Open Access Article
Open Access ArticleCocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours
David
Rudolph
a,
Myrto
Ischyropoulou
bc,
Juliana
Pfeifer
d,
Joanna
Napp
 *bc,
Ute
Schepers
*bc,
Ute
Schepers
 *d,
Frauke
Alves
*d,
Frauke
Alves
 *bce and
Claus
Feldmann
*bce and
Claus
Feldmann
 *a
*a
aInstitute for Inorganic Chemistry (IAC), Karlsruhe Institute of Technology (KIT), Engesserstraße 15, 76131 Karlsruhe, Germany. E-mail: claus.feldmann@kit.edu
bUniversity Medical Center Goettingen (UMG), Institute for Diagnostic and Interventional Radiology, Robert-Koch-Straße 40, 37075 Göttingen, Germany. E-mail: falves@gwdg.de; joanna.napp@med.uni-goettingen.de
cMax-Planck-Institute for Multidisciplinary Sciences (MPI-NAT), Translational Molecular Imaging, Hermann-Rein-Straße 3, 37075, Göttingen, Germany
dInstitute of Functional Interfaces, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: ute.schepers@kit.edu
eClinic of Hematology and Medical Oncology, University Medical Center Göttingen, Robert-Koch-Straße 40, 37075 Göttingen, Germany
First published on 2nd January 2024
Abstract
ITC/Toc@Gd2(FLP)3 core@shell nanocarriers with a chemotherapeutic cocktail of lipophilic irinotecan (ITC) as the particle core and hydrophilic fludarabine phosphate (FLP) in the particle shell are realized. They are prepared via a microemulsion approach with ITC dissolved in tocopherol (Toc) as droplet phase and stabilized by water-insoluble Gd2(FLP)3. The synthesis can be followed by zeta-potential analysis. X-ray powder diffraction, infrared spectroscopy, elemental analysis, thermogravimetry, and photometry show a drug load of 49 μg per mL ITC and 317 μg per mL FLP at a nanocarrier concentration of 1.5 mg mL−1. Size and structure are evidenced by electron microscopy, resulting in a total diameter of 45 ± 16 nm, an inner core of 40 ± 17 nm, and a shell of 3–8 nm. In vitro studies with different cancer cell lines (i.e., human melanoma/SK-Mel-28, cervical cancer/HeLa, mouse pancreatic cancer/Panc02 and KPC as well as human pancreatic cancer/Capan-1 cells) prove efficient nanocarrier uptake and promising cytostatic efficacy. Specifically for KPC cells, ITC/Toc@Gd2(FLP)3 nanocarriers show an increased efficacy, with half maximal inhibitory concentration (IC50: 4.2 μM) > 10 times lower than the free drugs (IC50: ITC: 47.7 μM, FLP: 143 μM). This points to the synergistic effect of the ITC/FLP drug cocktail in the nanocarriers and may result in a promising strategy to treat pancreatic ductal adenocarcinoma (PDAC).
Introduction
Oncology belongs to the very first areas of application, for which drug-loaded nanocarriers received clinical approval.1 Here, non-PEGylated liposomal doxorubicin (Myocet®) or PEGylated liposomal doxorubicin (CAELYX®, DOXIL®) are typical examples.2 Such nanocarrier formulations are expected to have specific advantages over the freely dissolved chemotherapeutics, including an increased drug accumulation in the tumour, the use of higher doses over shorter periods of time, less side effects, and/or a reduced off-target uptake.1,2a In difference to freely dissolved drugs, nanocarriers also offer the option of straightforward tracking for instantaneous assessment of drug delivery and targeting.3Current nanocarrier concepts often suffer from inadequate drug loading (often <20% of total nanocarrier mass), high material complexity, uncontrolled drug leakage, limited cell uptake, damage of cell membranes, unexpected toxicity and/or hypersensitivity.4 Typically, the active drug is encapsulated in certain matrix material such as organic polymers (e.g. polyethyleneglycol/PEG) or biopolymers (e.g., polysaccharides, polypeptides),5 liposomes or micelles,6 or inorganic matrices such as silica, iron oxides, and metal phosphates.7 Here, the matrix material usually represents the majority phase of the nanocarrier (often >80% of total nanocarrier mass) and – although not being an active drug – the matrix material may nevertheless cause toxic or allergic effects and needs to be biodegradable to ensure a complete release from the body. Moreover, current nanocarrier formulations predominately contain only a single drug. Nanocarriers with a combination of several cytostatic agents were yet barely addressed although state-of-the-art clinical tumour therapy relays on drug cocktails with two or more chemotherapeutics (e.g. FOLFIRINOX® with 5-fluorouracil, irinotecan and oxaliplatinum).7 Drug cocktails with two or more chemotherapeutics and different pharmacological properties for a simultaneous release at the site of the tumour with high concentration are highly desirable to reduce the therapeutic dose, to enhance the anticancer efficacy due to synergistic effects, and/or to minimize the risk of multi-drug resistance.8 To this regard, nanocarrier-based platforms can be highly promising.9
Aiming at novel chemotherapeutic nanocarriers, we here specifically aim at drug cocktails and achieving a high drug load of the nanocarriers (>50% of total nanocarrier mass).10 As a proof-of-the-concept, we have selected two chemotherapeutics with different properties: fludarabine phosphate (FLP) and irinotecan (ITC). FLP is highly hydrophilic, water soluble and blocks the enzymes ribonucleotide reductase and DNA polymerase, which are important for cell division and DNA synthesis.11 ITC inhibits topoisomerase I and is – in contrast to FLP – lipophilic and poorly soluble in water.12 Accordingly, FLP and ITC exhibit different mechanisms of action and are both known for high activity not only against pancreatic ductal adenocarcinoma (PDAC), but also against other solid tumors and blood cancers in both clinical and experimental settings.13 Especially for PDAC, which is the most common type of pancreatic cancer (90% of incidences) with a 5 years survival rate of only 8%,14 due to late detection, tumor heterogeneity, intrinsic chemoresistance and treatment failure.15 Thus, there is an urgent need for novel therapeutic strategies and novel multidrug delivery systems, which hold the promise to prolong survival and quality-of-life for chemotherapy-treated PDAC patients.
Experimental section
Nanocarrier synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 5 min). Thereafter, the centrifugate was resuspended in 10 mL of ammonium acetate solution (20 mg, 259 μmol) by ultrasonic irradiation (Bandelin Sonoplus HD 2070, Germany) with an amplitude of 50% for 3 min. Upon further ultrasonic treatment (1 min), a solution of H2(FLP) (10 mg, 27.4 μmol) was added over a period of 10 s.
000 × g, 5 min). Thereafter, the centrifugate was resuspended in 10 mL of ammonium acetate solution (20 mg, 259 μmol) by ultrasonic irradiation (Bandelin Sonoplus HD 2070, Germany) with an amplitude of 50% for 3 min. Upon further ultrasonic treatment (1 min), a solution of H2(FLP) (10 mg, 27.4 μmol) was added over a period of 10 s.
For fluorescence labelling of the nanocarriers, 50 μL of a solution with the nucleotide-based dyes Dyomics DUT 549-aadUTP or ATTO 647-aadUTP (50 nmol) was added to the H2(FLP) solution prior to injection. Finally, the suspension was adjusted to pH 8 by addition of 0.1 M NaOH and stirred for additional 10 min. After centrifugation (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 10 min), the fluorescence-labelled nanocarriers were centrifuged/redispersed from/in demineralized water. Finally, they were resuspended in sodium citrate solution (18.6 μmol) or dried in vacuum at room temperature to obtain powder samples.
000 × g, 10 min), the fluorescence-labelled nanocarriers were centrifuged/redispersed from/in demineralized water. Finally, they were resuspended in sodium citrate solution (18.6 μmol) or dried in vacuum at room temperature to obtain powder samples.
The synthesis of Toc@Gd2(AMP)3 nanoparticles (AMP: adenosinemonophosphate) as drug-free negative control followed the same synthesis procedure. It must be noticed that no ITC was dissolved in the microemulsion. Moreover, a solution of Na2(AMP) (9.5 mg; 27.4 μmol) in 5 mL of water was added instead of a solution of H2(FLP).
Analytical equipment
Cell studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and KPC murine PDAC cell lines18,19 as well as Capan-1 human cell line20 were used.
17 and KPC murine PDAC cell lines18,19 as well as Capan-1 human cell line20 were used.
MH-S cells were bought from ATCC (CRL-2019™)21 and Panc02 cells were kindly provided by Dr S. F. Pedersen (Section for Cell Biology and Physiology, Department of Biology, Faculty of Science, University of Copenhagen, Denmark). KPC cells (KPCbl6, clone 2.2) were kindly provided by Prof. Dr Volker Ellenrieder (Clinic for Gastroenterology, Gastrointestinal Oncology and Endocrinology, University Medical Center Göttingen, Germany). Capan-1 was bought from ATCC (HTB-79™).
MH-S cells were grown in RPMI medium supplemented with 10% fetal bovine serum (FBS, PAA Laboratories Gold), 0.1% mercaptoethanol, sodium pyruvate, L-glutamine, and D-glucose (Gibco), Panc02 were grown in Dulbecco's Modified Eagle Medium (DMEM, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, L-glutamine, and D-glucose (Gibco), KPC in 10% fetal bovine serum (FBS), 1% NEAA (non-essential amino acids), sodium pyruvate, L-glutamine, and D-glucose (Gibco) and Capan-1 in Iscove's Modified Dulbecco's Medium (IMDM) with 20% FBS sodium pyruvate, L-glutamine, and D-glucose (Gibco).
The cell line SK-Mel-28 is a cell line isolated from the skin of male patients with malignant melanoma. The HeLa cell line is the oldest human cell line derived from cervical cancer cells. Both types of cells were cultured in (DMEM, Thermo Fisher Scientific) supplemented with 10% (FBS, Thermo Fisher Scientific) and 1% penicillin/streptomycin (P/S, Thermo Fisher Scientific). All cells were cultivated at 37 °C under a humidified atmosphere of 5% CO2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 and allowed to attach for 4 h. After, the cells were treated with gradient concentrations according to the FLP content in the IOH-NPs, 10–100 μM of ITC/Toc@Gd2(FLP)3 and the corresponding amounts of reference IOH-NPs as well as control solutions with ITC, FLP, and the combination of both ITC and FLP. Cell confluence was measured over two weeks, using the live cell imaging system (Incucyte® ZOOM; Sartorius). Phase-contrast images (two images per well) were acquired every hour using a 10× objective. The concentration-dependent efficacy and the half-maximal inhibitory concentration (IC50) were calculated using GraphPad Prism 9 software, based on the cell confluence at 72 h.
000 cells per cm2 and allowed to attach for 4 h. After, the cells were treated with gradient concentrations according to the FLP content in the IOH-NPs, 10–100 μM of ITC/Toc@Gd2(FLP)3 and the corresponding amounts of reference IOH-NPs as well as control solutions with ITC, FLP, and the combination of both ITC and FLP. Cell confluence was measured over two weeks, using the live cell imaging system (Incucyte® ZOOM; Sartorius). Phase-contrast images (two images per well) were acquired every hour using a 10× objective. The concentration-dependent efficacy and the half-maximal inhibitory concentration (IC50) were calculated using GraphPad Prism 9 software, based on the cell confluence at 72 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 were plated on coverslips and incubated for different times (30 min, 5, 24, 48 h) with 12.5 ng mL−1 of the respective nanocarriers. The coverslips were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature and counterstained and mounted with 4′,6-diamidino-2-phenylindole (DAPI, 1
000 cells per cm2 were plated on coverslips and incubated for different times (30 min, 5, 24, 48 h) with 12.5 ng mL−1 of the respective nanocarriers. The coverslips were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature and counterstained and mounted with 4′,6-diamidino-2-phenylindole (DAPI, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Thermo Fisher Scientific, Germany). Fluorescence was visualized with a SP5 Leica TCS X confocal microscope (Leica Microsystems, Germany).
1000, Thermo Fisher Scientific, Germany). Fluorescence was visualized with a SP5 Leica TCS X confocal microscope (Leica Microsystems, Germany).
For the analysis of HeLa and SKMel28 cells, the cells were seeded at the density of 2 × 104 cells per well in an 8-well μSlide from IBIDI plate and cultured for 24 h. Afterwards, the cells were incubated for 1 day with the 50 μg per mL ITC/Toc@Gd2(FLP)3 nanocarriers, then stained for mitochondria (125 nM MitoTracker™ Green FM, Thermo Fisher Scientific, Germany) and nuclei (2 μg per mL Hoechst 33342, Thermo Fisher Scientific, Germany), and finally examined with fluorescence confocal microscope (STELLARIS 5, Leica Microsystems, Germany).
Results and discussion
Synthesis of ITC/Toc@Gd2(FLP)3 core@shell nanocarriers
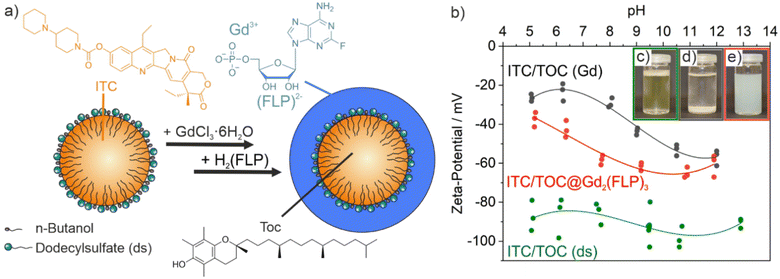
ITC/Toc@Gd2(FLP)3 nanocarriers with a cocktail of lipophilic ITC and hydrophilic FLP were synthesized via a microemulsion approach (Fig. 1). First of all, an oil-in-water microemulsion (o/w-ME) was prepared using α-tocopherol as the oil phase, ITC as a lipophilic chemotherapeutic agent as well as sodium dodecylsulfate (SDS) with dodecylsulfate (ds) serving as surfactant and n-butanol as co-surfactant (SDS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-butanol = 1
n-butanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Fig. 1a). α-Tocopherol – also known as vitamin E – has the advantage to be highly biocompatible.23 After mixing and equilibration, a transparent pale yellow microemulsion was obtained with the yellow colour indicating the presence of ITC (Fig. 1c).
2) (Fig. 1a). α-Tocopherol – also known as vitamin E – has the advantage to be highly biocompatible.23 After mixing and equilibration, a transparent pale yellow microemulsion was obtained with the yellow colour indicating the presence of ITC (Fig. 1c).
In order to stabilize the ITC-containing micellar droplet with a solid shell and in order to add FLP as the second chemotherapeutic agent, an aqueous solution of GdCl3 × 6H2O was added slowly. Gd3+ efficiently coordinates to the sulphate groups of ds. On the one hand, this can be directly visualized since changing the surface charge from the negative sulfonate groups of the surfactant to positively charged Gd3+ on the surface of the micelles results in a destabilization of the micelle, which causes a colourless fluffy precipitate (Fig. 1d). Moreover, the change of the surface charge can be followed by a zeta-potential analysis (Fig. 1b). Thus, the ITC-containing microemulsion exhibits a highly negative charge of −80 to −100 mV at pH 5–13, which relates to the anionic head groups of ds as the surfactant. After addition of Gd3+, the surface charge decreases to −20 to −50 mV along with the coordination of Gd3+ to the sulfate head groups of ds. The reduced surface charge and the cation-based surface termination are causative for the low colloidal stability of the suspension at this point of the synthesis.
As a next step, an aqueous solution of H2(FLP) was added within about 10 seconds to initiate the formation of a Gd2(FLP)3 shell around the ITC-filled micellar droplet (Fig. 1a). During the addition, the suspension was homogenized by sonification in order to prevent agglomeration and to ensure the formation of a uniform shell. Upon formation of a Gd2(FLP)3 shell, the surface charge returns to more negative values of −40 to −65 mV due to coordination of Gd3+ with the FLP phosphate groups and the surface termination with the highly polar FLP (Fig. 1b). Again, the course of the synthesis can be also followed with the naked eye since suspensions of the final ITC/Toc@Gd2(FLP)3 core@shell nanoparticles are colloidally highly stable due to negative surface charging (Fig. 1e). After purification by centrifugation and redispersion to remove dissolved salts and excess starting materials, aqueous ITC/Toc@Gd2(FLP)3 core@shell nanocarrier suspensions are colloidally stable and do not show precipitation over a period of several weeks.
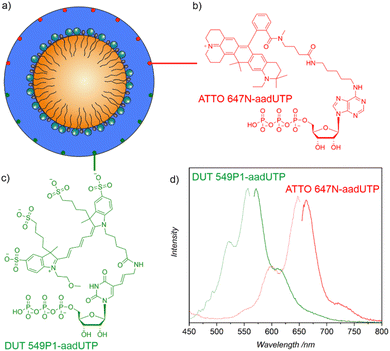
To allow a fluorescence detection of the ITC/Toc@Gd2(FLP)3 core@shell nanocarriers, small amounts of nucleotide-based dyes such as Dyomics DUT549P1-aadUTP or ATTO647N-aadUTP can be added together with FLP (Fig. 1). As both dyes contain triphosphate groups, they are encapsulated together with FLP in the nanocarrier shell (Fig. 2a). Successful incorporation of the dyes is confirmed qualitatively by the resulting blue (DUT 549) or pink (ATTO) colour of the nanocarrier suspensions. The presence of the dyes is validated by fluorescence spectroscopy (Fig. 2d). Thus, the nanocarrier suspensions show strong absorption at 450–550 nm (DUT 549) and 525–650 nm (ATTO 647). Green and red emission is observed at 565–725 nm (DUT 549) and 655–775 nm (ATTO 647) with maximum emission at 572 nm for DUT549P1-aadUTP-labelled nanocarriers and maximum emission at 664 nm for ATTO647N-aadUTP-labelled nanocarriers.
Chemical composition of ITC/Toc@Gd2(FLP)3 nanocarriers
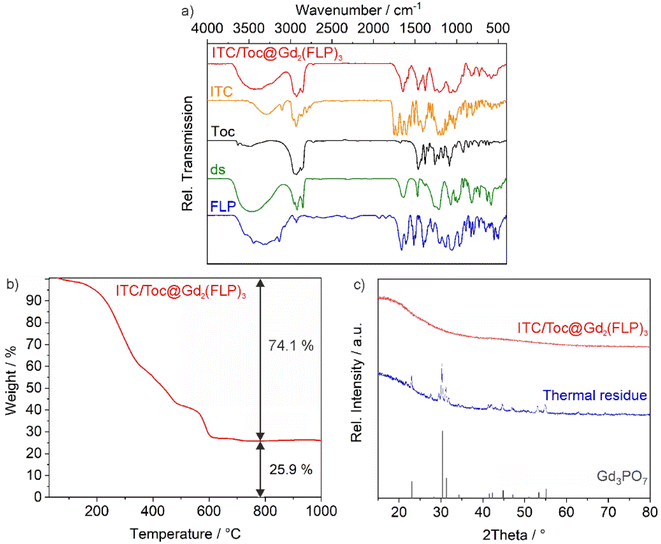
The chemical composition of the as-prepared ITC/Toc@Gd2(FLP)3 nanocarriers was examined by different methods, including X-ray powder diffraction (XRD), Fourier-transformed infrared (FT-IR) spectroscopy, thermogravimetry (TG), elemental analysis (EA), and photometry (Fig. 3 and 4). According to XRD, the nanocarriers are amorphous, which is to be expected for such organophosphates with voluminous anions and a synthesis at room temperature (Fig. 3c). Aiming at drug release, however, amorphous drug nanocarriers are considered to be advantageous as the dissolution rate is often enhanced in comparison to crystalline drug nanocarriers.24FT-IR spectroscopy, first of all, confirms the presence of phosphate-related vibrations with high intensity (ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1250, 1020 cm−1) and the characteristic carbon–nitrogen stretching vibrations of the purine ring (ν(C
O): 1250, 1020 cm−1) and the characteristic carbon–nitrogen stretching vibrations of the purine ring (ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N): 1610 cm−1) that are both related to FLP. Furthermore, the presence of Toc and ds is confirmed by carbon–hydrogen stretching vibrations of the alkyl chains (ν(C–H): 3000–2850 cm−1) and the characteristic sulfur–oxygen stretching vibrations (ν(S
N): 1610 cm−1) that are both related to FLP. Furthermore, the presence of Toc and ds is confirmed by carbon–hydrogen stretching vibrations of the alkyl chains (ν(C–H): 3000–2850 cm−1) and the characteristic sulfur–oxygen stretching vibrations (ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1300–1200 cm−1) (Fig. 3a). Vibrations of ITC are not visible as they are less characteristic and superimposed by other vibrations. Thermogravimetry (TG) is used to determine the total-organics content and indicates a more-or-less continuous thermal decomposition of the nanocarriers up to a temperature of 700 °C (Fig. 3b). After drying in vacuum to remove all surface-adsorbed water and solvents, a mass loss of 74.1 wt% is observed. A solid remain of 25.9 wt% occurred and was identified by XRD as Gd3PO7 (Fig. 3c). Furthermore, EA results in a content of 54.3% C, 7.1% H, 3.9% N, and 1.4% S. Hereof, the sulfur content can be assigned to ds as the sole S-containing compound. The N content stems from the drugs ITC and FLP. Considering these data, the as-prepared nanocarriers can be estimated to contain 3.0% ITC, 49.1% Toc, 11.6% ds, 18.7% FLP and 17.6% Gd, resulting in calculated values of 54% C, 7% H, 4% N, 2% S, a total weight loss of 76% and a solid residue of 24%. These calculated values are in good agreement with the experimental data (TG, EA) and also in agreement with the drug load obtained by photometry (see below).
O): 1300–1200 cm−1) (Fig. 3a). Vibrations of ITC are not visible as they are less characteristic and superimposed by other vibrations. Thermogravimetry (TG) is used to determine the total-organics content and indicates a more-or-less continuous thermal decomposition of the nanocarriers up to a temperature of 700 °C (Fig. 3b). After drying in vacuum to remove all surface-adsorbed water and solvents, a mass loss of 74.1 wt% is observed. A solid remain of 25.9 wt% occurred and was identified by XRD as Gd3PO7 (Fig. 3c). Furthermore, EA results in a content of 54.3% C, 7.1% H, 3.9% N, and 1.4% S. Hereof, the sulfur content can be assigned to ds as the sole S-containing compound. The N content stems from the drugs ITC and FLP. Considering these data, the as-prepared nanocarriers can be estimated to contain 3.0% ITC, 49.1% Toc, 11.6% ds, 18.7% FLP and 17.6% Gd, resulting in calculated values of 54% C, 7% H, 4% N, 2% S, a total weight loss of 76% and a solid residue of 24%. These calculated values are in good agreement with the experimental data (TG, EA) and also in agreement with the drug load obtained by photometry (see below).
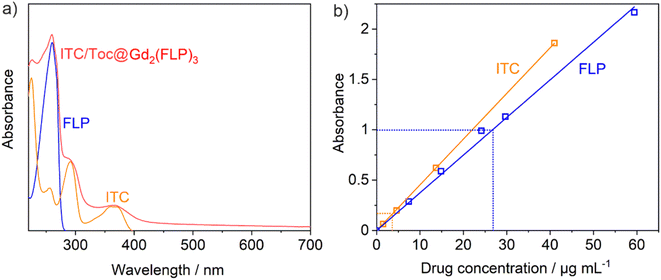
Even more important than the sum composition is the ITC and FLP content of the ITC/Toc@Gd2(FLP)3 nanocarriers, which was quantified by photometry (Fig. 4). To this concern, the characteristic absorption of ITC at 360 nm and of FLP at 260 nm were measured and compared to reference solutions with known concentration of ITC or FLP (Fig. 4a). For this purpose, calibration curves of both ITC dissolved in ds-stabilized ITC/Toc microemulsions and FLP dissolved in water were recorded (Fig. 4b). The calibration curves show an almost ideal linear dependence between the absorbance of ITC/FLP and the respective concentration in a range of 0–60 μg mL−1 (Fig. 4b). Based on the photometrical quantification, the as-prepared suspensions can be concluded to contain ITC/Toc@Gd2(FLP)3 nanocarriers with a concentration of c(ITC) = 49 μg mL−1 and c(FLP) = 317 μg mL−1 at a nanocarrier concentration of 1.5 mg mL−1. Here, it needs to be noticed that the superposition of the absorbance at 260 nm was tackled by subtracting the absorbance of ITC from the peak intensity at 260 nm to obtain the absorbance related to FLP. Furthermore, the as-prepared suspensions were diluted by a factor of 12 to guarantee a linear correlation of absorbance and concentration according to the Lambert–Beer law.16 The ratio of ITC and FLP of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 determined via photometry is also in agreement with the estimation based on the TG and EA results.
6.5 determined via photometry is also in agreement with the estimation based on the TG and EA results.
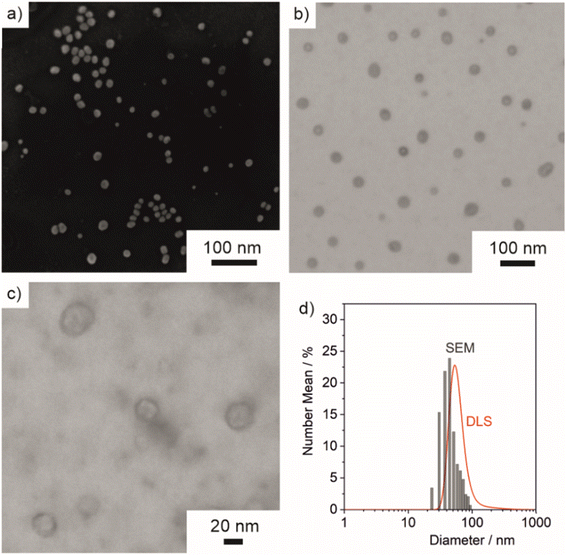
Size and structure of ITC/Toc@Gd2FLP3 nanocarriers
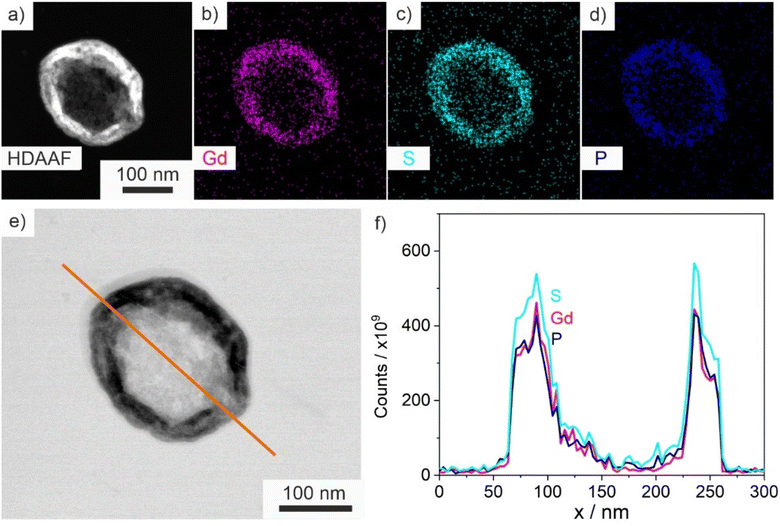
Size, size distribution, and structure of the as-prepared ITC/Toc@Gd2(FLP)3 nanocontainers were examined by dynamic light scattering (DLS) and electron microscopy. To this concern, DLS performed with aqueous suspensions lead to a mean hydrodynamic diameter of 63 ± 33 nm (Fig. 5d). A statistical evaluation of >250 nanoparticles on scanning electron microscopy (SEM) images resulted in a slightly smaller diameter of 45 ± 16 nm (Fig. 5a and d), which is in accordance with the larger hydrodynamic diameter stemming from DLS. Scanning transmission electron microscopy (STEM) images confirm the spherical shape and point to the presence of a core@shell structure (Fig. 5b and c). Based on STEM images with higher-resolution, furthermore, the inner cavity diameter as well as the wall thickness can be determined to 40 ± 17 nm and 3–8 nm, respectively.The core@shell-type structure and the chemical composition are further validated by energy-dispersive X-ray spectroscopy (EDXS). Thus, EDXS element mappings display a uniform distribution of gadolinium, phosphorous, and sulphur around an inner cavity (Fig. 6a–d). These elements reflect the presence of Gd2(FLP)3 as the nanocarrier shell. Furthermore, EDXS line scans along the orange line on HAADF-STEM images elucidate the core@shell structure with a characteristic dip of the elements Gd, P and S in the center of the nanocarrier (Fig. 6e and f). Here, it must be noticed that the ITC/Toc@Gd2(FLP)3 core@shell nanocarriers show rapid decomposition under the electron beam. This is due to the high organic content and the low conductivity of the nanocarriers as well as due to the bombardment with high-energy electrons causing local heating and charging. Due to the low stability, we have specifically selected larger ITC/Toc@Gd2(FLP)3 core@shell nanocarriers for EDXS area- and line-scans.
Cytostatic activity of ITC/Toc@Gd2(FLP)3 nanocarriers
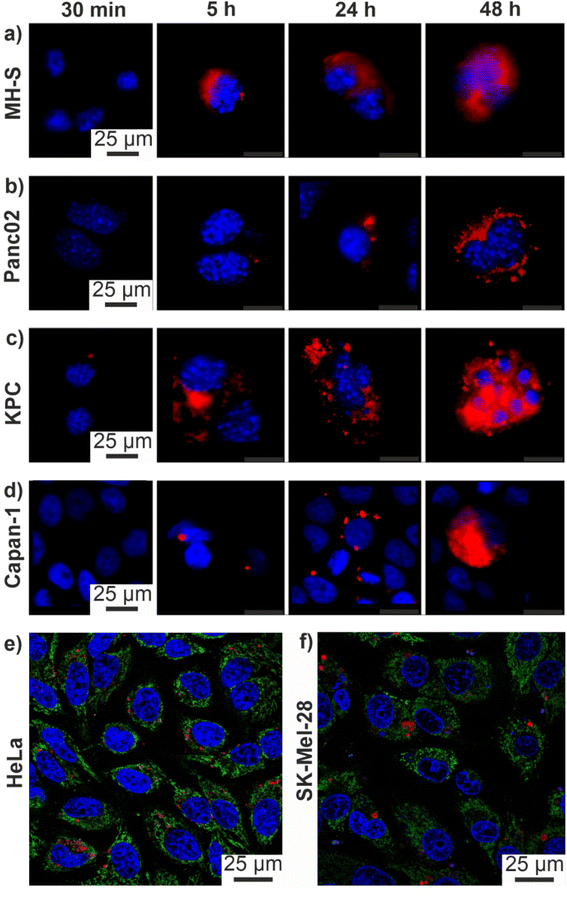
PDAC exhibits a specifically high probability for mutations, which results in a high genetic diversity of the respective cells. Due to the influence of different drugs on the susceptibility of the cells, these mutations can significantly complicate an effective therapy. For this reason, the efficacy of the ITC/Toc@Gd2(FLP)3 nanocarriers was tested in murine KPC and Panc02 cell lines. Panc02 is a well-established grade III adenocarcinoma model developed by chemical induction with 3-MCA (3-methylcholanthrene) in male C57BL/6 mice,17 With a mutation of the SMAD4 gene only, Panc02 cells are less relevant in regard of the mutational spectrum of clinically occurring PDAC, nevertheless they are important for preclinical studies to examine nanocarrier uptake and efficacy. Furthermore, KPC cells derived from a KPC mouse representing a transgenic model of PDAC were applied, which exhibit mutations of the KRAS and p53 genes, resulting in non-functional proteins. Therefore, the KPC cell line closely mimmicks the clinical situation, reflecting mutations often occurring for PDAC patients.To evaluate the efficacy of the as-prepared ITC/Toc@Gd2(FLP)3 nanocarriers, first of all, an effective uptake of the nanocarriers by tumour cells is essential. For this reason, the uptake of the nanocarriers was assessed by confocal microscopy with cancer cells with different origin (Fig. 7). To prevent premature death of the investigated cells, uptake studies were conducted with drug-free Toc@Gd2(AMP)3 nanoparticles. These reference nanoparticles (negative control) only contain Toc in the particle core (but no ITC) and Gd2(AMP)3 with adenosine monophosphate (AMP) as the particle shell. AMP is chemically similar to FLP but does not contain any fluorine at the uridine unit so that DNA reproduction is not blocked. Similar to ITC/Toc@Gd2(FLP)3, the Toc@Gd2(AMP)3 reference nanoparticles can be labelled either with DUT 549 or ATTO 647 as a fluorescent dye (Fig. 2).
To account for the genetic versatility of PDAC, the two mouse pancreatic cancer cell lines Panc02 and KPC, the human pancreatic cancer cell line Capan-1 as well as the MH-S macrophage cell line (positive control: murine alveolar macrophages known for efficient uptake of nanoparticles) were treated with Toc@Gd2(AMP)3 nanoparticles (12.5 ng mL−1). The cell uptake was analysed via confocal microscopy 0.5, 5, 24 and 48 h after incubation. ATTO647-labelled Toc@Gd2(AMP)3 can be clearly detected due to their intense red emission (Fig. 7a–d). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) showing blue emission. A time-dependent uptake of these nanocarriers is clearly visible and indicates a massive nanocarrier concentration not only in the MH-S macrophages (Fig. 7a) but also in all pancreatic cancer cells 48 h after incubation. Due to their high phagocytizing ability, nanocarrier accumulation in MH-S cells was already observed after 5 h. After 24 and 48 h, massive nanocarrier uptake is also observed in all PDAC cells (Fig. 7b–d). Among the PDAC cells, in particular, KPC cells show fast nanocarrier uptake with substantial amounts already observed after 5 h (Fig. 7c).
In addition to PDAC cells, the uptake of the ITC/Toc@Gd2(FLP)3 nanocarriers was also investigated on other tumour-cell lines to demonstrate their versatility also on a more general level. For this purpose, human skin melanoma cells (SK-Mel-28) and cervical cancer cells (HeLa) were selected and treated with the nanocarriers as well (Fig. 7e and f). Cell uptake was again investigated via confocal microscopy 72 h after incubation with suspensions of ATTO 647-labeled ITC/Toc@Gd2(FLP)3 nanocarriers (50 μg mL−1). With 50 μg mL−1 of the nanocarriers, here, a higher concentration was necessary as compared to the treatment of the pancreatic tumour cells (12.5 ng mL−1). Thereafter, the nanocarriers could be clearly visualized in SK-Mel-28 and HeLa cells based on their red fluorescence. The lower nanocarrier uptake in SK-Mel-28 and HeLa cells in comparison to Panc02, KPC, and Capan-1 cells, in fact, could give rise to a possible specificity to tumour cells with high phagocytic activity.
In all tested cells, the nanocarriers predominately accumulate in the perinuclear region (Fig. 7), especially at later time points, suggesting their presence in late endosomal/lysosomal vesicles. We have observed similar intracellular localization patterns also for other, comparable types of nanocarriers, suggesting an endocytic internalization pathway with late endosomal/lysosomal localization after 24 and 48 h, respectively.25
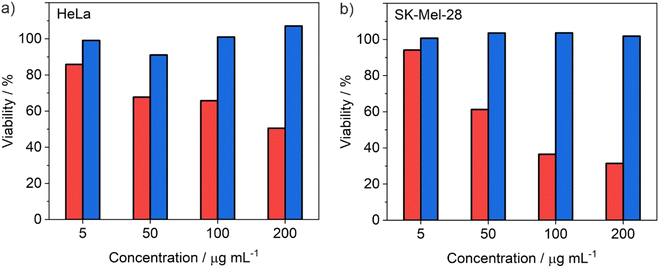
The ability of the ITC/Toc@Gd2(FLP)3 nanocarriers, loaded with the antitumour drugs ITC and FLP, to inhibit cell growth and proliferation of tumour cells was assessed by various in vitro studies. For an initial assessment of the cytotoxic activity, ITC/Toc@Gd2(FLP)3 nanocarriers were examined by MTT toxicity assays, using SK-Mel-28 and HeLa cells as standard cell types (Fig. 8). Herein, the viability of the respective cells was quantified by their ability to reduce the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) to formazan. After 72 h of incubation, a clear concentration-dependent effect (5–200 μg mL−1) is visible. ITC/Toc@Gd2(FLP)3 nanocarriers (red columns) lead to a strong decrease in cell viability with half-maximal inhibitory concentrations (IC50) of 75 μg mL−1 for SKMel28 (Fig. 8b) and 195 μg mL−1 for HeLa cells (Fig. 8a), whereas drug-free Toc@Gd2(AMP)3 nanocarriers (blue columns) as a negative control do not show any considerable effect. The latter points to the absence of non-specific toxic effects of the nanocarriers as such and their non-drug ingredients (i.e., Gd, Toc, ds).
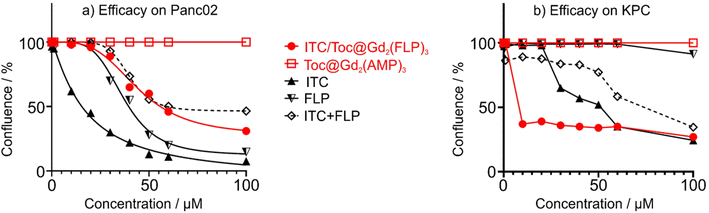
For in vitro evaluation of the nanocarrier efficacy, the Panc02 and KPC cells were plated out with a concentration of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 and treated with different concentrations of the ITC/Toc@Gd2(FLP)3 nanocarriers. Their confluence was measured via life-cell imaging once every hour (Fig. 9). The concentration-dependent efficacy was calculated after 72 h of incubation – a point in time when control cells reached 100% confluence. At concentrations ≥ 40 μM (Panc02) and ≥10 μM (KPC), the nanocarriers show high effectiveness in killing both cell types, leading to a significant reduction in confluence. Studies on Panc02 cells show a confluence decrease to 29% (Fig. 9a). In the case of KPC cells, confluence was even further decreased to only 24% (Fig. 9b). Whereas the efficacy of ITC/Toc@Gd2(FLP)3 nanocarriers on Panc02 cells is already higher compared to the combined free drugs ITC and FLP (as indicated by IC50, i.e. the concentration necessary to reduce cell confluence by 50%, Table 1), the most promising efficacy of ITC/Toc@Gd2(FLP)3 nanocarriers is on KPC cells as further evidenced by their IC50 (Table 1). For KPC cells, an IC50 of 4.2 μM was calculated after 72 h, which is >10 times lower than for the free drugs ITC (47.7 μM) and FLP (143 μM). This clearly implies the KPC cells to be more sensitive to the ITC/Toc@Gd2(FLP)3 nanocarrier formulation than to the corresponding free drugs. This also points to the synergistic effect of the ITC/FLP drug cocktail combined in a single type of nanocarriers. While these in vitro data already show the efficient uptake, the high efficacy as well as certain selectivity for specific tumor cells, the full potential of the ITC/Toc@Gd2(FLP)3 nanocarriers can only be unlocked from in vivo studies, which also consider selective nanocarrier delivery and potential side effects.
000 cells per cm2 and treated with different concentrations of the ITC/Toc@Gd2(FLP)3 nanocarriers. Their confluence was measured via life-cell imaging once every hour (Fig. 9). The concentration-dependent efficacy was calculated after 72 h of incubation – a point in time when control cells reached 100% confluence. At concentrations ≥ 40 μM (Panc02) and ≥10 μM (KPC), the nanocarriers show high effectiveness in killing both cell types, leading to a significant reduction in confluence. Studies on Panc02 cells show a confluence decrease to 29% (Fig. 9a). In the case of KPC cells, confluence was even further decreased to only 24% (Fig. 9b). Whereas the efficacy of ITC/Toc@Gd2(FLP)3 nanocarriers on Panc02 cells is already higher compared to the combined free drugs ITC and FLP (as indicated by IC50, i.e. the concentration necessary to reduce cell confluence by 50%, Table 1), the most promising efficacy of ITC/Toc@Gd2(FLP)3 nanocarriers is on KPC cells as further evidenced by their IC50 (Table 1). For KPC cells, an IC50 of 4.2 μM was calculated after 72 h, which is >10 times lower than for the free drugs ITC (47.7 μM) and FLP (143 μM). This clearly implies the KPC cells to be more sensitive to the ITC/Toc@Gd2(FLP)3 nanocarrier formulation than to the corresponding free drugs. This also points to the synergistic effect of the ITC/FLP drug cocktail combined in a single type of nanocarriers. While these in vitro data already show the efficient uptake, the high efficacy as well as certain selectivity for specific tumor cells, the full potential of the ITC/Toc@Gd2(FLP)3 nanocarriers can only be unlocked from in vivo studies, which also consider selective nanocarrier delivery and potential side effects.
| Treatment | Panc02 cells | KPC cells |
|---|---|---|
| ITC/Toc@Gd2(FLP)3 | 46.4 | 4.2 |
| Free ITC | 13.5 | 47.7 |
| Free FLP | 34.2 | 143.2 |
| Free ITC + FLP | 59.4 | 84.0 |
Conclusions
After single-drug chemotherapeutic nanocarriers having reached clinical approval, nanocarriers containing a cocktail of chemotherapeutics are the next step for nanocarrier-based tumour treatment. Such adjunctive approach can become most relevant for combinational therapy, particularly in treating with grim prognosis such as the pancreatic ductal adenocarcinoma (PDAC). This approach allows to simultaneously release high concentrations of drug cocktails at the tumor site, to enhance anticancer efficacy through synergistic or additive effects, to reduce side effects, and/or to minimize the risk of multi-drug resistance. In this regard, we present ITC/Toc@Gd2(FLP)3 core@shell nanocarriers with a chemotherapeutic cocktail of lipophilic irinotecan (ITC) and hydrophilic fludarabine phosphate (FLP) for the first time.ITC/Toc@Gd2(FLP)3 core@shell nanocarriers contain the lipophilic ITC (49 μg mL−1) as the particle core and the hydrophilic FLP (317 μg mL−1) in the particle shell. Size and structure of the nanocarriers are validated by different methods (XRD, FT-IR, TG, EA, photometry, SEM, STEM, HAADF-TEM, EDXS) and result in a total diameter of 45 ± 16 nm, an inner core diameter of 40 ± 17 nm, and a shell thickness of 3–8 nm. With a nanocarrier concentration of 1.5 mg mL−1, aqueous suspensions are colloidally stable over several weeks. For fluorescence detection in vitro, the nanocarriers are labelled with Dyomics DUT549P1-aadUTP or ATTO647N-aadUTP to result in green or red emission. The anti-tumour efficacy of ITC/Toc@Gd2(FLP)3 nanocarriers were studied in vitro with mouse and human pancreatic cancer cell lines (Panc02, KPC, Capan-1) and non-PDAC tumour cell lines (SK-Mel-28, HeLa). Specifically for the murine pancreatic KPC cancer cells, ITC/Toc@Gd2(FLP)3 nanocarriers show very promising efficacy with an inhibitory concentration (IC50: 4.2 μM) outperforming the free drugs (ITC: 47.7 μM, FLP: 143 μM) more than 10 times. This aligns with the impressively early uptake of the high amounts of the IOH-NPs by KPC cells as observed by fluorescence microscopy.
With these results, the core@shell chemotherapeutic cocktail concept can become a highly promising tool for future drug delivery strategies and combined chemotherapies especially for the treatment of pancreatic cancer with poor prognosis. Following the establishment of the synthesis strategy of core@shell dual-drug nanocarriers, an adjustment of drug content and drug synergistic ratio as well as a transfer to other drugs are intended and offer additional options for synthesis and material concepts.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
D. R., J. P., U. S. and C. F. thank the Deutsche Forschungsgemeinschaft (DFG) for funding within the Research Training Group 2039. Moreover, J. N. and C. F. are grateful to the DFG for funding with in the project “Synergistic Image-guided Nanoparticles for Drug Delivery (SIN-Drug)”. J. P. also thanks the Ministerium für Ernährung, Ländlichen Raum und Verbraucherschutz Baden-Württemberg (MLR) for financial support. U. S. thanks for funding under the Germany's Excellence Strategy via the Excellence Cluster “3D Matter Made to Order (EXC-2082/1-390761711)”. The work of J. P. and U. S. was also supported by the Helmholtz Program Materials Systems Engineering (MSE). Finally, the authors acknowledge Jacqueline Stier and Bärbel Heidrich for excellent assistance in experiments.References
- (a) F. Farjadian, A. Ghasemi, O. Gohari, A. Roointan, M. Karimi and M. R. Hamblin, Nanomedicine, 2019, 14, 93–126 CrossRef CAS PubMed; (b) D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- (a) Y. Barenholz, J. Controlled Release, 2012, 160, 117–134 CrossRef CAS PubMed; (b) W. J. Gradishar, S. Tjulandin, N. Davidson, H. Shaw, D. Heather, N. Desai, P. Bhar, M. Hawkins and J. O'Shaughnessy, J. Clin. Oncol., 2005, 23, 7794–7803 CrossRef CAS PubMed.
- (a) S. Kunjachan, J. Ehling, G. Storm, F. Kiessling and T. Lammers, Chem. Rev., 2015, 115, 10907–10937 CrossRef CAS PubMed; (b) T. L. Doane and C. Burda, Chem. Soc. Rev., 2012, 41, 2885–2911 RSC.
- (a) P. H. D. Nguyen, M. K. Jayasinghe, A. H. Le, B. Peng and M. T. N. Le, ACS Nano, 2023, 17, 5187–5210 CrossRef CAS PubMed; (b) K. Ulbrich, K. Hola, V. Subr, A. Bakandritsos, J. Tucek and R. Zboril, Chem. Rev., 2016, 116, 5338–5431 Search PubMed.
- (a) T. Senthilkumar, L. Zhou, Q. Gu, L. Liu, F. Lv and S. Wang, Angew. Chem., Int. Ed., 2018, 57, 13114–13119 CrossRef CAS PubMed; (b) C. D. Spicer, C. Jumeaux, B. Gupta and M. M. Stevens, Chem. Soc. Rev., 2018, 47, 3574–3620 Search PubMed; (c) K. Ulbrich, K. Hola, V. Subr, A. Bakandritsos, J. Tucek and R. Zboril, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- (a) T. M. Allen and P. R. Cullis, Adv. Drug Deliv. Rev., 2013, 65, 36–48 Search PubMed; (b) S. Parveen, R. Misra and S. K. Sahoo, Nanomedicine, 2012, 8, 147–166 CrossRef CAS PubMed.
- (a) A. Jozefczak, K. Kaczmarek and R. Bielas, Theranostics, 2021, 11, 10091–10113 CrossRef CAS PubMed; (b) M. Manzano and M. Vallet-Regi, Adv. Funct. Mater., 2020, 30, 1902634 Search PubMed; (c) S. Zhao, X. Yu, Y. Qian, W. Chen and J. Shen, Theranostics, 2020, 10, 6278–6309 CrossRef CAS PubMed.
- T. Conroy, C. Gavoille, E. Samalin, M. Ychou and M. Ducreux, Curr. Oncol. Rep., 2013, 15, 182–189 CrossRef CAS PubMed.
- A. Detappe, H. V. T. Nguyen, Y. Jiang, M. P. Agius, W. Wang, C. Mathieu, N. K. Su, S. L. Kristufek, D. J. Lundberg, S. Bhagchandani, I. M. Ghobrial, P. P. Ghoroghchian and J. A. Johnson, Nat. Nanotechnol., 2023, 18, 184–192 CrossRef CAS PubMed.
- (a) D. Rudolph, N. Redinger, K. Schwarz, F. Li, G. Hädrich, M. Cohrs, L. A. Dailey, U. E. Schaible and C. Feldmann, ACS Nano, 2023, 17, 9478–9486 CrossRef CAS PubMed; (b) M. Khorenko, A. Meschkov, J. Napp, J. Pfeifer, J. Stier, F. Alves, U. Schepers and C. Feldmann, J. Mater. Chem. B, 2023, 11, 3635–3649 RSC; (c) V. Rein, E. Zittel, K. Hagens, N. Redinger, U. Schepers, H. Mehlhorn, U. Schaible and C. Feldmann, Adv. Funct. Mater., 2019, 29, 1900543 CrossRef.
- W. Hiddemann, R. Rottmann, B. Wörmann, A. Thiel, M. Essink, C. Ottensmeier, M. Freund, T. Büchner and J. van de Loo, Ann. Hematol., 1991, 63, 1–4 CrossRef CAS PubMed.
- R. H. J. Mathijssen, R. J. van Alphen, J. Verweij, W. J. Loos, K. Nooter, G. Stoter and A. Sparreboom, Clin. Cancer Res., 2001, 7, 2182–2194 CAS.
- S. Stevanović, L. M. Draper, M. M. Langhan, T. E. Campbell, M. L. Kwong, J. R. Wunderlich, M. E. Dudley, J. C. Yang, R. M. Sherry, U. S. Kammula, N. P. Restifo, S. A. Rosenberg and C. S. Hinrichs, J. Clin. Oncol., 2015, 33, 1543–1550 CrossRef PubMed.
- (a) M. Orth, P. Metzger, S. Gerum, J. Mayerle, G. Schneider, C. Belka, M. Schnurr and K. Lauber, Radiat. Oncol., 2019, 14, 141–161 CrossRef PubMed; (b) T. Y. S. Le Large, M. F. Bijlsma, G. Kazemier, H. W. M. van Laarhoven, E. Giovannetti and C. R. Jimenez, Semin. Cancer Biol., 2017, 44, 153–169 CrossRef CAS PubMed.
- E. S. Christenson, E. Jaffee and N. S. Azad, Lancet Oncol., 2020, 21, e135 CrossRef CAS PubMed.
- T. G. Mayerhöfer, A. V. Pipa and J. Popp, ChemPhysChem, 2019, 20, 2748–2753 CrossRef PubMed.
- T. H. Corbett, B. J. Roberts, W. R. Leopold, J. C. Peckham, L. J. Wilkoff, D. P. Griswold and F. M. Schabel, Cancer Res., 1984, 44, 717–726 CAS.
- S. R. Hingorani, L. Wang, A. S. Multani, C. Combs, T. B. Deramaudt, R. H. Hruban, A. K. Rustgi, S. Chang and D. A. Tuveson, Cancer Cell, 2005, 7(5), 469–483 Search PubMed.
- J. W. Lee, C. A. Komar, F. Bengsch, K. Graham and G. L. Beatty, Curr. Protoc. Pharmacol., 2016, 73, 14.39.1–14.39.20 Search PubMed.
- A. P. Kyriazis, A. A. Kyriazis, D. G. Scarpelli, J. Fogh, M. S. Rao and R. Lepera, Am. J. Pathol., 1982, 106(2), 250–260 CAS.
- I. N. Mbawuike and H. B. Herscowitz, J. Leukoc. Biol., 1989, 46, 119–127 CrossRef CAS PubMed.
- W. S. Rasband, ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018, https://imagej.net/ij/ Search PubMed.
- A. Kamal-Eldin and L. A. Appelqvist, Lipids, 1996, 31, 671–701 CrossRef CAS PubMed.
- R. Jog and D. J. Burgess, J. Pharmaceut. Sci., 2017, 106, 39–65 CrossRef CAS PubMed.
- M. Ischyropoulou, K. Sabljo, L. Schneider, C. M. Niemeyer, J. Napp, C. Feldmann and F. Alves, Adv. Mater., 2023, 2305151 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |