Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Adsorption of gases on B12N12 and Al12N12 nanocages†
Remya
Geetha Sadasivan Nair
 *,
Arun Kumar
Narayanan Nair
*,
Arun Kumar
Narayanan Nair
 * and
Shuyu
Sun
*
* and
Shuyu
Sun
*
Physical Science and Engineering Division (PSE), Computational Transport Phenomena Laboratory, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: remya.nair@kaust.edu.sa; arun.narayanannair@kaust.edu.sa; shuyu.sun@kaust.edu.sa
First published on 1st April 2024
Abstract
Density functional theory (DFT) was used to investigate the adsorption of twenty-four gases (SiH4, H2, Cl2, F2, CF4, CH4, CF2Cl2, N2, CHF3, OCS, N2O, AsH3, CH3Cl, COCl2, C2H2, C2H4, H2Se, H2S, PH3, COF2, CH3F, HCHO, (CH3)2O, and CH3NH2) on B12N12 and Al12N12 nanocages. Most of the studied gases are weakly (strongly) adsorbed on the B12N12 (Al12N12) nanocage. However, AsH3, H2Se, H2S, PH3, CH3F, HCHO, (CH3)2O, and CH3NH2 are strongly adsorbed on the B12N12 nanocage and H2, F2, CF4, CH4, and CF2Cl2 are weakly adsorbed on the Al12N12 nanocage. The most negative-valued molecular electrostatic potential (MESP) minimum (Vmin) corresponds to the electron-rich region (e.g., lone pair and π-bond) in the molecule. An important observation is that the adsorption energies of the gases on the B12N12 and Al12N12 nanocages are well correlated with the MESP Vmin values of the gases. Substantial changes are found in the DFT reactivity indices like chemical potential and hardness of the B12N12 and Al12N12 nanocages, mainly due to the strong gas adsorption. The quantum theory of atoms in molecules analysis suggests the covalent nature of interactions only in the AsH3/B12N12, H2Se/B12N12, H2S/B12N12, and PH3/B12N12 systems.
1. Introduction
Air pollution due to rapid urbanization and economic developments is a major environmental concern. Methane (CH4) and nitrous oxide (N2O) are the most important anthropogenic contributors to the greenhouse effect after carbon dioxide (CO2).1,2 Enteric fermentation and the oil and gas industry have been identified as the two main sources of anthropogenic CH4. The use of nitrogen fertilizers is identified as an important source of nitrous oxide (N2O) emissions. Hydrogen sulfide (H2S) is a highly toxic gas released from sewage treatment processes, fuel burning, and petroleum extraction.3 Carbonyl sulfide (OCS) is released from the burning of biomass and fossil fuel, as well as petrochemical and gasification processes.4 Halomethanes are found both in marine environments and those of man-made origin, and some of them are widespread and hazardous.5,6 For example, the ultraviolet photolysis of refrigerants such as dichlorodifluoromethane (CF2Cl2) can damage the stratospheric ozone layer. Silane (SiH4) finds applications in the photovoltaic and semiconductor industries.7 Hydrogen selenide (H2Se) is used in the preparation of materials for battery applications.8 Chlorine (Cl2) can be used for the sterilization of drinking water.9 Fluorine (F2) plays a key role in the pharmaceutical industry.10 The pnictogen hydride gases phosphine (PH3)11 and arsine (AsH3)12 are used as a fumigant and in the semiconductor industry, respectively. Phosgene (COCl2) is of historical interest (chemical weapon in World War I) and currently used in the manufacture of polyurethanes, polycarbonates, dyestuff, pharmaceuticals, etc.13 The usage of carbonyl fluoride (COF2) as a cleaning gas could reduce the greenhouse gas emissions from the semiconductor industry.14 Formaldehyde (HCHO) is mainly used to synthesize resins.15 Dimethyl ether ((CH3)2O) can be used as an aerosol propellant and as an alternative to diesel fuel.16 Methylamine (CH3NH2) is used for fabricating perovskite solar cells.17 However, these gases could cause severe health and safety concerns. Hydrogen (H2) storage is a key issue in developing materials which could be utilized for fuel cell technology.18 Nitrogen (N2) is often used in modeling the adsorption process.19 There are growing interests in gas capture and storage necessitated by concerns arising from the demand to mitigate air pollution. Materials like metal–organic framework, zeolite, graphene, fullerene, carbon nanotube, polymer, clay, cyclo[n]carbon, etc. exhibit promising potential for applications in gas capture and storage.20–27There have been density functional theory (DFT) studies of the gas-adsorption on nanoparticles and nanosheets. Abbasi et al. found that the adsorption of NO2, CH2O and H2S molecules on the N-doped TiO2 anatase nanoparticles is energetically more favorable than the adsorption on the pristine ones.28–30 The adsorption behaviors of SOx molecules showed an improved adsorption ability for N-doped ZnO nanoparticles over undoped nanoparticles.31 The interaction of O3 and NO2 with the N-doped TiO2/ZnO nanocomposite was stronger than with the pristine one.32 The noble metal (Rh, Pt, Pd) decorated N-doped graphene may potentially be used as sensors for biogas detection.33 The B-doped stanene may be a good candidate for gas sensing.34,35 It was found that the O3, SO2, and SO3 molecules weakly interact with the MoS2 monolayer.36 The adsorption of CO and NO molecules on the doped MoS2 monolayers was more favorable in energy than that on the pristine monolayers.37
The gas-adsorption on fullerene-like B12N12 and Al12N12 nanocages has attracted a lot of interest in recent years.27,38–51 The synthesis of the fullerene-like B12N12 nanocage clusters has been reported.52 There have been reports of the synthesis of AlN nanotube and nanocone.53,54 The Al12N12 nanocage has been computed to be the most stable of the AlnNn (n = 2–41) nanocages.55 The DFT studies showed that the adsorption of CH4 has no significant effect on the electronic properties of the B12N12 nanocage.38 H2S showed a weak physisorption on the B12N12 nanocage.39 The formaldehyde adsorption induced considerable variation in the electronic properties of the B12N12 nanocage.40 The adsorption of N2 and F2 showed negligible changes on the electronic and structural properties of the B12N12 nanocage.41 Higher values of adsorption energy were obtained for CH3F than for CH3Cl on the B12N12 nanocage.42 NH3 molecule was adsorbed on the B12N12 nanocage with considerable adsorption energy, while the PH3 and AsH3 molecules were relatively weakly adsorbed.43 DFT studies showed that CH3Cl and CH3F strongly interact with the Al12N12 nanocage.44 It was found that H2S and OCS strongly interact with the Al12N12 nanocage.45 Acetylene and ethylene interact preferably with an Al atom rather than N atom of the Al12N12 nanocage.46 A very weak adsorption of H2 on the B12N12 and Al12N12 nanocages has been reported.47–49 It was found that dimethyl ether chemisorbed on the B12N12 and Al12N12 nanocages.50 The adsorption of phosgene molecules on the B12N12 (Al12N12) nanocage proceeds by way of physisorption (chemisorption).51 We recently investigated the adsorption process of NO, CO2, CO, and NH3 on the B12N12 and Al12N12 nanocages using DFT calculations.27 It was found that, for example, NH3 chemisorbed on the B12N12 and Al12N12 nanocages.27 However, adsorption of gases like SiH4 on the B12N12 and Al12N12 nanocages has yet to be studied.
In this study, we perform DFT calculations to understand the adsorption behavior of SiH4, H2, Cl2, F2, CF4, CH4, CF2Cl2, N2, CHF3, OCS, N2O, AsH3, CH3Cl, COCl2, C2H2, C2H4, H2Se, H2S, PH3, COF2, CH3F, HCHO, (CH3)2O, and CH3NH2 on the B12N12 and Al12N12 nanocages. Most of the studied gases are weakly (strongly) adsorbed on the B12N12 (Al12N12) nanocage. The most negative-valued molecular electrostatic potential (MESP) minimum (Vmin) corresponds to the electron-rich region (e.g., lone pair and π-bond) in the molecule.56–58 An important observation is that the adsorption energies of the gases on the B12N12 and Al12N12 nanocages are well correlated with the MESP Vmin values of the gases. Our results are expected to contribute to the exploration and development of promising adsorbents for gas capture and sequestration.
2. Computational details and calculations
We recently studied the adsorption of NO, CO2, CO, and NH3 on B12N12 and Al12N12 nanocages using DFT calculations.27 Here we study the adsorption of an additional 24 gases (SiH4, H2, Cl2, F2, CF4, CH4, CF2Cl2, N2, CHF3, OCS, N2O, AsH3, CH3Cl, COCl2, C2H2, C2H4, H2Se, H2S, PH3, COF2, CH3F, HCHO, (CH3)2O, and CH3NH2) on B12N12 and Al12N12 nanocages using DFT calculations. All the DFT calculations were performed with the Gaussian 16 code.59 All structures were optimized at the M062X/6-311G(d,p) level.60 The harmonic frequencies (all positive) were computed to confirm the minimal nature of the optimized structures. The MESP topographical analysis of molecules were performed at the M062X/6-311G(d,p) level. The MESP, V(r), is expressed as56–58 | (1) |
The DFT reactivity indices are given by61–66
 | (2) |
 | (3) |
 | (4) |
 | (5) |
The adsorption energy (Eads) is given by
| Eads = Egas/nanocage − (Enanocage + Egas) | (6) |
The adsorption free energy (Gads) is given by
| Gads = Ggas/nanocage − (Gnanocage + Ggas) | (7) |
 | ||
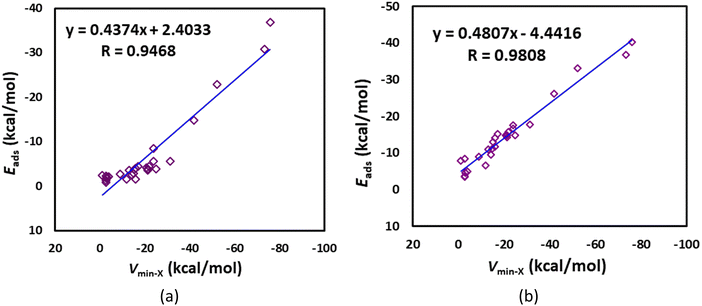
| Fig. 1 Comparison of our results for adsorption energies with literature values.38–51 | ||
Furthermore, Bader's quantum theory of atoms in molecules (QTAIM) analysis68 at the M062X/6-311G(d,p) level was performed using Multiwfn software.69 The covalent and noncovalent bonding scenario can be analyzed based on the value of ρ and its Laplacian (∇2ρ) at the bond critical point. Usually, for the covalent interaction, ρ is relatively high and ∇2ρ < 0, while for the non-covalent interaction (van der Waals, ionic, etc.), ρ is relatively low and ∇2ρ > 0.70,71 The electron localization function (ELF)72 and localized orbital locator (LOL)73 were calculated using the Multiwfn program.69 The relatively high values of ELF and LOL are indicative of covalent interactions. The non-covalent interaction (NCI) analysis74,75 was performed using the Multiwfn program69 and the results were visualized with the Gnuplot76 and visual molecular dynamics (VMD) software.77
3. Results and discussion
3.1. MESP
The MESP maps of the gas molecules are given in Fig. 2. A visual check of the MESP maps shows the presence of a blue region (most electron-rich region) in, for example, the formaldehyde, dimethyl ether, and methylamine molecules. The blue region is situated close to the O atoms of the formaldehyde and dimethyl ether molecules, and the N atom of the methylamine molecule. The MESP Vmin values of the gas molecules (represented as Vmin-X) are given in Table 1. The locations of the MESP Vmin for a few representative cases are given in Fig. S1 (ESI†). Here these Vmin-X values are in the range of −0.94 (SiH4) to −75.80 kcal mol−1 (CH3NH2). The MESP Vmin values of the diatomic gases follow the order: H2 < Cl2 < F2 < NO < N2 < CO. The MESP Vmin values of the triatomic molecules follow the order: OCS < CO2 < N2O < H2Se < H2S. The MESP Vmin value of silane is lower than that of methane (−3.07 kcal mol−1). The MESP Vmin values of the hydrocarbon gases follow the order: methane < acetylene < ethylene. The MESP Vmin values of the halomethanes follow the order: CF4 < CF2Cl2 < CHF3 < CH3Cl < CH3F. The MESP Vmin values of formaldehyde and its halogenated derivatives follow the order: phosgene < carbonyl fluoride < formaldehyde. The MESP Vmin value of dimethyl ether (−52.02 kcal mol−1) is lower than that of both ammonia (−73.23 kcal mol−1) and methylamine. The MESP Vmin values of the pnictogen hydrides and methylamine follow the order arsine < phosphine < ammonia < methylamine.| Adsorbate | V min-X | Adsorbate | V min-X |
|---|---|---|---|
| a Taken from ref. 27. | |||
| SiH4 | −0.94 | AsH3 | −15.88 |
| H2 | −2.64 | CH3Cl | −17.07 |
| Cl2 | −2.76 | COCl2 | −20.65 |
| F2 | −2.76 | C2H2 | −21.15 |
| CF4 | −2.95 | C2H4 | −21.27 |
| CH4 | −3.07 | H2Se | −21.77 |
| CF2Cl2 | −3.89 | H2S | −23.78 |
| NOa | −8.97 | PH3 | −23.91 |
| N2 | −11.92 | COF2 | −24.85 |
| CHF3 | −12.86 | CH3F | −31.31 |
| OCS | −14.12 | HCHO | −41.92 |
| CO2a | −14.87 | (CH3)2O | −52.02 |
| N2O | −15.19 | NH3a | −73.23 |
| COa | −15.88 | CH3NH2 | −75.80 |
Both of the B12N12 and Al12N12 nanocages consist of 6 tetragonal and 8 hexagonal rings27,78 (Fig. S2, ESI†). These nanocages have two distinct B–N or Al–N bonds. Here, the two hexagonal rings share the shorter B–N (1.44 Å) or Al–N bond (1.78 Å), and the tetragonal and the hexagonal rings share the longer B–N (1.48 Å) or Al–N bond (1.85 Å). A visual check of the MESP maps shows that the blue regions (most electron-rich regions) are situated close to the N atoms of the B12N12 and Al12N12 nanocages (see Fig. S2, ESI†). It may be noted that the MESP Vmin values of B12N12 and Al12N12 (represented as Vmin-C) are −20.77 and −49.07 kcal mol−1 respectively.27
3.2. Adsorption of gases on B12N12
The optimized structures of the gases adsorbed on the B12N12 nanocage are given in Fig. 3. The studied gases are preferably bound to the B atom rather than the N atom of the B12N12 nanocage. Notably, the F atom of the CF2Cl2, the C atom of the OCS, and the O atom of the N2O are bound to the B atom of the B12N12 nanocage. Typically, the studied gases are weakly adsorbed on the B12N12 nanocage. However, CO, AsH3, H2Se, H2S, PH3, CH3F, HCHO, (CH3)2O, NH3, and CH3NH2 are strongly adsorbed on the B12N12 nanocage. Typically, the gases strongly interact with the B12N12 nanocage at a smaller adsorption distance (dads). Here these dads values are in the range of 1.61 (CH3NH2) to 3.12 Å (Cl2) (Table 2). These findings are further validated by the adsorption energy of the gases on the B12N12 nanocage (see Table 2) and other structural changes (Table S1, ESI†). In all cases, the adsorption energy of the gases on the B12N12 nanocage is negative, suggesting that the adsorption reaction is exothermic in nature. Typically, the gases strongly interact with the B12N12 nanocage at higher negative adsorption energies. Here these Eads values are in the range of −0.83 (H2) to −36.84 kcal mol−1 (CH3NH2). The Eads values of the diatomic gases follow the order: H2 < F2 < N2 < CO< Cl2 < NO. The Eads values of the triatomic molecules follow the order: OCS < CO2 < N2O < H2Se < H2S. The Eads value of silane (−2.47 kcal mol−1) is higher than that of methane (−1.70 kcal mol−1). The Eads values of the hydrocarbon gases follow the order: methane < acetylene < ethylene. The Eads values of the halomethanes follow the order: CF4 < CF2Cl2 < CHF3 < CH3Cl < CH3F. The Eads values of formaldehyde and its halogenated derivatives follow the order: carbonyl fluoride < phosgene < formaldehyde. The Eads value of dimethyl ether (−22.86 kcal mol−1) is lower than that of both ammonia (−30.82 kcal mol−1) and methylamine. The Eads values of the pnictogen hydrides and methylamine follow the order arsine < phosphine < ammonia < methylamine. As shown in Fig. S3a (ESI†), these adsorption energies are found to be reasonably correlated with the adsorption distances (correlation coefficient of 0.742). Another important observation is that, as shown in Fig. 4a, these adsorption energies are well correlated with the MESP Vmin-X values of the gases (correlation coefficient of 0.9468). The angle of the hexagonal ring of the pristine B12N12 nanocage is 125°. This angle at the adsorption site decreases by at least 2° due to the adsorption of AsH3, H2Se, H2S, PH3, CH3F, HCHO, (CH3)2O, and CH3NH2 (see Table S1, ESI†).| Adsorbate | d ads | E ads | G ads | V min-C′ | ΔVmin-C |
|---|---|---|---|---|---|
| a Taken from ref. 27. | |||||
| SiH4 | 2.47 | −2.47 | 5.86 | −21.77 | −1.00 |
| H2 | 2.67 | −0.83 | 3.94 | −21.34 | −0.56 |
| Cl2 | 3.12 | −2.36 | 4.84 | −21.40 | −0.63 |
| F2 | 2.85 | −1.34 | 5.65 | −21.59 | −0.82 |
| CF4 | 2.61 | −2.08 | 6.60 | −21.90 | −1.13 |
| CH4 | 2.87 | −1.70 | 3.21 | −21.27 | −0.50 |
| CF2Cl2 | 2.56 | −2.20 | 7.32 | −22.84 | −2.07 |
| NOa | 2.54 | −2.77 | 3.46 | −22.90 | −2.13 |
| N2 | 2.71 | −1.60 | 3.27 | −22.21 | −1.44 |
| CHF3 | 2.56 | −3.57 | 5.74 | −24.47 | −3.70 |
| OCS | 3.06 | −2.65 | 4.77 | −23.85 | −3.07 |
| CO2a | 2.66 | −2.95 | 3.51 | −22.53 | −1.76 |
| N2O | 2.52 | −3.69 | 3.88 | −24.10 | −3.33 |
| COa | 1.79 | −1.65 | 7.64 | −27.92 | −7.15 |
| AsH3 | 2.27 | −4.05 | 6.27 | −30.94 | −10.17 |
| CH3Cl | 2.78 | −4.45 | 4.21 | −25.04 | −4.27 |
| COCl2 | 2.42 | −4.11 | 7.00 | −26.17 | −5.40 |
| C2H2 | 2.90 | −3.54 | 1.64 | −24.85 | −4.08 |
| C2H4 | 2.86 | −3.87 | 4.04 | −23.22 | −2.45 |
| H2Se | 2.42 | −4.44 | 3.87 | −28.68 | −7.91 |
| H2S | 2.20 | −5.64 | 4.45 | −29.81 | −9.04 |
| PH3 | 2.08 | −8.49 | 2.38 | −32.82 | −12.05 |
| COF2 | 2.42 | −3.91 | 5.35 | −26.42 | −5.65 |
| CH3F | 2.08 | −5.64 | 3.83 | −29.87 | −9.10 |
| HCHO | 1.65 | −14.76 | −2.16 | −33.63 | −12.86 |
| (CH3)2O | 1.61 | −22.86 | −9.57 | −34.20 | −13.43 |
| NH3a | 1.62 | −30.82 | −18.62 | −34.89 | −14.12 |
| CH3NH2 | 1.61 | −36.84 | −23.22 | −35.27 | −14.50 |
The entropic effects may also influence the adsorption processes. The analysis of Gads values (Table 2) shows that the adsorption of most of the gases on the B12N12 nanocage is endergonic, indicative of the weak interactions. However, the adsorption process is found to be exergonic for the HCHO/B12N12, (CH3)2O/B12N12, NH3/B12N12, and CH3NH2/B12N12 systems. As shown in Fig. S4a (ESI†), these adsorption free energies are also well correlated with the MESP Vmin-X values of the gases (correlation coefficient of 0.9041).
The MESP maps of the gas-adsorbed B12N12 nanocage are given in Fig. 5. A visual check shows substantial changes in the MESP feature of the isolated molecules, mainly due to the strong gas adsorption. The blue color observed in the B12N12 nanocage is more marked in the presence of, for example, formaldehyde, dimethyl ether, and methylamine. The electronic changes associated with the adsorption process could be understood by comparing the MESP Vmin of the isolated nanocage (Vmin-C) with that of the gas-adsorbed nanocage (represented as Vmin-C′) (see, e.g., Fig. S1, ESI†). We find that ΔVmin-C = Vmin-C′ − Vmin-C is negative for all the studied systems (Table 2). This implies that the B12N12 nanocage becomes electron-rich due to the adsorption process. A large magnitude of ΔVmin-C has been observed, mainly due to the strong gas adsorption. Here these ΔVmin-C values are in the range of −0.50 (CH4/B12N12 system) to −14.50 kcal mol−1 (CH3NH2/B12N12 system).
 | ||
| Fig. 5 MESP mapped onto 0.01 a.u. electron density isosurface of gases (a) SiH4, (b) H2, (c) Cl2, (d) F2, (e) CF4, (f) CH4, (g) CF2Cl2, (h) N2, (i) CHF3, (j) OCS, (k) N2O, (l) AsH3, (m) CH3Cl, (n) COCl2, (o) C2H2, (p) C2H4, (q) H2Se, (r) H2S, (s) PH3, (t) COF2, (u) CH3F, (v) HCHO, (w) (CH3)2O, and (x) CH3NH2 adsorbed on B12N12. The color code is the same as in Fig. 2. | ||
The adsorption process could affect the HOMO and LUMO energies (see, e.g., Fig. S5, ESI†), and therefore, the DFT reactivity indices μ, η, S, and ω (see eqn (2)–(5)). The electrophilicity index ω consists of both the ability of the system to obtain additional electronic charge (driven by μ2) and the resistance of the system to exchange electronic charge with the environment (driven by η). As a result, good electrophiles have higher (lower) values of μ(η). The DFT reactivity indices μ, η, S and ω of the B12N12 nanocage are −4.73 eV, 4.72 eV, 0.11 eV−1 and 2.37 eV, respectively.27 These DFT reactivity indices for the gas-adsorbed B12N12 nanocage are provided in Table 3. Substantial changes are found in these DFT reactivity indices, mainly due to the strong gas adsorption. The change in the reactivity index was calculated by taking the difference between the reactivity index of the gas-adsorbed nanocage and the reactivity index of the corresponding pristine nanocages. For example, the change in μ, Δμ, and change in η, Δη, for the CH4/B12N12 system are 0.02 and 0.00 eV, respectively. The corresponding values for the CH3NH2/B12N12 system are 0.73 and −0.15 eV respectively.
| Adsorbate | μ | Δμ | η | Δη | S | ΔS | ω | Δω |
|---|---|---|---|---|---|---|---|---|
| SiH4 | −4.72 | 0.01 | 4.71 | −0.01 | 0.11 | 0.00 | 2.37 | 0.00 |
| H2 | −4.71 | 0.02 | 4.72 | 0.00 | 0.11 | 0.00 | 2.35 | −0.02 |
| Cl2 | −5.99 | −1.26 | 3.44 | −1.28 | 0.15 | 0.04 | 5.22 | 2.85 |
| F2 | −5.45 | −0.72 | 3.97 | −0.75 | 0.13 | 0.02 | 3.75 | 1.38 |
| CF4 | −4.70 | 0.03 | 4.72 | 0.00 | 0.11 | 0.00 | 2.33 | −0.04 |
| CH4 | −4.71 | 0.02 | 4.72 | 0.00 | 0.11 | 0.00 | 2.34 | −0.03 |
| CF2Cl2 | −4.66 | 0.07 | 4.72 | 0.00 | 0.11 | 0.00 | 2.31 | −0.06 |
| N2 | −4.67 | 0.06 | 4.72 | 0.00 | 0.11 | 0.00 | 2.31 | −0.06 |
| CHF3 | −4.71 | 0.02 | 4.71 | −0.01 | 0.11 | 0.00 | 2.36 | −0.01 |
| OCS | −4.73 | 0.00 | 4.68 | −0.04 | 0.11 | 0.00 | 2.39 | 0.02 |
| N2O | −4.70 | 0.03 | 4.71 | −0.01 | 0.11 | 0.00 | 2.35 | −0.02 |
| AsH3 | −4.17 | 0.56 | 4.66 | −0.06 | 0.11 | 0.00 | 1.87 | −0.50 |
| CH3Cl | −4.62 | 0.11 | 4.71 | −0.01 | 0.11 | 0.00 | 2.27 | −0.10 |
| COCl2 | −5.14 | −0.41 | 4.14 | −0.58 | 0.12 | 0.01 | 3.19 | 0.82 |
| C2H2 | −4.60 | 0.13 | 4.70 | −0.02 | 0.11 | 0.00 | 2.25 | −0.12 |
| C2H4 | −4.59 | 0.14 | 4.71 | −0.01 | 0.11 | 0.00 | 2.23 | −0.14 |
| H2Se | −4.45 | 0.28 | 4.49 | −0.23 | 0.11 | 0.00 | 2.20 | −0.17 |
| H2S | −4.29 | 0.44 | 4.64 | −0.08 | 0.11 | 0.00 | 1.99 | −0.38 |
| PH3 | −4.10 | 0.63 | 4.66 | −0.06 | 0.11 | 0.00 | 1.80 | −0.57 |
| COF2 | −4.57 | 0.16 | 4.71 | −0.01 | 0.11 | 0.00 | 2.22 | −0.15 |
| CH3F | −4.46 | 0.27 | 4.71 | −0.01 | 0.11 | 0.00 | 2.11 | −0.26 |
| HCHO | −5.42 | −0.69 | 3.25 | −1.47 | 0.15 | 0.04 | 4.53 | 2.16 |
| (CH3)2O | −3.99 | 0.74 | 4.67 | −0.05 | 0.11 | 0.00 | 1.70 | −0.67 |
| CH3NH2 | −4.00 | 0.73 | 4.57 | −0.15 | 0.11 | 0.00 | 1.75 | −0.62 |
The results of the QTAIM analysis of the gas-adsorbed B12N12 nanocage are provided in Fig. S6 (ESI†) and Table 4. For the AsH3/B12N12, H2Se/B12N12, H2S/B12N12, and PH3/B12N12 systems, ρb values are in the range of 0.0491 (H2Se/B12N12 system) to 0.0888 a.u. (PH3/B12N12 system) and the corresponding ∇2ρb values are negative. The QTAIM analysis suggests the covalent nature of interactions in these systems. For the rest of the systems, ρb values are in the range of 0.0051 (Cl2/B12N12 system) to 0.1252 a.u. (CH3NH2/B12N12 system) and the corresponding ∇2ρb values are positive. The QTAIM analysis suggests the noncovalent nature of interactions in these systems. For all the systems, the values of ELF and LOL are in the ranges of 0.012–0.683 and 0.098–0.595 a.u., respectively (see Table 4). The values of ELF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >
> ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and LOL
0.5 and LOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >
> ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 in the AsH3/B12N12, H2Se/B12N12, H2S/B12N12, and PH3/B12N12 systems are also indicative of covalent interactions.
0.5 in the AsH3/B12N12, H2Se/B12N12, H2S/B12N12, and PH3/B12N12 systems are also indicative of covalent interactions.
| Adsorbate | ρ b | ∇2ρb | ELF | LOL | Adsorbate | ρ b | ∇2ρb | ELF | LOL |
|---|---|---|---|---|---|---|---|---|---|
| SiH4 | 0.010 | 0.027 | 0.051 | 0.189 | CH3Cl | 0.016 | 0.041 | 0.077 | 0.225 |
| H2 | 0.007 | 0.023 | 0.028 | 0.144 | COCl2 | 0.018 | 0.056 | 0.059 | 0.201 |
| Cl2 | 0.005 | 0.019 | 0.015 | 0.109 | C2H2 | 0.011 | 0.030 | 0.056 | 0.196 |
| F2 | 0.007 | 0.029 | 0.012 | 0.098 | C2H4 | 0.013 | 0.030 | 0.079 | 0.226 |
| CF4 | 0.012 | 0.041 | 0.032 | 0.153 | H2Se | 0.049 | −0.002 | 0.529 | 0.515 |
| CH4 | 0.007 | 0.023 | 0.020 | 0.126 | H2S | 0.064 | −0.030 | 0.516 | 0.508 |
| CF2Cl2 | 0.012 | 0.043 | 0.033 | 0.155 | PH3 | 0.089 | −0.092 | 0.588 | 0.545 |
| N2 | 0.011 | 0.037 | 0.034 | 0.159 | COF2 | 0.018 | 0.056 | 0.062 | 0.204 |
| CHF3 | 0.009 | 0.037 | 0.018 | 0.119 | CH3F | 0.031 | 0.080 | 0.104 | 0.254 |
| OCS | 0.008 | 0.027 | 0.030 | 0.150 | HCHO | 0.090 | 0.336 | 0.129 | 0.278 |
| N2O | 0.016 | 0.048 | 0.054 | 0.193 | (CH3)2O | 0.102 | 0.410 | 0.139 | 0.287 |
| AsH3 | 0.067 | −0.053 | 0.683 | 0.595 | CH3NH2 | 0.125 | 0.314 | 0.219 | 0.346 |
The NCI analysis provides the reduced density gradient (RDG) isosurface and the plots of the RDG versus sign(λ2)ρ, where λ2 is the second eigenvalue of the electron density Hessian matrix. The results of the NCI analysis of the gas-adsorbed B12N12 nanocage are provided in Fig. S7a and S8 (ESI†). The H-bond, van der Waals, and steric interactions can be visualized by blue, green, and red colors, respectively, in the NCI isosurface. Also, the H-bond (sign(λ2)ρ < 0), van der Waals (sign(λ2)ρ ≈ 0), and steric (sign(λ2)ρ > 0) interactions can be visualized as blue, green, and red colored spikes, respectively, in the RDG graph. The NCI results indicate the presence of van der Waals interactions between the gas molecules and the B12N12 nanocage, except for the AsH3/B12N12, H2Se/B12N12, H2S/B12N12, and PH3/B12N12 systems. Overall, the steric interactions between the gas molecules and the B12N12 nanocage increases as the size of the gas molecules increases.
3.3. Adsorption of gases on Al12N12
The optimized structures of the gases adsorbed on the Al12N12 nanocage are given in Fig. 6. The studied gases are preferably bound to the Al atom rather than the N atom of the Al12N12 nanocage. Notably, the F atom of the CF2Cl2, the O atom of the OCS, and the O atom of the N2O are bound to the Al atom of the Al12N12 nanocage. Typically, the studied gases are strongly adsorbed on the Al12N12 nanocage. However, H2, F2, CF4, CH4, CF2Cl2, and NO are weakly adsorbed on the Al12N12 nanocage. Typically, the gases strongly interact with the Al12N12 nanocage at a smaller adsorption distance (dads). Here these dads values are in the range of 1.92 ((CH3)2O) to 2.63 Å (AsH3) (Table 5). These findings are further validated by the adsorption energy of the gases on the Al12N12 nanocage (see Table 5) and other structure changes (Table S2, ESI†). In all cases, the adsorption energy of the gases on the Al12N12 nanocage is negative, suggesting that the adsorption reaction is exothermic in nature. Typically, the gases strongly interact with the Al12N12 nanocage at higher negative adsorption energies. Here these Eads values are in the range of −3.59 (F2) to −40.19 kcal mol−1 (CH3NH2). The Eads values of the diatomic gases follow the order: F2 < H2 < N2 < Cl2 < NO < CO. The Eads values of the triatomic molecules follow the order: OCS < CO2 < N2O < H2Se < H2S. The Eads value of silane (−7.84 kcal mol−1) is higher than that of methane (−4.65 kcal mol−1). The Eads values of the hydrocarbon gases follow the order: methane < acetylene < ethylene. The Eads values of the halomethanes follow the order: CF4 < CF2Cl2 < CHF3 < CH3Cl < CH3F. The Eads values of formaldehyde and its halogenated derivatives follow the order: carbonyl fluoride < phosgene < formaldehyde. The Eads value of dimethyl ether (−33.18 kcal mol−1) is lower than that of both ammonia (−36.83 kcal mol−1) and methylamine. The Eads values of the pnictogen hydrides and methylamine follow the order arsine < phosphine < ammonia < methylamine. As shown in Fig. S3b (ESI†), these adsorption energies are not well correlated with the adsorption distances (correlation coefficient of 0.293). Another important observation is that, as shown in Fig. 4b, these adsorption energies are well correlated with the MESP Vmin-X values of the gases (correlation coefficient of 0.9808). The angle of the hexagonal ring of the pristine Al12N12 nanocage is 125°. This angle at the adsorption site is almost unaffected by the adsorption of H2, F2, CF4, CH4, and CF2Cl2 (see Table S2, ESI†).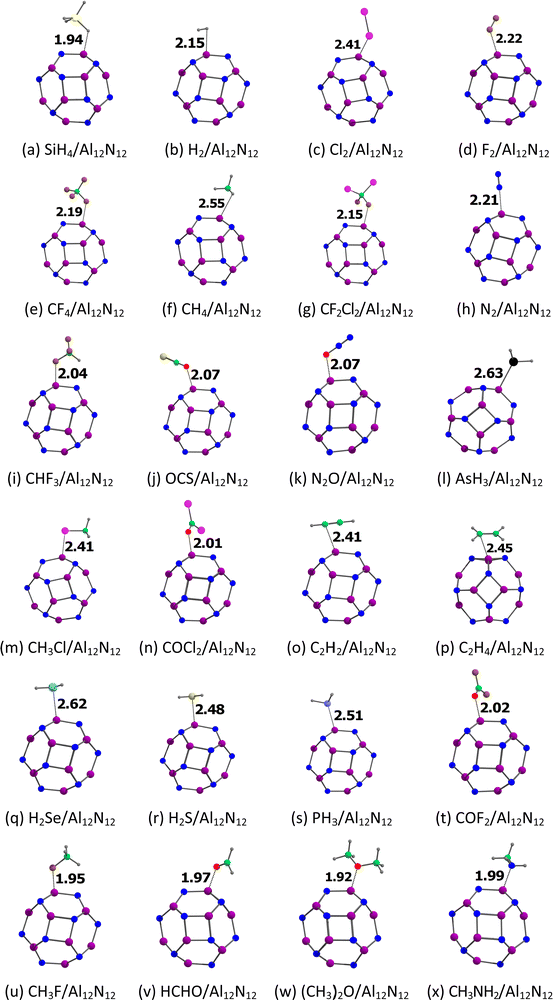 | ||
| Fig. 6 Optimized structures of gases (a) SiH4, (b) H2, (c) Cl2, (d) F2, (e) CF4, (f) CH4, (g) CF2Cl2, (h) N2, (i) CHF3, (j) OCS, (k) N2O, (l) AsH3, (m) CH3Cl, (n) COCl2, (o) C2H2, (p) C2H4, (q) H2Se, (r) H2S, (s) PH3, (t) COF2, (u) CH3F, (v) HCHO, (w) (CH3)2O, and (x) CH3NH2 adsorbed on Al12N12. The adsorption distances are given in Å. The color code is the same as in Fig. 3. In addition, the Al atom is denoted by the purple color. | ||
| Adsorbate | d ads | E ads | G ads | V min-C′ | ΔVmin-C |
|---|---|---|---|---|---|
| a Taken from ref. 27. | |||||
| SiH4 | 1.94 | −7.84 | 1.62 | −57.17 | −8.09 |
| H2 | 2.15 | −3.66 | 3.75 | −55.60 | −6.53 |
| Cl2 | 2.41 | −8.46 | 0.17 | −53.59 | −4.52 |
| F2 | 2.22 | −3.59 | 3.82 | −55.10 | −6.02 |
| CF4 | 2.19 | −4.54 | 5.11 | −56.79 | −7.72 |
| CH4 | 2.55 | −4.65 | 1.52 | −56.16 | −7.09 |
| CF2Cl2 | 2.15 | −4.94 | 4.89 | −57.92 | −8.85 |
| NOa | 2.22 | −9.02 | −0.40 | −56.98 | −7.91 |
| N2 | 2.21 | −6.69 | 1.34 | −57.10 | −8.03 |
| CHF3 | 2.04 | −10.94 | −0.87 | −57.42 | −8.35 |
| OCS | 2.07 | −9.50 | −0.53 | −60.37 | −11.30 |
| CO2a | 2.08 | −11.17 | −2.52 | −57.86 | −8.79 |
| N2O | 2.07 | −12.93 | −3.81 | −59.11 | −10.04 |
| COa | 2.20 | −11.68 | −3.04 | −57.23 | −8.16 |
| AsH3 | 2.63 | −14.12 | −4.29 | −60.05 | −10.98 |
| CH3Cl | 2.41 | −15.12 | −4.48 | −59.49 | −10.42 |
| COCl2 | 2.01 | −14.90 | −3.94 | −62.00 | −12.93 |
| C2H2 | 2.41 | −14.35 | −7.61 | −58.17 | −9.10 |
| C2H4 | 2.45 | −14.93 | −4.51 | −58.61 | −9.54 |
| H2Se | 2.62 | −15.80 | −6.29 | −59.36 | −10.29 |
| H2S | 2.48 | −16.74 | −7.26 | −59.30 | −10.23 |
| PH3 | 2.51 | −17.54 | −8.03 | −60.30 | −11.23 |
| COF2 | 2.02 | −14.81 | −4.39 | −60.74 | −11.67 |
| CH3F | 1.95 | −17.72 | −7.49 | −60.74 | −11.67 |
| HCHO | 1.97 | −26.09 | −14.15 | −62.94 | −13.87 |
| (CH3)2O | 1.92 | −33.18 | −21.28 | −61.43 | −12.36 |
| NH3a | 2.01 | −36.83 | −26.53 | −62.12 | −13.05 |
| CH3NH2 | 1.99 | −40.19 | −28.68 | −61.43 | −12.36 |
The analysis of Gads values (Table 5) shows that the adsorption of most of the gases on the Al12N12 nanocage is exergonic, indicative of the stronger interactions. However, the adsorption process is found to be endergonic for the SiH4/Al12N12, H2/Al12N12, Cl2/Al12N12, F2/Al12N12, CF4/Al12N12, CH4/Al12N12 CF2Cl2/Al12N12, and N2/Al12N12 systems. As shown in Fig. S4b (ESI†), these adsorption free energies are also well correlated with the MESP Vmin-X values of the gases (correlation coefficient of 0.9818).
The MESP maps of the gas-adsorbed Al12N12 nanocage are given in Fig. 7. A visual check shows substantial changes in the MESP feature of most of the isolated gas molecules due to the adsorption process. The blue region (most electron-rich region) in, for example, the formaldehyde, dimethyl ether, and methylamine molecules is less marked in the presence of Al12N12. We find that ΔVmin-C is negative for all the studied systems (Table 5). This implies that the Al12N12 nanocage becomes electron-rich due to the adsorption process. Here in most of the systems, a large magnitude of ΔVmin-C has been observed due to the adsorption process. These ΔVmin-C values are in the range of −4.52 (Cl2/Al12N12 system) to −13.87 kcal mol−1 (HCHO/Al12N12 system).
 | ||
| Fig. 7 MESP mapped onto 0.01 a.u. electron density isosurface of gases (a) SiH4, (b) H2, (c) Cl2, (d) F2, ® CF4, (f) CH4, (g) CF2Cl2, (h) N2, (i) CHF3, (j) OCS, (k) N2O, (l) AsH3, (m) CH3Cl, (n) COCl2, (o) C2H2, (p) C2H4, (q) H2S®(r) H2S, (s) PH3, (t) COF2, (u) CH3F, (v) HCHO, (w) (CH3)2O, and (x) CH3NH2 adsorbed on Al12N12. The color code is the same as in Fig. 2. | ||
The DFT reactivity indices μ, η, S and ω of the Al12N12 nanocage are −4.86 eV, 3.16 eV, 0.16 eV−1 and 3.74 eV, respectively.27 In most of the systems, substantial changes are observed in these DFT reactivity indices due to the adsorption process (Table 6). For example, Δμ and Δη for the CH4/Al12N12 system are 0.11 and 0.00 eV, respectively. The corresponding values for the CH3NH2/Al12N12 system are 0.43 and −0.03 eV respectively.
| Adsorbate | μ | Δμ | η | Δη | S | ΔS | ω | Δω |
|---|---|---|---|---|---|---|---|---|
| SiH4 | −4.69 | 0.17 | 3.16 | 0.00 | 0.16 | 0.00 | 3.49 | −0.25 |
| H2 | −4.75 | 0.11 | 3.18 | 0.02 | 0.16 | 0.00 | 3.54 | −0.20 |
| Cl2 | −5.32 | −0.46 | 2.80 | −0.36 | 0.18 | 0.02 | 5.06 | 1.32 |
| F2 | −5.44 | −0.58 | 2.52 | −0.64 | 0.20 | 0.04 | 5.87 | 2.13 |
| CF4 | −4.71 | 0.15 | 3.18 | 0.02 | 0.16 | 0.00 | 3.50 | −0.24 |
| CH4 | −4.75 | 0.11 | 3.16 | 0.00 | 0.16 | 0.00 | 3.57 | −0.17 |
| CF2Cl2 | −4.66 | 0.20 | 3.17 | 0.01 | 0.16 | 0.00 | 3.43 | −0.31 |
| N2 | −4.68 | 0.18 | 3.19 | 0.03 | 0.16 | 0.00 | 3.44 | −0.30 |
| CHF3 | −4.71 | 0.15 | 3.16 | 0.00 | 0.16 | 0.00 | 3.52 | −0.22 |
| OCS | −4.59 | 0.27 | 3.15 | −0.01 | 0.16 | 0.00 | 3.33 | −0.41 |
| N2O | −4.63 | 0.23 | 3.15 | −0.01 | 0.16 | 0.00 | 3.40 | −0.34 |
| AsH3 | −4.52 | 0.34 | 3.16 | 0.00 | 0.16 | 0.00 | 3.24 | −0.50 |
| CH3Cl | −4.60 | 0.26 | 3.16 | 0.00 | 0.16 | 0.00 | 3.34 | −0.40 |
| COCl2 | −4.93 | −0.07 | 2.74 | −0.42 | 0.18 | 0.02 | 4.43 | 0.69 |
| C2H2 | −4.59 | 0.27 | 3.16 | 0.00 | 0.16 | 0.00 | 3.33 | −0.41 |
| C2H4 | −4.60 | 0.26 | 3.15 | −0.01 | 0.16 | 0.00 | 3.35 | −0.39 |
| H2Se | −4.56 | 0.30 | 3.16 | 0.00 | 0.16 | 0.00 | 3.29 | −0.45 |
| H2S | −4.58 | 0.28 | 3.16 | 0.00 | 0.16 | 0.00 | 3.32 | −0.42 |
| PH3 | −4.51 | 0.35 | 3.16 | 0.00 | 0.16 | 0.00 | 3.21 | −0.53 |
| COF2 | −4.56 | 0.30 | 3.17 | 0.01 | 0.16 | 0.00 | 3.29 | −0.45 |
| CH3F | −4.53 | 0.33 | 3.14 | −0.02 | 0.16 | 0.00 | 3.27 | −0.47 |
| HCHO | −4.75 | 0.11 | 2.87 | −0.29 | 0.17 | 0.01 | 3.93 | 0.19 |
| (CH3)2O | −4.45 | 0.41 | 3.14 | −0.02 | 0.16 | 0.00 | 3.16 | −0.58 |
| CH3NH2 | −4.43 | 0.43 | 3.13 | −0.03 | 0.16 | 0.00 | 3.13 | −0.61 |
The results of the QTAIM analysis of the gas-adsorbed Al12N12 nanocage are provided in Fig. S9 (ESI†) and Table 7. For all the systems, ρb values are in the range of 0.0122 (CH4/Al12N12 system) to 0.0587 a.u. (CH3NH2/Al12N12 system) and the corresponding ∇2ρb values are positive. For all the systems, the values of ELF and LOL are in the ranges of 0.030–0.214 and 0.150–0.343 a.u., respectively (see Table 7). These results suggest the noncovalent nature of interactions in these systems. The results of the NCI analysis of the gas-adsorbed Al12N12 nanocage are provided in Fig. S7b and S10 (ESI†). The NCI results indicate the presence of van der Waals interactions between the gas molecules and the Al12N12 nanocage. Overall, the steric interactions between the gas molecules and the Al12N12 nanocage increase as the size of the gas molecules increases.
| Adsorbate | ρ b | ∇2ρb | ELF | LOL | Adsorbate | ρ b | ∇2ρb | ELF | LOL |
|---|---|---|---|---|---|---|---|---|---|
| SiH4 | 0.028 | 0.082 | 0.085 | 0.234 | CH3Cl | 0.040 | 0.165 | 0.081 | 0.229 |
| H2 | 0.020 | 0.063 | 0.058 | 0.198 | COCl2 | 0.042 | 0.276 | 0.048 | 0.184 |
| Cl2 | 0.048 | 0.134 | 0.214 | 0.343 | C2H2 | 0.029 | 0.085 | 0.093 | 0.243 |
| F2 | 0.021 | 0.100 | 0.034 | 0.157 | C2H4 | 0.029 | 0.069 | 0.116 | 0.266 |
| CF4 | 0.022 | 0.118 | 0.030 | 0.150 | H2Se | 0.032 | 0.082 | 0.111 | 0.261 |
| CH4 | 0.012 | 0.034 | 0.068 | 0.213 | H2S | 0.035 | 0.103 | 0.104 | 0.254 |
| CF2Cl2 | 0.024 | 0.137 | 0.030 | 0.151 | PH3 | 0.038 | 0.096 | 0.125 | 0.275 |
| N2 | 0.028 | 0.148 | 0.042 | 0.173 | COF2 | 0.040 | 0.258 | 0.048 | 0.184 |
| CHF3 | 0.032 | 0.208 | 0.037 | 0.164 | CH3F | 0.041 | 0.294 | 0.042 | 0.173 |
| OCS | 0.034 | 0.208 | 0.095 | 0.245 | HCHO | 0.049 | 0.325 | 0.059 | 0.200 |
| N2O | 0.036 | 0.212 | 0.047 | 0.183 | (CH3)2O | 0.056 | 0.395 | 0.060 | 0.201 |
| AsH3 | 0.033 | 0.072 | 0.128 | 0.277 | CH3NH2 | 0.059 | 0.335 | 0.082 | 0.230 |
Our results show that only AsH3, H2Se, H2S, PH3, CH3F, HCHO, (CH3)2O, and CH3NH2 are strongly adsorbed on the B12N12 nanocage. Furthermore, only H2, F2, CF4, CH4, and CF2Cl2 are weakly adsorbed on the Al12N12 nanocage. Overall, our results indicate that the Al12N12 nanoclusters may have a higher potential in gas storage and catalytic applications than the B12N12 nanoclusters. The stronger the adsorption of gas molecules, the more difficult it is for them to desorb from the surface.78 The gas-sensing behavior of the Al12N12 nanocage might have a severe influence on the reusability of gas sensors. The MESP of a molecule is a real physical property that can be determined experimentally by X-ray diffraction techniques or by computational methods.58 There is a strong linear correlation between the adsorption energies of the gas-adsorbed B12N12 and Al12N12 systems and the MESP Vmin values of the gases (see Fig. 4). This enables us to predict the adsorption energies once we know the MESP features of the gas molecules.
4. Conclusions
The adsorption of twenty-four gases (SiH4, H2, Cl2, F2, CF4, CH4, CF2Cl2, N2, CHF3, OCS, N2O, AsH3, CH3Cl, COCl2, C2H2, C2H4, H2Se, H2S, PH3, COF2, CH3F, HCHO, (CH3)2O, and CH3NH2) on the B12N12 and Al12N12 nanocages was studied using DFT calculations. Most of the studied gases are weakly adsorbed on the B12N12 nanocage. However, AsH3, H2Se, H2S, PH3, CH3F, HCHO, (CH3)2O, and CH3NH2 are strongly adsorbed on the B12N12 nanocage. The gases strongly interact with the nanocage usually at a smaller adsorption distance and a higher negative adsorption energy. For the gas-adsorbed B12N12 system, the dads values are in the range of 1.61 (CH3NH2) to 3.12 Å (Cl2), and the Eads values are in the range of −0.83 (H2) to −36.84 kcal mol−1 (CH3NH2). Here the adsorption energies are found to be reasonably correlated with the adsorption distances. Another important observation is that these adsorption energies are well correlated with the MESP Vmin values of the gases. Substantial changes are found in the DFT reactivity indices (μ, η, S and ω) of the B12N12 nanocage, mainly due to the strong gas adsorption. For example, Δμ and Δη for the CH4/B12N12 system are 0.02 and 0.00 eV, respectively. The corresponding values for the CH3NH2/B12N12 system are 0.73 and −0.15 eV respectively. The QTAIM analysis suggests the covalent nature of interactions in the AsH3/B12N12, H2Se/B12N12, H2S/B12N12, and PH3/B12N12 systems.Most of the studied gases are strongly adsorbed on the Al12N12 nanocage. However, H2, F2, CF4, CH4, and CF2Cl2 are weakly adsorbed on the Al12N12 nanocage. For the gas-adsorbed Al12N12 system, the dads values are in the range of 1.92 ((CH3)2O) to 2.63 Å (AsH3), and the Eads values are in the range of −3.59 (F2) to −40.19 kcal mol−1 (CH3NH2). These adsorption energies are also well correlated with the MESP Vmin-X values of the gases. In most of the systems, substantial changes are observed in the DFT reactivity indices (μ, η, S and ω) of the Al12N12 nanocage due to the gas adsorption. For example, Δμ and Δη for the CH4/Al12N12 system are 0.11 and 0.00 eV, respectively. The corresponding values for the CH3NH2/Al12N12 system are 0.43 and −0.03 eV respectively. The QTAIM analysis suggests the noncovalent nature of interactions in the gas-adsorbed Al12N12 systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is based upon work supported by the King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under award no. ORFS-2022-CRG11-5028. R. G. S. N. and A. K. N. N. would like to thank KAUST for providing the computational resources of the Shaheen II supercomputer.References
- U.S. EPA's report global mitigation of non-CO2 greenhouse gases: 2010–2030.
- H. Tian, et al. , Nature, 2020, 586, 248–256 CrossRef CAS PubMed.
- J. F. d S. Petruci and A. A. Cardoso, Anal. Chem., 2016, 88, 11714–11719 CrossRef CAS PubMed.
- P. D. N. Svoronos and T. J. Bruno, Ind. Eng. Chem. Res., 2002, 41, 5321–5336 CrossRef CAS.
- G. W. Gribble, J. Nat. Prod., 1992, 55, 1353–1395 CrossRef CAS.
- F. S. Rowland, Am. Sci., 1989, 77, 36–45 Search PubMed.
- J.-R. Chen, H.-Y. Tsai, S.-K. Chen, H.-R. Pan, S.-C. Hu, C.-C. Shen, C.-M. Kuan, Y.-C. Lee and C.-C. Wu, Proc. Safety Prog., 2006, 25, 237–244 CrossRef CAS.
- Y. N. Ko, S. H. Choi and Y. C. Kang, ACS Appl. Mater. Interfaces, 2016, 8, 6449–6456 CrossRef CAS PubMed.
- C. Winder, Environ. Res., 2001, 85, 105–114 CrossRef CAS PubMed.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- D. L. Sudakin, Hum. Exp. Toxicol., 2005, 24, 27–33 CrossRef CAS PubMed.
- D. Pakulska and S. Czerczak, Int. J. Occup. Med. Environ. Health, 2006, 19, 36–44 Search PubMed.
- J. Borak and W. F. Diller, J. Occup. Environ. Med., 2001, 43, 110–119 CrossRef CAS PubMed.
- Y. Mitsui, Y. Ohira, T. Yonemura, T. Takaichi, A. Sekiya and T. Beppu, J. Electrochem. Soc., 2004, 151, G297 CrossRef CAS.
- T. Salthammer, S. Mentese and R. Marutzky, Chem. Rev., 2010, 110, 2536–2572 CrossRef CAS PubMed.
- T. H. Fleisch, A. Basu, M. J. Gradassi and J. G. Masin, Stud. Surf. Sci. Catal., 1997, 107, 117–125 CrossRef CAS.
- S. R. Raga, Y. Jiang, L. K. Ono and Y. Qi, Energy Technol., 2017, 5, 1750–1761 CrossRef CAS.
- D. Mori and K. Hirose, Int. J. Hydrogen Energy, 2009, 34, 4569–4574 CrossRef CAS.
- C. Nguyen and D. D. Do, Langmuir, 1999, 15, 3608–3615 CrossRef CAS.
- V. K. Gupta and T. A. Saleh, Environ. Sci. Pollut. Res. Int., 2013, 20, 2828–2843 CrossRef CAS PubMed.
- S. Gadipelli and Z. X. Guo, Prog. Mater. Sci., 2015, 69, 1–60 CrossRef CAS.
- N. Rangnekar, N. Mittal, B. Elyassi, J. Caro and M. Tsapatsis, Chem. Soc. Rev., 2015, 44, 7128–7154 RSC.
- Y. Yang, A. K. Narayanan Nair and S. Sun, J. Phys. Chem. C, 2020, 124, 16478–16487 CrossRef CAS.
- Y. Yang, A. K. Narayanan Nair and S. Sun, Ind. Eng. Chem. Res., 2021, 60, 7729–7738 CrossRef CAS.
- A. K. Narayanan Nair, R. Cui and S. Sun, ACS Earth Space Chem., 2021, 5, 2599–2611 CrossRef CAS.
- R. Geetha Sadasivan Nair, A. Kumar Narayanan Nair and S. Sun, J. Mol. Liq., 2023, 122923 CrossRef CAS.
- R. Geetha Sadasivan Nair, A. K. Narayanan Nair and S. Sun, Energy Fuels, 2023, 37, 14053–14063 CrossRef CAS.
- A. Abbasi and J. Jahanbin Sardroodi, Environ. Sci.: Nano, 2016, 3, 1153–1164 RSC.
- A. Abbasi, J. J. Sardroodi and A. R. Ebrahimzadeh, Surf. Sci., 2016, 654, 20–32 CrossRef CAS.
- A. Abbasi and J. J. Sardroodi, Surf. Interfaces, 2017, 8, 15–27 CrossRef CAS.
- A. Abbasi and J. Jahanbin Sardroodi, New J. Chem., 2017, 41, 12569–12580 RSC.
- A. Abbasi and J. J. Sardroodi, J. Nanostruct. Chem., 2017, 7, 345–358 CrossRef CAS.
- C. Zhao and H. Wu, Appl. Surf. Sci., 2018, 435, 1199–1212 CrossRef CAS.
- A. Abbasi and J. J. Sardroodi, Comput. Theor. Chem., 2018, 1125, 15–28 CrossRef CAS.
- A. Abbasi and J. J. Sardroodi, Phys. E, 2019, 108, 382–390 CrossRef CAS.
- A. Abbasi and J. J. Sardroodi, Appl. Surf. Sci., 2019, 469, 781–791 CrossRef CAS.
- A. Abbasi, A. Abdelrasoul and J. J. Sardroodi, Adsorption, 2019, 25, 1001–1017 CrossRef CAS.
- J. Beheshtian, A. A. Peyghan, Z. Bagheri and M. Kamfiroozi, Struct. Chem., 2012, 23, 1567–1572 CrossRef CAS.
- K. Kalateh and A. Abdolmanafi, Int. J. New Chem., 2015, 2, 1–7 Search PubMed.
- E. Shakerzadeh, Phys. E, 2016, 78, 1–9 CrossRef CAS.
- A. Bahrami, M. B. Qarai and N. L. Hadipour, Comput. Theor. Chem., 2017, 1108, 63–69 CrossRef CAS.
- A. S. Rad, Semiconductors, 2017, 51, 134–138 CrossRef CAS.
- S. Larki, E. Shakerzadeh, E. C. Anota and R. Behjatmanesh-Ardakani, Chem. Phys., 2019, 526, 110424 CrossRef CAS.
- A. Shokuhi Rad, D. Zareyee, V. Pouralijan Foukolaei, B. Kamyab Moghadas and M. Peyravi, Mol. Phys., 2016, 114, 3143–3149 CrossRef CAS.
- L. Saedi, Z. Javanshir, S. Khanahmadzadeh, M. Maskanati and M. Nouraliei, Mol. Phys., 2020, 118, e1658909 CrossRef.
- A. S. Rad and K. Ayub, Mater. Chem. Phys., 2017, 194, 337–344 CrossRef CAS.
- M. Y. Mehboob, R. Hussain, F. Younas, S. Jamil, M. M. A. Iqbal, K. Ayub, N. Sultana and M. R. S. A. Janjua, J. Clust. Sci., 2023, 34, 1237–1247 CrossRef CAS.
- A. S. Rad and K. Ayub, Vacuum, 2016, 133, 70–80 CrossRef CAS.
- Q. Wang, Q. Sun, P. Jena and Y. Kawazoe, ACS Nano, 2009, 3, 621–626 CrossRef CAS PubMed.
- A. S. Rad, J. Theory Comput. Chem., 2018, 17, 1850013 CrossRef CAS.
- R. Padash, M. Rahimi-Nasrabadi, A. Shokuhi Rad, A. Sobhani-Nasab, T. Jesionowski and H. Ehrlich, J. Clust. Sci., 2019, 30, 203–218 CrossRef CAS.
- T. Oku, A. Nishiwaki and I. Narita, Sci. Technol. Adv., 2004, 5, 635–638 CrossRef CAS.
- C. Balasubramanian, S. Bellucci, P. Castrucci, M. De Crescenzi and S. V. Bhoraskar, Chem. Phys. Lett., 2004, 383, 188–191 CrossRef CAS.
- C. Liu, Z. Hu, Q. Wu, X. Wang, Y. Chen, H. Sang, J. Zhu, S. Deng and N. Xu, J. Am. Chem. Soc., 2005, 127, 1318–1322 CrossRef CAS PubMed.
- H.-S. Wu, F.-Q. Zhang, X.-H. Xu, C.-J. Zhang and H. Jiao, J. Phys. Chem. A, 2003, 107, 204–209 CrossRef CAS.
- G. S. Remya and C. H. Suresh, New J. Chem., 2018, 42, 3602–3608 RSC.
- G. S. Remya and C. H. Suresh, New J. Chem., 2019, 43, 14634–14642 RSC.
- C. H. Suresh, G. S. Remya and P. K. Anjalikrishna, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1601 CAS.
- M. J. Frisch, et al., Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford, CT, 2016.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- H. Chermette, J. Comput. Chem., 1999, 20, 129–154 CrossRef CAS.
- R. G. Parr, R. A. Donnelly, M. Levy and W. E. Palke, J. Chem. Phys., 1978, 68, 3801–3807 CrossRef CAS.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, Molecules, 2016, 21, 748 CrossRef PubMed.
- P. K. Chattaraj, U. Sarkar and D. R. Roy, Chem. Rev., 2006, 106, 2065–2091 CrossRef CAS PubMed.
- R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- R. G. Parr, L. v Szentpály and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- R. F. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- P. A. Hunt, C. R. Ashworth and R. P. Matthews, Chem. Soc. Rev., 2015, 44, 1257–1288 RSC.
- S. Khan, H. Sajid, K. Ayub and T. Mahmood, J. Mol. Liq., 2020, 316, 113860 CrossRef CAS.
- A. Savin, R. Nesper, S. Wengert and T. F. Fässler, Angew. Chem., Int. Ed. Engl., 1997, 36, 1808–1832 CrossRef CAS.
- A. V. Afonin, V. A. Semenov and A. V. Vashchenko, Phys. Chem. Chem. Phys., 2021, 23, 24536–24540 RSC.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J. P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625–632 CrossRef PubMed.
- T. Williams and C. Kelley, GNUplot: an interactive plotting program, 1998 Search PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graph., 1996, 14, 33–38 CrossRef CAS PubMed.
- A. L. Pereira Silva and J. d J. G. Varela Júnior, Inorg. Chem., 2023, 62, 1926–1934 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional details of DFT analysis. See DOI: https://doi.org/10.1039/d3nj05703h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |