DOI:
10.1039/D4NA00160E
(Paper)
Nanoscale Adv., 2024,
6, 3961-3977
NiII-containing L-glutamic acid cross-linked chitosan anchored on Fe3O4/f-MWCNT: a sustainable catalyst for the green reduction and one-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes†
Received
26th February 2024
, Accepted 28th May 2024
First published on 25th June 2024
Abstract
In this research, new and eye-catching catalytic applications of the nickelII (NiII) nanoparticles (NPs)-containing L-glutamic acid cross-linked chitosan anchored on magnetic carboxylic acid-functionalized multi-walled carbon nanotube (Fe3O4/f-MWCNT-CS-Glu/NiII) system, which was characterized by Fourier transform infrared (FT-IR), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), SEM-based energy-dispersive X-ray (EDX) and elemental mapping, inductively coupled plasma-optical emission spectrometry (ICP-OES), thermogravimetric analysis (TGA), differential thermal analysis (DTA), and vibrating sample magnetometry (VSM), have been introduced for the environmentally benign and efficient reduction and one-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes in water at 60 °C under an air atmosphere. It is worth noting that the NiII-containing hybrid nanocatalyst, in the mentioned organic reactions, showed short reaction times, high yields of the desired products, acceptable turnover numbers (TONs) and turnover frequencies (TOFs), and also satisfactory magnetic recycling and reusability performance even after ten times of reuse. As another significant point, all the titled organic transformations have been carried out in water as an entirely favorable and green solvent for chemical reactions.
1. Introduction
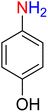
The reduction (or hydrogenation) and conversion of aromatic nitro compounds, some of which are very hazardous1 due to their carcinogenicity, non-biodegradability, and high toxicity, are used in a wide range of industrial and academic applications, such as environmental and water remediation, fuels, petroleum refining, explosives, batteries, dyes and pigments, rubber, photographic chemicals, agrochemicals, and also medicinal chemistry.2 It is worth noting that aryl amines and N-aryl acetamides, which are among the most straightforward and practical compounds resulting from the chemical transformation of nitroarenes, are abundant in pharmaceutical and biological structures (Fig. 1). For example, as shown in Fig. 1, the mentioned structures existed in the backbone of amprenavir (human immunodeficiency virus 1 (HIV-1) protease inhibitor), bromfenac (non-steroidal anti-inflammatory drug (NSAID)), sparfloxacin (antibiotic), mocetinostat (histone deacetylase 1 (HDAC-1) inhibitor), nomifensine (norepinephrine-dopamine reuptake (NDR) inhibitor), garsorasib (KRAS(G12C) inhibitor), acetaminophen (non-opioid analgesic and antipyretic), and trametinib (mitogen-activated protein kinase kinases 1 and 2 (MEK-1 and MEK-2) inhibitor). In 2023, a boronate-based oxidant-responsive derivative of acetaminophen with IUPAC name (4-((4-acetamidophenoxy)methyl)phenyl)-boronic acid (Fig. 1, compound I) was reported as a proinhibitor of myeloperoxidase (MPO).3 On the other hand, 4-acetamidophenyl(S)-2-(4-isobutylphenyl)propanoate (Fig. 1, compound II) was reported as a potential anti-nociceptive compound.4 In another research paper, which was published in 2023, a new paracetamol-containing scaffold with IUPAC name N-(4-((5-(((1-(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-1,3,4-oxadiazol-2-yl)methoxy)phenyl)acetamide (Fig. 1, compound III) was reported as a cyclooxygenase-2 (COX-2) inhibitor.5
 |
| | Fig. 1 Representative examples of drugs and drug-like compounds bearing aryl amine and N-aryl acetamide. | |
In the past two or three decades, heterogeneous catalytic approaches have been picked as the logical synthetic methods for converting nitroarenes to beneficial compounds such as aryl amines and N-aryl acetamides. To this purpose, the fabrication of environmentally benign and capable catalytic systems has a remarkable impact on the mentioned organic transformations from the green chemistry point of view. It should be noted that the green chemistry principles have greatly influenced the design of chemical reactions in recent years.6 Based on green chemistry protocols, the design of magnetically recoverable nanocatalysts as brand-new heterogeneous catalytic systems is a suitable alternative for organic synthesis. In this regard, preparing and (or) designing an applicable and stable platform (and or supporter) for the catalyst scaffold construction are crucial. Chitosan (CS), as a pseudo-natural polysaccharide that is typically obtained from the alkaline N-deacetylation of chitin, is an outstanding biomacromolecule for developing new types of environmentally benign catalytic platforms and systems due to its unique, attractive, and natural properties such as biodegradability, biocompatibility, renewability, non-toxicity, affordability, simple recyclability, stability to air and moisture, thermal and chemical stability, high surface area, and insolubility in most of the organic solvents as well as aqueous reaction mediums (except in acidic aqueous solutions), and many others.7 Furthermore, the presence of diverse functional groups in the backbone of chitosan, including hydroxyl, amino, acetamido, and ether, along with chiral centers, makes chitosan an exceptional framework that can play many roles in catalysis science, especially the role of an excellent chelating and or coordinator agent for different metals, ions, and nanoparticles.7 On the other hand, carbon nanomaterials are a prevalent choice as the best platform for developing catalytic systems since they can be manufactured in various physical structures and shapes with vital properties such as mechanical strength, pore dispersion, and thermal and chemical stability.8 One of the well-known nanocarbons in science, especially in catalysis, is multi-walled carbon nanotubes (MWCNTs).9
Solvent is another significant factor from the green chemistry point of view. To this end, a list based on greenness has been introduced for known solvents in chemical reactions, and interestingly, ranked first in the mentioned list is water, and from most aspects, it is an ideal solvent for organic synthesis.10
In continuation of our research program on catalytic organic transformations,11,12 and likewise, due to the importance of introducing new environmentally benign protocols to the conversion of nitroarenes to valuable organic compounds, herein we wish to report green and efficient strategies for the reduction and one-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes using the Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite as a powerful magnetically recoverable nanocatalytic system (Fig. 2).
 |
| | Fig. 2 Catalytic applications of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite. | |
2. Results and discussion
2.1. Preparation of the hybrid Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite
We started our work with the preparation of the hybrid Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite system according to our previous published paper in RSC Advances (Fig. 3).11 Briefly, in step one, we used a sequential one-pot five-component strategy for the fabrication of L-glutamic acid cross-linked chitosan supported on magnetic carboxylic acid-functionalized multi-walled carbon nanotube (Fe3O4/f-MWCNT-CS-Glu) (Fig. 3). After that, in the second step, by using nickelII nitrate hexahydrate (Ni(NO3)2·6H2O) in the H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CH2OH (1
CH3CH2OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture under ultrasonic conditions, we immobilized the nickelII (NiII) nanoparticles into the matrix of the prepared Fe3O4/f-MWCNT-CS-Glu nanocomposite to achieve the desired Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite (Fig. 3). It should be noted that the structure of the mentioned nanocomposite was previously fully characterized and examined by our group using Fourier transform infrared (FT-IR) (Fig. 4), powder X-ray diffraction (PXRD) (Fig. 5), scanning electron microscopy (SEM) (Fig. 6), transmission electron microscopy (TEM) (Fig. 7), SEM-based energy-dispersive X-ray spectroscopy (EDX) (Fig. 8) and elemental mapping (Fig. 9), inductively coupled plasma-optical emission spectrometry (ICP-OES), thermogravimetric analysis (TGA) (Fig. 10), differential thermal analysis (DTA) (Fig. 11), and vibrating sample magnetometry (VSM) (Fig. 12) analyses.11
2) mixture under ultrasonic conditions, we immobilized the nickelII (NiII) nanoparticles into the matrix of the prepared Fe3O4/f-MWCNT-CS-Glu nanocomposite to achieve the desired Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite (Fig. 3). It should be noted that the structure of the mentioned nanocomposite was previously fully characterized and examined by our group using Fourier transform infrared (FT-IR) (Fig. 4), powder X-ray diffraction (PXRD) (Fig. 5), scanning electron microscopy (SEM) (Fig. 6), transmission electron microscopy (TEM) (Fig. 7), SEM-based energy-dispersive X-ray spectroscopy (EDX) (Fig. 8) and elemental mapping (Fig. 9), inductively coupled plasma-optical emission spectrometry (ICP-OES), thermogravimetric analysis (TGA) (Fig. 10), differential thermal analysis (DTA) (Fig. 11), and vibrating sample magnetometry (VSM) (Fig. 12) analyses.11
 |
| | Fig. 3 Preparation pathway of the hybrid Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 4 FT-IR spectra of the Fe3O4, Fe3O4/f-MWCNT, Fe3O4/f-MWCNT-CS, Fe3O4/f-MWCNT-CS-Glu, and Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposites.11 | |
 |
| | Fig. 5 PXRD patterns of the Fe3O4, Fe3O4/f-MWCNT and Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposites.11 | |
 |
| | Fig. 6 SEM images of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 7 TEM images of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 8 SEM-based EDX diagram of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 9 SEM-based elemental mapping of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 10 TGA diagram of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 11 DTA diagram of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite.11 | |
 |
| | Fig. 12 VSM curves of the Fe3O4, Fe3O4/f-MWCNT, and Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposites.11 | |
2.2. Reduction of nitroarenes to the corresponding aryl amines
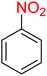
It is worth noting that the reduction (or hydrogenation) of nitroarenes is presently considered a benchmark reaction to test metal (or metal oxide)-containing nanocatalytic systems. In this regard, and after preparation of the hybrid Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite, we started our work with reduction of nitrobenzene (PhNO2) to aniline (PhNH2) using 2 mmol of sodium borohydride (NaBH4) as a mild reducing agent in water at 60 °C in the presence of 5, 7, and 10 mg of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocatalyst (Table 1, entries 1–3), and it was found that increasing the amount of the nanocatalyst has no superior effect on the reaction and in all cases the reaction was completed in 4 minutes. Notably, at room temperature, the mentioned reaction has a conversion rate of 50%, even after 60 minutes (Table 1, entry 4). Also, we screened the effect of various organic solvents, including CH3OH, CH3CH2OH, CH3CN, n-hexane, and CH2Cl2, on the stated model reduction reaction in the presence of 5 mg of the mentioned NiII-containing nanocatalyst at 60 °C (Table 1, entries 5–9), and the results were utterly unsatisfactory. After the suitable reaction conditions have been established (Table 1, entry 1), the general efficiency of this protocol is delineated for the reduction of various nitroarenes (Table 2), and interestingly, all the reactions were completed rapidly with high yields. On the other hand, in these reactions, the turnover numbers (TONs) and turnover frequencies (TOFs) of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocatalyst were calculated and are listed in Table 2. Remarkably, the results of TONs and TOFs were acceptable and satisfactory.
Table 1 Optimization experiments for the reduction of PhNO2 to PhNH2 with NaBH4 catalyzed by the hybrid Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite
|

|
| Entry |
Catalyst (mg) |
Solvent |
Temperature conditions |
Time (min) |
Conversion (%) |
| 1 |
5 |
H2O |
60 °C |
4 |
100 |
| 2 |
7 |
H2O |
60 °C |
4 |
100 |
| 3 |
10 |
H2O |
60 °C |
4 |
100 |
| 4 |
5 |
H2O |
Room temperature |
60 |
50 |
| 5 |
5 |
CH3OH |
Reflux |
120 |
35 |
| 6 |
5 |
CH3CH2OH |
Reflux |
120 |
35 |
| 7 |
5 |
CH3CN |
Reflux |
120 |
20 |
| 8 |
5 |
n-Hexane |
Reflux |
120 |
10 |
| 9 |
5 |
CH2Cl2 |
Reflux |
120 |
0 |
Table 2 Reduction of nitroarenes to corresponding aryl amines using NaBH4 catalyzed by the Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite in watera
2.3. One-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes to the corresponding aryl acetamides
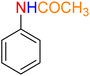
As a matter of fact, the amide bond is one of the most common and precious functional elements that is abundantly present in the structure of drugs, agrochemicals, peptides, proteins, alkaloids, and many others.13 In this context, introducing efficient, straightforward, and simple synthetic strategies for the construction of amide bonds is important. Also, acetylation of amines, especially aryl amines, is an effortless approach to the construction of an amide bond, and is extensively used in chemistry labs and the chemical industry.14 It is imperative to bear in mind that nitroarenes are much cheaper than aryl amines in terms of price, and not only are one of the most important substrates for the preparation of aryl amines through a reduction process, but they are also toxic, and their transformation into other useful substances is valuable. Consequently, designing new protocols for the synthesis of aryl amides via straightforward one-pot reductive amidation (especially acetylation) of nitroarenes without isolation of the aryl amine intermediate is interesting. Furthermore, from the green chemistry point of view, one-pot reactions are recognized as a powerful tool in modern synthetic organic chemistry, which leads to a clear and tangible decrease in the consumption of reagents, auxiliaries, catalysts and or promoters, and solvents and consequently causes minimization of waste, energy, and time.15 In this regard, and after obtaining the successful strategy for the reduction of nitroarenes, we decided to introduce a new one-pot two-step reductive Schotten–Baumann-type acetylation approach for the efficient and green synthesis of N-aryl acetamides from aromatic nitro compounds. To this purpose, in the second step of the mentioned one-pot organic transformation (viz. Schotten–Baumann-type acetylation), we used 1 mmol of acetic anhydride (Ac2O) as an acetylating agent under the same temperature conditions (60 °C). As shown in Table 3, we successfully prepared diverse N-aryl acetamide derivatives, and it was found that the acetylation step is faster than the reduction step. Notably, according to the amount of catalyst used, our presented one-pot protocol has acceptable reaction times, yields, and TON and TOF values for the mentioned two-step organic transformation (Table 3). Furthermore, a plausible and concise mechanism for the current one-pot two-step reductive Schotten–Baumann-type acetylation reaction in the presence of the as-prepared NiII-containing hybrid nanocatalyst is depicted in Scheme 1.
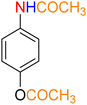
Table 3 One-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes catalyzed by the Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite in watera
 |
| | Scheme 1 Plausible and concise mechanism for the one-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes catalyzed by the Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite. | |
2.4. Recoverability, reusability, and hot filtration test experiments of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite
In the last stage of the present work, the recyclability and reusability of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite and its hot filtration test have been evaluated. In this regard, it seems necessary to point out two points. First, in some cases, chitosan-based catalytic systems suffer from mechanical and thermal stability that modification of chitosan (CS) through an additional process (such as a cross-linking process) not only improves but also can lead to the decrease of metal leaching.16 The thermal stability of the mentioned hybrid catalytic system not only is unique for the current organic transformations, which were carried out at 60 °C, but also is excellent even for most chemical reactions because, as shown in the TGA diagram, the structure of this nanocomposite is completely stable up to 225 °C, and just a 1% weight loss was observed below 240 °C, which may be related to the solvent and moisture evaporations (Fig. 10). Second, the VSM analysis demonstrated that the saturation magnetization (Ms) amount of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocatalyst was 19.743 emu g−1 which is suitable for a high magnetic recycling and reusability performance (Fig. 12). The recyclability and reusability experiments of the as-prepared superparamagnetic NiII-containing nanocatalyst, which were carried out on the reduction of PhNO2 (1 mmol) to PhNH2 using NaBH4 (2 mmol) in the presence of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII (5 mg) nanocatalyst in water at 60 °C as a model reaction, revealed satisfactory results even after ten runs (Fig. 13, section a). On the other hand, the hot filtration test was also performed for the further investigation upon leaching of the NiII NPs in the aforementioned model reaction, and it was found that separation of the whole of the Fe3O4/f-MWCNT-CS-Glu/NiII nanocatalytic system from the mentioned reaction environment (when the conversion rate was 50%) caused no substantial improvement in the reaction process, which clearly confirmed satisfactory stability of the NiII NPs in the Fe3O4/f-MWCNT-CS-Glu matrix (Fig. 13, section b). Moreover, the PXRD pattern (Fig. 13, section c) and a TEM image (Fig. 13, section d) of the recovered Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite after the sixth recycling step have been demonstrated, which shows that the structure of the mentioned NiII-containing nanocomposite remained intact. Furthermore, the leaching test results obtained by ICP-OES measurements revealed that the amount of nickel (Ni) decreased from 12.3 w% to 11.2 w% after the sixth run.
 |
| | Fig. 13 Recoverability and reusability experiments (a) and hot filtration test (b) of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocatalyst, along with the PXRD pattern (c) and TEM image (d) of the recovered Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite. | |
2.5. A comparative study
To demonstrate the efficiency and capability of our new green synthetic protocols on the reduction and one-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes in the presence of the as-prepared hybrid Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite as an influential catalytic system in water, they have been compared with some of the previously reported procedures. As shown in Table 4, the obtained results clearly demonstrated that the current green synthetic strategies have a suitable place in terms of efficiency and greenness compared to the previously published protocols.
Table 4 Comparison of the catalytic activity of the as-prepared Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite with literature samples reported on reduction and one-pot reductive acetylation of nitrobenzene
| Part B |

|
| Entry |
Reaction conditions |
Time |
Yield |
Ref. |
| B1 |
Fe3O4/f-MWCNT-CS-Glu/NiII (5 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; 60 °C |
5 min |
98% |
|
| B2 |
Fe3O4@SiO2@KCC-1@MPTMS@CuII (10 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; 60 °C |
7 min |
95% |
12b
|
| B3 |
(7 wt%) Pd/C (30 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; reflux |
8 min |
88% |
12c
|
| B4 |
Cu(Hdmg)2 (10 mol%); NaBH4 (3 mmol); EtOAc; 60 °C |
170 min |
97% |
12f
|
| B5 |
CuFe2O4 (48 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; reflux |
11 min |
97% |
12i
|
| B6 |
Ni2B@Cu2O (54 mg); NaBH4 (2.5 mmol); Ac2O (1 mmol); wet-solvent-free grinding; 40 °C |
2 min |
97% |
12k
|
| B7 |
Ni2B@CuCl2 (52 mg); NaBH4 (2.5 mmol); Ac2O (1 mmol); wet-solvent-free grinding; 40 °C |
3 min |
97% |
12k
|
| B8 |
[C4(DABCO)2] NiCl4 (80 mg); NaBH4 (3.5 mmol); H2O; Ac2O (1 mmol); 70 °C |
2 min |
98% |
24
|
| B9 |
rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) (20 mg); NH2NH2·H2O (2 mmol); Ac2O (2 mmol); CH3CH2OH; reflux |
15 min |
97% |
28
|
| B9 |
CuFe2O4@SiO2@PTMS@Tu@NiII (20 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; 65 °C |
7 min |
94% |
31
|
| B10 |
(2 wt%) Pd/(5 wt%) Sn–Al2O3 (50 mg); H2 atmosphere; Ac2O (1 mmol); H2O; r.t. |
3 h |
98% |
35
|
3. Conclusions
In this paper, we successfully demonstrated that entrapping NiII nanoparticles within a matrix of L-glutamic acid cross-linked chitosan supported on magnetic carboxylic acid-functionalized multi-walled carbon nanotube causes a unique hybrid earth-abundant transition metal-containing nanocomposite (Fe3O4/f-MWCNT-CS-Glu/NiII) as an effective and sustainable nanocatalytic system for the environmentally benign and efficient reduction and one-pot two-step reductive Schotten–Baumann-type acetylation of nitroarenes in water at 60 °C under an air atmosphere. The salient features of the presented synthetic protocols are short reaction times, high yields of the desired products, acceptable TONs and TOFs, and satisfactory recyclability and reusability of the catalyst. Notably, research to find and develop new and green nanocatalytic systems containing various earth-abundant transition metals to achieve highly efficient protocols for the conversion of hazardous aromatic nitro compounds to corresponding aryl amines, aryl acetamides, and or other valuable organic compounds is currently underway in our research group.
Data availability
Data will be made available on request.
Author contributions
Hossein Mousavi: conceptualization; methodology; software; validation; formal analysis; investigation; resources; data curation; writing – original draft; writing – review & editing; visualization; supervision; project administration. Behzad Zeynizadeh: conceptualization; methodology; validation; resources; data curation; supervision; project administration; funding acquisition. Morteza Hasanpour Galehban: conceptualization; methodology; validation; formal analysis; investigation; resources; data curation.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully appreciate the financial support of this work by the Research Council of Urmia University.
References
-
(a) D.-S. Zhang, Y. Liu, X. Ren, F. Geng, Y.-Z. Zhang, Y. Baikeli, M. Yang, Z. Liu, Y. Wang, X. Zhang and L. Geng, Colloids Interface Sci. Commun., 2023, 56, 100730 CrossRef CAS;
(b) M. Shahid, Z. H. Farooqi, R. Begum, M. Arif, W. Wu and A. Irfan, Crit. Rev. Anal. Chem., 2020, 50, 513–537 CrossRef CAS PubMed;
(c) P. Kovacic and R. Somanathan, J. Appl. Toxicol., 2014, 34, 810–824 CrossRef CAS PubMed;
(d) S. Sun, Z. Zhang, S. Li, J. Le, H. Qian, X. Yin, Y. Liu, W. Yang and Y. Chen, J. Mater. Sci., 2023, 58, 5587–5598 CrossRef CAS;
(e) W. Zhao, T. Wang, B. Wang, R. Wang, Y. Xia, M. Liu and L. Tian, Colloids Surf. A: Physicochem. Eng. Asp., 2023, 658, 130677 CrossRef CAS;
(f) L. Zhang, X. Deng, M. Hu, Y. Zhu, Z. Zhu, J. Wang, Z. Yao and H. Zhang, Colloids Surf. A: Physicochem. Eng. Asp., 2023, 672, 131735 CrossRef CAS;
(g) T. Huang, G. Sun, L. Zhao, N. Zhang, R. Zhong and Y. Peng, Int. J. Mol. Sci., 2021, 22, 8557 CrossRef CAS PubMed;
(h) S. Kazemi Movahed, P. Jafari and S. Mallakpour, J. Environ. Eng., 2023, 11, 110426 Search PubMed;
(i) S. Aghajani, M. Mohammadikish and M. Khalaji-Verjani, Langmuir, 2023, 39, 8484–8493 CrossRef CAS PubMed;
(j) S. Nooriyan, A. Bezaatpour, A. Nuri, M. Amiri and S. Nouhi, Catal. Commun., 2023, 178, 106671 CrossRef CAS;
(k) S. Zhang, Q. Liu, L. Zhong, J. Jiang, X. Luo, X. Hu, Q. Liu and Y. Lu, J. Environ. Sci., 2024, 138, 458–469 CrossRef CAS PubMed;
(l) N. Y. Baran, M. Çalişkan, A. Özpala and T. Baran, Int. J. Biol. Macromol., 2024, 262, 130134 CrossRef CAS PubMed;
(m) C. C. Naik, D. P. Kamat and S. K. Gaonkar, Int. J. Biol. Macromol., 2024, 286, 131752 CrossRef PubMed;
(n) A. Khalil, A. Khan, T. Kamal, A. A. P. Khan, S. B. Khan, M. T. S. Chani, K. A. Alzahrani and N. Ali, Int. J. Biol. Macromol., 2024, 262, 129986 CrossRef CAS PubMed;
(o) N. Y. Baran, J. Organomet. Chem., 2024, 1008, 123047 CrossRef.
-
(a) M. C. Manjula, S. Manjunatha, K. L. Nagashree, M. Shivanna, M. P. Rao, N. Nanda and P. Ramachandra, ChemistrySelect, 2023, 8, e202300936 CrossRef CAS;
(b) S. Sghajani and M. Mohammadikish, Langmuir, 2022, 38, 8686–8695 CrossRef PubMed;
(c) J. Song, Z.-F. Huang, L. Pan, K. Li, X. Zhang, L. Wang and J.-J. Zou, Appl. Catal., B, 2018, 227, 386–408 CrossRef CAS;
(d) S. Kumar and S. K. Maurya, J. Org. Chem., 2023, 88, 8690–8702 CrossRef CAS PubMed;
(e) X. Li, J. An, Z. Gao, C. Xu, Y. Cheng, S. Li, L. Li and B. Tang, Chem. Sci., 2023, 14, 3554–3561 RSC;
(f) S. Karimi, M. Gholinejad, R. Khezri, J. M. Sansano, C. Nájera and M. Yus, RSC Adv., 2023, 13, 8101–8113 RSC;
(g) H. Jiang, G. Yuan, Z. Cui, Z. Zhao, Z. Dong, J. Zhang, Y. Cong and X. Li, Ind. Eng. Chem. Res., 2023, 62, 13355–13367 CrossRef CAS;
(h) H. Yu, J. Liu, Q. Wan, G. Zhao, E. Gao, J. Wang, B. Xu, G. Zhao and X. Fan, Mol. Catal., 2023, 540, 113045 CrossRef CAS;
(i) Z. Ma, J. Chen, M. Chen, L. Dong, W. Mao, Y. Long and J. Ma, Mol. Catal., 2023, 547, 113372 CrossRef CAS;
(j) S. Taheri, M. M. Heravi and A. Saljooqi, Sci. Rep., 2023, 13, 17566 CrossRef CAS PubMed;
(k) Y. Wei, S. Wang, Y. Zhang, M. Li, J. Hu, Y. Liu, J. Li, L. Yu, R. Huang and D. Deng, ACS Catal., 2023, 13, 15824–15832 CrossRef CAS;
(l) Y. Wang, X.-Y. Ye and G.-Z. Han, Colloids Surf. A: Physicochem. Eng. Asp., 2024, 682, 132869 CrossRef CAS;
(m) J. Wu, W. Lang, H. Li, K. Du, J. Deng, S. Zhao, W. Zhang, Z. Peng and Z. Liu, ACS Sustainable Chem. Eng., 2023, 11, 14960–14968 CrossRef CAS;
(n) J. Ma, X. Mao, C. Hu, X. Wang, W. Gong, D. Liu, R. Long, A. Du, H. Zhao and Y. Xiong, J. Am. Chem. Soc., 2024, 146, 970–978 CrossRef CAS PubMed;
(o) A. Singh, O. Singh, A. Maji, S. Singh, N. Singh, P. K. Maji and K. Ghosh, Mol. Catal., 2024, 555, 113835 CrossRef CAS;
(p) W. Gao, Y. Gao, B. Liu, J. kang, Z. Zhang, M. Zhang and Y. Zou, RSC Adv., 2024, 14, 5055–5060 RSC;
(q) Z. Kefayati, M. Malmir and M. M. Heravi, Res. Chem. Intermed., 2024, 50, 127–146 CrossRef CAS.
- K. Pierzchała, J. Pięta, M. Pięta, M. Rola, J. Zielonka, A. Sikora, A. Marcinek and R. Michalski, Chem. Res. Toxicol., 2023, 36, 1398–1408 Search PubMed.
- M. E. González-Trujano, G. Urbie-Figueroa, S. Hidalgo-Figueroa, A. L. Martínez, M. Déciga-Campos and G. Navarrete-Vazquez, Biomed. Pharmacother., 2018, 101, 553–562 CrossRef PubMed.
- M. M. Alam, N. I. Alsenani, A. A. Abdelhamid, A. Ahmad, O. A. Baothman, S. A. Hosawi, H. Altayeb, M. S. Nadeem, V. Ahmad, S. Nazreen and A. A. Elhenawy, Arch. Pharm., 2024, 357, 2300340 CrossRef CAS PubMed.
-
(a) J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed;
(b) H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC;
(c) T. Keijer, V. Bakker and J. C. Slootweg, Nat. Chem., 2019, 11, 190–195 CrossRef CAS PubMed;
(d) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC;
(e) M. Rimaz, Z. Jalalian, H. Mousavi and R. H. Prager, Tetrahedron Lett., 2016, 57, 105–109 CrossRef CAS;
(f) M. Rimaz, H. Mousavi, P. Keshavarz and B. Khalili, Curr. Chem. Lett., 2015, 4, 159–168 CrossRef;
(g) M. Rimaz, H. Mousavi, M. Behnam and B. Khalili, Curr. Chem. Lett., 2016, 5, 145–154 CrossRef;
(h) M. Rimaz, H. Mousavi, B. Khalili and F. Aali, J. Chin. Chem. Soc., 2018, 65, 1389–1397 CrossRef CAS;
(i) M. Rimaz, H. Mousavi, B. Khalili and L. Sarvari, J. Iran. Chem. Soc., 2019, 16, 1687–1701 CrossRef CAS;
(j) N. Khaleghi, M. Esmkhani, M. Noori, N. Dasyafteh, M. Khalili Ghomi, M. Mahdavi, M. H. Sayahi and S. Javanshir, Nanoscale Adv., 2024, 6, 2337–2349 RSC.
-
(a) H. Mousavi, Int. J. Biol. Macromol., 2021, 186, 1003–1166 CrossRef CAS PubMed;
(b) Á. Molnár, Coord. Chem. Rev., 2019, 388, 126–171 CrossRef;
(c) A. Dhakshinamoorthy, M. Jacob, N. S. Vignesh and P. Varalakshmi, Int. J. Biol. Macromol., 2021, 167, 807–833 CrossRef CAS PubMed;
(d) A. Brik, M. El Kadiri, T. El Assimi, P. Dambruoso, R. Beniazza, G. Gouhier, A. El Kadib and M. Lahcini, Mol. Catal., 2023, 548, 113422 CrossRef CAS;
(e) P. Kaur, K. K. Gurjar, T. Arora, D. Bharti, M. Kaur, V. Kumar, J. Parkash and R. Kumar, Mol. Catal., 2023, 550, 113582 CrossRef CAS;
(f) M. Çalişkan and T. Baran, J. Organomet. Chem., 2022, 963, 122284 CrossRef;
(g) R. Beiranvand and M. G. Dekamin, Heliyon, 2023, 9, e16315 CrossRef CAS PubMed;
(h) M. Zeyadi and Y. Q. Almulaiky, Heliyon, 2023, 9, e21169 CrossRef CAS PubMed;
(i) F. Zhao, H. Yang, Z. Gao, H. Liu, P. Wu, B. Li, H. Yu and J. Shao, Heliyon, 2024, 10, e23563 CrossRef CAS PubMed;
(j) P. L. Deena, S. J. Selvaraj and K. J. Thomas, Curr. Chem. Lett., 2024, 13, 359–366 CrossRef.
-
(a) D. S. Su, S. Perathoner and G. Centi, Chem. Rev., 2013, 113, 5782–5816 CrossRef CAS PubMed;
(b) M. E. Khan, Nanoscale Adv., 2021, 3, 1887–1900 RSC;
(c) J. Zhu, A. Holmen and D. Chen, ChemCatChem, 2013, 5, 378–401 CrossRef CAS.
-
(a) B. Majumdar, S. Mandani, T. Bhattacharya, D. Sarma and T. K. Sarma, J. Org. Chem., 2017, 82, 2097–2106 CrossRef CAS PubMed;
(b) H. Saeidiroshan and L. Moradi, J. Organomet. Chem., 2019, 893, 1–10 CrossRef CAS;
(c) M. Gholinejad, H. Esmailoghli and F. Khosravi, J. Organomet. Chem., 2022, 963, 122295 CrossRef CAS;
(d) S. R. Mathapati, R. C. Alange, C. B. S. Mol, S. S. Bhande and A. H. Jadhav, Res. Chem. Intermed., 2022, 48, 4901–4928 CrossRef CAS;
(e) N. Karimi Hezarcheshmeh and J. Azizian, Mol. Divers., 2022, 26, 2011–2024 CrossRef PubMed;
(f) K. A. Wilson, L. A. Picinich and A. R. Siamaki, RSC Adv., 2023, 13, 7818–7827 RSC;
(g) D. Geedkar, A. Kumar and P. Sharma, J. Heterocycl. Chem., 2020, 57, 4331–4347 CrossRef CAS;
(h) M. Abdoli, N. Nami and Z. Hossaini, J. Heterocycl. Chem., 2021, 58, 523–533 CrossRef CAS;
(i) L. Hasani, E. Ezzatzadeh and Z. Hossaini, J. Heterocycl. Chem., 2023, 60, 2023–2035 CrossRef CAS;
(j) E. Ezzatzadeh, M. Mohammadi, M. Ghambarian and Z. Hossaini, Appl. Organomet. Chem., 2023, 37, e7252 CrossRef CAS;
(k) E. Ezzatzadeh, M. Mohammadi, Z. Hossaini and S. Khandan, ChemistrySelect, 2023, 8, e202302491 CrossRef CAS;
(l) E. Ezzatzadeh, S. Soleimani-Amiri, Z. Hossaini and K. Khandan Barani, Front. Chem., 2022, 10, 1001707 CrossRef CAS PubMed;
(m) E. Ezzatzadeh, Z. Hossaini, S. Majedi and F. H. S. Hussain, Polycycl. Aromat. Comp., 2023, 43, 4707–4728 CrossRef CAS;
(n) M. Jahandar Lashaki, R. Hajinasiri, Z. Hossaini and N. Nami, Polycycl. Aromat. Comp., 2023, 43, 6088–6106 CrossRef CAS;
(o) M. M. Kadhim, N. Tabarsaei, M. Ghorchibeigi and A. Sadeghi Meresht, Polycycl. Aromat. Comp., 2023, 43, 5785–5806 CrossRef CAS;
(p) S. Soleimani-Amiri, M. Mohammadi, N. Faal Hamedani and B. Dehbandi, Polycyclic Aromat. Compd., 2023, 43, 6046–6075 CrossRef CAS;
(q) Z. Azizi, M. Ghazvini, S. Afrashteh and Z. Hossaini, Polycycl. Aromat. Comp., 2024, 44, 2508–2534 CrossRef CAS;
(r) A. Moazeni Bistgani, L. Moradi and A. Dehghani, Catal. Commun., 2023, 182, 106755 CrossRef;
(s) A. R. Hajipour and Z. Khorasani, Catal. Commun., 2016, 77, 1–4 CrossRef CAS;
(t) D. Zeng, F. Wang, M. Bian, Y. Yang, W. Deng and R. Qiu, J. Organomet. Chem., 2024, 1004, 122943 CrossRef CAS;
(u) M. Lashanizadegan, F. Kamali, M. Ghiasi and H. Mirzazadeh, J. Mol. Struct., 2024, 1295, 136606 CrossRef CAS;
(v) L. Hasani, E. Ezzatzadeh and Z. Hossaini, Mol. Divers., 2024 DOI:10.1007/s11030-023-10803-7 , in press..
-
(a) C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC;
(b) D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC;
(c) V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. V. C. Long, M. Escribà-Gelonch, J. O. Tejada, S. Linke and K. Sunmacher, Green Chem., 2022, 24, 410–437 RSC;
(d) C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed;
(e) F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clarck, T. J. Farmer, A. J. Hunt, C. R. McElory and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef;
(f) H. Mousavi, B. Zeynizadeh and M. Rimaz, Bioorg. Chem., 2023, 135, 106390 CrossRef CAS PubMed;
(g) B. Zeynizadeh, H. Mousavi and F. Mohammad Aminzadeh, J. Mol. Struct., 2022, 1255, 131926 CrossRef CAS;
(h) R. N. Butler and A. C. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed;
(i) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed;
(j) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC;
(k) M. Rimaz, J. Khalafy, H. Mousavi, S. Bohlooli and B. Khalili, J. Heterocycl. Chem., 2017, 54, 3174–3186 CrossRef CAS;
(l) M. Rimaz, J. Khalafy and H. Mousavi, Res. Chem. Intermed., 2016, 42, 8185–8200 CrossRef CAS;
(m) Y. Jain, P. Gupta, P. Yadav and M. Kumari, ACS Omega, 2019, 4, 3582–3592 CrossRef CAS PubMed;
(n) Y. Jain, M. Kumari and R. Gupta, Tetrahedron Lett., 2019, 60, 1215–1220 CrossRef CAS.
- M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, RSC Adv., 2022, 12, 16454–16478 RSC.
-
(a) M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, J. Mol. Struct., 2023, 1271, 134017 CrossRef;
(b) M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, RSC Adv., 2022, 12, 11164–11189 RSC;
(c) B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Res. Chem. Intermed., 2021, 47, 3289–3312 CrossRef CAS;
(d) R. Bakhshi, B. Zeynizadeh and H. Mousavi, J. Chin. Chem. Soc., 2020, 67, 623–637 CrossRef CAS;
(e) B. Zeynizadeh, H. Mousavi and F. Sepehraddin, Res. Chem. Intermed., 2020, 46, 3361–3382 CrossRef CAS;
(f) B. Zeynizadeh, H. Mousavi and S. Zarrin, J. Chin. Chem. Soc., 2019, 66, 928–933 CrossRef CAS;
(g) B. Zeynizadeh, F. Sepehraddin and H. Mousavi, Ind. Eng. Chem. Res., 2019, 58, 16379–16388 CrossRef CAS;
(h) B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Res. Chem. Intermed., 2019, 45, 3329–3357 CrossRef CAS;
(i) B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Green Process. Synth., 2019, 8, 742–755 CAS;
(j) H. Mousavi, B. Zeynizadeh, R. Younesi and M. Esmati, Aust. J. Chem., 2018, 71, 595–609 CrossRef CAS;
(k) B. Zeynizadeh, R. Younesi and H. Mousavi, Res. Chem. Intermed., 2018, 44, 7331–7352 CrossRef CAS.
-
(a) P. Patinl, Q. Zheng, K. Kurpiewsk and A. Dömling, Nat. Commun., 2023, 14, 5807 CrossRef PubMed;
(b) A. Darvishi, H. Bakhshi and A. Heydari, RSC Adv., 2022, 12, 16535–16543 RSC;
(c) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC;
(d) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed;
(e) S. Kumari, A. V. Carmona, A. K. Tiwari and P. C. Trippier, J. Med. Chem., 2020, 63, 12290–12358 CrossRef CAS PubMed;
(f) S. S. R. Gupta, A. V. Nakhate, G. P. Deshmukh, S. Periasamy, P. S. Samudrala, S. K. Bhargava and M. L. Kantam, ChemistrySelect, 2018, 3, 8436–8443 CrossRef CAS;
(g) M. Lubberink, W. Finnigan and S. L. Flitsch, Green Chem., 2023, 25, 2958–2970 RSC;
(h) Y. Ge, W. Huang, S. Ahrens, A. Spannenberg, R. Jackstell and M. Beller, Nat. Synth., 2024, 3, 202–213 CrossRef;
(i) Q. Yan, Q.-J. Yuan, A. Shatskiy, G. R. Alvey, E. V. Stepanova, J.-Q. Liu, M. D. Kärkäs and X.-S. Wang, Org. Lett., 2024, 26, 3380–3385 CrossRef CAS PubMed.
- F. Piazzolla and A. Temperini, Tetrahedron Lett., 2018, 59, 2615–2621 CrossRef CAS.
-
(a) B. H. Rostein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef PubMed;
(b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed;
(c) F. Sutanto, S. Shaabani, C. G. Neochoritis, T. Zarganes-Tzitzikas, P. Patil, E. Ghonchepour and A. Dömling, Sci. Adv., 2021, 7, eabd9307 CrossRef CAS PubMed;
(d) D. Hurtado-Rodríguez, A. Salinas-Torres, H. Rojas, D. Becerra and J.-C. Castillo, RSC Adv., 2022, 12, 35158–35176 RSC;
(e) M. Mazloum Farsi Baf, B. Akhlaghinia, Z. Zarei and S. S. E. Ghodsinia, ChemistrySelect, 2020, 5, 15195–15208 CrossRef;
(f) M. Rimaz, B. Khalili, G. Khatyal, H. Mousavi and F. Aali, Aust. J. Chem., 2017, 70, 1274–1284 CrossRef CAS;
(g) M. Rimaz, H. Mousavi, L. Nikpey and B. Khalili, Res. Chem. Intermed., 2017, 43, 3925–3937 CrossRef CAS;
(h) M. Rimaz, H. Mousavi, M. Behnam, L. Sarvari and B. Khalili, Curr. Chem. Lett., 2017, 6, 55–68 CrossRef;
(i) D. Zeleke and T. Damena, Results Chem., 2024, 7, 101283 CrossRef CAS;
(j) N. Yaghmaeiyan, M. Mirzaei and A. Bamoniri, Results Chem., 2023, 5, 100696 CrossRef CAS;
(k) S. Handique and P. Sharma, Results Chem., 2023, 5, 100781 CrossRef CAS;
(l) S. S. Bhalodiya, M. P. Parmar, D. B. Upadhyay, C. D. Patel, D. P. Vala, D. Rajani and H. M. Patel, Results Chem., 2024, 7, 101304 CrossRef CAS;
(m) M. Rimaz and H. Mousavi, Turk. J. Chem., 2013, 37, 252–261 CAS;
(n) J. Aboonajmi, M. Mohammadi, F. Panahi, M. Aberi and H. Sharghi, RSC Adv., 2023, 13, 24789–24794 RSC;
(o) A. Ahmad, S. Rao and N. S. Shetty, RSC
Adv., 2023, 13, 28798–28833 RSC;
(p) M. Karimi, A. Ramazani, S. Sajjadifar and S. Rezayati, RSC Adv., 2023, 13, 29121–29140 RSC;
(q) F. Mohammad Aminzadeh and B. Zeynizadeh, Nanoscale Adv., 2023, 5, 4499–4520 RSC;
(r) H. Mousavi, J. Mol. Struct., 2022, 1251, 131742 CrossRef CAS;
(s) M. Rimaz, H. Mousavi, L. Ozzar and B. Khalili, Res. Chem. Intermed., 2019, 45, 2673–2694 CrossRef CAS;
(t) H. Mousavi, M. Rimaz and B. Zeynizadeh, ACS Chem. Neurosci., 2024, 15, 1828–1881 CrossRef CAS PubMed.
-
(a) Y. Zhang, B. Han, Z. Zhang, X. Zhao, W. Li, B. Li and L. Zhu, Green Chem., 2023, 25, 6253–6262 RSC;
(b) Y. L. Nunes, F. L. de Menezes, I. G. de Sousa, A. L. G. Cavalcante, F. T. T. Cavalcante, K. da Silva Moreira, A. L. B. de Oliveira, G. F. Mota, J. E. da Silva Souza, I. R. de Aguiar Falcão, T. G. Rocha, R. B. R. Valério, P. B. A. Fechine, M. C. M. de Souza and J. C. S. dos Santos, Int. J. Biol. Macromol., 2021, 181, 1124–1170 CrossRef CAS PubMed;
(c) M. Nourmohammadi, S. Rouhani, S. Azizi, M. Maaza, T. A. M. Msagati, S. Rostamnia, M. Hatami, S. Khaksar, E. Zarenezhad, H. W. Jang and M. Shokouhimehr, Mater. Today Commun., 2021, 29, 102798 CrossRef CAS.
- C. Sharma, A. K. Srivastava, A. Soni, S. Kumari and R. K. Joshi, RSC Adv., 2020, 10, 32516–32521 RSC.
- V. S. Sypu, M. Bhaumik, K. Raju and A. Maity, J. Colloid Interface Sci., 2021, 581, 979–989 CrossRef CAS PubMed.
- S. Yang, Z.-H. Zhang, Q. Chen, M.-Y. He and L. Wang, Appl. Organomet. Chem., 2018, 32, e4132 CrossRef.
- M. Pashaei and E. Mehdipour, Appl. Organomet. Chem., 2018, 32, e4226 CrossRef.
- M. Piri, M. M. Heravi, A. Elhampour and F. Nemati, J. Mol. Struct., 2021, 1242, 130646 CrossRef CAS.
- A. K. Srivastava, H. Khandaka and R. K. Joshi, SynOpen, 2023, 7, 121–129 CrossRef CAS.
- Z. Moradi and A. Ghorbani-Choghamarani, Sci. Rep., 2023, 13, 7645 CrossRef CAS PubMed.
- N. Seyedi, F. Shirini and H. Tajik, J. Mol. Struct., 2023, 1285, 135547 CrossRef CAS.
- M. Dabiri, A. Mnachekanian Salmasi, N. Salarinejad and S. Kazemi Movahed, J. Mol. Struct., 2023, 1287, 135609 CrossRef CAS.
- P. Mohammadi, M. M. Heravi, L. Mohammadi and A. Saljooqi, Sci. Rep., 2023, 13, 17375 CrossRef CAS PubMed.
- M. Z. Sarker, M. M. Rahman, H. Minami, M. S. I. Sarker and H. Ahmad, Colloids Surf. A: Physicochem. Eng. Asp., 2023, 668, 131447 CrossRef CAS.
- M. Bayzidi and B. Zeynizadeh, RSC Adv., 2022, 12, 15020–15037 RSC.
- R. Taheri-Ledari, S. S. Mirmohammadi, K. Valadi, A. Maleki and A. E. Shalan, RSC Adv., 2020, 10, 43670–43681 RSC.
- A. Capperucci, M. Clemente, A. Cenni and D. Tanini, ChemSusChem, 2023, 16, e202300086 CrossRef CAS PubMed.
- B. Zeynizadeh, Z. Shokri and M. Hasanpour Galehban, Appl. Organomet. Chem., 2019, 33, e4771 CrossRef.
- M. Esmaeilzadeh, S. Sadjadi and Z. Salehi, Int. J. Biol. Macromol., 2020, 150, 441–448 CrossRef CAS PubMed.
- J. Kaushik, C. Sharma, N. K. Lamba, P. Sharma, G. S. Das, K. M. Tripathi, R. K. Joshi and S. K. Sonkar, Langmuir, 2023, 39, 12865–12877 CrossRef CAS PubMed.
- D. Sharma, P. Choudhary, S. Kumar and V. Krishnam, J. Colloid Interface Sci., 2024, 657, 449–462 CrossRef CAS PubMed.
- V. Bilakanti, N. Gutta, V. K. Velisoju, M. Dumpalapally, S. Inkollu, N. Nama and V. Akula, React. Kinet. Mech. Catal., 2020, 130, 347–362 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *,
Behzad
Zeynizadeh
*,
Behzad
Zeynizadeh
 and
Morteza
Hasanpour Galehban
and
Morteza
Hasanpour Galehban

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CH2OH (1
CH3CH2OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture under ultrasonic conditions, we immobilized the nickelII (NiII) nanoparticles into the matrix of the prepared Fe3O4/f-MWCNT-CS-Glu nanocomposite to achieve the desired Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite (Fig. 3). It should be noted that the structure of the mentioned nanocomposite was previously fully characterized and examined by our group using Fourier transform infrared (FT-IR) (Fig. 4), powder X-ray diffraction (PXRD) (Fig. 5), scanning electron microscopy (SEM) (Fig. 6), transmission electron microscopy (TEM) (Fig. 7), SEM-based energy-dispersive X-ray spectroscopy (EDX) (Fig. 8) and elemental mapping (Fig. 9), inductively coupled plasma-optical emission spectrometry (ICP-OES), thermogravimetric analysis (TGA) (Fig. 10), differential thermal analysis (DTA) (Fig. 11), and vibrating sample magnetometry (VSM) (Fig. 12) analyses.11
2) mixture under ultrasonic conditions, we immobilized the nickelII (NiII) nanoparticles into the matrix of the prepared Fe3O4/f-MWCNT-CS-Glu nanocomposite to achieve the desired Fe3O4/f-MWCNT-CS-Glu/NiII nanocomposite (Fig. 3). It should be noted that the structure of the mentioned nanocomposite was previously fully characterized and examined by our group using Fourier transform infrared (FT-IR) (Fig. 4), powder X-ray diffraction (PXRD) (Fig. 5), scanning electron microscopy (SEM) (Fig. 6), transmission electron microscopy (TEM) (Fig. 7), SEM-based energy-dispersive X-ray spectroscopy (EDX) (Fig. 8) and elemental mapping (Fig. 9), inductively coupled plasma-optical emission spectrometry (ICP-OES), thermogravimetric analysis (TGA) (Fig. 10), differential thermal analysis (DTA) (Fig. 11), and vibrating sample magnetometry (VSM) (Fig. 12) analyses.11










![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaBH4 (mmol)
NaBH4 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst (mg). Yields refer to isolated pure products. TON (turnover number) = [(mol of the product formed)/(mol of the catalyst used)]. TOF (turnover frequency) = [(mol of the product formed)/(mol of the catalyst used) × (time)]. The TON and TOF values were calculated based on the existing amount of nickel (Ni) in the as-prepared nanocatalyst (in 5 mg of the hybrid nanocatalyst, 0.616 mg (or 0.010495217 mmol) of Ni exists).
catalyst (mg). Yields refer to isolated pure products. TON (turnover number) = [(mol of the product formed)/(mol of the catalyst used)]. TOF (turnover frequency) = [(mol of the product formed)/(mol of the catalyst used) × (time)]. The TON and TOF values were calculated based on the existing amount of nickel (Ni) in the as-prepared nanocatalyst (in 5 mg of the hybrid nanocatalyst, 0.616 mg (or 0.010495217 mmol) of Ni exists).


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaBH4 (mmol)
NaBH4 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst (mg)
catalyst (mg)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ac2O (mmol). Yields refer to isolated pure products. TON (turnover number) = [(mol of the product formed)/(mol of the catalyst used)]. TOF (turnover frequency) = [(mol of the product formed)/(mol of the catalyst used) × (time)]. The TON and TOF values were calculated based on the existing amount of nickel (Ni) in the as-prepared nanocatalyst (in 5 mg of the hybrid nanocatalyst, 0.616 mg (or 0.010495217 mmol) of Ni exists).
Ac2O (mmol). Yields refer to isolated pure products. TON (turnover number) = [(mol of the product formed)/(mol of the catalyst used)]. TOF (turnover frequency) = [(mol of the product formed)/(mol of the catalyst used) × (time)]. The TON and TOF values were calculated based on the existing amount of nickel (Ni) in the as-prepared nanocatalyst (in 5 mg of the hybrid nanocatalyst, 0.616 mg (or 0.010495217 mmol) of Ni exists).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1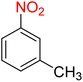

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1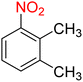

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3
H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 45 °C
1); 45 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); r.t.
1); r.t.






