) YbLH2 YbLH2 |
3.05 ± 0.04 | — | — | — | — | — | — |
YbL(OH) + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) YbL YbL |
11.84 ± 0.05 | — | 12.24 | 10.3237 | — | — | — |
L + Eu ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) EuL EuL |
20.1324 | 11.20 | — | — | 17.3 | 19.6 | 22.5 |
EuL + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) EuLH EuLH |
4.6924 | — | — | — | — | — | — |
EuLH + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) EuLH2 EuLH2 |
2.7624 | — | — | — | — | — | — |
L + Ca ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) CaL CaL |
8.29 ± 0.01 | — | 8.42 | 9.5536 | 10.5 | 13.1 | 10.7 |
CaL + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) CaLH CaLH |
— | — | 4.83 | 3.9236 | — | — | — |
L + Zn ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ZnL ZnL |
18.57 ± 0.17 | — | 16.97 | 18.9136 | 16.5 | 19.4 | 18.3 |
ZnL + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ZnLH ZnLH |
4.79 ± 0.18 | — | 4.04 | 3.9136 | — | — | — |
ZnLH + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ZnLH2 ZnLH2 |
3.86 ± 0.19 | — | 3.15 | 3.5436 | — | — | — |
ZnLH2 + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ZnLH3 ZnLH3 |
2.96 ± 0.17 | — | 1.34 | — | — | — | — |
ZnL(OH) + H ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ZnL ZnL |
8.52 ± 0.17 | — | 11.63 | — | — | — | — |
The spectrophotometric data relative to the complex formation (Fig. 3a) were best fitted by a speciation model including the formation of four Yb species (YbL, YbLH, YbLH2 and YbL(OH), Table 1) whose calculated molar absorbances are reported in Fig. S3 (ESI†). The speciation diagram of the Yb(III) complexes is shown in Fig. 4b, along with the molar absorbance at 332 nm (ε332) as a function of pH. A marked decrease in absorbances at 313 and 332 nm between pH 2 and 4 is present in correspondence with the YbLH2 and YbLH (Fig. 4b) species. At pH > 4, the YbL complex starts to form, coinciding with an overall rise in ε332, and continues until the pH reaches 10. Subsequently, a decrease in ε332 and ε313 is observed as the YbL(OH) species begins to form.
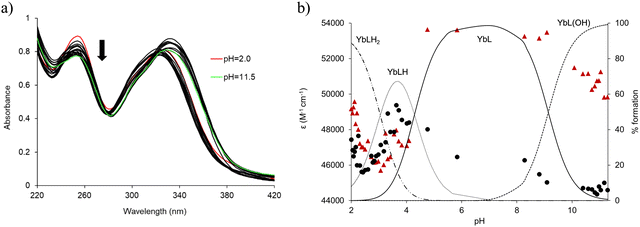 | ||
| Fig. 4 (a) UV-vis absorption spectra changes during the acid–base titration of the ligand L (0.016 mM) in the presence of an equimolar quantity of Yb(III); (b) evolution of the molar absorbance (ε) at λ = 313 (●) and 332 (▲) nm during the titration and species distribution relative to the total metal in solution calculated on the basis of the equilibrium constants in Table 1. Charges are omitted for clarity. | ||
The formation constant obtained for the YbL species is comparable to that of other Ln(III) complexes with similar acyclic ligands containing picolinate chelating groups,24,36 and compatible with the formation of an octadentate complex. The stability of the complexes with L experiences a slight increase from Eu(III) to Yb(III), as a consequence of the increasing charge density of the metal ion due to lanthanide contraction38,39 as often found for Ln(III) complexes with similar open-chain ligands (Fig. 3 and Table 1).40,41 Under physiological pH (7.4) and a L/Yb molar ratio of 1, the YbL species is largely dominant (>98%, Fig. 4). The formation constants obtained for the complexes with other metal ions of biological importance Ca(II) and Zn(II) are significantly lower than those found for Yb(III) (Table 1 and Fig. S4, ESI†). The selectivity, similar to that previously found for the complexes formed with octapa and CHXoctapa36 with Zn(II) and slightly higher for Ca(II) (Table 1), supports the application of YbL in biological media.
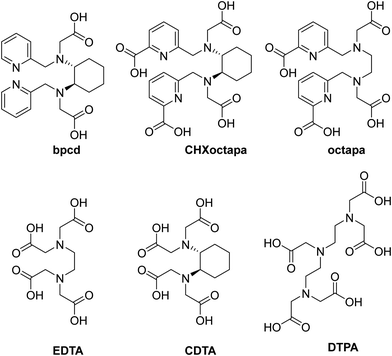
The possible structures of the related Eu(III) and Sm(III) complexes in solution were previously proposed on the basis of DFT calculations.24 In the case of Yb(III), the complexes result to be isostructural with those obtained previously for Y(III), with the water molecule retained in the coordination sphere. This result was not predictable, as the decrease of the ionic radius of the lanthanide can cause a decrease of the coordination number, as observed in complexes with macrocyclic polyaminocarboxylic ligands.42 As previously found,24 the trans-O,O and trans-N,N isomers (Fig. 5a and b respectively) are practically equal in energy (ΔE < 0.1 kcal mol−1), while the cis-O,O–N,N isomer (Fig. 5c) (4.2 kcal mol−1) is more stable, and therefore it can be proposed as the dominant species in solution.
Photophysical properties
The absorption (λmax, εmax), emission [2F5/2 lifetime of Yb(III)] and chiroptical properties [CD (gabs) and CPL (glum)] of the Yb complexes have been studied in water and methanol solutions and all the relevant data are reported in Table 2.| Complex | Solvent | λ max (nm) | ε max (M−1 cm−1) | τ (μs) | g abs | g lum |
|---|---|---|---|---|---|---|
| a = Deuterated water. b = Deuterated methanol. | ||||||
| (S,S)-[YbL]Cl | H2O | 333 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.0 | 0.0002 (259 nm) | −0.008 (970 nm) |
| 979 | 4 | (8.6)a | 0.0002 (302 nm) | 0.024 (984 nm) | ||
| −0.0001 (948 nm) | −0.034 (1021 nm) | |||||
| 0.0003 (982 nm) | ||||||
| MeOH | 336 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3.4 | 0.0003 (259 nm) | ||
| 979 | 9 | (10.2)b | 0.0004 (285 nm) | 0.015 (980 nm) | ||
| −0.001 (942 nm) | −0.038 (1023 nm) | |||||
| 0.003 (978 nm) | ||||||
| (R,R)-[YbL]Cl | H2O | 333 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.9 | −0.0002 (259 nm) | 0.017 (970 nm) |
| 979 | 5 | (8.5)a | −0.0003 (302 nm) | −0.017 (984 nm) | ||
| 0.0002 (948 nm) | 0.043 (1021 nm) | |||||
| −0.0004 (982 nm) | ||||||
| MeOH | 336 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3.3 | −0.0004 (259 nm) | ||
| 979 | 11 | (9.5)b | −0.0005 (285 nm) | −0.013 (980 nm) | ||
| 0.001 (942 nm) | 0.038 (1023 nm) | |||||
| −0.003 (978 nm) | ||||||
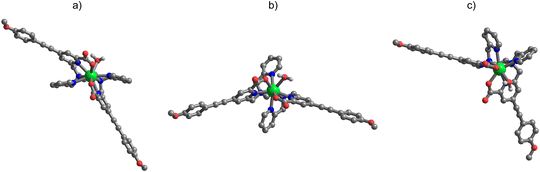
The absorption spectra, in general independent of the nature of the lanthanide ions, are identical to the ones recorded for the Eu and Sm-based counterparts.24 In water, they show two strong absorption peaks at 254 nm (ε around 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and 336 nm (ε around 44
000 M−1 cm−1) and 336 nm (ε around 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–47
000–47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) (Fig. 6).
000 M−1 cm−1) (Fig. 6).
The transition at 245 nm is related to the pyridine ring absorption17 whilst the broad and structureless band centered at 336 nm is assigned to the ILCT transition. The typical 2F5/2 ← 2F7/2 Yb(III) absorption band in the NIR spectral range around 980 nm is also present (Fig. 6, up, right). Due to the parity forbidden nature of the f–f lanthanide(III) transition, the molar extinction coefficient (around 5 M−1 cm−1) is four orders of magnitude lower, compared to the transitions in the UV range discussed above. Almost identical conclusions can be drawn when the absorption spectra were collected in methanol (Fig. S5, ESI†).
While the absorption spectra in both the UV/Vis and the NIR regions are identical for the two complex enantiomers, their differential absorption of left- and right-handed CP light was demonstrated by electronic circular dichroism (ECD) measurements. In both methanol and aqueous solutions, the spectra exhibit the characteristic mirror image structure for the two enantiomers (Fig. 6, bottom and Fig. S5, ESI†). In addition to the ligand-based effect observable in the UV/Vis spectral range, the ECD was also investigated in the NIR region using concentrated solutions (c ≈ 1.5 M).
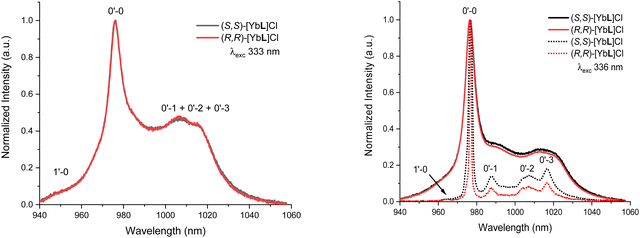
As for the complexes in aqueous solution, upon excitation at 333 nm (in the ILCT transition), the typical NIR emission pattern of Yb(III) is induced (Fig. 7, left).
This demonstrated the occurrence of an antenna effect involving the picolinate-based conjugated chromophore. At room temperature, the Yb(III) emission is broad and poorly resolved with a shoulder at higher energy assigned to the “hot band” originated for thermally populated mJ’ excited micro-states (1′–0). In order to increase the resolution, low temperature (77 K) measurements were conducted in an organic glass. In this condition, the hot band disappeared and the spectra are more resolved with the clear observation of four distinct transitions (0′ → 0–4) as expected for the 2F7/2 ground state crystal field splitting (Fig. 7, right). Despite the low temperature, a close inspection of this spectrum reveals the presence of a broader band in the 1000–1015 nm range with respect to the 0′–1 and 0′–3 transitions. This can be due to the presence of 1′ → (1/2/3) transitions that, even though very weak, can contribute to broaden the peak attributed to the 0′–2 peak. Alternatively, another possible explanation is that the vibronic coupling, quite important in Yb(III) electronic emission and absorption spectra, can affect the 0′–2 transition more in these complexes.
The photophysical properties of the S,S-[GdL]Cl complex have been recently published in order to determine the energy diagram of the coordinating ligand.24 These data revealed an energy level of the relaxed excited ILCT state [E(ILCT*)] is at about 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1, whilst the triplet state associated with the phosphorescence of the Gd(III) complex shows an energy position at 20
500 cm−1, whilst the triplet state associated with the phosphorescence of the Gd(III) complex shows an energy position at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 cm−1. A comparison with the energy levels of the accepting Yb(III) state (in the 10
830 cm−1. A comparison with the energy levels of the accepting Yb(III) state (in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–11
000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 range) clearly indicates the presence of a big energy gap between the donor and acceptor levels, which could negatively impact the ligand-to-metal energy transfer efficiency.
000 cm−1 range) clearly indicates the presence of a big energy gap between the donor and acceptor levels, which could negatively impact the ligand-to-metal energy transfer efficiency.
Nevertheless, the sensitization of the Yb(III) emission is not usually accounted for in the frame of the classical Förster–Dexter theory, precisely when a large mismatch between the involved electronic levels is present. So far, three mechanisms have been invoked in this context. The first one occurs by an internal redox process involving the Yb(III)/Yb(II) redox couple and the excited state of the ligand43 while the second proceeds via an internal conversion inside the energy levels of the Yb(III)-ligand system considered as a single chromophore.44 The third mechanism foresees the involvement of a ligand-to-metal charge transfer (LMCT) state.45–47 In the present case, the available information does not allow us to discriminate among the three proposed mechanisms and the in-depth study of this aspect is beyond the scope of the present contribution.
The luminescence decay curve of the Yb(III) 2F5/2 excited state can be fitted by a single exponential function in all the employed solvents (water, deuterated water, methanol, deuterated methanol; Fig. 8 and Fig. S6, ESI†).
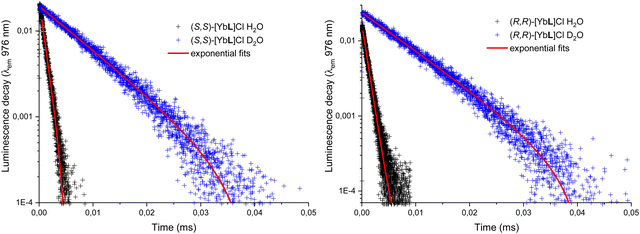 | ||
| Fig. 8 Luminescence decay of the 2F5/2 level of Yb(III) for both the enantiomers (S,S) (left) and (R,R) (right) of [YbL]Cl complex in water and deuterated water. Excitation at 333 nm. | ||
For both water and methanol, the calculated 2F5/2 lifetime increases significantly when the deuterated solvent is used (Table 1). This is in agreement with the presence of solvent molecules in the inner coordination sphere of the complexes capable of quenching the Yb(III) excited state by means of a non radiative multiphonon relaxation (MPR) process.48 This process is favored by the presence of one (in the case of methanol) or two (in the case of water) sources of high energy (O–H) vibrations, with a concomitant decrease in the lifetime. In contrast, O–D vibrations in the close proximity of the metal ion are much less efficient in the quenching of Yb(III) luminescence and the value of the excited state lifetime is higher. It is possible to estimate q and m, that are the number of water and methanol molecules in the inner coordination sphere, by using the equations 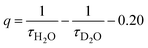 and
and 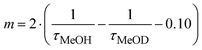 , where τ is the 2F5/2 observed lifetimes in μs.49 We calculate the values of q = 1 and m = 0.2, indicating that no solvent molecules are bound to the metal center in the case of methanol. In contrast, one water molecule is present in the first coordination sphere, as observed in the DFT minimum energy structure reported in Fig. 3. The change of Yb(III) coordination number (9 in water, 8 in methanol) could explain the observed differences in the chiroptical properties, such as NIR-ECD spectra (Fig. 6 and Fig. S5, ESI†) and NIR-CPL spectra (Fig. 9).
, where τ is the 2F5/2 observed lifetimes in μs.49 We calculate the values of q = 1 and m = 0.2, indicating that no solvent molecules are bound to the metal center in the case of methanol. In contrast, one water molecule is present in the first coordination sphere, as observed in the DFT minimum energy structure reported in Fig. 3. The change of Yb(III) coordination number (9 in water, 8 in methanol) could explain the observed differences in the chiroptical properties, such as NIR-ECD spectra (Fig. 6 and Fig. S5, ESI†) and NIR-CPL spectra (Fig. 9).
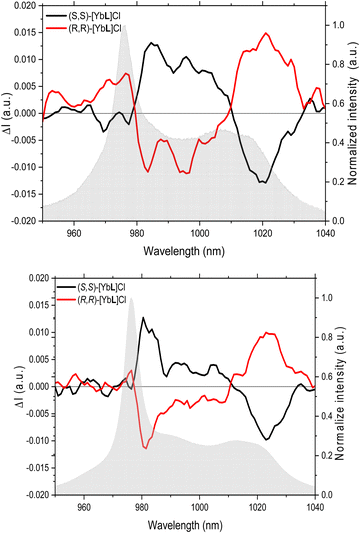 | ||
| Fig. 9 CPL spectra of (S,S)-[YbL]Cl and (R,R)-[YbL]Cl complexes in water (up) and in methanol (bottom) emitting in the near infrared region. Excitation at 365 nm. | ||
The capability of the enantiopure complexes to emit circularly polarized luminescence was studied by means of a home-made setup.34 Indeed, upon excitation at 365 nm (in the ILCT transition), the two enantiomers of the [YbL]Cl complex show a NIR CPL mirror image, both in water and methanol (Fig. 9).
The extent of the luminescence chiroptical activity, quantified by the values of glum (in the 0.01–0.04 range; Table 1) is in line with already reported examples of Yb(III) chiral complexes.11,50 As we recently demonstrated for the ligand L,24 the same excited state involved in the sensitization process of Eu(III) and Sm(III) luminescence (and Yb(III) in the present contribution) can be efficiently populated by both 1P and 2P excitation (at 337 nm and 720 nm, respectively). Hence, the emission of the complex does not depend on the nature of the sensitization process and the corresponding excitation source (1P or 2P excitation). Transferring this approach on the respective chiroptical properties, we can reasonably assume that (even though we do not directly prove that) the same CPL signal can be triggered upon both 1P and 2P excitation. The possibility of obtaining CPL at NIR wavelengths upon excitation in the same spectral range (720 nm) paves the way for the design of a new generation of lanthanide(III) complexes as potential NIR-to-NIR chiroptical probes for biomedical applications.
Conclusions
In this contribution, new Yb(III)-based enantiomeric complexes are synthesized and characterized from a thermodynamic and a spectroscopic point of view. The obtained high stability of the YbL species and selectivity with respect to Zn(II) and Ca(II) prevent the competition and release of the metal ion in the biological medium, which is an essential feature of a metal complex to be potentially employed in bioimaging experiments.DFT calculations suggest that among the three possible isomeric complexes, differing by the stereochemistry at sp3 N atoms of ligand L (trans-OO, trans-NN and cis-OO,NN), the latter one is dominant in aqueous solution. It is noteworthy that the Yb(III) luminescence in the NIR is circularly polarized (the highest glum value is |0.04| at 1021 nm) and can be efficiently sensitized upon excitation of the ligand into the picolinate-based absorption band at about 330 nm. As it is well known that this antenna exhibits a good two-photon cross-section (around 110 GM, at 700 nm), the Yb(III) luminescence can also be potentially triggered by excitation in the NIR spectral region. As the same electronic transition of the picolinate antenna, showing a charge transfer (CT) character, can be triggered by 2P absorption and the Ln(III) emission is not affected by the nature of the sensitization process (1P or 2P excitation), we can reasonably assume that the observed CPL signal can be obtained upon both 1P (in the UV range) and 2P (in the NIR range) excitation of the ligand. Our Yb(III)-based chiroptical complex is expected to play an important role in emerging chiroptical applications in the biological field, such as the study of live cell chiral molecular interactions. All that could be also applied in in vivo experiments, as [YbL]Cl can completely work in the so-called biological windows.
Author contributions
OM, AM and FP: conceptualization, writing the original draft; SM, AS and MS: investigation and methodology; SR and FB: supervision and data curation; LG, BB, AB-L, YG and SG: formal analysis and validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SM, SR, MS, AM and FP thank the Italian Ministry of University and Research for the received funds [PRIN (Progetti di Ricerca di Rilevante Interesse Nazionale, Bando 2022 PNRR) project “TheCURA”, Grant No. P20222TPZS]. SM, SR and FP gratefully also thank the Facility “Centro Piattaforme Tecnologiche” of the University of Verona for access to the instrumentation. Funding from the University of Verona is gratefully acknowledged. Article processing charges were supported by the special fund at the University of Verona dedicated to Open Access publications. AM and MS acknowledge the University of Udine for Research financed with European Union funds – NextGenerationEU—MSCA grants D.M. 737/2021—CUPG25F21003390007. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank the Agence Nationale de la Recherche (SMMCPL ANR-19-CE29-0012-02) for financial support and a grant to AS.References
- H.-Y. Wong, W.-S. Lo, K.-H. Yim and G.-L. Law, Chem, 2019, 5, 3058–3095 CAS.
- Y. Kitagawa, S. Wada, M. D. J. Islam, K. Saita, M. Gon, K. Fushimi, K. Tanaka, S. Maeda and Y. Hasegawa, Commun. Chem., 2020, 3, 1–5 CrossRef PubMed.
- O. G. Willis, F. Zinna and L. Di Bari, Angew. Chem., Int. Ed., 2023, 62, e202302358 CrossRef CAS PubMed.
- O. G. Willis, F. Petri, G. Pescitelli, A. Pucci, E. Cavalli, A. Mandoli, F. Zinna and L. Di Bari, Angew. Chem., Int. Ed., 2022, 61, e202208326 CrossRef CAS PubMed.
- N. F. M. Mukthar, N. D. Schley and G. Ung, J. Am. Chem. Soc., 2022, 144, 6148–6153 CrossRef CAS PubMed.
- B.-A. N. Willis, D. Schnable, N. D. Schley and G. Ung, J. Am. Chem. Soc., 2022, 144, 22421–22425 CrossRef CAS PubMed.
- O. G. Willis, A. Pucci, E. Cavalli, F. Zinna and L. Di Bari, J. Mater. Chem. C, 2023, 11, 5290–5296 RSC.
- K. Dhbaibi, M. Grasser, H. Douib, V. Dorcet, O. Cador, N. Vanthuyne, F. Riobé, O. Maury, S. Guy, A. Bensalah-Ledoux, B. Baguenard, G. L. J. A. Rikken, C. Train, B. Le Guennic, M. Atzori, F. Pointillart and J. Crassous, Angew. Chem., Int. Ed., 2023, 62, e202215558 CrossRef CAS PubMed.
- B. Lefeuvre, C. A. Mattei, J. F. Gonzalez, F. Gendron, V. Dorcet, F. Riobé, C. Lalli, B. Le Guennic, O. Cador, O. Maury, S. Guy, A. Bensalah-Ledoux, B. Baguenard and F. Pointillart, Chem. – Eur. J., 2021, 27, 7362–7366 CrossRef CAS PubMed.
- J. A. Adewuyi, N. D. Schley and G. Ung, Chem. – Eur. J., 2023, 29, e202300800 CrossRef CAS PubMed.
- E. Cavalli, C. Nardon, O. G. Willis, F. Zinna, L. Di Bari, S. Mizzoni, S. Ruggieri, S. C. Gaglio, M. Perduca, C. Zaccone, A. Romeo and F. Piccinelli, Chem. – Eur. J., 2022, 28, e202200574 CrossRef CAS PubMed.
- O. G. Willis, F. Zinna, G. Pescitelli, C. Micheletti and L. Di Bari, Dalton Trans., 2022, 51, 518–523 RSC.
- L. Wang, Z. Yao, W. Huang, T. Gao, P. Yan, Y. Zhou and H. Li, Inorg. Chem. Front., 2023, 10, 3664–3674 RSC.
- J. Yuasa, T. Ohno, H. Tsumatori, R. Shiba, H. Kamikubo, M. Kataoka, Y. Hasegawa and T. Kawai, Chem. Commun., 2013, 49, 4604–4606 RSC.
- S. Orsini, F. Zinna, T. Biver, L. D. Bari and I. Bonaduce, RSC Adv., 2016, 6, 96176–96181 RSC.
- M. Leonzio, A. Melchior, G. Faura, M. Tolazzi, M. Bettinelli, F. Zinna, L. Arrico, L. Di Bari and F. Piccinelli, New J. Chem., 2018, 42, 7931–7939 RSC.
- M. Leonzio, A. Melchior, G. Faura, M. Tolazzi, F. Zinna, L. Di Bari and F. Piccinelli, Inorg. Chem., 2017, 56, 4413–4422 CrossRef CAS PubMed.
- P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed.
- E. Cavalli, S. Ruggieri, S. Mizzoni, C. Nardon, M. Bettinelli and F. Piccinelli, Results Chem., 2022, 4, 100388 CrossRef CAS.
- F. Piccinelli, S. Mizzoni, G. Zanella, S. C. Gaglio, M. Perduca, A. Romeo, S. Ruggieri, C. Nardon and E. Cavalli, Molecules, 2023, 28(5), 2251 CrossRef CAS PubMed.
- A. D’Aléo, A. Bourdolle, S. Brustlein, T. Fauquier, A. Grichine, A. Duperray, P. L. Baldeck, C. Andraud, S. Brasselet and O. Maury, Angew. Chem., Int. Ed., 2012, 51, 6622–6625 CrossRef PubMed.
- M. Soulié, F. Latzko, E. Bourrier, V. Placide, S. J. Butler, R. Pal, J. W. Walton, P. L. Baldeck, B. Le Guennic, C. Andraud, J. M. Zwier, L. Lamarque, D. Parker and O. Maury, Chem. – Eur. J., 2014, 20, 8636–8646 CrossRef PubMed.
- N. Hamon, A. Roux, M. Beyler, J.-C. Mulatier, C. Andraud, C. Nguyen, M. Maynadier, N. Bettache, A. Duperray, A. Grichine, S. Brasselet, M. Gary-Bobo, O. Maury and R. Tripier, J. Am. Chem. Soc., 2020, 142, 10184–10197 CrossRef CAS PubMed.
- S. Mizzoni, S. Ruggieri, A. Sickinger, F. Riobé, L. Guy, M. Roux, G. Micouin, A. Banyasz, O. Maury, B. Baguenard, A. Bensalah-Ledoux, S. Guy, A. Grichine, X.-N. Nguyen, A. Cimarelli, M. Sanadar, A. Melchior and F. Piccinelli, J. Mater. Chem. C, 2023, 11, 4188–4202 RSC.
- A. I. Vogel and J. Mendham, Vogel's textbook of quantitative chemical analysis, Prentice Hall, 2000 Search PubMed.
- F. Endrizzi, P. Di Bernardo, P. L. Zanonato, F. Tisato, M. Porchia, A. A. Isse, A. Melchior and M. Tolazzi, Dalton Trans., 2017, 46, 1455–1466 RSC.
- A. C. Mendonça, A. F. Martins, A. Melchior, S. M. Marques, S. Chaves, S. Villette, S. Petoud, P. L. Zanonato, M. Tolazzi, C. S. Bonnet, É. Tóth, P. Di Bernardo, C. F. G. C. Geraldes and M. A. Santos, Dalton Trans., 2013, 42, 6046–6057 RSC.
- G. Gran, Analyst, 1952, 77, 661–670 RSC.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739–1753 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev.A03, Wallingford, CT Search PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1989, 90, 1730–1734 CrossRef CAS.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- B. Baguenard, A. Bensalah-Ledoux, L. Guy, F. Riobé, O. Maury and S. Guy, Nat. Commun., 2023, 14, 1065 CrossRef CAS PubMed.
- R. M. Smith and A. E. Martell, Sci. Total Environ, 1987, 64, 125–147 CrossRef CAS.
- F. Lucio-Martínez, Z. Garda, B. Váradi, F. K. Kálmán, D. Esteban-Gómez, É. Tóth, G. Tircsó and C. Platas-Iglesias, Inorg. Chem., 2022, 61, 5157–5171 CrossRef PubMed.
- M. de Guadalupe Jaraquemada–Peláez, X. Wang, T. J. Clough, Y. Cao, N. Choudhary, K. Emler, B. O. Patrick and C. Orvig, Dalton Trans., 2017, 46, 14647–14658 RSC.
- J. A. Peters, K. Djanashvili, C. F. G. C. Geraldes and C. Platas-Iglesias, Coord. Chem. Rev., 2020, 406, 213146 CrossRef CAS.
- P. Di Bernardo, A. Melchior, M. Tolazzi and P. L. Zanonato, Coord. Chem. Rev., 2012, 256, 328–351 CrossRef CAS.
- M. Regueiro-Figueroa, D. Esteban-Gómez, A. de Blas, T. Rodríguez-Blas and C. Platas-Iglesias, Chem. – Eur. J., 2014, 20, 3974–3981 CrossRef CAS PubMed.
- P. K. Tse and J. E. Powell, Inorg. Chem., 1985, 24, 2727–2730 CrossRef CAS.
- M. Woods, K. M. Payne, E. J. Valente, B. E. Kucera and V. G. Young Jr., Chem. – Eur. J., 2019, 25, 9997–10005 CrossRef CAS PubMed.
- W. D. Horrocks, J. P. Bolender, W. D. Smith and R. M. Supkowski, J. Am. Chem. Soc., 1997, 119, 5972–5973 CrossRef CAS.
- C. Reinhard and H. U. Güdel, Inorg. Chem., 2002, 41, 1048–1055 CrossRef CAS PubMed.
- V. A. Ilichev, A. V. Rozhkov, R. V. Rumyantcev, G. K. Fukin, I. D. Grishin, A. V. Dmitriev, D. A. Lypenko, E. I. Maltsev, A. N. Yablonskiy, B. A. Andreev and M. N. Bochkarev, Dalton Trans., 2017, 46, 3041–3050 RSC.
- A. Masuya-Suzuki, S. Goto, T. Kambe, R. Karashimada, Y. Kubota and N. Iki, ChemistryOpen, 2021, 10, 46–55 CrossRef CAS PubMed.
- Y.-J. Liang, F. Liu, Y.-F. Chen, X.-J. Wang, K.-N. Sun and Z. Pan, Light: Sci. Appl., 2016, 5, e16124 CrossRef CAS PubMed.
- E. Kreidt, C. Kruck and M. Seitz, in Handbook on the Physics and Chemistry of Rare Earths, ed. J.-C. G. Bünzli and V. K. Pecharsky, Elsevier, 2018, vol. 53, pp. 35–79 Search PubMed.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. G. Williams and M. Woods, J. Chem. Soc., Perkin Trans. 2, 1999, 493–504 RSC.
- F. Zinna, L. Arrico and L. D. Bari, Chem. Commun., 2019, 55, 6607–6609 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj00550c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |