Open Access Article
Open Access ArticleMXene and Xene: promising frontier beyond graphene in tissue engineering and regenerative medicine
Moon Sung
Kang†
a,
Hee Jeong
Jang†
a,
Hyo Jung
Jo
a,
Iruthayapandi Selestin
Raja
b and
Dong-Wook
Han
 *ab
*ab
aDepartment of Cogno-Mechatronics Engineering, College of Nanoscience and Nanotechnology, Pusan National University, Busan 46241, Republic of Korea. E-mail: nanohan@pusan.ac.kr
bBIO-IT Fusion Technology Research Institute, Pusan National University, Busan 46241, Republic of Korea
First published on 16th November 2023
Abstract
The emergence of 2D nanomaterials (2D NMs), which was initiated by the isolation of graphene (G) in 2004, revolutionized various biomedical applications, including bioimaging and -sensing, drug delivery, and tissue engineering, owing to their unique physicochemical and biological properties. Building on the success of G, a novel class of monoelemental 2D NMs, known as Xenes, has recently emerged, offering distinct advantages in the fields of tissue engineering and regenerative medicine. In this review, we focus on the comparison of G and Xene materials for use in fabricating tissue engineering scaffolds. After a brief introduction to the basic physicochemical properties of these materials, recent representative studies are classified in terms of the engineered tissue, i.e., bone, cartilage, neural, muscle, and skin tissues. We analyze several methods of improving the clinical potential of Xene-laden scaffolds using state-of-the-art fabrication technologies and innovative biomaterials. Despite the considerable advantages of Xene materials, critical concerns, such as biocompatibility, biodistribution and regulatory challenges, should be considered. This review and collaborative efforts should advance the field of Xene-based tissue engineering and enable innovative, effective solutions for use in future tissue regeneration.
Introduction
Recently, interest in 2D nanomaterials (2D NMs) has increased owing to their potential applicability in a wide range of biomedical fields.1 It began when André Geim and Kostya Novoselov successfully isolated graphene (G) in 2004,2 and subsequently, derivatives of G, such as G oxide (GO) and reduced GO (rGO), with numerous functional groups and lattice defects, were introduced.3–5 The G derivatives have garnered significant attention in the biomedical field, particularly in bioimaging,6,7 -sensing,8,9 and -robotics,14–16 theranostics,10,11 drug delivery,12 and tissue engineering,13 owing to their special physicochemical and biological properties. The application of G in the fields of tissue engineering and regenerative medicine has enabled the tailoring of the biological and mechanical properties of native materials by introducing binding sites for further bio-functionalization with biological molecules. Additional properties, such as conductivity for regulating cell behavior, proliferation, and differentiation, which promote specific tissue regeneration, may also be introduced.17–19Notably, extensive research on G enabled the development of other functional 2D NMs. The remarkable progress reported with respect to G over the past few decades has led to novel categories of functional 2D NMs, i.e., transition metal dichalcogenides, transition metal oxides, layered double hydroxides, hexagonal BN, and metal–organic frameworks.20 MXenes, which are 2D NMs comprising transition metal carbides and nitrides, were first discovered by Gogotsi et al. in 2011.21 They exhibit remarkable features in converting photothermal energy, rendering them well-suited for applications in photonic hyperthermia treatment within the second near-infrared (NIR) biowindow (NIR-II biowindow), facilitating deep tissue penetration.22,23 The range of metals available for use within MXenes renders them useful contrast agents in computed tomography (CT) and magnetic resonance imaging. The remarkable electrical conductivity and numerous functional groups of MXenes, which are highly promising for use in the fields of tissue engineering and regenerative medicine, merit attention in this regard.24–27
Subsequently, a novel category of monoelemental 2D materials emerged, known as Xenes. The component elements primarily occur in the main groups of the periodic table, i.e., IIIA (B, Al, Ga, and In), IVA (Si, Ge, Sn, and Pd), VA (P, As, Sb, and Bi), and VIA (Se and Te).28 Following a nomenclature akin to that of G, these monoelemental 2D materials are typically named by combining the elemental names with the suffix “-ene,” yielding names such as borophene, germanene, and tellurene. Xenes exhibit rapid responses to external stimuli (e.g., NIR laser irradiation or pH changes) owing to their ultrathin 2D structures.29 This enables the triggered and/or controlled release of loaded molecules, rendering them suitable for use in the targeted delivery of multi-responsive therapeutics to desired sites.30 Additionally, the chemical properties of Xenes, including their tunable, versatile surface chemistries, enable the adsorption of various biomolecules, proteins, and cyto-/chemokines, facilitating regenerative medicinal applications.31–33
In this review, we explore the emerging era of MXenes and other types of Xene materials that display potential for use in the fields of tissue engineering and regenerative medicine (Fig. 1). We analyze the distinctive attributes of Xenes, which surpass those of G, and their potential applications in engineering and restoring various tissues, including bone, cartilage, nerves, muscles, and skin. Moreover, we address the opportunities and challenges associated with the utilization of 2D NMs beyond G in the context of the future clinical and industrial applications of Xenes.
Classification and physicochemical characteristics of Xenes
This section provides a concise overview of commonly employed Xenes, including their synthetic techniques and typical features. Based on the positions of their elements in the periodic table, Xenes may be classified as follows: Group III (borophene and gallenene), Group IV (silicene, germanene, and stanene), Group V (phosphorene, arsenene, antimonene, and bismuthene), and Group VI (selenene and tellurene).29G derivatives
G serves as a representative 2D NM and displays numerous structural features characteristic of 2D NMs. Its structure comprises a single layer of C atoms covalently bonded in a flat, regular hexagonal pattern via sp2 bonds.34 Conversely, GO exhibits the same hexagonal C atom arrangement but contains sp3 C atoms bonded to functional groups above or below the plane of the NM, resulting in a rougher structure, accompanied by pronounced local polarity. GO is a non-stoichiometric compound of C, O, and H in a variable ratio, which is influenced by the processing methods used. It contains abundant O-containing functional groups, including epoxide, carbonyl, hydroxyl, and phenol groups, which are introduced during chemical exfoliation.35 These defects alter the inert G structure, resulting in unique properties that enable diverse applications as sensors, photovoltaics, membranes, purification materials, etc.36 rGO lies in between G and GO in terms of structure. It may be produced by reducing GO via electrochemical, microwave, and photo-assisted thermal methods, which eliminate most functional groups and partially restore sp2 hybridization.37,38 rGO exhibits excellent light absorption characteristics across the entire spectrum, with even a single layer displaying the capacity to absorb a substantial amount of light at visible and NIR wavelengths.39MXenes
MXenes are synthesized by selectively removing specific atomic layers from parent MAX materials using HF or a combination of a potent acid and fluoride salt.40 Their structural formula is M(n+1)XnTx, with M representing early transition metals, such as Ti, V, Zr, and Nb, X representing C and/or N, and T representing surface functional groups, such as –O, –OH, and –F. Varying the atomic layer number in the unit cell yields the typical structures M2XTx, M3X2Tx, and M4X3Tx.41 Initially, MXenes were investigated for use in energy conversion and storage systems due to their high theoretical capacity and electrical conductivity, but recently they have received significant attention in the field of photocatalysis.42 Their exceptional physicochemical characteristics may be ascribed to several factors. Wet chemical etching generates an abundance of functional groups, facilitating close contact between the MXene and biological system or material.43 Adjustments in surface chemistry enable the tuning of the bandgap alignment of the MXene. The conductive metal cores within the layered structure endow the MXene with excellent metallic conductivity and electron-accepting capacity. Consequently, MXenes emerged as strong candidates among 2D materials, and they have been thoroughly explored for use in various photocatalytic applications, including H2O splitting, CO2 reduction, pollutant degradation, and N2 fixation.42 Additionally, the simple surface modification of an MXene improves the in vivo performance by reducing toxicity, enhancing colloidal stability, and prolonging circulation within the body.44Group III Xene (borophene)
B is one of the most chemically complex elements due to its trivalent electronic configuration.45 This complexity disrupts the octet rule, resulting in an unusual electron-poor bonding pattern, where the electrons of B and its compounds are shared among ≥3 atoms.46 In its bulk form, pure B exhibits significant structural diversity, with 5–16 different polymorphs with highly complex unit cells.47 Borophene, which is a unique 2D B sheet, displays remarkable properties, including a tunable anisotropic structure, metallic behavior, optical transparency, and potential high-temperature superconductivity (10–20 K).48–50 Various synthetic methods, such as physical vapor deposition (PVD), mechanical cleavage, etching, and liquid-phase exfoliation, have been successfully employed in producing borophene.51,52Group IV Xenes (silicene, germanene, and stanene)
Silicene displays a non-planar buckled honeycomb configuration in its monolayer form, which is distinct from the sp2-hybridized C atoms within bulk graphite.53 It should exhibit G-like Dirac fermions and semi-metallic properties, along with enhanced spin–orbit coupling effects due to its lower symmetry.54,55 Theoretical insights suggest that silicene may exhibit topologically nontrivial electronic states, gate-tunable bandgaps, and spin-polarized edge states, rendering it suitable for use in devices such as tunable transistors and photodetectors.56 Experimental synthetic methods, such as PVD and chemical exfoliation, have been successfully employed in producing silicene.57 Germanene, akin to silicene, features a buckled honeycomb structure,58 which is characterized by Ge atoms forming a corrugated 2D layer structure, driven by its enhanced spin–orbit coupling due to its heavier atomic nature compared to that of Si.59 This attribute endows germanene with stronger topological insulator properties. Common methods of synthesizing germanene include mechanical cleavage and PVD.60 Similar to silicene and germanene, stanene displays a buckled honeycomb lattice structure with stable π⋯π bonding within the atomic plane.61 The distinct feature of stanene is its strong spin–orbit coupling, which deviates from the behavior of graphene and results in a bandgap opening of approximately 0.1 eV, with topologically nontrivial states at the edges of the material.62 Stanene, which is a promising topological insulator, has been experimentally synthesized using PVD.63Group V Xene (phosphorene)
P exhibits various allotropes, including gaseous P, black P (BP), blue P, white P, violet P, and red P. BP, which is the most thermodynamically stable allotrope, has been studied extensively, particularly its 2D variant, referred to as phosphorene. Phosphorene exhibits an orthorhombic structure characterized by parallel atomic layers that are puckered in a double-floor arrangement.64 Every P atom within an atomic layer bears 5 valence electrons and engages in covalent bonding with adjacent P atoms.65 At an elevated pressure, BP displays the capacity to undergo transition to a semi-metallic β phase, which is characterized by a double-layered rhombohedral structure comprising ruffled, interlocked, six-membered rings.64,66 Due to its versatility, BP is one of the most studied Xenes after G, and various experimental methods have been developed for use in phosphorene synthesis, including mechanical cleavage,67 liquid-phase exfoliation,68 etching,69 chemical vapor deposition,70 PVD,71 and wet-chemistry techniques.72Tissue regeneration using MXenes and Xenes: comparison with G derivatives
Bone and cartilage tissue engineering using G, MXenes, and Xenes
| Applications | Xene materials | Formulations | Test species | Biocompatibility and therapeutic applications | Enhanced osteogenic markers | Outlooks | Ref. |
|---|---|---|---|---|---|---|---|
| Bone | rGO | rGO-Ti substrate | hMSCs | High viability at 4 mg mL−1 rGO-coated substrates | ALP | Osteogenic properties of rGO can be applied for dental and orthopedic bone implants | 76 |
| Mineralization nodule formation | |||||||
| GO | GO-Alg/Gel hydrogel | hMSCs | 90% viability at 2 mg mL−1 | COL1A2, SOST, ALP, BGLAP, and PHEX | Potential ex vivo model for critical-sized defects | 97 | |
| Enhanced spreading and proliferation for 42 d | Mineralization nodule formation | ||||||
| Stress fiber expression | |||||||
| PGBC nanocomposites | SD rat primary osteoblasts | 95–100% viability at 0.5 w/w% | ALP, OPN, and Smad5 | Viable orthopaedic materials for clinical application | 78 | ||
| New Zealand rabbit | Sustained attachment and proliferation with no apoptosis during 7 d | BV/TV, TbN, TbSp | |||||
| No hematological toxicity | |||||||
| GO-coated PLA film | MC3T3-E1 cells | 82% viability at 0.1 mg mL−1 | Bone mineral density | Customizable structured GBR implant for dental regeneration | 98 | ||
| Enhanced migration, adhesion, spreading, and proliferation for 5 d | BV/TV, Tb.Th, Tb.Sp, and Tb.N | ||||||
| High membrane integrity with no intracellular ROS generation | |||||||
| Nb2C MXene | 3DP NBGS lattices | Saos-2 cells | 90% cell viability at 1 mg mL−1 | NIR-assisted tumor suppression | Photothermal properties in the NIR-II biowindow offer effective treatment of bone malignancy and osteosarcoma | 79 | |
| NIR-radiated photonic hypothermia | Neovascularization | ||||||
| Col1, OCN, RUNX2, and OPN | |||||||
| BV/TV, BMD, and lowered porosity | |||||||
| Ti3C2Tx MXene | MXene films | MC3T3-E1 cells | Enhanced adhesion, actin filament spreading, and proliferation on free-standing MXene films | ALP, OCN, and OPN | Biocompatibility, osteoinductivity, and osseointegration of MXene NPs are highlighted for GBR therapy | 80 | |
| No noticeable inflammatory cell infiltration and necrosis | Bone volume fracture, Tb.Th, and Tb.Sp | ||||||
| New bone volume | |||||||
| MXene/RSF hydrogels | BMSCs | MXene < 8 mg mL−1 was cytocompatible | RUNX-2, Col-1, OCN, COL-1, and ALP | Piezoresistive and electroactive properties promote direct osteogenesis by activating the Ca2+/CALM signaling pathway | 81 | ||
| RAW264.7 cells | MXene supported angiogenesis, upregulation of immune regulatory markers with M2 polarization | Ca2+/CALM signaling pathway | |||||
| HUVECs | ES enhanced neovascularization and macrophage polarization | ||||||
| Sprague–Dawley rats | |||||||
| BP | BP-CNTpega injectable gel | MC3T3-E1 cells | No cell viability at 0.4 mg mL−1 BP | ALP and OCN | Enhanced mechanical strength, electrical conductivity, and continuous phosphate ion release for bone regeneration therapy | 85 | |
| Rabbits | Enhanced adhesion and proliferation | CSF-1, GDF10, VEGF-A, SMAD1, CSF3, NOG, and INTB1 | |||||
| ES supported osteogenesis and cell growth both in vitro and in vivo | Osseointegration in bone defects | ||||||
| BP-GelMA/U-Arg-PEA hydrogel | hDPSCs | No significant cytotoxicity up to 300 mg mL−1 | Col1, BMP4, and RUNX2 | Supplying extra phosphorus for effective bone regeneration | 86 | ||
| New Zealand rabbits | Supported cell adhesion, spreading, and migration | Accelerated new bone formation | |||||
| ALP and mineralization nodule formation | |||||||
| BP/GelMA-CaP hydrogel | hMSCs | No cytotoxicity up to 0.1 w/v% BP | RUNX2, ALP, OCN, and OPN | Multifunctional therapeutic BP nanocomposite offering photothermal antibacterial capability and bone regeneration | 87 | ||
| Saos-2 cells | NIR-assisted anti-bacterial effects | ALP and mineralization nodule formation | |||||
| SD rats | BMD, BV/TV, and BV | ||||||
| New bone formation | |||||||
| Silicene | SNSs@AIPH/CPC scaffold | BMSCs | No cytotoxicity up to 29 μmol mL−1 | ALP and mineralization nodule formation | Thermodynamic properties under NIR-II irradiation promoting sequential and multistage bone regeneration | 93 | |
| HUVECs | NIR radiation promoted proliferation and angiogenesis | OPN, BSP, RUNX2, OXS | |||||
| SD rats | Lama5, Tgfb3, Col15a1, Fgf10, Ramp1, Bmp2, Smad3, Bmp6, Wnt5b, Sema3a, and Smad1 | ||||||
| New bone formation with enhanced BMD, BV/TV, and Tb.N | |||||||
| H-Si TCP scaffold | BMSCs | ROS scavenging effects | SP7, BMP2, SPP1, and ALP | Orchestrating scaffold degradation and bone regeneration in a spatiotemporal manner through osteoimmunomodulation for treating large bone defects | 94 | ||
| Raw264.7 cells | No cytotoxicity up to 50 μg mL−1 | TGFβ and BMP signaling pathway | |||||
| SD rats | Supported adhesion and proliferation of BMSCs | Mineralization nodule formation | |||||
| Immunoregulatory effects on Raw264.7 and M2 polarization | New bone formation with less fibrous encapsulation and more osseous tissue accumulation | ||||||
| H-Si@HAp-Ti | BMSCs | ROS scavenging and autophagy activation | SOD1, OCN, OPN, and OSX | Fracture healing by regulating the acidic microenvironment suppressing oxidative stress and facilitating osteogenic differentiation | 96 | ||
| MC3T3-E1 cells | No hemotoxicity | ALP activity and mineralization | |||||
| SD rats | Supported cell adhesion and proliferation up to 1.2 mg mL−1 | Osseointegration with enhanced BMD and BV/TV |
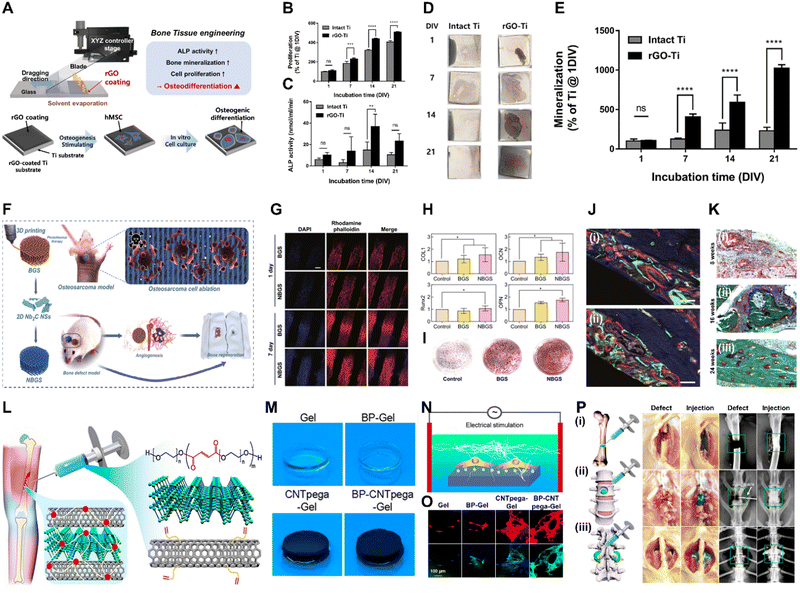 | ||
| Fig. 2 Xene-based tissue engineering scaffolds for use in bone regeneration. (A)–(E) rGO-coated Ti substrates for use in osteogenic differentiation of hMSCs. (A) Fabrication of the rGO-Ti substrates. (B) Proliferation and (C) ALP activity of hMSCs within 21 d. (D) Digital images and (E) quantified results of the ARS staining of the hMSCs cultured on the rGO-Ti substrate within 21 d. Data reproduced from ref. 76. Copyright Springer Nature 2021. (F)–(K) 3DP NBGS lattices for use in osteosarcoma phototherapy and osteogenesis. (F) Fabrication and study scheme of the 3DP NBGS lattices. (G) Immunofluorescence-stained images of the hBMSCs on BGS/NBGS (blue: nucleus and red: F-actin). (H) Relative expression of osteogenic genes. (I) ARS staining of hBMSCs cultured on different groups. (J) New woven bone around the scaffold at wk 8 at spots (i) 1 and (ii) 2. (K) Goldner trichrome staining of the regenerated tissue in the NBGS group at wks (i) 8, (ii) 16, and (iii) 24. Data reproduced from ref. 79. Copyright Springer Nature 2021. (L)–(P) BP-CNTpega injectable gel for use in bone tissue engineering. (L) Schematic and (M) digital microscopy image of the fabricated BP-CNTpega gel. (N) Schematic and (O) immunofluorescence-stained images of ES-promoted cell proliferation. (P) Capacity of the injectable BP-CNTpega-gels to fill various bone defects. Photograph and X-ray images of rabbit (i) femur and (ii) vertebral body and (iii) posterolateral spinal fusion. Data reproduced from ref. 85. Copyright American Chemical Society 2020. The scale bars represent (J) 250, (G) and (K) 200, and (O) 100 μm, and the asterisks denote statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns: not significant). | ||
Owing to their biofunctionalities, which are due to their numerous functional groups and elemental nature, MXene nanoparticles (NPs) were recently utilized in bone tissue engineering. Yin et al. developed Nb2C MXene-functionalized scaffolds, which were combined with NIR-radiation osteosarcoma phototherapy in restoring bone defects via angio- and osteogenesis (Fig. 2F–K).79 Bioactive glass (BGS) was reinforced with Nb2C MXenes to fabricate NBGS bioinks. These Nb2C-MXene nanosheets (NSs) exhibit unique photonic responses in the NIR-II biowindow, rendering them effective in killing bone cancer cells, with deep tissue penetration. Additionally, as the Nb-based components of the Nb2C MXenes are degraded, they stimulate the growth and migration of blood vessels in the vicinity of the bone defect. This enhances the delivery of O2, nutrients, and immune cells, accelerating scaffold degradation and providing space for bone remodeling. Furthermore, Ca and PO43− released during scaffold degradation support the mineralization of new bone tissue. Therefore, the multimodal properties of the NBGS scaffolds may facilitate bone regeneration with anticancer effects, rendering them promising biomaterials for use in treating bone tumors. Zhang et al. assessed the biocompatibility and osteogenic potential of a Ti3C2Tx MXene in in vitro and in vivo settings to elucidate its suitability for use in bone tissue engineering.80 They produced flexible MXene films using the MILD method and conducted in vitro and in vivo studies. In vitro studies using MC3T3-E1 cells revealed that the MXene films significantly enhanced early-stage osteogenic differentiation, as indicated by the increased ALP activity and expression of osteogenic genes. The subcutaneous implantation of the MXene films in rats demonstrated their excellent biocompatibility, with mild inflammatory responses and tissue integration. In a rat calvarial defect model, the MXene films adhered well to the bone tissue, whereas the positive control Ti membranes exhibited reduced mechanical compatibility over time. Micro-CT revealed that the MXene films promoted significant, uniform new bone formation in the defect area, which was guided by the films. Histological analysis confirmed mature bone regeneration on the MXene films without inflammatory reactions. Overall, this study provided valuable insights into the biocompatibility and osteoinductive properties of Ti3C2Tx MXenes, thereby supporting their application in bone regeneration. In a study conducted by Hu et al., a Ti3C2 MXene-incorporated regenerated silk fibroin (RSF) hydrogel was prepared and used in elucidating the role of electrical stimulation (ES) in bone regeneration.81 ES accelerates the development, specialization, and multiplication of various cell types, including stem cells (SCs).82 RSF, which is sourced from Bombyx mori silkworms, is an extensively studied biopolymer known for its exceptional mechanical strength, biocompatibility, and controllable biodegradability.83 The MXene and ES enhanced osteogenic differentiation, based on increased ALP activity and Alizarin red S (ARS) staining, in addition to the upregulation of osteogenic markers (i.e., runt-related transcription factor 2 (RUNX2), Col-1, osteocalcin (OCN), COL-1, and ALP) at the protein and messenger ribonucleic acid (mRNA) levels. ES also promotes M2 macrophage polarization and indirectly enhances the osteogenic differentiation of BMSCs. The MXene/RSF hydrogels combined with ES promoted neovascularization in vitro, enhancing the migration and tube formation capacity of human umbilical vein endothelial cells (HUVECs). Additionally, the use of MXene/RSF hydrogels resulted in significant levels of bone regeneration, mineralization, and angiogenesis in cranial defect models, with enhanced levels of M2 macrophage polarization, good biocompatibility, and gradual levels of degradation. Ribonucleic acid (RNA) sequencing indicated that ES upregulated genes related to biomineral tissue development and the Ca signaling pathway, particularly the CALM gene, suggesting that ES-induced osteogenic effects are associated with the activation of Ca2+/CALM signaling within BMSCs.
BP is one of the most advantageous biomaterials for use in tissue engineering, because P is involved in various cell signal cascades, in addition to metabolism, membrane and nucleic acid structures, mineralized matrix formation, and enzyme catalysis.84 Liu et al. engineered a BP-infused C nanotube-polyethylene glycol-acrylate (BP-CNTpega) injectable hydrogel for use in repairing irregular tissue defects by enhancing the mechanical strength, electrical conductivity, and PO43− release to support bone tissue regeneration (Fig. 2L–P).85 The BP-CNTpega hydrogel exhibited a PO43− release profile with an initial burst release, followed by a slower release due to BP oxidation. Cell viability and proliferation were significantly enhanced within the nanocomposite gel, likely due to the endogenous PO43− release and improved mechanical properties. ES was applied to enhance cellular growth and osteogenesis in MC3T3 preosteoblasts, leading to significantly increased levels of cell proliferation, ALP activity, OCN content, and expression of multiple osteogenic genes. The BP-CNTpega hydrogel effectively filled the defect sites in rabbit models, according to X-ray visualization, highlighting its potential for use in diverse bone defect repair applications. In a study conducted by Huang et al., BP was incorporated into a photopolymerizable hydrogel comprising gelatin methacrylamide (GelMA) and cationic arginine-based unsaturated poly(ester amide)s (U-Arg-PEAs), denoted the BP-GelMA/U-Arg-PEA hydrogel.86 The prepared hydrogels exhibited photoresponsive levels of PO43− release upon exposure to 808 nm NIR irradiation and enhanced levels of in vitro mineralization and retained superior mechanical properties, even after 15 d of immersion in simulated body fluid. The BP-GelMA/U-Arg-PEA hydrogel stimulated the enhanced mineralization of human dental pulp SCs (hDPSCs). Western blot analysis and the enzyme-linked immunosorbent assay and reverse transcription quantitative polymerase chain reaction (rt-qPCR) confirmed elevated levels of osteogenic markers, such as Col-1, bone morphogenetic protein 4 (BMP4), and RUNX2, within the hDPSCs treated with BPN-containing hydrogels. This suggests that the presence of Ca-free P within these hydrogels was crucial in promoting osteogenic differentiation via the BMP–RUNX2 pathway. In vivo evaluation of rabbit calvarial defects demonstrated enhanced bone regeneration, leading to the formation of mature bone within 12 weeks (wk), as indicated by histological analysis, vascular formation, and the expression of osteogenic markers. Meanwhile, Miao et al. fabricated a BP/GelMA-CaP hydrogel via 3D printing and assessed its in vitro and in vivo osteogenic properties.87 CaP reinforces crosslinked networks and enhances multiple bioactivities of hydrogels.88,89 The BP/GelMA-CaP hydrogel displayed significant photothermal properties under NIR irradiation, leading to a substantial increase in temperature that diminished the activities and viability of Saos-2 osteosarcoma cells and Staphylococcus aureus. In vitro assays using hMSCs revealed significantly upregulated expression of osteogenesis-related genes, including OCN and OPN, and increased ALP activity. Immunofluorescence assays revealed increased expression of the RUNX2, ALP, OCN, and OPN proteins within the BP/GelMA-CaP hydrogel, and increased Ca deposition was observed using ARS staining. In vivo compatibility and therapeutic performance were assessed using a rat cranial defect model, which indicated excellent biocompatibility for subcutaneous implantation and significantly enhanced new bone formation, as revealed by micro-CT, histomorphometric analysis, and hematoxylin and eosin (H&E) staining.
Si, as the second most abundant element on Earth, is highly regarded for its biocompatibility. It is a key component in various nanomedicines and biomaterials due to its roles in collagen and elastin syntheses and its presence in essential bodily components, such as bone and hair.90 Silicene exhibits exceptional characteristics, such as a large surface area and high biocompatibility, photothermal conversion in the NIR biowindow, and biodegradability, rendering it an attractive candidate for use in biomedical engineering.91,92 In this context, Ni et al. engineered Ca3(PO4)2 cement (CPC) containing SiO2-silicene@2,2′-azobis(2-(2-imidazolin-2-yl) propane) (SNSs@AIPH/CPC), with inherent thermodynamic properties and osteoinductive activity.93In vitro studies using HUVECs revealed that SNSs@AIPH/CPC treatment with NIR-II irradiation led to increased cell proliferation, migration, and tube formation and the upregulation of vascular endothelial growth factor (VEGF). In vivo studies revealed denser vascular networks and enhanced VEGF expression around the bone defect sites after NIR-II irradiation. Additionally, SNSs@AIPH/CPC significantly enhanced the early-stage osteogenic differentiation and late-stage mineralization of BMSCs. High-throughput RNA sequencing and pathway analysis revealed that SNSs@AIPH upregulated genes associated with the tumor growth factor β and BMP signaling pathways, which are critical in osteogenesis. In vivo studies using a rat cranial defect model indicated that SNSs@AIPH/CPC-NIR-II exhibited outstanding osteoinductive properties, significantly improving the bone mineral density and volume and Tb.N compared to the other groups. Polychrome fluorescent labeling confirmed the strong osteogenic capacity, particularly with NIR-II activation, and immunohistochemical staining supported the enhancement of new bone formation. Lin et al. introduced a hydrogenated-Si NSs (H-Si NSs)-functionalized β-tricalcium phosphate (H-Si TCP) scaffold for use in healing bone defects.94 Due to their capacity to scavenge excess ROS, the H-Si TCP scaffolds promoted M2 macrophage polarization, with up- and downregulation of the M2 and M1 markers, respectively. The H-Si TCP scaffolds upregulated the expression of key osteogenic markers, including BMP2, ALP, secreted phosphoprotein 1, and SP7, in the early and late stages of osteogenesis and promoted ALP activity and Ca deposition. This may be attributed to the sustained release of ionic Si, which activates osteogenic signaling pathways and supports biomineralization. In vivo studies revealed that the H-Si TCP scaffolds induced well-coordinated immune responses with reduced fibrous encapsulation, monocyte infiltration, and pathological fibrosis aided by foreign-body giant cells. In vivo, H-Si TCP significantly accelerated osteogenesis by promoting osteoblastic differentiation and activation, as indicated by the increased osteogenic activity and histological evidence of more activated osteoblasts, leading to the enhancement of new bone formation. It also significantly promoted bone remodeling by providing Ca, P, and Si, leading to improved bone repair and mineralization, as indicated by micro-CT and histological analysis of the improved bone volume and density and Tb.Th. Hydroxyapatite (HAp) exhibits the advantages of excellent biocompatibility and the capacity to integrate with natural bone tissue, rendering it a preferred material for use in bone implants and coatings.95 Yuan et al. anchored 2D H-Si NSs onto HA-coated Ti substrates to develop H-Si@HAp-Ti implants.96 The study investigated the influence of the H-Si@HA coating on BMSCs and MC3T3 cells, highlighting the role of Si ion release from the H-Si NSs in enhancing cell proliferation, osteogenic differentiation, and the expression of osteogenic markers. The results suggest that Si ions are critical in promoting osteogenic processes, including enhanced Ca deposition, activation of Wnt signaling, and ROS scavenging. The study investigated the in vivo osteogenesis and bone fracture-healing properties of H-Si@HA@Ti in a rat tibial fracture model. H-Si@HA@Ti significantly enhanced fracture healing, bone formation and mineral density, and the maximum bending load compared to the other groups. Additionally, the composite coating was biosafe and inhibited osteoclast differentiation and activity with a low attrition rate during implantation, indicating strong binding to the metal substrate. Gene set enrichment analysis showed enrichment in pathways related to Ca channel regulation, hormone binding, and signaling, suggesting the role of H-Si in promoting osteogenesis. Furthermore, a Venn plot identified 90 upregulated mRNAs associated exclusively with H-Si, with hub mRNAs, such as ADAD1 and AQP2, potentially influencing autophagy-related osteogenesis.
| Applications | Xene materials | Formulations | Test species | Biocompatibility and therapeutic applications | Enhanced chondrogenic markers | Outlooks | Ref. |
|---|---|---|---|---|---|---|---|
| Cartilage | G and pGO | G-BMSCs biocomposite | BMSCs | 50 μg mL−1 G and pGO did not induce cytotoxicity while 25 μg mL−1 GO induced long-term toxicity | Col2, Alcian blue, Sox9, and ACAN | Construction of cartilage tissues optimizing G concentrations and porosity | 100 |
| GO | GelMA-PEGDA-GO hydrogel | BMSCs | 0.25 mg mL−1 GO induced high cell spreading and proliferation | Alcian blue stain | Chondrogenic differentiation with abundant ECM production for cartilage regeneration | 102 | |
| Col2, GAG, SOX9, ACAN | |||||||
| ACG/GO scaffold | hADMSCs | 1 mg mL−1 GO enhanced cell viability and proliferation | ACAN, SOX9, Col2 | Bioconjugated nanocomposite bioink showcasing excellent printability, cytocompatibility, and chondroinductive capability | 104 | ||
| GO-ACM hydrogel | Rabbit BMSCs | 2 mg mL−1 GO promoted cell adhesion and proliferation | Alcian blue, Safranin O, Col2, GAG, ACAN, SOX9 | 3D acellular cartilage scaffold promising treatment of articular cartilage injuries | 106 | ||
| SD rats | Suppressed macrophage activation in vivo | Col1, Col2, toluidine blue, and Safranin O in vivo | |||||
| New Zealand white rabbits | |||||||
| Ti3C2Tx MXene | CFOM/PLLA scaffold | Mouse BMSCs | NIR-irradiated localized hyperthermia of MXene exhibited antibacterial effects on P. Aeruginosa and S. Aureus | Alcian blue stain | NIR-triggered antimicrobial properties with photothermal and photodynamic effects for clinical treatment of tracheal injuries | 107 | |
| MXene did not hinder cell viability and proliferation | ACAN, Col2, SOX9 |
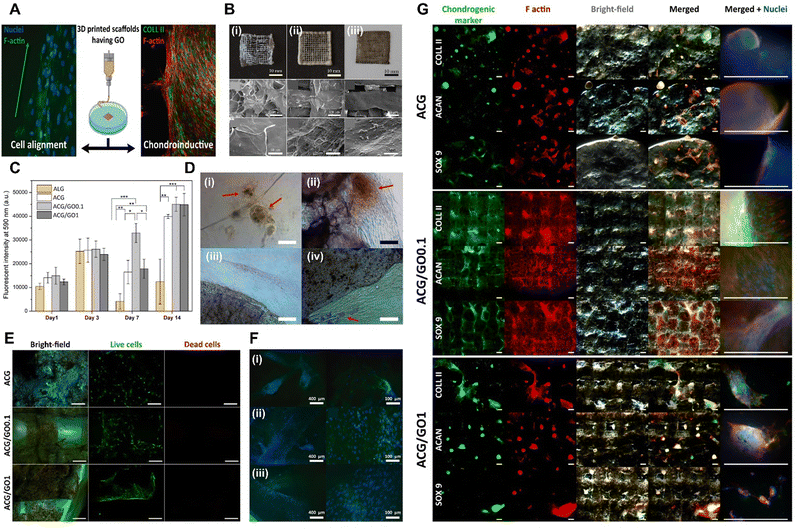 | ||
| Fig. 3 Xene-based tissue engineering scaffolds for use in cartilage regeneration. (A) Schematic of the chondroinductive ACG/GO scaffold. (B) Digital and SEM images of the lyophilized 3D printed (i) ACG, (ii) ACG/GO0.1, and (iii) ACG/GO1 inks. (C) Proliferation of the hADMSCs within 14 d and (D) optical microscopy images at 7 d on (i) Alg, (ii) ACG, (iii) ACG/GO0.1, and (iv) ACG/GO1. (E) Live/dead assay of the hADMSCs seeded on each scaffold at 7 d. (F) Fluorescence microscopy images of the hADMSCs at 7 d seeded on the (i) ACG, (ii) ACG/GO0.1, and (iii) ACG/GO1 scaffolds (green: F-actin and blue: nucleus). (G) Fluorescence microscopy images of the chondrogenic markers (green), including Col-2, ACAN, and SOX9, within the 3D printed scaffolds: ACG (top), ACG/GO0.1 (middle), and ACG/GO1 (bottom), after 28 d of culture (red: F-actin and blue: nucleus). Data reproduced from ref. 104. Copyright American Chemical Society 2020. The scale bars represent 10 mm, 300 μm and 50 μm for (B) upper, middle, and lower layers, respectively; and (D) 100 μm; (E) 200 μm; (F) 400 μm (left) and 100 μm (right); and (G) 500 μm. The asterisks denote statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns: not significant). | ||
To date, despite the significant potential of Xene materials in the field of cartilage tissue engineering, only a single relevant study has been presented. Qian et al. developed an NIR-triggered antimicrobial CuFe2O4-MXene heterojunction via the formation of CuFe2O4 on a Ti3C2 MXene, which was then uniformly incorporated into poly(L-lactic acid) (PLLA) and utilized in generating a tracheal scaffold (CFOM/PLLA).107 The NIR-irradiated CFOM/PLLA tracheal scaffolds displayed powerful antibacterial performances, with respective antibacterial rates of 96.49% and 95.33% for S. aureus and Pseudomonas aeruginosa. Additionally, these scaffolds exhibited excellent antibiofilm activities and levels of disruption of bacterial membranes, consumption of glutathione, and generation of ROS, leading to bacterial death. Moreover, the CFOM/PLLA scaffolds released low concentrations of Cu and Fe ions to promote chondrogenic differentiation in BMSCs, as indicated by the increased levels of GAG deposition and the upregulated expression of chondrogenic genes, including ACAN, Col-2, and SOX9. Thus, the fabricated CFOM/PLLA tracheal scaffolds display potential for use in clinical applications because of their excellent antibacterial and regenerative properties.
Neuromuscular tissue engineering using G, MXenes, and Xenes
| Applications | Xene materials | Formulations | Test species | Biocompatibility and therapeutic applications | Enhanced neurogenic markers | Outlooks | Ref. |
|---|---|---|---|---|---|---|---|
| Neural tissues | rGO | PCL/Gel-rGO nanofiber | – Rat ENPCs | – 10 w/v% rGO enhanced cell viability | – MAP2, vimentin | Customized GO to modulate the properties of electrospun nanofibers for neural tissue engineering | 110 |
| Ap/PLCL-rGO nanofiber | – ES, SCs, and PC12 cells | – 2 mg mL−1 rGO-incorporated matrices with ES enhanced cell viability and proliferation | – NGF, PMP22, and Krox20 | Promising peripheral nerve repair and regeneration by promoting neural cell behaviors in vitro and facilitating nerve repair in vivo | 114 | ||
| – SD rats | – Neurite length and neurogenic differentiation | ||||||
| – In vivo nerve regeneration with enhanced nerve conduction velocity | |||||||
| – Schwann cell population, myelination, and axon diameter | |||||||
| – S100, NF200, and GFAP | |||||||
| adECM-PLA/PCL-rGO nanofiber | – NE-4C cells | – 1.5 wt% rGO did not hinder cell viability and proliferation | – Tuj1, GFAP, and MAP2 | The adECM functionalized with PDA-rGO guiding neural stem cell adhesion, migration, and spontaneous neuronal differentiation | 115 | ||
| – NSCs | |||||||
| Ti3C2Tx MXene | MXene-PCL NGCs | – RSCs | – 40 mg mL−1 MXene coating did not hinder cell viability and proliferation | – Functional and histological regeneration of sciatic nerve | Promoting nerve regeneration, angiogenesis, and the transmission of physiological neural electric signals for peripheral nerve injury repair | 117 | |
| – SD rats | – No main organ damage | – Gastrocnemius muscle regeneration | |||||
| – Myelinated axon | |||||||
| – S100, MBP | |||||||
| – Tuj1 and NF200 | |||||||
| – CD34 and CD31 | |||||||
| MXene-matrigel hydrogel | – SGNs | – 300 μg mL−1 MXene did not hinder cell growth and proliferation | – Neurite length with growth cone and filopodia expression | Promoting neural network formation when combined with a low-frequency cochlear implant, for patients with sensorineural hearing loss through cochlear implantation | 118 | ||
| – Wild-type mice | – EAS stimulated neural differentiation | – Synapsin-1, PSD95 | |||||
| – Calcium oscillations | |||||||
| – NGS on neurogenic markers | |||||||
| MXene-PLLA nanofiber | – Mouse NSCs | – 250 μg mL−1 MXene NPs were cytocompatible | – Neurite guidance with enhanced branch number, length, DCI, and intersection | Electrical conductivity, surface functionality, and biocompatibility, promoting NSC neurogenesis, and neurite outgrowth, making it a promising substrate for NSC engineering | 119 | ||
| – Anti-inflammatory reaction with less foreign body responses | – Nestin, Ki67, GFAP, and Tuj1 | ||||||
| – Synaptophysin and synaptic puncta | |||||||
| – PI3K-Akt and Ca-signal pathway | |||||||
| Lam-MXene film | – Mouse NSCs | – MXene-coating did not hinder cell viability, proliferation, and membrane integrity | – Nestin and Tuj1 | Interface for regulating NSCs with electrically conductive interface for biosystems and future clinical applications | 120 | ||
| – Neuronal differentiation, neurite length, branches, and dendrite protrusion | |||||||
| – Synapsin-1, PSD95 | |||||||
| BP | PLCL/Lam/BP nanofiber | – HT22 cell | – Cell viability was decreased at ≥ 62.5 μg mL−1 | – Neurofilament heavy polypeptide | Neuritogenesis in hippocampal neurons for potential neural tissue engineering and regeneration | 122 | |
| – 0.4 g BP-laden matrices enhanced cell proliferation | – Neurite length and guidance | ||||||
| – DCX, NeuN, MAP2, and Nestin | |||||||
| GelMA-BP@PDA nanofiber | – Rat BMSCs | – 0.3 mg mL−1 BP@PDA with ES did not induce cytotoxicity | – Nestin, Tuj1, GFAP, and MAP2 | Electrical conductivity and neural differentiation of MSCs offering potential applications in tissue engineering for electroactive tissues | 123 | ||
| – SD rats | – Cell spreading and proliferation were enhanced | ||||||
| – ES supported neurogenic differentiation | |||||||
| E@BP hydrogel | – Rat primary neuron | – 100 μg mL−1 BP alleviated inflammatory effects, reduced apoptosis, and promoted proliferation | – BBB score | Spinal cord injury repair by reducing inflammation and promoting neuronal regeneration through the activation of the AKT signaling pathway | 124 | ||
| – SCI rats | – 100 μg mL−1 BP showed no toxicity on blood and main organs | – CSPG, NeuN, MAP2, and GFAP | |||||
| – Spinal cord tissue regeneration | |||||||
| – PDK1/AKT/GSK3 and PDK1/AKT/14-3-3/BAD pathways |
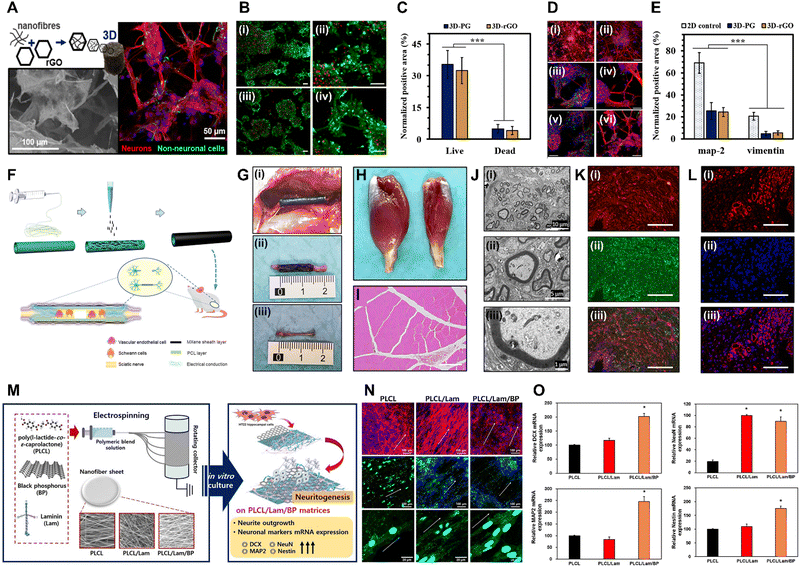 | ||
| Fig. 4 Xene-based tissue engineering scaffolds for use in neural tissue regeneration. (A)–(E) PCL/Gel-rGO nanofiber scaffolds. (A) Schematic and representative fluorescence images of neurons differentiated on the PCL/Gel-rGO nanofiber scaffolds. (B) Live/dead assay and (C) quantified results of the ENMCs cultured on (i) PCL/Gel and (ii) PCL/Gel-rGO. (D) Immunofluorescence staining and (E) quantified results of the ENMCs cultured on (i) PCL/Gel and (ii) PCL/Gel-rGO (red: MAP2, green: vimentin, and blue: cell nuclei). Data reproduced from ref. 110. Copyright American Chemical Society 2020. (F)–(L) MXene-PCL NGCs. (F) Schematic of the fabrication of MXene-PCL NGCs for use in neural regeneration. (G) Morphologies of the MXene-PCL NGCs and regenerated nerve. (i) Digital image captured at 12 wk after implantation. (ii) MXene-PCL NCGs and (iii) regenerated nerves extracted from SD rats. Representative (H) optical and (I) H&E staining images of the gastrocnemius muscle at 12 wk post-operation. (J) TEM images of the cross-sections of the nerves regenerated using the MXene-PCL conduit: (i) Low-, (ii) medium-, and (iii) high-magnification images. (K) Immunofluorescence staining of S100 (red) and MBP (green) of the MXene-PCL group. (L) Immunofluorescence staining of CD34 (red) and 4′,6-diamidino-2-phenylindole (blue) of the MXene-PCL group. Data reproduced from ref. 117. Copyright Frontiers 2022. (M)–(O) PLCL/Lam/BP nanofiber matrices for use in neural tissue engineering. (M) Schematic of the electrospinning of the PLCL/Lam/BP matrices and the enhanced neuritogenesis of the HT22 cells. (N) Immunofluorescence staining of the HT22 cells cultured within each matrix (red: F-actin, blue: nucleus, and green: neurofilament). (O) Relative levels of mRNA expression of DCX, NeuN, MAP2, and nestin. Data reproduced from ref. 122. Copyright Elsevier 2020. The scale bars represent (I) and (K) 200 μm, (A-left, N and L) 100 μm, (A-right, D, B and C) 50 μm, (J-i) 10 μm, (J-ii) 5 μm, and (J-iii) 1 μm, and the asterisks denote statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns: not significant). | ||
Owing to their high electrical conductivity derived from their 2D structures and elemental nature, MXenes have also gained considerable attention in the field of neural tissue engineering.116 Nan et al. developed Ti3C2Tx MXene-coated electrospun PCL NGCs to enhance neurite regeneration and angiogenesis (Fig. 4F–L).117 The MXene-PCL NGCs exhibited excellent biocompatibility, with no significant toxicity toward Schwann cells, good levels of cell attachment, and no abnormalities in major organs when postoperatively evaluated in rats at wk 12. The NGCs remained structurally intact but displayed some surface degradation, which did not affect their biocompatibility or levels of nerve regeneration. At 12 wk post-implantation, the MXene-PCL NGCs significantly enhanced gastrocnemius muscle and muscle mass restoration and increased the muscle fiber diameters. Functional recovery of the sciatic nerve, as assessed using the sciatic functional index and electrophysiological analysis, was excellent when using the MXene-PCL NGCs, and immunohistological and -fluorescence analyses revealed that these NGCs contributed to nerve fiber regeneration and myelination. Additionally, the MXene-PCL group displayed increased neovascularization, as indicated by the higher microvessel density compared to that of the PCL group, with comparable results to those of the autograft group. These findings indicate the potential of the MXene-PCL NGCs for use in promoting nerve regeneration and functional recovery. Meanwhile, Liao et al. introduced an ES setup prepared by integrating a cochlear implant with a conductive Ti3C2Tx MXene-matrigel hydrogel, which was utilized to culture spiral ganglion neurons (SGNs) and expose them to ES delivered via the cochlear implant.118 At concentrations of 40–100 μg mL−1, the MXene-matrigel hydrogel promoted neurite outgrowth and induced significant increases in the growth cone areas and filopodia numbers of the SGNs. Additionally, the cochlear implant-MXene-matrigel hydrogel-electroacoustic stimulation (EAS) system enhanced the neurite development of the SGNs, resulting in longer neurites, larger growth cone areas, and increased filopodia numbers, without inducing neurotoxicity. The system also promoted the formation of neural networks among the SGNs without affecting the expression or co-localization of synapsin 1 and postsynaptic density protein 95, indicating mature potential synapses. This system also increases the number of SGN synapses and enhances the Ca oscillations within the SGNs, potentially accelerating signal transmission and promoting neural network formation. RNA sequencing analysis revealed differential gene expression between the control and EAS groups, with genes related to ion transmembrane transport, synaptic transmission and plasticity, and cell adhesion significantly affected, suggesting that the system regulates various aspects of SGN behavior and function. Zhu et al. introduced Ti3C2Tx MXene-coated PLLA nanofiber (MXene-PLLA) matrices for use in NSC applications.119 The prepared MXene-PLLA matrices increased the mRNA expression levels of Tuj1 and GFAP in NSCs with the maturation of the NSC-derived neurons and astrocytes (i.e., neurite branching, extended branching length, and increased dendritic complexity indices and synaptic densities). Furthermore, Kyoto Encyclopedia of Genes and Genomes analysis highlighted involvement in neuroactive ligand–receptor interactions and signaling pathways related to NSC self-renewal and Ca modulation. In vivo analysis indicated that the MXene-PLLA matrices exhibited excellent in vivo biocompatibility as they reduced foreign body responses, inflammation, and fibrotic scar formation, while enhancing matrix stability and slowing degradation, rendering them promising for use in nerve tissue engineering. Guo et al. investigated the neurogenic effects of a laminin-coated Ti3C2Tx MXene (Lam-MXene) film using HT22 hippocampal neuronal cells.120 The Lam-MXene film significantly enhanced the differentiation of NSCs to neurons, as indicated by an increased neuron content, extended neurite length, higher numbers of branch points and tips, and an improved expression of neurogenic markers (PSD95 and nestin). However, no discernible effect on synaptic development in the NSC-derived neurons was observed. In Ca imaging studies, the NSCs cultured on the Lam-MXene film exhibited spontaneous Ca spikes with high frequencies and synchronization. Moreover, ES supported the Lam-MXene film by enhancing NSC adhesion, distribution, terminal extension, and metabolism.
Meanwhile, BP aids the intercellular interactions and recovery of peripheral nerves due to its high electrical conductivity.121 Kang et al. developed Lam- and BP-coated PLCL (PLCL/Lam/BP) nanofiber matrices to enhance the neuritogenesis of HT22 hippocampal cells (Fig. 4M–O).122 Due to the hydrophilic nature of BP and Lam, the fabricated PLCL/Lam/BP matrices displayed enhanced hydrophilicity and decreased nanofiber diameters, which are favorable for neural cell growth. The levels of cell proliferation on 7 d were significantly increased in the PLCL/Lam/BP matrices, whereas no difference in initial cell adhesion was observed. HT22 cells cultured within the PLCL/Lam/BP matrices exhibited increased neurite length, with clear expression of neurofilament heavy chains. Based on mRNA analysis, doublecortin X (DCX), NeuN, MAP2, and nestin were significantly upregulated within the PLCL/Lam/BP matrices, indicating the neurogenic potential of the BP-incorporated nanofiber matrix. Xu et al. introduced a PDA-modified and BP-laden GelMA (GelMA-BP@PDA) hydrogel to promote the neural differentiation of rat BMSCs.123 ES at 100 mV cm−1 was applied to mesenchymal SCs seeded on the GelMA-BP@PDA hydrogels, resulting in increased expression of the early neural markers nestin, Tuj1, MAP2, and GFAP, suggesting that the rat BMSCs were differentiated into neurogenic lineage. Four wks after subcutaneous implantation of the GelMA-BP@PDA hydrogel into SD rats, accelerated in vivo degradation was observed owing to enhanced cell infiltration and the presence of biological enzymes. Additionally, histological staining confirmed the excellent cytocompatibility of the hydrogel, with cells infiltrating and surrounding it over time, suggesting that the prepared GelMA-BP@PDA hydrogel combined with ES was favorable for use in in vitro neural tissue engineering and in vivo applications. Xie et al. fabricated BP quantum dot containing epigallocatechin-3-gallate (E@BP) hydrogels that target the protein kinase B (Akt) signaling pathway in spinal cord injury repair.124 E@BP significantly improved motor function recovery in SCI rats, as indicated by their higher Basso–Beattie–Bresnahan scores and improved performances in inclined plate studies. Furthermore, E@BP treatment reduced apoptosis and promoted neural regeneration in injured neurons, as indicated by the decrease in the number of caspase-3-positive and propidium iodide/annexin double-positive neurons and the improved mitochondrial morphology. Additionally, E@BP facilitation of neural regeneration was indicated by the increase in the number of 5-ethynyl-2′-deoxyuridine-positive neurons and cell cycle progression. E@BP repaired the spinal cord tissue by reducing inflammation and promoting neuronal survival. Finally, the study investigated the molecular mechanisms underlying the effects of E@BP, revealing that it regulates the phosphoinositide-dependent kinase-1 (PDK1)/Akt/glycogen synthase kinase 3 and PDK1/Akt/14-3-3/Bcl-2-associated death promoter pathways, promotes cell cycle progression, and reduces apoptosis in SCI rats.
| Applications | Xene materials | Formulations | Test species | Biocompatibility and therapeutic applications | Enhanced myogenic markers | Outlooks | Ref. |
|---|---|---|---|---|---|---|---|
| Skeletal muscle | GO | PU-nGO nanofiber | – C2C12 cells | – 8 w/w% GO promoted cell adhesion, spreading, and proliferation | – MHC, α-actinin, MyoG, and MyoD | Flexibility, mechanical properties, and capacity for stimulating myogenic differentiation for skeletal muscle engineering | 130 |
| – Dynamic tensional stimuli facilitated myogenesis | |||||||
| GO/GHPA hydrogel | – C2C12 cells | – 10 μg mL−1 GO promoted cell growth and proliferation | – MHC and aligned myotube formation | 3D printable GO-laden bioink for customizable skeletal muscle-mimetic constructs | 132 | ||
| – MyoD and MyoG | |||||||
| Cardiac muscle | rGO | rGO-Col scaffold | – HUVECs | – 90 μg mL−1 rGO enhanced viability, spreading, and proliferation | – In vivo host cell migration | Enhanced mechanical properties and ability to upregulate cardiac gene expression | 133 |
| – Rat CMs | – In vitro and in vivo angiogenesis | – Cx43, Actn4, and TrpT-2 | |||||
| – Cardiac muscle regeneration | |||||||
| GO | GO-PEI scaffold | – HL-1 cells | – GO coating maintained good viability and adhesion | – Cx43, Nkx 2.5, and intercellular interaction | Biocompatibility and cardiac cell growth with electrical signal conduction for cardiac tissue engineering and in vitro cardiac tissue models | 134 | |
| Skeletal muscle | Ti3C2Tx MXene | – MXene/AuNP/GelMA hydrogel | – C2C12 cells | – 0.05 mg mL−1 MXene induced high cell viability and proliferation | – MHC | AuNPs and MXene improved printability, conductivity, and cellular differentiation, showing potential for muscle tissue engineering applications | 136 |
| – Myotube maturation indexes | |||||||
| – MXene-F127 hydrogel | – Raw264.7 cells | – 80 μg mL−1 MXene induced high cell viability | – MHC | Antioxidation, anti-inflammation, and angiogenesis properties for skeletal muscle repair by regulating cell niche | 140 | ||
| – C2C12 cells | – Antioxidant and anti-inflammatory effects | – Myotube maturation indexes | |||||
| – HUVECs | – Angiogenic effects | – MyoD, MyoG, and MHC | |||||
| – SD rats | – In vivo regeneration of myofibers and capillary | ||||||
| Cardiac muscle | Ti3C2Tx MXene | – MXene-PEG hydrogel | – iCMs | – High viability and proliferation on MXene constructs | – MYH7, TNNT2, SERCA2, and GJA1 | Clinically relevant cardiac patches for myocardial infarction treatment, addressing the need for ordered structure and electroconductivity in cardiac patches | 141 |
| – Ca2+ transient kinetics | |||||||
| – Spontaneous beating | |||||||
| – MXene-Col film | – C3H10 cells | – 60 w/w% MXene induced cell metabolic activity and proliferation | – CMs count and spreading | Conductive biohybrid platform for cardiac tissue engineering, showing improved electrical conductivity and cell growth | 142 | ||
| – Rat CMs | – ES stimulated maturation and elongation of iCMs | – cTnT, sarcomeric α-actinin, and Cx43 | |||||
| – iCMs | – Antibacterial properties on S. aureus | – action potential |
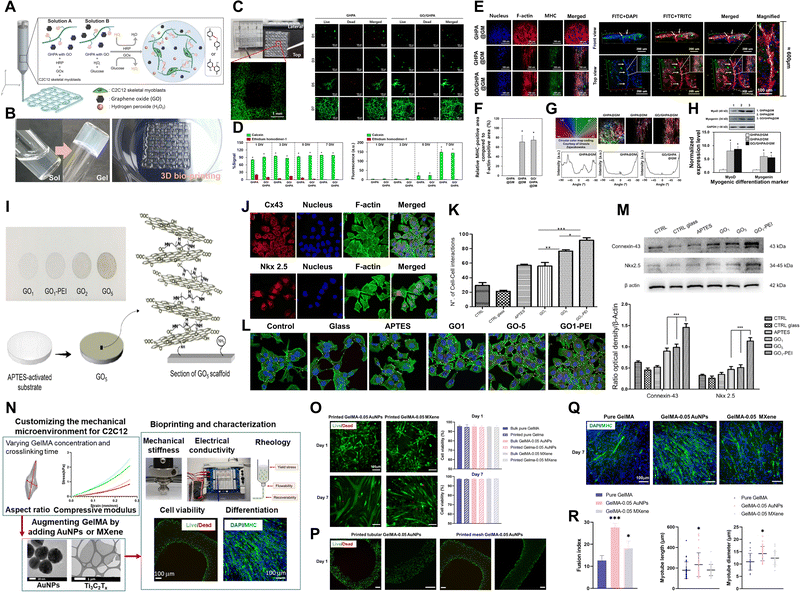 | ||
| Fig. 5 Xene-based tissue engineering approaches for use in muscle tissue regeneration. (A)–(H) GO@GHPA printable bioink for use in skeletal muscle tissue regeneration. (A) Schematic of the in situ crosslinkable GO@GHPA hydrogel. (B) Digital images of in situ crosslinking and the 3D printed construct. (C) Live/dead assay within 7 d and (D) quantification. (E) Immunofluorescence staining of the C2C12 myoblasts cultured within the GO@GHPA bioinks (blue: nucleus, red: F-actin, and green: MHC). (F) Relative expression of the MHC-positive area. (G) Cell alignment quantification. (H) Relative levels of mRNA expression of the myogenic differentiation markers MyoD and MyoG. Data reproduced from ref. 132. Copyright American Chemical Society 2021. (I)–(M) GO-PEI scaffolds for use in cardiac muscle engineering. (I) Schematic of GO-PEI scaffold fabrication. (J) Immunofluorescence staining images of Cx43 (upper panel) and the expression of Nkx 2.5 (lower panel) in HL-1 cells (blue: nucleus and green: F-actin), with (K) quantification. (L) Immunofluorescence staining of Cx43 (green) in the different groups (blue: nucleus). (M) Levels of protein expression of Cx43 and Nkx 2.5 in the different groups. Data reproduced from ref. 134. Copyright American Chemical Society 2023. (N)–(R) MXene/AuNP/GelMA hydrogel for use in skeletal muscle regeneration. (N) Schematic of MXene/AuNP/GelMA hydrogel formation and characterization of the bioprinted constructs. (O) Live/dead assay and quantification of the C2C12 myoblasts cultured within the printed constructs. (P) Low-magnification results of the live/dead assays of the constructs, which were printed differently. (Q) Immunofluorescence staining of the C2C12 myoblasts cultured within the printed constructs (blue: nucleus and green: MHC). (R) Myotube maturation parameters, including fusion index and myotube length and diameter. Data reproduced from ref. 136. Copyright American Chemical Society 2021. The scale bars represent (C-left) 1 mm and (E-right) 200 μm and (C-right, E-left, N, O and Q) 100 μm, and the asterisks denote statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns: not significant). | ||
Skin tissue engineering using G, MXenes, and Xenes
Skin tissue engineering involves preparing artificial substitutes that replicate human skin, primarily driven by the crucial role of the skin as a protective barrier against various environmental threats.143 This field employs advanced tissue engineering techniques to develop skin substitutes for use as sophisticated models in research and in applications such as wound healing. A key challenge is the ex vivo expansion of cells, while preserving their normal properties, for use in transplantation or in vitro studies.144 Owing to its remarkable characteristics, such as its substantial surface area-to-volume ratio, mechanical durability, antimicrobial properties, and capacity to facilitate collagen crosslinking, G is used in the field of skin tissue engineering (Table 5).145,146| Applications | Xene materials | Formulations | Test species | Biocompatibility and therapeutic applications | Enhanced wound regeneration markers | Outlooks | Ref. |
|---|---|---|---|---|---|---|---|
| Skin | GO | ADM-GO-PEG/Que scaffold | – MSCs | – 50 μM GO-PEG/Que supported high cell viability, proliferation, and spreading with reduced apoptosis | – Accelerated regeneration on diabetic wounds | Improved diabetic wound healing for potential drug delivery, stem cell therapies, and tissue engineering applications | 147 |
| – Diabetic ICR mice | – Reduced in vivo inflammation | – Re-epithelialization | |||||
| – Dermal regeneration | |||||||
| – Collagen deposition and neovascularization | |||||||
| – Skin appendage regeneration | |||||||
| – Col1, Col3, and α-SMA | |||||||
| rGO | UC-GSC scaffold | – MEF cells | – Antibacterial activity against S. aureus and Pseudomonas | – In vivo wound closure | Efficient antibacterial properties and positive impact on cutaneous wound healing | 151 | |
| – Rat | – Support proliferation | – Re-epithelialization | |||||
| – Dermal regeneration | |||||||
| – Granulation tissue and collagen fiber formation | |||||||
| – Neovascularization | |||||||
| Ti3C2Tx MXene | T-RMF-vitamin E nanofiber | – BMSCs | – T-RMF nanofiber matrices supported cell adhesion, spreading, and proliferation | – CD31, VEGF, and PCNA | Drug delivery and temperature-responsive wound-healing capability for future clinical applications | 154 | |
| – Kunming mice | – No toxicity on main organs | – In vivo wound closure | |||||
| – NIR-irradiated wound healing | – Re-epithelialization | ||||||
| – Dermal regeneration | |||||||
| BP | HA-DA@BP hydrogel | – GES-1 cells | – ES-supported cell behaviors and antibacterial properties | – In vivo wound closure | Reversible phase transformation under electrical stimulus offering promising applications in skin tissue engineering with adjustable mechanical properties, controlled drug release, and electrical antibacterial activity | 155 | |
| – Kunming mice | – Enhanced cell viability and wound healing | – Re-epithelialization | |||||
| – No hemolytic activity | – Dermal regeneration | ||||||
| – No erythema or edema after transplantation | |||||||
| – In vitro antibacterial property against E. coli | |||||||
| – Prevention of in vivo bacterial inflammation | |||||||
| Borophene | B-FENG nanogenerator | – L929 cells | – Enhanced cell spreading, proliferation, and in vitro wound healing | – In vivo wound closure | B-TENG with potential applications in mechanical energy harvesting, medical assistive systems, gait phase sensing, and wound monitoring and therapy, showing promise for wearable healthcare technology | 157 | |
| – SD rats | – ES support wound healing | – Re-epithelialization | |||||
| – Dermal regeneration | |||||||
| Stanene | Sn@PEI hydrogel | – 3T3 and L02 cells | – 150 μg mL−1 Sn@PEI hydrogel maintained high cell viability | – In vivo wound closure | Stanene treatment for drug-resistant bacterial infections and wound healing | 158 | |
| – HUVECs | – US induced antibacterial activity against S. aureus and MRSA | – Dermal regeneration | |||||
| – BALB/c mice | – Hemocompatibility | – Granulation tissue with collagen deposition | |||||
| – In vivo activation of inflammatory cells | – In vivo cell migration | ||||||
| – ES support wound healing | |||||||
| Germanene | CS/GeNCs0.8 hydrogel | – HeLa and NIH3T3 cells | – No toxicity up to 2 mg mL−1 | – In vivo wound closure | Germanene nanocrystals for photothermal antibacterial properties and wound healing capability | 161 | |
| – Kunming mice | – NIR-irradiated antibacterial activity against E. coli and S. aureus | – Re-epithelialization | |||||
| – Hemocompatibility | – Dermal regeneration | ||||||
| – No toxicity on main organs | |||||||
| In vivo suppression of bacterial colony |
Chu et al. fabricated a PEGylated, GO-mediated, quercetin (Que)-modified acellular dermal matrix (ADM; ADM-GO-PEG/Que) hybrid scaffold for use in skin tissue engineering and diabetic wound healing.147 The GO-PEG composite displays the capacity to efficiently deliver and release various substances, including proteins, gene therapeutics, imaging agents, chemotherapy drugs, and anti-cancer medications, while enabling precise control over release.148–150 The use of the ADM-GO-PEG/Que hybrid scaffold resulted in accelerated wound healing, with a faster rate of wound closure compared to those of other groups, reaching 87.34% closure by 21 d. Histological analysis revealed Col deposition, neovascularization, and the regeneration of skin appendages, including mature vessels and hair follicles, in the ADM-GO-PEG/Que scaffold-treated group on 14 d. The ADM-GO-PEG/Que scaffold enhanced Col synthesis and capillary formation and supported the formation of granulation tissue, rendering it effective in promoting the healing of diabetic wounds. In a study conducted by Esmaeili et al., nanofibrous scaffolds, denoted UC-GSC scaffolds, which were prepared using PU and cellulose acetate and contained rGO/Ag nanocomposites, were used to exploit the strong antibacterial properties of rGO/Ag.151 The prepared UC-GSC scaffolds displayed similar rates of H2O vapor transmission compared to those observed in other studies and biocompatibility with cells, indicating their viability for use in tissue engineering. Additionally, the scaffolds exhibited significant antibacterial activities against Gram-negative and -positive bacteria. The histological findings revealed that the UC-GSC scaffold exhibited significant improvements in wound healing, including epidermal hyperplasia, granulation tissue formation, collagen fiber deposition, and neovascularization, indicating its potential for use in promoting wound healing.
MXenes may undergo degradation via their interactions with H2O and O2, indicating that their distinctive photothermal and biodegradable attributes may be exploited practically in wound healing.152,153 Jin et al. developed temperature-responsive MXene nanobelt fibers (T-RMFs) loaded with vitamin E (T-RMF-vitamin E) for use in wound healing (Fig. 6).154 These T-RMFs exhibited photothermal properties that could be controlled using NIR irradiation, which facilitated the release of vitamin E in wound healing. The experimental results indicated that the photothermal properties of the T-RMFs were controlled under NIR irradiation, thereby ensuring safe increases in temperature. In vitro and in vivo studies confirmed the excellent biocompatibility of the T-RMFs, which promoted cell attachment and proliferation. Furthermore, the T-RMFs, which could release vitamin E under NIR exposure, significantly upregulated cluster of differentiation 31 (CD31), VEGF, and proliferating cell nuclear antigen (PCNA), which are markers of stimulated wound healing and angiogenesis. Notably, these therapeutic effects were realized with no adverse effects on the main organs, highlighting the potential of T-RMFs for use in safe, effective wound healing. BP may be utilized in skin tissue engineering by incorporating it into conductive hydrogels. Liu et al. prepared a conductive hydrogel, denoted HA-DA@BP, using hyaluronic acid (HA) and dopamine (DA), which was designed to produce antibacterial effects on wounds with the aid of ES.155 The experimental results confirmed that the BP nanosheets within the hydrogel retained their original structures during degradation, and BP incorporation significantly enhanced the conductivity of the hydrogel, enabling effective electrical antibacterial activity against Escherichia coli when exposed to ES. Further in vitro studies were conducted using normal human gastric epithelial cells (GES-1), which revealed an excellent biocompatibility and the promotion of cell proliferation and migration under ES. In a mouse wound model, the HA-DA@BP hydrogel combined with ES significantly accelerated wound healing, reduced bacterial counts, and mitigated inflammation, indicating its potential for in vivo application as a wound dressing, with disinfection and tissue regeneration capacities.
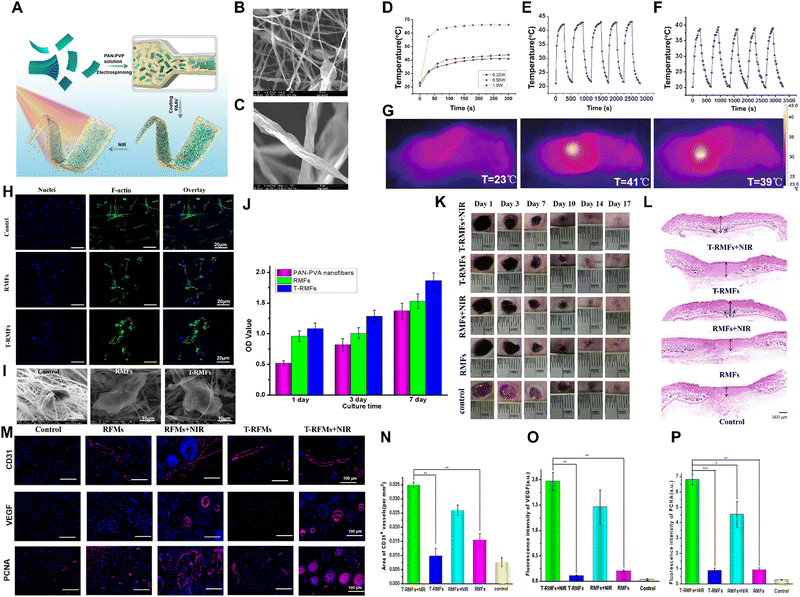 | ||
Fig. 6 Xene-based tissue engineering approaches for use in skin tissue regeneration. (A) Schematic of the fabrication of the T-RMF-vitamin E nanofiber matrices. (B) Low- and (C) high-magnification SEM images of the RMFs. (D) Heating profiles of the RMFs under 0.33, 0.50, and 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W NIR light. Temperature changes of the (E) RMFs and (F) T-RMFs over 5 on/off cycles. (G) Thermal images of each group. (H) Immunofluorescence staining (blue: nucleus and green: F-actin), (I) SEM images, and (J) proliferation of the BMSCs cultured on the different nanofiber groups. (K) Representative photographs and (L) H&E staining images of the skin wounds of the various groups. (M) Immunofluorescence staining of CD31, VEGF, and PCNA of the BMSCs cultured on the different groups with or without NIR irradiation. Quantitative levels of expression of (N) CD31, (O) VEGF, and (P) PCNA. Data reproduced from ref. 154. Copyright Springer Nature 2021. The scale bars represent (M) 100 μm, (A-upper, H) 20 μm, (I) 10 μm, and (A-lower) 5 μm, and the asterisks denote statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns: not significant). W NIR light. Temperature changes of the (E) RMFs and (F) T-RMFs over 5 on/off cycles. (G) Thermal images of each group. (H) Immunofluorescence staining (blue: nucleus and green: F-actin), (I) SEM images, and (J) proliferation of the BMSCs cultured on the different nanofiber groups. (K) Representative photographs and (L) H&E staining images of the skin wounds of the various groups. (M) Immunofluorescence staining of CD31, VEGF, and PCNA of the BMSCs cultured on the different groups with or without NIR irradiation. Quantitative levels of expression of (N) CD31, (O) VEGF, and (P) PCNA. Data reproduced from ref. 154. Copyright Springer Nature 2021. The scale bars represent (M) 100 μm, (A-upper, H) 20 μm, (I) 10 μm, and (A-lower) 5 μm, and the asterisks denote statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns: not significant). | ||
Meanwhile, interest in borophene has been significant owing to its intricate layered structure, pronounced anisotropy, and exceptional electron transport and mechanical characteristics, but the number of studies exploring its potential in the field of tissue engineering is limited.156 Chen et al. reported the utilization of a borophene/ecoflex nanocomposite-based triboelectric nanogenerator (B-TENG) in energy harvesting, medical devices, and wound healing.157 The flexible B-TENG functioned based on triboelectricity, producing an electrical output via charge transfer upon contact with polyester, and it maintained its stability and durability, even after extensive evaluation and mechanical strain. The B-TENG was incorporated into a smart keyboard and robotic system for use in upper-limb medical assistance for disabled users; it was also applied in a lower-limb gait phase visualization platform to detect and display various gait phases. In terms of biological assessment, in vitro studies with L929 fibroblasts showed that the B-TENG could enhance cell proliferation and migration. Animal models also demonstrated the effectiveness of the B-TENG in promoting wound healing, with reduced wound sizes and improved levels of tissue regeneration. Stanene NSs (SnSNs) are semiconductors that may exhibit thickness-dependent adjustable bandgaps which render them favorable for use in cancer photothermal therapy.63 Tao et al. introduced SnSNs-assembled thermosensitive poly(D,L-lactide)-PEG-poly(D,L-lactide) for use in preparing Sn@hydrogels to exploit their sonodynamic antibacterial activities.158 Sn@PEI exhibited excellent sonodynamic properties, generating ROS effectively under ultrasound (US) irradiation, leading to antibacterial activity. Sn@PEI displayed high antibacterial efficiencies against S. aureus and methicillin-resistant S. aureus when exposed to US, whereas no significant cytotoxicity was observed toward normal cells. In vivo analysis revealed accelerated wound healing (full regeneration on 14 d) in mice after the transplantation of the ES-supported Sn@PEI hydrogel. The hydrogel also exhibited a potent antibacterial activity, drastically reducing the levels of bacterial contamination of infected skin wounds. Germanene displays considerable potential as a photothermal agent for use in treating infected wounds due to its efficient photothermal conversion, while its biocompatibility and low toxicity render it promising for use in clinical applications.159,160 Therefore, Wang et al. developed a CS hydrogel embedded with hydroxy-functionalized germanene nanocrystals (GeNCs) via the crosslinking of CS and zinc acetate and self-assembly with the GeNCs to yield the photothermal wound-healing hydrogel CS/GeNCs0.8.161In vitro studies revealed that the CS/GeNCs0.8 hydrogels effectively adsorbed and killed Gram-positive S. aureus and Gram-negative E. coli when combined with NIR laser irradiation. SEM revealed that the NIR-irradiated CS/GeNCs hydrogels caused significant protein and nucleic acid leakage by disrupting membrane integrity. Additionally, the CS/GeNCs0.8 hydrogels exhibited excellent hemostatic properties and negligible hemolytic effects on red blood cells. In vivo studies in mice indicated that the CS/GeNCs0.8 hydrogel effectively inhibited bacterial infection and promoted wound healing, with no significant toxicity or adverse effects on body mass or the major organs, indicating its potential as a photothermal antibacterial platform for use in clinical applications.
Conclusion and future perspectives of MXene and Xene materials in tissue engineering
The achievements of G have demonstrated the feasibility of producing stable, ultra-thin layers of van der Waals materials, consisting of just one or a few atoms, using diverse techniques for fabricating single-layer, few-layer, and multi-layer assemblies in solution, on surfaces, and at a wafer scale.162,163 In recent decades, there has been great achievement led by pioneers in the synthesis, characterization, and applications of 2D nanomaterials.164 For instance, P. M. Ajayan led the way in the large-scale production and analysis of single layers of hexagonal boron nitride, while A. Kis and his research team achieved the synthesis of single-layer MoS2 for use in optoelectronics and energy harvesting applications.165,166 These advancements in 2D nanomaterials have led to increased utilization of these materials in bioengineering research, exploiting their distinctive physicochemical and biological properties.167This review extensively compares the research progress of Xene-material-based tissue engineering with that of G-based materials, which have been extensively studied over the last decade. Section 2 provides an overview of the fundamental physicochemical characteristics of G, MXenes, and group III–V Xene materials. In the subsequent section, we review recent experimental advancements in Xene-based tissue engineering and regenerative medicine for different tissue types, i.e., bone, cartilage, neural, muscle, and skin tissues. The versatility of fabrication techniques (e.g., 3D bioprinting and electrospinning) and cell sources (e.g., SCs and primary cells) enabled researchers to produce numerous scaffolds and tissue mimetics with various structures and biofunctionalities. Each study was reviewed to explore the innovative combinations of Xene materials with other biomaterials and their capacity to promote tissue maturation from a mechanistic perspective.
The goal of tissue engineering and regenerative medicine is to prepare biomimetic constructs or matrices that provide biochemical and structural support to cells, induce the expression of intrinsic phenotypes, and facilitate differentiation to mature tissues. G-based research laid the foundation for 2D NM-based tissue engineering and serves as a valuable starting point for the subsequent development of MXene and Xene materials. Despite the promising progress, several critical challenges remain unresolved. Safety and biocompatibility are primary concerns, as the biocompatibility of most Xene materials has not been fully elucidated, necessitating further studies and long-term clinical investigations under various conditions. Understanding the interactions between Xene materials and the body (i.e., biodistribution, organ accumulation, clearance pathways, inflammatory reactions, and potential genotoxicity) is crucial in exploring their future in vivo applications.168 Moreover, engineered scaffolds exhibit unique complexities compared to conventional grafts, rendering the following of regulatory guidelines and processes challenging. Additionally, the multitude of active components and fabrication parameters may unpredictably affect reactions within the human body.
To address these limitations, further interdisciplinary studies and clinical trials are required to elucidate long-term efficacy and potential side effects. Standardization of culture conditions and quality control are essential to ensure the safety, efficacy, and reproducibility of Xene-based engineered scaffolds. For future clinical applications of Xene materials, it is crucial to understand the unique properties of various elemental composites and surface reactivity with the biological system in relation to their fabrication processes and structures. The ability to tailor their exterior surface functional groups enhances their adaptability for therapeutic and imaging purposes. Successful translation of preclinical findings into clinical settings, demonstrated by ongoing clinical trials, will pave the way for the acceptance and implementation of these nanomaterials in personalized medicine. Integrating nanotechnology with artificial intelligence (AI)-based methods for drug combination and dosing optimization holds great potential for enhancing the efficacy and clinical feasibility of Xene-based biomedical applications. In conclusion, researchers with diverse backgrounds should advance the field of Xene-based tissue engineering by addressing these challenges and considering the proposed suggestions.
Author contributions
Conceptualization: Moon Sung Kang, Hee Jeong Jang, Dong-Wook Han. Funding acquisition and project administration: Dong-Wook Han. Resources: Hyo Jung Jo, Iruthayapandi Selestin Raja. Supervision: Dong-Wook Han. Writing – original draft: Moon Sung Kang, Hee Jeong Jang. Writing – review & editing: Dong-Wook Han.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (the Ministry of Science and ICT (MSIT)) (No. 2021R1A2C2006013), the Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Ministry of Trade, Industry, and Energy (MOTIE, Korea) (No. 20014399), the Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (Ministry of Education-MOTIE) (No. P0022108, Next Generation bio-health industry Innovation Talent Training Program), and Specialization Project of Pusan National University.References
- S. Rahman and Y. Lu, Nanoscale Horiz., 2022, 7, 849–872 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D.-E. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- M. K. Kumawat, M. Thakur, R. B. Gurung and R. Srivastava, ACS Sustainable Chem. Eng., 2017, 5, 1382–1391 CrossRef CAS.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48, 3686–3699 RSC.
- L. Wang, W. Li, B. Wu, Z. Li, S. Wang, Y. Liu, D. Pan and M. Wu, Chem. Eng. J., 2016, 300, 75–82 CrossRef CAS.
- M. T. Hwang, M. Heiranian, Y. Kim, S. You, J. Leem, A. Taqieddin, V. Faramarzi, Y. Jing, I. Park and A. M. van der Zande, Nat. Commun., 2020, 11, 1543 CrossRef CAS PubMed.
- Z. Gu, S. Zhu, L. Yan, F. Zhao and Y. Zhao, Adv. Mater., 2019, 31, 1800662 CrossRef PubMed.
- G. Cellot, S. Vranic, Y. Shin, R. Worsley, A. F. Rodrigues, C. Bussy, C. Casiraghi, K. Kostarelos and J. R. McDearmid, Nanoscale Horiz., 2020, 5, 1250–1263 RSC.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS PubMed.
- Y. Qian, X. Zhao, Q. Han, W. Chen, H. Li and W. Yuan, Nat. Commun., 2018, 9, 323 CrossRef PubMed.
- Y.-T. Kwon, Y.-S. Kim, S. Kwon, M. Mahmood, H.-R. Lim, S.-W. Park, S.-O. Kang, J. J. Choi, R. Herbert and Y. C. Jang, Nat. Commun., 2020, 11, 3450 CrossRef CAS PubMed.
- R. D. Rodriguez, A. Khalelov, P. S. Postnikov, A. Lipovka, E. Dorozhko, I. Amin, G. V. Murastov, J.-J. Chen, W. Sheng and M. E. Trusova, Mater. Horiz., 2020, 7, 1030–1041 RSC.
- W. Shao, L. Zhang, Z. Jiang, M. Xu, Y. Chen, S. Li and C. Liu, Nanoscale Horiz., 2022, 7, 1411–1417 RSC.
- K. Tadyszak, J. K. Wychowaniec and J. Litowczenko, Nanomaterials, 2018, 8, 944 CrossRef PubMed.
- S. R. Shin, B. Aghaei-Ghareh-Bolagh, T. T. Dang, S. N. Topkaya, X. Gao, S. Y. Yang, S. M. Jung, J. H. Oh, M. R. Dokmeci and X. Tang, Adv. Mater., 2013, 25, 6385–6391 CrossRef CAS PubMed.
- N. Dubey, R. Bentini, I. Islam, T. Cao, A. H. Castro Neto and V. Rosa, Stem Cells Int., 2015, 2015, 804213 Search PubMed.
- T. Hu, X. Mei, Y. Wang, X. Weng, R. Liang and M. Wei, Sci. Bull., 2019, 64, 1707–1727 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- S. Pan, J. Yin, L. Yu, C. Zhang, Y. Zhu, Y. Gao and Y. Chen, Adv. Sci., 2020, 7, 1901511 CrossRef CAS PubMed.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef CAS PubMed.
- S. Iravani and R. S. Varma, Mater. Adv., 2021, 2, 2906–2917 RSC.
- Y. Zhong, S. Huang, Z. Feng, Y. Fu and A. Mo, J. Biomed. Mater. Res., Part A, 2022, 110, 1840–1859 CrossRef CAS PubMed.
- R. Huang, X. Chen, Y. Dong, X. Zhang, Y. Wei, Z. Yang, W. Li, Y. Guo, J. Liu and Z. Yang, ACS Appl. Bio Mater., 2020, 3, 2125–2131 CrossRef CAS PubMed.
- R. Yang, S. Wen, S. Cai, W. Zhang, T. Wu and Y. Xiong, Nanoscale Horiz., 2023, 8(10), 1333–1344 RSC.
- H. Qiao, H. Liu, Z. Huang, R. Hu, Q. Ma, J. Zhong and X. Qi, Energy Environ. Mater., 2021, 4, 522–543 CrossRef CAS.
- W. Tao, N. Kong, X. Ji, Y. Zhang, A. Sharma, J. Ouyang, B. Qi, J. Wang, N. Xie and C. Kang, Chem. Soc. Rev., 2019, 48, 2891–2912 RSC.
- W. Tao, X. Ji, X. Zhu, L. Li, J. Wang, Y. Zhang, P. E. Saw, W. Li, N. Kong and M. A. Islam, Adv. Mater., 2018, 30, 1802061 CrossRef PubMed.
- M. Qiu, A. Singh, D. Wang, J. Qu, M. Swihart, H. Zhang and P. N. Prasad, Nano Today, 2019, 25, 135–155 CrossRef CAS.
- H. Huang, W. Feng and Y. Chen, Chem. Soc. Rev., 2021, 50, 11381–11485 RSC.
- S. Zhu, Y. Liu, Z. Gu and Y. Zhao, Adv. Drug Delivery Rev., 2022, 114420 CrossRef CAS PubMed.
- M. C. Shin, M. S. Kang, R. Park, S. Y. Chae, D.-W. Han and S. W. Hong, Appl. Surf. Sci., 2021, 561, 150115 CrossRef CAS.
- B. Konkena and S. Vasudevan, J. Phys. Chem. Lett., 2012, 3, 867–872 CrossRef CAS PubMed.
- L. Sun, Chin. J. Chem. Eng., 2019, 27, 2251–2260 CrossRef CAS.
- D. Chimene, D. L. Alge and A. K. Gaharwar, Adv. Mater., 2015, 27, 7261–7284 CrossRef CAS PubMed.
- R. Tarcan, O. Todor-Boer, I. Petrovai, C. Leordean, S. Astilean and I. Botiz, J. Mater. Chem. C, 2020, 8, 1198–1224 RSC.
- Y. Shen, S. Yang, P. Zhou, Q. Sun, P. Wang, L. Wan, J. Li, L. Chen, X. Wang and S. Ding, Carbon, 2013, 62, 157–164 CrossRef CAS.
- Y. Guan, S. Jiang, Y. Cong, J. Wang, Z. Dong, Q. Zhang, G. Yuan, Y. Li and X. Li, 2D Mater., 2020, 7, 025010 CrossRef CAS.
- B. Zazoum, Mater. Sci. Forum, 2022, 1053, 77–82 Search PubMed.
- Q. Zhong, Y. Li and G. Zhang, Chem. Eng. J., 2021, 409, 128099 CrossRef CAS.
- X. Li, X. Yin, S. Liang, M. Li, L. Cheng and L. Zhang, Carbon, 2019, 146, 210–217 CrossRef CAS.
- M. Soleymaniha, M. A. Shahbazi, A. R. Rafieerad, A. Maleki and A. Amiri, Adv. Healthcare Mater., 2019, 8, 1801137 CrossRef PubMed.
- Q. Guo, K. Wu, Z. Shao, E. T. Basore, P. Jiang and J. Qiu, Adv. Opt. Mater., 2019, 7, 1900322 CrossRef.
- H. Braunschweig, P. Constantinidis, T. Dellermann, W. C. Ewing, I. Fischer, M. Hess, F. R. Knight, A. Rempel, C. Schneider and S. Ullrich, Angew. Chem., Int. Ed., 2016, 55, 5606–5609 CrossRef CAS PubMed.
- A. R. Oganov, J. Chen, C. Gatti, Y. Ma, Y. Ma, C. W. Glass, Z. Liu, T. Yu, O. O. Kurakevych and V. L. Solozhenko, Nature, 2009, 457, 863–867 CrossRef CAS PubMed.
- S. Qi, J. Liu, Y. Li and M. Zhao, Phys. Rev. B, 2022, 106, 165204 CrossRef CAS.
- N. Wächter, C. Munson, R. Jarošová, I. Berkun, T. Hogan, R. C. Rocha-Filho and G. M. Swain, ACS Appl. Mater. Interfaces, 2016, 8, 28325–28337 CrossRef PubMed.
- M. I. Eremets, V. V. Struzhkin, H.-K. Mao and R. J. Hemley, Science, 2001, 293, 272–274 CrossRef CAS PubMed.
- A. J. Mannix, Z. Zhang, N. P. Guisinger, B. I. Yakobson and M. C. Hersam, Nat. Nanotechnol., 2018, 13, 444–450 CrossRef CAS PubMed.
- X. Ji, N. Kong, J. Wang, W. Li, Y. Xiao, S. T. Gan, Y. Zhang, Y. Li, X. Song and Q. Xiong, Adv. Mater., 2018, 30, 1803031 CrossRef PubMed.
- A. Molle, C. Grazianetti, L. Tao, D. Taneja, M. H. Alam and D. Akinwande, Chem. Soc. Rev., 2018, 47, 6370–6387 RSC.
- H. J. Zandvliet, Nano Today, 2014, 9, 691–694 CrossRef CAS.
- C.-C. Liu, W. Feng and Y. Yao, Phys. Rev. Lett., 2011, 107, 076802 CrossRef PubMed.
- A. J. Mannix, B. Kiraly, M. C. Hersam and N. P. Guisinger, Nat. Rev. Chem., 2017, 1, 0014 CrossRef CAS.
- H. Lin, W. Qiu, J. Liu, L. Yu, S. Gao, H. Yao, Y. Chen and J. Shi, Adv. Mater., 2019, 31, 1903013 CrossRef PubMed.
- A. Acun, L. Zhang, P. Bampoulis, M. V. Farmanbar, A. van Houselt, A. Rudenko, M. Lingenfelder, G. Brocks, B. Poelsema and M. Katsnelson, J. Phys.: Condens. Matter, 2015, 27, 443002 CrossRef CAS PubMed.
- C. Si, J. Liu, Y. Xu, J. Wu, B.-L. Gu and W. Duan, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 115429 CrossRef.
- S. Noreen, M. B. Tahir, A. Hussain, T. Nawaz, J. U. Rehman, A. Dahshan, M. Alzaid and H. Alrobei, Int. J. Hydrogen Energy, 2022, 47, 1371–1389 CrossRef CAS.
- Y. Shaidu and O. Akin-Ojo, Comput. Mater. Sci., 2016, 118, 11–15 CrossRef CAS.
- P. Garg, I. Choudhuri, A. Mahata and B. Pathak, Phys. Chem. Chem. Phys., 2017, 19, 3660–3669 RSC.
- J. Ouyang, L. Zhang, L. Li, W. Chen, Z. Tang, X. Ji, C. Feng, N. Tao, N. Kong and T. Chen, Nano-Micro Lett., 2021, 13, 1–18 CrossRef PubMed.
- S. Zhang, S. Guo, Z. Chen, Y. Wang, H. Gao, J. Gómez-Herrero, P. Ares, F. Zamora, Z. Zhu and H. Zeng, Chem. Soc. Rev., 2018, 47, 982–1021 RSC.
- M. Sun, W. Tang, Q. Ren, S.-K. Wang, J. Yu and Y. Du, Appl. Surf. Sci., 2015, 356, 110–114 CrossRef CAS.
- X. Yu, W. Liang, C. Xing, K. Chen, J. Chen, W. Huang, N. Xie, M. Qiu, X. Yan and Z. Xie, J. Mater. Chem. A, 2020, 8, 12887–12927 RSC.
- W. Lu, H. Nan, J. Hong, Y. Chen, C. Zhu, Z. Liang, X. Ma, Z. Ni, C. Jin and Z. Zhang, Nano Res., 2014, 7, 853–859 CrossRef CAS.
- V. Sresht, A. A. Padua and D. Blankschtein, ACS Nano, 2015, 9, 8255–8268 CrossRef CAS PubMed.
- S. Lee, Y. Lee, L. P. Ding, K. Lee, F. Ding and K. Kim, ACS Nano, 2022, 16, 12822–12830 CrossRef CAS PubMed.
- L. Qiu, J. Dong and F. Ding, Nanoscale, 2018, 10, 2255–2259 RSC.
- M. Zhang, G. M. Biesold and Z. Lin, Chem. Soc. Rev., 2021, 50, 13346–13371 RSC.
- M. Batmunkh, M. Bat-Erdene and J. G. Shapter, Adv. Mater., 2016, 28, 8586–8617 CrossRef CAS PubMed.
- M. S. Kang, R. Park, H. J. Jo, Y. C. Shin, C.-S. Kim, S.-H. Hyon, S. W. Hong, J. Oh and D.-W. Han, Cells, 2023, 12, 1448 CrossRef CAS PubMed.
- Y. C. Shin, J.-H. Bae, J. H. Lee, I. S. Raja, M. S. Kang, B. Kim, S. W. Hong, J.-B. Huh and D.-W. Han, Biomater. Res., 2022, 26, 11 CrossRef CAS PubMed.
- X. Wu, X. Liu, J. Wang, J. Huang and S. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 39009–39017 CrossRef CAS PubMed.
- M. S. Kang, S. J. Jeong, S. H. Lee, B. Kim, S. W. Hong, J. H. Lee and D.-W. Han, Biomater. Res., 2021, 25, 1–9 CrossRef PubMed.
- M. S. Kang, J. Jang, H. J. Jo, W.-H. Kim, B. Kim, H.-J. Chun, D. Lim and D.-W. Han, Biomolecules, 2022, 13, 55 CrossRef PubMed.
- Q.-C. Tan, X.-S. Jiang, L. Chen, J.-F. Huang, Q.-X. Zhou, J. Wang, Y. Zhao, B. Zhang, Y.-N. Sun and M. Wei, Mater. Today Bio, 2023, 18, 100500 CrossRef CAS PubMed.
- J. Yin, S. Pan, X. Guo, Y. Gao, D. Zhu, Q. Yang, J. Gao, C. Zhang and Y. Chen, Nano-Micro Lett., 2021, 13, 1–18 Search PubMed.
- J. Zhang, Y. Fu and A. Mo, Int. J. Nanomed., 2019, 10091–10103 CrossRef CAS PubMed.
- Z.-C. Hu, J.-Q. Lu, T.-W. Zhang, H.-F. Liang, H. Yuan, D.-H. Su, W. Ding, R.-X. Lian, Y.-X. Ge and B. Liang, Bioact. Mater., 2023, 22, 1–17 CAS.
- T. F. Cabada, M. Ruben, A. El Merhie, R. P. Zaccaria, A. Alabastri, E. M. Petrini, A. Barberis, M. Salerno, M. Crepaldi and A. Davis, Nanoscale Horiz., 2023, 8, 95–107 RSC.
- P. Bhattacharjee, B. Kundu, D. Naskar, H.-W. Kim, T. K. Maiti, D. Bhattacharya and S. C. Kundu, Acta Biomater., 2017, 63, 1–17 CrossRef CAS PubMed.
- K. Soetan, C. Olaiya and O. Oyewole, Afr. J. Food Sci., 2010, 4, 200–222 CAS.
- X. Liu, M. N. George, L. Li, D. Gamble, A. L. Miller II, B. Gaihre, B. E. Waletzki and L. Lu, ACS Biomater. Sci. Eng., 2020, 6, 4653–4665 CrossRef CAS PubMed.
- K. Huang, J. Wu and Z. Gu, ACS Appl. Mater. Interfaces, 2018, 11, 2908–2916 CrossRef PubMed.
- Y. Miao, X. Shi, Q. Li, L. Hao, L. Liu, X. Liu, Y. Chen and Y. Wang, Biomater. Sci., 2019, 7, 4046–4059 RSC.
- T. Xin, Y. Gu, R. Cheng, J. Tang, Z. Sun, W. Cui and L. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 41168–41180 CrossRef CAS PubMed.
- E. R. Janeček, J. R. McKee, C. S. Tan, A. Nykänen, M. Kettunen, J. Laine, O. Ikkala and O. A. Scherman, Angew. Chem., Int. Ed., 2015, 54, 5383–5388 CrossRef PubMed.
- C. T. Price, K. J. Koval and J. R. Langford, Int. J. Endocrinol., 2013, 2013, 316783 Search PubMed.
- F. Wang, H. Duan, R. Zhang, H. Guo, H. Lin and Y. Chen, Nanoscale, 2020, 12, 17931–17946 RSC.
- C. He, L. Yu, L. Ding, H. Yao, Y. Chen and Y. Hao, Biomaterials, 2020, 255, 120181 CrossRef CAS PubMed.
- N. Ni, M. Ge, R. Huang, D. Zhang, H. Lin, Y. Ju, Z. Tang, H. Gao, H. Zhou and Y. Chen, Adv. Healthcare Mater., 2023, 12, 2203107 CrossRef CAS PubMed.
- Z. Lin, Z. Chen, Y. Chen, N. Yang, J. Shi, Z. Tang, C. Zhang, H. Lin and J. Yin, Exploration, 2023, 3, 20220149 CrossRef PubMed.
- H. Zhou and J. Lee, Acta Biomater., 2011, 7, 2769–2781 CrossRef CAS PubMed.
- B. Yuan, M. Xue, Y. Zhao, Q. Guo, G. Zheng, Z. Xu, F. Li, X. Chen, Z. Chen and J. Shi, Nano Today, 2023, 52, 101959 CrossRef CAS.
- J. Zhang, H. Eyisoylu, X.-H. Qin, M. Rubert and R. Müller, Acta Biomater., 2021, 121, 637–652 CrossRef CAS PubMed.
- H. J. Jang, M. S. Kang, W.-H. Kim, H. J. Jo, S.-H. Lee, E. J. Hahm, J. H. Oh, S. W. Hong, B. Kim and D.-W. Han, Nanoscale Adv., 2023, 5(14), 3619–3628 RSC.
- N. Amiryaghoubi, M. Fathi, J. Barar, N. Noroozi-Pesyan, H. Omidian and Y. Omidi, J. Drug Delivery Sci. Technol., 2023, 104437 CrossRef CAS.
- W. C. Lee, C. H. Lim, Kenry, C. Su, K. P. Loh and C. T. Lim, Small, 2015, 11, 963–969 CrossRef CAS PubMed.
- Y. Liu, G. Zhou and Y. Cao, Engineering, 2017, 3, 28–35 CrossRef CAS.
- X. Zhou, M. Nowicki, H. Cui, W. Zhu, X. Fang, S. Miao, S.-J. Lee, M. Keidar and L. G. Zhang, Carbon, 2017, 116, 615–624 CrossRef CAS.
- E. Axpe and M. L. Oyen, Int. J. Mol. Sci., 2016, 17, 1976 CrossRef PubMed.
- F. Olate-Moya, L. Arens, M. Wilhelm, M. A. Mateos-Timoneda, E. Engel and H. Palza, ACS Appl. Mater. Interfaces, 2020, 12, 4343–4357 CrossRef CAS PubMed.
- F. Pati, J. Jang, D.-H. Ha, S. Won Kim, J.-W. Rhie, J.-H. Shim, D.-H. Kim and D.-W. Cho, Nat. Commun., 2014, 5, 3935 CrossRef CAS PubMed.
- M. Gong, J. Sun, G. Liu, L. Li, S. Wu and Z. Xiang, Mater. Sci. Eng., C, 2021, 119, 111603 CrossRef CAS PubMed.
- G. Qian, L. Zhang, Y. Shuai, X. Wu, Z. Zeng, S. Peng and C. Shuai, Appl. Surf. Sci., 2023, 614, 156108 CrossRef CAS.
- A. Halim, K.-Y. Qu, X.-F. Zhang and N.-P. Huang, ACS Biomater. Sci. Eng., 2021, 7, 3503–3529 CrossRef CAS PubMed.
- P. Gupta, P. Rathi, R. Gupta, H. Baldi, Q. Coquerel, A. Debnath, H. G. Derami, B. Raman and S. Singamaneni, Nanoscale Horiz., 2023, 8(11), 1537–1555 RSC.
- A. F. Girao, J. Sousa, A. Dominguez-Bajo, A. Gonzalez-Mayorga, I. Bdikin, E. Pujades-Otero, N. Casan-Pastor, M. J. Hortigüela, G. Otero-Irurueta and A. Completo, ACS Appl. Mater. Interfaces, 2020, 12, 38962–38975 CrossRef CAS PubMed.
- S. A. Sydlik, S. Jhunjhunwala, M. J. Webber, D. G. Anderson and R. Langer, ACS Nano, 2015, 9, 3866–3874 CrossRef CAS PubMed.
- O. Akhavan, E. Ghaderi, E. Abouei, S. Hatamie and E. Ghasemi, Carbon, 2014, 66, 395–406 CrossRef CAS.
- X. Li, Y. Ren, Y. Xue, Y. Zhang and Y. Liu, Nanoscale Horiz., 2023, 8(10), 1313–1332 RSC.
- J. Wang, Y. Cheng, L. Chen, T. Zhu, K. Ye, C. Jia, H. Wang, M. Zhu, C. Fan and X. Mo, Acta Biomater., 2019, 84, 98–113 CrossRef CAS PubMed.
- D. M. da Silva, N. Barroca, S. C. Pinto, Â. Semitela, B. M. de Sousa, P. A. Martins, L. Nero, I. Madarieta, N. García-Urkia and F.-J. Fernández-San-Argimiro, Chem. Eng. J., 2023, 472, 144980 CrossRef.
- C. Qiao, H. Wu, X. Xu, Z. Guan and W. Ou-Yang, Adv. Mater. Interfaces, 2021, 8, 2100903 CrossRef CAS.
- L.-P. Nan, Z. Lin, F. Wang, X.-H. Jin, J.-Q. Fang, B. Xu, S.-H. Liu, F. Zhang, Z. Wu and Z.-F. Zhou, Front. Bioeng. Biotechnol., 2022, 10, 850650 CrossRef PubMed.
- M. Liao, Y. Hu, Y. Zhang, K. Wang, Q. Fang, Y. Qi, Y. Shen, H. Cheng, X. Fu and M. Tang, ACS Nano, 2022, 16, 16744–16756 CrossRef CAS PubMed.
- Y. D. Zhu, X. Y. Ma, L. P. Li, Q. J. Yang, F. Jin, Z. N. Chen, C. P. Wu, H. B. Shi, Z. Q. Feng and S. K. Yin, Adv. Healthcare Mater., 2023, 2300731 CrossRef CAS PubMed.
- R. Guo, M. Xiao, W. Zhao, S. Zhou, Y. Hu, M. Liao, S. Wang, X. Yang, R. Chai and M. Tang, Acta Biomater., 2022, 139, 105–117 CrossRef CAS PubMed.
- Y. Qian, W.-E. Yuan, Y. Cheng, Y. Yang, X. Qu and C. Fan, Nano Lett., 2019, 19, 8990–9001 CrossRef CAS PubMed.
- M. S. Kang, S.-J. Song, J. H. Cha, Y. Cho, H. U. Lee, S.-H. Hyon, J. H. Lee and D.-W. Han, J. Ind. Eng. Chem., 2020, 92, 226–235 CrossRef CAS.
- C. Xu, Y. Xu, M. Yang, Y. Chang, A. Nie, Z. Liu, J. Wang and Z. Luo, Adv. Funct. Mater., 2020, 30, 2000177 CrossRef CAS.
- D.-M. Xie, C. Sun, Q. Tu, S. Li, Y. Zhang, X. Mei and Y. Li, J. Tissue Eng., 2023, 14, 20417314231180033 CrossRef PubMed.
- S. Ostrovidov, S. Salehi, M. Costantini, K. Suthiwanich, M. Ebrahimi, R. B. Sadeghian, T. Fujie, X. Shi, S. Cannata and C. Gargioli, Small, 2019, 15, 1805530 CrossRef PubMed.
- F. Weinberger, I. Mannhardt and T. Eschenhagen, Circ. Res., 2017, 120, 1487–1500 CrossRef CAS PubMed.
- Y. C. Shin, S.-J. Song, J. H. Lee, R. Park, M. S. Kang, Y. B. Lee, S. W. Hong and D.-W. Han, Sci. Adv. Mater., 2020, 12, 474–480 CrossRef CAS.
- S. H. Kang, Y. C. Shin, E. Y. Hwang, J. H. Lee, C.-S. Kim, Z. Lin, S. H. Hur, D.-W. Han and S. W. Hong, Mater. Horiz., 2019, 6, 1066–1079 RSC.
- Y. C. Shin, S. H. Kang, J. H. Lee, B. Kim, S. W. Hong and D.-W. Han, J. Biomater. Sci., Polym. Ed., 2018, 29, 762–774 CrossRef CAS PubMed.
- S. B. Jo, U. Erdenebileg, K. Dashnyam, G.-Z. Jin, J.-R. Cha, A. El-Fiqi, J. C. Knowles, K. D. Patel, H.-H. Lee and J.-H. Lee, J. Tissue Eng., 2020, 11, 2041731419900424 Search PubMed.
- Y. C. Shin, C. Kim, S.-J. Song, S. Jun, C.-S. Kim, S. W. Hong, S.-H. Hyon, D.-W. Han and J.-W. Oh, Nanotheranostics, 2018, 2, 144 CrossRef PubMed.
- M. S. Kang, J. I. Kang, P. Le Thi, K. M. Park, S. W. Hong, Y. S. Choi, D.-W. Han and K. D. Park, ACS Macro Lett., 2021, 10, 426–432 CrossRef CAS PubMed.
- M. H. Norahan, M. Amroon, R. Ghahremanzadeh, M. Mahmoodi and N. Baheiraei, J. Biomed. Mater. Res., Part A, 2019, 107, 204–219 CrossRef CAS PubMed.
- S. Pilato, S. Moffa, G. Siani, F. Diomede, O. Trubiani, J. Pizzicannella, D. Capista, M. Passacantando, P. Samorì and A. Fontana, ACS Appl. Mater. Interfaces, 2023, 15, 14077–14088 CAS.
- B. Salesa, P. Ferrús-Manzano, A. Tuñón-Molina, A. Cano-Vicent, M. Assis, J. Andrés and Á. Serrano-Aroca, Chem. – Biol. Interact., 2023, 382, 110646 CrossRef CAS PubMed.
- S. Boularaoui, A. Shanti, M. Lanotte, S. Luo, S. Bawazir, S. Lee, N. Christoforou, K. A. Khan and C. Stefanini, ACS Biomater. Sci. Eng., 2021, 7, 5810–5822 CrossRef CAS PubMed.
- S. H. Lee, M. S. Kang, S. Jeon, H. J. Jo, S. W. Hong, B. Kim and D.-W. Han, Heliyon, 2023, 9, e14490 CrossRef CAS PubMed.
- J. Liu, W. Lu, X. Lu, L. Zhang, H. Dong and Y. Li, Nano Res., 2022, 1–9 Search PubMed.
- J. Zhang, Y. Wang, N. Ding, P. Ma, Z. Zhang and Y. Liu, Colloids Interface Sci. Commun., 2023, 56, 100733 CrossRef CAS.
- T. Li, J. Ma, W. Wang and B. Lei, Adv. Healthcare Mater., 2023, 12, 2201862 CrossRef CAS PubMed.
- G. Basara, M. Saeidi-Javash, X. Ren, G. Bahcecioglu, B. C. Wyatt, B. Anasori, Y. Zhang and P. Zorlutuna, Acta Biomater., 2022, 139, 179–189 CrossRef CAS PubMed.
- G. A. Asaro, M. Solazzo, M. Suku, D. Spurling, K. Genoud, J. G. Gonzalez, F. J. O. Brien, V. Nicolosi and M. G. Monaghan, npj 2D Mater. Appl., 2023, 7, 44 CrossRef CAS.
- Z. Han, M. Yuan, L. Liu, K. Zhang, B. Zhao, B. He, Y. Liang and F. Li, Nanoscale Horiz., 2023, 8, 422–440 RSC.
- F. Groeber, M. Holeiter, M. Hampel, S. Hinderer and K. Schenke-Layland, Adv. Drug Delivery Rev., 2011, 63, 352–366 CrossRef CAS PubMed.
- I. S. Raja, H. J. Jang, M. S. Kang, K. S. Kim, Y. S. Choi, J.-R. Jeon, J. H. Lee and D.-W. Han, Multifaceted Biomedical Applications of Graphene, 2022, pp. 89–105 Search PubMed.
- I. S. Raja, H. J. Jang, M. S. Kang, K. S. Kim, Y. S. Choi, J.-R. Jeon, J. H. Lee and D.-W. Han, in Advances in Experimental Medicine and Biology, ed. D. -W. Han and S. W. Hong, Springer, Berlin, 1st edn, 2022, ch. 3, pp. 89–105 Search PubMed.
- J. Chu, P. Shi, W. Yan, J. Fu, Z. Yang, C. He, X. Deng and H. Liu, Nanoscale, 2018, 10, 9547–9560 RSC.
- H. Shen, M. Liu, H. He, L. Zhang, J. Huang, Y. Chong, J. Dai and Z. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 6317–6323 CrossRef CAS PubMed.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S.-T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318–3323 CrossRef CAS PubMed.
- L. Feng, X. Yang, X. Shi, X. Tan, R. Peng, J. Wang and Z. Liu, Small, 2013, 9, 1989–1997 CrossRef CAS PubMed.
- E. Esmaeili, T. Eslami-Arshaghi, S. Hosseinzadeh, E. Elahirad, Z. Jamalpoor, S. Hatamie and M. Soleimani, Int. J. Biol. Macromol., 2020, 152, 418–427 CrossRef CAS PubMed.
- X. Xu, S. Wang, H. Wu, Y. Liu, F. Xu and J. Zhao, Colloids Surf., B, 2021, 207, 111979 CrossRef CAS PubMed.
- C. Liang, J. He, Y. Cao, G. Liu, C. Zhang, Z. Qi, C. Fu and Y. Hu, J. Biol. Eng., 2023, 17, 39 CrossRef CAS PubMed.
- L. Jin, X. Guo, D. Gao, C. Wu, B. Hu, G. Tan, N. Du, X. Cai, Z. Yang and X. Zhang, NPG Asia Mater., 2021, 13, 24 CrossRef CAS.
- W. Liu, Z. Tao, Y. Chen, Y. Zhu, L. Zhang and A. Dong, Adv. Healthcare Mater., 2023, 2301817 CrossRef CAS PubMed.
- P. Ranjan, J. M. Lee, P. Kumar and A. Vinu, Adv. Mater., 2020, 32, 2000531 CrossRef CAS PubMed.
- S. W. Chen, S. M. Huang, H. S. Wu, W. P. Pan, S. M. Wei, C. W. Peng, I. C. Ni, B. T. Murti, M. L. Tsai and C. I. Wu, Adv. Sci., 2022, 9, 2201507 CrossRef CAS PubMed.
- N. Tao, Z. Zeng, Y. Deng, L. Chen, J. Li, L. Deng and Y.-N. Liu, Chem. Eng. J., 2023, 456, 141109 CrossRef CAS.
- J. Ouyang, C. Feng, X. Ji, L. Li, H. K. Gutti, N. Y. Kim, D. Artzi, A. Xie, N. Kong and Y. N. Liu, Angew. Chem., 2019, 131, 13539–13544 CrossRef.
- C. Feng, J. Ouyang, Z. Tang, N. Kong, Y. Liu, L. Fu, X. Ji, T. Xie, O. C. Farokhzad and W. Tao, Matter, 2020, 3, 127–144 CrossRef.
- X. Wang, X. Sun, T. Bu, K. Xu, L. Li, M. Li, R. Li and L. Wang, Int. J. Biol. Macromol., 2022, 221, 1558–1571 CrossRef CAS PubMed.
- S. Guo, S. Garaj, A. Bianco and C. Ménard-Moyon, Nat. Rev. Phys., 2022, 4, 247–262 CrossRef CAS.
- G. Reina, J. M. González-Domínguez, A. Criado, E. Vázquez, A. Bianco and M. Prato, Chem. Soc. Rev., 2017, 46, 4400–4416 RSC.
- B. Ma, C. Martín, R. Kurapati and A. Bianco, Chem. Soc. Rev., 2020, 49, 6224–6247 RSC.
- L. Song, L. Ci, H. Lu, P. B. Sorokin, C. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Nano Lett., 2010, 10, 3209–3215 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- R. Kurapati, K. Kostarelos, M. Prato and A. Bianco, Adv. Mater., 2016, 28, 6052–6074 CrossRef CAS PubMed.
- R. Kurapati, S. P. Mukherjee, C. Martín, G. Bepete, E. Vázquez, A. Pénicaud, B. Fadeel and A. Bianco, Angew. Chem., Int. Ed., 2018, 57, 11722–11727 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to the paper. |
| This journal is © The Royal Society of Chemistry 2024 |