Open Access Article
Open Access ArticleRevealing the synergistic effect of Ni single atoms and adjacent 3d metal doped Ni nanoparticles in electrocatalytic CO2 reduction†
Yingjie
Liu‡
a,
Zhaohui
Wu‡
a,
Sha
Bai
a,
Tianyang
Shen
a,
Qian
Li
a,
Guihao
Liu
a,
Xiaoliang
Sun
a,
Yihang
Hu
a,
Ziheng
Song
a,
Jinfeng
Chu
*a and
Yu-Fei
Song
 *ab
*ab
aState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: jfchu@163.com; songyf@mail.buct.edu.cn; Fax: +86 10 64431832; Tel: +86 10 64431832
bQuzhou Institute for Innovation in Resource Chemical Engineering, Quzhou 324000, Zhejiang Province, P. R. China
First published on 18th March 2024
Abstract
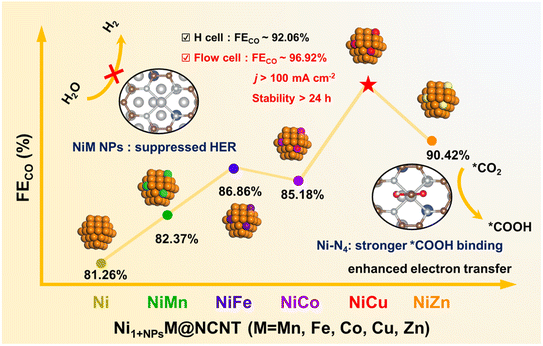
Herein, we report the successful fabrication of a series of transition metal doped Ni nanoparticles (NPs) coordinated with Ni single atoms in nitrogen-doped carbon nanotubes (denoted as Ni1+NPsM-NCNTs, M = Mn, Fe, Co, Cu and Zn; Ni1 = Ni single atom). X-ray absorption fine structure reveals the coexistence of Ni single atoms with Ni–N4 coordination and NiM NPs. When applied for electrocatalytic CO2RR, the Ni1+NPsM-NCNT compounds show the Faradaic efficiency of CO (FECO) with a volcano-like tendency of Mn < Fe ≈ Co < Zn < Cu, in which the Ni1+NPsCu-NCNT exhibits the highest FECO of 96.92%, a current density of 171.25 mA cm−2 and a sustainable stability over 24 hours at a current density of 100 mA cm−2, outperforming most reported examples in the literature. Detailed experiments and theoretical calculations reveal that for Ni1+NPsCu-NCNTs, the electron transfer from NiCu NPs to Ni single atoms strengthens the adsorption of *COOH intermediates. Moreover, the d-band center of Ni–N in Ni1+NPsCu-NCNT is upshifted, providing stronger binding with the reaction intermediates of *COOH, whereas the NiCu NPs increase the Gibbs free energy change of the Volmer step, suppressing the competitive HER.
Introduction
Due to the burning of fossil fuels and human activities, excessive emission of CO2 has increased in the past decades, which quickens the pace of global warming and the rise of sea level.1–4 Electrochemical CO2RR is an efficient method to make use of the greenhouse gas CO2 for its clean energy source and operational convenience.5–7 Among multiple products in the CO2RR, the two-electron product CO stands out as one of the most economically favorable products per unit of electrical energy input in the CO2 reduction reaction (CO2RR). It also serves as a crucial industrial feedstock in Fischer–Tropsch synthesis for producing multi-carbon chemicals. Despite its importance, the CO2RR to CO faces several challenges, including the activation of CO2 and slow hydrogenation kinetics, resulting in a high energy barrier for the overall electrocatalytic reaction. In addition, the overpotential of the CO2RR is higher than that of the competing hydrogen evolution reaction (HER), which makes the selectivity of the CO2RR hard to control.8–12 Therefore, it's of vital importance to fabricate an efficient catalyst to promote CO2RR to CO.Single atom catalysts (SACs) with high utilization of atoms have emerged as a variety of promising materials in the CO2RR and their distinct active sites are ascertained to be effective sites to produce CO.13–15 In recent years, SACs containing Mn,16 Fe,17–19 Co,20,21 Ni,22,23 Cu24 and Zn25,26 have been widely applied for the CO2RR and been demonstrated as outstanding catalysts with high selectivity. However, during the CO2RR, CO2 and H2O need to be simultaneously activated. SACs with uniform single designated active sites may not offer the optimized performance, which calls for new strategies for catalyst design.27,28 Metal nanoparticles, such as Ni and Fe, are capable of promoting H2O splitting, which can accelerate proton-feeding to generate the key intermediate *COOH.29,30 Recently, Ren et al. reported that the high conductivity of Ni NPs can modulate the electronic structure of single sites, which would further promote the adsorption of *COOH intermediates.31 However, excessive Ni NPs would promote the HER, resulting in much more H2 production and low CO selectivity. Hence, it's of great importance to promote the CO2 activation and to restrain the side HER by rational design of catalysts in order to guarantee the kinetic balance and performance of CO2RR to CO.32,33
Herein, we report the pyrolysis of the mixture of NiMAl-LDH (M = Mn, Fe, Co, Cu and Zn) and melamine, which results in the formation of the co-existence of 3d transition metal doped Ni NPs and Ni single atoms that are encapsulated by N-doped carbon nanotubes (denoted as Ni1+NPsM@NCNT, M = Mn, Fe, Co, Cu and Zn). The electron density on Ni single atoms in Ni1+NPsM@NCNT can be successfully regulated by introducing 3d transition metals in Ni NPs. The Ni1+NPsCu@NCNT exhibits the best FECO of 92.06% and a satisfying stability over 24 h. Moreover, when applied in a flow cell, the Ni1+NPsCu@NCNT achieves a current density of 200 mA cm−2 and a FECO above 90% over a wide range of 0.38 V to −1.08 V (vs. RHE). The DFT calculation proves that the NiM NPs can not only promote electron transfer to the active single Ni sites, but also upshift the 3d orbital of Ni, thus improving the intrinsic activity of Ni atoms.
Results and discussion
Synthesis and characterization
The Ni1+NPsM@NCNT compounds are synthesized according to the literature with slight modification.33 Typically, NiMAl-LDHs (M = Mn, Fe, Co, Cu, Zn) are synthesized by the coprecipitation method, whose X-ray diffraction (XRD) patterns show characteristic diffraction peaks corresponding to (00l) facets that match well with LDHs (Fig. S1†). Melamine is chosen as the source of carbon and nitrogen and then mixed with NiMAl-LDHs for the following calcination at 650 °C.31 Acid-leaching is applied to remove the acid soluble impurities of Ni1+NPsM@NCNTs. The XRD pattern (Fig. S2†) shows diffraction peaks corresponding to (002) of graphite and (111) and (200) of Ni metal, which preliminarily prove the existence of Ni NPs and that structural modification caused by M doping is negligible. As presented in Fig. S3,† Raman spectra display a D band at about 1350 cm−1 and a G band at 1600 cm−1, which can be attributed to amorphous and ordered sp2 carbon, respectively.32,34 Intensity ratios between the two bands (ID/IG) can measure the defect degree of carbon-based materials. ID/IG values of Ni1+NPsM@NCNT compounds are similar, indicating their unaffected graphitization degree. As shown in Fig. S4,† scanning electron microscopy (SEM) proves that Ni1+NPsM@NCNT compounds are composed of carbon nanotubes. In addition, transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) present NPs encapsulated in the carbon shell. As a result, the diameter of carbon nanotubes and the size of trapped NPs are measured to range from 10 nm to 20 nm (Fig. 1a, c and S5†). As shown in Fig. 1d, the lattice fringes of the NPs in Ni1+NPsCu@NCNT are calculated to be 0.207 nm, which is slightly larger than that of pure Ni NPs (0.202 nm, Fig. 1b), matching well with NiCu.35 Moreover, energy dispersive X-ray spectroscopy (EDX) elemental mapping images (Fig. 1e and S6†) reveal that C and N are uniformly distributed in the catalyst. Ni signals not only aggregate in the NPs, but also uniformly distribute throughout the carbon nanotube, demonstrating the uniform distribution of Ni single atoms. On the other hand, Cu can only be detected in the NP. In addition, the EDX line scan results in Fig. S7† reveal that both Cu and Ni can be detected in the NP but no Cu signal can be detected in NCNTs. These results preliminarily prove the basically non-existing Cu single sites compared to Ni single atoms and successfully synthesized NiCu NPs.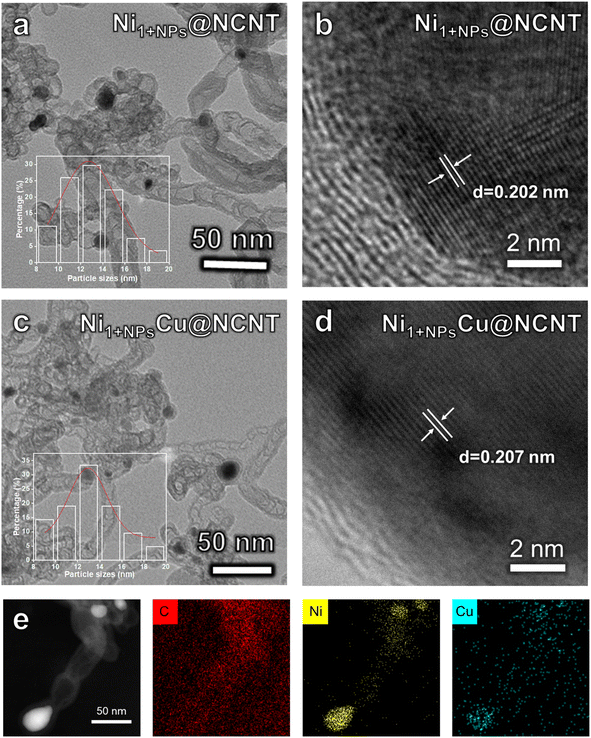 | ||
| Fig. 1 TEM and High-resolution transmission electron microscopy (HRTEM) images of (a and b) Ni1+NPs@NCNT and (c and d) Ni1+NPsCu@NCNT; (e) EDX elemental mapping images of the Ni1+NPsCu@NCNT. | ||
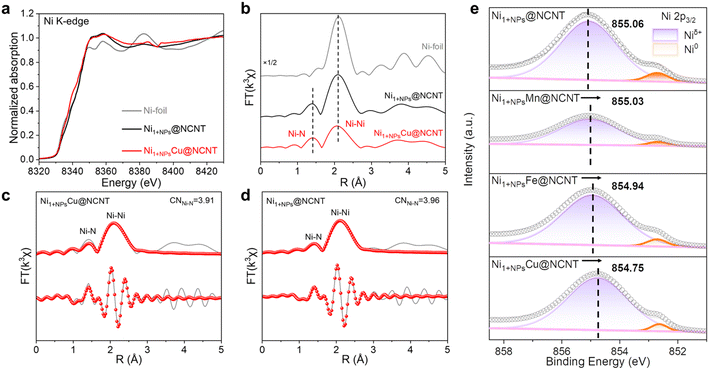
X-ray absorption fine structure (XAFS) analysis is conducted to examine the electronic structures and chemical configurations of the metal elements. Ni K-edge X-ray absorption near-edge spectroscopy (XANES) spectra are displayed in Fig. 2a; the absorption edges of Ni1+NPsCu@NCNT and Ni1+NPs@NCNT are located nearly the same as that of Ni-foil, which proves that metallic Ni is the dominating species in NCNTs. Nevertheless, the oscillations of Ni1+NPsCu@NCNT and Ni1+NPs@NCNT in the E space are different from that of Ni-foil, suggesting that the coordination environments are not exactly alike. The extended XAFS (EXAFS) were Fourier transformed (FT) k3-weighted to further detect the fine structure of the samples (Fig. 2b). For Ni1+NPsCu@NCNT and Ni1+NPs@NCNT, both coordination shells of Ni–N (1.49 Å) and Ni–Ni (2.04 Å) can be observed, Furthermore, the coordination number (CN) of Ni–N is obtained from the EXFAS fitting results and the average values in Ni1+NPsCu@NCNT and Ni1+NPs@NCNT are determined to be 3.91 and 3.96, respectively (Fig. 2c and d). The EXAFS fitting results show that the first shell can be attributed to the Ni–N4 coordination, which is in good agreement with the reported literature.22,35 The EXAFS results demonstrate the co-existence of Ni NPs and single atoms, which is in good line with the TEM results. On the other hand, in Cu K-edge XANES, the pre-edge of Ni1+NPsCu@NCNT performs basically the same as that of Cu foil (Fig. S9†), indicating that Cu mainly exist as metallic species. Such results agree well with EDX mapping results to affirm that Cu mainly exists in the NPs rather than single sites coordinated with N.
X-ray photoelectron spectroscopy (XPS) is then applied to investigate the elementary composition of Ni1+NPsM@NCNT. As shown in Fig. S11a,† the N 1s spectra of Ni1+NPs@NCNT and Ni1+NPsM@NCNT (M = Mn, Fe, Cu) are located at similar binding energy and display identical shape features, which can be deconvoluted into five species: pyridinic N (398.9 eV), Ni–N (400.3 eV), pyrrolic N (400.4 eV), graphitic N (401.6 eV) and oxidized N (403.3 eV).36 Meanwhile, the quantitative integral reveals that the contents of the N species are also similar in the four samples. In Fig. S11b† and 2e, the Ni 2p2/3 spectra can be split into two peaks assigned to Niδ+ (0< δ < 2) and metallic Ni0, respectively. Notably, the Niδ+ peak shifts as follows: Ni1+NPs@NCNT (855.06 eV) > Ni1+NPsMn@NCNT (855.03 eV) > Ni1+NPsFe@NCNT (854.94 eV) > Ni1+NPsCu@NCNT (854.75 eV). These demonstrate that electrons are transferred from NiM NPs to the Niδ+ atoms and result in electron accumulation on the Ni–N4 sites.
CO2RR performance
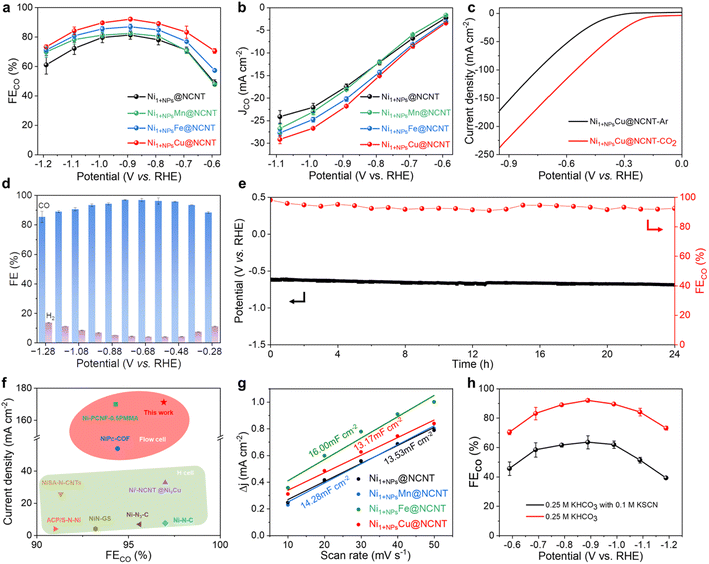
The as-prepared Ni1+NPsM@NCNT catalysts are then applied to electrochemical CO2RR to evaluate their catalytic performance, which is first operated in an H-type cell. The linear sweep voltammetry (LSV) curves taken in 0.25 M KHCO3 saturated with Ar or CO2 are compared in Fig. S12;† all six samples show great enhancement in current density when CO2 is injected into the electrolyte, indicating that the catalysts possess great capability for CO2 reduction. FECO values of all catalysts under potentials ranging from −0.59 to −1.19 V (vs. RHE) are then measured and presented in Fig. S14a† and 3a. The gas chromatography (GC) and 1H nuclear magnetic resonance (NMR) analyses are applied to detect the gaseous and liquid products, respectively (Fig. S13†). No other products except for CO and H2 can be traced. All the Ni1+NPsM@NCNT compounds show a higher FECO than that of Ni1+NPs@NCNT and display a tendency of Mn < Fe ≈ Co < Zn < Cu. Among them, the Ni1+NPsCu@NCNT outperforms others by a wide margin and reaches the highest FECO of 92.06% at −0.89 V (vs. RHE). The partial current density of CO is then calculated and shown in Fig. S14b† and 3b, where Ni1+NPsCu@NCNT surpasses Ni1+NPs@NCNT (17.5 mA cm−2) with an outstanding value of 21.8 mA cm−2 at −0.89 (vs. RHE).To meet the industrial demand for abundant CO production, the CO2RR of Ni1+NPsCu@NCNT is then tested in a flow cell equipped with gas diffusion electrodes (GDEs). The current density reaches up to 200 mA cm−2 at −0.84 V (vs. RHE) in 1 M KOH with a CO2 flow rate of 14 mL min−1 (Fig. 3e). Moreover, a high CO selectivity of Ni1+NPsCu@NCNT can also be extended to a wider potential window. As shown in Fig. 3f, a high FECO of above 90% is achieved under a wide range of applied potentials from −0.38 V to −1.08 V (vs. RHE), which reaches the maximum 96.92% at −0.78 V (vs. RHE). Such performance exhibits competitive superiority in the CO2RR when compared with other state-of-the-art catalysts with Ni single atoms (Fig. 3g). The stability of Ni1+NPsCu@NCNT is further tested to prove its practical significance for industrial application (Fig. 3h). After the galvanostatic measurement at 100 mA cm−2 for 24 h, Ni1+NPsCu@NCNT maintains a FECO of above 91% throughout the period and the applied potential only exhibits a slight increase from −0.61 V to −0.69 V (vs. RHE). XRD and TEM patterns of Ni1+NPsCu@NCNT after the reaction display consistent diffraction peaks and morphology (Fig. S16†), illustrating the sustainable durability of Ni1+NPsCu@NCNT.
To elucidate the better CO2RR performance of Ni1+NPsCu@NCNT, electrochemical surface areas (ECSA) of Ni1+NPsM@NCNT are investigated. ECSAs are obtained from the electrochemical double-layer capacitance (Cdl) by measuring cycle curves at scan speeds which increase equally (Fig. S15† and 3c). As a result, approximate values are obtained for Ni1+NPs@NCNT, Ni1+NPsMn@NCNT, Ni1+NPsFe@NCNT and Ni1+NPsCu@NCNT, indicating that the superior performance is more likely to originate from active species rather than the surface area available.37 Thiocyanate ions (SCN−) are then added into the electrolyte as an inhibitor to block the metal-N sites, thus illustrating their activity for the CO2RR.25 As expected, the FECO exhibits a significant decline after the addition of SCN− (Fig. 3d), confirming Ni–N4 sites to be active centers for the CO2RR. On the other hand, NiNPs@NCNT without Ni single atoms is in favor of the HER and the CO production is negligible, explaining that NPs play a role in *H generation for proton feeding.
DFT calculation
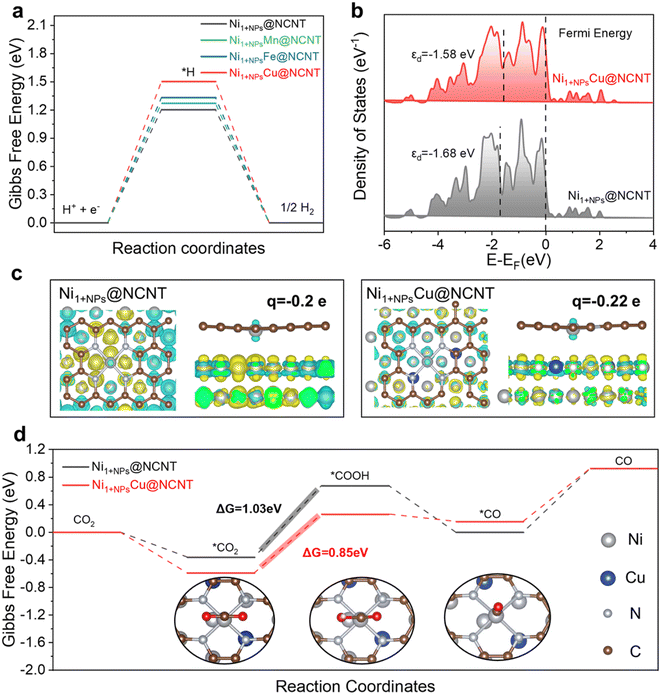
To shed light on the enhanced CO-produced CO2RR performance of Ni1+NPsCu@NCNT, the DFT calculation is further carried out. The free energy profile of the competitive HER is calculated first. All the Gibbs free energy barriers (ΔGH*) of the Volmer step (H+ + e− → H*) are increased after 3d transition metal doping, revealing the suppression of the HER process. The ΔGH* increases in the following order: Ni (1.20 eV) < NiMn (1.27 eV) < NiFe (1.33 eV) < NiCu (1.50 eV), which is in good agreement with the sequence of FEH2 decrease in Fig. 3, suggesting that ΔGH* can be an effective indicator for the inhibition of the HER, and thus the Ni1+NPsCu@NCNT with the highest H* energy barrier among these samples has a most favorable selectivity for CO.Further electronic structure analysis is employed to understand the Cu doping from an atomistic perspective. The d-band center (εd) has been identified as a descriptor of intermediate binding in electrocatalytic systems.38 The εd upshift of Ni 3d orbitals in Ni1+NPsCu@NCNT (−1.68 eV) compared with Ni1+NPs@NCNT (−1.58 eV) suggests the less occupancy of anti-bonding states, and thus stronger intermediate binding strength is promisingly obtained. The contour of electron density difference shows that the electron is transferred from Ni SAs to NPs (Fig. 4c). Then Mulliken charge analysis is used for further quantitative analysis.39,40 It is found that the Mulliken charge of the Ni single site in Ni1+NPsCu@NCNT (−0.22e) is more negative than that in Ni1+NPs@NCNT (−0.2e), demonstrating more electrons are accumulated on Ni–N4 in Ni1+NPsCu@NCNT, which is consistent with the XPS data. Electron accumulation can also enhance the binding of the reaction site to form the key intermediates. The stronger affinity of the substrate and intermediate will be helpful for the sufficient utilization of active sites and reaction kinetics. Thus, the Gibbs free energy profile of the overall CO2-to-CO reduction process is calculated to understand the function of Cu doping for the overall pathway. The Ni1+NPsCu@NCNT shows a lower ΔG for the first CO2 activation step, indicating CO2 activation. For the formation step of key intermediate COOH* (CO2* + H+ + e− → COOH*), the free energy barrier decreases to 0.85 eV after Cu doping, which is obviously less than that of pristine Ni1+NPs@NCNT (1.03 eV), suggesting a more thermodynamically favorable CO production. For the CO desorption process, the CO can be desorbed from the surface of Ni1+NPsCu@NCNT with a lower energy barrier (0.77 eV) compared with Ni1+NPs@NCNT (0.93 eV), which is in line with the optimal FECO and efficient CO production (Fig. 5).
Conclusion
In summary, we make use of one-step pyrolysis to successfully fabricate a series of Ni1+NPsM (M = Mn, Fe, Co, Cu, Zn) NPs and Ni single atoms that are encapsulated in NCNTs. The HRTEM images confirm the existence of NiM NPs with a size of 10–20 nm. EXAFS further demonstrates the fine structure of the as-prepared Ni1+NPs@NCNTs: Ni single atoms are coordinated to N atoms with a similar Ni–N4 structure. XPS proves the lower valence of Ni in Ni1+NPsM@NCNT compounds than that in Ni1+NPs@NCNT. The FECO values of the as-prepared Ni1+NPsM@NCNT compounds display a volcanic tendency where Ni1+NPsCu@NCNT reaches the highest value of 92.06% in an H-cell. When applied in a flow cell, the Ni1+NPsCu@NCNT exhibits an FECO of 96.92% and a current density of 171.25 mA cm−2 at −0.78 V (vs. RHE). During the galvanostatic test under 100 mA cm−2, a long-lasting stability of 24 h is achieved. DFT calculation reveals that the NPs can induce electron accumulation on the Ni–N sites, thus promoting the activation of CO2 and the stabilization of the *COOH intermediate. Meanwhile, the d-band center of Ni–N is also upshifted, offering stronger binding with reaction intermediates. In addition, NPs can also increase the Gibbs free energy change of the Volmer step, thus suppressing the competitive HER.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22288102, 22178019, 22208013, and 22378012) and the Fundamental Research Funds for the Central Universities (XK1802-6, XK1803-05, and XK1902). We thank the 1W1B station in Beijing Synchrotron Radiation Facility (BSRF) and BL11B station in Shanghai Synchrotron Radiation Facility (SSRF) for XAFS measurement.References
- S. Chu, Y. Cui and N. Liu, The path towards sustainable energy, Nat. Mater., 2016, 16(1), 16–22 CrossRef PubMed.
- E. S. Sanz-Perez, C. R. Murdock, S. A. Didas and C. W. Jones, Direct Capture of CO2 from Ambient Air, Chem. Rev., 2016, 116(19), 11840–11876 CrossRef CAS PubMed.
- X. Meng, J. Yang, C. Zhang, Y. Fu, K. Li, M. Sun, X. Wang, C. Dong, B. Ma and Y. Ding, Light-Driven CO2 Reduction over Prussian Blue Analogues as Heterogeneous Catalysts, ACS Catal., 2021, 12(1), 89–100 CrossRef.
- B. Li, M. Chen, Q. Hu, J. Zhu, X. Yang, Z. Li, C. Hu, Y. Li, P. Ni and Y. Ding, Facilely tunable dodecahedral polyoxometalate framework loaded with mono- or bimetallic sites for efficient photocatalytic CO2 reduction, Appl. Catal., B, 2024, 346, 123733 CrossRef CAS.
- D. T. Whipple and P. J. A. Kenis, Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction, J. Phys. Chem. Lett., 2010, 1(24), 3451–3458 CrossRef CAS.
- J. Liu, P. Li, J. Bi, S. Jia, Y. Wang, X. Kang, X. Sun, Q. Zhu and B. Han, Switching between C2+ Products and CH4 in CO2 Electrolysis by Tuning the Composition and Structure of Rare-Earth/Copper Catalysts, J. Am. Chem. Soc., 2023, 145(42), 23037–23047 CrossRef CAS PubMed.
- L. Zhang, J. Feng, L. Wu, X. Ma, X. Song, S. Jia, X. Tan, X. Jin, Q. Zhu, X. Kang, J. Ma, Q. Qian, L. Zheng, X. Sun and B. Han, Oxophilicity-Controlled CO2 Electroreduction to C2+ Alcohols over Lewis Acid Metal-Doped Cuδ+ Catalysts, J. Am. Chem. Soc., 2023, 145(40), 21945–21954 CrossRef CAS PubMed.
- O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley and E. H. Sargent, What Should We Make with CO2 and How Can We Make It?, Joule, 2018, 2(5), 825–832 CrossRef CAS.
- C. Chen, J. F. Khosrowabadi Kotyk and S. W. Sheehan, Progress toward Commercial Application of Electrochemical Carbon Dioxide Reduction, Chem, 2018, 4(11), 2571–2586 CAS.
- N. H. Han, P. Ding, L. He, Y. Li and Y. Li, Promises of Main Group Metal-Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate, Adv. Energy Mater., 2020, 10, 1902338 CrossRef CAS.
- S. Verma, B. Kim, H. R. Jhong, S. Ma and P. J. Kenis, A Gross-Margin Model for Defining Technoeconomic Benchmarks in the Electroreduction of CO2, ChemSusChem, 2016, 9(15), 1–9 CrossRef PubMed.
- Q. Lu, C. Chen, Q. Di, W. Liu, X. Sun, Y. Tuo, Y. Zhou, Y. Pan, X. Feng, L. Li, D. Chen and J. Zhang, Dual Role of Pyridinic-N Doping in Carbon-Coated Ni Nanoparticles for Highly Efficient Electrochemical CO2 Reduction to CO over a Wide Potential Range, ACS Catal., 2022, 12(2), 1364–1374 CrossRef CAS.
- B. Lu, Q. Liu and S. Chen, Electrocatalysis of Single-Atom Sites: Impacts of Atomic Coordination, ACS Catal., 2020, 10(14), 7584–7618 CrossRef CAS.
- T. N. Nguyen, M. Salehi, Q. V. Le, A. Seifitokaldani and C. T. Dinh, Fundamentals of Electrochemical CO2 Reduction on Single-Metal-Atom Catalysts, ACS Catal., 2020, 10(17), 10068–10095 CrossRef.
- Y. Wang, H. Su, Y. He, L. Li, S. Zhu, H. Shen, P. Xie, X. Fu, G. Zhou, C. Feng, D. Zhao, F. Xiao, X. Zhu, Y. Zeng, M. Shao, S. Chen, G. Wu, J. Zeng and C. Wang, Advanced Electrocatalysts with Single-Metal-Atom Active Sites, Chem. Rev., 2020, 120(21), 12217–12314 CrossRef CAS PubMed.
- B. Zhang, J. Zhang, J. Shi, D. Tan, L. Liu, F. Zhang, C. Lu, Z. Su, X. Tan, X. Cheng, B. Han, L. Zheng and J. Zhang, Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction, Nat. Commun., 2019, 10(1), 2980 CrossRef PubMed.
- J. Gu, C.-S. Hsu, L. Bai, H. M. Chen and X. Hu, Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO, Science, 2019, 364, 1091–1094 CrossRef CAS PubMed.
- K. Li, S. Zhang, X. Zhang, S. Liu, H. Jiang, T. Jiang, C. Shen, Y. Yu and W. Chen, Atomic Tuning of Single-Atom Fe-N-C Catalysts with Phosphorus for Robust Electrochemical CO2 Reduction, Nano Lett., 2022, 22(4), 1557–1565 CrossRef CAS PubMed.
- X. Sun, Y. Tuo, C. Ye, C. Chen, Q. Lu, G. Li, P. Jiang, S. Chen, P. Zhu, M. Ma, J. Zhang, J. H. Bitter, D. Wang and Y. Li, Phosphorus Induced Electron Localization of Single Iron Sites for Boosted CO2 Electroreduction Reaction, Angew. Chem., Int. Ed., 2021, 60(44), 23614–23618 CrossRef CAS PubMed.
- Y. Pan, R. Lin, Y. Chen, S. Liu, W. Zhu, X. Cao, W. Chen, K. Wu, W. C. Cheong, Y. Wang, L. Zheng, J. Luo, Y. Lin, Y. Liu, C. Liu, J. Li, Q. Lu, X. Chen, D. Wang, Q. Peng, C. Chen and Y. Li, Design of Single-Atom Co-N5 Catalytic Site: A Robust Electrocatalyst for CO2 Reduction with Nearly 100% CO Selectivity and Remarkable Stability, J. Am. Chem. Soc., 2018, 140(12), 4218–4221 CrossRef CAS PubMed.
- X. Wang, Z. Chen, X. Zhao, T. Yao, W. Chen, R. You, C. Zhao, G. Wu, J. Wang, W. Huang, J. Yang, X. Hong, S. Wei, Y. Wu and Y. Li, Regulation of Coordination Number over Single Co Sites: Triggering the Efficient Electroreduction of CO2, Angew. Chem., Int. Ed., 2018, 57(7), 1944–1948 CrossRef CAS PubMed.
- L. Jiao, W. Yang, G. Wan, R. Zhang, X. Zheng, H. Zhou, S. H. Yu and H. L. Jiang, Single-Atom Electrocatalysts from Multivariate Metal-Organic Frameworks for Highly Selective Reduction of CO2 at Low Pressures, Angew. Chem., Int. Ed., 2020, 59(46), 20589–20595 CrossRef CAS PubMed.
- X. Rong, H. J. Wang, X. L. Lu, R. Si and T. B. Lu, Controlled Synthesis of a Vacancy-Defect Single-Atom Catalyst for Boosting CO2 Electroreduction, Angew. Chem., Int. Ed., 2020, 59(5), 1961–1965 CrossRef CAS PubMed.
- A. Guan, Z. Chen, Y. Quan, C. Peng, Z. Wang, T.-K. Sham, C. Yang, Y. Ji, L. Qian, X. Xu and G. Zheng, Boosting CO2 Electroreduction to CH4via Tuning Neighboring Single-Copper Sites, ACS Energy Lett., 2020, 5(4), 1044–1053 CrossRef CAS.
- J. Chen, Z. Li, X. Wang, X. Sang, S. Zheng, S. Liu, B. Yang, Q. Zhang, L. Lei, L. Dai and Y. Hou, Promoting CO2 Electroreduction Kinetics on Atomically Dispersed Monovalent Zn(I) Sites by Rationally Engineering Proton-Feeding Centers, Angew. Chem., Int. Ed., 2022, 61(7), e202111683 CrossRef CAS PubMed.
- F. Yang, P. Song, X. Liu, B. Mei, W. Xing, Z. Jiang, L. Gu and W. Xu, Highly Efficient CO2 Electroreduction on ZnN4-based Single-Atom Catalyst, Angew. Chem., Int. Ed., 2018, 57(38), 12303–12307 CrossRef CAS PubMed.
- X. Li, W. Bi, M. Chen, Y. Sun, H. Ju, W. Yan, J. Zhu, X. Wu, W. Chu, C. Wu and Y. Xie, Exclusive Ni-N4 Sites Realize Near-Unity CO Selectivity for Electrochemical CO2 Reduction, J. Am. Chem. Soc., 2017, 139(42), 14889–14892 CrossRef CAS PubMed.
- A. Pedersen, J. Barrio, A. Li, R. Jervis, D. J. L. Brett, M. M. Titirici and I. E. L. Stephens, Dual-Metal Atom Electrocatalysts: Theory, Synthesis, Characterization, and Applications, Adv. Energy Mater., 2021, 12(3), 2102715 CrossRef.
- T. N. Huan, N. Ranjbar, G. Rousse, M. Sougrati, A. Zitolo, V. Mougel, F. Jaouen and M. Fontecave, Electrochemical Reduction of CO2 Catalyzed by Fe-N-C Materials: A Structure-Selectivity Study, ACS Catal., 2017, 7(3), 1520–1525 CrossRef CAS.
- Y. Cheng, S. Zhao, B. Johannessen, J. P. Veder, M. Saunders, M. R. Rowles, M. Cheng, C. Liu, M. F. Chisholm, R. De Marco, H. M. Cheng, S. Z. Yang and S. P. Jiang, Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction, Adv. Mater., 2018, 30(13), 1706287 CrossRef PubMed.
- W. Ren, X. Tan, C. Jia, A. Krammer, Q. Sun, J. Qu, S. Smith, A. Schueler, X. Hu and C. Zhao, Electronic Regulation of Ni Single Atom by Confined Ni Nanoparticles for Energy-Efficient CO2 Electroreduction, Angew. Chem., Int. Ed., 2022, e202203335 CAS.
- W. Liu, P. Bai, S. Wei, C. Yang and L. Xu, Gadolinium Changes the Local Electron Densities of Nickel 3d Orbitals for Efficient Electrocatalytic CO2 Reduction, Angew. Chem., Int. Ed., 2022, e202201166 CAS.
- T. Zhang, X. Han, H. Yang, A. Han, E. Hu, Y. Li, X. Q. Yang, L. Wang, J. Liu and B. Liu, Atomically Dispersed Nickel(I) on an Alloy-Encapsulated Nitrogen-Doped Carbon Nanotube Array for High-Performance Electrochemical CO2 Reduction Reaction, Angew. Chem., Int. Ed., 2020, 59(29), 12055–12061 CrossRef CAS PubMed.
- S. Liang, Q. Jiang, Q. Wang and Y. Liu, Revealing the Real Role of Nickel Decorated Nitrogen-Doped Carbon Catalysts for Electrochemical Reduction of CO2 to CO, Adv. Energy Mater., 2021, 11(36), 2101477 CrossRef CAS.
- S. Bai, L. Tan, C. Ning, G. Liu, Z. Wu, T. Shen, L. Zheng and Y. F. Song, Revealing the Kinetic Balance between Proton-Feeding and Hydrogenation in CO2 Electroreduction, Small, 2023, e2300581 CrossRef PubMed.
- S. Li, M. Ceccato, X. Lu, S. Frank, N. Lock, A. Roldan, X.-M. Hu, T. Skrydstrup and K. Daasbjerg, Incorporation of nickel single atoms into carbon paper as self-standing electrocatalyst for CO2 reduction, J. Mater. Chem. A, 2021, 9(3), 1583–1592 RSC.
- Q. Zhang, P. Kumar, X. Zhu, R. Daiyan, N. M. Bedford, K. H. Wu, Z. Han, T. Zhang, R. Amal and X. Lu, Electronically Modified Atomic Sites Within a Multicomponent Co/Cu Composite for Efficient Oxygen Electroreduction, Adv. Energy Mater., 2021, 11(17), 2100303 CrossRef CAS.
- L. Zhang, J. Feng, S. Liu, X. Tan, L. Wu, S. Jia, L. Xu, X. Ma, X. Song, J. Ma, X. Sun and B. Han, Atomically Dispersed Ni-Cu Catalysts for pH-Universal CO2 Electroreduction, Adv. Mater., 2023, e2209590 CrossRef PubMed.
- X. Chen, X. T. Wang, J. B. Le, S. M. Li, X. Wang, Y. J. Zhang, P. Radjenovic, Y. Zhao, Y. H. Wang, X. M. Lin, J. C. Dong and J. F. Li, Revealing the role of interfacial water and key intermediates at ruthenium surfaces in the alkaline hydrogen evolution reaction, Nat. Commun., 2023, 14(1), 5289 CrossRef CAS PubMed.
- X. Liao, R. Lu, L. Xia, Q. Liu, H. Wang, K. Zhao, Z. Wang and Y. Zhao, Density Functional Theory for Electrocatalysis, Energy Environ. Mater., 2021, 5(1), 157–185 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00167b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |