Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A poly-δ-decalactone (PDL) based nanoemulgel for topical delivery of ketoconazole and eugenol against Candida albicans†
Prashant
Dubey
a,
Ankaj
Kumar
 a,
Klaudi K.
Vaiphei
a,
Klaudi K.
Vaiphei
 a,
Sargun
Basrani
b,
Ashwini
Jadhav
b,
Carl-Eric
Wilen
c,
Jessica M.
Rosenholm
d,
Kuldeep K.
Bansal
a,
Sargun
Basrani
b,
Ashwini
Jadhav
b,
Carl-Eric
Wilen
c,
Jessica M.
Rosenholm
d,
Kuldeep K.
Bansal
 cd,
Rudra
Chakravarti
e,
Dipanjan
Ghosh
cd,
Rudra
Chakravarti
e,
Dipanjan
Ghosh
 e and
Arvind
Gulbake
e and
Arvind
Gulbake
 *a
*a
aDepartment of Pharmaceutics, National Institute of Pharmaceutical Education and Research, Guwahati, Assam 781101, India. E-mail: arvind@niperguwahati.in; arvind.gulbake@gmail.com
bDepartment of Medical Biotechnology, CIR, D.Y. Patil Education Society, Institution Deemed to be University, Kolhapur, India
cLaboratory of Molecular Science and Engineering, Åbo Akademi University, Aurum, Henrikinkatu 2, 20500 Turku, Finland. E-mail: kuldeep.bansal@abo.fi
dPharmaceutical Sciences Laboratory, Faculty of Science and Engineering, Åbo Akademi University, Turku 20520, Finland
eDepartment of Natural Products, National Institute of Pharmaceutical Education and Research, Kolkata, India
First published on 2nd August 2024
Abstract
This study aimed to investigate the potential of poly-δ-decalactone (PDL) and a block copolymer (methoxy-poly(ethylene glycol)-b-poly-δ-decalactone (mPEG-b-PDL)) in the topical delivery of ketoconazole (KTZ) and eugenol (EUG) against Candida albicans. The nanoemulsion (NE) was studied for its significant factors and was optimized using the design of experiments (DOE) methodologies. A simple robust nanoprecipitation method was employed to successfully produce a nanoemulsion (KTZ–EUG–NE). The spherical globules exhibited rough surfaces, explaining the adsorption of mPEG-b-PDL onto PDL. The sustained drug release effects were governed by the amorphous nature of PDL. KTZ–EUG–NE was further used to develop a 1% w/v Carbopol-940-based nanoemulgel (KTZ–EUG–NE gel). The optimal rheological and spreadability properties of the developed nanoemulgel explain the ease of topical applications. Ex vivo permeation and retention studies confirmed the accumulation of KTZ–EUG–NE at different layers of the skin when applied topically. The cytotoxicity of the developed NE in human keratinocyte (HaCaT) cells demonstrated the utility of this newly explored nanocarrier in reducing the cell toxicity of KTZ. The higher antifungal activities of KTZ–EUG–NE at 19.23-fold lower concentrations for planktonic growth and 4-fold lower concentrations for biofilm formation than coarse drugs explain the effectiveness of the developed NE.
1. Introduction
Globally, the frequency of fungal infections is increasing continuously, and more than thirty-five million people are currently affected by superficial fungal infections.1 Among such, infections caused by the Candida species, especially Candida albicans, human fungal pathogens account for more of the reported worldwide deaths by fungi. It occurs at the superficial layer of the nails, skin, and hair (superficial mycosis) and can also spread to inner tissues.2 Various drugs (azoles, amphotericin B, allylamines, and echinocandins) have been employed for the treatment of Candida albicans. However, repeated use of such medicines leads to the development of resistance to single antifungal agents. Combination therapy is required to produce additive or synergistic effects at lower concentrations that can restrict the development of drug resistance against Candida albicans.3 Ketoconazole (KTZ) is a broad-spectrum imidazole drug with antifungal activity. Poor water solubility limits the antifungal potential of such drug, necessitating the use of drug carriers that can efficiently enhance the therapeutic potential of such agent.4 The existing conventional topical KTZ formulations are creams, gels, and lotions. However, all such formulations either have less skin penetration (creams and gels) or less contact time with the targeted area (lotions). Advanced nanotechnology-driven KTZ formulations, including nanostructured lipid carriers (NLCs), solid lipid nanoparticles (SLNs), poly(lactic-co-glycolic acid nanoparticles (PLGA NPs), and nanocomplexes, have been explored by researchers.5–8 KTZ has also been studied for combination approaches employing oils to produce nanoemulsions and microemulsion-based nanoemulgels.9,10 Eugenol (EUG), a phenolic aromatic oil belonging to the allylbenzene class, has been reported to have antifungal properties. The development of formulations containing such essential oils as bioactive agents has been restricted owing to their volatile nature, instability, and low bioavailability.11,12 The benefits of eugenol as a therapeutic agent have been studied through cubosomal and nanoemulsion-based approaches.13,14 Using KTZ and EUG as therapeutic agents can result in synergistic or potentiating effects. However, an effective drug nanocarrier is required to address the challenges of combining KTZ and eugenol for effective antifungal therapeutic applications.Extensive research has been conducted on nanogel systems containing nanoemulsions (NEs). Nanoemulsions are isotropic and kinetically stable systems, in which the oil phases are stabilized by thin layers of emulsifying agents.15,16 The advancement in NEs has led to the development of polymeric nanoemulsions prepared from a polymer (PDL) as the viscous oil and a block copolymer (mPEG-b-PDL) as the surfactant. NEs prepared from such a viscous oily polymer exhibit physical stability and no sign of Ostwald ripening, as discussed in various literature.17–20 Polymers and copolymers have been explored as drug carriers (micelles and nanoemulsions).21–23
This study reports the first topical application of a nanoemulgel bearing a PDL-based nanoemulsion. Although the carrier system has been well studied to encapsulate hydrophobic moieties, mostly one drug, this study aims to incorporate dual drugs, i.e., KTZ and EUG. The lipophilic and amorphous forms of PDL may enhance drug permeation through the skin and retention in the dermis or epidermis regions, thus providing an effective antifungal effect.24,25 The present investigation involves the quality by design (QbD)-driven development of a polymeric nanoemulsion using KTZ and EUG. The nanoemulgel was prepared and evaluated for topical antifungal applications by employing ex vivo skin permeation and retention studies. Finally, the in vitro antifungal activities were studied to define the effectiveness of the novel copolymer NE in topical applications.
2. Materials and methods
Materials
PDL and mPEG-b-PDL were synthesized according to the procedure reported by Bansal et al. at Åbo Akademi University.18,22 Eugenol and dialysis membranes were purchased from Sigma-Aldrich Inc. (St. Louis, MO, U.S.A). KTZ, Carbopol-940, and Poloxamer-407 were purchased from Yarrow Chem Pvt. Ltd. (Maharashtra, India). Acetone, acetonitrile, and methanol were purchased from Merck Life Sciences Pvt. Ltd. (Maharashtra, India). All the materials used in the study had the highest purity grade >95%.Methodology
| Independent variables as factors | Dependent variables as responses | ||||
|---|---|---|---|---|---|
| Critical factors | Altered levels | Response | Name | Units | |
| Low | High | ||||
| −1 | +1 | Y 1 | Globule size | nm | |
| A: Surfactant (mg) | 10 | 20 | Y 2 | PDI | Value |
| B: Drug (mg) | 5 | 15 | Y 3 | Zeta potential | mV |
| C: Amplitude (%) | 30 | 40 | Y 4 | Drug content | % |
Characterization of KTZ–EUG–NE
Total drug content
The amount of KTZ and EUG in the NE was determined by employing the direct method of drug determination. Briefly, 1 mL of KTZ–EUG–NE was dissolved in 4 mL of methanol under probe sonication to break each globule. The sample was centrifuged, and the supernatant was filtered, diluted, and analyzed using the developed RP-HPLC method. The detection of KTZ and EUG was carried out using acetonitrile, methanol, and phosphate buffer (pH 5.5) as the mobile phase (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and a Phenomenex Luna (C18, 5 μm, 240 × 4 mm) column as the stationary phase. The mobile phase was run at a flow rate of 1 mL min−1 and injection volumes were taken as 10 μL for quantifying each sample.34
20) and a Phenomenex Luna (C18, 5 μm, 240 × 4 mm) column as the stationary phase. The mobile phase was run at a flow rate of 1 mL min−1 and injection volumes were taken as 10 μL for quantifying each sample.34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da MWCO) was activated in EDTA solution before the release study. Briefly, a 1 mL sample (a coarse suspension of KTZ–EUG and KTZ–EUG–NE) was placed in a dialysis bag (DB) clipped at both ends. The DB was placed in an acceptor compartment (AC) that consisted of physiologically relevant media (phosphate buffer at pH 5.5 with 10% methanol). The process parameters employed were a speed of 100 rpm and a temperature of 37 ± 0.5 °C. At regular intervals of time (0.25, 0.5, 1, 2, 4, 8, 12, and 24 h), aliquots of 1 mL were withdrawn and replaced with the same media to maintain the sink conditions. To determine the amount of drug, all samples were filtered through 0.22 μm syringe filters and analyzed using the developed HPLC method.34 The experiment was performed in triplicate, and the data are presented as mean ± SD.
000 Da MWCO) was activated in EDTA solution before the release study. Briefly, a 1 mL sample (a coarse suspension of KTZ–EUG and KTZ–EUG–NE) was placed in a dialysis bag (DB) clipped at both ends. The DB was placed in an acceptor compartment (AC) that consisted of physiologically relevant media (phosphate buffer at pH 5.5 with 10% methanol). The process parameters employed were a speed of 100 rpm and a temperature of 37 ± 0.5 °C. At regular intervals of time (0.25, 0.5, 1, 2, 4, 8, 12, and 24 h), aliquots of 1 mL were withdrawn and replaced with the same media to maintain the sink conditions. To determine the amount of drug, all samples were filtered through 0.22 μm syringe filters and analyzed using the developed HPLC method.34 The experiment was performed in triplicate, and the data are presented as mean ± SD.
Preparation and characterization of KTZ–EUG–NE–Gel
Evaluation of the gel
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The sample was further filtered, diluted, and examined for total drug content. The experiment was performed in triplicate, and the data were reported as mean ± SD.40
000 rpm for 30 min. The sample was further filtered, diluted, and examined for total drug content. The experiment was performed in triplicate, and the data were reported as mean ± SD.40
Ex vivo skin permeation studies
The ex vivo permeation and skin retention studies were performed on fresh pig ear skin, and the detailed methodology is mentioned in the ESI (Sections 1.2 and 1.3).†Statistical data
Most experiments were performed in triplicate, and the resulting data are presented as mean ± standard deviation (SD). The analysis of binary data was conducted using Design Expert (Version 13), DD solver, and Graph Pad Prism 9.1.0 (GraphPad software, USA). A P-value < 0.05 was considered significant in the whole experiment.3. Results and discussion
Synthesis and characterization of polymers
The synthesis and purification of PDL and mPEG-b-PDL were confirmed by 1H NMR (ESI Fig. S1†), and the results were in accordance with previously reported values.22 According to NMR, the molecular weight of the homopolymer was calculated to be 5.2 K using proton integrals at 4.9, 5.1, and 7.3 ppm. Similarly, the molecular weight of the copolymer was calculated to be 8.7 K using proton integrals at 0.9, 3.4 and 4.9 ppm.Experimental design for developing KTZ–EUG–NE
The CQA and CMA governing the quality target product profile are depicted in Fig. 1. The CQAs considered in the study were taken on the basis of the literature and initial trials. The concentrations of drugs and surfactant were selected as CMAs, whereas the sonication amplitude was selected as the CPP to generate a polymeric NE using the nano-precipitation method. The QTPP ensures the safety, efficacy, and applicability of the finished product. ESI Table S1† explains the main QTPP parameters used to generate the polymeric NE.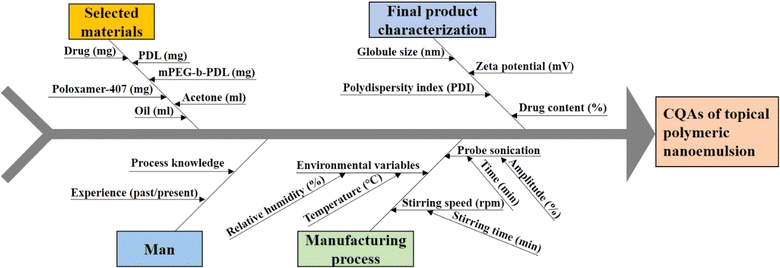 | ||
| Fig. 1 Ishikawa fish-bone diagram to showcase the potential cause-and-effect of polymeric nanoemulsion. | ||
DOE for the optimization of KTZ–EUG–NE
The CCD design was used to optimize KTZ–EUG–NE by employing twenty runs (Table 2). Variations in the globule size (nm), polydispersity index (PDI), zeta potential (mV), and % drug content were examined by response surface methodology that explains the correlations among each factor in the design of the polymeric NE. All data were statistically analyzed and the best-fit model was determined for the independent variables of the polymeric NE.44,45 After 20 successful runs, all responses (Y1, Y2, Y3, and Y4) exhibited a quadratic model with significant p values and higher R2 values. The values of lack of fit (P > 0.05), predicted R2, and adequate precision were found to be in good agreement, demonstrating that the models are reliable and can be used to navigate the design space.| Run | Surfactant (A) (mg) | Amplitude (B) (%) | Drug (C) (mg) | Globule size (nm) (Y1) | PDI (Y2) | Zeta potential (Y3) (mV) | Drug content (Y4) (%) |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 35 | 10 | 69.07 | 0.2 | −3.59 | 83.99 |
| 2 | 15 | 35 | 10 | 77.05 | 0.2 | −2.3 | 86.69 |
| 3 | 20 | 40 | 5 | 157.7 | 0.25 | 1.48 | 74.41 |
| 4 | 20 | 30 | 15 | 233.1 | 0.4 | −1.11 | 91.03 |
| 5 | 10 | 40 | 5 | 129 | 0.15 | −3.3 | 69.73 |
| 6 | 15 | 35 | 10 | 62.19 | 0.16 | −3.5 | 86.3 |
| 7 | 15 | 35 | 1.5 | 80.98 | 0.24 | −0.766 | 59.68 |
| 8 | 15 | 35 | 10 | 68 | 0.18 | −2.85 | 85.35 |
| 9 | 23.4 | 35 | 10 | 137 | 0.3 | 1.04 | 87.04 |
| 10 | 15 | 26.5 | 10 | 194 | 0.28 | −9.78 | 76.37 |
| 11 | 15 | 35 | 10 | 62 | 0.16 | −4.2 | 83.47 |
| 12 | 6.5 | 35 | 10 | 142 | 0.3 | −4.9 | 77.83 |
| 13 | 15 | 43.4 | 10 | 130.1 | 0.23 | −4.9 | 88.53 |
| 14 | 10 | 40 | 15 | 102.2 | 0.4 | −8.25 | 88.48 |
| 15 | 20 | 40 | 15 | 179 | 0.35 | −0.361 | 90.01 |
| 16 | 20 | 30 | 5 | 102 | 0.363 | −0.335 | 70.23 |
| 17 | 15 | 35 | 18.4 | 173 | 0.537 | −3.43 | 92.03 |
| 18 | 15 | 35 | 10 | 60.31 | 0.18 | −2.72 | 84.67 |
| 19 | 10 | 30 | 15 | 215.4 | 0.464 | −6.2 | 84.3 |
| 20 | 10 | 30 | 5 | 131 | 0.3 | −5.25 | 64.29 |
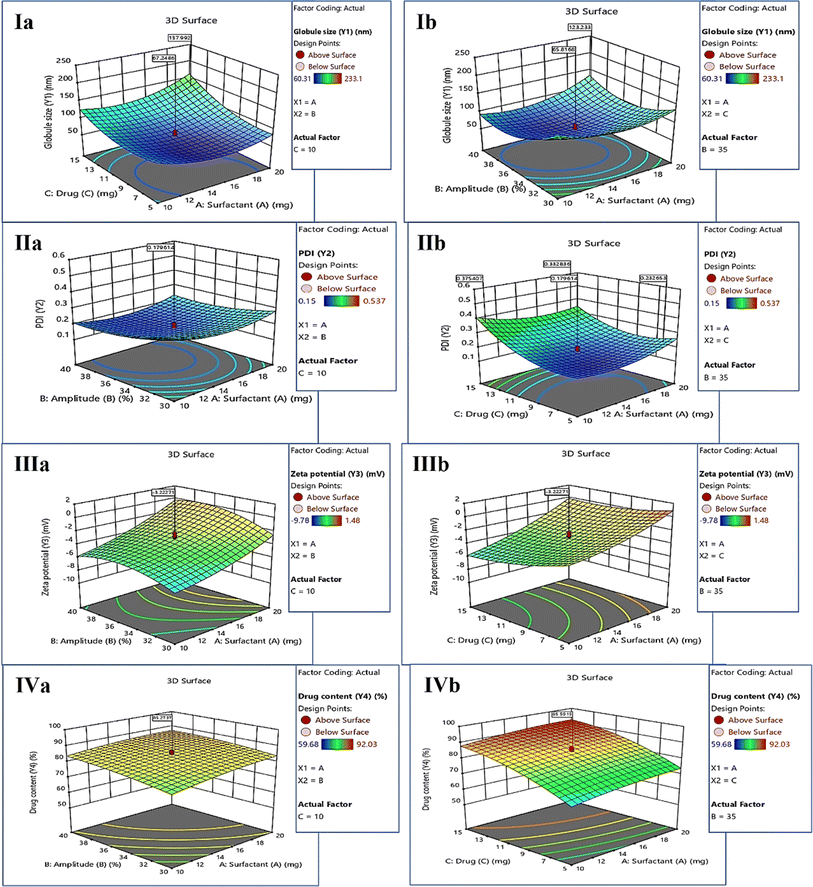
The ANOVA table was obtained for each response, explaining the important and significant factors that must be considered during the development of KTZ–EUG–NE. Each response was explained for its correlation with independent factors using coded equations. The average globule size of KTZ–EUG–NE was found to be in the range of 60.31 ± 0.04 to 233.1 ± 0.43 nm. The significant factors obtained were B, C, BC, A2, B2, and C2, as given in ESI Table S2.† The coded equations obtained for each response explained the correlations among these factor to generate the desired responses.
| Y1 (globule size) = 66.16 + 6.28A − 16.19B + 26.71C + 14.60AB + 11.85AC − 27.63BC + 27.67A2 + 35.64B2 + 23.24C2 | (1) |
The positive sign in the equations (eqn (1)–(4)) denotes a direct correlation between the response and the factors; however, the negative sign indicates an indirect or negative correlation. Significant terms were considered according to the coded equation to justify the correlation. An inverse correlation was found between the globule size (Y1) and amplitude (B) owing to the presence of sonic waves for the reduction of globule size (eqn (1)). However, the concentration of drugs showed a positive correlation with globule size, governing the nanoprecipitation of free drug when taken at higher concentrations. The increase in globule size was also governed by the square effect of A, B, and C, as shown in Table S2.† The correlation was represented by employing 3D surface plots for the globule size as a response. As shown in Fig. 2(Ia), a lower concentration of the drug decreases the globule size because of the solubility differences in the drugs. The increase in surfactant concentration decreases the globule size, as can be visualized in Fig. 2(Ib), which also explains the negative correlation between the globule size and amplitude of sonication.
The average polydispersity index (PDI) of KTZ–EUG–NE after twenty runs was found to be in the range of 0.15 ± 0.03 to 0.537 ± 0.23. The significant factors examined by ANOVA were B, C, AC, A2, B2, and C2, as given in ESI Table S3.† The coded equation generated to study the correlation between PDI responses and factors is given below (eqn (2))
| Y2 (PDI) = 0.1796 + 0.0036A − 0.0338B + 0.0769C + 0.0064AB − 0.0346AC + 0.0186BC + 0.0450A2 + 0.0290B2 + 0.0762C2 | (2) |
According to the equation, an increase in the sonication amplitude decreases the PDI value, producing uniform globules. However, a positive correlation was observed between the additive effect of amplitude (B2) and PDI (Y2) values owing to coalescence resulting from the decreased globule size. The concentration of KTZ and EUG was found to be another significant factor that directly correlated with an increase in the PDI. The increase in the PDI value was also governed by an increase in the concentration of surfactant, as depicted in the equation and 3D response plots of Fig. 2(IIa) and (IIb).
The average zeta potential (ZP) of KTZ–EUG–NE was found to be in the range of −9.78 to 1.48 mV. For ZP, the response significant terms considered were A, C, and B2, as given in ESI Table S4.†
| Y3 (zeta potential) = −3.22 + 2.39A + 0.7814B − 0.9516C + 0.3330AB + 0.4105AC − 0.6332BC + 0.6386A2 − 1.27B2 + 0.5792C2 | (3) |
An inverse correlation was found between the zeta potential (Y3) and surfactant-2 (A) owing to the amphiphilic nature that was responsible for the neutralization of the zeta potential (eqn (3)). The equation also explained the negative correlation between the zeta potential and the concentration of the drugs employed in the study. The correlation was visualized using 3D response surface plots, as shown in the plots of Fig. 2(IIIa) and (IIIb).
The average KTZ content from KTZ–EUG–NE was found to be in the range of 59.68 ± 0.56% to 92.03 ± 0.37%. The factors that significantly affected the KTZ content of KTZ–EUG–NE were found to be A, B, C, and C2, as shown in ESI Table S5.† According to eqn (4), an increase in the surfactant and drug concentration increases the drug content of the NE owing to an increase in the solubility of KTZ in the presence of surfactant. The higher amplitude also increases the drug content of the NE because of the high energy-driven entrapment of KTZ in the polymeric NE. The correlation of each factor can be visualized using the 3D surface plots in Fig. 2(IVa) and (IVb).
| Y4 (% drug content) = 85.11 + 2.52A + 2.43B + 9.49C − 0.8075AB − 0.2950AC − 0.8075BC − 1.12A2 − 1.12B2 − 3.45C2 | (4) |
Optimization of KTZ–EUG–NE
Statistical optimization was conducted by employing the desirability function (D). To achieve the QTPP for the polymeric NE, optimization was carried out using solutions from a design expert. The formulations were prepared according to the optimized protocol (CPP), and responses were recorded (CQA). The predicted values for each response Y1–Y4 were recorded and compared with experimental values to further calculate the prediction error percentage, which was found to be <20% (Table 3).
A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
Response variables | Experimental value | Predicted value | Predicted error (%) |
|---|---|---|---|---|
| Surfactant (A), 15 mg | Globule size (Y1) | 68.91 | 66.15 | 4.17 |
| Amplitude (B), 35% | PDI (Y2) | 0.191 | 0.17 | 12.35 |
| Drug (C), 10 mg | Zeta potential (Y3) | −3.36 | −3.2 | 5.1 |
| % drug content (Y4) | 84.33 | 85.10 | 3.1 |
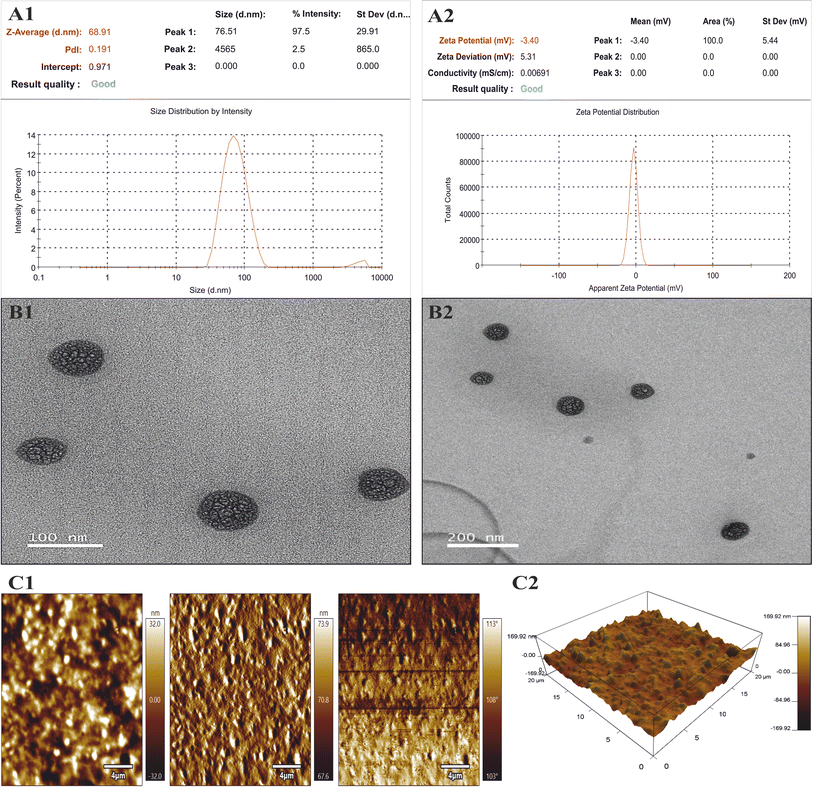
Physicochemical characterization of KTZ–EUG–NE
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, 1031.95 cm−1 for the stretching of the aliphatic ether group (C–O), 1244.13 cm−1 for the stretching of the cyclic ether (C–O), 1510 cm−1 for aromatic asymmetric stretching (C
O stretching, 1031.95 cm−1 for the stretching of the aliphatic ether group (C–O), 1244.13 cm−1 for the stretching of the cyclic ether (C–O), 1510 cm−1 for aromatic asymmetric stretching (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1584 cm−1 for aromatic symmetric stretching (C
C), 1584 cm−1 for aromatic symmetric stretching (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), and 3119 cm−1 for (
C), and 3119 cm−1 for (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching). EUG showed characteristic peaks at 3521 cm−1 (OH stretching), 1365 cm−1 (isopropyl group) and 1516 cm−1 (C
C–H stretching). EUG showed characteristic peaks at 3521 cm−1 (OH stretching), 1365 cm−1 (isopropyl group) and 1516 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), which was similar to a previously reported study.46 The polymer (PDL) had characteristic peaks at 2928.10 cm−1 (C–H) and 1166.71 cm−1 (C–O). Peaks at 2877.01 cm−1 (C–H stretching), 1729.65 cm−1 (C
C), which was similar to a previously reported study.46 The polymer (PDL) had characteristic peaks at 2928.10 cm−1 (C–H) and 1166.71 cm−1 (C–O). Peaks at 2877.01 cm−1 (C–H stretching), 1729.65 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1102.11 cm−1 (aliphatic O–CH2–CH2) were observed for mPEG-b-PDL. P-407 had characteristic peaks at 2882.18 cm−1 (C–H) and 1353.30 cm−1 (CH–CH3). In the case of blank NE, some distinctive peaks of the excipients were observed at 1108.22 cm−1, 1730.74 cm−1 (C
O), and 1102.11 cm−1 (aliphatic O–CH2–CH2) were observed for mPEG-b-PDL. P-407 had characteristic peaks at 2882.18 cm−1 (C–H) and 1353.30 cm−1 (CH–CH3). In the case of blank NE, some distinctive peaks of the excipients were observed at 1108.22 cm−1, 1730.74 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2876.66 cm−1 (C–H stretching), and 1342.18 cm−1 (CH–CH3), except for those of the drug and oil. The spectrum of KTZ–EUG–NE showed similar peaks to the blank spectrum; however, the peak at 1520 cm−1 may be governed by the presence of the aromatic asymmetric stretching (C
O), 2876.66 cm−1 (C–H stretching), and 1342.18 cm−1 (CH–CH3), except for those of the drug and oil. The spectrum of KTZ–EUG–NE showed similar peaks to the blank spectrum; however, the peak at 1520 cm−1 may be governed by the presence of the aromatic asymmetric stretching (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of KTZ or EUG. The observed findings may illustrate the entrapment of KTZ and EUZ inside the polymeric PDL.47
C) of KTZ or EUG. The observed findings may illustrate the entrapment of KTZ and EUZ inside the polymeric PDL.47
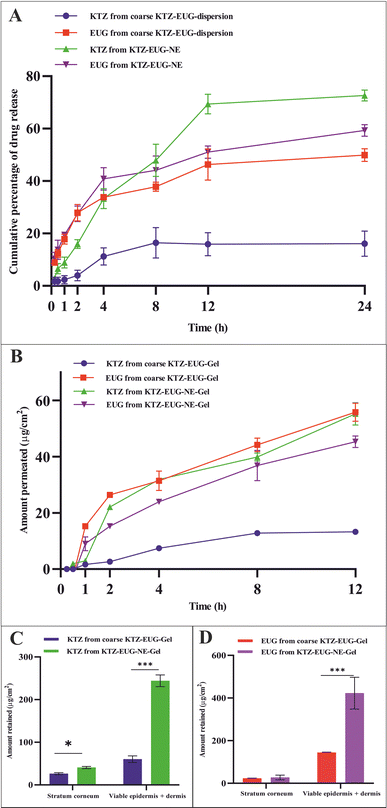
The cumulative percentage of drug release data was fitted into different release kinetic models, viz., zero-order, first-order, Higuchi, and Korsmeyer–Peppas. The best-fit model was determined on the basis of a higher regression coefficient (R2) and lower AIC values. The data for KTZ and EUG release from KTZ–EUG–NE were best fitted to the Korsmeyer–Peppas model with the highest R2 values of 0.965 and 0.964, respectively. Moreover, the Korsmeyer–Peppas model showed the lowest AIC values of 45.12 and 39.15 for KTZ and EUG, respectively, as depicted in ESI Table S6.† The release exponent values were found to be in the range of 0.45–0.89, explaining that the NE follows anomalous (non-Fickian) diffusion of drug release.
Characterization of KTZ–EUG–NE–Gel
The NE-loaded gel was evaluated for physical properties (color, texture, pH, and homogeneity). NE–Gel prepared using a 1% w/v Carbopol concentration possessed a whitish appearance with greater homogeneity and no indications of grittiness or lumps (ESI Fig. S3†). However, NE–Gel prepared using a 2% w/v Carbopol concentration showed a clumsy appearance and was thus excluded from further evaluation. The prepared NE–Gel possessed a pH value of 6.2 ± 0.04, which is essential for avoiding irritation and ease of topical application against fungal infection.48 The content of KTZ and EUG in 1% w/v Carbopol KTZ–EUG–NE–Gel was found to be 81.32 ± 0.23% and 83.24 ± 0.95%, respectively. The observed concentration of drugs explained the uniformity of the NE-loaded gel.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 ± 11.92 mPa s, which depicted the viscous nature of the prepared gel.
942 ± 11.92 mPa s, which depicted the viscous nature of the prepared gel.
Ex vivo skin permeation and retention studies
The skin permeability of KTZ–EUG–NE was assessed using an ex vivo permeation study (Fig. 4(B)). The amount of KTZ permeated through the 3.14 cm2 area of the skin was found to be 18.44 ± 2.97 μg and 71.28 ± 1.54 μg from coarse KTZ–EUG–Gel and KTZ–EUG–NE–Gel, respectively. A 5-fold higher transdermal flux (Jss) accounted for KTZ–EUG–NE–Gel (1.819 μg h−1 cm−2) compared to coarse KTZ–Gel (0.371 μg h−1 cm−2) might be due to the nanosize range and lipophilic nature of the PDL, which helps in drug penetration. The values of the permeability coefficient (Kp) for KTZ–EUG–NE–Gel and coarse KTZ–EUG–Gel were found to be 0.047 × 10−3 h−1 cm−2 and 0.4 × 10−2 h−1 cm−2, respectively.In addition, the lipophilic nature of EUG helps to attain greater permeation, as reported in various articles.49 The study depicted Jss values for EUG permeated from coarse KTZ–EUG–Gel and KTZ–EUG–NE–Gel as 1.41 μg h−1 cm−2 and 3.22 μg h−1 cm−2, respectively, suggesting a 2-fold higher permeation. The Kp values for coarse KTZ–EUG–Gel and KTZ–EUG–NE–Gel were determined to be 0.23 × 10−2 h−1 cm−2 and 0.5 × 10−2 h−1 cm−2, respectively.
After 12 h of application on the skin, KTZ–EUG–NE–Gel was evaluated for ex vivo retention on the superficial layer of the skin (the stratum corneum and the viable epidermis or dermis) (Fig. 4(C) and (D)). The poorly water-soluble KTZ when loaded in the polymeric NE exhibited 1.59 times higher retention at the stratum corneum and 4.03 times higher retention in the viable epidermis and dermis layers in contrast to coarse KTZ–EUG–Gel. However, EUG, owing to its lipophilic nature, restricts the retention in the superficial layer when applied in the form of coarse KTZ–EUG–Gel. It was the polymeric NE that retained a greater amount (2.93-fold) of EUG when compared with coarse KTZ–EUG–Gel in the viable epidermis or dermis. The findings from the study explain that the presence of the drug in the upper layers of the skin could provide a better treatment of superficial mycoses (Candida albicans).
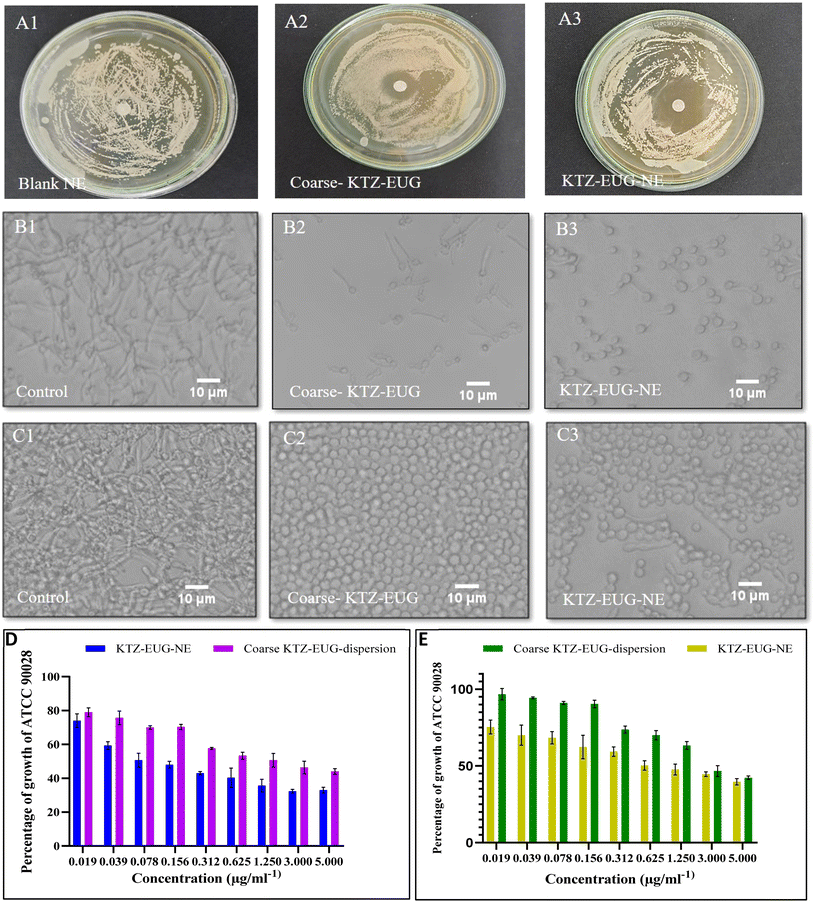
The Candida albicans infection mainly occurs in the top layer of the skin, i.e., the epidermis, as reported in various studies.50 After the successful completion of an ex vivo permeation study, higher fluorescence was observed in the epidermis after 12 h, as shown in ESI Fig. S6.† The layer is generally lipophilic in nature, and owing to the lipophilic character of PDL, lipid depots may form that can retain the drug for a longer time. In contrast to the brightfield view, the fluorescent view depicted the presence of FITC in the epidermis region. However, a tiny fraction of fluorescence was observed in the dermis region of the skin, which may be due to the hydrophilic properties of the dermis layer.51 The amorphous characteristics of the PDL may govern the permeation of FITC into the dermis layer; thus, a lower fraction was detected.
4. Conclusion
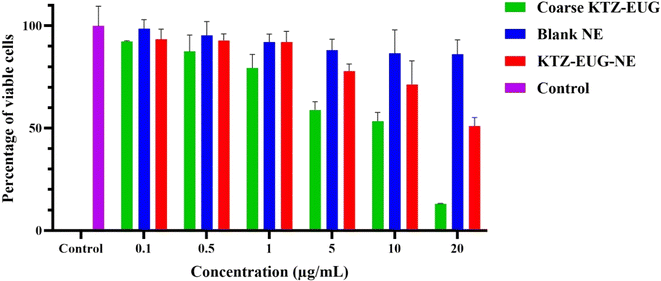
Every year, approximately 150 million people are infected with Candida albicans. The drug combination of KTZ and EUG was successfully investigated using a newly explored polymeric NE. Renewable PDL and mPEG-b-PDL based NEs were produced using a central composite design through Design Expert® (version 13) software. The concentration of surfactant, drugs, and sonication amplitude were found to be crucial factors in the development of KTZ–EUG–NE. The QbD-driven development of the NE results in globule size < 100 nm, PDI < 0.3, and a total drug content for KTZ and EUZ of 80–85%. The rough-surfaced spherical NE was governed by the adsorption of mPEG-b-PDL on the globule, as confirmed by AFM and TEM analysis. The in vitro drug release study results depicted an initial fast release, followed by a sustained release effect with a non-Fickian diffusion-release model for KTZ and EUG. A 1% w/v gel was successfully developed and evaluated for physical characteristics, pH, and drug content. The optimal spreadability and viscosity of the KTZ–EUG–NE-based nanoemulgel explained the ease of topical application. The higher retention of KTZ and EUG from KTZ–EUG–NE onto the skin, mainly in the epidermis layer, concluded the localization of drugs to facilitate antifungal activity at the disease site. KTZ–EUG–NE was found to be effective in inhibiting planktonic growth and yeast to hyphal morphogenesis at significantly lower concentrations than the coarse KTZ–EUG dispersion. The inhibition of biofilm formation by KTZ–EUG–NE explains the importance of developing KTZ–EUG–NE. Moreover, the in vitro cell line and irritation studies depicted the safety and non-irritant nature of PDL and mPEG-b-PDL-based NEs for topical applications. The overall findings showed the potential of KTZ and EUG based polymeric nanoemulsions for topical delivery of antifungal agents. Further preclinical studies on dermatophytosis animal models are required to evaluate the antifungal efficacy that will strengthen the role of such biocompatible PDL and mPEG-b-PDL-based NEs.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors report no conflict of interest related to the manuscript.Acknowledgements
This research was partly funded by the Business Finland Research-to-Business project Jasmine PRO (1609/31/2021). The authors would like to acknowledge the National Institute of Pharmaceutical Education & Research (NIPER) Guwahati (Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, India) for extending facilities during this manuscript writing. Dr Gulbake would like to thank the Science and Engineering Research Board (SERB), (EEQ/2022/000950) New Delhi, India, for extending facilities to write the manuscript. Author RC would like to acknowledge DST-INSPIRE for providing him with fellowship. This study is part of the activities of the ÅAU Foundation (SÅA) funded Centre of Excellence in Research “Materials-driven solutions for combating antimicrobial resistance (MADNESS)” and to the strategic research profiling area “Solutions for Health” at Åbo Akademi University. The authors would like to acknowledge CIF and NECBH, IIT-Guwahati (BT/NER/143/SP44675/2023) for providing TEM and AFM facilities. The authors also would like to acknowledge Dr Jagdish Kumar Balani (Veterinary officer) for extending research facilities for the ex vivo permeation studies.References
- M. Shahid, A. Hussain, A. A. Khan, A. M. Alanazi, A. L. Alaofi, M. Alam and M. Ramzan, Antifungal Cationic Nanoemulsion Ferrying Miconazole Nitrate with Synergism to Control Fungal Infections: In Vitro, Ex Vivo, and In Vivo Evaluations, ACS Omega, 2022, 7, 13343–13353 CrossRef CAS PubMed.
- E. M. Alyahya, K. Alwabsi, A. E. Aljohani, R. Albalawi, M. El-Sherbiny, R. Ahmed, Y. Mortagi and M. Qushawy, Preparation and Optimization of Itraconazole Transferosomes-Loaded HPMC Hydrogel for Enhancing Its Antifungal Activity: 2^3 Full Factorial Design, Polymers, 2023, 15, 995 CrossRef CAS PubMed.
- M. C. Fisher, A. Alastruey-Izquierdo, J. Berman, T. Bicanic, E. M. Bignell, P. Bowyer, M. Bromley, R. Brüggemann, G. Garber, O. A. Cornely, S. J. Gurr, T. S. Harrison, E. Kuijper, J. Rhodes, D. C. Sheppard, A. Warris, P. L. White, J. Xu, B. Zwaan and P. E. Verweij, Tackling the emerging threat of antifungal resistance to human health, Nat. Rev. Microbiol., 2022, 20, 557–571 CrossRef CAS PubMed.
- F. Teng, P. Deng, Z. Song, F. Zhou and R. Feng, Enhanced effect in combination of curcumin- and ketoconazole-loaded methoxy poly(ethylene glycol)-poly(ε-caprolactone) micelles, Biomed. Pharmacother., 2017, 88, 43–51 CrossRef CAS PubMed.
- A. Kumar, D. Panwar, V. Bhavana, P. Thakor, P. K. Singh and N. K. Mehra, Lipid-Based Nanomaterials: A Brief Note on Composition, Development, and Drug Delivery Applications, in Nanomaterial-Based Drug Delivery Systems: Therapeutic and Theranostic Applications, ed. C. V. Pardeshi, Springer International Publishing, Cham, 2023, pp. 65–98 Search PubMed.
- S. Sadozai, S. Khan, N. Karim, D. Becker, N. Steinbrück, S. Gier, A. Baseer, F. Breinig, G. Kickelbick and M. Schneider, Ketoconazole loaded nanoparticles and its synergism against Candida albicans when combined with silver nanoparticles, J. Drug Delivery Sci. Technol., 2020, 56, 101574 CrossRef CAS.
- N. Dudhipala and A. A. Ay, Amelioration of ketoconazole in lipid nanoparticles for enhanced antifungal activity and bioavailability through oral administration for management of fungal infections, Chem. Phys. Lipids, 2020, 232, 104953 CrossRef CAS PubMed.
- M. Ramzan, S. Gourion-Arsiquaud, A. Hussain, J. S. Gulati, Q. Zhang, S. Trehan, V. Puri, B. Michniak-Kohn and I. P. Kaur, In vitro release, ex vivo penetration, and in vivo dermatokinetics of ketoconazole-loaded solid lipid nanoparticles for topical delivery, Drug Delivery Transl. Res., 2022, 12, 1659–1683 CrossRef CAS PubMed.
- I. Ahmad, M. Farheen, A. Kukreti, O. Afzal, M. H. Akhter, H. Chitme, S. Visht, A. S. A. Altamimi, M. A. Alossaimi, E. R. Alsulami, M. Jaremko and A. H. Emwas, Natural Oils Enhance the Topical Delivery of Ketoconazole by Nanoemulgel for Fungal Infections, ACS Omega, 2023, 8, 28233–28248 CrossRef CAS PubMed.
- N. Tiwari, A. Sivakumar, A. Mukherjee and N. Chandrasekaran, Enhanced antifungal activity of Ketoconazole using rose oil based novel microemulsion formulation, J. Drug Delivery Sci. Technol., 2018, 47, 434–444 CrossRef CAS.
- M. Didehdar, Z. Chegini and A. Shariati, Eugenol: A novel therapeutic agent for the inhibition of Candida species infection, Front. Pharmacol, 2022, 13, 872127 CrossRef CAS PubMed.
- E. Schmidt, L. Jirovetz, K. Wlcek, G. Buchbauer, V. Gochev, T. Girova, A. Stoyanova and M. Geissler, Antifungal Activity of Eugenol and Various Eugenol-Containing Essential Oils against 38 Clinical Isolates of Candida albicans, J. Essent. Oil Bear. Plants, 2013, 10, 421–429 CrossRef.
- C. L. Putta, S. N. R. Rahman, P. Chakraborty and T. Shunmugaperumal, Development, systematic optimisation and biofilm disruption activity of eugenol-based nanosized emulsions stabilised with Tween 80, J. Microencapsulation, 2023, 1–17 Search PubMed.
- H. A. Elgendy, A. M. A. Makky, Y. E. Elakkad, R. M. Ismail and N. F. Younes, Syringeable atorvastatin loaded eugenol enriched PEGylated cubosomes in situ gel for the intra-pocket treatment of periodontitis: statistical optimization and clinical assessment, Drug Delivery, 2023, 30, 2162159 CrossRef PubMed.
- S. Grijalvo and C. Rodriguez-Abreu, Polymer nanoparticles from low-energy nanoemulsions for biomedical applications, Beilstein J. Nanotechnol., 2023, 14, 339–350 CrossRef CAS PubMed.
- T. Liu, Z. Gao, W. Zhong, F. Fu, G. Li, J. Guo and Y. Shan, Preparation, Characterization, and Antioxidant Activity of Nanoemulsions Incorporating Lemon Essential Oil, Antioxidants, 2022, 11, 650 CrossRef CAS PubMed.
- T. J. Wooster, M. Golding and P. Sanguansri, Impact of oil type on nanoemulsion formation and Ostwald ripening stability, Langmuir, 2008, 24, 12758–12765 CrossRef CAS PubMed.
- J. Wik, K. K. Bansal, T. Assmuth, A. Rosling and J. M. Rosenholm, Facile methodology of nanoemulsion preparation using oily polymer for the delivery of poorly soluble drugs, Drug Delivery Transl. Res., 2020, 10, 1228–1240 CrossRef CAS PubMed.
- J. Pyrhönen, K. Bansal, R. Bhadane, C.-E. Wilen, O. Salo-Ahen and J. Rosenholm, Molecular Dynamics Prediction Verified by Experimental Evaluation of the Solubility of Different Drugs in Poly(decalactone) for the Fabrication of Polymeric Nanoemulsions, Adv. NanoBiomed Res., 2021, 2, 2100072 CrossRef.
- S. Maru, J. Verma, C. E. Wilen, J. M. Rosenholm and K. K. Bansal, Attenuation of celecoxib cardiac toxicity using Poly(δ-decalactone) based nanoemulsion via oral route, Eur. J. Pharm. Sci., 2023, 190, 106585 CrossRef CAS PubMed.
- K. K. Bansal, J. Gupta, A. Rosling and J. M. Rosenholm, Renewable poly(δ-decalactone) based block copolymer micelles as drug delivery vehicle: in vitro and in vivo evaluation, Saudi Pharm. J., 2018, 26, 358–368 CrossRef PubMed.
- K. Bansal, D. Kakde, L. Purdie, D. Irvine, S. Howdle, G. Mantovani and C. Alexander, New Biomaterials from Renewable Resources - Amphiphilic Block Copolymers from δ-Decalactone, Polym. Chem., 2015, 6, 7196–7210 RSC.
- K. K. Bansal, E. Özliseli, G. K. Saraogi and J. M. Rosenholm, Assessment of Intracellular Delivery Potential of Novel Sustainable Poly(δ-decalactone)-Based Micelles, Pharmaceutics, 2020, 12, 726 CrossRef CAS PubMed.
- K. Inoue, K. Ogawa, J. Okada and K. Sugibayashi, Enhancement of skin permeation of ketotifen by supersaturation generated by amorphous form of the drug, J. Controlled Release, 2005, 108, 306–318 CrossRef CAS PubMed.
- M. Sala, R. Diab, A. Elaissari and H. Fessi, Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications, Int. J. Pharm., 2018, 535, 1–17 CrossRef CAS PubMed.
- P. Negi, B. Singh, G. Sharma, S. Beg and O. P. Katare, Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation, J. Microencapsulation, 2015, 32, 419–431 CrossRef CAS PubMed.
- E. Lambert and J. M. Janjic, Quality by design approach identifies critical parameters driving oxygen delivery performance in vitro for perfluorocarbon based artificial oxygen carriers, Sci. Rep., 2021, 11, 5569 CrossRef CAS PubMed.
- S. F. Tan, H. R. Masoumi, R. A. Karjiban, J. Stanslas, B. P. Kirby, M. Basri and H. B. Basri, Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability, Ultrason. Sonochem., 2016, 29, 299–308 CrossRef CAS PubMed.
- Y. Shi, H. Li, J. Li, D. Zhi, X. Zhang, H. Liu, H. Wang and H. Li, Development, optimization and evaluation of emodin loaded nanoemulsion prepared by ultrasonic emulsification, J. Drug Delivery Sci. Technol., 2015, 27, 46–55 CrossRef CAS.
- M. M. Ahmed, M. K. Anwer, F. Fatima, A. S. Alali, M. A. Kalam, A. Zafar, S. Alshehri and M. M. Ghoneim, Development of Apremilast Nanoemulsion-Loaded Chitosan Gels: In Vitro Evaluations and Anti-Inflammatory and Wound Healing Studies on a Rat Model, Gels, 2022, 8, 253 CrossRef CAS PubMed.
- S. Abbas, E. Karangwa, M. Bashari, K. Hayat, X. Hong, H. R. Sharif and X. Zhang, Fabrication of polymeric nanocapsules from curcumin-loaded nanoemulsion templates by self-assembly, Ultrason. Sonochem., 2015, 23, 81–92 CrossRef CAS PubMed.
- M. H. Teaima, M. A. Eltabeeb, M. A. El-Nabarawi and M. M. Abdellatif, Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting contraceptive: in vitro, ex vivo, and in vivo evaluation, Drug Delivery, 2022, 29, 2549–2560 CrossRef CAS PubMed.
- A. Rosso, G. Lollo, Y. Chevalier, N. Troung, C. Bordes, S. Bourgeois, O. Maniti, T. Granjon, P.-Y. Dugas, S. Urbaniak and S. Briançon, Development and structural characterization of a novel nanoemulsion for oral drug delivery, Colloids Surf., A, 2020, 593, 124614 CrossRef CAS.
- S. N. Chilamakuri, A. Kumar, A. G. Nath, A. Gupta, S. Selvaraju, S. Basrani, A. Jadhav and A. Gulbake, Development and In Vitro Evaluation of Eugenol-Based Nanostructured Lipid Carriers for Effectual Topical Treatment Against C. albicans, J. Pharm. Sci., 2023, 113, 772–784 CrossRef PubMed.
- A. Kumar, B. Valamla, P. Thakor, P. S. Chary, N. Rajana and N. K. Mehra, Development and evaluation of nanocrystals loaded hydrogel for topical application, J. Drug Delivery Sci. Technol., 2022, 74, 103503 CrossRef CAS.
- D. Verma, P. S. Thakur, S. Padhi, T. Khuroo, S. Talegaonkar and Z. Iqbal, Design expert assisted nanoformulation design for co-delivery of topotecan and thymoquinone: Optimization, in vitro characterization and stability assessment, J. Mol. Liq., 2017, 242, 382–394 CrossRef CAS.
- M. Pourmadadi, M. Ahmadi, M. Abdouss, F. Yazdian, H. Rashedi, M. Navaei-Nigjeh and Y. Hesari, The synthesis and characterization of double nanoemulsion for targeted Co-Delivery of 5-fluorouracil and curcumin using pH-sensitive agarose/chitosan nanocarrier, J. Drug Delivery Sci. Technol., 2022, 70, 102849 CrossRef CAS.
- H. Amoozegar, A. Ghaffari, M. Keramati, S. Ahmadi, S. Dizaji, F. Moayer, I. Akbarzadeh, M. Abazari, M. razzaghi-abyaneh and H. Bakhshandeh, A novel formulation of simvastatin nanoemulsion gel for infected wound therapy: In vitro and in vivo assessment, J. Drug Delivery Sci. Technol., 2022, 72, 103369 CrossRef CAS.
- X. Du, M. Hu, G. Liu, B. Qi, S. Zhou, K. Lu, F. Xie, X. Zhu and Y. Li, Development and evaluation of delivery systems for quercetin: A comparative study between coarse emulsion, nano-emulsion, high internal phase emulsion, and emulsion gel, J. Food Eng., 2022, 314, 110784 CrossRef CAS.
- K. R. Vijaya Rani, S. Rajan, M. Bhupathyraaj, R. K. Priya, N. Halligudi, M. A. Al-Ghazali, S. B. Sridhar, J. Shareef, S. Thomas, S. M. Desai and P. D. Pol, The Effect of Polymers on Drug Release Kinetics in Nanoemulsion In Situ Gel Formulation, Polymers, 2022, 14, 427 CrossRef CAS PubMed.
- V. K. Rapalli, S. Sharma, A. Roy, A. Alexander and G. Singhvi, Solid lipid nanocarriers embedded hydrogel for topical delivery of apremilast: In vitro, ex vivo, dermatopharmacokinetic and anti-psoriatic evaluation, J. Drug Delivery Sci. Technol., 2021, 63, 102442 CrossRef CAS.
- A. D. R. Teixeira, A. V. Quaresma, R. T. Branquinho, S. Santos, J. T. Magalhães, F. Silva, M. B. F. Marques, S. A. L. Moura, A. P. M. Barboza, M. G. F. Araújo and G. R. D. Silva, Miconazole-loaded nanoparticles coated with hyaluronic acid to treat vulvovaginal candidiasis, Eur. J. Pharm. Sci., 2023, 188, 106508 CrossRef CAS PubMed.
- R. L. Pereira, F. I. Leites, K. Paese, R. M. Sponchiado, C. B. Michalowski, S. S. Guterres and E. E. Schapoval, Hydrogel containing adapalene- and dapsone-loaded lipid-core nanocapsules for cutaneous application: development, characterization, in vitro irritation and permeation studies, Drug Dev. Ind. Pharm., 2016, 42, 2001–2008 CrossRef CAS PubMed.
- A. Sivaraman and A. K. Banga, Quality by design approaches for topical dermatological dosage forms, Res. Rep. Transdermal Drug Delivery, 2015, 4, 9–21 CrossRef.
- G. Nikaeen, S. Yousefinejad, S. Rahmdel, F. Samari and S. Mahdavinia, Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity, Sci. Rep., 2020, 10, 9642 CrossRef CAS PubMed.
- Z. Yang, Y. Chai, D. Zhou, X. Yao and H. Ji, Mechanism for efficient separation of eugenol and eugenol acetate with β -cyclodextrin as a selective solvent, Supramol. Chem., 2019, 31, 1–9 CrossRef.
- A. Nawaz, M. S. Latif, M. A. Alnuwaiser, S. Ullah, M. Iqbal, M. Alfatama and V. Lim, Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery, Gels, 2022, 8, 412 CrossRef CAS PubMed.
- T. Ramasamy, S. Umadevi, H. Ruttala and S. Shanmugam, Development of solid lipid nanoparticles enriched hydrogels for topical delivery of anti-fungal agent, Macromol. Res., 2012, 20, 682–692 CrossRef CAS.
- E. Makuch, A. Nowak, A. Günther, R. Pełech, Ł. Kucharski, W. Duchnik and A. Klimowicz, Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives, AMB Express, 2020, 10, 187 CrossRef CAS PubMed.
- A. Raz-Pasteur, Y. Ullmann and I. Berdicevsky, The pathogenesis of Candida infections in a human skin model: scanning electron microscope observations, ISRN Dermatol., 2011, 2011, 150642 CAS.
- M. S. Roberts, H. S. Cheruvu, S. E. Mangion, A. Alinaghi, H. A. E. Benson, Y. Mohammed, A. Holmes, J. van der Hoek, M. Pastore and J. E. Grice, Topical drug delivery: History, percutaneous absorption, and product development, Adv. Drug Delivery Rev., 2021, 177, 113929 CrossRef CAS PubMed.
- F. J. Osonga, G. Eshun, S. Kalra, I. Yazgan, L. Sakhaee, R. Ontman, S. Jiang and O. A. Sadik, Influence of Particle Size and Shapes on the Antifungal Activities of Greener Nanostructured Copper against Penicillium italicum, ACS Agric. Sci. Technol., 2022, 2, 42–56 CrossRef CAS.
- G. H. Kathwate, R. B. Shinde and S. M. Karuppayil, Antiepileptic Drugs Inhibit Growth, Dimorphism, and Biofilm Mode of Growth in Human Pathogen Candida albicans, Assay Drug Dev. Technol., 2015, 13, 307–312 CrossRef CAS PubMed.
- D. A. Sanchez, D. Schairer, C. Tuckman-Vernon, J. Chouake, A. Kutner, J. Makdisi, J. M. Friedman, J. D. Nosanchuk and A. J. Friedman, Amphotericin B releasing nanoparticle topical treatment of Candida spp. in the setting of a burn wound, Nanomedicine, 2014, 10, 269–277 CrossRef CAS PubMed.
- A. Panácek, M. Kolár, R. Vecerová, R. Prucek, J. Soukupová, V. Krystof, P. Hamal, R. Zboril and L. Kvítek, Antifungal activity of silver nanoparticles against Candida spp, Biomaterials, 2009, 30, 6333–6340 CrossRef PubMed.
- A. C. S. Oliveira, P. M. Oliveira, M. Cunha-Filho, T. Gratieri and G. M. Gelfuso, Latanoprost Loaded in Polymeric Nanocapsules for Effective Topical Treatment of Alopecia, AAPS PharmSciTech, 2020, 21, 305 CrossRef CAS PubMed.
- L. A. Balestrin, T. Kreutz, F. N. S. Fachel, J. Bidone, N. E. Gelsleichter, L. S. Koester, V. L. Bassani, E. Braganhol, C. L. Dora and H. F. Teixeira, Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential, Pharmaceutics, 2021, 13, 1241 CrossRef CAS PubMed.
- N. Ruscinc, R. A. Massarico Serafim, C. Almeida, C. Rosado and A. R. Baby, Challenging the safety and efficacy of topically applied chlorogenic acid, apigenin, kaempferol, and naringenin by HET-CAM, HPLC-TBARS-EVSC, and laser Doppler flowmetry, Front. Chem., 2024, 12, 1400881 CrossRef CAS PubMed.
- B. da Costa, B. Pippi, S. J. Berlitz, A. R. Carvalho, M. L. Teixeira, I. C. Külkamp-Guerreiro, S. F. Andrade and A. M. Fuentefria, Evaluation of activity and toxicity of combining clioquinol with ciclopirox and terbinafine in alternative models of dermatophytosis, Mycoses, 2021, 64, 727–733 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00176a |
| This journal is © The Royal Society of Chemistry 2024 |