Open Access Article
Open Access ArticleDesign, synthesis, and structure–activity relationship studies of 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole derivatives as necroptosis inhibitors†
Zechen
Jin‡
ac,
Yang
Dai‡
bc,
Yinchun
Ji
b,
Xia
Peng
b,
Wenhu
Duan
acd,
Jing
Ai
*bc and
Hefeng
Zhang
 *a
*a
aSmall-Molecule Drug Research Center, Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203, China. E-mail: zhanghefeng1@simm.ac.cn
bCancer Research Center, Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai, 201203, China. E-mail: jai@simm.ac.cn
cSchool of Pharmacy, University of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing, 100049, China
dShandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, Yantai, Shandong 264117, China
First published on 21st June 2024
Abstract
The development of necroptosis inhibitors has emerged as a promising strategy to effectively mitigate necroptosis-related inflammatory diseases, neurodegenerative diseases, and cancers. In this paper, we reported a series of 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole derivatives as potent necroptosis inhibitors. The representative compound 26 displayed potent anti-necroptotic activity in both human and mouse cellular assays and exhibited potent inhibitory activity against receptor-interacting protein kinase 1 (RIPK1). In vivo pharmacokinetic studies were performed to determine the oral exposure of compound 26. Finally, molecular docking elucidated that compound 26 could effectively bind to the allosteric pocket of RIPK1 and serve as a type III inhibitor. Taken together, our findings highlighted that compound 26 represented a promising lead compound for future necroptosis inhibitor development.
Introduction
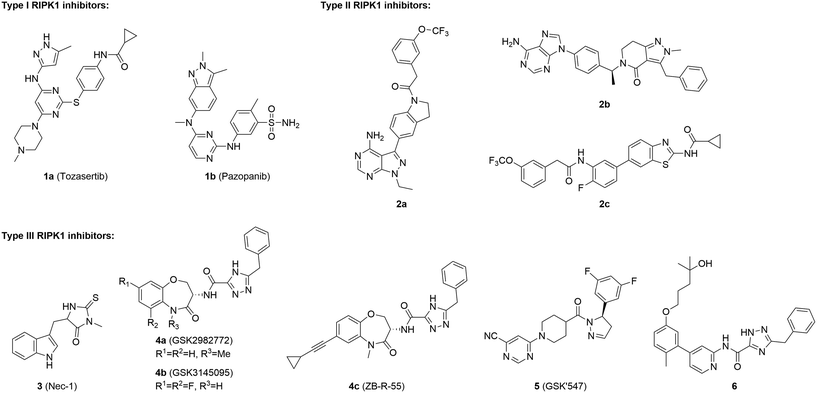
Necroptosis is an important form of programmed cell death (PCD). Although necroptosis shares certain characteristics with necrosis, it is precisely regulated by the receptor-interacting protein kinase (RIPK) family.1 Generally, necroptosis is activated when apoptosis pathways are compromised. In the classic tumor necrosis factor receptor-α (TNF-α)-mediated necroptosis signaling pathway, RIPK1 is initially ubiquitinated and plays a central role in complex I.2 When RIPK1 is deubiquitinated and complex I is disassociated, RIPK1 translocates to complex IIa/IIb to initiate PCD.3,4 With the deficiency of caspase-8 in the cytoplasm, RIPK1, RIPK3, and mixed lineage kinase domain-like protein (MLKL) join together to form necrosomes, where RIPK1 triggers cascade phosphorylation and eventually initiates necroptosis (Fig. 1).4 Although necroptosis is essential for some physiological processes such as nerve development, abnormal necroptosis could lead to various diseases.5 Necroptosis upregulation has been implicated in autoimmune disorders such as systemic lupus erythematosus, neurodegenerative diseases including Alzheimer's disease and stroke-induced brain injury.6–8 Necroptosis also promotes several cancers such as glioblastoma, lung cancer, pancreatic ductal adenocarcinoma, and ovarian carcinoma.9–12 The development of necroptosis inhibitors may offer promising therapeutic strategies for the treatment of these diseases.The development of RIPK1-targeted necroptosis inhibitors has aroused increased attention in recent years. Basing on different binding modes, current RIPK1 inhibitors are categorized into three classes: type I, II, and III (Fig. 2). Type I RIPK1 inhibitors are ATP-competitive inhibitors that interact with the DLG-in (Asp-Leu-Gly-in) conformation of RIPK1. Multitarget kinase inhibitors tozasertib (1a) and pazopanib (1b) are type I RIPK1 inhibitors with submicromolar level potency.13,14 Type II RIPK1 inhibitors interact with DLG-out RIPK1 by occupying both the hinge region pocket and the allosteric pocket (Fig. 3A), thus exhibiting elevated bioactivity and selectivity. Compounds 2a and 2b are orally bioavailable type II RIPK1 inhibitors with favorable selectivity profiles.15,16 A series of benzothiazole-based type II RIPK1 inhibitors were reported recently, and the representative compound 2c demonstrated potent anti-necroptotic efficacy both in vitro and in vivo.17–19 Type III inhibitors occupy the allosteric pocket of RIPK1 (Fig. 3B) and feature exceptional inhibitory potency and selectivity. Nec-1 (3) is the first type III selective RIPK1 inhibitor tool compound.20 GSK2982772 (4a) has progressed into phase II clinical trials for treating several autoimmune diseases.21,22 GSK3145095 (4b) has advanced into phase II clinical studies for cancer treatment (terminated).23,24 Several analogs of 4a, such as ZB-R-55 (4c), have been reported recently.25–27 GSK′547 (5) stands out as a potent inhibitor against both human and murine RIPK1 (hRIPK1 and mRIPK1).28 Compound 6 is another type III RIPK1 inhibitor which demonstrates strong anti-necroptotic activity against human-derived HT29 cells.29
 | ||
| Fig. 3 Co-crystal structures and binding modes of the RIPK1 kinase domain complexed with (A) aminoisoquinoline-based type II inhibitor (PDB ID: 4NEU) or (B) type III inhibitor 4b (PDB ID: 6RLN). | ||
In this paper, we reported a series of novel 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole-based type III RIPK1 inhibitors that displayed remarkable anti-necroptotic activity in both human and murine cells.
Results and discussion
Chemistry
The synthetic routes for compounds 7–20 are shown in Scheme 1. First, phenolic compounds S1a–c were treated with methyl 4-bromobutanoate to obtain carboxylic acid esters S2a–c. Treatment of esters S2a–c with methylmagnesium bromide afforded intermediates S3a–c, which underwent Miyaura borylation and Suzuki coupling reactions to yield amines S5a–g. Finally, amines S5a–g were condensed with corresponding acids S7a–h to afford the target compounds 7–20.Compounds 21–28 were obtained via similar routes (Scheme 2). Phenol ethers S8a–e were obtained through nucleophilic substitution reactions. Then intermediates S8a–e underwent Suzuki coupling reactions with (2-aminopyridin-4-yl)boronic acid to give amines S9a–e. Finally, amines S9a–e were condensed with corresponding acids S7a, S11a or S11b to afford the target compounds 21–28.
Design rationale and structure–activity relationship (SAR) studies
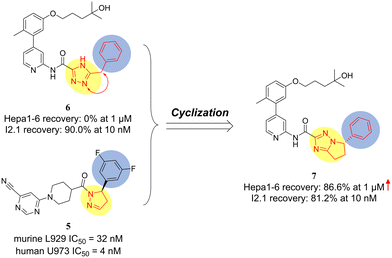
Compound 6 with good human cellular activity (HT29 IC50 < 100 nM in the literature) was selected at the starting point of our research.29 The anti-TNF-α-induced necroptosis activity of compounds was evaluated using both human I2.1 cells and murine Hepa1-6 cells. Despite compound 6 demonstrating potent anti-necroptotic activity against I2.1 cells (90.0% recovery at 10 nM), it exhibited no recovery activity against Hepa1-6 cells (0% recovery at 1 μM). The species selectivity of compound 6 impedes its further biological evaluations in rodents, which should be addressed. It is noteworthy that GSK2982772, another RIPK1 inhibitor which features the 3-benzyl-4H-1,2,4-triazole moiety as the allosteric fragment, also exhibited species selectivity between hRIPK1 and mRIPK1 (hRIPK1 IC50 = 16 nM, mRIPK1 IC50 = 2.5 μM).21 It was reported that such species difference might be attributed to the diminished flexibility of residues near the allosteric regions in mRIPK1.26,28 This evidence shows us that the allosteric fragment of compound 6 requires further optimization to develop novel necroptosis inhibitors that are active for both human and murine cells.Fig. 4 demonstrates our design rationale. Our attention was drawn to the hRIPK/mRIPK inhibitor GSK′547. The 5-phenyl-4,5-dihydro-1H-pyrazol-1-yl allosteric moiety of GSK′547 should be critical for its mRIPK1 inhibitory activity.28 We adopted a cyclization design strategy and incorporated the allosteric structural feature of GSK′547 into compound 6. As a result, the 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole derivative 7 was designed. Encouragingly, compound 7 showed a marked increase in anti-necroptotic activity for murine Hepa1-6 cells (86.6% recovery at 1 μM) while retaining potent anti-necroptotic efficacy for human I2.1 cells. Following this breakthrough, we conducted systematic SAR studies for compound 7. Those compounds achieving over 75% recovery rate in I2.1 cells at 10 nM were further tested to determine their anti-necroptotic activities in Hepa1-6 cells.
Initially, we explored the terminal phenyl ring at the R3-position and synthesized compounds 8–12 (Table 1). Introduction of a fluorine substituent at the ortho (compound 8), meta (compound 9), or para (compound 10) position of the phenyl led to a slight decrease in activity. This result suggested that additional substituents on the phenyl ring were unfavorable. Replacing the phenyl ring with cycloalkyls (compounds 11 and 12) maintained the activity, indicating that the R3 group interacts with the RIPK1 protein primarily through hydrophobic interactions, rather than π–π stacking. Considering both activity and molecular weight, unsubstituted phenyl was selected as the optimal substitution pattern for the R3 group.
| Compd | R 1 | R 2 | R 3 | X 1 | X 2 | I2.1 recovery (%) at 10 nM | Hepa1-6 recovery (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 μM | 100 nM | 10 nM | |||||||
| a These data are the mean ± SD values of at least two assays. b N.D. refers to not determined. | |||||||||
| 6 | — | — | — | — | — | 90.0 ± 14.1 | <0 | N.D.b | N.D. |
| 7 | CH3 | H |

|
CH | H | 81.2 ± 17.3 | 86.6 ± 4.3 | 26.1 ± 3.5 | 5.5 ± 0.1 |
| 8 | CH3 | H |

|
CH | H | 62.7 ± 5.1 | N.D. | ||
| 9 | CH3 | H |

|
CH | H | 58.1 ± 3.4 | N.D. | ||
| 10 | CH3 | H |

|
CH | H | 60.1 ± 1.4 | N.D. | ||
| 11 | CH3 | H |

|
CH | H | 61.2 ± 1.5 | N.D. | ||
| 12 | CH3 | H |

|
CH | H | 73.2 ± 19.4 | N.D. | ||
| 13 | CH3 | H |
|
CH | F(trans) | 98.9 ± 1.6 | 104.2 ± 0.6 | 41.3 ± 1.3 | 6.3 ± 1.3 |
| 14 | CH3 | H |

|
CH | F(cis) | 80.6 ± 0.6 | 110.0 ± 4.5 | 107.6 ± 5.3 | 100.5 ± 3.3 |
| 15 | CH3 | F |

|
CH | H | 58.1 ± 16.5 | N.D. | ||
| 16 | CH3 | Cl |

|
CH | H | 56.2 ± 9.1 | N.D. | ||
| 17 | CH3 | CH3 |

|
CH | H | 72.7 ± 5.2 | N.D. | ||
| 18 | CH3 | H |

|
N | H | 53.6 ± 6.2 | N.D. | ||
| 19 | Cl | H |

|
CH | H | 80.6 ± 0.6 | 2.9 ± 0.1 | N.D. | N.D. |
| 20 | H | H |

|
CH | H | 86.1 ± 19.7 | 2.2 ± 0.7 | N.D. | N.D. |
Next, we introduced a fluorine substituent at the 7′-position of the 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole moiety and afforded cis–trans-isomers 13 and 14 (Table 1). Both compounds 13 and 14 showed enhanced activity in Hepa1-6 cells, suggesting that 7′-fluoro might contribute to additional van der Waals interactions with mRIPK1. The cis-isomer 13 exhibited over 100-fold improved potency in Hepa1-6 cells compared to compound 7. Consequently, the cis-substituted fluorine at the 7′-position was chosen as the preferred substitution pattern for this segment.
We next explored the SARs of the pyridine–phenyl moiety (compounds 15–20, Table 1). Introduction of a halogen atom (15 and 16) or methyl group (17) at the R2-position resulted in a slight loss in activity. This indicated that the R2-position might be close to the pocket surface and could not accommodate additional substituents. Replacing the pyridine ring with pyridazine led to decreased potency (18). At the R1-position, replacing the methyl group of compound 7 with a chlorine substituent (19) or removing the methyl group (20) diminished the activity in Hepa1-6 cells. The R1-methyl group was presumed to restrain the appropriate dihedral angle of the pyridine–phenyl moiety.
We also investigated the SAR of the R4 group (Table 2). Replacing the R4 group of compound 7 with methyl (21) or cyanopropyl (24) resulted in decreased potency, which might be due to the reduced solubility of these compounds. Compounds 22 and 23 with hydrophilic groups as R4 substituents exhibited elevated potency in both I2.1 and Hepa1-6 cells. Given the potential metabolic risk of the hydroxyl group, the 2-(tetrahydro-2H-pyran-4-yl)ethyl group of compound 23 was identified as the optimal substituent for the R4 group.
| Compd | R 4 | I2.1 recovery (%) at 10 nM | Hepa1-6 recovery (%) | ||
|---|---|---|---|---|---|
| 1 μM | 100 nM | 10 nM | |||
| a These data are the mean ± SD values of at least two assays. b N.D. refers to not determined. | |||||
| 21 | CH3 | 73.7 ± 1.8 | N.D.b | ||
| 22 |
|
98.2 ± 1.9 | 94.1 ± 0.5 | 10.9 ± 1.3 | 3.8 ± 1.3 |
| 23 |

|
91.9 ± 8.2 | 96.6 ± 5.2 | 10.7 ± 2.5 | 3.7 ± 1.6 |
| 24 |

|
35.8 ± 5.8 | N.D. | ||
Ultimately, we combined the advantageous substitution patterns of compounds 7, 14, and 23 and performed appropriate derivatizations to synthesize compounds 25–28 with absolute configurations. Additionally, we tested their RIPK1 inhibitory activities at a concentration of 1 μM (Table 3). The results indicated that compounds 25 and 27 with “R,R” chiral centers had no RIPK1 inhibitory effect. This consequence highlighted the critical impact of the absolute configuration on this series of compounds: the 5′-(R) configuration should have impeded the extension of the 5′-phenyl into the hydrophobic pocket of RIPK1. In contrast, the “S,S”-configured compounds 26 and 28 showed satisfactory RIPK1 inhibitory activity and cellular recovery efficacy. Compound 26 which features a morpholine fragment at the R4-substituent exhibited superior activity in I2.1 cells. Therefore, compound 26 was selected for further studies.
| Compd | R 4 | Stereo (5′,7′) | RIPK1 inhibition (%) at 1 μM | I2.1 recovery (%) at 10 nM | Hepa1-6 recovery (%) at 10 nM |
|---|---|---|---|---|---|
| a These data are the mean ± SD values of at least two assays. b N.D. refers to not determined. | |||||
| 25 |

|
R,R | <0 | N.D.b | N.D. |
| 26 |

|
S,S | 79.1 ± 5.2 | 97.9 ± 3.0 | 102.4 ± 0.2 |
| 27 |

|
R,R | <0 | N.D. | N.D. |
| 28 |
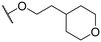
|
S,S | 78.8 ± 1.7 | 70.5 ± 9.1 | 111.4 ± 2.1 |
Pharmacokinetic properties of compound 26
We evaluated the pharmacokinetic properties of compound 26 in SD rats (N = 3) at a dose of 3 mg kg−1 (p.o.). The plasma concentrations of compound 26 were monitored for 24 h. As shown in Table 4, compound 26 reached a low peak plasma concentration (Cmax) of 8.90 ng mL−1 at 0.67 h. The elimination half-life (T1/2) of compound 26 was determined to be 1.91 h, suggesting a moderate duration of presence in the systemic circulation. Additionally, the area under the curve (AUC), a critical measure of the drug's overall presence in the plasma over time, was calculated to be 15.2 h ng mL−1.| Compd (3 mg kg−1) | T 1/2 (h) | T max (h) | C max (ng mL−1) | AUClast (h ng mL−1) |
|---|---|---|---|---|
| a N = 3, data are shown as mean values. | ||||
| 26 | 1.91 | 0.67 | 8.90 | 15.2 |
Molecular docking
Molecular docking was conducted to elucidate the binding mode of compound 26. Fig. 5 illustrates that compound 26 effectively occupied the allosteric hydrophobic pocket of RIPK1 and formed two critical hydrogen bonds with ASP156. The 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole ring and the 7′-fluoro substituent interacted with RIPK1 primarily through hydrophobic interactions. Additionally, the morpholine fragment formed two salt bridge interactions with ASN99 and GLU142. The pyridine–phenyl moiety adopted a cross conformation and acted as a linker connecting the allosteric segment with the solvent moiety. In summary, compound 26 was a potential type III RIPK1 inhibitor. | ||
| Fig. 5 Proposed binding mode of compound 26 with RIPK1 (PDB 6NYH). Interactions of compound 26 (green) and amino acid residues (magenta) are shown. | ||
Conclusion
The development of necroptosis inhibitors has attracted widespread attention for their potential applications in autoimmune diseases, neurodegenerative diseases, and cancers. In this study, we started with the human-cell specific necroptosis inhibitor compound 6 and employed a cyclization design strategy to synthesize a series of 6,7-dihydro-5H-pyrrolo[1,2-b][1,2,4]triazole derivatives as potent necroptosis inhibitors. These compounds exhibited potent anti-necroptotic activity against both human and murine cells. Systematic SAR studies led to the optimum compound 26. Compound 26 demonstrated robust anti-necroptotic activity in both human I2.1 and murine Hepa1-6 cells and significantly inhibited RIPK1 enzymatic activity. In vivo pharmacokinetic evaluation demonstrated that compound 26 displayed relatively low oral exposure. Molecular docking revealed that compound 26 served as a potential type III RIPK1 inhibitor and effectively bound to the allosteric pocket of RIPK1. In summary, compound 26 represents a promising lead compound for future development of necroptosis inhibitors.Experimental section
Chemistry
Unless specifically stated, all reagents and solvents were obtained from commercial sources and used without further purification. Reactions were generally carried out under an argon atmosphere unless otherwise stated. NMR spectra were obtained on Varian Mercury 400 or Bruker AVANCE NMR 400, 500, 600 spectrometers. 1H and 13C NMR spectra were obtained at 400/600 MHz and 126/151 MHz, respectively. The chemical shifts were reported in ppm relative to trimethylsilane or deuterated solvents (chloroform-d, DMSO-d6, methanol-d4, acetone-d6) as internal standards. The observed splitting patterns were designated as “s” for singlet, “d” for doublet, “t” for triplet, “q” for quartet, and “m” for multiplet, respectively. Mass spectrometric analysis was performed using an Agilent 6110 Quadrupole LC/MS in ESI-MS mode. High-resolution mass spectrometry (HR-MS) was conducted on an Agilent G6520 Q-TOF spectrometer (ESI-MS mode). Purity assessment of all compounds (>95%) was performed using an Agilent Infinity 1260 high-performance liquid chromatography (HPLC) system.Preparation procedure of compounds 7–28
Intermediates S6g, S6h, S10a and S10b were synthesized according to the literature.31,32
Compounds 8–28 were synthesized via similar routes to compound 7.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 58%. 1H NMR (400 MHz, methanol-d4) δ 8.38 (d, J = 5.1 Hz, 1H), 8.27 (s, 1H), 7.48–7.39 (m, 1H), 7.28–7.16 (m, 5H), 6.92 (dd, J = 8.4, 2.7 Hz, 1H), 6.82 (d, J = 2.7 Hz, 1H), 5.86 (dd, J = 8.6, 5.8 Hz, 1H), 4.01 (t, J = 6.4 Hz, 2H), 3.42–3.36 (m, 1H), 3.28–3.11 (m, 2H), 2.82–2.72 (m, 1H), 2.24 (s, 3H), 1.93–1.81 (m, 2H), 1.67–1.62 (m, 2H), 1.23 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.8, 160.7, 160.0 (1JC–F = 247.0 Hz), 157.2, 157.0, 150.9 (2JC–F = 58.0 Hz), 148.3, 139.5, 131.8, 130.7 (3JC–F = 8.4 Hz), 128.9 (4JC–F = 3.0 Hz), 126.1, 126.0 (3JC–F = 12.5 Hz), 124.9, 120.7, 116.0, 115.9, 114.9, 114.6, 113.8, 68.5, 68.3, 56.1, 34.0, 29.3, 24.0, 20.6, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2563.
V) as a white solid, yield 58%. 1H NMR (400 MHz, methanol-d4) δ 8.38 (d, J = 5.1 Hz, 1H), 8.27 (s, 1H), 7.48–7.39 (m, 1H), 7.28–7.16 (m, 5H), 6.92 (dd, J = 8.4, 2.7 Hz, 1H), 6.82 (d, J = 2.7 Hz, 1H), 5.86 (dd, J = 8.6, 5.8 Hz, 1H), 4.01 (t, J = 6.4 Hz, 2H), 3.42–3.36 (m, 1H), 3.28–3.11 (m, 2H), 2.82–2.72 (m, 1H), 2.24 (s, 3H), 1.93–1.81 (m, 2H), 1.67–1.62 (m, 2H), 1.23 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.8, 160.7, 160.0 (1JC–F = 247.0 Hz), 157.2, 157.0, 150.9 (2JC–F = 58.0 Hz), 148.3, 139.5, 131.8, 130.7 (3JC–F = 8.4 Hz), 128.9 (4JC–F = 3.0 Hz), 126.1, 126.0 (3JC–F = 12.5 Hz), 124.9, 120.7, 116.0, 115.9, 114.9, 114.6, 113.8, 68.5, 68.3, 56.1, 34.0, 29.3, 24.0, 20.6, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2563.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 56%. 1H NMR (400 MHz, methanol-d4) δ 8.38 (d, J = 5.2 Hz, 1H), 8.28 (s, 1H), 7.51–7.41 (m, 1H), 7.23 (d, J = 8.4 Hz, 1H), 7.18 (d, J = 5.2 Hz, 1H), 7.17–7.06 (m, 3H), 6.92 (dd, J = 8.4, 2.8 Hz, 1H), 6.83 (d, J = 2.7 Hz, 1H), 5.69–5.62 (m, 1H), 4.01 (t, J = 6.4 Hz, 2H), 3.30–3.07 (m, 3H), 2.78–2.68 (m, 1H), 2.25 (s, 3H), 1.92–1.82 (m, 2H), 1.69–1.60 (m, 2H), 1.23 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.9, 162.4 (1JC–F = 244.0 Hz), 160.7, 157.1 (2JC–F = 32.0 Hz), 151.1, 150.7, 148.3, 142.0 (3JC–F = 7.1 Hz), 139.5, 131.8, 130.9 (3JC–F = 8.0 Hz), 126.1, 123.0, 120.7, 115.3, 115.2, 114.9, 114.6, 113.8, 113.7 (2JC–F = 21.0 Hz), 68.5, 68.3, 60.4, 35.2, 29.3, 24.0, 20.6, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2560.
V) as a white solid, yield 56%. 1H NMR (400 MHz, methanol-d4) δ 8.38 (d, J = 5.2 Hz, 1H), 8.28 (s, 1H), 7.51–7.41 (m, 1H), 7.23 (d, J = 8.4 Hz, 1H), 7.18 (d, J = 5.2 Hz, 1H), 7.17–7.06 (m, 3H), 6.92 (dd, J = 8.4, 2.8 Hz, 1H), 6.83 (d, J = 2.7 Hz, 1H), 5.69–5.62 (m, 1H), 4.01 (t, J = 6.4 Hz, 2H), 3.30–3.07 (m, 3H), 2.78–2.68 (m, 1H), 2.25 (s, 3H), 1.92–1.82 (m, 2H), 1.69–1.60 (m, 2H), 1.23 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.9, 162.4 (1JC–F = 244.0 Hz), 160.7, 157.1 (2JC–F = 32.0 Hz), 151.1, 150.7, 148.3, 142.0 (3JC–F = 7.1 Hz), 139.5, 131.8, 130.9 (3JC–F = 8.0 Hz), 126.1, 123.0, 120.7, 115.3, 115.2, 114.9, 114.6, 113.8, 113.7 (2JC–F = 21.0 Hz), 68.5, 68.3, 60.4, 35.2, 29.3, 24.0, 20.6, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2560.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 55%. 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 8.42 (d, J = 5.1 Hz, 1H), 8.10 (d, J = 1.3 Hz, 1H), 7.38 (dd, J = 8.7, 5.4 Hz, 2H), 7.30–7.19 (m, 4H), 6.93 (dd, J = 8.4, 2.7 Hz, 1H), 6.80 (d, J = 2.7 Hz, 1H), 5.67 (t, J = 7.2 Hz, 1H), 4.19 (s, 1H), 3.97 (t, J = 6.6 Hz, 2H), 3.26–3.01 (m, 4H), 2.19 (s, 3H), 1.80–1.70 (m, 2H), 1.52–1.43 (m, 2H), 1.10 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.6, 162.0 (1JC–F = 244.0 Hz), 160.6, 157.1 (2JC–F = 31.0 Hz), 151.1, 150.7, 148.3, 139.5, 135.4, 131.8, 129.1 (3JC–F = 8.1 Hz), 126.1, 120.7, 115.8, 115.6, 114.9, 114.6, 113.7, 68.5, 68.3, 60.4, 35.3, 29.3, 24.0, 20.6, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2562.
V) as a white solid, yield 55%. 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 8.42 (d, J = 5.1 Hz, 1H), 8.10 (d, J = 1.3 Hz, 1H), 7.38 (dd, J = 8.7, 5.4 Hz, 2H), 7.30–7.19 (m, 4H), 6.93 (dd, J = 8.4, 2.7 Hz, 1H), 6.80 (d, J = 2.7 Hz, 1H), 5.67 (t, J = 7.2 Hz, 1H), 4.19 (s, 1H), 3.97 (t, J = 6.6 Hz, 2H), 3.26–3.01 (m, 4H), 2.19 (s, 3H), 1.80–1.70 (m, 2H), 1.52–1.43 (m, 2H), 1.10 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.6, 162.0 (1JC–F = 244.0 Hz), 160.6, 157.1 (2JC–F = 31.0 Hz), 151.1, 150.7, 148.3, 139.5, 135.4, 131.8, 129.1 (3JC–F = 8.1 Hz), 126.1, 120.7, 115.8, 115.6, 114.9, 114.6, 113.7, 68.5, 68.3, 60.4, 35.3, 29.3, 24.0, 20.6, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2562.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 52%. 1H NMR (400 MHz, methanol-d4) δ 8.39 (d, J = 5.1 Hz, 1H), 8.30 (s, 1H), 7.23 (d, J = 8.4 Hz, 1H), 7.18 (dd, J = 5.2, 1.4 Hz, 1H), 6.91 (dd, J = 8.5, 2.7 Hz, 1H), 6.83 (d, J = 2.7 Hz, 1H), 4.43–4.34 (m, 1H), 4.00 (t, J = 6.4 Hz, 2H), 3.02–2.93 (m, 2H), 2.89–2.80 (m, 2H), 2.66–2.53 (m, 1H), 2.25 (s, 3H), 2.03–1.75 (m, 8H), 1.75–1.60 (m, 4H), 1.51–1.41 (m, 2H), 1.23 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.2, 160.0, 157.3, 156.9, 151.1, 150.7, 148.3, 139.6, 131.8, 126.1, 120.6, 114.9, 114.6, 113.7, 68.5, 68.3, 62.3, 41.1, 29.3, 28.2, 27.7, 27.1, 25.7, 25.4, 24.0, 20.5, 19.0. HRMS: calcd for C30H40N5O3 [M + H]+, 518.3131; found, 518.3124.
V) as a white solid, yield 52%. 1H NMR (400 MHz, methanol-d4) δ 8.39 (d, J = 5.1 Hz, 1H), 8.30 (s, 1H), 7.23 (d, J = 8.4 Hz, 1H), 7.18 (dd, J = 5.2, 1.4 Hz, 1H), 6.91 (dd, J = 8.5, 2.7 Hz, 1H), 6.83 (d, J = 2.7 Hz, 1H), 4.43–4.34 (m, 1H), 4.00 (t, J = 6.4 Hz, 2H), 3.02–2.93 (m, 2H), 2.89–2.80 (m, 2H), 2.66–2.53 (m, 1H), 2.25 (s, 3H), 2.03–1.75 (m, 8H), 1.75–1.60 (m, 4H), 1.51–1.41 (m, 2H), 1.23 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.2, 160.0, 157.3, 156.9, 151.1, 150.7, 148.3, 139.6, 131.8, 126.1, 120.6, 114.9, 114.6, 113.7, 68.5, 68.3, 62.3, 41.1, 29.3, 28.2, 27.7, 27.1, 25.7, 25.4, 24.0, 20.5, 19.0. HRMS: calcd for C30H40N5O3 [M + H]+, 518.3131; found, 518.3124.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 70%. 1H NMR (400 MHz, acetone-d6) δ 9.59 (s, 1H), 8.40 (d, J = 5.1 Hz, 1H), 8.35 (d, J = 1.4 Hz, 1H), 7.24 (d, J = 8.4 Hz, 1H), 7.16 (dd, J = 5.2, 1.6 Hz, 1H), 6.92 (dd, J = 8.4, 2.8 Hz, 1H), 6.86 (d, J = 2.7 Hz, 1H), 4.43 (td, J = 8.0, 4.8 Hz, 1H), 4.03 (t, J = 6.6 Hz, 2H), 3.30 (s, 1H), 3.00–2.91 (m, 3H), 2.58–2.47 (m, 1H), 2.36–2.26 (m, 1H), 2.24 (s, 3H), 2.03–1.96 (m, 1H), 1.93–1.84 (m, 2H), 1.81–1.72 (m, 1H), 1.68–1.45 (m, 8H), 1.19 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.0, 159.9, 157.3, 156.9, 151.1, 150.7, 148.3, 139.6, 131.8, 126.2, 120.6, 114.9, 114.6, 113.6, 68.5, 68.3, 61.7, 43.7, 30.2, 29.3, 28.8, 28.0, 25.0, 24.6, 24.0, 20.3, 19.0. HRMS: calcd for C29H38N5O3 [M + H]+, 504.2975; found, 504.2966.
V) as a white solid, yield 70%. 1H NMR (400 MHz, acetone-d6) δ 9.59 (s, 1H), 8.40 (d, J = 5.1 Hz, 1H), 8.35 (d, J = 1.4 Hz, 1H), 7.24 (d, J = 8.4 Hz, 1H), 7.16 (dd, J = 5.2, 1.6 Hz, 1H), 6.92 (dd, J = 8.4, 2.8 Hz, 1H), 6.86 (d, J = 2.7 Hz, 1H), 4.43 (td, J = 8.0, 4.8 Hz, 1H), 4.03 (t, J = 6.6 Hz, 2H), 3.30 (s, 1H), 3.00–2.91 (m, 3H), 2.58–2.47 (m, 1H), 2.36–2.26 (m, 1H), 2.24 (s, 3H), 2.03–1.96 (m, 1H), 1.93–1.84 (m, 2H), 1.81–1.72 (m, 1H), 1.68–1.45 (m, 8H), 1.19 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.0, 159.9, 157.3, 156.9, 151.1, 150.7, 148.3, 139.6, 131.8, 126.2, 120.6, 114.9, 114.6, 113.6, 68.5, 68.3, 61.7, 43.7, 30.2, 29.3, 28.8, 28.0, 25.0, 24.6, 24.0, 20.3, 19.0. HRMS: calcd for C29H38N5O3 [M + H]+, 504.2975; found, 504.2966.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 42%. 1H NMR (400 MHz, chloroform-d) δ 9.56 (s, 1H), 8.43–8.40 (m, 1H), 8.37 (d, J = 5.1 Hz, 1H), 7.47–7.41 (m, 3H), 7.23–7.17 (m, 3H), 7.08 (dd, J = 5.2, 1.5 Hz, 1H), 6.88 (dd, J = 8.3, 2.8 Hz, 1H), 6.84 (d, J = 2.7 Hz, 1H), 6.13 (dd, 2JH–F= 55.4 Hz, J = 6.2 Hz, 1H), 5.78 (td, J = 6.6, 3.1 Hz, 1H), 4.01 (t, J = 6.3 Hz, 2H), 3.52–3.38 (m, 1H), 3.17–3.01 (m, 2H), 2.83 (d, J = 0.6 Hz, 3H), 2.27 (s, 3H), 1.96–1.86 (m, 2H), 1.70–1.66 (m, 2H), 1.28 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 161.7, 158.7 (2JC–F = 22 Hz), 156.9, 151.1, 150.6, 148.3, 139.5, 137.3, 131.8, 128.9, 128.8, 127.3, 126.1, 120.8, 114.9, 114.6, 114.1, 83.4 (1JC–F = 178.0 Hz), 68.5, 68.3, 60.4, 44.1 (2JC–F = 23.0 Hz), 38.2, 29.3, 24.0, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2559.
V) as a white solid, yield 42%. 1H NMR (400 MHz, chloroform-d) δ 9.56 (s, 1H), 8.43–8.40 (m, 1H), 8.37 (d, J = 5.1 Hz, 1H), 7.47–7.41 (m, 3H), 7.23–7.17 (m, 3H), 7.08 (dd, J = 5.2, 1.5 Hz, 1H), 6.88 (dd, J = 8.3, 2.8 Hz, 1H), 6.84 (d, J = 2.7 Hz, 1H), 6.13 (dd, 2JH–F= 55.4 Hz, J = 6.2 Hz, 1H), 5.78 (td, J = 6.6, 3.1 Hz, 1H), 4.01 (t, J = 6.3 Hz, 2H), 3.52–3.38 (m, 1H), 3.17–3.01 (m, 2H), 2.83 (d, J = 0.6 Hz, 3H), 2.27 (s, 3H), 1.96–1.86 (m, 2H), 1.70–1.66 (m, 2H), 1.28 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 161.7, 158.7 (2JC–F = 22 Hz), 156.9, 151.1, 150.6, 148.3, 139.5, 137.3, 131.8, 128.9, 128.8, 127.3, 126.1, 120.8, 114.9, 114.6, 114.1, 83.4 (1JC–F = 178.0 Hz), 68.5, 68.3, 60.4, 44.1 (2JC–F = 23.0 Hz), 38.2, 29.3, 24.0, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2559.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 38%. 1H NMR (400 MHz, chloroform-d) δ 9.53 (s, 1H), 8.38 (s, 1H), 8.34 (d, J = 4.8 Hz, 1H), 7.39 (s, 3H), 7.17 (d, J = 8.1 Hz, 1H), 7.05 (d, J = 4.7 Hz, 1H), 6.85 (d, J = 8.1 Hz, 1H), 6.81 (s, 1H), 6.05 (dd, 2JH–F= 55.9 Hz, J = 7.0 Hz, 1H), 5.51 (s, 1H), 4.02–3.96 (m, 2H), 3.75–3.58 (m, 1H), 3.07–2.91 (m, 1H), 2.23 (s, 3H), 1.92–1.83 (m, 2H), 1.68–1.63 (m, 2H), 1.25 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 23.0 Hz), 156.9, 151.1, 150.6, 148.3, 139.5, 138.3, 131.8, 129.0, 128.7, 126.7, 126.1, 120.8, 114.9, 114.6, 114.1, 83.2 (1JC–F = 178.0 Hz), 68.5, 68.3, 60.1, 43.0 (2JC–F = 22.0 Hz), 29.3, 24.0, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2564.
V) as a white solid, yield 38%. 1H NMR (400 MHz, chloroform-d) δ 9.53 (s, 1H), 8.38 (s, 1H), 8.34 (d, J = 4.8 Hz, 1H), 7.39 (s, 3H), 7.17 (d, J = 8.1 Hz, 1H), 7.05 (d, J = 4.7 Hz, 1H), 6.85 (d, J = 8.1 Hz, 1H), 6.81 (s, 1H), 6.05 (dd, 2JH–F= 55.9 Hz, J = 7.0 Hz, 1H), 5.51 (s, 1H), 4.02–3.96 (m, 2H), 3.75–3.58 (m, 1H), 3.07–2.91 (m, 1H), 2.23 (s, 3H), 1.92–1.83 (m, 2H), 1.68–1.63 (m, 2H), 1.25 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 23.0 Hz), 156.9, 151.1, 150.6, 148.3, 139.5, 138.3, 131.8, 129.0, 128.7, 126.7, 126.1, 120.8, 114.9, 114.6, 114.1, 83.2 (1JC–F = 178.0 Hz), 68.5, 68.3, 60.1, 43.0 (2JC–F = 22.0 Hz), 29.3, 24.0, 19.0. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2564.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 55%.1H NMR (400 MHz, acetone-d6) δ 9.64 (s, 1H), 8.33 (d, J = 1.4 Hz, 1H), 8.30 (d, J = 5.6 Hz, 1H), 7.43–7.33 (m, 3H), 7.32–7.24 (m, 3H), 6.96 (dd, J = 8.4, 2.7 Hz, 1H), 6.88 (d, J = 2.7 Hz, 1H), 5.65 (dd, J = 8.3, 5.9 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.40–3.28 (m, 2H), 3.25–3.14 (m, 1H), 3.14–3.03 (m, 1H), 2.73 (s, 1H), 2.15 (s, 3H), 1.91–1.82 (m, 2H), 1.62–1.56 (m, 2H), 1.18 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.1, 156.8, 153.1 (1JC–F = 249.0 Hz), 147.1, 139.3, 138.1 (3JC–F = 16.0 Hz), 136.2 (2JC–F = 27.0 Hz), 133.6, 131.4, 128.9, 128.4, 127.1, 126.7, 115.6, 115.3, 115.1, 68.5, 68.4, 61.1, 35.4, 29.3, 23.9, 20.6, 18.4. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2566.
V) as a white solid, yield 55%.1H NMR (400 MHz, acetone-d6) δ 9.64 (s, 1H), 8.33 (d, J = 1.4 Hz, 1H), 8.30 (d, J = 5.6 Hz, 1H), 7.43–7.33 (m, 3H), 7.32–7.24 (m, 3H), 6.96 (dd, J = 8.4, 2.7 Hz, 1H), 6.88 (d, J = 2.7 Hz, 1H), 5.65 (dd, J = 8.3, 5.9 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.40–3.28 (m, 2H), 3.25–3.14 (m, 1H), 3.14–3.03 (m, 1H), 2.73 (s, 1H), 2.15 (s, 3H), 1.91–1.82 (m, 2H), 1.62–1.56 (m, 2H), 1.18 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.1, 156.8, 153.1 (1JC–F = 249.0 Hz), 147.1, 139.3, 138.1 (3JC–F = 16.0 Hz), 136.2 (2JC–F = 27.0 Hz), 133.6, 131.4, 128.9, 128.4, 127.1, 126.7, 115.6, 115.3, 115.1, 68.5, 68.4, 61.1, 35.4, 29.3, 23.9, 20.6, 18.4. HRMS: calcd for C30H33FN5O3 [M + H]+, 530.2567; found, 530.2566.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 52%. 1H NMR (400 MHz, acetone-d6) δ 9.64 (s, 1H), 8.44 (s, 1H), 8.27 (s, 1H), 7.44–7.34 (m, 3H), 7.30 (d, J = 7.3 Hz, 2H), 7.25 (d, J = 8.6 Hz, 1H), 6.95 (dd, J = 8.4, 2.6 Hz, 1H), 6.78 (s, 1H), 5.66 (t, J = 7.3 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.39 (s, 1H), 3.27–3.17 (m, 2H), 3.16–3.09 (m, 1H), 1.91–1.84 (m, 2H), 1.62–1.57 (m, 2H), 1.18 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.5, 157.3, 156.7, 149.5, 149.4, 147.5, 139.3, 137.2, 131.2, 128.9, 128.4, 126.8, 126.4, 125.3, 115.2, 115.0, 114.3, 68.5, 68.3, 61.1, 35.4, 29.3, 23.9, 20.6, 18.2. HRMS: calcd for C30H33ClN5O3 [M + H]+, 546.2272; found, 546.2263.
V) as a white solid, yield 52%. 1H NMR (400 MHz, acetone-d6) δ 9.64 (s, 1H), 8.44 (s, 1H), 8.27 (s, 1H), 7.44–7.34 (m, 3H), 7.30 (d, J = 7.3 Hz, 2H), 7.25 (d, J = 8.6 Hz, 1H), 6.95 (dd, J = 8.4, 2.6 Hz, 1H), 6.78 (s, 1H), 5.66 (t, J = 7.3 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.39 (s, 1H), 3.27–3.17 (m, 2H), 3.16–3.09 (m, 1H), 1.91–1.84 (m, 2H), 1.62–1.57 (m, 2H), 1.18 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.5, 157.3, 156.7, 149.5, 149.4, 147.5, 139.3, 137.2, 131.2, 128.9, 128.4, 126.8, 126.4, 125.3, 115.2, 115.0, 114.3, 68.5, 68.3, 61.1, 35.4, 29.3, 23.9, 20.6, 18.2. HRMS: calcd for C30H33ClN5O3 [M + H]+, 546.2272; found, 546.2263.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 43%. 1H NMR (400 MHz, acetone-d6) δ 9.52 (s, 1H), 8.28 (s, 1H), 8.11 (s, 1H), 7.45–7.38 (m, 3H), 7.35–7.29 (m, 2H), 7.25 (d, J = 8.4 Hz, 1H), 6.92 (dd, J = 8.4, 2.8 Hz, 1H), 6.72 (d, J = 2.7 Hz, 1H), 5.67 (dd, J = 8.3, 5.9 Hz, 1H), 4.02 (t, J = 6.6 Hz, 2H), 3.44–3.34 (m, 1H), 3.27–3.18 (m, 2H), 3.18–3.08 (m, 1H), 2.81 (s, 3H), 2.75 (s, 1H), 2.02 (s, 3H), 1.91–1.85 (m, 2H), 1.64–1.59 (m, 2H), 1.20 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.6, 160.7, 157.0, 156.7, 150.9, 148.9, 148.7, 139.3, 131.2, 128.9, 128.4, 127.4, 126.7, 125.9, 114.3, 113.9, 113.3, 68.5, 68.2, 61.0, 35.4, 29.3, 23.9, 20.6, 18.3, 15.8. HRMS: calcd for C31H36N5O3 [M + H]+, 526.2818; found, 526.2813.
V) as a white solid, yield 43%. 1H NMR (400 MHz, acetone-d6) δ 9.52 (s, 1H), 8.28 (s, 1H), 8.11 (s, 1H), 7.45–7.38 (m, 3H), 7.35–7.29 (m, 2H), 7.25 (d, J = 8.4 Hz, 1H), 6.92 (dd, J = 8.4, 2.8 Hz, 1H), 6.72 (d, J = 2.7 Hz, 1H), 5.67 (dd, J = 8.3, 5.9 Hz, 1H), 4.02 (t, J = 6.6 Hz, 2H), 3.44–3.34 (m, 1H), 3.27–3.18 (m, 2H), 3.18–3.08 (m, 1H), 2.81 (s, 3H), 2.75 (s, 1H), 2.02 (s, 3H), 1.91–1.85 (m, 2H), 1.64–1.59 (m, 2H), 1.20 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.6, 160.7, 157.0, 156.7, 150.9, 148.9, 148.7, 139.3, 131.2, 128.9, 128.4, 127.4, 126.7, 125.9, 114.3, 113.9, 113.3, 68.5, 68.2, 61.0, 35.4, 29.3, 23.9, 20.6, 18.3, 15.8. HRMS: calcd for C31H36N5O3 [M + H]+, 526.2818; found, 526.2813.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 28%. 1H NMR (400 MHz, acetone-d6) δ 10.13 (s, 1H), 9.70 (d, J = 2.5 Hz, 1H), 8.33 (d, J = 2.5 Hz, 1H), 7.46–7.35 (m, 3H), 7.32–7.23 (m, 3H), 7.06 (d, J = 2.6 Hz, 1H), 6.96 (d, J = 8.7 Hz, 1H), 5.66 (t, J = 7.1 Hz, 1H), 4.04 (t, J = 6.6 Hz, 2H), 3.43–3.31 (m, 1H), 3.30 (s, 1H), 3.26–3.17 (m, 1H), 3.16–3.06 (m, 1H), 2.31 (s, 3H), 1.92–1.85 (m, 2H), 1.64–1.58 (m, 2H), 1.19 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.9, 161.0, 160.4, 158.8, 156.9, 142.4, 139.4, 138.2, 137.7, 131.9, 128.9, 128.4, 127.2, 126.8, 115.4, 115.3, 115.2, 68.5, 68.3, 61.1, 35.5, 29.3, 24.0, 20.5, 19.0. HRMS: calcd for C29H33N6O3 [M + H]+, 513.2614; found, 513.2605.
V) as a white solid, yield 28%. 1H NMR (400 MHz, acetone-d6) δ 10.13 (s, 1H), 9.70 (d, J = 2.5 Hz, 1H), 8.33 (d, J = 2.5 Hz, 1H), 7.46–7.35 (m, 3H), 7.32–7.23 (m, 3H), 7.06 (d, J = 2.6 Hz, 1H), 6.96 (d, J = 8.7 Hz, 1H), 5.66 (t, J = 7.1 Hz, 1H), 4.04 (t, J = 6.6 Hz, 2H), 3.43–3.31 (m, 1H), 3.30 (s, 1H), 3.26–3.17 (m, 1H), 3.16–3.06 (m, 1H), 2.31 (s, 3H), 1.92–1.85 (m, 2H), 1.64–1.58 (m, 2H), 1.19 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.9, 161.0, 160.4, 158.8, 156.9, 142.4, 139.4, 138.2, 137.7, 131.9, 128.9, 128.4, 127.2, 126.8, 115.4, 115.3, 115.2, 68.5, 68.3, 61.1, 35.5, 29.3, 24.0, 20.5, 19.0. HRMS: calcd for C29H33N6O3 [M + H]+, 513.2614; found, 513.2605.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 49%. 1H NMR (400 MHz, acetone-d6) δ 9.61 (s, 1H), 8.47–8.39 (m, 2H), 7.46 (d, J = 8.4 Hz, 1H), 7.43–7.36 (m, 3H), 7.33–7.30 (m, 2H), 7.25 (dd, J = 5.3, 1.4 Hz, 1H), 7.07–7.02 (m, 2H), 5.67 (dd, J = 8.3, 6.0 Hz, 1H), 4.08 (t, J = 6.6 Hz, 2H), 3.44–3.28 (m, 2H), 3.26 (s, 1H), 3.23–3.07 (m, 2H), 1.94–1.85 (m, 2H), 1.65–1.57 (m, 2H), 1.19 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.8, 157.2, 150.7, 148.5, 148.4, 139.3, 138.2, 131.0, 128.9, 128.4, 126.8, 121.8, 120.8, 116.7, 116.5, 113.9, 68.8, 68.5, 61.1, 35.4, 29.3, 23.8, 20.6. HRMS: calcd for C29H31ClN5O3 [M + H]+, 532.2115; found, 532.2109.
V) as a white solid, yield 49%. 1H NMR (400 MHz, acetone-d6) δ 9.61 (s, 1H), 8.47–8.39 (m, 2H), 7.46 (d, J = 8.4 Hz, 1H), 7.43–7.36 (m, 3H), 7.33–7.30 (m, 2H), 7.25 (dd, J = 5.3, 1.4 Hz, 1H), 7.07–7.02 (m, 2H), 5.67 (dd, J = 8.3, 6.0 Hz, 1H), 4.08 (t, J = 6.6 Hz, 2H), 3.44–3.28 (m, 2H), 3.26 (s, 1H), 3.23–3.07 (m, 2H), 1.94–1.85 (m, 2H), 1.65–1.57 (m, 2H), 1.19 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.8, 157.2, 150.7, 148.5, 148.4, 139.3, 138.2, 131.0, 128.9, 128.4, 126.8, 121.8, 120.8, 116.7, 116.5, 113.9, 68.8, 68.5, 61.1, 35.4, 29.3, 23.8, 20.6. HRMS: calcd for C29H31ClN5O3 [M + H]+, 532.2115; found, 532.2109.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 58%. 1H NMR (400 MHz, acetone-d6) δ 9.59 (s, 1H), 8.64 (s, 1H), 8.41 (d, J = 5.3 Hz, 1H), 7.50–7.38 (m, 5H), 7.37–7.30 (m, 4H), 7.08 (d, J = 8.3 Hz, 1H), 5.69 (t, J = 7.3 Hz, 1H), 4.13 (t, J = 6.5 Hz, 2H), 3.45–3.33 (m, 1H), 3.29 (d, J = 1.5 Hz, 1H), 3.25–3.09 (m, 2H), 2.81–2.69 (m, 1H), 1.99–1.88 (m, 2H), 1.70–1.61 (m, 2H), 1.22 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 159.3, 157.2, 151.3, 149.5, 148.9, 139.3, 138.8, 130.5, 128.9, 128.4, 126.8, 118.9, 118.2, 115.3, 112.9, 111.1, 68.5, 68.4, 61.1, 35.4, 29.3, 23.9, 20.6. HRMS: calcd for C29H32N5O3 [M + H]+, 498.2505; found, 498.2502.
V) as a white solid, yield 58%. 1H NMR (400 MHz, acetone-d6) δ 9.59 (s, 1H), 8.64 (s, 1H), 8.41 (d, J = 5.3 Hz, 1H), 7.50–7.38 (m, 5H), 7.37–7.30 (m, 4H), 7.08 (d, J = 8.3 Hz, 1H), 5.69 (t, J = 7.3 Hz, 1H), 4.13 (t, J = 6.5 Hz, 2H), 3.45–3.33 (m, 1H), 3.29 (d, J = 1.5 Hz, 1H), 3.25–3.09 (m, 2H), 2.81–2.69 (m, 1H), 1.99–1.88 (m, 2H), 1.70–1.61 (m, 2H), 1.22 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 159.3, 157.2, 151.3, 149.5, 148.9, 139.3, 138.8, 130.5, 128.9, 128.4, 126.8, 118.9, 118.2, 115.3, 112.9, 111.1, 68.5, 68.4, 61.1, 35.4, 29.3, 23.9, 20.6. HRMS: calcd for C29H32N5O3 [M + H]+, 498.2505; found, 498.2502.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 53%. 1H NMR (400 MHz, acetone-d6) δ 9.58 (s, 1H), 8.39 (d, J = 5.1 Hz, 1H), 8.32 (d, J = 1.4 Hz, 1H), 7.44–7.35 (m, 3H), 7.34–7.29 (m, 2H), 7.25 (d, J = 8.5 Hz, 1H), 7.15 (dd, J = 5.2, 1.5 Hz, 1H), 6.92 (dd, J = 8.5, 2.8 Hz, 1H), 6.85 (d, J = 2.8 Hz, 1H), 5.69–5.64 (m, 1H), 3.81 (s, 3H), 3.44–3.33 (m, 1H), 3.27–3.17 (m, 1H), 3.16–3.05 (m, 1H), 2.80 (s, 1H), 2.79–2.69 (m, 1H), 2.23 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.5, 157.2, 151.1, 150.7, 148.3, 139.6, 139.3, 131.7, 128.9, 128.4, 126.8, 126.3, 120.7, 114.3, 114.1, 113.7, 61.1, 55.2, 35.4, 20.6, 19.0. HRMS: calcd for C25H24N5O2 [M + H]+, 426.1930; found, 426.1925.
V) as a white solid, yield 53%. 1H NMR (400 MHz, acetone-d6) δ 9.58 (s, 1H), 8.39 (d, J = 5.1 Hz, 1H), 8.32 (d, J = 1.4 Hz, 1H), 7.44–7.35 (m, 3H), 7.34–7.29 (m, 2H), 7.25 (d, J = 8.5 Hz, 1H), 7.15 (dd, J = 5.2, 1.5 Hz, 1H), 6.92 (dd, J = 8.5, 2.8 Hz, 1H), 6.85 (d, J = 2.8 Hz, 1H), 5.69–5.64 (m, 1H), 3.81 (s, 3H), 3.44–3.33 (m, 1H), 3.27–3.17 (m, 1H), 3.16–3.05 (m, 1H), 2.80 (s, 1H), 2.79–2.69 (m, 1H), 2.23 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.5, 157.2, 151.1, 150.7, 148.3, 139.6, 139.3, 131.7, 128.9, 128.4, 126.8, 126.3, 120.7, 114.3, 114.1, 113.7, 61.1, 55.2, 35.4, 20.6, 19.0. HRMS: calcd for C25H24N5O2 [M + H]+, 426.1930; found, 426.1925.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 45%. 1H NMR (400 MHz, acetone-d6) δ 9.58 (s, 1H), 8.39 (d, J = 5.1 Hz, 1H), 8.32 (s, 1H), 7.45–7.34 (m, 3H), 7.33–7.28 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.16 (dd, J = 5.1, 1.6 Hz, 1H), 6.95 (dd, J = 8.5, 2.8 Hz, 1H), 6.88 (d, J = 2.6 Hz, 1H), 5.74–5.63 (m, 1H), 3.83 (s, 2H), 3.72 (s, 1H), 3.42–3.32 (m, 1H), 3.28–3.18 (m, 1H), 3.18–3.07 (m, 1H), 2.79–2.74 (m, 1H), 2.23 (s, 3H), 1.28 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.2, 151.1, 150.7, 148.3, 139.5, 139.3, 131.8, 128.9, 128.4, 126.8, 126.5, 126.2, 120.6, 115.1, 114.8, 113.7, 76.3, 68.6, 61.1, 35.4, 26.6, 20.6, 19.0. HRMS: calcd for C28H30N5O3 [M + H]+, 484.2349; found, 484.2347.
V) as a white solid, yield 45%. 1H NMR (400 MHz, acetone-d6) δ 9.58 (s, 1H), 8.39 (d, J = 5.1 Hz, 1H), 8.32 (s, 1H), 7.45–7.34 (m, 3H), 7.33–7.28 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.16 (dd, J = 5.1, 1.6 Hz, 1H), 6.95 (dd, J = 8.5, 2.8 Hz, 1H), 6.88 (d, J = 2.6 Hz, 1H), 5.74–5.63 (m, 1H), 3.83 (s, 2H), 3.72 (s, 1H), 3.42–3.32 (m, 1H), 3.28–3.18 (m, 1H), 3.18–3.07 (m, 1H), 2.79–2.74 (m, 1H), 2.23 (s, 3H), 1.28 (s, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.2, 151.1, 150.7, 148.3, 139.5, 139.3, 131.8, 128.9, 128.4, 126.8, 126.5, 126.2, 120.6, 115.1, 114.8, 113.7, 76.3, 68.6, 61.1, 35.4, 26.6, 20.6, 19.0. HRMS: calcd for C28H30N5O3 [M + H]+, 484.2349; found, 484.2347.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 38%. 1H NMR (400 MHz, acetone-d6) δ 9.58 (s, 1H), 8.39 (d, J = 5.1 Hz, 1H), 8.32 (s, 1H), 7.45–7.34 (m, 3H), 7.34–7.29 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.15 (dd, J = 5.2, 1.5 Hz, 1H), 6.92 (dd, J = 8.3, 2.8 Hz, 1H), 6.86 (d, J = 2.7 Hz, 1H), 5.66 (dd, J = 8.3, 6.0 Hz, 1H), 4.08 (t, J = 6.4 Hz, 2H), 3.92–3.81 (m, 2H), 3.43–3.26 (m, 3H), 3.26–3.16 (m, 1H), 3.16–3.07 (m, 1H), 2.79–2.67 (m, 1H), 2.23 (s, 3H), 1.75–1.63 (m, 4H), 1.32–1.25 (m, 3H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.2, 156.8, 151.1, 150.7, 148.3, 139.5, 139.3, 131.7, 128.9, 128.4, 126.8, 126.2, 120.7, 114.9, 114.7, 113.7, 67.0, 65.1, 61.1, 35.6, 35.4, 32.6, 31.5, 20.6, 19.0. HRMS: calcd for C31H34N5O3 [M + H]+, 524.2662; found, 524.2660.
V) as a white solid, yield 38%. 1H NMR (400 MHz, acetone-d6) δ 9.58 (s, 1H), 8.39 (d, J = 5.1 Hz, 1H), 8.32 (s, 1H), 7.45–7.34 (m, 3H), 7.34–7.29 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.15 (dd, J = 5.2, 1.5 Hz, 1H), 6.92 (dd, J = 8.3, 2.8 Hz, 1H), 6.86 (d, J = 2.7 Hz, 1H), 5.66 (dd, J = 8.3, 6.0 Hz, 1H), 4.08 (t, J = 6.4 Hz, 2H), 3.92–3.81 (m, 2H), 3.43–3.26 (m, 3H), 3.26–3.16 (m, 1H), 3.16–3.07 (m, 1H), 2.79–2.67 (m, 1H), 2.23 (s, 3H), 1.75–1.63 (m, 4H), 1.32–1.25 (m, 3H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.2, 156.8, 151.1, 150.7, 148.3, 139.5, 139.3, 131.7, 128.9, 128.4, 126.8, 126.2, 120.7, 114.9, 114.7, 113.7, 67.0, 65.1, 61.1, 35.6, 35.4, 32.6, 31.5, 20.6, 19.0. HRMS: calcd for C31H34N5O3 [M + H]+, 524.2662; found, 524.2660.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 20
methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 53%. 1H NMR (400 MHz, methanol-d4) δ 8.35 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.43–7.33 (m, 3H), 7.29–7.20 (m, 3H), 7.18–7.12 (m, 1H), 6.93 (dd, J = 8.3, 2.7 Hz, 1H), 6.84 (d, J = 2.7 Hz, 1H), 5.60 (t, J = 7.2 Hz, 1H), 4.08 (t, J = 5.9 Hz, 2H), 3.27 (s, 1H), 3.18 (dd, J = 9.4, 4.4 Hz, 1H), 3.14–3.05 (m, 1H), 2.76–2.67 (m, 1H), 2.64 (t, J = 7.1 Hz, 2H), 2.22 (s, 3H), 2.16–2.05 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.2, 156.5, 151.0, 150.7, 148.3, 139.6, 139.3, 131.8, 128.9, 128.4, 126.8, 120.6, 120.3, 115.0, 114.7, 113.7, 65.9, 61.1, 35.4, 24.7, 20.6, 19.0, 13.3. HRMS: calcd for C28H27N6O2 [M + H]+, 479.2195; found, 479.2193.
V) as a white solid, yield 53%. 1H NMR (400 MHz, methanol-d4) δ 8.35 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.43–7.33 (m, 3H), 7.29–7.20 (m, 3H), 7.18–7.12 (m, 1H), 6.93 (dd, J = 8.3, 2.7 Hz, 1H), 6.84 (d, J = 2.7 Hz, 1H), 5.60 (t, J = 7.2 Hz, 1H), 4.08 (t, J = 5.9 Hz, 2H), 3.27 (s, 1H), 3.18 (dd, J = 9.4, 4.4 Hz, 1H), 3.14–3.05 (m, 1H), 2.76–2.67 (m, 1H), 2.64 (t, J = 7.1 Hz, 2H), 2.22 (s, 3H), 2.16–2.05 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 162.7, 160.6, 157.2, 156.5, 151.0, 150.7, 148.3, 139.6, 139.3, 131.8, 128.9, 128.4, 126.8, 120.6, 120.3, 115.0, 114.7, 113.7, 65.9, 61.1, 35.4, 24.7, 20.6, 19.0, 13.3. HRMS: calcd for C28H27N6O2 [M + H]+, 479.2195; found, 479.2193.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 15
methanol = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 40%. 1H NMR (600 MHz, methanol-d4) δ 8.37 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.46–7.37 (m, 3H), 7.33–7.29 (m, 2H), 7.23 (d, J = 8.4 Hz, 1H), 7.19–7.15 (m, 1H), 6.95–6.91 (m, 1H), 6.85 (d, J = 2.7 Hz, 1H), 6.15 (dd, 2JH–F= 56.3 Hz, J = 7.1 Hz, 1H), 5.70–5.64 (m, 1H), 4.15 (t, J = 5.5 Hz, 2H), 3.84–3.74 (m, 1H), 3.71 (t, J = 4.7 Hz, 4H), 2.90–2.83 (m, 1H), 2.81 (t, J = 5.4 Hz, 2H), 2.60 (t, J = 4.7 Hz, 4H), 2.23 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.7, 151.0, 150.6, 148.3, 139.5, 138.3, 131.8, 129.0, 128.7, 126.7, 126.4, 120.8, 114.9, 114.8, 114.1, 83.2 (1JC–F = 178.0 Hz), 66.2, 65.5, 60.1, 57.0, 53.6, 43.0 (2JC–F = 22.0 Hz), 19.0. HRMS: calcd for C30H32FN6O3 [M + H]+, 543.2520; found, 543.2518.
V) as a white solid, yield 40%. 1H NMR (600 MHz, methanol-d4) δ 8.37 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.46–7.37 (m, 3H), 7.33–7.29 (m, 2H), 7.23 (d, J = 8.4 Hz, 1H), 7.19–7.15 (m, 1H), 6.95–6.91 (m, 1H), 6.85 (d, J = 2.7 Hz, 1H), 6.15 (dd, 2JH–F= 56.3 Hz, J = 7.1 Hz, 1H), 5.70–5.64 (m, 1H), 4.15 (t, J = 5.5 Hz, 2H), 3.84–3.74 (m, 1H), 3.71 (t, J = 4.7 Hz, 4H), 2.90–2.83 (m, 1H), 2.81 (t, J = 5.4 Hz, 2H), 2.60 (t, J = 4.7 Hz, 4H), 2.23 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.7, 151.0, 150.6, 148.3, 139.5, 138.3, 131.8, 129.0, 128.7, 126.7, 126.4, 120.8, 114.9, 114.8, 114.1, 83.2 (1JC–F = 178.0 Hz), 66.2, 65.5, 60.1, 57.0, 53.6, 43.0 (2JC–F = 22.0 Hz), 19.0. HRMS: calcd for C30H32FN6O3 [M + H]+, 543.2520; found, 543.2518.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 15
methanol = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 43%. 1H NMR (600 MHz, methanol-d4) δ 8.37 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.46–7.37 (m, 3H), 7.33–7.29 (m, 2H), 7.23 (d, J = 8.4 Hz, 1H), 7.19–7.15 (m, 1H), 6.95–6.91 (m, 1H), 6.85 (d, J = 2.7 Hz, 1H), 6.15 (dd, 2JH–F= 56.3 Hz, J = 7.1 Hz, 1H), 5.70–5.64 (m, 1H), 4.15 (t, J = 5.5 Hz, 2H), 3.84–3.74 (m, 1H), 3.71 (t, J = 4.7 Hz, 4H), 2.90–2.83 (m, 1H), 2.81 (t, J = 5.4 Hz, 2H), 2.60 (t, J = 4.7 Hz, 4H), 2.23 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.7, 151.0, 150.6, 148.3, 139.5, 138.3, 131.8, 129.0, 128.7, 126.7, 126.4, 120.8, 114.9, 114.8, 114.1, 83.2 (1JC–F = 178.0 Hz), 66.2, 65.5, 60.1, 57.0, 53.6, 43.0 (2JC–F = 22.0 Hz), 19.0. HRMS: calcd for C30H32FN6O3 [M + H]+, 543.2520; found, 543.2515.
V) as a white solid, yield 43%. 1H NMR (600 MHz, methanol-d4) δ 8.37 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.46–7.37 (m, 3H), 7.33–7.29 (m, 2H), 7.23 (d, J = 8.4 Hz, 1H), 7.19–7.15 (m, 1H), 6.95–6.91 (m, 1H), 6.85 (d, J = 2.7 Hz, 1H), 6.15 (dd, 2JH–F= 56.3 Hz, J = 7.1 Hz, 1H), 5.70–5.64 (m, 1H), 4.15 (t, J = 5.5 Hz, 2H), 3.84–3.74 (m, 1H), 3.71 (t, J = 4.7 Hz, 4H), 2.90–2.83 (m, 1H), 2.81 (t, J = 5.4 Hz, 2H), 2.60 (t, J = 4.7 Hz, 4H), 2.23 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.7, 151.0, 150.6, 148.3, 139.5, 138.3, 131.8, 129.0, 128.7, 126.7, 126.4, 120.8, 114.9, 114.8, 114.1, 83.2 (1JC–F = 178.0 Hz), 66.2, 65.5, 60.1, 57.0, 53.6, 43.0 (2JC–F = 22.0 Hz), 19.0. HRMS: calcd for C30H32FN6O3 [M + H]+, 543.2520; found, 543.2515.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 15
methanol = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 40%. 1H NMR (600 MHz, methanol-d4) δ 8.38 (d, J = 5.1 Hz, 1H), 8.26 (s, 1H), 7.46–7.39 (m, 3H), 7.33 (d, J = 7.4 Hz, 2H), 7.23 (d, J = 8.5 Hz, 1H), 7.18 (d, J = 5.0 Hz, 1H), 6.91 (d, J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.16 (dd, 2JH–F= 56.2 Hz, J = 7.1 Hz, 1H), 5.69 (d, J = 8.3 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.97–3.90 (m, 2H), 3.86–3.75 (m, 1H), 3.43 (t, J = 11.8 Hz, 2H), 2.87 (dd, J = 26.6, 15.2 Hz, 1H), 2.24 (s, 3H), 1.83 (s, 1H), 1.76–1.68 (m, 4H), 1.40–1.28 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.9, 151.1, 150.6, 148.3, 139.5, 138.2, 131.8, 129.0, 128.7, 126.7, 126.2, 120.8, 114.9, 114.7, 114.1, 83.2 (1JC–F = 178.0 Hz), 67.0, 65.1, 60.1, 43.0 (2JC–F = 22.0 Hz), 35.6, 32.6, 31.5, 19.0. HRMS: calcd for C31H33FN5O3 [M + H]+, 542.2567; found, 542.2560.
V) as a white solid, yield 40%. 1H NMR (600 MHz, methanol-d4) δ 8.38 (d, J = 5.1 Hz, 1H), 8.26 (s, 1H), 7.46–7.39 (m, 3H), 7.33 (d, J = 7.4 Hz, 2H), 7.23 (d, J = 8.5 Hz, 1H), 7.18 (d, J = 5.0 Hz, 1H), 6.91 (d, J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.16 (dd, 2JH–F= 56.2 Hz, J = 7.1 Hz, 1H), 5.69 (d, J = 8.3 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.97–3.90 (m, 2H), 3.86–3.75 (m, 1H), 3.43 (t, J = 11.8 Hz, 2H), 2.87 (dd, J = 26.6, 15.2 Hz, 1H), 2.24 (s, 3H), 1.83 (s, 1H), 1.76–1.68 (m, 4H), 1.40–1.28 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.9, 151.1, 150.6, 148.3, 139.5, 138.2, 131.8, 129.0, 128.7, 126.7, 126.2, 120.8, 114.9, 114.7, 114.1, 83.2 (1JC–F = 178.0 Hz), 67.0, 65.1, 60.1, 43.0 (2JC–F = 22.0 Hz), 35.6, 32.6, 31.5, 19.0. HRMS: calcd for C31H33FN5O3 [M + H]+, 542.2567; found, 542.2560.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 15
methanol = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, V
1, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) as a white solid, yield 39%. 1H NMR (600 MHz, methanol-d4) δ 8.38 (d, J = 5.1 Hz, 1H), 8.26 (s, 1H), 7.46–7.39 (m, 3H), 7.33 (d, J = 7.4 Hz, 2H), 7.23 (d, J = 8.5 Hz, 1H), 7.18 (d, J = 5.0 Hz, 1H), 6.91 (d, J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.16 (dd, 2JH–F= 56.2 Hz, J = 7.1 Hz, 1H), 5.69 (d, J = 8.3 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.97–3.90 (m, 2H), 3.86–3.75 (m, 1H), 3.43 (t, J = 11.8 Hz, 2H), 2.87 (dd, J = 26.6, 15.2 Hz, 1H), 2.24 (s, 3H), 1.83 (s, 1H), 1.76–1.68 (m, 4H), 1.40–1.28 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.9, 151.1, 150.6, 148.3, 139.5, 138.2, 131.8, 129.0, 128.7, 126.7, 126.2, 120.8, 114.9, 114.7, 114.1, 83.2 (1JC–F = 178.0 Hz), 67.0, 65.1, 60.1, 43.0 (2JC–F = 22.0 Hz), 35.6, 32.6, 31.5, 19.0. HRMS: calcd for C31H33FN5O3 [M + H]+, 542.2567; found, 542.2565.
V) as a white solid, yield 39%. 1H NMR (600 MHz, methanol-d4) δ 8.38 (d, J = 5.1 Hz, 1H), 8.26 (s, 1H), 7.46–7.39 (m, 3H), 7.33 (d, J = 7.4 Hz, 2H), 7.23 (d, J = 8.5 Hz, 1H), 7.18 (d, J = 5.0 Hz, 1H), 6.91 (d, J = 8.5 Hz, 1H), 6.82 (s, 1H), 6.16 (dd, 2JH–F= 56.2 Hz, J = 7.1 Hz, 1H), 5.69 (d, J = 8.3 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.97–3.90 (m, 2H), 3.86–3.75 (m, 1H), 3.43 (t, J = 11.8 Hz, 2H), 2.87 (dd, J = 26.6, 15.2 Hz, 1H), 2.24 (s, 3H), 1.83 (s, 1H), 1.76–1.68 (m, 4H), 1.40–1.28 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 161.8, 158.7 (2JC–F = 22.0 Hz), 156.9, 156.9, 151.1, 150.6, 148.3, 139.5, 138.2, 131.8, 129.0, 128.7, 126.7, 126.2, 120.8, 114.9, 114.7, 114.1, 83.2 (1JC–F = 178.0 Hz), 67.0, 65.1, 60.1, 43.0 (2JC–F = 22.0 Hz), 35.6, 32.6, 31.5, 19.0. HRMS: calcd for C31H33FN5O3 [M + H]+, 542.2567; found, 542.2565.
Biology
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shanghai Institute of Materia Medica and approved by the Animal Ethics Committee of Shanghai Institute of Materia Medica (approval no. 2023-01-YY-26).
Author contributions
Z. J. and Y. D. contributed equally to this article. Z. J. and Y. D.: investigation, methodology, writing – original draft. Y. J. and X. P.: investigation, methodology. W. D.: conceptualization, supervision, writing – review & editing. H. Z. and J. A.: conceptualization, investigation, supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests.Acknowledgements
This work was supported by China Postdoctoral Science Foundation (Certificate Number: 2023T160663), Institutes for Drug Discovery and Development, Chinese Academy of Sciences (No. SIMM0320231002), the Natural Science Foundation of China for Innovation Research Group (No. 81821005), the Project of Shanghai Institute of Materia Medica, Chinese Academy of Sciences (No. SIMM0120231001) and the Shandong Laboratory Program (SYS202205).References
- K. Yamanaka, Y. Saito, T. Yamamori, Y. Urano and N. Noguchi, J. Biol. Chem., 2011, 286, 24666–24673 CrossRef CAS PubMed.
- R. L. Ang, M. Chan and A. T. Ting, Front. Cell Dev. Biol., 2019, 7, 163 CrossRef PubMed.
- W. Wu, X. Wang, N. Berleth, J. Deitersen, N. Wallot-Hieke, P. Böhler, D. Schlütermann, F. Stuhldreier, J. Cox, K. Schmitz, S. Seggewiß, C. Peter, G. Kasof, A. Stefanski, K. Stühler, A. Tschapek, A. Gödecke and B. Stork, Cell Rep., 2020, 31, 107547 CrossRef CAS PubMed.
- N. Festjens, T. Vanden Berghe, S. Cornelis and P. Vandenabeele, Cell Death Differ., 2007, 14, 400–410 CrossRef CAS PubMed.
- D. Bertheloot, E. Latz and B. S. Franklin, Cell. Mol. Immunol., 2021, 18, 1106–1121 CrossRef CAS PubMed.
- C. Xu, J. Wu, Y. Wu, Z. Ren, Y. Yao, G. Chen, E. F. Fang, J. H. Noh, Y. U. Liu, L. Wei, X. Chen and J. Sima, Theranostics, 2021, 11, 9452–9469 CrossRef CAS PubMed.
- L. Jin, P. Liu, M. Yin, M. Zhang, Y. Kuang and W. Zhu, J. Dermatol. Sci., 2020, 99, 146–151 CrossRef CAS PubMed.
- A. Q. Chen, Z. Fang, X. L. Chen, S. Yang, Y. F. Zhou, L. Mao, Y. P. Xia, H. J. Jin, Y. N. Li, M. F. You, X. X. Wang, H. Lei, Q. W. He and B. Hu, Cell Death Dis., 2019, 10, 487 CrossRef PubMed.
- L. He, K. Peng, Y. Liu, J. Xiong and F. F. Zhu, Onco Targets Ther, 2013, 6, 1539–1543 CAS.
- S. Park, K. J. Hatanpaa, Y. Xie, B. E. Mickey, C. J. Madden, J. M. Raisanen, D. B. Ramnarain, G. Xiao, D. Saha, D. A. Boothman, D. Zhao, R. M. Bachoo, R. O. Pieper and A. A. Habib, Cancer Res., 2009, 69, 2809–2816 CrossRef CAS PubMed.
- Q. Wang, W. Chen, X. Xu, B. Li, W. He, M. T. Padilla, J. H. Jang, T. Nyunoya, S. Amin, X. Wang and Y. Lin, Carcinogenesis, 2013, 34, 2119–2128 CrossRef CAS PubMed.
- L. Seifert, G. Werba, S. Tiwari, N. N. Giao Ly, S. Alothman, D. Alqunaibit, A. Avanzi, R. Barilla, D. Daley, S. H. Greco, A. Torres-Hernandez, M. Pergamo, A. Ochi, C. P. Zambirinis, M. Pansari, M. Rendon, D. Tippens, M. Hundeyin, V. R. Mani, C. Hajdu, D. Engle and G. Miller, Nature, 2016, 532, 245–249 CrossRef CAS PubMed.
- S. Hofmans, L. Devisscher, S. Martens, D. Van Rompaey, K. Goossens, T. Divert, W. Nerinckx, N. Takahashi, H. De Winter, P. Van Der Veken, V. Goossens, P. Vandenabeele and K. Augustyns, J. Med. Chem., 2018, 61, 1895–1920 CrossRef CAS PubMed.
- A. Fauster, M. Rebsamen, K. V. Huber, J. W. Bigenzahn, A. Stukalov, C. H. Lardeau, S. Scorzoni, M. Bruckner, M. Gridling, K. Parapatics, J. Colinge, K. L. Bennett, S. Kubicek, S. Krautwald, A. Linkermann and G. Superti-Furga, Cell Death Dis., 2015, 6, e1767 CrossRef CAS PubMed.
- Halia Therapeutics Inc., WO Pat., 2022212326A1, 2022 Search PubMed.
- Y. Qin, D. Li, C. Qi, H. Xiang, H. Meng, J. Liu, S. Zhou, X. Gong, Y. Li, G. Xu, R. Zu, H. Xie, Y. Xu, G. Xu, Z. Zhang, S. Chen, L. Pan, Y. Li and L. Tan, Acta Pharm. Sin. B, 2024, 14, 319–334 CrossRef CAS PubMed.
- J. J. Fang, H. Z. Yao, C. Zhuang and F. E. Chen, J. Med. Chem., 2023, 66, 15288–15308 CrossRef CAS PubMed.
- Y. Sun, L. Xu, H. Shao, D. Quan, Z. Mo, J. Wang, W. Zhang, J. Yu, C. Zhuang and K. Xu, J. Med. Chem., 2022, 65, 14957–14969 CrossRef CAS PubMed.
- H. Zhang, L. Xu, X. Qin, X. Chen, H. Cong, L. Hu, L. Chen, Z. Miao, W. Zhang, Z. Cai and C. Zhuang, J. Med. Chem., 2019, 62, 6665–6681 CrossRef CAS PubMed.
- A. Degterev, Z. Huang, M. Boyce, Y. Li, P. Jagtap, N. Mizushima, G. D. Cuny, T. J. Mitchison, M. A. Moskowitz and J. Yuan, Nat. Chem. Biol., 2005, 1, 112–119 CrossRef CAS PubMed.
- P. A. Harris, S. B. Berger, J. U. Jeong, R. Nagilla, D. Bandyopadhyay, N. Campobasso, C. A. Capriotti, J. A. Cox, L. Dare, X. Dong, P. M. Eidam, J. N. Finger, S. J. Hoffman, J. Kang, V. Kasparcova, B. W. King, R. Lehr, Y. Lan, L. K. Leister, J. D. Lich, T. T. MacDonald, N. A. Miller, M. T. Ouellette, C. S. Pao, A. Rahman, M. A. Reilly, A. R. Rendina, E. J. Rivera, M. C. Schaeffer, C. A. Sehon, R. R. Singhaus, H. H. Sun, B. A. Swift, R. D. Totoritis, A. Vossenkämper, P. Ward, D. D. Wisnoski, D. Zhang, R. W. Marquis, P. J. Gough and J. Bertin, J. Med. Chem., 2017, 60, 1247–1261 CrossRef CAS PubMed.
- D. Tompson, M. Whitaker, R. Pan, G. Johnson, T. Fuller, V. Zann, L. McKenzie, K. Abbott-Banner, S. Hawkins and M. Powell, Pharm. Res., 2022, 39, 153–165 CrossRef CAS PubMed.
- P. A. Harris, J. M. Marinis, J. D. Lich, S. B. Berger, A. Chirala, J. A. Cox, P. M. Eidam, J. N. Finger, P. J. Gough, J. U. Jeong, J. Kang, V. Kasparcova, L. K. Leister, M. K. Mahajan, G. Miller, R. Nagilla, M. T. Ouellette, M. A. Reilly, A. R. Rendina, E. J. Rivera, H. H. Sun, J. H. Thorpe, R. D. Totoritis, W. Wang, D. Wu, D. Zhang, J. Bertin and R. W. Marquis, ACS Med. Chem. Lett., 2019, 10, 857–862 CrossRef CAS PubMed.
- GlaxoSmithKline, Study of GSK3845097 in previously treated participants with advanced synovial sarcoma and myxoid/round cell liposarcoma, https://www.clinicaltrials.gov/study/NCT05943990.
- X. Yang, H. Lu, H. Xie, B. Zhang, T. Nie, C. Fan, T. Yang, Y. Xu, H. Su, W. Tang and B. Zhou, Angew. Chem., Int. Ed., 2022, 61, e202114922 CrossRef CAS PubMed.
- P. A. Harris, B. W. King, D. Bandyopadhyay, S. B. Berger, N. Campobasso, C. A. Capriotti, J. A. Cox, L. Dare, X. Dong, J. N. Finger, L. C. Grady, S. J. Hoffman, J. U. Jeong, J. Kang, V. Kasparcova, A. S. Lakdawala, R. Lehr, D. E. McNulty, R. Nagilla, M. T. Ouellette, C. S. Pao, A. R. Rendina, M. C. Schaeffer, J. D. Summerfield, B. A. Swift, R. D. Totoritis, P. Ward, A. Zhang, D. Zhang, R. W. Marquis, J. Bertin and P. J. Gough, J. Med. Chem., 2016, 59, 2163–2178 CrossRef CAS PubMed.
- M. Yoshikawa, M. Saitoh, T. Katoh, T. Seki, S. V. Bigi, Y. Shimizu, T. Ishii, T. Okai, M. Kuno, H. Hattori, E. Watanabe, K. S. Saikatendu, H. Zou, M. Nakakariya, T. Tatamiya, Y. Nakada and T. Yogo, J. Med. Chem., 2018, 61, 2384–2409 CrossRef CAS PubMed.
- P. A. Harris, N. Faucher, N. George, P. M. Eidam, B. W. King, G. V. White, N. A. Anderson, D. Bandyopadhyay, A. M. Beal, V. Beneton, S. B. Berger, N. Campobasso, S. Campos, C. A. Capriotti, J. A. Cox, A. Daugan, F. Donche, M. H. Fouchet, J. N. Finger, B. Geddes, P. J. Gough, P. Grondin, B. L. Hoffman, S. J. Hoffman, S. E. Hutchinson, J. U. Jeong, E. Jigorel, P. Lamoureux, L. K. Leister, J. D. Lich, M. K. Mahajan, J. Meslamani, J. E. Mosley, R. Nagilla, P. M. Nassau, S. L. Ng, M. T. Ouellette, K. K. Pasikanti, F. Potvain, M. A. Reilly, E. J. Rivera, S. Sautet, M. C. Schaeffer, C. A. Sehon, H. Sun, J. H. Thorpe, R. D. Totoritis, P. Ward, N. Wellaway, D. D. Wisnoski, J. M. Woolven, J. Bertin and R. W. Marquis, J. Med. Chem., 2019, 62, 5096–5110 CrossRef CAS PubMed.
- Beijing Scitech-MQ Pharmaceuticals Ltd., WO Pat., 2020151589A1, 2020 Search PubMed.
- Genentech Inc., CN Pat., 110023313A, 2019 Search PubMed.
- Hoffmann-La Roche, Genentech Inc., WO Pat., 2019012063A1, 2019 Search PubMed.
- Genentech Inc., Hoffmann-La Roche, WO Pat., 2022212809A1, 2022 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00265b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |