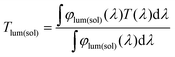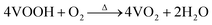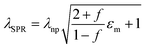Open Access Article
Open Access ArticleKirkendall effect induced ultrafine VOOH nanoparticles and their transformation into VO2(M) for energy-efficient smart windows†
Liangfei
Wu‡
a,
Antonio
Teng‡
b,
Ming
Li
 *ac,
Liang
Li
*ac,
Liang
Li
 ac,
Zhulin
Huang
ac,
Zhulin
Huang
 ac,
Xinyang
Li
ac,
Xinyang
Li
 a,
Jie
Yu
a,
Sichao
Xu
a,
Fengxia
Zou
ac,
Andy
Zou
d,
Jinghui
Zhang
ef,
Tao
Jiang
ef,
Ye
Xin
g,
Xiaoye
Hu
a,
Jie
Yu
a,
Sichao
Xu
a,
Fengxia
Zou
ac,
Andy
Zou
d,
Jinghui
Zhang
ef,
Tao
Jiang
ef,
Ye
Xin
g,
Xiaoye
Hu
 *ac and
Guanghai
Li
*ac and
Guanghai
Li
 *ac
*ac
aKey Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, P. R. China. E-mail: liming@issp.ac.cn; hxy821982@issp.ac.cn; ghli@issp.ac.cn
bContiTech ChinaRubber & Plastics Technology Ltd, Changshu 215500, P. R. China
cUniversity of Science and Technology of China, Hefei 230026, P. R. China
dBenecke Changshun Auto Trim Co., Ltd., Zhangjiagang 215632, P. R. China
eKey Laboratory of Atmospheric Optics, Anhui Institute of Optics and Fine Mechanics, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, P. R. China
fAdvanced Laser Technology Laboratory of Anhui Province, Hefei 230037, P. R. China
gNaval Research Institute, Beijing 102442, P. R. China
First published on 4th December 2023
Abstract
Vanadium dioxide (VO2) has received widespread attention for application in energy-efficient smart windows because of its distinct thermochromic property in the near-infrared region during the reversible metal–insulator phase transition. In this study, lepidocrocite VOOH ultrafine nanoparticles (NPs) with a diameter less than 30 nm were prepared by a mild and efficient hydrothermal method, and the Kirkendall effect played a vital role in the growth of the VOOH NPs. It was found that VOOH could be transformed into VO2via a subsequent annealing treatment during which the size and morphology of VOOH are well preserved even though the annealing temperature is up to 500 °C. The ultrafine VO2 NPs are crucial for achieving excellent nanothermochromic performance with a luminous transmittance (Tlum) up to 56.45% and solar modulation ability (ΔTsol) up to 14.95%. The environmental durability is well improved by coating VO2 NPs with an SiO2 shell as confirmed via progressive oxidation and acid corrosion experiments. Meanwhile, the Tlum of the VO2@SiO2 film is further increased from 56.45% to 62.29% while the ΔTsol remained unchanged. This integrated thermochromic performance presents great potential for the development of VO2-based smart windows.
New conceptsVO2 has a unique dynamic thermochromic property that can be exploited in energy-efficient smart windows with the aim of maintaining comfortable living surroundings without the excessive use of air-conditioning. Hydrothermal synthesis is a powerful method to obtain high-performance VO2 nanostructures for nanothermochromic coatings, but it still suffers from time-consumption and high reaction temperatures. In this study, newly reported lepidocrocite VOOH nanoparticles of about 25 nm were prepared for the first time via an efficient and mild hydrothermal route. The Kirkendall effect was found to be a critically important feature in the formation of ultrafine VOOH NPs. The phase transition from VOOH to VO2(M) was studied by annealing in order to obtain the thermochromic performance. Both the reduced particle size and localized surface plasmon resonance (LSPR) absorption gave rise to the enhanced thermochromic property of VO2 with a Tlum of 56.45% and ΔTsol of 14.95%. After coating VO2 with an amorphous SiO2 shell, the Tlum was further increased up to 62.29% while the ΔTsol had no degradation. The outstanding thermochromic performance and enhanced environmental durability of VO2 will pave the way forward for the development of energy-efficient smart windows. |
1. Introduction
Building energy consumption accounts for ∼40% of total global energy consumption, and a major part of this energy (∼60%) is lost through windows in the building because of the superabundant solar radiation transmitted through the window increasing the indoor cooling and heating loads for air conditioners in summer and winter, respectively.1–3 Therefore, the use of smart windows that can dynamically regulate the amount of solar transmission in response to an external stimulus could have a significant positive effect on energy savings. Diverse smart windows have been achieved using mechano, thermo, electro, or photo stimuli. The thermochromic smart window provides a new, intriguing option owing to its structural simplicity and does not require any additional energy consumption to achieve more energy savings.4–6VO2 is the most widely studied thermochromic material and exhibits an obvious reversible phase transition from a monoclinic (M) insulator phase to a rutile (R) metallic phase at a critical temperature of ∼340 K. The structural phase transition is accompanied by a dramatic change of optical property: VO2(R) is highly reflective to infrared radiation, while VO2(M) exhibits optical transparency in the infrared band.7,8 It is worth mentioning that the phase transition can block heat radiation without shading the glazing because the luminous transmittance is of nearly the same value for both VO2(R) and VO2(M). In addition, the percolative nature of the thermally-induced phase transition in VO2 allows for designing devices with a self-adaptive property, eliminating the need for human intervention or additional energy supply for external control. Hence, thermochromic smart windows based on VO2 provide real intelligent control over infrared modulation. For practical applications, both the luminous transmittance (Tlum) and solar modulation ability (ΔTsol) are important parameters that determine the thermochromic performance of VO2 coatings used in smart windows. An ideal thermochromic coating should be highly transparent in the visible region, and the variation of infrared modulation associated with the phase change should be as high as possible. In other words, the higher both Tlum and ΔTsol, the better the application prospects of VO2 coatings. Unfortunately, a higher Tlum is usually at the expense of an extremely low ΔTsol and vice versa. Therefore, the major challenge for VO2-based smart windows is to simultaneously improve the Tlum (> 60%) and ΔTsol (> 12%).
Several strategies have been deployed to overcome the trade-off of ΔTsol and Tlum, such as porous films, nanothermochromic coatings, bio-inspired structure, multilayer anti-reflective film, and so on.9–11 Most experimental data and theoretical results demonstrate that, of the nanothermochromic coatings, VO2 NPs dispersed in a glass or polymer matrix outperformed all the other methods since the subwavelength VO2 NPs can reduce light scattering and increase transmittance. In this case, the particle size, dispersibility and crystallinity of VO2 NPs play a vital role in affecting the optical properties of VO2-based smart windows. Additionally, the ultrafine VO2 NPs exhibit superior modulation of ΔTsol and Tlum due to plasmonic effects when the NPs are in their metallic state. Thus, reducing the particle size and improving the crystallinity of VO2 have been the focus of recent research. However, to date, a simple and practical method for producing ultrafine high-quality VO2 NPs is still lacking, especially as it is in high demand to selectively control the size, dispersibility and degree of crystallinity of VO2.
V exhibits a wide range of oxidation states, and VO2 has a number of polymorphic forms, such as VO2(M), VO2(M2), VO2(R), VO2(A), VO2(B), and VO2(D). Thus, the choice of preparation method is particularly important to obtain the specific thermochromic phase of VO2(M) or VO2(R). The hydrothermal method has been shown to be the most suitable method to synthesize high-quality VO2 NPs with controllable crystal structure, morphology and crystallinity by adjusting simple parameters such as reaction temperature, precursor concentration, pH and hydrothermal time. But, in most cases, the hydrothermal products are usually a metastable phase of VO2(B) or VO2(A).12,13 Even though a few reports have studied one-step hydrothermal synthesis of VO2(M), the preparation conditions were harsh, such as a higher reaction temperature (>240 °C) and a longer hydrothermal time (>24 h). Hence, some special intermediate phases like VO2(D), VO2(P) and VC2H4O3 have been prepared first by mild hydrothermal method and then an extra annealing process is applied to obtain VO2(M).14–16 It is important to highlight that the mild hydrothermal conditions and annealing process are both beneficial to obtaining the nanostructure as well as improving the crystallinity of VO2(M), which provides an alternative route to overcome the Achilles heels of overgrowth and low crystallization of VO2 NPs. Amongst the above-mentioned special intermediate phases, VOOH exhibits a number of polymorphic forms, such as α-VOOH, montroseite VOOH and lepidocrocite VOOH. However, nearly all studies about them and their derivatives focus on the electrochemical fields, such as lithium-ion batteries, water electrolysis and supercapacitors.17–20 Moreover, as an intermediate product, all reported VOOH are larger than 100 nm in size, which is far from being able to meet the needs of VO2-based smart windows. Thus, it is meaningful to prepare ultrafine VOOH NPs and investigate their phase transformation behavior from VOOH to VO2(M), which could develop and enrich the mild strategy for preparing nanothermochromic VO2(M).
In this paper, we demonstrated a mild hydrothermal method to prepare lepidocrocite VOOH NPs with diameters less than 40 nm and the Kirkendall effect was responsible for the formation of the ultrafine NPs. Importantly, the VOOH NPs could be converted to VO2(M) with no significant size change during the annealing treatment even though the annealing temperature was up to 500 °C. The nanothermochromic films on glass substrates were prepared by incorporating VO2(M) NPs into a PVP (polyvinylpyrrolidone) matrix, which presented a Tlum of 56.45% and ΔTsol of 14.95%. Moreover, the optical performance and environmental durability could be further enhanced by coating a SiO2 shell on the VO2(M) NPs. The as-prepared VO2@SiO2/PVP films exhibited outstanding thermochromic performance with an enhanced Tlum of 62.29% and ΔTsol of 14.91%, showing great potential for practical application in energy-efficient smart windows.
2. Experimental section
Materials
The solvents and chemicals, including ammonium metavanadate (NH4VO3), hydrochloric acid (HCl, 37%), hydrazine hydrate aqueous solution (H4N2·H2O, 85%), formic acid (HCOOH, 88 wt%), ammonia water (NH3·H2O, 28 wt%), tetraethyl orthosilicate (TEOS), and PVP K30, were used without further purification.Preparation of lepidocrocite VOOH ultrafine NPs
Lepidocrocite VOOH ultrafine NPs were prepared via hydrothermal method. Briefly, 0.234 g NH4VO3 was added into 30 mL deionized water under magnetic stirring at room temperature until the solution became transparent. Later, 2 ml 1 M HCl, 1 ml HCOOH and 2 ml H4N2·H2O were added in turn. After stirring for 30 min, the suspension was hydrothermally treated at 160 °C for 6 h. The black product was collected by centrifugation, washed with ethanol and dried at 60 °C in vacuum. The specific values for the constant parameters for different gradient experiments are shown in Fig. S1 (ESI†).Preparation of core–shell VO2(M)@SiO2 NPs
In order to transform VOOH into VO2(M), the VOOH NPs were heated at 500 °C for 1 h in a vacuum of ∼1 Pa. The obtained blue-black powder was dispersed into 60 ml ethanol and sonicated for 1 h. 10 ml deionized water, 4 ml 28 wt% concentrated ammonia solution and 0.2 (S1), 0.5 (S2) or 1 ml (S3) TEOS were added sequentially and slowly into the above dispersions under stirring for 1 h. Finally, the product was washed with ethanol and collected by centrifugation.Preparation of VO2-based coatings
0.27 g VO2(M) or VO2@SiO2, 0.27 g PVP K30, and 10 ml ethanol were well mixed by means of ball milling. Before spin coating, the glass substrates (25 × 25 mm2) were cleaned with acetone, ethanol and deionized water sequentially to remove surface contamination and were finally dried at room temperature. Then, 0.2 ml dispersion was deposited on the glass and then spin-coated at a speed of 1500 rpm for 30 s. The thickness of the film can be changed by varying the number of spinning times. The preparation process is illustrated in Fig. 1a.Characterization
The morphology and phase structure of the NPs were examined using field-emission scanning electron microscopy (FESEM, Hitachi SU8020) and X-ray diffraction with a Cu Ka1 line (XRD, PANalytical X’Pert). High resolution TEM analysis was carried out using an image aberration corrected TEM (JEOL JEM-2010). A low accelerating voltage of 80 kV was used in order to avoid beam irradiation induced damage. The phase transition behaviors were analyzed using a differential scanning calorimeter (DSC, PerkinElmer) at a heating rate of 10 °C min−1 in a flowing nitrogen atmosphere. Optical transmission spectra were recorded using a UV-3600 spectrophotometer (Shimadzu UV3600-MPC3100) equipped with a temperature controller.To assess the visual and energy saving performance of all the films, the integral luminous transmittance (Tlum, 380–780 nm) and solar transmittance (Tsol, 250–2500 nm) were calculated using the following equation,
| Tsol = Tsol,lt − Tsol,ht |
Simulation
The three-dimensional finite difference time domain (FDTD) method was used to simulate the optical performance of VO2@SiO2. The radius of VO2 was 20 nm and the shell thickness was 11 nm. PML (perfectly matched layer) boundary conditions were set for the Z direction. Periodic boundary conditions were applied in the X and Y directions. The plane wave (300 nm–2500 nm) was incident perpendicular to the structure along the Z direction. Power monitors were placed at fixed Z positions below the VO2 to detect the transmitted beam intensity.3. Results and discussion
Lepidocrocite ultrafine VOOH NPs
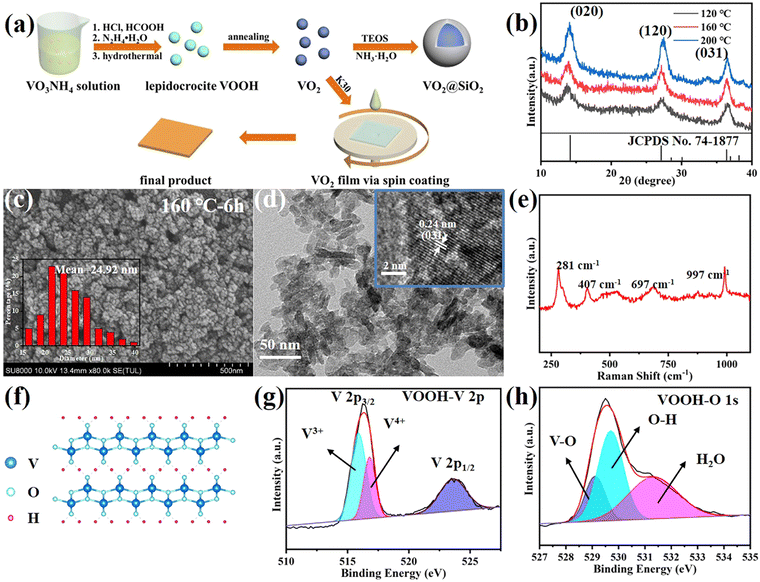
In our work, by adjusting some hydrothermal parameters, including the volume of HCOOH as well as the hydrothermal temperature and reaction time, it was possible to alter the characteristics of the VOOH particles, such as size, morphology and crystalline phase. To study the synthesis temperature's influence on the hydrothermal products, a gradient experiment was performed by varying the synthesis temperature from 80 °C to 200 °C with a temperature interval of 40 °C. Fig. 1b displays the XRD patterns of VOOH NPs prepared under different hydrothermal temperatures. The diffraction peaks at 2θ = 14°, 27° and 36.4° matched well with the (020), (120) and (031) crystalline planes of lepidocrocite FeOOH (JCPDS card no. 74–1877), and no other phases of vanadium oxides were detected, indicating lepidocrocite VOOH NPs were prepared successfully.21 It was clear that the diffraction peaks became stronger with a higher temperature due to the enhanced crystallinity. In the SEM images (Fig. 1c and Fig. S2a, b, ESI†), all three VOOH NPs with uniform size <40 nm were clearly discernable and the one obtained at 160 °C presented the smallest average size of 24.92 nm (measured by Nano Measurer 1.2 software). Notably, Fig. S2c, d (ESI†) indicated VOOH NPs could not be obtained with a lower hydrothermal temperature of 80 °C. The ultrafine VOOH NPs were further confirmed by the TEM images shown in Fig. 1d, of which the high-resolution TEM image indicated that the lattice spacing is 0.24 nm, consistent with the (031) plane of VOOH. Considering the similar shapes and sizes between the samples synthesized in these experiments, it could be concluded that the synthesis temperature in a wide range had a limited effect on the morphology and size of the obtained VOOH NPs. For further characterization, the structure of VOOH was determined from Raman spectroscopy, as seen in Fig. 1e, and the characteristic layer structure was observed at 281 cm−1, which was consistent with the crystal structure of VOOH shown in Fig. 1f.19 The oxidation state of VOOH was investigated by X-ray photoelectron spectroscopy (XPS). For the valence states of V, in addition to V3+ (515.9 eV) from the V 2p spectra of VOOH, V4+ (516.8 eV) could be also found, suggesting that the V3+ was partially oxidized due to exposure to air (Fig. 1g). In the O 1s spectrum of VOOH, the three peaks located at 529, 529.6 and 531.3 eV were assigned to O2− in V–O, O–H and H2O, respectively (Fig. 1h). Combined with the above results, it could be concluded that lepidocrocite VOOH ultrafine NPs were obtained after the hydrothermal reaction.Besides the reaction temperature, the influence of the addition of HCOOH on the morphology of VOOH was also evaluated during the hydrothermal process. We fixed the total volume of acid at 3 ml and changed the volume of HCOOH from 0 to 2 ml. The SEM images of the different hydrothermal products are shown in Fig. S3a–d (ESI†). In Fig. S3a (ESI†), micron-sized hollow spheres can be found when no HCOOH was added and this was a common shape of VOOH in previous research work.17 As the amount of HCOOH increased, the size of the particles decreased gradually. However, the particle size increased inversely when the HCOOH content was greater than 1 ml. Particularly, when the amount of HCOOH was 2 ml, the resulting hydrothermal product was irregular micron-sized particles. Moreover, the SEM images of the solid precipitates before hydrothermal treatment with different volumes of HCOOH are displayed in Fig. S4a–d (ESI†). Fig. S4e (ESI†) shows the digital photographs of the corresponding hydrothermal precursor solution before and after centrifugation. It was noteworthy that, when the content of HCOOH was 2 ml, there was no precipitate formed and the solution was a clear azure color, which may be attributed to the presence of aqueous tetravalent vanadium in the form of vanadyl ions (VO2+).22 At the same time, a small amount of VO2+ was generated when the HCOOH content was 1.5 ml, which can be inferred from the color of the precursor solution after centrifugation. In this case, the coexistence of VO2+ and solid precipitates may be responsible for the paradoxical increase in the size of hydrothermal product. Otherwise, by comparing the SEM images before and after hydrothermal treatment, it can be assumed that there was a positive correlation between the size of the hydrothermal product and the hydrothermal precursor, while the main function of HCOOH was to reduce the size of the precursor, thus promoting the formation of ultrafine VOOH NPs.
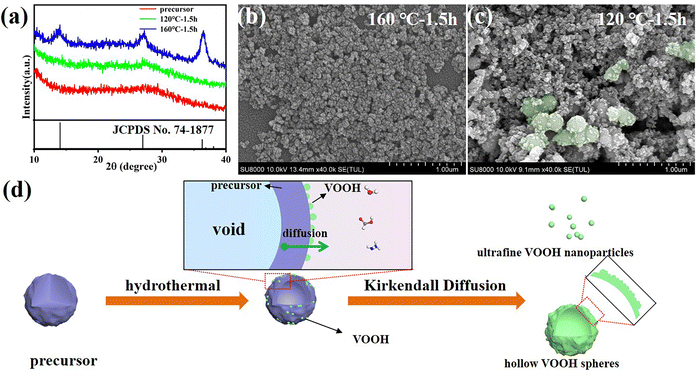
The reaction time was also examined for insight into the formation process of VOOH NPs. The XRD patterns in Fig. 2a suggested that 1.5 h was enough for the formation of lepidocrocite VOOH when the hydrothermal temperature was 160 °C, even though ultrafine VOOH NPs had a tendency to agglomerate (Fig. 2b). It gave us a hint that the hydrothermal time could be further shortened. However, the precursors could not be totally converted into VOOH when the hydrothermal temperature was 120 °C, because the amorphous peak at ∼28° belonging to the amorphous precursor was maintained (Fig. 2a). As shown in Fig. 2c, some VOOH NPs can be found adhered to the precursor surface, indicating that the VOOH NPs generated at the precursor/solution interface. Based on the above characterization, a diagram to depict the formation of ultrafine VOOH NPs is illustrated in Fig. 2d. At the beginning of the hydrothermal process, the hydrolyzation reaction at the precursor/solution interface occurred to form a loosely packed VOOH shell. This process was coupled with a continuous outward flow of the core structure of the precursor based on the Kirkendall effect, where voids can be formed due to differences in diffusion direction and speed between different ions in the synthesis process.23–26 The Kirkendall effect usually occurs in one-step, relatively facile processes that do not require template removal to prepare hollow-structured nanomaterials, but it seemed to be somewhat different in this work. For precursors with larger particle sizes, there was sufficient core mass to sustain the transport process and a relatively compact shell was formed which corresponded to the SEM image in Fig. S3a (ESI†). For precursors with smaller particle sizes, the core mass was insufficient to create effective connections between the initial VOOH NPs randomly distributed on the surface of the precursor, leading to the generation of isolated VOOH NPs. At the same time, a higher hydrothermal temperature not only helped to accelerate the reaction process, but also provided more active sites for the reaction, further reducing the particle size of VOOH. The increase in particle size of VOOH at a hydrothermal temperature of 200 °C might be related to the solid-solution-solid mechanism, which induces the anisotropic growth of VOOH to form a flake structure and is confirmed in Fig. S5 (ESI†).
Transformation from VOOH to VO2(M)
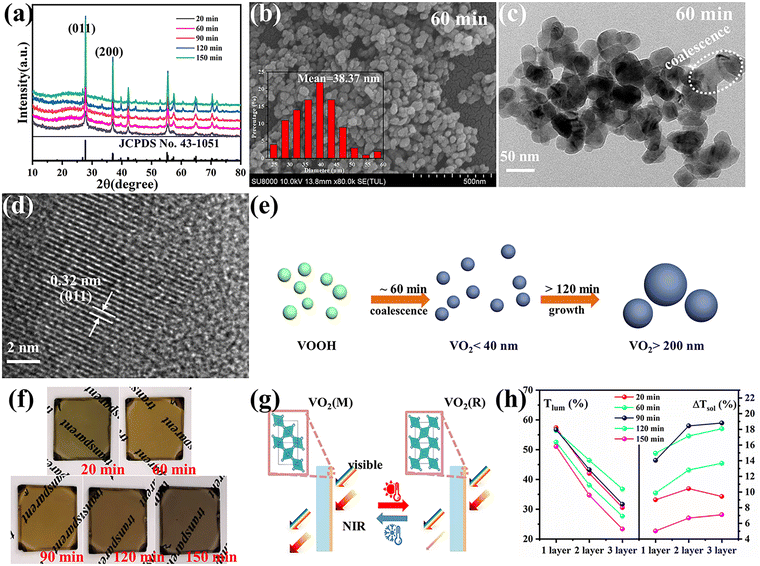
VOOH can react with O2 to form VO2 according to the following formula.In accordance with the above equation, appropriate oxygen content is necessary to obtain VO2. Therefore, the VOOH NPs were thermally annealed under a vacuum of ∼1 Pa at a temperature of 500 °C. Different annealing times were applied to study the phase structure and morphology evolution during vacuum annealing. Fig. 3a displays the XRD patterns of samples with an annealing time increase from 20 to 150 min. All diffraction peaks matched well with the standard card JCPDS No. 43-1051, indicating that the pure monoclinic VO2(M) phase was successfully synthesized.27,28 The SEM images of VO2 NPs with different annealing times are shown in Fig. 3b and Fig. S6a–d (ESI†). It was found that the diameter of VO2 NPs increased with prolonged annealing time and that significant coalescence events only occurred with annealing times above 2 h. Fig. S7 (ESI†) and Fig. 3c present the TEM images of VO2 NPs annealed for 20 and 60 min, respectively. Most of the VO2 NPs were isolated with a few particles starting to coalesce (circled by the white line), but the particle size was still small enough to be considered ultrafine VOOH. Fig. 3d reveals the crystalline structure of VO2 with clear lattice fringes. The calculated interplanar spacing was ∼0.32 nm, which matched well with the lattice spacing of the (011) plane.29,30 In order to investigate the structural phase change from VOOH to VO2, different annealing temperatures and vacuum degrees were also employed. Fig. S8a (ESI†) displays the XRD patterns of VO2 with different annealing temperatures. It was found that VOOH was first transformed into VO(A) and then to VO2(M) when the annealing temperature increased from 350 °C to 450 °C. The SEM images of the annealed products at different annealing temperatures are displayed in Fig. S8b–d (ESI†). All three samples presented similar sizes, suggesting that annealing temperature had a minor effect on the morphologies. Fig. S9a (ESI†) displays the XRD patterns of VO2 with different vacuum degrees. O2 was necessary for the transformation from VOOH to VO2, thus only V3O5 was obtained at 0 Pa. VO2(M) was obtained when the vacuum degree was increased to 3 Pa or 6 Pa. However, the particle sizes of the prepared VO2 were about 150 nm under these conditions, which was not conducive to optical performance. Fig. S10 (ESI†) further confirms that different hydrothermal products could also be successfully transformed into VO2 after annealing at 500 °C with a vacuum degree of 1 Pa for 1 h. The phase transition property of the VO2 powder was further analyzed by DSC, as shown in Fig. S11 (ESI†). When VOOH was annealed for 20 min, the phase transition temperature of Tc was 64.62 °C which increased to ∼80 °C when VOOH was annealed for a longer time. That was because the defects generated during the transformation process from VOOH to VO2 and the defect density were reduced with the increase of annealing time. Thus, a relatively large supercooling/superheating degree was necessary to drive the phase transition, which resulted in a large thermal hysteresis (the temperature gap between the exothermic peak and endothermic peak). It is worth noting that when the heat treatment time was 120 min, multiple exothermic peaks emerged, suggesting the coexistence of different types of VO2 NPs, which is consistent with the corresponding SEM images in Fig. S6c (ESI†). Based on the above characterization, it could be concluded that the formation and coalescence of VO2 occur simultaneously (Fig. 3e). As long as the annealing treatment time was controlled properly, VO2 with good crystallinity and fine particle size less than 40 nm could be obtained easily, which is crucial for optimizing the optical properties of VO2-based thermochromic films.
For practicality, VO2 NPs were dispersed into a PVP matrix and then coated onto glass substrates to form VO2-based films. Fig. 3f displays the digital images of the VO2/PVP films. Both shorter (20 min) and longer (120 and 150 min) annealing times for VO2 caused the films to become dark. The two cases in this study were due to the presence of residual VOOH for the former and the strong Mie scattering from the large particle size of VO2 for the latter.13 An appropriate annealing time (60 and 90 min) led to the film exhibiting the typical yellow-brown color belonging to VO2(M). The SEM image for the surface morphology of the VO2/PVP-60 min film is presented in Fig. S12a (ESI†) and the white dots are VO2 grains. The inset AFM image in Fig. S12a (ESI†) displays a surface roughness of 11.87 nm for the film. The cross-sectional image in Fig. S12b (ESI†) further reveals the thickness of the film to be around 400 nm. The optical modulation properties of the VO2/PVP films were investigated to evaluate their potential for usage in smart windows by measuring the transmittance spectra at 25 °C and 100 °C (Fig. S13a–e, ESI†). The corresponding Tlum and ΔTsol are summarized in Fig. 3h and Fig. S14 (ESI†). For all VO2 films, the thicker films exhibited larger ΔTsol in the infrared spectral region and a decreased Tlum in visible light. For VO2 obtained with an annealing time of 60 min, Tlum and ΔTsol are (56.45%, 14.95%), (46.45%, 17.15%) and (36.77%, 18.08%) for 1, 2 and 3 times of spin coating, respectively, exhibiting excellent optical properties. When the annealing time of VO2 was extended to 150 min, the LSPR absorption edge (near 1200 nm) almost disappeared and the optical properties of the film dropped significantly, mainly due to the increase in VO2 particle size. It is well-known that the M phase scatters light more strongly due to its larger refractive index, which can cause lower transmittance than the R phase. As result of this, there was a crosspoint in the transmittance curves found in Fig. S13d and e (ESI†) (circled by dashed line) which reduced ΔTsol. Nevertheless, if the sizes of the VO2(M) NPs were small enough, the difference in the light scattering between the M phase and R phase became negligible and the crosspoint disappeared (Fig. S13a–c, ESI†). Otherwise, the excitation of LSPR induced by sub-100 nm VO2 NPs reduced the near-infrared region transmittance at high temperature, which was crucial to enhancing the optical performances of VO2-based nanothermochromic films.16,31,32
The optical properties of VO2@SiO2 composite film
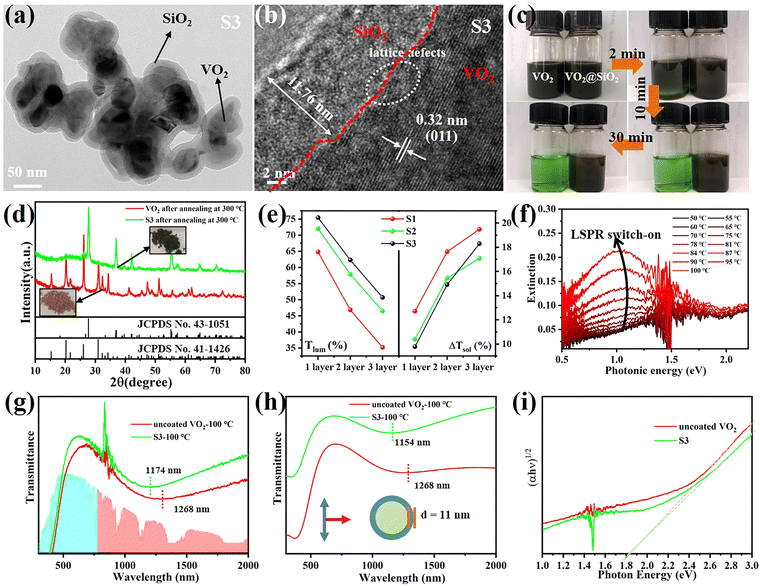
VO2 (M) is not thermodynamically stable in air and will be oxidized to V2O5 gradually over a long time. Coating a protective SiO2 layer onto the surfaces of VO2 particles is an effective way to solve the chemical and mechanical stability problem. This core–shell structure is optically transparent and can decrease the scattering caused by the refractive index mismatch between the nanoparticles and their polymer matrix.33 Therefore, VO2@SiO2 core–shell NPs were prepared using the Stöber procedure, in which the shell thickness of the SiO2 was controlled by the amount of TEOS used. The TEM images in Fig. 4a and Fig. S15 (ESI†) indicate that the thickness of the SiO2 shell increased from 5.71 nm to 11.76 nm with the increased TEOS. From Fig. 4b, it can found that the SiO2 shell is amorphous and the (011) crystal plane of VO2(M) with an interplanar spacing of 0.32 nm was observed. Some lattice defects were observed which may be attributed to partial etching during the coating process. The enhancement of the acid corrosion resistance of the VO2@SiO2 NPs was confirmed using a comparison experiment. The uncoated VO2 and S3 were added into a 1 M HCl solution at room temperature and Fig. 4c displays the color change of the suspension solution with time duration. The time-dependent transmittance at 550 nm of uncoated VO2 and S3 suspension is shown in Fig. S16 (ESI†). The uncoated VO2 was quickly etched and the suspension changed to green after 2 min and became completely transparent at about 10 min, indicating that the VO2 NPs were dissolved in the acid solution. In contrast, S3 remained stable in acidic solutions from 0 min to 30 min and no visible color change was detected. The anti-oxidation of both samples was also investigated by annealing treatment in air. Fig. 4d shows the XRD patterns of uncoated VO2 and S3 after annealing at 300 °C for 2 h in air. The uncoated VO2 nanoparticle was almost totally oxidized to V2O5 and a yellow powder was obtained. For S3, there was no noticeable change in the XRD pattern and the color of the sample remained blue-black. Thus, coating an inert SiO2 shell onto the surface of VO2 dramatically improves the environmental durability and extends the lifetime of VO2-based devices. Fig. S17a (ESI†) displays the DSC curves of VO2@SiO2 with various shell thicknesses. It was found that the Tc was reduced from 78.06 °C for uncoated VO2 to 75.27, 72.47 and 72.11 °C for S1, S2 and S3, respectively. The incorporation of Si in the interface of VO2 may increase the concentration of defects (see Fig. 4b), resulting in a decreased phase transition temperature. The SEM image of the surface morphology of S3/PVP film is presented in Fig. S17b (ESI†) and it was rougher than the surface of the VO2/PVP film. The AFM image in the inset in Fig. S17b (ESI†) shows that the surface roughness was 47.309 nm and the cross-sectional image in Fig. S17c (ESI†) reveals that the thickness of the film was around 520 nm, while both values were larger than those of VO2/PVP film.The transmittance spectra of VO2@SiO2/PVP films at different temperatures are plotted in Fig. S18a–c (ESI†) and the calculated Tlum and ΔTsol are summarized in Fig. 4e and Fig. S19 (ESI†). VO2@SiO2 with thinner shells exhibited higher ΔTsol but lower Tlum when the same spin coating time was applied. For S3, the Tlum was 62.29% and the ΔTsol was 14.91%, much higher than those of uncoated VO2 NPs, demonstrating an enhancement of both Tlum and ΔTsol and indicating a great potential for practical application in energy-efficient smart windows. Fig. 4f demonstrates the temperature-dependent LSPR on–off character and the enhanced LSPR intensity at higher temperatures due to the emergence of intermediate states during the metal–insulator transition. Furthermore, the existence of sub-50 nm VO2 NPs was another key to the generation of LSPR. The LSPR peaks of S3 and uncoated VO2 were at ∼1174 and ∼1268 nm, respectively (Fig. 4g). The blue-shift was mainly attributed to the SiO2 shell and this was confirmed by the finite-difference time-domain (FDTD) simulations (Fig. 4h). According to the effective medium theory, the wavelength of the LSPR peak can be given by
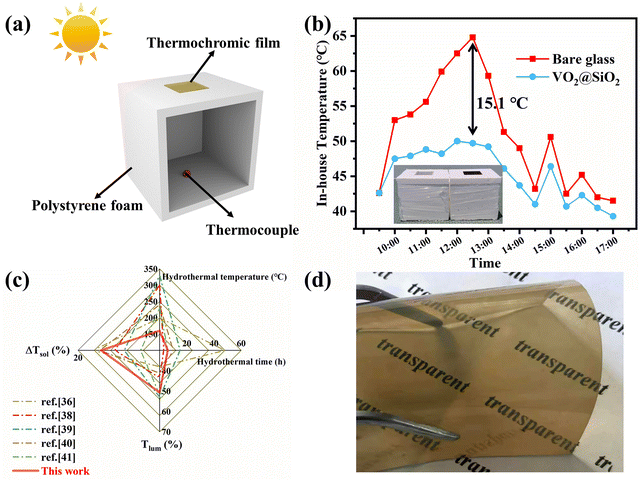
To evaluate the energy-saving effect of VO2@SiO2 thermochromic film under outdoor conditions, we built a simple house model using polystyrene foam and pasted thermochromic film in its roof, as depicted in Fig. 5a. The temperature inside the house was monitored by a thermocouple and another reference model was mounted with a bare glass window. The temperature change curve of the test environment is displayed in Fig. S20a (ESI†). Fig. 5b shows that the in-house temperatures of both model houses increased and reached the maximum temperature at noon with continuous exposure to solar irradiation. Notably, the in-house temperature of the model with the thermochromic film increased more slowly and a maximum 15.1 °C temperature difference was seen between these two models, indicating the effective solar energy regulation of VO2@SiO2 film. The model house experiment was also conducted in winter and the results are displayed in Fig. S20b and c (ESI†). Taking into account both annual heating and annual cooling energy, this film may do well in reducing energy consumption all year round. Fig. 5c displays recent reports on the ΔTsol and Tlum of VO2 obtained under different hydrothermal times and temperatures. For the one-step hydrothermal method, a critical hydrothermal temperature above 240 °C or even 300 °C is essential.38,39 For the two-step hydrothermal method, the hydrothermal temperature could be lower than 240 °C but needs to be at least 200 °C.36,40,41 It is obvious the hydrothermal conditions of our work were mild and effective. Moreover, as for the thermochromic performance, the prepared VO2@SiO2/PVP film achieved a good balance between ΔTsol (18.30%) and Tlum (50.69%), which is desirable for the application of VO2-based films as smart windows. Fig. 5d shows the VO2@SiO2/PVP film cast onto a PET substrate, indicating the potential application of VO2 in some promising flexible devices.
4. Conclusion
In summary, we reported an efficient and mild hydrothermal method for the preparation of lepidocrocite VOOH ultrafine NPs. The influence of hydrothermal conditions on the hydrothermal products was studied and the characterizations confirmed that the formation of VOOH NPs was induced by a Kirkendall diffusion process. The obtained VOOH could be further transformed into VO2(M) after annealing treatment, leading to well crystalline VO2 NPs with optimum thermochromic and plasmonic properties. Coating VO2 NPs with an inert SiO2 shell not only improved the environmental tolerance of VO2, but also enhanced the optical modulation of the VO2 film, which was attributed to the blue-shift of the LSPR peak. The optimal ΔTsol and Tlum were 14.91% (∼15%) and 62.29% (> 60%), offering new opportunities for VO2-based energy-efficient smart windows.Author contributions
L. W. and A. T. fabricated the samples, finished the data measurement and prepared the manuscript. M. L., X. H. and G. L. conceived the idea and revised the paper. L. L., Z. H., X. L., J. Y, S. X. and F. Z. coordinated this study and checked the literature. A. Z., J. Z., T. J. and Y. X. discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the HFIPS Director's Fund (Grant No. YZJJ202202-CX, YZJJ2022QN28, YZJJ202308-TS, YZJJ202312-TS, YZJJ-GGZX-2022-01); Zhulin Huang would like to thank the Natural Science Foundation of China (Grant No. 52222208, 52072373).References
- S. Wang, T. Jiang, Y. Meng, R. Yang, G. Tan and Y. Long, Science, 2021, 374, 1501–1504 CrossRef PubMed.
- Q. Lei, W. Yu, G. Xie, Y. Li, C. Wu, G. Jiang, Y. Zhou and H. Xie, Sol. RRL, 2023, 7, 2200990 CrossRef.
- G. Chen, K. Wang, J. Yang, J. Huang, Z. Chen, J. Zheng, J. Wang, H. Yang, S. Li, Y. Miao, W. Wang, N. Zhu, X. Jiang, Y. Chen and J. Fu, Adv. Mater., 2023, 35, 2211716 CrossRef PubMed.
- H. Zhang, J. Feng, F. Sun, D. Zhou, G. Cao, S. Wang, X. Hu, J. Ma, F. Su, Y. Tian and Y. Tian, Adv. Mater. Technol., 2023, 8, 2201688 CrossRef.
- J. Liu, R. Yang, J. Zhang, Q. Tao, A. Li, Z. Liu, Y. Su and Y. Liu, Sol. Energy Mater. Sol. Cells, 2023, 249, 112048 CrossRef CAS.
- E. Poloni, A. Rafsanjani, V. Place, D. Ferretti and A. R. Studart, Adv. Mater., 2022, 34, 2104874 CrossRef CAS.
- M. Liu, X. Li, L. Li, L. Li, S. Zhao, K. Lu, K. Chen, J. Zhu, T. Zhou, C. Hu, Z. Lin, C. Xu, B. Zhao, G. Zhang, G. Pei and C. Zou, ACS Nano, 2023, 17, 9501–9509 CrossRef CAS PubMed.
- T. Zhang and Q. Li, J. Solid State Chem., 2022, 311, 123117 CrossRef CAS.
- J. Zhang, X. Sun, T. Wang, W. Xu, G. Luo, Y. Wang and C. Zhou, Opt. Mater., 2023, 136, 113498 CrossRef CAS.
- Y. Ke, Y. Tan, C. Feng, C. Chen, Q. Lu, Q. Xu, T. Wang, H. Liu, X. Liu, J. Peng and Y. Long, Appl. Energy, 2022, 315, 119053 CrossRef CAS.
- Z. Li, C. Cao, M. Li, L. Wang, D. Zhu, F. Xu, A. Huang, P. Jin, L. Yu and X. Cao, ACS Appl. Mater. Interfaces, 2023, 15, 9401–9411 CrossRef CAS PubMed.
- L. Zhang, J. Yao, Y. Guo, F. Xia, Y. Cui, B. Liu and Y. Gao, Ceram. Int., 2018, 44, 19301–19306 CrossRef CAS.
- C. Wang, H. Xu, C. Wang, T. Liu, S. Yang, Y. Nie, X. Guo, X. Ma and X. Jiang, J. Alloys Compd., 2021, 877, 159888 CrossRef CAS.
- L. Liu, F. Cao, T. Yao, Y. Xu, M. Zhou, B. Qu, B. Pan, C. Wu, S. Wei and Y. Xie, New J. Chem., 2012, 36, 619–625 RSC.
- S. Guan, A. Rougier, O. Viraphong, D. Denux, N. Penin and M. Gaudon, Inorg. Chem., 2018, 57, 8857–8865 CrossRef CAS.
- K. Li, M. Li, C. Xu, Y. Luo and G. Li, J. Alloys Compd., 2020, 816, 152655 CrossRef CAS.
- C. Z. Wu, Y. Xie, L. Y. Lei, S. Q. Hu and C. Z. OuYang, Adv. Mater., 2006, 18, 1727–1732 CrossRef CAS.
- J. Zhang, R. Cui, C. Gao, L. Bian, Y. Pu, X. Zhu, X. A. Li and W. Huang, Small, 2019, 15, 1904688 CrossRef CAS.
- H. Shi, H. Liang, F. Ming and Z. Wang, Angew. Chem., Int. Ed., 2017, 56, 573–577 CrossRef CAS PubMed.
- J. Yao, H. Zhang, Z. Zhao, Z. Zhu, J. Yao, X. Zheng and Y. Yang, Dalton Trans., 2021, 50, 3867–3873 RSC.
- W. Xiao, S. Oh, T. V. M. Sreekanth, J. Kim and K. S. Yoo, ACS Appl. Mater. Interfaces, 2022, 14, 34802–34813 CrossRef.
- X. Cao, N. Wang, J. Y. Law, S. C. J. Loo, S. Magdassi and Y. Long, Langmuir, 2014, 30, 1710–1715 CrossRef.
- C. Wu, X. Zhang, B. Ning, J. Yang and Y. Xie, Inorg. Chem., 2009, 48, 6044–6054 CrossRef PubMed.
- Y. Xu, L. Zheng and Y. Xie, Dalton Trans., 2010, 39, 10729–10738 RSC.
- M. Fan, D. Liao, M. F. A. Aboud, I. Shakir and Y. Xu, Angew. Chem., Int. Ed., 2020, 59, 8247–8254 CrossRef PubMed.
- B. Shen, L. Huang, J. Shen, X. Hu, P. Zhong, C. Y. Zheng, C. Wolverton and C. A. Mirkin, ACS Nano, 2023, 17, 4642–4649 CrossRef.
- J. Pi, C.-B. Li, R.-Y. Sun, L.-Y. Li, F. Wang, F. Song, J.-M. Wu, X.-L. Wang and Y.-Z. Wang, Compos. Commun., 2022, 32, 101167 CrossRef CAS.
- Z. Li, S. Zhao, Z. Shao, H. Jia, A. Huang, P. Jin and X. Cao, Chem. Eng. J., 2022, 447, 137556 CrossRef CAS.
- X. Li, C. Cao, C. Liu, W. He, K. Wu, Y. Wang, B. Xu, Z. Tian, E. Song, J. Cui, G. Huang, C. Zheng, Z. Di, X. Cao and Y. Mei, Nat. Commun., 2022, 13, 7819 CrossRef CAS PubMed.
- L. H. Molloro, S. Tain, N. Belachew, K. A. Owusu and X. Zhao, RSC Adv., 2021, 11, 13556–13563 RSC.
- W. Li, S. Ji, K. Qian and P. Jin, J. Colloid Interface Sci., 2015, 456, 166–173 CrossRef CAS.
- X. Wang, M. Li, Q. Wang, J. Zhang, J. Shi, Y. Lu and G. Li, Eur. J. Inorg. Chem., 2020, 1783–1789 CrossRef CAS.
- Y.-Q. Li, S.-Y. Fu, Y. Yang and Y.-W. Mai, Chem. Mater., 2008, 20, 2637–2643 CrossRef CAS.
- J. Song, Y. Zhao, L. Sun, Q. Luo, H. Xu, C. Wang, H. Xin, W. Wu and F. Ma, Ceram. Int., 2022, 48, 15868–15876 CrossRef CAS.
- S. Long, X. Cao, R. Huang, F. Xu, N. Li, A. Huang, G. Sun, S. Bao, H. Luo and P. Jin, ACS Appl. Mater. Interfaces, 2019, 11, 22692–22702 CrossRef CAS PubMed.
- Z. Du, M. Li, F. Zou, Y. Song, S. Xu, L. Wu, L. Li and G. Li, ACS Appl. Nano Mater., 2022, 5, 12972–12979 CrossRef CAS.
- Y. Chen, X. Zeng, J. Zhu, R. Li, H. Yao, X. Cao, S. Ji and P. Jin, ACS Appl. Mater. Interfaces, 2017, 9, 27784–27791 CrossRef CAS.
- X. Zhao, J. Sun, Z. Guo, J. Su, T. Liu, R. Hu, W. Yao and X. Jiang, Chem. Eng. J., 2022, 446, 137308 CrossRef CAS.
- Z. Chen, Y. Tang, A. Ji, L. Zhang and Y. Gao, ACS Appl. Nano Mater., 2021, 4, 4048–4054 CrossRef CAS.
- J. Kang, J. Liu, F. Shi, Y. Dong, X. Song, Z. Wang, Z. Tian, J. Xu, J. Ma and X. Zhao, Appl. Surf. Sci., 2022, 573, 151507 CrossRef CAS.
- Z. Zhao, Y. Liu, D. Wang, C. Ling, Q. Chang, J. Li, Y. Zhao and H. Jin, Sol. Energy Mater. Sol. Cells, 2020, 209, 110443 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01393f |
| ‡ The authors have equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |