Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green synthesis of a thermo/photochromic doped cellulose polymer: a biocompatible film for potential application in cold chain visual tracking†
A.
Azzali‡§
 ,
M. F.
Di Filippo§
,
L.
Bertuccioli
,
S.
Lilburn¶
,
S.
Panzavolta
,
M. F.
Di Filippo§
,
L.
Bertuccioli
,
S.
Lilburn¶
,
S.
Panzavolta
 *,
F.
Grepioni
*,
F.
Grepioni
 and
S.
d'Agostino
and
S.
d'Agostino
 *
*
Department of Chemistry “Giacomo Ciamician”– The University of Bologna, via F. Selmi 2, 40126, Bologna, Italy. E-mail: silvia.panzavolta@unibo.it; simone.dagostino2@unibo.it
First published on 31st May 2024
Abstract
To mitigate food losses and ensure a robust cold chain in transportation, sensors play a pivotal role in swiftly and visibly monitoring storage conditions. The most commonly used indicators for reporting temperature violations are based on devices capable of signaling when a threshold temperature has been reached or exceeded or on disposable colorimetric sensors. A potential alternative, which uses reusable colorimetric sensors, may come from utilizing systems capable of displaying reversible color changes upon temperature variations; in this regard, molecules exhibiting thermo- and photochromic properties such as N-salicylideneaniline derivatives (anils) have emerged as promising candidates due to the simplicity of their synthesis and their ability to respond to temperature and light stimuli. In this study we have synthesized a family of anils through mechanochemistry, focusing on H/F substituents on the bromoaniline residue. The compounds were fully characterized using XRD and thermal techniques, and their thermo- and photochromic properties were explored via infrared spectroscopy. Among the series, the most suitable compound, i.e., a photochromic one showing a neat color change (from white to red/orange) quickly naked eye-detectable and whose back reaction is slow or virtually negligible at low temperatures, was identified and incorporated into a carboxymethyl cellulose (CMC) biopolymer matrix to produce a composite film, which was further characterized via XRD, thermal analyses and mechanical tests. The selected compound maintained its photochromic behavior upon embedding, and UV irradiation induced a color change in the film from colorless to red, while reversibility was evaluated at different temperatures (−19 °C, 4 °C and RT) using UV-vis spectroscopy. The composite film maintained a deep red color at −19 °C and 4 °C for seven weeks, while rapidly reversing to white/yellowish at room temperature, making it a suitable candidate for the development of sensors for cold chain transport and scenarios requiring rapid visual inspection of storage conditions.
Design, System, ApplicationCold chain trackers are systems designed to monitor and track perishable goods' temperature and environmental conditions throughout the supply chain, from production to distribution and storage. Nowadays, most trackers are divided into devices and colorimetric indicators for visual inspection. Designing novel cold chain trackers with visual inspection capabilities is paramount. These trackers allow real-time feedback on the condition of goods, enabling quick identification of potential issues. In this work, we used molecular design to synthesize a family of crystalline thermo- and photo-chromic pigments and the possibility of incorporating them within a biodegradable GRAS biopolymer matrix to create tags of the composite material to be used as indicators. Tests at various storage temperatures were also performed. We believe that this work can potentially contribute to developing novel cold-chain trackers for visual inspection working on a broader range of temperatures. |
1. Introduction
Food waste can be defined as the collection of products discarded from the agri-food chain. These products are still edible and potentially suitable for human consumption; however, they are destined for elimination or disposal due to economic and aesthetic reasons or to their proximity to the consumption/expiration date. Data relating to this practice, unfortunately widespread, are disconcerting; it is estimated that 30 to 50% of the food produced never reaches the consumer's table.1,2The loss and waste of food is not only an ethical issue, but also involves a significant waste of resources in the production process.2–4
The ‘cold chain’ is vital for perishable goods and pharmaceuticals, ensuring quality and safety from production to sale by controlling the temperature during transportation and storage. Proper temperature management minimizes spoilage risk, extends shelf life, and guarantees product safety.
According to the Hazard Analysis and Critical Control Points (HACCP) standards, foods are categorized by temperature requirements: chilled (0 °C to 15 °C), frozen (−18 °C to −25 °C), and deep-frozen (below −25 °C). For instance, meat and cheese should be stored at 4–6 °C, while fruits and vegetables require temperatures below 8 °C.5 The global cold chain market was valued at $270 billion in 2021 and is projected to grow at a compound annual rate (CAGR) of 18.6% from 2023 to 2030.6
Maintaining the right temperature for each food prevents deterioration of the nutritional and organoleptic properties, while defrosting and refreezing can cause the growth and multiplication of undesirable microorganisms and bacteria.7–9 The same treatment is applied to pharmaceutical products stored at a constant temperature.10–16
To date, the most commonly used indicators for reporting temperature violations are based on radio frequency identification (RFID) and time–temperature indicator (TTI) tags, capable of signaling when a given threshold temperature has been reached or exceeded,17–20 or disposable colorimetric sensors.21,22 Digital devices are costly and complex, often unsuitable for individual products. On the other hand, colorimetric sensors offer affordable and user-friendly monitoring without the need for read-out devices or trained personnel,21,22 whereas most of the indicators on the market are based on pigments/mixtures that degrade with increasing temperature, posing, thus, limits on reutilization. Alternatives based on nanoparticles that exhibit fluorescence changes due to temperature variations have been proposed recently in the literature;21–24 however, their synthetic procedures and implementation into devices remain pretty challenging. Therefore, finding affordable and easy-to-synthesize materials for colorimetric sensors in the cold chain is crucial. In this regard, thermo- and photochromic molecules are promising candidates since they undergo a color change when heated above a threshold temperature or upon irradiation with visible or UV light.14,25,26
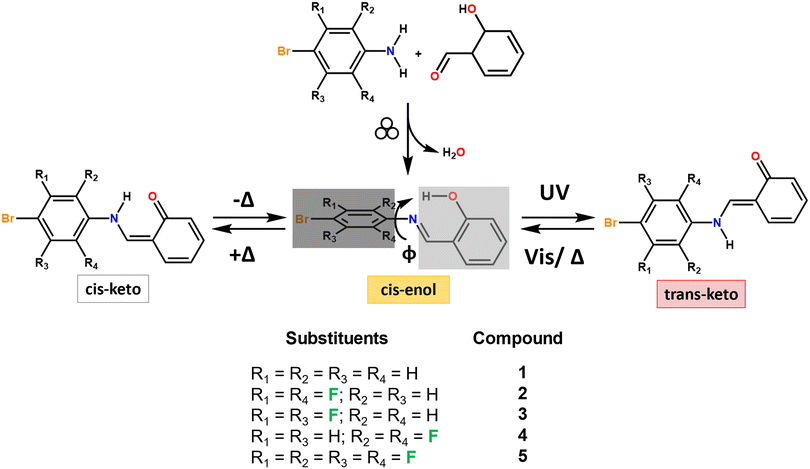
Among thermo- and photochromic compounds, N-salicylideneanilines, also named anils, and their derivatives have received particular attention due to the ease of preparation and their ability to switch, depending on the stimulus applied, between three different forms: a pale yellow enol form, a colorless cis-keto form, and an orange-red trans-keto form,26–28 as depicted in Fig. 1.
In the solid state, most anil compounds have a thermochromic behavior, whereas photochromism is rarely observed in these systems. As a rule of thumb, photochromism is usually observed for non-planar anils, characterized by a dihedral angle φ ≥ 30° between the aromatic ring planes. Quasi-planar conformations (φ ≤ 20°) tend to exhibit thermochromic features, whereas for φ values between 20° and 30°, the compound can display both behaviors (see Fig. 1).27,29–32 However, a number of studies have shown the limit of such an empirical rule.33–36 Photochromism is the preferred choice for developing a suitable sensor for the cold-chain, especially when the color change, e.g., from white to red/orange, is marked and easily naked eye-detectable, and the back reaction is slow or virtually negligible at low temperature. In contrast, thermochromic solids tend to quickly change their color with temperature variations.
According to the proposed “pedal-motion” mechanism of cis–trans photoisomerization, photochromism should be observed only when there is sufficient reaction volume for the molecule to undergo the isomerization process within the crystal.31,37
The main strategies currently used to design and modify materials properties rely either on a crystal engineering approach,28,30,38–40i.e. a search for co-crystals or polymorphs, or on covalent modifications, namely, the introduction of substituents able to act as space closers/openers41,42 or to alter the torsional angle φ,43,44 thus enabling the cis–trans photoisomerization. In this regard, functionalizing the aniline residue offers several possibilities.
Intermolecular C–F⋯H–C interactions occurring in the crystal structures of organofluorines45,46 play an important role in directing supramolecular assembly in chemical and biological systems.45,47–49
Moreover, in contrast to the C–F⋯F–C ones, whose directionality is insignificant,45,50 they can be exploited to lock in place the adjacent bromoaniline residue through the establishment of self-complementary interactions, which, in combination with the repulsive character of the C–H⋯H–C and C–F⋯F–C interactions, can result in a change of the torsional angle (φ) which can be exploited to favoring thermo or photochromism.
Additionally, the enlargement of the electropositive σ-hole localized on the Br-atom,51–53 through the increasing number of F-atoms on the aniline residue, may give rise to halogen bonding interactions (XB)54,55 of the type X⋯X,56,57 thus influencing the relative molecular arrangement within the solid products.
The aim of this research was manifold: on the one hand, we were interested in synthesizing a new family of anils bearing different numbers of H/F substituents on the bromoaniline residue (see Fig. 1) and seeing how the H/F ratio affects the chromic features in solids. Five anil compounds were synthesized via mechanochemistry, with an eye on green chemistry principles,58,59 and thoroughly characterized through a combination of X-ray diffraction (XRD), thermal techniques including differential scanning calorimetry and thermogravimetric analysis (DSC and TGA), and infrared spectroscopy (FTIR). The best candidate was then selected and embedded in carboxymethyl cellulose (CMC), a non-toxic, biodegradable and GRAS (generally recognized as safe) biopolymer; the photochromic composite film thus obtained was further characterized via XRD, thermal and mechanical techniques.
UV irradiation of the composite film resulted in a color change from the yellowish cis-enol form to the red trans-keto form stable at −19 °C and 4 °C; the reverse process was monitored with UV-vis spectroscopy at −19 °C, 4 °C and room temperature. At RT, the color fades completely in approximately four days, making, de facto, the selected compound a suitable pigment to be used in the cold chain for long transportation or in all cases for which a rapid visual inspection of the storage conditions is desirable.
2. Materials and methods
All reactants and reagents were purchased from Sigma-Aldrich and used without further purification. Reagent grade solvents and Milli-Q water were used. All acronyms and compound names are listed in Table 1.| 1 | 4-Bromo-N-salicylideneaniline |
| 2 | 4-Bromo-2,5-difluoro-N-salicylideneaniline |
| 3 | 4-Bromo-3,5-difluoro-N-salicylideneaniline |
| 4 | 4-Bromo-2,6-difluoro-N-salicylideneaniline |
| 5 | 4-Bromo-2,3,5,6-tetrafluoro-N-salicylideneaniline |
| CMC | Carboxymethyl cellulose |
| CMC-5@x% | Carboxymethyl cellulose with 5 incorporated at x% in weight (x = 1, 5, 10, and 15) |
| NMR | Nuclear magnetic resonance spectroscopy |
| SCXRD | Single crystal X-ray diffraction |
| PXRD | Powder X-ray diffraction |
| TGA | Thermogravimetric analysis |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| UV-vis | Ultraviolet and visible electronic absorption spectroscopy |
| HSM | Hot stage microscopy |
| HACCP | Hazard analysis and critical control points |
2.1 Synthesis
For the synthesis of compounds 1–5, 100 μL (0.95 mmol) of salicylaldehyde was co-ground manually for ca. 20–30 minutes in a ball-milling apparatus (Retsch MM 200) operating at 20 Hz for 20 minutes with one equivalent (0.95 mmol) of the corresponding bromoaniline. When the reaction did not reach completion, a slight excess of aldehyde was added, to compensate for the loss of aldehyde due to evaporation during the manual grinding process. Recrystallizing of the so-obtained powders from hot ethanolic solutions left to cool down slowly in the dark afforded single crystals suitable for XRD.2.2 NMR spectroscopy
1HNMR and 13CNMR spectra were recorded on a Varian INOVA 400 (400 MHz) spectrometer using, as a solvent, chloroform-d1 (DMSO-d6) bought from Sigma-Aldrich. See Fig. S1–S5.†2.3 CMC-5@15% film preparation
300 mg of carboxymethyl cellulose sodium salt (CMCNa) was dissolved in 6 mL of Milli-Q water (5% w/v) and stirred overnight. Subsequently, 45 mg of compound 5, previously ball-milled at 5 Hz for 90 minutes, was added and the resulting suspension was left under stirring for another 3 hours. The mixture was then ultrasonicated in an ELMASONIC S15H bath filled with water operating at a frequency of 37 kHz for 5 minutes to increase the homogeneity of the dispersed polycrystalline powders, and finally cast into a petri dish with a diameter of 10 cm. Upon evaporation of the solvent, films with a thickness of about 100 μm were obtained. The film thickness was measured using a hand-held digital micrometer (Mitutoyo, Japan) at six different positions in each specimen to an accuracy of 0.001 mm. The obtained films had comparable thicknesses.2.4 Crystal structure determination
Single-crystal data for compounds 1–5 were collected at RT on an Oxford X'Calibur S CCD diffractometer equipped with a graphite monochromator (Mo-Kα radiation, λ = 0.71073 Å). In the case of crystals showing twinning, reflection data were integrated with the default configuration for twinned crystals of the CrysAlisPro Software. Subsequent structural solution and refinement were performed using the HKLF4 file containing non-overlapped reflections. The structures were solved with SHELXT60 by intrinsic phasing and refined on F2 with SHELXL61 implemented in the Olex2 software62 by full-matrix least squares refinement. HOH atoms were either directly located or, when not possible, added in calculated positions; HCH atoms for all compounds were added in calculated positions and refined riding on their respective carbon atoms. All non-hydrogen atoms were anisotropically refined and the rigid-body RIGU restraints applied.61 Data collection and refinement details are listed in Table S1.† The program Mercury63 was used for molecular graphics and calculation of intermolecular interactions.2.5 Powder XRD measurements
For phase identification and variable temperature purposes, powder X-ray diffractograms were collected in the Bragg–Brentano geometry, in the 2θ range of 5–40°, on a PANalytical X'Pert PRO automated diffractometer equipped with an X'Celerator detector and using Cu Kα radiation without a monochromator (step size 0.02°; time per step 20 s; 0.04 rad Soller; 40 mA × 40 kV). The program Mercury63 was used to calculate the powder X-ray patterns based on single crystal data collected in this work or retrieved from the CSD.64 In all cases, the identity between polycrystalline samples and single crystals was always verified by comparing experimental and calculated powder diffraction patterns (see Fig. S6–S9†).2.6 Thermogravimetric analysis measurements
TGA measurements were performed with a PerkinElmer TGA7 instrument in the temperature range of 40–300 °C, under a nitrogen flow at a heating rate of 5.00 °C min−1.2.7 Differential scanning calorimetry measurements
DSC measurements were performed with a PerkinElmer Pyris Diamond DSC instrument. Samples (3–5 mg) were placed in open aluminum pans. Heating was carried out for all samples at 5.00 °C min−1 under a N2 atmosphere.2.8 Hot stage optical microscopy
HSM analyses were carried out using a Linkam TMS94 device connected to a Linkam LTS350 platinum plate and equipped with polarizing filters. Images were collected with a NIKON DS FI3 camera, from an Olympus BX41 stereomicroscope.2.9 Fourier-transform infrared spectroscopy
FTIR spectra were recorded in transmission mode on a Bruker Alpha FTIR spectrometer. 1–2 mg of each sample was finely dispersed in ca. 200 mg of dry KBr pellets with a diameter of 1.3 cm.2.10 Mechanical properties
Tensile tests were performed on strip-shaped samples (5 mm width and 40 mm length) using a 4465 Instron dynamometer equipped with a 100 N load cell. The thickness was evaluated with a Mitutoyo micrometer. Stress–strain curves were recorded at a crosshead speed of 5 mm min−1 using the software Bluehill Universal for Windows. At least seven specimens were tested for each composition. The stress at break (σb) and the strain at break (εb) were evaluated and reported with the standard deviation.2.11 Solid-state UV-irradiation
Samples in KBr pellets or polymeric films placed at a distance of 5 cm were irradiated for 10 minutes using a UV-LED LED EnginLZ1-10UV00-0000 centered at λ = 365 nm (FWHM = 25 nm) and operated at 4.5 V and 700 mA with a radiant flux (φ) of 1200 mw.2.12 Ultraviolet-visible spectroscopy
The UV-vis electronic absorption spectra of the CMC polymeric films containing finely dispersed photochromic compound 5 were recorded using a Varian CARY 5 UV-vis spectrophotometer in the range of 300–800 nm. The films were cut into 1 cm wide rectangular strips and then inserted into the instrument sample holder. The color fading kinetics were studied utilizing commercial refrigerators/freezers set at 30% relative humidity (RH). The fading trials were replicated twice using distinct film samples. Irradiation cycles were employed to rule out UV-induced degradation and gauge the reproducibility of the photochromic reaction, monitoring absorbance at 470 nm in single-point mode. Additionally, irradiation cycles were employed to evaluate errors in UV-vis measurements, resulting in a standard deviation (σ) of ±0.002.3. Results and discussion
3.1 Mechanochemical synthesis and powder diffraction
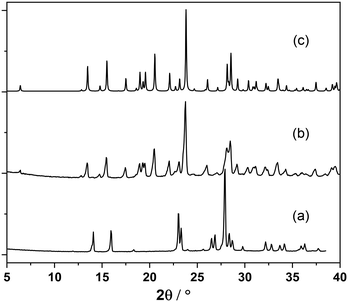
In a typical mechanochemical synthesis, salicylaldehyde and the respective bromoaniline derivatives were manually co-ground, and the progress of the reaction was monitored by powder X-ray diffraction (XRD) analysis; no solvent addition was required to facilitate the reaction, as the bromoaniline derivatives are liquid. The resulting solids were then recrystallized in the minimum amount of solvent, and the single crystals obtained were analyzed via XRD.In general, the experimental powder XRD patterns of all five mechanochemistry outcomes are significantly different from those of the reagents and can be superimposed to the ones calculated on the basis of the structures obtained from single crystal X-ray diffraction (see Fig. S6–S9†); we can therefore conclude that the synthesis by grinding is successful for all five compounds selected. In some syntheses, the patterns obtained by grinding contained some small peaks attributable to the reactant, and this is possibly due to the volatility of salicylaldehyde at RT; in those cases, adding a slight excess of salicylaldehyde was necessary to obtain the desired product in the pure form. On the other hand, when the same syntheses were carried out in a ball milling apparatus, no salicylaldehyde excess was required, and a quantitative reaction could be obtained with a stoichiometric ratio of the reactants. Fig. 2 depicts the PXRD patterns of the reactants and mechanochemical product in the case of compound 5.
3.2 Structural and chromic features
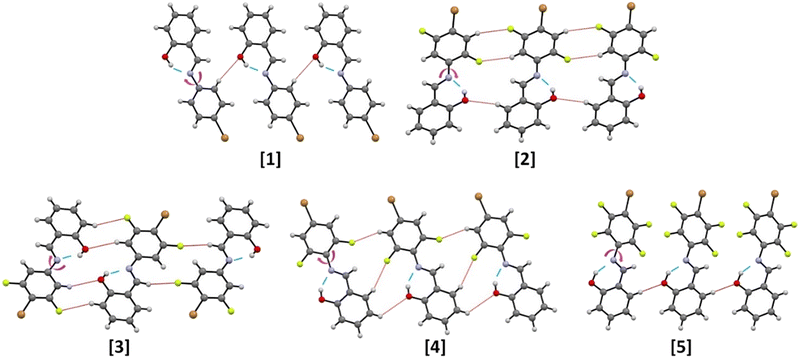
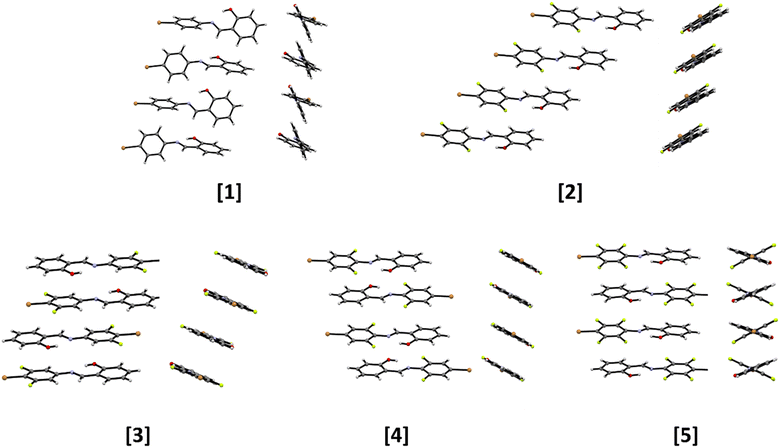
Single crystal diffraction reveals that the molecular structures of the five obtained compounds are all quite similar. An analysis of the C–O and C–N distances confirms the formation of the more thermodynamically stable cis-enol form in all cases, and, as expected, all compounds feature the presence of an intramolecular hydrogen bond65,66 between the –OH and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functions; besides this, also weak intermolecular interactions of types C–H⋯O and C–F⋯H are detected (see Fig. 3 and Table 2). Despite the increasing number of F atoms, along the series 1–5, no occurrence of significant X⋯X bonds is observed. Instead, this feature affects the stacking pattern of the molecules, see Fig. 4. Compounds 1 and 2 form head-to-head piles, whereas in 3, 4 and 5 there are head-to-tail stackings.
N– functions; besides this, also weak intermolecular interactions of types C–H⋯O and C–F⋯H are detected (see Fig. 3 and Table 2). Despite the increasing number of F atoms, along the series 1–5, no occurrence of significant X⋯X bonds is observed. Instead, this feature affects the stacking pattern of the molecules, see Fig. 4. Compounds 1 and 2 form head-to-head piles, whereas in 3, 4 and 5 there are head-to-tail stackings.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond distances (Å), and torsional angles (°) in crystalline 1–5 calculated from the program Mercury64
N bond distances (Å), and torsional angles (°) in crystalline 1–5 calculated from the program Mercury64
| Entry | O(H)⋯N | C(H)⋯O | C(H)⋯F | C–OH | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
φ | Expected behavior |
|---|---|---|---|---|---|---|---|
| 1 | 2.593(9) | 3.427(9) | — | 1.35(1) | 1.29(1) | 38 | Photochromic |
| 2 | 2.58(2) | 3.37(2) | 3.48(2) | 1.34(2) | 1.28(2) | 5 | Thermochromic |
| 3 | 2.624(5) | 3.484(5) | 3.523(5) | 1.343(5) | 1.263(5) | 4 | Thermochromic |
| 4 | 2.596(3) | 3.535(5) | 3.273(5) | 1.343(5) | 1.270(5) | 4 | Thermochromic |
| 5 | 2.619(7) | 3.565(9) | — | 1.353(9) | 1.282(9) | 47 | Photochromic |
In general, the interplay between weak intermolecular interactions and stacking accounts for the modification of crystal packing and torsional angle (φ).
3.3 Thermal and photochromic properties
As shown by thermal analyses (see Fig. S10 and S11† for TGA and DSC traces, respectively, and Table S2† for DSC data), all compounds present good stability, with an endothermic peak after 100 °C corresponding to melting, whereas degradation starts at ca. 150 °C in each case.The thermo- and photochromic properties were verified by lowering the temperature and irradiating with UV (λ = 365 nm) single crystal samples of compounds 1–5. As witnessed by the slight thermal fading (pale yellow → colorless), all five compounds possess a weak thermochromic character (see Fig. 5 for compound 5).
As expected, compounds 1 and 5, with a torsional angle greater than 20° (Table 2), confirm the hypotheses previously made and, upon UV irradiation, show a very intense and rapid color variation, from white/yellowish to red, associated with the conversion into the corresponding trans-keto forms. Compounds 2 and 4, which possess planar structures, are not photochromic; in contrast, compound 3, also planar, displays only a weak color change after prolonged (>30 min) UV irradiation. After irradiation, all specimens were again analyzed via X-ray diffraction: despite the color variation, visible to the naked eye, no significant changes were found in the crystal structures, as the unit cell parameters and C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond distances were the same as those observed before irradiation.
N bond distances were the same as those observed before irradiation.
Photochromic compounds 1, 3, and 5 were irradiated and maintained at 100 K for single crystal analysis. However, no structural changes were detected. The authors suppose therefore that the interconversion between the species is only superficial: the UV light can only radiate through the first few layers of the crystals, and conversion to the trans-keto form is accompanied by some amorphization.
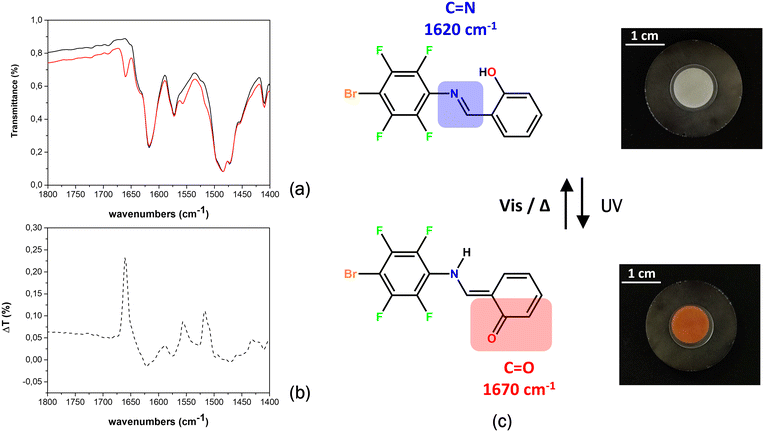
Infrared spectroscopy (FTIR) was then used to assess the formation of the trans-keto form responsible for the red coloring in photochromic compounds. The three photochromic compounds were dispersed in KBr pellets (ca. 2% in weight) and subjected to UV irradiation (LED at 365 nm) to evaluate the presence of the trans-keto form within their crystals.
In compound 1, the back reaction is fast, and the trans-keto form reverts quickly to the cis-enol form; persistence of the red color can only be observed upon steady UV irradiation. Conversion to the keto-form in solid 3 could only be observed by visual inspection, as the product is likely present in quantities below the detectable limit. On the other hand, the red color observed with compound 5 is maintained for about 90 minutes when the solid is stored under ambient light and temperature conditions. As shown in Fig. 6, the color change is indeed accompanied by a marked variation in its spectrum: the band at 1620 cm−1 (ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching of the enol form) decreases, while the appearance of a band at 1670 cm−1, the region where the stretching of conjugated C
N stretching of the enol form) decreases, while the appearance of a band at 1670 cm−1, the region where the stretching of conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O falls,29,67 confirms the formation of the trans-keto tautomer product. After thermal treatment (80 °C, 20 min) or after exposure to visible light (447 nm), the original color is instantaneously recovered, therefore the process is reversible (see the movie in the ESI†). Fig. S14† shows the behavior for the other compounds.
O falls,29,67 confirms the formation of the trans-keto tautomer product. After thermal treatment (80 °C, 20 min) or after exposure to visible light (447 nm), the original color is instantaneously recovered, therefore the process is reversible (see the movie in the ESI†). Fig. S14† shows the behavior for the other compounds.
3.4 Composite cellulose film preparation
Based on this preliminary screening, we concluded that compound 5 is the best candidate to incorporate in the CMC-polymeric film to obtain a membrane with photochromic properties. To this end, a polycrystalline sample of 5 was finely ground for 90 minutes with ball milling at a frequency of 5 Hz to maximize the homogeneity of the crystalline powder, which was then incorporated in the polymeric matrix.By varying the amount of compound 5, it was possible to optimize the color intensity obtained upon irradiation with visible light: four different weight percentages of 5 (1, 5, 10 and 15%) were suspended in CMC, providing four different composite film formulations labelled CMC-5@1%, CMC-5@5%, CMC-5@10% and CMC-5@15%, respectively. These films were irradiated at λ = 365 nm for 10 minutes, with the UV lamp positioned at 5 cm from the film; the films were then stored at 4 °C up to 30 days.
As shown in Fig. 7, only in the films CMC-5@10% and CMC-5@15% the red color was intense and persistent over time: samples stored at 4 °C for 30 days fully retained their coloring. Given the higher intensity of red staining and the better homogeneity of 5 in the film, the CMC-5@15% composite was selected for further testing.
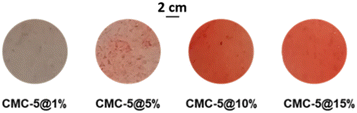 | ||
| Fig. 7 Micrographs of CMC-based films containing, from left to right, increasing amounts of compound 5, showing how composite film homogeneity is reached at higher concentrations. | ||
Fig. 8a compares the thermograms for a pristine CMC film, a composite CMC-5@15%, and pure 5, showing that the photochromic compound has been successfully embedded in the polymeric film. In the thermogravimetric plot for the composite, three weight losses can be detected: the first one at around 50 °C corresponding to the evaporation of surface water; the second one starting at 130 °C, attributable to the degradation of crystalline 5, and the third one beginning at 260 °C due to carboxymethyl cellulose degradation. DSC measurements were conducted to study the thermal behavior of photochromic film CMC-5@15% between the temperature range of −25 °C and 200 °C (Fig. S12†). The results of the DSC measurements were consistent with TGA, indicating that the thermal behavior of the film is a superimposition of the behaviors of its two components, i.e., dehydration of CMC followed by the melting of compound 5. At the same time, no significant processes were observed at lower temperatures. Heating and cooling cycles within the same range were also performed on CMC-5@15%. The reproducibility of the traces ensures that any possibility of sample degradation can be dismissed (Fig. S14†). Powder XRD measurements (Fig. 8b) further confirm the doping of 5 in the CMC membrane, as all the characteristic peaks of pure crystalline 5 can be observed in the measured pattern, despite the preferred orientation of the powders in the cellulose film affecting intensities in the PXRD pattern.
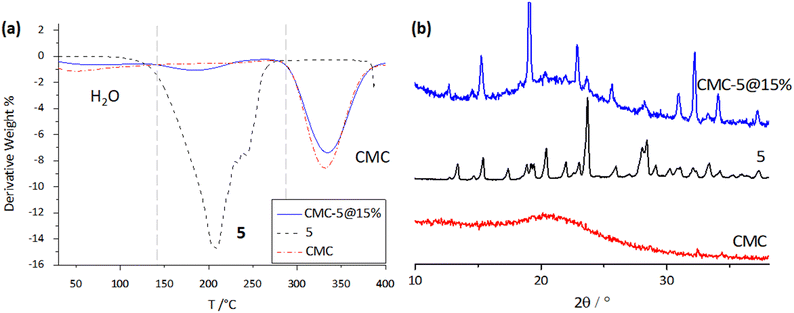 | ||
| Fig. 8 (a) TGA thermograms (left) and (b) powder XRD patterns for the pristine CMC film, pure 5, and the CMC-5@15% composite. | ||
The mechanical properties of the pristine CMC and of the film obtained after mixing of the biopolymer with 5 have been evaluated by tensile stress–strain tests. By comparing the stress–strain curves (Fig. S15†), the similar shape and the complete superimposition at a few percent of deformation indicate that the presence of CMC dominates the mechanical behavior, and the crystal addition predominantly reduces the stress at break (29 ± 9 MPa and 55 ± 6 MPa, for CMC and CMC-5@15%, respectively) of the films without affecting their brittleness. The mechanical characteristics necessary for the proposed application are thus preserved.
3.5 Photochromic features in the composite film
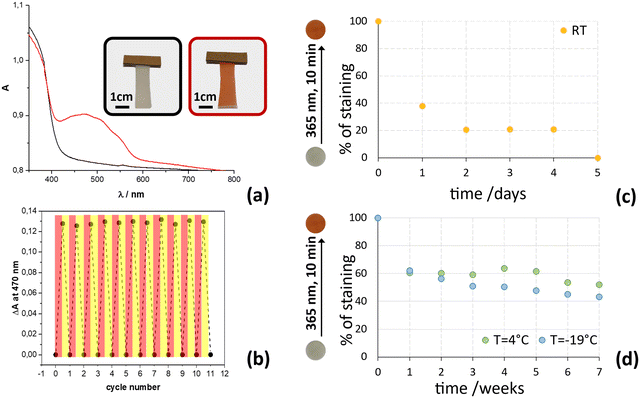
The absorption spectra of CMC-5@15%, before and after UV irradiation, were also registered and are shown in Fig. 9. The non-irradiated film shows a distinct broad absorption band extending below λ = 430, which, in analogy with literature reports,32,34,68,69 can be attributed to the n–π* absorption of the cis-enol form and to the absorption of the CMC. UV irradiation causes an increase of the absorption in the λ = 400–600 nm region, and the presence of the band at 470 nm indicates the presence of the cis-keto tautomer, in agreement with the photochromic behavior observed in FTIR spectroscopy.The absorption spectrum, initially recorded before irradiation at 365 nm, undergoes complete restoration when the film is irradiated again at room temperature with a blue LED emitting light at 447 nm. To evaluate the compound's stability and reproducibility, 11 cycles were executed, as illustrated in Fig. 9.
Thermal fading of staining was further evaluated with UV-vis spectroscopy by monitoring the absorption intensity at 470 nm of irradiated CMC-5@15% film strips, stored at three different temperatures (−19 °C, 4 °C and RT); in all cases the relative humidity, RH, was 30%. Remarkably, even at sub-freezing temperatures (−19 °C), the color change induced by irradiation at 365 nm was easily noticeable to the naked eye. Importantly, there was no ice formation that obscured its visibility or affecting the composite film under sub-freezing conditions, contrasting with occurrences in other reported systems.70 The thermal fading curves at different temperatures are reported in Fig. 9b and reveal that the composite film CMC-5@15% can maintain its staining after irradiation with UV light for several weeks only if stored at temperatures below 4 °C. Indeed, the film strips preserved at −19 °C and 4 °C have maintained high staining (>40%) even after 7 weeks (50 days). In contrast, when the film is stored at room temperature (about 19 °C), its coloring decreases much more quickly, reaching a reduction to less than 40% in absorption values already after one day and of 20% within 2 days, showing equal to pre-irradiation values in about 5 days.
These results indicate that the photochromic film exhibits comparable performance at temperatures of −19 °C and 4 °C, lacking the ability to effectively discriminate temperature differences below 4 °C. In contrast, the film demonstrates consistent and reliable color changes in response to room temperature fluctuations, suggesting that it could be an excellent candidate as a sensor for monitoring refrigerated products within the 0–15 °C temperature range. Taking advantage of the film's ability to visually indicate temperature excursions, companies can improve quality control measures and ensure compliance with temperature sensitive storage requirements in the cold chain.
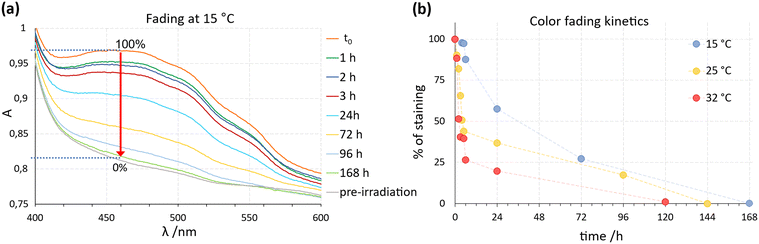
To obtain information on the effect of temperature changes over time on the film's staining changes and to evaluate its applicability as a sensor at a set of temperatures commonly encountered, further experiments were carried out. The photochromic film samples were subjected to irradiation followed by storage in the dark at 15, 25, and 32 °C. The hourly evaluation of color fading using UV-vis measurements was recorded post-irradiation by monitoring changes in the intensity of the peak centered at 470 nm.
The peak intensity at 470 nm recorded at t0 (film just irradiated) was considered as 100%, while that of the curve recorded after the complete fading of the staining (which overlaps that of the non-irradiated film) has been taken as 0%, as shown in Fig. 10 for the film at 15 °C.
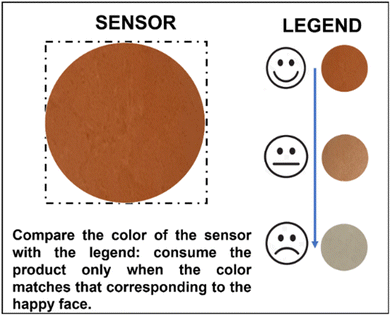
The color fading kinetics revealed significant variations depending on temperature conditions. A pronounced change in film staining occurred within the initial hours of exposure to different temperatures. At 32 °C, the film showed a 50% reduction in staining already within 2 hours, and within 4 h if stored at 25 °C, while it took about 1 day at 15 °C. These results confirm previous observations at room temperature (about 19 °C) and emphasize the potential of the film to visually indicate thermal history. Indeed, these results suggest that photochromic films can serve as effective indicators of temperature excursions in cold chain applications: a staining decrease below 75% of the initial intensity is readily discernible to the naked eye, enabling quick assessment of temperature deviations. Leveraging this property, we propose a labelling system incorporating the photochromic film as a ‘sensor’ on product packaging or containers (Fig. 11).
The legend presents three different intensities: one relative to the maximum intensity of coloration, equivalent to 100% (just-irradiated film), one relative to 50% of the sensor intensity, while the third one indicates the intensity of coloration when the film has already faded. By comparing the film's staining with the predefined legend, users can easily ascertain if temperature guidelines have been maintained. For instance, matching the ‘happy face’ color indicates compliance, while matching the ‘neutral face’ suggests deviations, such as prolonged exposure to above 15 °C for approximately 24 hours or within the range of 25–32 °C for 2–4 hours. This innovative approach holds promise for enhancing cold chain management practices and safeguarding product quality.
The unique properties of photochromic films open a multitude of potential applications in cold chain management: a promising path is their use in national or same-day ground transportation services. The ease of preparation and low production costs associated with these materials make them particularly attractive to small and medium-sized enterprises looking for cost-effective solutions to monitor their transport operations and ensure compliance with cold chain management.
Compared to existing sensors on the market, which can be prohibitive for small businesses, photochromic films, thanks to the low costs of raw materials and ease of preparation, are attractive for small and medium-sized enterprises looking for cost-effective solutions to monitor their transport operations and ensure compliance with cold chain management. This accessibility and ease of use, coupled with their reliability in indicating temperature excursions, position them as a turning point for companies aiming to support the integrity of their goods during transit.
In addition, the scalability of production processes for CMC films71 could facilitate integration into existing supply chain workflows. Whether tracking perishable goods over short or long distances, these films offer a versatile solution that can be adapted to various transport modes and logistics scenarios.
4. Conclusions
The outcomes of this study are in line with the goal stated in the introduction, that is to investigate and explore new thermo- and photochromic compounds that can be utilized as active components for making temperature colorimetric indicators. These types of indicators can help to visually track the cold chain and mitigate food losses caused by improper maintenance of the cold chain during transportation.To this end, five salicylaldehyde derivatives (1–5) bearing different ratios of H/F substituents on the aniline residue were designed and synthesized via mechanochemical methods and thoroughly characterized via XRD and thermal analyses. All solids showed pale and reversible thermochromism whereas crystalline 1, 3, and 5 showed photochromic properties when irradiated under UV light, with a marked change in color attributed to the interconversion from the cis-enol to the trans-keto tautomer, as confirmed by infrared spectroscopy (FTIR). Regarding the aim of this research, compound 5 showed the best performances in terms of color change, persistency and fading dependence on the ambient temperature. Compound 5 was, further, embedded in a non-toxic, biocompatible carboxy methyl cellulose polymer, and a composite photochromic film was produced. As the color hue and intensity remained dependent on the external temperature and the changes were appreciable by simple visual inspection, the composite was tested for potential application in the cold chain by studying the color fading at three different temperatures: −19 °C, 4 °C and RT. The results showed that, when stored below 4 °C, the composite film retained its red color for over 7 weeks. At room temperature (19 °C), however, a rapid decline in color intensity occurred, from 40% of the initial value after 1 day. Further experiments were performed at constant temperatures (15 °C, 25 °C, and 32 °C) to simulate material exposure under varied environmental conditions. Results showed that the fading rate increased proportionally with temperature, with a 50% reduction in coloration observed within 2 hours at 32 °C, within 4 hours at 25 °C, and 22 hours (about 1 day) at 15 °C.
These characteristics make this photochromic film potentially suitable for the transport of refrigerated products, which generally require a transport temperature between 0 and 15 °C, and it can represent a potential alternative to electronic devices such as radio frequency identification (RFID) and time–temperature indicators (TTIs) or to other non-reusable colorimetric sensors based on degradable pigments or nanoparticles.
Overall, these results demonstrate, as a proof of concept, that films doped with photochromic compounds can be proposed as materials for controlling cold chains in food or drug transportation.
However, the doped photochromic film, presented herein, was studied under laboratory conditions, and further development, e.g., toxicity assessment, scaling up over the gram scale, and tests under real conditions, is required prior to be effectively applied in the cold chain, whereas future optimizations of the formulation could allow the obtainment of a suitable material capable of indicating temperature changes even below 4 °C. Work is ongoing to develop new materials and expand the temperature range detection.
Author contributions
SDA, FG, SP: conceptualization, manuscript preparation and supervision; AA, MFDL, LB, SL: experimentation and data collection; FG: funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the University of Bologna (RFO-Scheme).References
- J. Parfitt, M. Barthel and S. Macnaughton, Philos. Trans. R. Soc., B, 2010, 365, 3065–3081 CrossRef PubMed.
- FAO, Global food losses and food waste: extent, causes and prevention, Food and Agriculture Organization of the United Nations, Rome, 2011 Search PubMed.
- ISPRA AMBIENTE, Rapporto spreco alimentare: un approcio sistemico per la prevenzione e la riduzione strutturali, 2018 Search PubMed.
- J. Lundqvist, C. de Fraiture and D. Molden, Saving Water: From Field to Fork – Curbing Losses and Wastage in the Food Chain, SIWI Policy Brief, 2008 Search PubMed.
- EU, Regulation (EC) No 883/2004 of the European Parliament and of the Council of 29 April 2004, Macmillan Education UK, London, 2015 Search PubMed.
- Grand View Research, Cold Chain Market Size, Share & Trends Analysis Report By Type (Storage, Transportation, Packaging, Monitoring Components), By Temperature Range, By Application, By Region, And Segment Forecasts, 2024 - 2030, 2024 Search PubMed.
- T. Gao, Y. Tian, Z. Zhu and D.-W. Sun, Trends Food Sci. Technol., 2020, 99, 311–322 CrossRef CAS.
- J.-W. Han, M. Zuo, W.-Y. Zhu, J.-H. Zuo, E.-L. Lü and X.-T. Yang, Trends Food Sci. Technol., 2021, 109, 536–551 CrossRef CAS.
- Q.-S. Ren, K. Fang, X.-T. Yang and J.-W. Han, Trends Food Sci. Technol., 2022, 119, 133–151 CrossRef CAS.
- N. A. Pambudi, A. Sarifudin, I. M. Gandidi and R. Romadhon, Energy Rep., 2022, 8, 955–972 CrossRef.
- C. M. Hanson, A. M. George, A. Sawadogo and B. Schreiber, Vaccine, 2017, 35, 2127–2133 CrossRef PubMed.
- B. F. Hibbs, E. Miller, J. Shi, K. Smith, P. Lewis and T. T. Shimabukuro, Vaccine, 2018, 36, 553–558 CrossRef PubMed.
- W. Huang, X. Wang, J. Xia, Y. Li, L. Zhang, H. Feng and X. Zhang, Trends Food Sci. Technol., 2023, 133, 189–204 CrossRef CAS.
- S. Rossi, M. Simeoni and A. Quaranta, Dyes Pigm., 2021, 184, 108879 CrossRef CAS.
- T. N. Tikhonova, D. Cohen-Gerassi, Z. A. Arnon, Y. Efremov, P. Timashev, L. Adler-Abramovich and E. A. Shirshin, ACS Appl. Mater. Interfaces, 2022, 14, 55392–55401 CrossRef CAS PubMed.
- Y. Xiao, Z. Tang, X. Huang, W. Chen, J. Zhou, H. Liu, C. Liu, N. Kong and W. Tao, Chem. Soc. Rev., 2022, 51, 3828–3845 RSC.
- J. Xia, W. Huang, X. Wang, Z. Zhu, M. Zhang and X. Zhang, Food Control, 2023, 154, 109976 CrossRef.
- B. Ye, J. Chen, H. Ye, Y. Zhang, Q. Yang, H. Yu, L. Fu and Y. Wang, Food Chem., 2022, 373, 131448 CrossRef CAS PubMed.
- M. Fattori, C. A. M. Da Costa, J. Fijn, E. Genco, P. Harpe, E. Cantatore and M. Charbonneau, in 2020 IEEE Custom Integrated Circuits Conference (CICC), IEEE, Boston, MA, USA, 2020, pp. 1–4 Search PubMed.
- S. Wang, X. Liu, M. Yang, Y. Zhang, K. Xiang and R. Tang, Packag. Technol. Sci., 2015, 28, 839–867 CrossRef CAS.
- G. Cui, M. Li, A. Qileng, H. Yang, M. Chen, W. Liu and Y. Liu, J. Mater. Chem. C, 2023, 11, 10843–10851 RSC.
- Y.-C. Wang, L. Lu and S. Gunasekaran, Biosens. Bioelectron., 2017, 92, 109–116 CrossRef CAS PubMed.
- A. Navrotskaya, D. Aleksandrova, M. Chekini, I. Yakavets, S. Kheiri, E. Krivoshapkina and E. Kumacheva, ACS Nano, 2022, 16, 8641–8650 CrossRef CAS PubMed.
- Y. Zou, J. Guo, Y. Liu, Y. Du, Y. Pu and D. Wang, Polym. Chem., 2023, 14, 1275–1281 RSC.
- M. Maafi, Molecules, 2008, 13, 2260–2302 CrossRef CAS PubMed.
- Y. Li, H. Li, W. Jin, X. Xu, H. Liu, Y. Ding, G. Wang, T. Zhang, Q. Peng, J. He, Q. Hu, L. Pan and K. Li, Dyes Pigm., 2022, 202, 110295 CrossRef CAS.
- E. Hadjoudis and I. M. Mavridis, Chem. Soc. Rev., 2004, 33, 579–588 CAS.
- N. Baus Topić and D. Cinčić, Cryst. Growth Des., 2023, 23, 6795–6804 CrossRef.
- A. Carletta, F. Spinelli, S. d'Agostino, B. Ventura, M. R. Chierotti, R. Gobetto, J. Wouters and F. Grepioni, Chem. – Eur. J., 2017, 23, 5317–5329 CrossRef CAS PubMed.
- K. M. Hutchins, S. Dutta, B. P. Loren and L. R. MacGillivray, Chem. Mater., 2014, 26, 3042–3044 CrossRef CAS.
- H. Sugiyama and H. Uekusa, CrystEngComm, 2018, 20, 2144–2151 RSC.
- J. Harada, T. Fujiwara and K. Ogawa, J. Am. Chem. Soc., 2007, 129, 16216–16221 CrossRef CAS PubMed.
- F. Robert, A. D. Naik, B. Tinant, R. Robiette and Y. Garcia, Chem. – Eur. J., 2009, 15, 4327–4342 CrossRef CAS PubMed.
- A. Carletta, X. Buol, T. Leyssens, B. Champagne and J. Wouters, J. Phys. Chem. C, 2016, 120, 10001–10008 CrossRef CAS.
- A. A. Dar and A. A. Ganie, Cryst. Growth Des., 2020, 20, 3888–3897 CrossRef CAS.
- R. Koibuchi, I. Yoshikawa and H. Houjou, J. Phys. Chem. A, 2022, 126, 4164–4175 CrossRef CAS PubMed.
- J. Harada and K. Ogawa, Chem. Soc. Rev., 2009, 38, 2244–2252 RSC.
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342–8356 CrossRef CAS PubMed.
- D. Braga, F. Grepioni, L. Maini and S. d'Agostino, IUCrJ, 2017, 4, 369–379 CrossRef CAS PubMed.
- C. B. Aakeröy, N. R. Champness and C. Janiak, CrystEngComm, 2009, 12, 22–43 RSC.
- I. O. Staehle, B. Rodríguez-Molina, S. I. Khan and M. A. Garcia-Garibay, Cryst. Growth Des., 2014, 14, 3667–3673 CrossRef CAS.
- A. Carletta, M. Colaço, S. R. Mouchet, A. Plas, N. Tumanov, L. Fusaro, B. Champagne, S. Lanners and J. Wouters, J. Phys. Chem. C, 2018, 122, 10999–11007 CrossRef CAS.
- M. Sliwa, S. Létard, I. Malfant, M. Nierlich, P. G. Lacroix, T. Asahi, H. Masuhara, P. Yu and K. Nakatani, Chem. Mater., 2005, 17, 4727–4735 CrossRef CAS.
- J. Quertinmont, L. Maschio, A. Datta and B. Champagne, J. Phys. Chem. C, 2020, 124, 24451–24459 CrossRef CAS.
- E. O. Levina, I. Y. Chernyshov, A. P. Voronin, L. N. Alekseiko, A. I. Stash and M. V. Vener, RSC Adv., 2019, 9, 12520–12537 RSC.
- V. R. Hathwar, T. S. Thakur, R. Dubey, M. S. Pavan, T. N. Guru Row and G. R. Desiraju, J. Phys. Chem. A, 2011, 115, 12852–12863 CrossRef CAS PubMed.
- V. R. Thalladi, H.-C. Weiss, D. Bläser, R. Boese, A. Nangia and G. R. Desiraju, J. Am. Chem. Soc., 1998, 120, 8702–8710 CrossRef CAS.
- A. Sudheendranath, K. A. Sandeep, K. Avinash, P. Venugopalan, A. Kumar and S. P. Thomas, Cryst. Growth Des., 2023, 23, 4013–4024 CrossRef CAS.
- T. S. Thakur, M. T. Kirchner, D. Bläser, R. Boese and G. R. Desiraju, CrystEngComm, 2010, 12, 2079–2085 RSC.
- B. K. Saha, A. Saha and S. A. Rather, Cryst. Growth Des., 2017, 17, 2314–2318 CrossRef CAS.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- P. J. Costa, Phys. Sci. Rev., 2019, 2(111), 20170136 Search PubMed.
- P. Politzer, J. S. Murray, T. Clark and G. Resnati, Phys. Chem. Chem. Phys., 2017, 19, 32166–32178 RSC.
- P. Metrangolo, F. Meyer, T. Pilati, G. Resnati and G. Terraneo, Angew. Chem., Int. Ed., 2008, 47, 6114–6127 CrossRef CAS PubMed.
- P. Metrangolo and G. Resnati, Chem. – Eur. J., 2001, 7, 2511–2519 CrossRef CAS PubMed.
- P. Metrangolo and G. Resnati, IUCrJ, 2014, 1, 5–7 CrossRef CAS PubMed.
- G. R. Desiraju and R. Parthasarathy, J. Am. Chem. Soc., 1989, 111, 8725–8726 CrossRef CAS.
- H. Mutlu and L. Barner, Macromol. Chem. Phys., 2022, 223, 2200111 CrossRef CAS.
- R. Cernansky, Nature, 2015, 519, 379–380 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. a. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood, J. Appl. Crystallogr., 2020, 53, 226–235 CrossRef CAS PubMed.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- D. Braga, S. d'Agostino and F. Grepioni, Organometallics, 2012, 31, 1688–1695 CrossRef CAS.
- M. Jabłoński, Molecules, 2021, 26, 6319 CrossRef PubMed.
- S. d'Agostino, F. Spinelli, E. Boanini, D. Braga and F. Grepioni, Chem. Commun., 2016, 52, 1899–1902 RSC.
- T. Fujiwara, J. Harada and K. Ogawa, J. Phys. Chem. B, 2004, 108, 4035–4038 CrossRef CAS.
- G. M. Mercier, K. Robeyns and T. Leyssens, Cryst. Growth Des., 2016, 16, 3198–3205 CrossRef CAS.
- R. Chatterjee, U. Chaudhari and S. Anand, Adv. Funct. Mater., 2023, 33, 2206301 CrossRef CAS.
- J. F. Reynes, V. Isoni and F. García, Angew. Chem., Int. Ed., 2023, 62, e202300819 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystal data tables, powder XRD patterns, thermal analyses, FTIR spectra, and stress–strain curves. CCDC 2306305–2306309. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4me00055b |
| ‡ Present address: Department of Inorganic Chemistry – University of Granada, Avenida de la Fuente Nueva S/N 18071 Granada, Spain. |
| § These authors contributed equally to this work. |
| ¶ Present address: University College Cork, T12 YN60, Cork, Ireland. |
| This journal is © The Royal Society of Chemistry 2024 |