In vitro nanomaterial testing: unveiling biases through biomolecular corona influence†
Fangfang
Cao
National University of Singapore, Singapore. E-mail: cffdc@nus.edu.sg
Abstract
This article highlights the recent work of Castagnola, Armirotti, et al. (Nanoscale Horiz., 2024, https://doi.org/10.1039/D3NH00510K) on demonstrating that the widespread use of 10% fetal bovine serum in an in vitro assay cannot recapitulate the complexity of in vivo systemic administration.
Currently, nanomaterials are attracting significant attention in the field of biomedicine. However, once these nanomaterials are utilized for in vivo treatments they interact with the surrounding physiological environment, leading to the adsorption of various biomolecules onto their surfaces, forming a biomolecular corona (BMC) and thereby influencing the performance and behavior of the nanomaterials. Presently, the in vitro studies of nanomaterials primarily involve dispersing the nanoparticles in 10% fetal bovine serum (FBS) and then evaluating their toxicity and therapeutic effects. However, this evaluation method is insufficient as it cannot accurately simulate the conditions of human blood. Moreover, this practical issue remains unresolved to date.
To validate the series of biases existing in established experimental practices and to advance the fields of nanomedicine and nanotoxicology, the study by Castagnola, Armirotti, et al. (https://doi.org/10.1039/D3NH00510K) investigated two nanomaterial types with vastly different physicochemical properties commonly used in biomedicine. The research compared the molecular and biological biases resulting from the mismatch between nanomaterials dispersed in 10% FBS (utilized for in vitro biological assays) and whole human plasma (HP, closer to in vivo administration schemes) (Fig. 1). Through comparative analysis using proteomics, lipidomics, high-throughput multi-parameter in vitro screening, and single-molecule feature analysis, it was demonstrated that the dynamic changes in BMC composition are material dependent and that cell viability, transport pathways, and autophagic cascades are influenced by the presence or absence of pre-formed BMCs. These findings underscore the potential limitations of nanomaterial in vitro testing in accurately representing real in vivo conditions. Therefore, it is necessary to establish new shared protocols to enhance the accuracy and predictive capability of nanomaterial testing.
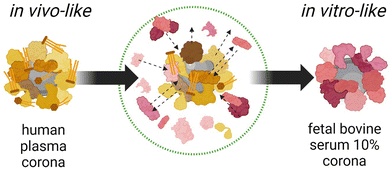 | ||
| Fig. 1 Schematic illustrating the molecular and biological biases arising from the well-known in vitro/in vivo mismatch in nanomedicine due to the biomolecular corona. Reproduced from https://doi.org/10.1039/d3nh00510k with permission from the Royal Society of Chemistry. | ||
In summary, this study confirms the biases that may exist when using standard in vitro conditions for nanomaterial toxicology assessments, reminding us of the need to establish a comprehensive experimental framework to generate and support new knowledge in the field of biologically relevant nanomaterial interactions. For instance, integrating advanced predictive tools such as artificial intelligence and machine learning will enable nanotoxicology and nanomedicine to progress towards personalized solutions for precision healthcare.
Footnote |
| † This content was originally published on our blogs platform at https://blogs.rsc.org/nh/2024/04/15/in-vitro-nanomaterial-testing-unveiling-biases-through-biomolecular-corona-influence/. |
| This journal is © The Royal Society of Chemistry 2024 |

