A reflection on ‘Highly dispersible polypyrrole nanospheres for advanced nanocomposite ultrafiltration membranes’
Cheng-Wei
Lin
 a and
Richard B.
Kaner
a and
Richard B.
Kaner
 *ab
*ab
aDepartment of Chemistry and Biochemistry and California NanoSystems Institute, University of California, Los Angeles, California 90095, USA. E-mail: kaner@chem.ucla.edu
bDepartment of Materials Science and Engineering, University of California, Los Angeles, California 90095, USA
First published on 10th May 2024
Abstract
Conducting polymers are unique polymers that conduct electricity, but do not typically serve as the active material for water filtration membranes. In 2014, our group presented an integrated nanocomposite of conjugated polypyrrole and polysulfone to make ultrafiltration membranes (Y. Liao, T. P. Farrell, G. R. Guillen, M. Li, J. A. T. Temple, X.-G. Li, E. M. V. Hoek and R. B. Kaner, Mater. Horiz., 2014, 1, 58–64, https://doi.org/10.1039/C3MH00049D). This reflection briefly reviews the impacts in the field of conjugated polymer-based water filtration membranes, and the significance on the subsequent research in our group.
Previously, our group created ultrafiltration membranes made by blending a highly dispersible form of the conducting polymer polypyrrole with commercially available polysulfone. Polypyrrole nanospheres were synthesized using conventional oxidative polymerization, with the addition of a trace amount of 2,4-diaminodiphenylamine as an initiator. Solutions of polysulfone and polypyrrole dissolved in N-methly-2-pyrrolidone were cast on a nonwoven polyester fabric, followed by precipitation through nonsolvent induced phase separation (NIPS) with water at room temperature. The resulting membranes possessed smooth and shiny surfaces, and finger-like microstructures as water transporting channels. In terms of performance, the nanocomposite membranes showed significant improvements in hydrophilicity, permeability, thermal stability, and recovered permeability after bovine serum albumin (BSA) fouling, while retaining over 80% of BSA rejection. This work, featured on the front cover for the first issue of Materials Horizons in 2014 (https://doi.org/10.1039/C3MH00049D),1 successfully intertwined the expertise of our research group in conducting polymers and water filtration membranes. The leading author, Dr Yaozu Liao, is now a Professor at Donghua University in China. Since then, our research has focused on the development of conducting polymer-based water filtration membranes,2 eventually leading to two successful spin-off companies in the fields of water filtration membranes and biomedical applications (Fig. 1).
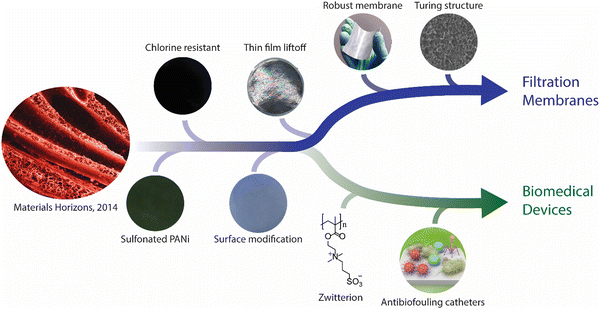 | ||
| Fig. 1 Our membrane research progressing towards filtration membranes and biomedical devices. (Reprinted with permission from ref. 22 Copyright 2013; ref. 29 Copyright 2019; ref. 30 Copyright 2020. American Chemical Society.) | ||
In revisiting this work, we found out that the incorporation of polypyrrole has had a strong impact on membrane fabrication with hybrid materials and the advancement of electrically conductive membranes (ECMs).3 Researchers have constructed membranes with composites of polymer matrices and functional materials, such as graphene, graphene oxide, halloysite, ferric oxide, and carbon nanotubes, to achieve enhanced hydrophilicity, antifouling, thermal stability, chlorine resistance, and biocidal activity.4–12 Despite the gentle decrease in sieving ability, deploying functional materials in polymer composites has significantly leveraged diverse functionalities of ultrafiltration membranes. On the other hand, ECMs have been attracting increasing interest owing to their ability to actively repel and remove foulants with a variety of electrochemical behaviors at surfaces, e.g. electrophoresis, electrostatic repulsion and adsorption, electroosmosis, electrolysis, and electrochemical oxidation and reduction, when voltages are applied.13–15 The facile preparation approach of this work demonstrated the feasibility and effectiveness of incorporating organic, inorganic, and carbon-derived electrically conductive fillers into membranes while keeping membrane performances up to the mark.16–20 For instance, Duan et al. reported an ultrafiltration membrane made of polysulfone and polyaniline coated carbon nanotubes, where the voltage-driven (3 V) in situ cleaning process effectively destroyed organic foulants, rendering much higher flux recoveries.21
Since our Materials Horizons publication, we have demonstrated significant improvements in hydrophilicity and chlorine resistance of membranes through the molecular engineering of conducting polyaniline. By functionalizing polyaniline with sulfonated groups under extremely acidic conditions, the resulting hydrophilic ultrafiltration composite membranes possessed higher flux (up to 207.4 L m−2 h−1 at 20 psi), lower flux decline (16%), negligible loss in the rejection rate of bovine serum albumin (BSA), and superior flux recovery (95%) compared to a pure polysulfone commercial membrane. The superhydrophilic and antifouling properties are attributed to the zwitterionic nature of the sulfonated polyaniline, where the hydroxyls in the sulfonic groups exhibit negative charges and protonated imines exhibit positive charges on the polymer backbone.22 In another study, we discovered that the addition of hydroxyethyl groups on the nitrogen of polyaniline not only inhibited gelation at high loading concentrations by eliminating intermolecular hydrogen bond formation, but also showed a high resistance to chlorine attack. The as-made 30 wt% poly(n-hydroxyethyl aniline) ultrafiltration membrane is hydrophilic with a contact angle of 36°; thus, showing less adhesion of E. coli and much lower BSA fouling (11% flux decline and 91% flux recovery) compared to pristine polyaniline membranes. The membrane possessed a permeability of 108.7 Lmh per bar and a 70.2% BSA rejection rate even after soaking in 250 ppm of sodium hypochlorite solution for 30 days, while a pristine polyaniline membrane completely lost its filtering ability within 2 days.23
The aforementioned research efforts in conjunction with our development of a simple chemical method to make polyaniline nanofibers led to a start-up company called Polycera, Inc. This start-up company developed from a close collaboration with Dr Eric Hoek, who was an assistant professor in the Department of Civil and Environmental Engineering at the University of California, Los Angeles (UCLA) at the time. Our first project, funded by Abraxis Biosciences, was to develop membranes to accelerate kidney dialysis. With our experience in start-up companies (Fibron, Inc. for Kaner and NanoH2O for Hoek) we founded Polycera, Inc. The name, Polycera, came from the properties of polyaniline, which is an inexpensive polymer, that acts like an expensive ceramic membrane in regard to separations and stability. First, the rigid backbone of polyaniline renders superior mechanical properties and is able to form composites.24 Second, polyaniline can survive temperatures above 300 °C based on thermogravimetric analysis, i.e., high environmental stability.25 Third, polyaniline remains intact when exposed to extremely acidic or basic conditions, i.e., a much wider pH compatibility.26
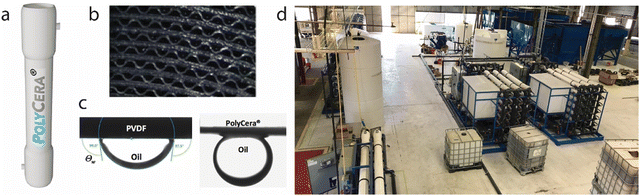
After talking with nephrologists and learning that the human body cannot take much faster kidney dialysis rates, Polycera, Inc., enlightened by the Deepwater Horizon oil spill in the Gulf of Mexico, turned its attention to fracking. Hydraulic fracturing, commonly called fracking, is a drilling method that involves high-pressure injection of water into underground oil and natural gas deposits. Unfortunately, for every barrel of oil extracted, 7–10 barrels of oily wastewater are produced. Reinjecting this oily wastewater back into the well is now outlawed as it often leads to ground water contamination, so the wastewater must be cleaned. The idea of using polyaniline membranes for oil–water separations led Water Planet, LLC to acquire Polycera, Inc. and they named their membrane PolyCera® (Fig. 2a), used to perfect the last part of their oil–water separation process. The corrugated feed channels in the membrane module (Fig. 2b) feature higher influent suspended solids, and increased tangential flow and shearing force to prevent fouling. The oleophobic and hydrophilic membrane surface (Fig. 2c) renders superior separation of water from oil. Water Planet demonstrated the ability to scale up the membrane fabrication (Fig. 2d) while reducing the overall oil clean-up costs by up to 40%. After Water Planet was acquired by PSP.US, PolyCera® membranes are now operating in over 100 oil installations worldwide.27
Next our research group developed a novel technique for creating ultrathin membranes called thin-film liftoff (T-FLO). Inspired by the process of peeling graphene from graphite using Scotch tape,28 this technique uses glue-like polymeric epoxies that serve as the supporting layer to delaminate a pre-cast active layer from a smooth substrate often coated with an Epoxease. This “bottom-up” approach, in contrast to the conventional “top-down” method, offers a non-destructive and facile route to construct an active layer of a thin film composite (TFC) membrane that previously was not possible to be made by conventional techniques. Another great advantage of the T-FLO technique is the formation of chemical bonds between the supporting and active layers, creating very durable membranes. Our first publication using T-FLO demonstrated that this method can be universally applied to organic solvent nanofiltration based on polybenzimidazole as the active layer. We also demonstrated N2/CO2 gas separation based on polyaniline as the active layer.29 In subsequent work, we showed how to precisely tune the thickness of a graphene oxide active layer (down to 32 nm) for organic dye removal. The thin graphene oxide (GO) layer possessed flexibility and stayed intact even after soaking in water for 30 days, while a vacuum filtered GO membrane disintegrated within 3 days while soaking in water. Notably, the T-FLO GO membrane demonstrated a nearly 100% enhancement in fracture toughness in a tensile test (force applied perpendicular to the membrane surface) compared to conventional GO membranes. Owing to the chemical bond formation between the epoxy supporting layer and the GO active layer, the T-FLO GO membrane can operate normally in a dead-end cell test at 30 psi even when installed backwards.30
Besides composite membranes, our group has been interested in controlling surfaces for use in technological applications. Back in 2014, our research group reported a scalable process to modify reverse osmosis (RO) membranes by employing perfluorophenyl azide chemistry.31 Polyethylene glycol (PEG) was chemically grafted onto RO membranes through highly reactive phenylnitrene radicals upon exposure to UV light.32 The resulting modified membranes showed a decreased contact angle (35° for modified membranes vs. 63° for unmodified ones) resulting in 20 times less adhesion of the bacterium E. coli. Thereafter, in light of our previous research efforts with the conjugated aniline tetramer,33–36 we successfully modified commercial polyethersulfone ultrafiltration membrane surfaces with aniline tetramer through similar techniques. The modified membranes demonstrated reversible doping and de-doping processes with the characteristic blue color under neutral and basic environments, and a green color upon exposure to an acidic environment. The surface grafting of short chain aniline tetramers granted more water affinity (contact angle of ∼45°) than pristine polyethersulfone membranes (contact angle of ∼80°) within a pH range of 1 to 11. More importantly, the modified membranes exhibited two orders of magnitude lower adhesion of E. coli. compared to a pristine membrane.37
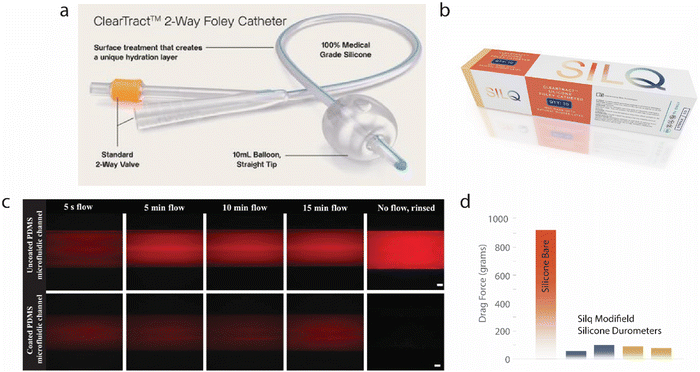
We then created a company called Hydrophilix, Inc. to focus on roll-to-roll manufacturing based on surface modifications. At the early stage, Hydrophilix, Inc. resolved the insufficient wetting issues between non-polar polyolefin separators and polar organic electrolytes in lithium-ion batteries.38 We then changed the name of Hydrophilix to Silq Technologies Corp., with a new focus on advanced biomedical applications (https://www.silq.tech/). Using the zwitterionic molecular anchoring technique, we achieved drastically improved hydrophilicity on a wide spectrum of polymeric substrates, including PDMS, nylon 66, polystyrene, polyvinyl chloride, and polyethylene. Silq Technologies applied for and received Food and Drug Administration (FDA) clearance for Foley Catheters (called Silq ClearTract™) (Fig. 3a and b). The modified PDMS substrate, given its wide array of applications in the medical device industry, was shown to have increased hydrophilicity as demonstrated by contact angle, reduced protein adsorption, reduced bacterial adhesion, a low coefficient of friction (as tested by ATSM D1894), and excellent biocompatibility (Fig. 3c and d).39 An initial clinical evaluation was performed with 16 patients receiving the Silq ClearTract™ Foley catheter and on responding to a questionnaire, 13 elected to continue using the Silq catheter after the trial ended. Further clinical studies are currently being performed (NCT04841226, NCT05931887) and are expected to yield results which enable Silq to expand its product lines.
Silq Technologies has also been given clearance to use their catheters in suprapubic and nephrostomy applications which are commonly needed by chronically catheterized individuals who are especially susceptible to Catheter Associated Urinary Tract Infections (CAUTIs). There are over 1 million CAUTIs in the United States annually, representing one of the most common forms of device associated infections. Silq intends to focus their efforts on chronically catheterized patient populations and will develop additional products utilizing the zwitterionic molecular anchoring technology with the aim of reducing medical device associated complications.
Looking into the future, we have been paying close attention to advances in self-cleaning processes using electrically conductive membranes. We have even attempted to shrink GO and carbon nanotube membranes with liquid ammonia in order to increase the electrical conductivity of the membranes, but this has so far not succeeded.40,41 Currently, we are working on the development of conductive framework membranes for ultrahigh pressure filtrations, membranes for critical elements separations, as well as resolving the epoxy infiltration issue with T-FLO.
Conflicts of interest
R. B. K. has an equity interest in Silq Technologies, Corp., which has licensed technology from UCLA.Acknowledgements
The authors would like to thank the Dr Myung Ki Hong Endowed Chair in Materials Innovation at UCLA and Silq Technologies, Inc. for financial support. The authors thank Ethan Rao for helpful discussions.References
- Y. Liao, T. P. Farrell, G. R. Guillen, M. Li, J. A. T. Temple, X.-G. Li, E. M. V. Hoek and R. B. Kaner, Mater. Horiz., 2014, 1, 58–64 RSC.
- C.-W. Lin, S. Xue, C. Ji, S.-C. Huang, V. Tung and R. B. Kaner, Nano Lett., 2021, 21, 3699–3707 CrossRef CAS PubMed.
- N. H. Barbhuiya, U. Misra and S. P. Singh, Environ. Sci.: Water Res. Technol., 2021, 7, 671–705 RSC.
- H. Luo, Y. V. Kaneti, Y. Ai, Y. Wu, F. Wei, J. Fu, J. Cheng, C. Jing, B. Yuliarto, M. Eguchi, J. Na, Y. Yamauchi and S. Liu, Adv. Mater., 2021, 33, 2007318 CrossRef CAS PubMed.
- V. R. Pereira, A. M. Isloor, A. A. Ahmed and A. F. Ismail, New J. Chem., 2015, 39, 703–712 RSC.
- M. S. Algamdi, I. H. Alsohaimi, J. Lawler, H. M. Ali, A. M. Aldawsari and H. M. A. Hassan, Sep. Purif. Technol., 2019, 223, 17–23 CrossRef CAS.
- M. Kumar, N. Sreedhar, M. A. Jaoude and H. A. Arafat, ACS Appl. Mater. Interfaces, 2020, 12, 1617–1627 CrossRef CAS PubMed.
- G. P. S. Ibrahim, A. M. Isloor, A. Moslehyani and A. F. Ismail, J. Water Process Eng., 2017, 20, 138–148 CrossRef.
- H.-P. Xu, W.-Z. Lang, X. Yan, X. Zhang and Y.-J. Guo, J. Membr. Sci., 2014, 467, 142–152 CrossRef CAS.
- R. S. Hebbar, A. M. Isloor, A. K. Zulhairun, M. Sohaimi Abdullah and A. F. Ismail, J. Taiwan Inst. Chem. Eng., 2017, 72, 244–252 CrossRef CAS.
- R. S. Hebbar, A. M. Isloor, K. Ananda, M. S. Abdullah and A. F. Ismail, New J. Chem., 2017, 41, 4197–4211 RSC.
- R. Saranya, M. Kumar, R. Tamilarasan, A. F. Ismail and G. Arthanareeswaran, J. Chem. Technol. Biotechnol., 2016, 91, 748–761 CrossRef CAS.
- F. Ahmed, B. S. Lalia, V. Kochkodan, N. Hilal and R. Hashaikeh, Desalination, 2016, 391, 1–15 CrossRef CAS.
- K. Wang, L. Xu, K. Li, L. Liu, Y. Zhang and J. Wang, J. Membr. Sci., 2019, 570–571, 371–379 CrossRef CAS.
- F. Meng, S. Zhang, Y. Oh, Z. Zhou, H.-S. Shin and S.-R. Chae, Water Res., 2017, 114, 151–180 CrossRef CAS PubMed.
- B. Khorshidi, J. Hajinasiri, G. Ma, S. Bhattacharjee and M. Sadrzadeh, J. Membr. Sci., 2016, 500, 151–160 CrossRef CAS.
- Z. Zhang, G. Huang, Y. Li, X. Chen, Y. Yao, S. Ren, M. Li, Y. Wu and C. An, Chem. Eng. J., 2022, 427, 131987 CrossRef CAS.
- X.-T. Yuan, L. Wu, H.-Z. Geng, L. Wang, W. Wang, X.-S. Yuan, B. He, Y.-X. Jiang, Y. Ning, Z.-R. Zhu and J. Li, J. Water Process Eng., 2021, 40, 101903 CrossRef.
- H. Kim, K. Kim and S. J. Lee, NPG Asia Mater., 2017, 9, e445 CrossRef CAS.
- Z.-Y. Guo, X.-S. Yuan, H.-Z. Geng, L.-D. Wang, L.-C. Jing and Z.-Z. Gu, Appl. Nanosci., 2018, 8, 1597–1606 CrossRef CAS.
- W. Duan, A. Ronen, S. Walker and D. Jassby, ACS Appl. Mater. Interfaces, 2016, 8, 22574–22584 CrossRef CAS PubMed.
- B. T. McVerry, J. A. T. Temple, X. Huang, K. L. Marsh, E. M. V. Hoek and R. B. Kaner, Chem. Mater., 2013, 25, 3597–3602 CrossRef CAS.
- X. Huang, B. T. McVerry, C. Marambio-Jones, M. C. Y. Wong, E. M. V. Hoek and R. B. Kaner, J. Mater. Chem. A, 2015, 3, 8725–8733 RSC.
- K. Tzou and R. V. Gregory, Synth. Met., 1993, 55, 983–988 CrossRef CAS.
- V. G. Kulkarni, L. D. Campbell and W. R. Mathew, Synth. Met., 1989, 30(3), 321–325 CrossRef CAS.
- L. Brožová, P. Holler, J. Kovářová, J. Stejskal and M. Trchová, Polym. Degrad. Stab., 2008, 93, 592–600 CrossRef.
- R. B. Kaner, Chemist, 2021, 92, 1–7 Search PubMed.
- A. K. Geim, Angew. Chem., Int. Ed., 2011, 50, 6966–6985 CrossRef CAS PubMed.
- B. McVerry, M. Anderson, N. He, H. Kweon, C. Ji, S. Xue, E. Rao, C. Lee, C.-W. Lin, D. Chen, D. Jun, G. Sant and R. B. Kaner, Nano Lett., 2019, 19, 5036–5043 CrossRef CAS PubMed.
- S. Xue, C. Ji, M. D. Kowal, J. C. Molas, C.-W. Lin, B. T. McVerry, C. L. Turner, W. H. Mak, M. Anderson, M. Muni, E. M. V. Hoek, Z.-L. Xu and R. B. Kaner, Nano Lett., 2020, 20, 2209–2218 CrossRef CAS PubMed.
- B. T. McVerry, M. C. Y. Wong, K. L. Marsh, J. A. T. Temple, C. Marambio-Jones, E. M. V. Hoek and R. B. Kaner, Macromol. Rapid Commun., 2014, 35, 1528–1533 CrossRef CAS PubMed.
- S. J. Pastine, D. Okawa, B. Kessler, M. Rolandi, M. Llorente, A. Zettl and J. M. J. Fréchet, J. Am. Chem. Soc., 2008, 130, 4238–4239 CrossRef CAS PubMed.
- Y. Wang, H. D. Tran, L. Liao, X. Duan and R. B. Kaner, J. Am. Chem. Soc., 2010, 132, 10365–10373 CrossRef CAS PubMed.
- Y. Wang, J. A. Torres, A. Z. Stieg, S. Jiang, M. T. Yeung, Y. Rubin, S. Chaudhuri, X. Duan and R. B. Kaner, ACS Nano, 2015, 9, 9486–9496 CrossRef CAS PubMed.
- C.-W. Lin, R. L. Li, S. Robbennolt, M. T. Yeung, G. Akopov and R. B. Kaner, Macromolecules, 2017, 50, 5892–5897 CrossRef CAS.
- C.-W. Lin, W. H. Mak, D. Chen, H. Wang, S. Aguilar and R. B. Kaner, ACS Catal., 2019, 9, 6596–6606 CrossRef CAS.
- C.-W. Lin, S. Aguilar, E. Rao, W. H. Mak, X. Huang, N. He, D. Chen, D. Jun, P. A. Curson, B. T. McVerry, E. M. V. Hoek, S.-C. Huang and R. B. Kaner, Chem. Sci., 2019, 10, 4445–4457 RSC.
- E. Rao, B. McVerry, A. Borenstein, M. Anderson, R. S. Jordan and R. B. Kaner, ACS Appl. Energy Mater., 2018, 1, 3292–3300 CrossRef CAS.
- B. McVerry, A. Polasko, E. Rao, R. Haghniaz, D. Chen, N. He, P. Ramos, J. Hayashi, P. Curson, C. Wu, P. Bandaru, M. Anderson, B. Bui, A. Sayegh, S. Mahendra, D. D. Carlo, E. Kreydin, A. Khademhosseini, A. Sheikhi and R. B. Kaner, Adv. Mater., 2022, 34, 2200254 CrossRef CAS PubMed.
- C.-W. Lin, Z. Yang, A. Huang, X. Chang, C. Wang, F. Yang, C. Wei, M. Thiel, Y. Katsuyama, L. Jin, D. Jassby and R. B. Kaner, RSC Appl. Interfaces, 2024, 1, 194–205 RSC.
- C. K. F. Hermann, J. Chem. Educ., 1997, 74, 1357 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |