Ethanol purification enables high-quality α-phase FAPbI3 perovskite microcrystals for commercial photovoltaic applications†
Hyun Seo
Kim
 ab,
Hyun-Sung
Yun
a,
Chae-Eun
Seo
ab,
Hyun-Sung
Yun
a,
Chae-Eun
Seo
 b,
Soo
Bin Yoo
a,
Bong Joo
Kang
b,
Soo
Bin Yoo
a,
Bong Joo
Kang
 *a,
Eui Hyuk
Jung
*a,
Eui Hyuk
Jung
 *b and
Nam Joong
Jeon
*b and
Nam Joong
Jeon
 *a
*a
aDivision of Advanced Materials, Korea Research Institute of Chemical Technology (KRICT), 141 Gajeong-ro, Yuseong-gu, Daejeon 34114, Republic of Korea. E-mail: bjkang@krict.re.kr; njjeon@krict.re.kr
bDepartment of Energy Engineering, Korea Institute of Energy Technology (KENTECH), 21 KENTECH-gil, Naju, 58330, Republic of Korea. E-mail: ehjung@kentech.ac.kr
First published on 17th April 2024
Abstract
Reliable quality and sustainable processes must be developed for commodities to enter the commercial stage. For next-generation photovoltaic applications such as perovskite solar cells, it is essential to manufacture high-quality photoactive perovskites via eco-friendly processes. We demonstrate that ethanol, an ideal green solvent, can be applied to yield efficient alpha-phase FAPbI3 perovskite microcrystals.
New conceptsIn this work, an eco-friendly solvent process for synthesizing alpha-phase FAPbI3 perovskite microcrystals is proposed. A variety of organic solvents were used for the washing of strongly-bound solvent molecules, which is a crucial step to obtain high-quality perovskite microcrystals, and systematically compared in terms of the physical properties and environmental hazards of solvents. The perovskite microcrystals purified using eco-friendly ethanol, which possesses a chemical environment most similar to that of the main reaction solvent, exhibited superior properties compared with those based on other solvents. In the film state, it shows lower Urbach energy, higher photoluminescence intensity, and prolonged carrier lifetime, indicating effectively controlled trap states. When applied to the solar cells, the ethanol-assisted perovskite microcrystals result in more efficient devices and improved reproducibility than the other solvent-processed perovskite microcrystals. The results presented in this study pave the way for a new approach to commercialization, potentially reducing environmental and human hazards during mass production while improving reproducibility and photovoltaic performance. |
Introduction
Organic–inorganic hybrid perovskites have been consistently found to exhibit remarkable potential with respect to feasible bandgap tunability, high absorptivity, long charge-carrier diffusion length, simple defect control, and varied processibility.1–4 A consensus among the academic and industrial communities recognizes perovskites as a promising material for exploitation in next-generation optoelectronics.5–7 Perovskite solar cells (PSCs) were rapidly investigated on the laboratory scale within their first decade8,9 and are currently entering the transition stage corresponding to the technology readiness level (TRL 5).10 In order for PSCs to effectively reach the commercial development level, we must consider how to implement the mass production of high-grade perovskite materials with good reliability and minimal quality difference from batch to batch.Moreover, the use of sustainable processes to produce the perovskites should also be taken into account. Hybrid perovskites are formed by a solution-based preparation process, and the usage of organic solvents is the key issue in developing sustainable processes. In general, huge quantities of various organic solvents are utilized at chemical industrial sites to synthesize the reagents and purify the reactants by means of extraction, chromatography, filtration, and so on.11,12 However, it has been found that the chemical wastes from the solvent process can detrimentally influence environmental health and safety (EHS),13 as well as result in an economic cost to recycle or incinerate them.14,15 Since the release of a report by the United Nations in 1987, humanity has considered sustainable development without adversely affecting future generations in regards to climate, environment, and natural resources.16,17 One of the considerations is the diminution or elimination of the usage of toxic and unsustainable solvents by replacing them with ‘green solvents’ in processes. The terminology ‘green solvent’ reflects not only the minimization of the direct EHS impact of a chemical process, but also the indirect effects, such as the energy demand and cost of production, storage, recycling, or waste treatment after usage.18
In preparing the perovskite solutions to fabricate a thin film, it has been reported that devices derived from synthesized perovskite powders are more stable and efficient than those from mixtures of precursors of organic cation halides and lead halides.19–24 The synthesis of the powders requires various organic solvents such as toluene, diethyl ether, or acetonitrile to purify the reactant of unreacted chemicals, impurities, and reaction solvents. These solvents are classified as being of concern or unsuitable for use in industry.13 Although numerous efforts have recently demonstrated that the usage of a green solvent for the preparation of perovskite solutions is effective,21,25,26 there are few examples of the replacement of the usage of undesirable solvents with green solvents in the perovskite synthetic process.
We sought to determine an appropriate green solvent to obtain high-quality perovskite microcrystals (MCs) of alpha-phase formamidinium lead triiodide (FAPbI3), which has a bandgap of 1.48 eV close to the theoretical optimum value and high stability against thermal stress.27,28 We envisioned that ethanol could be the best choice for manufacturing photoactive FAPbI3 MCs through a sustainable process. The reason is that this solvent is considered to be not only a highly recommended green solvent by several pharmaceutical companies (e.g. Pfizer & AstraZeneca) based on EHS criteria,13 but also has the ability to be produced by renewable resources as a known biofuel.
In this work, we investigated a sustainable way to synthesize high-quality α-phase FAPbI3 perovskite MCs using green solvents. We realized that a compatible chemical environment during the purification process is one of the crucial parameters for efficiently eliminating residues. The perovskite MCs purified with EtOH during chemical synthesis show superior crystallinity with controlled trap density in the thin film state, which is advantageous for eliminating detrimental non-radiative recombination pathways, as confirmed by structural and optical analyses. Furthermore, perovskite solar cells fabricated from the resulting α-FAPbI3 MCs provided enhanced and reliable photovoltaic performance, with significantly reduced voltage loss in particular. These cells exhibited light-intensity-dependent voltage output with a high diode ideal factor, enabling substantial voltage generation under sunlight irradiation.
Results and discussion
The synthesis process of FAPbI3 perovskite MCs is shown schematically in Fig. 1. Lead(II) iodide (PbI2) (90 g, 0.195 mol) and formamidinium iodide (FAI) (33.6 g, 0.195 mol) were fully dissolved in 100 mL of 2-methoxy ethanol (2ME) at room temperature using a magnetic stirrer. After the dissolution of the precursors was completed, the temperature of the solution was elevated to 120 °C by immersion in an oil bath to precipitate FAPbI3 MCs utilizing the retrograde solubility of the perovskite.29,30 After the precipitation reaction, the supernatant was carefully removed. The resulting FAPbI3 precipitate was then purified four times with the washing solvent (5 mL g−1) by intensively shaking the mixture for 3 min to eliminate residues. Subsequently, the solvent with undesirable substances was completely removed by vacuum filtration for 20 min. The reactant was annealed at 170 °C for an additional 20 min under ambient conditions to completely convert the FAPbI3 MCs with an alpha-phase crystalline structure. For other washing solvents, identical synthetic parameters were applied.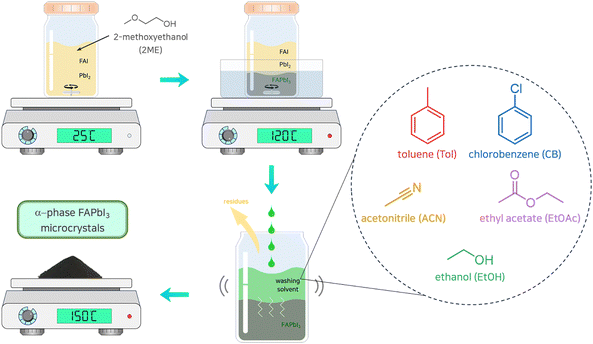 | ||
| Fig. 1 Schematic diagram of the purification process during the synthesis of α-phase FAPbI3 perovskite microcrystals using washing solvents. | ||
As reported in previous literature,31 in which it was claimed that the physical properties of the processing solvents determine the types of soluble species, we hypothesized that physical properties such as the dielectric constant (εr) and Gutmann donor number (DN) (Table S1, ESI†) may also have a significant effect on the purification process. Among the investigated solvents, EtOH has εr and DN values that are well-matched with those of 2ME, indicating that EtOH could be the most suitable washing solvent to eliminate impurities dissolved in 2ME.
For the as-fabricated perovskite thin films obtained from FAPbI3 MCs processed with the selected organic solvents on quartz substrates under about 20% relative humidity, the film thickness and absorbance characteristics were comparable regardless of the kind of MCs. The ‘Precursor’ sample indicates a mixture of FAI and PbI2 with equivalent stoichiometry (Fig. S1a, ESI†).
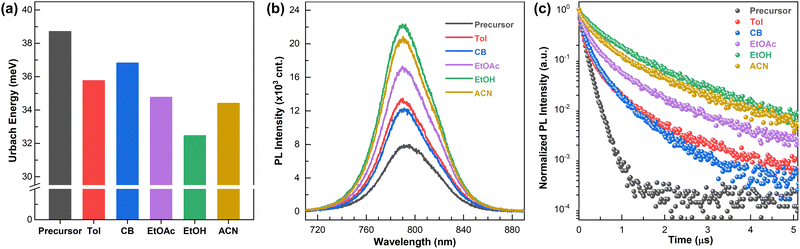
We estimated the properties of the perovskite thin films fabricated using the FAPbI3 MCs. First, the Urbach energies (EU), which are used to quantify the energetic disorder in the band edges, were compared. Fig. 2a shows the EU values evaluated by nonlinear fitting of the band edge in the ultraviolet-visible absorption spectra using the following equation: 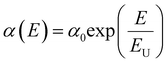 , where α is the absorption coefficient and E is the photon energy hν.32 The values of EU indicate that the synthesized perovskite MCs retain less non-radiative recombination centers in the thin films as compared to the precursor. In addition, the trend was found that the EU of the perovskite MC decreases as the physical properties of the corresponding processing solvent become more similar to those of the reaction solvent (2ME). In particular, the EtOH-assisted FAPbI3 MC exhibits the lowest EU. The crystalline properties of perovskite thin films were analyzed using X-ray diffraction (XRD) measurements (Fig. S2, ESI†). All the samples exhibited similar diffraction peaks; the prominent peak at 14° was assigned to cubic perovskite structure (001) planes, and a distinct PbI2 peak was not observed.33 For the perovskite MCs processed using EtOH, the full width at half maximum (FWHM) of the peak is narrower, which could result from a larger crystalline size with fewer embedded crystal defects.34 These crystalline characteristics of perovskite films could lead to the improved performance and long-term stability.35 From a comparison of the scanning electron microscopy (SEM) images of the perovskite films, it can be seen that the processing solvent used for the FAPbI3 MCs does not strongly influence the morphology of the perovskite film (Fig. S3, ESI†). Additionally, the absence of significant changes in the composition of the perovskite MCs was confirmed through EDS, EA, and DLS measurements for the three solvents, excluding the possibility of overfiltration of the FAPbI3 components during the purification process (Fig. S4, ESI†).
, where α is the absorption coefficient and E is the photon energy hν.32 The values of EU indicate that the synthesized perovskite MCs retain less non-radiative recombination centers in the thin films as compared to the precursor. In addition, the trend was found that the EU of the perovskite MC decreases as the physical properties of the corresponding processing solvent become more similar to those of the reaction solvent (2ME). In particular, the EtOH-assisted FAPbI3 MC exhibits the lowest EU. The crystalline properties of perovskite thin films were analyzed using X-ray diffraction (XRD) measurements (Fig. S2, ESI†). All the samples exhibited similar diffraction peaks; the prominent peak at 14° was assigned to cubic perovskite structure (001) planes, and a distinct PbI2 peak was not observed.33 For the perovskite MCs processed using EtOH, the full width at half maximum (FWHM) of the peak is narrower, which could result from a larger crystalline size with fewer embedded crystal defects.34 These crystalline characteristics of perovskite films could lead to the improved performance and long-term stability.35 From a comparison of the scanning electron microscopy (SEM) images of the perovskite films, it can be seen that the processing solvent used for the FAPbI3 MCs does not strongly influence the morphology of the perovskite film (Fig. S3, ESI†). Additionally, the absence of significant changes in the composition of the perovskite MCs was confirmed through EDS, EA, and DLS measurements for the three solvents, excluding the possibility of overfiltration of the FAPbI3 components during the purification process (Fig. S4, ESI†).
Steady-state photoluminescence (ssPL) emission spectra and time-resolved photoluminescence (trPL), which show optical properties related to carrier recombination and dynamics, support the trend in the Urbach energy of the perovskite thin films. All samples showed a symmetric PL spectrum centered at ∼790 nm, which corresponds to the band-edge transition for the FAPbI3-based perovskite thin film (Fig. 2b). The PL intensities of the synthesized perovskite films were significantly greater than that of the control sample. The PL intensity of the EtOH-based perovskite thin film is the strongest among the samples, being about three times that of the precursor-based perovskite thin film. The elevated PL intensity observed in synthesized perovskite samples indicates that a higher concentration of charge carrier density remains within the perovskite bulk, which is attributed to reduced carrier loss through non-radiative recombination,36 which is one of the crucial parameters in solar cell technology to improve device performance.
The enhancement of the PL intensity was further confirmed by charge-carrier recombination dynamics over a nanoseconds-to-microseconds time scale, in which low-order recombination processes are predominantly observed.37 The trPL results measured using the time-correlated single-photon counting (TCSPC) method are presented in Fig. 2c. We confirmed the prolonged recombination dynamics of the synthesized perovskite samples compared to the control sample. The corresponding carrier lifetimes were obtained from the fitting of the PL decay curves38 (Table S2, ESI†). The perovskite film fabricated by the EtOH-assisted MCs showed an average lifetime of 793 ns, which is significantly longer than that of the control film (105 ns). The results imply that the controlled defect density reduces the mono-molecular recombination rate based on long-range Coulombic interactions.39 As the highest PL intensity and the longest carrier lifetime were observed in the film fabricated from the EtOH-assisted MCs, it could be inferred that the matching physical properties between the reaction and washing solvents leads to the effective elimination of undesirable chemicals while retaining the necessary components, such as intact organic halides.
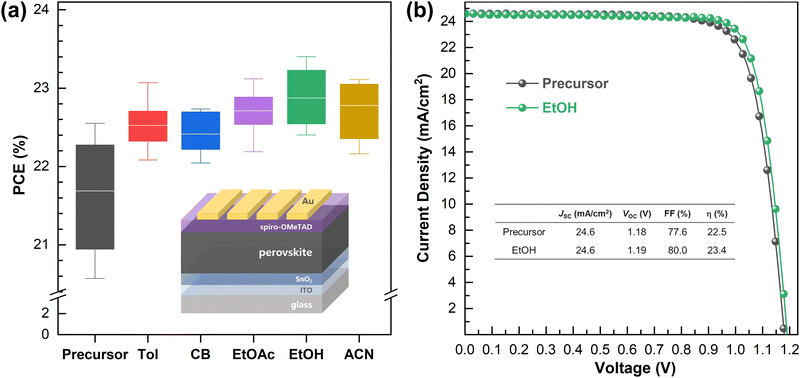
To investigate the effects of the processing solvent on the photovoltaic properties, we fabricated PSC devices with the architecture ITO/SnO2/(FAPbI3)0.95(MAPbBr3)0.05/spiro-OMeTAD/Au. The box diagrams of the statistical distribution of power conversion efficiency (PCE), open-circuit voltage (VOC), fill factor (FF), and short-circuit current density (JSC) measured under simulated AM 1.5G are displayed in Fig. 3 and Fig. S5 (ESI†). Enhanced PCEs in the purified FAPbI3-based solar cells with reduced standard deviation can obviously be seen as compared with the precursor-based solar cells. The reduced standard deviation in the performances for the purified FAPbI3-based device indicates that the purification process not only improves the PCE, but also facilitates high reproducibility of the performance. The photovoltaic performance trends are consistent with the properties of the perovskite thin film, showing the best performance using EtOH. The averaged values of VOC, JSC, FF, and PCE for the control (mixture of precursors, labeled as “Precursor”) were 1.159 V, 24.35 mA cm−2, 77.01%, and 21.69%, respectively, and those of the target (perovskite MCs washed using EtOH, labeled as “EtOH”), improved to 1.187 V, 24.47 mA cm−2, 78.65%, and 22.88%, respectively. We attribute the enhancement of the PCE to major improvements in VOC originating from less VOC deficit due to the crystallinity (XRD), reduced trap and defect density (EU and ssPL), and prolonged recombination dynamics (trPL). Within the error bar, no major difference was observed in JSC. Additionally, the J–V characteristics of the best-performing device based on the control and target are shown in Fig. 3b. The values determined from the curve were a PCE of 22.5%, VOC of 1.18 V, JSC of 24.6 mA cm−2 and FF of 77.6% for the control, and a PCE of 23.4%, VOC of 1.19 V, JSC of 24.6 mA cm−2, and FF of 80.0% for the target, signifying that the EtOH processing method is well suited for energy harvesting applications. The JSC values of the control and target devices agree very well with the integrated current from the external quantum efficiency (EQE) spectra (Fig. S6, ESI†).
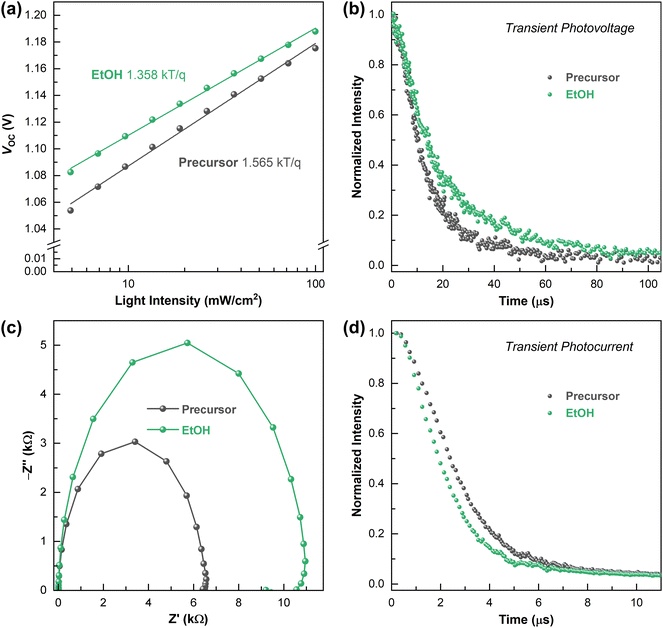
To elucidate the performance improvement, the VOC was plotted as a function of the light intensity (Fig. 4a), which provided more information on the charge recombination in the PSC devices. The diode ideal factor (n) can be calculated by fitting the slope of the curve using the following equation: 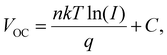 where n is the ideal factor related to single-molecule recombination, I is the light intensity, k is Boltzmann's constant, T is the device temperature, and q is the charge constant.40 It has been reported that n can vary between 1 and 3 for PSCs, depending on the recombination. In general, an ideality factor of n ≈ 1 indicates that bimolecular recombination is dominant, and n ≈ 2 implies trap-assisted recombination.41,42 The target device exhibited a higher VOC value than the control device under all light intensities. The fitted ideal factors of the control and target PSCs were 1.565 and 1.358. The low slope efficiency of the target device, which is closer to that of an ideal diode, indicates the effective suppression of trap-assisted non-radiative recombination in the systems due to the reduction of the surface defect states of the perovskite film based on EtOH-assisted MCs.43,44 The JSC as a function of the light intensity fits the power law equation JSC ∝ Iα, where α is an exponential factor; deviation from α = 1 is due to recombination loss45 (Fig. S7a, ESI†). The α values of the target and control plots are 0.999 and 0.998, respectively, which are both close to 1, implying that the charge collection efficiency is independent of the processing solvent, that both possess sufficient electron and hole mobility, and that there are non-space-charge-limited photocurrents.46,47 In addition, as the light intensity decreases to levels below 0.1 Sun, we noted a VOC of about 1.1 V and efficient charge collection for the target device, which is promising for indoor photovoltaic applications.
where n is the ideal factor related to single-molecule recombination, I is the light intensity, k is Boltzmann's constant, T is the device temperature, and q is the charge constant.40 It has been reported that n can vary between 1 and 3 for PSCs, depending on the recombination. In general, an ideality factor of n ≈ 1 indicates that bimolecular recombination is dominant, and n ≈ 2 implies trap-assisted recombination.41,42 The target device exhibited a higher VOC value than the control device under all light intensities. The fitted ideal factors of the control and target PSCs were 1.565 and 1.358. The low slope efficiency of the target device, which is closer to that of an ideal diode, indicates the effective suppression of trap-assisted non-radiative recombination in the systems due to the reduction of the surface defect states of the perovskite film based on EtOH-assisted MCs.43,44 The JSC as a function of the light intensity fits the power law equation JSC ∝ Iα, where α is an exponential factor; deviation from α = 1 is due to recombination loss45 (Fig. S7a, ESI†). The α values of the target and control plots are 0.999 and 0.998, respectively, which are both close to 1, implying that the charge collection efficiency is independent of the processing solvent, that both possess sufficient electron and hole mobility, and that there are non-space-charge-limited photocurrents.46,47 In addition, as the light intensity decreases to levels below 0.1 Sun, we noted a VOC of about 1.1 V and efficient charge collection for the target device, which is promising for indoor photovoltaic applications.
This effect was also supported by a prolonged transient photovoltage (TPV) measurement under 0.3-Sun illumination (Fig. 4b), which provided the time constant of photogenerated carrier recombination in the operating devices. The TPV lifetime, which is determined as the time needed to reach 1/e intensity, was 19.70 μs for the target device, which is a 45% improvement over that of the control (13.47 μs). The prolonged carrier lifetime of the PSCs indicates that non-radiative recombination was effectively suppressed under open-circuit conditions.48,49 This result is in good agreement with the enhanced VOC and is consistent with the ssPL and trPL results shown above for the film characterization.
Electrochemical impedance spectroscopy (EIS) is another way to investigate charge-carrier recombination (Fig. 4c). Nyquist plots under dark conditions with a bias near VOC were obtained for the target and the control devices. Since the size of the semicircle corresponds to the recombination resistance (Rrec), the approximately 70% larger semicircle for the target device than the control device indicates that the charge-carrier recombination rate at the interface is reduced.50,51 Moreover, reduced series resistance (RS) was observed for the target device in the high-frequency region (Fig. S7b, ESI†). The increased Rrec can contribute to an increase in VOC, while the reduced RS can improve the FF, and is therefore conducive to the enhancement of the overall performance of PSCs.52
A slightly improved FF was also observed in the transient photocurrent (TPC) traces, which give information about charge transfer for PSCs (Fig. 4d). The TPC lifetime of the target device was 2.46 μs, which is a 20% reduction compared to that of the control device (3.02 μs). The purification with the green solvent facilitates efficient charge transfer and minimizes recombination losses at the interface in the device structure. These clear correlations provide a compelling explanation for the enhanced photovoltaic performance achieved through our approach.
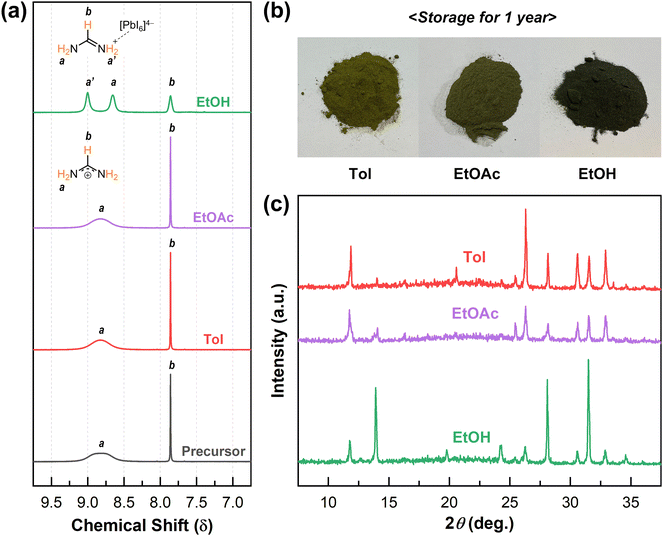
In the context of the material distribution procedure for commercial PSC production, the perovskite MCs should have a long-term shelf life after the materials are produced. Unfortunately, the photoactive α-phase of FAPbI3 undergoes a transition to the yellow δ-phase at room temperature caused by the intrinsic lattice strain, even in the absence of external inputs such as thermal, radiation, or humidity stress.53 We investigated the effects of the organic solvents utilized in the processing of FAPbI3 MCs on the structural durability. We anticipated that the chemical environment of the FAPbI3 MCs could provide clues to disclose the relationship with the phase transition. For this, we conducted proton nuclear magnetic resonance (1H-NMR) measurements of the FAPbI3 solutions dissolved in dimethyl sulfoxide-d6 (Fig. 5a). These could demonstrate the chemical environment of FAI in the perovskite by analyzing the two kinds of protons in the FAI molecule, which are a proton bound at a carbon atom (–CH) and the others bound at two nitrogen atoms (–NH2). Typically, an individual FAI molecule shows a sharp single peak corresponding to the –NH2 protons, since the positive charge on the molecule is delocalized across the N–C–N connection.54,55 When FAI and PbI2 are dissolved together with an identical stoichiometry, the peak of the –NH2 protons is broadened due to an interaction between the two species.54 Furthermore, some literature has reported that the α-FAPbI3 MC solution exhibits two split peaks corresponding to the –NH2 protons, while the mixture of the precursors shows a single peak.55 We deduced that the split peaks might indicate that one of the two nitrogen atoms with a positive charge is locked to [PbI6]4− and the resonance of FAI disappears in the perovskite structure; subsequently, even though the perovskite is dissolved in a solvent, the ionic binding will still be maintained. In the cases of the precursor, Tol, and EtOAc samples, the –NH2 protons showed a broad single peak, while the EtOH sample exhibited two distinct –NH2 proton peaks. Based on these results, we surmise that FAPbI3 MCs processed with EtOH include tighter interactions between organic cations and lead halide anions in the perovskite structure.
A comparison of the appearance of the FAPbI3 MCs stored under dark and ambient conditions supported the interpretation by NMR analysis (Fig. 5b). The as-prepared FAPbI3 MCs showed the black α-phase regardless of the processing solvent used. After storing them for a year, the MCs processed with EtOAc and Tol had gradually transformed into the photoinactive yellow phase, while the MCs processed with EtOH still exhibited the black phase. To confirm this phenomenon, we performed XRD measurements of the MCs (Fig. 5c). The XRD results demonstrate that the MCs prepared using EtOAc and Tol were mainly converted to the δ-phase, while the major portion of the MCs prepared using EtOH remained in the α-phase. Therefore, in terms of phase stability, the EtOH-assisted FAPbI3 MCs have excellent merits to ensure an extended expiration date.
We further conducted a techno-economic analysis of the synthesis of the FAPbI3 perovskite (Table S1, ESI†). When only the cost per product unit for the different washing solvents is considered, ethanol cannot provide greater competitiveness than toluene or ethyl acetate. However, when we consider the life-cycle assessment of the organic solvents (EtOH, Tol and EtOAc), toluene and ethyl acetate consume more energy in their life-cycle, indicating that they require a higher cost for continuous usage than ethanol.14 Furthermore, in terms of EHS, when using ethanol and ethyl acetate, further installations to protect against solvent leakage are not a serious concern, while toluene usage should be accompanied by an additional capture installation for unpredictable leakage.
In conclusion, we have presented an eco-friendly purification solvent for the synthesis of perovskite MCs. In particular, we investigated the correlation between the physical properties, such as dielectric constant and Gutmann donor number of the washing solvent, the crystallinity of the thin film state, and the photovoltaic parameters of the solar cell. Generally, perovskite MCs processed by EtOH exhibit higher crystallinity and optical properties due to the purification of residues, which are better than those of the precursor mixture. Compared with those prepared using toluene, chlorobenzene, ethyl acetate, and acetonitrile, the ethanol-assisted FAPbI3 MCs exhibit the smallest structural disorder and trap density, showing the highest PL intensity and the longest PL lifetime as measured using optical spectroscopy. The as-prepared devices fabricated from the resulting perovskite materials exhibit trends consistent with those of the thin films. As a result, the EtOH device exhibited improved efficiency and reliability with significantly high VOC (1.191 V) and PCE (23.4%) values on the ITO/glass substrate. Its enhanced photovoltaic characteristics were elucidated using electrical spectroscopy, which indicated its superior ideality factor, charge collection, photogenerated carrier recombination and transfer, and recombination resistance. In addition, the ethanol-assisted FAPbI3 MCs were confirmed to have robust storage stability for more than one year. These findings offer deeper insights into future directions for the sustainable mass-production of perovskite materials with reliable quality, paving the way for the commercialization of next-generation perovskite technologies.
Author contributions
All authors developed the concept for this work through discussion (conceptualization). H. S. K. conducted experiments and analysis including material synthesis, device fabrication, UV-Vis absorption, XRD, NMR, EDS, EA, and DLS. H.-S. Y., S. B. Y., and C.-E. S. supported the experiments (investigation & formal analysis). B. J. K. analyzed the photophysical characteristics including ssPL and trPL, and device physics including TPV, TPC, and EIS, and wrote the draft (investigation & writing). E. H. J. gathered and trimmed the data, constructed figures to visualize the prominence of this work, and wrote the draft (visualization & writing). N. J. J., E. H. J., B. J. K. supervised this work (supervision).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2022R1C1C1010741 (Young Researcher Program) & RS-2023-00283070 (Nano & Material Technology Development Project-Nano Connect)). This research was supported by POSCO Science Fellowship of POSCO TJ Park Foundation.References
- Y. Zhao and K. Zhu, Chem. Soc. Rev., 2016, 45, 655–689 RSC.
- R. E. Brandt, V. Stevanović, D. S. Ginley and T. Buonassisi, MRS Commun., 2015, 5, 265–275 CrossRef CAS.
- H. Zhang, L. Pfeifer, S. M. Zakeeruddin, J. Chu and M. Grätzel, Nat. Rev. Chem., 2023, 7, 632–652 CrossRef CAS PubMed.
- N.-G. Park and K. Zhu, Nat. Rev. Mater., 2020, 5, 333–350 CrossRef CAS.
- J. Song, Q. Shang, X. Deng, Y. Liang, C. Li, X. Liu, Q. Xiong and Q. Zhang, Adv. Mater., 2023, 35, 2302170 CrossRef CAS PubMed.
- Y. Wu, J. Feng, Z. Yang, Y. Liu and S. Liu, Adv. Sci., 2023, 10, 2205536 CrossRef CAS PubMed.
- X.-K. Liu, W. Xu, S. Bai, Y. Jin, J. Wang, R. H. Friend and F. Gao, Nat. Mater., 2021, 20, 10–21 CrossRef CAS PubMed.
- https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf, DOI: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf.
- M. A. Green, E. D. Dunlop, G. Siefer, M. Yoshita, N. Kopidakis, K. Bothe and X. Hao, Prog. Photovolt. Res. Appl., 2023, 31, 3–16 CrossRef.
- L. McGovern, E. Alarcón-Lladó, E. C. Garnett, B. Ehrler and B. van der Zwaan, ACS Energy Lett., 2023, 8, 4862–4866 CrossRef CAS PubMed.
- United States Environmental Protection Agency (EPA), Toxics Release Inventory (TRI) Program: TRI Toxics Tracker (accessed: Jan. 2024), https://edap.epa.gov/public/extensions/TRIToxicsTracker/TRIToxicsTracker.html#continue.
- J. H. Clark, A. Hunt, C. Topi, G. Paggiola and J. Sherwood, Sustainable Solvents: Perspectives from Research, Business and International Policy, The Royal Society of Chemistry, 2017 Search PubMed.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Process, 2016, 4, 7 CrossRef.
- UN General Assembly, Report of the World Commission on Environment and Development: Our Common Future, 1987.
- T. Welton, Proc. R. Soc. A, 2015, 471, 20150502 CrossRef PubMed.
- F. Pena-Pereira, A. Kloskowski and J. Namieśnik, Green Chem., 2015, 17, 3687 RSC.
- Y. Zhang, S. Seo, S. Y. Lim, Y. Kim, S.-G. Kim, D.-K. Lee, S.-H. Lee, H. Shin, H. Cheong and N.-G. Park, ACS Energy Lett., 2020, 5, 360–366 CrossRef CAS.
- G. S. Shin, Y. Zhang and N.-G. Park, ACS Appl. Mater. Interfaces, 2020, 12, 15167–15174 CrossRef CAS PubMed.
- Y. Wang, Z. Shi, Y. Wang, Q. U. Khan, X. Li, L. Deng, Y. Pan, X. Zhang, Y. Yang, X. Yue, T. Hu, F. Liu, H. Wang, C. Li, K. Liu, W. Yuan, C. Cong, A. Yu and Y. Zhan, Adv. Mater., 2023, 35, 2302298 CrossRef CAS PubMed.
- O. E. Solis, C. Fernández-Saiz, J. M. Rivas, D. Esparza, S.-H. Turren-Cruz, B. Julián-López, P. P. Boix and I. Mora-Seró, Electrochim. Acta, 2023, 439, 141701 CrossRef CAS.
- F. Fei, L. Gu, Y. Xu, K. Du, X. Zhou, X. Dong, X. Chen, N. Yuan, S. Wang and J. Ding, ACS Appl. Mater. Interfaces, 2022, 14, 52960–52970 CrossRef CAS PubMed.
- T. N. Mandal, J. H. Heo, S. H. Im and W.-S. Kim, Small, 2023, 19, 2305246 CrossRef CAS PubMed.
- H.-S. Yun, H. W. Kwon, M. J. Paik, S. Hong, J. Kim, E. Noh, J. Park, Y. Lee and S. Il Seok, Nat. Energy, 2022, 7, 828–834 CrossRef CAS.
- Y. Miao, M. Ren, Y. Chen, H. Wang, H. Chen, X. Liu, T. Wang and Y. Zhao, Nat. Sustainable, 2023, 6, 1465–1473 CrossRef.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982–988 RSC.
- Z. Zheng, S. Wang, Y. Hu, Y. Rong, A. Mei and H. Han, Chem. Sci., 2022, 13, 2167–2183 RSC.
- M. I. Saidaminov, A. L. Abdelhady, G. Maculan and O. M. Bakr, Chem. Commun., 2015, 51, 17658–17661 RSC.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. Maculan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nat. Commun., 2015, 6, 7586 CrossRef PubMed.
- P. Zhu, D. Wang, Y. Zhang, Z. Liang, J. Li, J. Zeng, J. Zhang, Y. Xu, S. Wu, Z. Liu, X. Zhou, B. Hu, F. He, L. Zhang, X. Pan, X. Wang, N.-G. Park and B. Xu, Science, 2024, 383, 524–531 CrossRef CAS PubMed.
- J. Park, J. Kim, H.-S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2023, 616, 724–730 CrossRef CAS PubMed.
- L.-Q. Xie, L. Chen, Z.-A. Nan, H.-X. Lin, T. Wang, D.-P. Zhan, J.-W. Yan, B.-W. Mao and Z.-Q. Tian, J. Am. Chem. Soc., 2017, 139, 3320–3323 CrossRef CAS PubMed.
- H. Guo, G. W. Yoon, Z. J. Li, Y. Yun, S. Lee, Y.-H. Seo, N. J. Jeon, G. S. Han and H. S. Jung, Adv. Energy Mater., 2024, 14, 2302743 CrossRef CAS.
- G. Tumen-Ulzii, C. Qin, D. Klotz, M. R. Leyden, P. Wang, M. Auffray, T. Fujihara, T. Matsushima, J.-W. Lee, S.-J. Lee, Y. Yang and C. Adachi, Adv. Mater., 2020, 32, 1905035 CrossRef CAS PubMed.
- D. W. deQuilettes, M. Laitz, R. Brenes, B. Dou, B. T. Motes, S. D. Stranks, H. J. Snaith, V. Bulović and D. S. Ginger, Pure Appl. Chem., 2020, 92, 697–706 CrossRef CAS.
- M. Stolterfoht, C. M. Wolff, J. A. Márquez, S. Zhang, C. J. Hages, D. Rothhardt, S. Albrecht, P. L. Burn, P. Meredith, T. Unold and D. Neher, Nat. Energy, 2018, 3, 847–854 CrossRef CAS.
- H. Kim, J. Lee, B. Kim, H. R. Byun, S. H. Kim, H. M. Oh, S. Baik and M. S. Jeong, Sci. Rep., 2019, 9, 15461 CrossRef PubMed.
- C. Zhang, S. Mahadevan, J. Yuan, J. K. W. Ho, Y. Gao, W. Liu, H. Zhong, H. Yan, Y. Zou, S.-W. Tsang and S. K. So, ACS Energy Lett., 2022, 7, 1971–1979 CrossRef CAS.
- M. Bernechea, N. Cates, G. Xercavins, D. So, A. Stavrinadis and G. Konstantatos, Nat. Photon., 2016, 10, 521–525 CrossRef CAS.
- T. Singh and T. Miyasaka, Adv. Energy Mater., 2018, 8, 1700677 CrossRef.
- C. Fei, B. Li, R. Zhang, H. Fu, J. Tian and G. Cao, Adv. Energy Mater., 2017, 7, 1602017 CrossRef.
- M. Stolterfoht, C. M. Wolff, Y. Amir, A. Paulke, L. Perdigón-Toro, P. Caprioglio and D. Neher, Energy Environ. Sci., 2017, 10, 1530–1539 RSC.
- J. Liu, J. Wu, W. Sun, X. Wang, Y. Du, G. Li, Q. Chen, F. Cai, S. Zhu and Z. Lan, ACS Appl. Energy Mater., 2021, 4, 13471–13481 CrossRef CAS.
- F. Zhang, W. Shi, J. Luo, N. Pellet, C. Yi, X. Li, X. Zhao, T. J. S. Dennis, X. Li, S. Wang, Y. Xiao, S. M. Zakeeruddin, D. Bi and M. Grätzel, Adv. Mater., 2017, 29, 1606806 CrossRef PubMed.
- L. J. A. Koster, V. D. Mihailetchi, H. Xie and P. W. M. Blom, Appl. Phy. Lett., 2005, 87, 203502 CrossRef.
- D. Bi, L. Yang, G. Boschloo, A. Hagfeldt and E. M. J. Johansson, J. Phys. Chem. Lett., 2013, 4, 1532–1536 CrossRef CAS PubMed.
- W.-Q. Wu, Z. Yang, P. N. Rudd, Y. Shao, X. Dai, H. Wei, J. Zhao, Y. Fang, Q. Wang, Y. Liu, Y. Deng, X. Xiao, Y. Feng and J. Huang, Sci. Adv., 2019, 5, eaav8925 CrossRef CAS PubMed.
- Z. S. Wang, F. Ebadi, B. Carlsen, W. C. H. Choy and W. Tress, Small Methods, 2020, 4, 2000290 CrossRef.
- N. Mohammadian, A. Moshaii, A. Alizadeh, S. Gharibzadeh and R. Mohammadpour, J. Phys. Chem. Lett., 2016, 7, 4614–4621 CrossRef CAS PubMed.
- C. Ma and N.-G. Park, Chem, 2020, 6, 1254–1264 CAS.
- H. Kim, D. Y. Lee, J. Lim, J. Kim, J. Park, J. Seidel, J. S. Yun and S. I. Seok, Adv. Energy Mater., 2023, 13, 2301046 CrossRef CAS.
- Y. An, J. Hidalgo, C. A. R. Perini, A.-F. Castro-Méndez, J. N. Vagott, K. Bairley, S. Wang, X. Li and J.-P. Correa-Baena, ACS Energy Lett., 2021, 6, 1942–1969 CrossRef CAS.
- M. P. U. Haris, J. Xia, S. Kazim, Z. Molenda, L. Hirsch, T. Buffeteau, D. M. Bassani, M. K. Nazeeruddin and S. Ahmad, Cell Rep. Phys. Sci., 2023, 4, 101304 CrossRef CAS PubMed.
- Y. Huang, J. Liang, Z. Zhang, Y. Zheng, X. Wu, C. Tian, Z. Zhou, J. Wang, Y. Yang, A. Sun, Y. Liu, C. Tang, Z. Chen and C.-C. Chen, Small Methods, 2022, 6, 2200933 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00061g |
| This journal is © The Royal Society of Chemistry 2024 |