Open Access Article
Open Access ArticleRSM optimization of Friedel–Crafts C-acylation of para-fluorophenol over the catalysis of phosphomolybdic acid encapsulated in MIL-53 (Fe) metal organic frameworks
Ahmad
Nikseresht
 *a,
Reza
Mehravar
a and
Masoud
Mohammadi
*a,
Reza
Mehravar
a and
Masoud
Mohammadi
 b
b
aDepartment of Chemistry, Payame Noor University, PO BOX 19395-4697, Tehran, Iran
bDepartment of Chemistry, Faculty of Science, Ilam University, P.O. Box 69315516, Ilam, Iran. E-mail: a_nik55@yahoo.com; ahmad.nikseresht@pnu.ac.ir; Tel: +98-918-8418754
First published on 6th May 2024
Abstract
In this research, a heterogeneous acid catalyst was synthesized by room temperature encapsulation of phosphomolybdic acid (PMA) in the pores of the MIL-53 (Fe) metal organic framework (MOF) under ultrasonic conditions. Then the catalytic activity of PMA@MIL-53 (Fe) was investigated in Friedel–Crafts C-acylation of para-fluorophenol, and this procedure was optimized using response surface methodology based on central composite design (RSM-CCD). The impact of critical reaction parameters including reaction duration, catalyst dosage, and PMA amount in the catalyst was optimized, leading to the formation of the target product in excellent yield at a short reaction time.
1. Introduction
The Friedel–Crafts reactions are widely used in organic synthesis for the introduction of new substituents onto aromatic rings.1 Among these reactions, Friedel–Crafts alkylation and acylation are two of the most commonly employed methods.2 While both reactions have their own merits and limitations, the choice of reaction largely depends on the nature of the substituent to be introduced.2 In recent years, Friedel–Crafts acylation has gained increasing attention as a suitable alternative to Friedel–Crafts alkylation in mono-substitution of aromatic rings.3 This is due to the fact that Friedel–Crafts acylation offers several advantages over Friedel–Crafts alkylation, including higher selectivity, milder reaction conditions, and fewer side reactions.4 This acylation method involves the electrophilic substitution of an aromatic ring with an acyl group. This reaction has been extensively studied since its discovery in the late 19th century and has become a key tool for the preparation of a wide range of organic compounds, including in the fields of pharmaceuticals, agrochemicals, and materials science.5 The success of this reaction relies on the use of Lewis acid catalysts, such as aluminum chloride, and a variety of acylating agents, including acid chlorides, anhydrides, and esters.4 Despite its broad applicability, the Friedel–Crafts acylation mechanism is still subject to debate, with various proposals for the formation of the key intermediates and transition states. Based on the recent experimental and computational studies that shed light on the reaction mechanism, this process probably is thought to involve the creation of a complex between a Lewis acid and a chlorine atom from the acid chloride, which then leads to the formation of an acylium ion through the breaking of the C–Cl bond. This reactive intermediate has a positively charged carbon and is stabilized through resonance, which acts as an electrophile and reacts with the arene to produce the mono-acylated product through formation of a new carbon–carbon bond, which is an aryl ketone.6–8Aluminum chloride is a popular catalyst in aromatic substitution reactions due to its effectiveness. However, it has some drawbacks, such as instability and temperature sensitivity. Additionally, aluminum chloride can ignite combustibles and react with water and wet air to produce hazardous hydrogen chloride gas and heat. Furthermore, this Lewis acid can cause equipment deterioration and generate a significant amount of waste during the deactivation of the catalytic complex.4 In recent times, a variety of catalysts have been employed in this reaction. The entire process generates a significant amount of hazardous waste that is challenging to separate from the target product.9,10 Solid acid catalysts that are recyclable and environment-friendly, such as metal–organic frameworks (MOFs), are preferred because of their advantages. This will decrease the impact of environmental and economic issues.
Metal–organic frameworks (MOFs), a series of porous materials with adjustable chemical and physical characteristics, are one of the most widely used materials for the heterogenization of solid acids.11–16 These materials' adaptable structure enables a wide range of chemical processes, such as catalysis, photocatalysis, electrocatalysis, adsorption, separation, sensing, drug administration, and organic transformation.16–20 The acidity of MOFs can be tuned by changing the metal node or ligand's functional group, allowing for the selective activation of specific functional groups in the reactants.21 In particular, supported phosphomolybdic acid catalysts have been used in various organic reactions, including esterification, transesterification, aldol condensation, Friedel–Crafts acylation, etc.22–28 We have just recently achieved the synthesis of a phosphomolybdic acid-based catalyst that is prepared by encapsulation of phosphomolybdic acid groups in the MIL-53 (Fe) MOF pores by employing a simple one-pot approach.11 This acid was characterized as having a high level of thermal stability, a large surface area, efficient catalytic activity, and reusability.11 Herein, we demonstrate the catalytic application of this solid acid catalyst for selective Friedel–Crafts C-acylation of para-fluorophenol utilizing acetyl chloride as an effective acylating agent.
2. Experimental
2.1. Materials
The Merck Millipore company supplied all of the components and solvents, which were then put to use without undergoing any additional purification steps.2.2. General procedure for the encapsulation of phosphotungstic acid in MIL-53 (Fe) (PTA@MIL-53 (Fe))
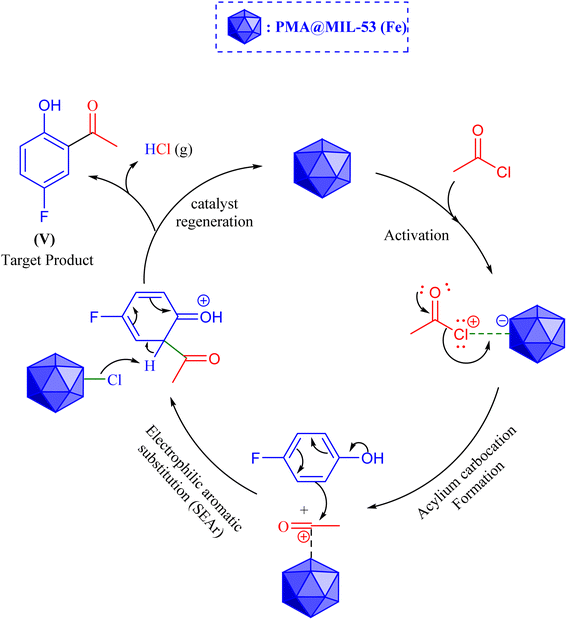
According to the findings that were obtained from our previous report,11 PMA@MIL-53 (Fe) was synthesized via employing a one-pot ultrasonic assisted procedure. In this sense, the standard process involves dissolving 1 mmol of FeCl3·6H2O, 1 mmol of 1,4-benzendicarboxylic acid (1,4-BDC) and varying quantities of phosphomolybdic acid hydrate (PMA) (0.01 and 0.02 g) in 5 mL of dimethylformamide (DMF). Afterwards, the mixture was placed in an ultrasonic bath for 15 minutes at a frequency of 37 kHz and an output power of 240 W. The temperature of the bath was kept at room temperature throughout the process. Following that, the product was filtered with a Büchner funnel, the obtained powder was washed using DMF under Vacuum filtration and, then, it was dried in a vacuum at a temperature of 150 °C for 15 h (Scheme 1).2.3. General procedure for the Friedel–Crafts C-acylation of para-fluorophenol over the catalysis of PMA@MIL-53 (Fe) MOF
A mixture of 4-fluorophenol (7 mmol), acetyl chloride (7 mmol), and 30–200 mg of PMA@MIL-53 (Fe) as the catalyst comprising various levels of PMA (5–30%) was put into a 25 mL round bottom flask and, then, it was stirred at room temperature for 10–50 minutes. After a specified time period based on the test parameters, the catalyst was collected by centrifugation from the diluted mixture. It was then extracted with ethyl acetate, washed, and dried, and the solvent was evaporated. Finally, the crude products were purified using a silica gel plate (Scheme 2).3. Results and discussion
3.1. Characterization
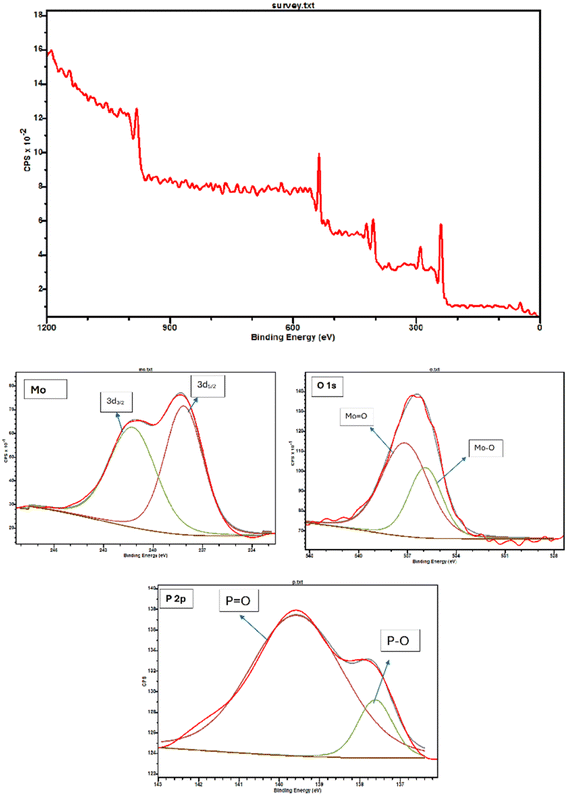
The as-prepared phosphomolybdic acid encapsulated within the MIL-53 (Fe) MOF was previously subjected to thorough characterization in our laboratory. This involved employing a range of analytical techniques such as Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), energy-dispersive X-ray spectroscopy (EDS), inductively coupled plasma optical emission spectrometry (ICP-OES), X-ray mapping, field emission scanning electron microscopy (FE-SEM), high-resolution transmission electron microscopy (HR-TEM), and nitrogen adsorption–desorption analysis.11 In this report, our primary aim was to provide a more comprehensive characterization of the PMA@MIL-53 (Fe) MOF. To achieve this, we ensured thorough completion of catalyst characterization, including XPS analysis of pure phosphomolybdic acid for the purpose of comparison.The XPS analysis depicted in Fig. 1 illustrates the elemental composition and chemical bonding of phosphomolybdic acid (PMA). Consisting primarily of phosphorus, molybdenum, and oxygen, this compound reveals distinctive peaks corresponding to its constituent elements and their bonding configurations. In the spectrum, the characteristic peaks for the P–O and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds within PMA emerge prominently at 137.6 and 139.5 eV, respectively. Molybdenum, predominantly existing in the +6 oxidation state, exhibits discernible signals for Mo: 3d3/2 and Mo: 3d5/2 at 241.1 and 238.1 eV, respectively. Likewise, the Mo–O and Mo
O bonds within PMA emerge prominently at 137.6 and 139.5 eV, respectively. Molybdenum, predominantly existing in the +6 oxidation state, exhibits discernible signals for Mo: 3d3/2 and Mo: 3d5/2 at 241.1 and 238.1 eV, respectively. Likewise, the Mo–O and Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds manifest distinct peaks at 537.1 eV and 535.8 eV, elucidating the chemical environment surrounding molybdenum within the compound. These findings align closely with our prior investigations on PMA@MIL-53 (Fe) MOF by XPS analysis11 and provide conclusive evidence regarding the presence of phosphomolybdic acid within the catalyst structure. The agreement between these results and previous reports underscores the reliability of our analytical methodology and reinforces our understanding of PMA's role in catalytic processes.
O bonds manifest distinct peaks at 537.1 eV and 535.8 eV, elucidating the chemical environment surrounding molybdenum within the compound. These findings align closely with our prior investigations on PMA@MIL-53 (Fe) MOF by XPS analysis11 and provide conclusive evidence regarding the presence of phosphomolybdic acid within the catalyst structure. The agreement between these results and previous reports underscores the reliability of our analytical methodology and reinforces our understanding of PMA's role in catalytic processes.
3.2. Design expert
The CCD method is more effective than other RSM methods because it covers a larger operability region and accurately predicts the center point, regardless of missing data points. Thus, Friedel–Crafts C-acylation of para-fluorophenol using the PMA@MIL-53 (Fe) MOF catalyst was designed with the CCD technique, focusing on reaction time, catalyst amount, and PMA weight percent as key parameters affecting para-fluorophenol conversion and acylated product yield. The experiment was conducted at room temperature in line with green chemistry principles, and five-level coded factors (, 1, 0, +1, +) were considered with corresponding values presented in Table 1. The parameter ranges for the initial experiment design were selected based on preliminary tests.| Run | Independent variables | Response | ||
|---|---|---|---|---|
| Time (min) | Catalyst loading (mg) | PMA (wt%) | Yielda (%) | |
| a Isolated yield. | ||||
| 1 | 18 | 165.54 | 24.93 | 59 |
| 2 | 42 | 164.46 | 24.93 | 67 |
| 3 | 30 | 115 | 17.50 | 79 |
| 4 | 10 | 115 | 17.50 | 26 |
| 5 | 30 | 200 | 17.50 | 73.1 |
| 6 | 30 | 115 | 5.00 | 15 |
| 7 | 30 | 115 | 17.50 | 81.8 |
| 8 | 18 | 64.46 | 24.93 | 15.5 |
| 9 | 18 | 165.54 | 10.07 | 29 |
| 10 | 42 | 165.54 | 10.07 | 18 |
| 11 | 42 | 64.46 | 10.07 | 34 |
| 12 | 42 | 165.54 | 24.93 | 96.3 |
| 13 | 18 | 64.46 | 10.07 | 9 |
| 14 | 50 | 115 | 17.50 | 81.8 |
| 15 | 30 | 115 | 30.00 | 81 |
| 16 | 30 | 30 | 17.50 | 29 |
| 17 | 30 | 115 | 17.50 | 76 |
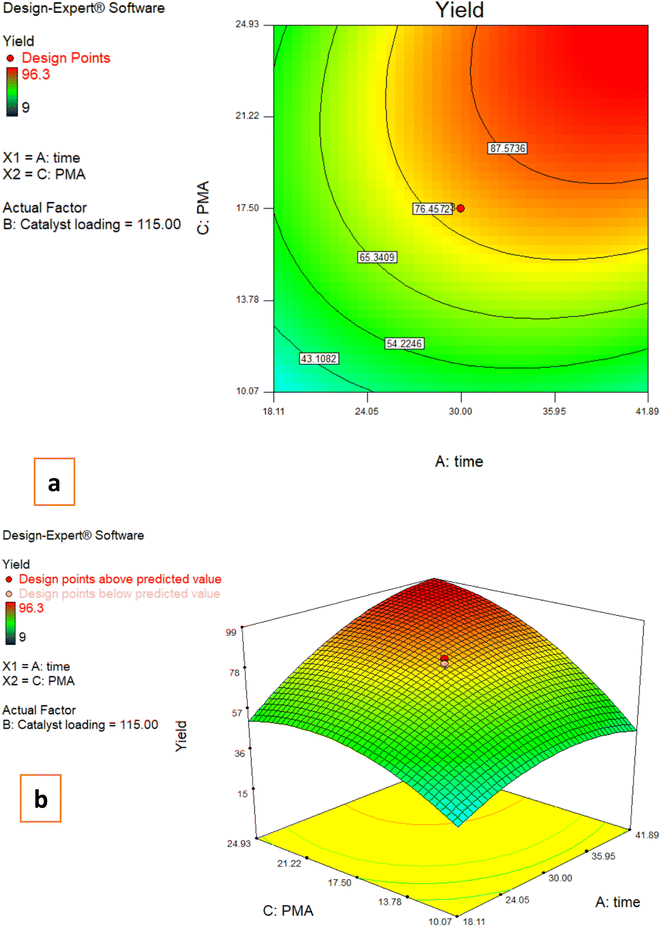
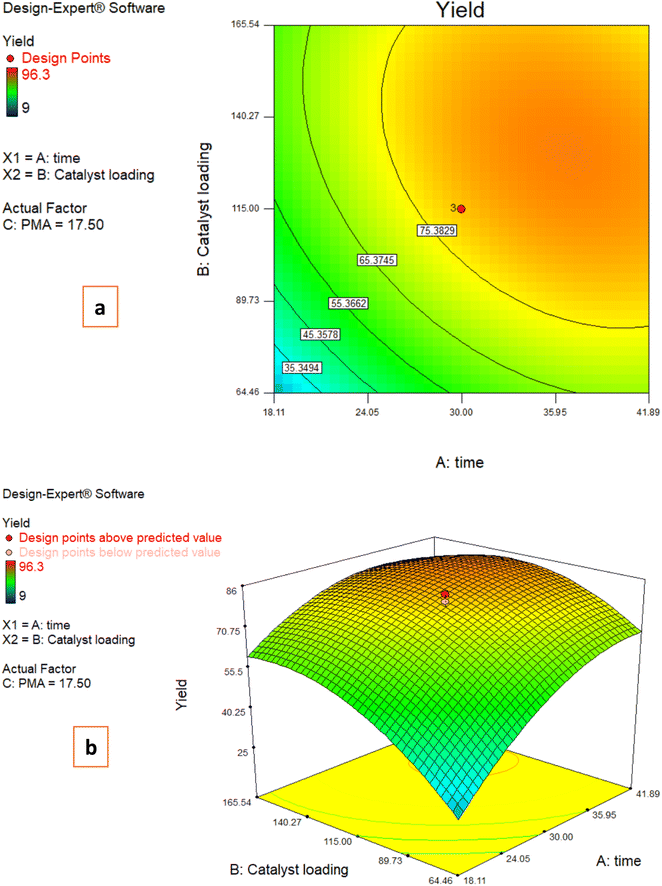
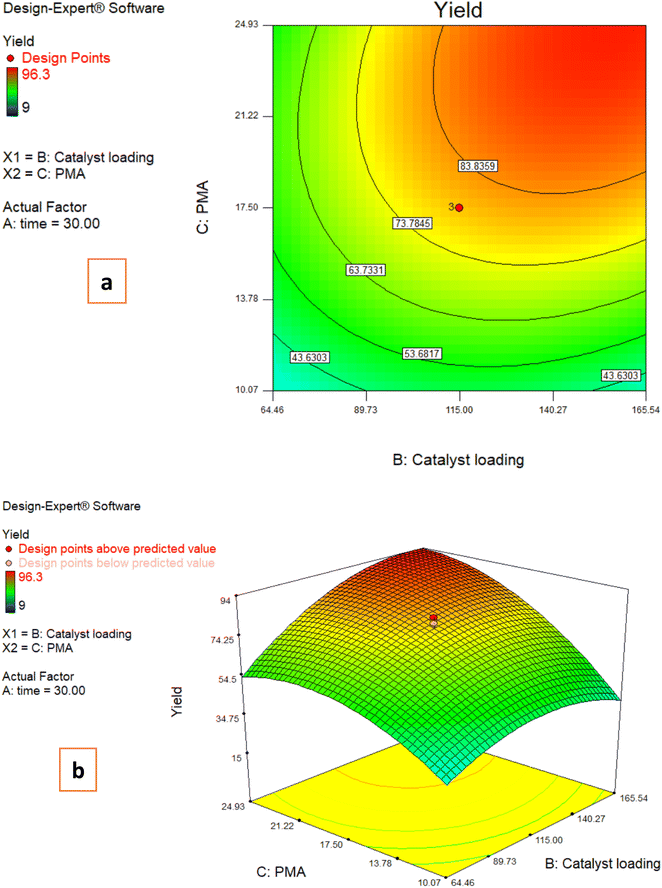
3.3. Effects of reaction parameters on the C-acylated yield
The following plots were made in order to demonstrate the impacts of two factors having an influence on the response while the third variable remains unchanged.3.4. Model development
The RSM was applied to create a mathematical model and analyze how the variables and their interactions affected the response. Additionally, a trivariate CCD was employed to determine the impact of different factors on the amount of C-acylated produced. To predict the yield, an equation was suggested.| Yield (%) in Fries rearrangement reaction = 79.47 + 14.40 × time + 11.05 × catalyst loading + 18.95 × PMA − 6.28time × catalyst loading + 9.35time × PMA + 8.60B × PMA − 10.70 × time2 − 11.70 × catalyst loading2 − 12.78 × PMA2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 003, and the value of R2-adj, which indicates the degree of correlation between the experimental results and the predicted results, is equal to 13
003, and the value of R2-adj, which indicates the degree of correlation between the experimental results and the predicted results, is equal to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008. These values show that the proposed model is very efficient in predicting the behavior of the system in the range of operating parameters used in the design.
008. These values show that the proposed model is very efficient in predicting the behavior of the system in the range of operating parameters used in the design.
| Source | Sum of squares | Dfa | Mean square | F-Valueb | p-Value (prob > F) c | |
|---|---|---|---|---|---|---|
| a Degree of freedom. b Evaluate the model's similarity to the residual (error) variance. c Chances of discovering the measured F value under the null hypothesis (significant at p < 0.05 and not significant at higher values). | ||||||
| Model | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969.26 969.26 |
9 | 1525.14 | 28.00 | 0.0001 | Significant |
| A-Reaction time | 2831.46 | 1 | 2831.46 | 51.08 | 0.0002 | |
| B-Catalyst loading | 1668.84 | 1 | 1668.84 | 30.10 | 0.0009 | |
| C-PMA | 4904.25 | 1 | 4904.25 | 88.47 | <0.0001 | |
| AB | 315.01 | 1 | 315.01 | 5.68 | 0.0486 | |
| AC | 699.38 | 1 | 699.38 | 12.62 | 0.0093 | |
| BC | 591.68 | 1 | 591.68 | 10.67 | 0.0137 | |
| A 2 | 1289.76 | 1 | 1289.76 | 23.27 | 0.0019 | |
| B 2 | 1544.21 | 1 | 1544.21 | 27.86 | 0.0012 | |
| C 2 | 1841.87 | 1 | 1841.87 | 33.23 | 0.0007 | |
| Residual | 388.04 | 7 | 55.43 | |||
| Lack of fit | 371.22 | 5 | 74.24 | 8.82 | 0.1049 | Not significant |
| Pure error | 16.83 | 2 | 8.41 | |||
| Cor. total | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357.31 357.31 |
16 | ||||
As can be seen from the proposed model and the results of Table 2, among the parameters affecting the process, besides the specific effect of the reaction time and PTA percentage parameters with value-F values equal to 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 and 88
008 and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001, the catalyst amount (30
001, the catalyst amount (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010) has a more prominent role. In order to predict the effects of the studied parameters on the value of oscillation, this software presented the following equations:
010) has a more prominent role. In order to predict the effects of the studied parameters on the value of oscillation, this software presented the following equations:
| Yield (%) = 79.47 + 14.40 × time + 11.05 × catalyst loading + 18.95 × PMA − 6.28time × catalyst l + 9.35time × PMA + 8.60B × PMA − 10.70 × time2 − 11.70 × catalyst loading2 − 12.78 × PMA |
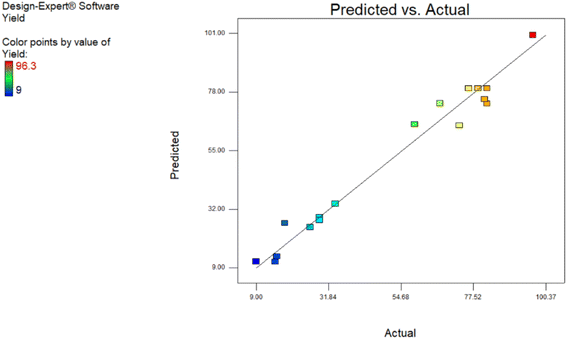
The comparison between the predicted data from the software and the experimental data is shown in Fig. 5. This comparison shows that the proposed model has well predicted the study of mutual effects between factors and also their effects on the C-acylation process.
4. Conclusions
In this study, we utilized phosphomolybdic acid encapsulated in MIL-53 (Fe) as a heteropolyacid catalyst for the first time in the Friedel–Crafts acylation of para-fluorophenol. We investigated and optimized various parameters such as catalyst loading, PMA percentage, and reaction time using the response surface method. Our analysis showed that time and PMA percentage had the greatest effect on the production process. The optimal conditions for achieving the best efficiency were found to be 115 mg of catalyst containing 5.11 wt% of PMA for 30 minutes. Importantly, experiments on catalyst regeneration demonstrated its stability, and the yield of reactions did not vary considerably even after the catalyst was reused seven times.Data availability
All data and materials in this publication are available by contacting the corresponding author.Author Contributions
Reza Mehravar (the MSc student) performed most of the practical laboratory work as part of his master's thesis supervised by Dr Ahmad Nikseresht. Ahmad Nikseresht (supervisor, PhD) designed and coordinated the study, software, conceptualization, and edited the final version and submitted the manuscript for publication. Masoud Mohammadi (PhD) contributed to conceptualization, software, writing, review, and editing of the manuscript draft.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the Payame Noor University, Tehran, Iran.References
- M. A. Hadj and N. Masurier, Recent advances in aza Friedel-Crafts reaction: strategies for achiral and stereoselective synthesis, Org. Chem. Front., 2023, 10(7), 1847–1866, 10.1039/d2qo02018a.
- T. Ahmad, S. Khan and N. Ullah, Recent Advances in the Catalytic Asymmetric Friedel-Crafts Reactions of Indoles, ACS Omega, 2022, 7(40), 35446–35485, DOI:10.1021/acsomega.2c05022.
- R. B. Leveson-Gower and G. Roelfes, Biocatalytic Friedel-Crafts Reactions, ChemCatChem, 2022, 14(18), e202200636, DOI:10.1002/cctc.202200636.
- N. Taheri, M. Dinari and M. Asgari, Recent Applications of Porous Organic Polymers Prepared via Friedel-Crafts Reaction under the Catalysis of AlCl3: A Review, ACS Appl. Polym. Mater., 2022, 4(9), 6288–6302, DOI:10.1021/acsapm.2c00927.
- A. Sumita and T. Ohwada, Friedel-Crafts-Type Acylation and Amidation Reactions in Strong Brønsted Acid: Taming Superelectrophiles, Molecules, 2022, 27(18), 5984, DOI:10.3390/molecules27185984.
- V. Kumar, W. B. Turnbull and A. Kumar, Review on Recent Developments in Biocatalysts for Friedel–Crafts Reactions, ACS Catal., 2022, 12(17), 10742–10763, DOI:10.1021/acscatal.2c01134.
- M. Rachwalski, A. Buchcic-Szychowska and S. Leśniak, Recent advances in selected asymmetric reactions promoted by chiral catalysts: Cyclopropanations, friedel–crafts, mannich, michael and other zinc-mediated processes—an update, Symmetry, 2021, 13(10), 1762, DOI:10.3390/sym13101762.
- D. Gaviña, M. Escolano, J. Torres, G. Alzuet-Piña, M. Sánchez-Roselló and C. del Pozo, Organocatalytic Enantioselective Friedel-Crafts Alkylation Reactions of Pyrroles, Adv. Synth. Catal., 2021, 363(14), 3439–3470, DOI:10.1002/adsc.202100313.
- K. K. Gangu and S. B. Jonnalagadda, A Review on Metal-Organic Frameworks as Congenial Heterogeneous Catalysts for Potential Organic Transformations, Front. Chem., 2021, 9, 747615, DOI:10.3389/fchem.2021.747615.
- Y. N. Nayak, S. Nayak, Y. F. Nadaf, N. S. Shetty and S. L. Gaonkar, Zeolite catalyzed friedel-crafts reactions: A review, Lett. Org. Chem., 2020, 17(7), 491–506, DOI:10.2174/1570178616666190807101012.
- A. Nikseresht, R. Bagherinia, M. Mohammadi and R. Mehravar, Phosphomolybdic acid hydrate encapsulated in MIL-53 (Fe): a novel heterogeneous heteropoly acid catalyst for ultrasound-assisted regioselective nitration of phenols, RSC Adv., 2023, 13(1), 674–687, 10.1039/D2RA07077D.
- M. Koolivand, M. Nikoorazm, A. Ghorbani-Choghamarani and M. Mohammadi, A novel cubic Zn-citric acid-based MOF as a highly efficient and reusable catalyst for the synthesis of pyranopyrazoles and 5-substituted 1H-tetrazoles, Appl. Organomet. Chem., 2022, 36(6), 2641–2663, DOI:10.1002/aoc.6656.
- A. Ghorbani-Choghamarani, Z. Taherinia and M. Mohammadi, Facile synthesis of Fe3O4@GlcA@Ni-MOF composites as environmentally green catalyst in organic reactions, Environ. Technol. Innovation, 2021, 24, 102050, DOI:10.1016/j.eti.2021.102050.
- N. Hussain-Khil, A. Ghorbani-Choghamarani and M. Mohammadi, A new silver coordination polymer based on 4,6-diamino-2-pyrimidinethiol: synthesis, characterization and catalytic application in asymmetric Hantzsch synthesis of polyhydroquinolines, Sci. Rep., 2021, 11(1), 15657, DOI:10.1038/s41598-021-94846-6.
- C. Li, H. Lv, K. Yang and X. Zhang, Robust Fluorine-Functionalized {Ln5}-Organic Frameworks for Excellent Catalytic Performance on Cycloaddition of CO2 with Epoxides and Knoevenagel Condensation, ACS Appl. Mater. Interfaces, 2023, 15(29), 35052–35061, DOI:10.1021/acsami.3c06804.
- B. Zhao, C. Li, T. Hu and X. Zhang, Nanoporous {Pb3}-Organic Framework for Catalytic Cycloaddition of CO2 with Epoxides and Knoevenagel Condensation, ACS Appl. Nano Mater., 2023, 6(24), 23196–23206, DOI:10.1021/acsanm.3c04586.
- H. Keypour, J. Kouhdareh, S. Alavinia, K. Rabiei, M. Mohammadi and A. Maryamabadi, et al., Post-synthetic modification of dual-porous UMCM-1-NH2 with palladacycle complex as an effective heterogeneous catalyst in Suzuki and Heck coupling reactions, J. Organomet. Chem., 2023, 989, 122646, DOI:10.1016/j.jorganchem.2023.122646.
- F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour and H. Zeynali, Copper(II) Schiff-Base Complex Modified UiO-66-NH2(Zr) Metal-Organic Framework Catalysts for Knoevenagel Condensation-Michael Addition-Cyclization Reactions, Inorg. Chem., 2022, 61(12), 4825–4841, DOI:10.1021/acs.inorgchem.1c03284.
- J. Yin, W. Li, W. Li, L. Liu, D. Zhao and X. Liu, et al., Heterometallic ZnHoMOF as a dual-responsive luminescence sensor for efficient detection of hippuric acid biomarker and nitrofuran antibiotics, Molecules, 2023, 28(17), 6274, DOI:10.3390/molecules28176274.
- F. Wang, D. Zhao, W. Li, H. Zhang, B. Li and T. Hu, et al., Rod-shaped units based cobalt(II) organic framework as an efficient electrochemical sensor for uric acid detection in serum, Microchem. J., 2023, 185, 108154, DOI:10.1016/j.microc.2022.108154.
- A. M. Naseri, M. Zarei, S. Alizadeh, S. Babaee, M. A. Zolfigol and D. Nematollahi, et al., Synthesis and application of [Zr-UiO-66-PDC-SO3H]Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods, Sci. Rep., 2021, 11(1), 16817, DOI:10.1038/s41598-021-96001-7.
- A. Nikseresht, A. Daniyali, M. Ali-Mohammadi, A. Afzalinia and A. Mirzaie, Ultrasound-assisted biodiesel production by a novel composite of Fe(III)-based MOF and phosphotangestic acid as efficient and reusable catalyst, Ultrason. Sonochem., 2017, 37, 203–207, DOI:10.1016/j.ultsonch.2017.01.011.
- A. K. Dizaji, B. Mokhtarani and H. R. Mortaheb, Deep and fast oxidative desulfurization of fuels using graphene oxide-based phosphotungstic acid catalysts, Fuel, 2019, 236, 717–729, DOI:10.1016/j.fuel.2018.09.076.
- C. Peinado, J. M. Campos-Martin and S. Rojas, Phosphotungstic acid catalysed bioethylene synthesis under industrially relevant conditions, React. Chem. Eng., 2023, 8(4), 815–823, 10.1039/D2RE00354F.
- H. Zhu and R. Wang, Phosphotungstic acid-promoted Mn-Fe bimetal oxide with high sulfur resistance for low-temperature selective catalytic reduction of nitrogen oxides with NH3, J. Alloys Compd., 2023, 936, 168272, DOI:10.1016/j.jallcom.2022.168272.
- Z. Liu, X. Peng, Y. Xu, T. Tang, S. Wei and B. Liu, et al., Macromolecular structural characteristics and functional potential of tobacco stalk lignin from the phosphotungstic acid-assisted delignification process, Biomass Bioenergy, 2023, 170, 106706, DOI:10.1016/j.biombioe.2023.106706.
- H. Luo, Y. Gu, D. Liu and Y. Sun, Advances in oxidative desulfurization of fuel oils over mofs-based heterogeneous catalysts, Catalysts, 2021, 11(12), 1557, DOI:10.3390/catal11121557.
- S. Parak, A. Nikseresht, M. Alikarami and S. Ghasemi, RSM optimization of biodiesel production by a novel composite of Fe(III)-based MOF and phosphomolybdic acid, Res. Chem. Intermed., 2022, 48(9), 3773–3793, DOI:10.1007/s11164-022-04783-w.
- N. X. Wang, A. G. Yu, G. X. Wang, X. H. Zhang, Q. S. Li and Z. Li, Synthesis of ( S , R , R , R )-α,α′-Iminobis(methylene)bis(6-fluoro-3 H ,4 H -dihydro-2 H -1-benzopyran-2-methanol), Synthesis, 2007, 2007(8), 1154–1158, DOI:10.1055/s-2007-965993.
- R. Murashige, Y. Hayashi, S. Ohmori, A. Torii, Y. Aizu and Y. Muto, et al., Comparisons of O-acylation and Friedel–Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonic acid: effective synthesis of optically active homotyrosines, Tetrahedron, 2011, 67(3), 641–649, DOI:10.1016/j.tet.2010.11.047.
| This journal is © The Royal Society of Chemistry 2024 |