Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A high-throughput effector screen identifies a novel small molecule scaffold for inhibition of ten-eleven translocation dioxygenase 2
Shubhendu
Palei
,
Jörn
Weisner
,
Melina
Vogt
,
Rajesh
Gontla
,
Benjamin
Buchmuller
,
Christiane
Ehrt
,
Tobias
Grabe
,
Silke
Kleinbölting
,
Matthias
Müller
,
Guido H.
Clever
*,
Daniel
Rauh
* and
Daniel
Summerer
*
Department of Chemistry and Chemical Biology, TU Dortmund University and, Drug Discovery Hub Dortmund (DDHD), Zentrum für Integrierte Wirkstoffforschung (ZIW), Otto-Hahn Str. 4a, 44227 Dortmund, Germany. E-mail: guido.clever@tu-dortmund.de; daniel.rauh@tu-dortmund.de; daniel.summerer@tu-dortmund.de
First published on 29th January 2024
Abstract
Correction for ‘A high-throughput effector screen identifies a novel small molecule scaffold for inhibition of ten-eleven translocation dioxygenase 2’ by Shubhendu Palei et al., RSC Med. Chem., 2022, 13, 1540–1548, https://doi.org/10.1039/D2MD00186A.
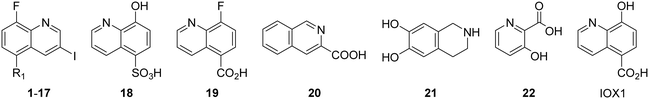
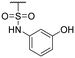
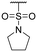
The authors would like to correct the activity data reported in Table 1 and adjust the associated conclusions. Compound 2, a simple sulfonic acid-based quinoline scaffold, was synthesized via hydrolysis of the corresponding sulfonic acid chloride, and an IC50 of 2.3 μM for the inhibition of the iron/αKG-dependent dioxygenase hTET2 was obtained from a MALDI-based inhibition assay. Compound 2 is structurally related to the known broad-spectrum iron/αKG-dependent dioxygenase inhibitor IOX-1 that features an iron-chelating 8-hydroxyquinoline (8HQ) core. In contrast, 2 bears a fluorine substituent in the 8-position that is expected to significantly attenuate iron affinity (supported by DFT calculations). This led to the conclusion that 2 acts as an isostere of IOX1 with low iron affinity, making it a starting point for TET2 inhibitors without this property. However, a later re-synthesis of 2 using a different starting material yielded an inactive compound. After consultation with the manufacturer, the authors suspect trace impurities of undefined polychlorinated quinoline species in the sulfonic acid chloride to be responsible for the observed activity, but attempts to purify these impurities proved unsuccessful. The authors thus conclude that the activity data of the compounds obtained via this sulfonic acid chloride are not trustable, and apologize for any confusion this data may have caused.
The authors conducted additional SAR, revealing that the sulfonic acid group of 2 can replace the carboxyl group of IOX1, leading to a simple sulfonic acid-based quinoline 18 with a virtually identical IC50. In contrast, a replacement of IOX1's 8-hydroxyl group with a fluorine atom led to inactivity (19). The authors further identified and validated other IOX1-derived compounds without the 8HQ core that nevertheless showed micromolar IC50 values, such as isoquinoline-3-carboxylic acid (20), tetrahydroisoquinoline-6,7-diol (21), and 3-hydroxypicolinic acid (22). This combined data indicates that the 8HQ core is essential for hTET2 inhibition by IOX1 itself, whereas it is not essential for IOX1-derived compounds in general, providing new starting points for TET2 inhibitors without the 8HQ core.
An updated version of Table 1 is included here.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2024 |