Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Trehalose in cryopreservation. Applications, mechanisms and intracellular delivery opportunities
Alex
Murray
ab,
Peter
Kilbride
c and
Matthew I.
Gibson
 *abcde
*abcde
aDepartment of Chemistry, University of Warwick, CV4 7AL, UK
bDivision of Biomedical Sciences, Warwick Medical School, University of Warwick, CV4 7AL, UK
cAsymptote, Cytiva, Chivers Way, Cambridge, CB24 9BZ, USA
dDepartment of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: Matt.gibson@manchester.ac.uk
eManchester Institute of Biotechnology, University of Manchester, 131 Princess Street, Manchester, M1 7DN, UK
First published on 19th July 2024
Abstract
Cryopreservation is crucial to fields including immune and stem cell therapies, reproductive technology, blood banking, regenerative medicine and across all biotechnology. During cryopreservation, cryoprotectants are essential to protect cells from the damage caused by exposure to freezing temperatures. The most common penetrating cryoprotectants, such as DMSO and glycerol do not give full recovery and have a cytotoxicity limit on the concentration which can be applied. The non-reducing disaccharide trehalose has been widely explored and used to supplement these, inspired by its use in nature to aid survival at extreme temperatures and/or desiccation. However, trehalose has challenges to its use, particular its low membrane permeability, and how its protective role compares to other sugars. Here we review the application of trehalose and its reported benefit and seek to show where chemical tools can improve its function. In particular, we highlight emerging chemical methods to deliver (as cargo, or via selective permeation) into the intracellular space. This includes encapsulation, cell penetrating peptides or (selective) modification of hydroxyls on trehalose.
Introduction
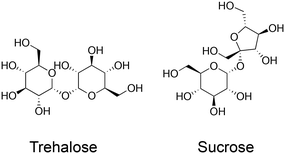
Cryopreservation is the practice of storing biological materials at sub-zero temperatures to halt metabolism and degradation. Cryopreservation is deployed for a vast range of biological materials from proteins,1 cells,2 tissues,3,4 mRNA vaccines,5 and potentially organs.6,7 Cryopreservation is essential in cell culture as it allows for the routine storage of cell lines, reducing the need for continuous culture, which would otherwise lead to phenotype drift as well as being practically challenging. It also has medical applications such as transporting stem cells,8 and blood for transfusion.9 Current cryopreservation techniques, whilst successful, are not perfect: a proportion of cells always die during the process and their functionality is often reduced. One of the major limiting factors in cryopreservation is cryoprotectant toxicity; cryopreservation requires the use of cryoprotectants (including but not limited to solvents) such as DMSO or glycerol which are penetrating cryoprotectants. These can have adverse effects when the thawed cells are transplanted into patients.10 Cryoprotectant toxicity also limits the concentration of cryoprotectant that can be used. The mechanism of cryoprotectant action is concentration-dependent but there becomes a point where further increases in concentration lead to toxicity that outweighs any protective effects. This has motivated research into non-/lower toxicity cryoprotectants.Trehalose (α-D-glucopyranosyl-(1 → 1)-α-D-glucopyranoside) is a water-soluble non-reducing disaccharide made up of two glucose subunits, joined by a 1,1-glycosidic bond.11 It has 8 hydrogen bond donors and 11 hydrogen bond acceptors. Trehalose is biosynthesised by a wide variety of non-mammalian organisms as an energy source and is used in some organisms to protect against freezing and desiccation, allowing them to survive over winter or in other harsh environments. These organisms include the rice water weevil Lissorhoptrus oryzophilus,12 the codling moth Cydia pomonella13 and some tardigrade species such as Macrobiotus richtersi.14 In the past, trehalose was expensive to make, but the development of an efficient manufacturing process in 1994 made this much cheaper, leading to increased research interest.15 Trehalose has two proposed protective mechanisms, which have been termed the ‘vitrification hypothesis’ and the ‘water replacement hypothesis’. These mechanisms are usually discussed in the context of preservation by desiccation or anhydrobiosis in nature but are also applicable to cryopreservation. It is crucial to note that trehalose (and other disaccharides) are non-penetrating cryoprotectants in contrast to the widely used DMSO or glycerol. Fig. 1 summarises the two key methods in cryopreservation relating to vitrification and slow-freezing, which necessitate different concentrations of cryoprotectant and freezing rates, and where problems can occur. Fig. 2 shows the chemical structure of trehalose compared to sucrose.
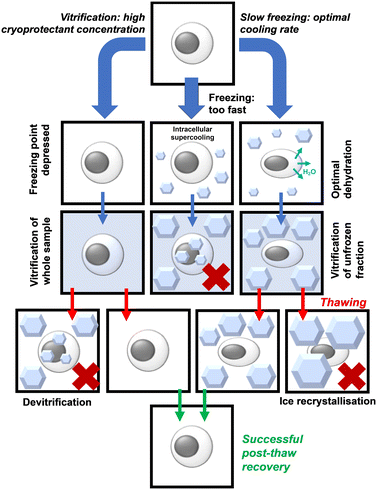 | ||
| Fig. 1 Schematic of the vitrification verses slow freezing pathways, indicating where failure can occur. | ||
The vitrification hypothesis
The vitrification hypothesis holds that trehalose protects biological materials by forming a high-viscosity glass-like state. The transition of a liquid trehalose solution to a glassy state is achieved by concentrating the trehalose through either desiccation or freeze-induced dehydration. During cryopreservation by freezing, the cells survive within the vitrified fraction of the sample.16 Trehalose has several properties that contribute to the formation of this fraction: adding trehalose to a solution increases its glass transition temperature (Tg),17 resulting in vitrification at a higher temperature. Trehalose is also a kosmotrope,18 it orders water molecules around itself, altering the structure of the surrounding hydrogen bond network in such a way that it cannot form ice.19 This network is over three hydration shells wide,20 so each molecule of trehalose can prevent many molecules of water from freezing.21 Trehalose has been found to reduce the size of ice crystals in a concentration-dependent manner.22The water replacement hypothesis
Under normal conditions, proteins and other cell components are stabilised/hydrated by bound water. The water replacement hypothesis holds that trehalose stabilises these components once the water content is sufficiently low by acting as a replacement for this water, Fig. 3.23,24 During cryopreservation, trehalose protects proteins from cold denaturation,25 and cryoprotectant toxicity.26 Trehalose also lowers the temperature at which the membrane gel-to-liquid crystal phase transition occurs.27,28 During desiccation, trehalose stabilises the cell membrane by hydrogen bonding to phospholipids,29 so trehalose may play a similar role in stabilising the cell membrane during cryopreservation. In addition to stabilisation, trehalose likely also counteracts solute concentration damage when it is used at sufficient concentrations, as would any biocompatible, water-soluble, small molecule.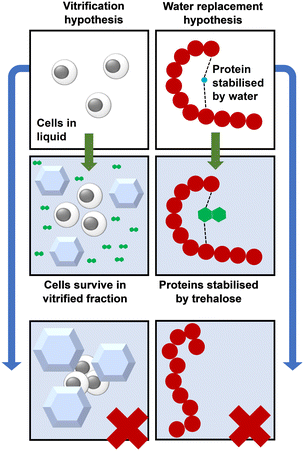 | ||
| Fig. 3 Vitrification compared to water replacement for protein storage using trehalose. The bottom panels show ice formation and protein denaturation in the absence of trehalose. | ||
Trehalose is a polar molecule (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ∼ −3.7), which alongside its large number of hydrogen bond donors/acceptors, means it does not cross the cell plasma membrane and hence cannot provide intracellular cryoprotection. Thus, it is usually used as an extracellular cryoprotectant, but recent advances now allow its intracellular delivery. This review will firstly discuss trehalose as a cryoprotectant for different cell types and provide a critical comparison to other sugars, highlighting cases where trehalose does not offer additional benefit. Secondly, methods to deliver trehalose into the intracellular space and the impact on cryopreservation will be explored.
P ∼ −3.7), which alongside its large number of hydrogen bond donors/acceptors, means it does not cross the cell plasma membrane and hence cannot provide intracellular cryoprotection. Thus, it is usually used as an extracellular cryoprotectant, but recent advances now allow its intracellular delivery. This review will firstly discuss trehalose as a cryoprotectant for different cell types and provide a critical comparison to other sugars, highlighting cases where trehalose does not offer additional benefit. Secondly, methods to deliver trehalose into the intracellular space and the impact on cryopreservation will be explored.
Trehalose as a (primarily) extracellular cryoprotectant
The need for improved cryoprotectants has resulted in research into sugars as a supplement or replacement for traditional penetrating cryoprotectants such as DMSO, ethylene glycol, propylene glycol and glycerol. Trehalose has gained particular attention due to its unique properties among the sugars, which will be discussed in more detail later.Trehalose has been tested as a cryoprotectant in an extensive range of cell types and other biological materials. In most studies, trehalose is supplemented into the standard penetrating cryoprotectant for that cell type – typically 10% DMSO. It is observed that this increases cell recovery and/or improves post-thaw proliferation and other functional outcomes. A recurring trend throughout these studies is that trehalose has an optimal concentration – usually between 100 mM to 400 mM – beyond which adding further trehalose becomes detrimental to post-thaw outcome.30–32 The detrimental effect of higher concentrations is likely due to osmotic pressure rather than through any specific trehalose toxicity. Importantly, trehalose can often lower the amount of penetrating cryoprotectant required for cryopreservation while providing an equivalent or better post-thaw outcome.
Trehalose is not cell penetrative and cannot normally cross the cell membranes and enter the intracellular space unaided, but occasionally, extracellular trehalose can be used without solvent cryoprotectants,33–35 suggesting the presence of intracellular trehalose, which can enter the cell through endocytosis or a membrane phase transition during cooling. Whilst this section is about the extracellular use of trehalose, small amounts of trehalose are likely to be present intracellularly in many of these studies. These concepts are discussed in later in this review. Fig. 4 shows an overview of this, and Fig. 5 and 6 demonstrate the individual mechanisms. A summary of trehalose supplementation to extracellular media and its impact is made below spanning a range of cryopreservation scenarios to allow the reader to compare and contrast the benefits.
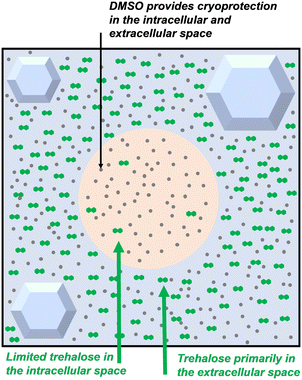 | ||
| Fig. 4 Schematic of localisation of trehalose compared to DMSO during cryopreservation (green hexagons = trehalose, grey dots = DMSO). | ||
 | ||
| Fig. 6 Engineered and formulated trehalose increases passive uptake by membrane diffusion followed by esterase action. | ||
Stem cell preservation
For murine (mouse) spermatogonial stem cells, adding 50 mM trehalose to the standard 10% DMSO freezing media improved viability after storage for one week (90% vs. 76%).36 Adding 200 mM trehalose did not improve viability, but did improve long-term proliferation (49% vs. 28%). For murine microencapsulated mesenchymal stem cells, 5% DMSO, 10%, glycerol, 10%, 5% trehalose, or any combination of trehalose and less than 10% DMSO, all resulted in lower recovery than 10% DMSO.37 For mesenchymal stem cells DMSO can be partially replaced with trehalose or poly(ethylene glycol) (PEG), although trehalose was not more effective than PEG, which might suggest that lower DMSO was tolerated in this model.38Human pluripotent stem cells: cryopreservation can be improved by replacing the standard 10% DMSO with 500 mM trehalose and 10% glycerol. Relative viability was increased by approximately 20–30% depending on the exact cell type. Phenotype and functionality were maintained.39 This raises a particular point that DMSO-free cryopreservation is possible, but in many cases achieved by adding a different penetrating solvent in its place.
Human umbilical cord blood stem cells: in Chen et al.30 2.5% DMSO and 30 mM trehalose was at least as good as 10% EG and 2% DMSO, or 10% DMSO and 2.0% dextran-40 at maintaining viability. Trehalose also slightly reduced the amount of post-thaw apoptosis. In Zhang et al.40 freezing mediums containing 146 mM trehalose + 10% or 5% DMSO resulted in proliferation that was as high as the non-frozen control group. Trehalose has also been successfully used in combination with taurine and catalase for cord blood stem cell preservation.41
Human peripheral blood stem cells: in Martinetti et al.35 the use of 1 M trehalose alone gave higher long- and short-term viability than DMSO alone, or DMSO combined with trehalose. The 1 M trehalose concentration used here is much higher than the typical optimal concentration of 100–400 mM in most cell types; it is possible that the slow addition of freezing media to the cells in this study allowed a higher concentration of trehalose to be reached without causing too much osmotic stress. For marrow and blood stem cells Scheinkönig et al.42 found that 500 mM trehalose provides a similar level of cryoprotection to DMSO.
Human adipose-derived stem cells: cryopreservation with 250 mM trehalose alone resulted in very poor viability compared to 5% DMSO (11% vs. 75%).43 In Pu et al.32 200 mM trehalose allowed the reduction of DMSO to 3.3% while maintaining good cryoprotection.
Spermatozoa
Spermatozoa are distinct from other cell types in that they have a much larger surface area to volume ratio, have a more densely packed cytoplasm, and contain less freezable water, which makes them less vulnerable to intracellular ice formation.44 Thus, optimal spermatozoa cryopreservation differs from that of more typical cells, with protocols often preferring fast freezing or vitrification to slow freezing.45 Trehalose has been added to the “freezing extender” (a term referring to a freezing media used for spermatozoa) and tested in the cryopreservation of spermatozoa from many different animals.Rabbit: spermatozoa vitrification with 4% DMSO was improved with the addition of 100 mM trehalose as measured by increased motility (43% vs. 25%), membrane integrity (45% vs. 24%) and mitochondrial membrane integrity (53% vs. 28%). Trehalose also decreased ROS and improved catalase function.46
Ram: post-thaw outcome was improved in multiple studies, with the optimal trehalose concertation typically being 50–100 mM.47–53 Some studies found that higher concentrations of around 200 mM are ineffective,54,55 while others have found positive effects at higher concentrations.56,57 These differences may be due to the total osmolality of the solution; in an extender with a high initial osmolality, less trehalose can be added before the total osmolality becomes too high and dehydrates the cells. Trehalose has also been combined with low-density lipoprotein for use as an extender.58
Equine: trehalose has had mixed results in equine spermatozoa, one study reported a benefit to using a small amount of trehalose.59 Other studies reported that trehalose does not improve post-thaw outcome.60–62
Other animals: trehalose also improved cryopreservation outcomes for the spermatozoa of goats,63,64 carp,65 and oysters.66 Trehalose did not provide benefit for the cryopreservation of European brown hare spermatozoa.67
Tissue cryopreservation
Tissue cryopreservation is significantly more challenging than for individual cells (or e.g. spheroids) for several reasons. The large volume to surface area to ratio makes it difficult for a cryoprotectant to homogeneously permeate the tissue, and to ensure a uniform cooling rate. Extracellular ice formation is particularly damaging to the tissue macrostructure – unlike individual cells which can move freely within the media, allowing them to fit within the unfrozen portion of the sample. Several studies have found a cryoprotective benefit to using trehalose: in one study, the viability of adipose tissue was increased by 80% through the addition of 250 mM trehalose to an unspecified standard freezing media.68 In another study, the addition of 500 mM trehalose to 10% DMSO increased the post-thaw live cell count of human foetal skin (65% vs. 44%), improved post-thaw morphology, graft necrosis, and shrinking.69 Trehalose can also reduce post-thaw tissue pigmentation.70 Dog tracheas have been preserved for transplant in 10% DMSO and 100 mM trehalose, resulting in a 100% animal and graft survival rate (n = 6) after 184 days when transplanted. This study did not report cell recovery or viability.71 The viability of bovine calf testicular tissue, cryopreserved with 10% DMSO, was higher with increasing trehalose concentrations of up to 438 mM.72Other cell types
Human: viability of hepatocytes was improved (63% vs. 47%) with the addition of 200 mM trehalose to 10% DMSO.73 Trehalose and salidroside increased the survival of red blood cells (RBCs) after cryopreservation. However, adding salidroside to glycerol is slightly more effective than replacing glycerol with trehalose 61% vs. 56% respectively.73 Trehalose and catalase protect the proteins on the surface of human hematopoietic cells.74Non-human: in murine embryos, 1.5 M glycerol and 100 mM trehalose was the optimal cryoprotectant combination for freezing. Interestingly at 2.0 M glycerol, increasing trehalose concentration increases viability in a dose-dependent manner, even beyond the optimal trehalose concentration at optimal and suboptimal glycerol concentrations. This suggests that glycerol prevents damage by preventing dehydration damage, and/or trehalose protects cells from glycerol toxicity.75 For Trypanosoma brucei, studies have found that 400 mM trehalose in host blood, or 200 mM trehalose in vitro, without DMSO or glycerol, is optimal for cryopreservation. In these studies, the replacement of 7.5% DMSO with trehalose alone resulted in drastic increases in survival rate, depending on the form of the trypanosome.76,77 In porcine spermatogonia stem cells, 200 mM trehalose alone, compared to 10% DMSO, did not significantly increase the recovery of testis cells, but did increase proliferation capacity and the recovery of germ cells from thawed testis cells and tissue.78 In ecto-mycorrhizal basidiomycetes, trehalose addition to DMSO improves post-thaw viability of most sub-types.79 Finally, trehalose has been used to cryopreserve multiple cell types by pre-freeze dehydration.80
However, there are a number of ways in which trehalose appears to be a better cryoprotectant than sucrose: in solution, trehalose forms stronger hydrogen bonds with water than sucrose,87 and becomes associated with more molecules of unfrozen water, in this way each molecule of trehalose is able to disrupt the hydrogen bonding network of a greater number of water molecules, preventing them from forming ice.88,89 Trehalose has superior ice growth inhibiting properties compared with sucrose.90 Unlike sucrose, trehalose is able to displace water bound to the carbonyl groups of phospholipid membranes, displacing more water.91 Trehalose has a larger hydrated volume than sucrose, which is correlated with protein protection, and more sucrose is needed to provide the same level of protein protection as trehalose.21,92 Trehalose and sucrose cause a dynamic slowing effect on the surrounding water network, this effect is stronger in trehalose.93 Some of these effects may occur because sucrose has more intramolecular hydrogen bonding, resulting in fewer and/or weaker intermolecular hydrogen bonds to interact with water.88 Trehalose solutions have a higher glass transition temperature than sucrose solutions,94,95 however, a lower concentration of sucrose is needed to vitrify in aqueous solution at any given cooling rate.96 During freeze-drying where water content is very low and samples are stored at room temperature, trehalose would be the superior stabiliser as it would be more likely to remain above its glass transition temperature, even if it absorbed a small amount of water. Conversely, during cryopreservation, sucrose may be more useful (at least with regard to its vitrifying properties) in both vitrification, and in slow freezing where vitrification occurs due to freeze concentration. Sucrose also has greater solubility in water,97 making it less likely to precipitate out of solution at high concentrations and low temperatures. In some organisms that use trehalose as a cryoprotectant, antifreeze proteins may be used to mitigate this solubility problem.98 A summary of the comparative advantages of trehalose and sucrose can be seen in Table 1. While trehalose is – in theory – a better cryoprotectant than other sugars, the results of experimental cryopreservation studies where trehalose has been directly compared to other sugars are mixed. Discussed below are cryopreservation studies where trehalose has been tested and found to be a better, worse, or equivalent cryoprotectant compared to other sugars.
| Advantages of trehalose | Advantages of sucrose |
|---|---|
| Higher Tg at any given concentration (106 °C vs. 60 °C for the pure sugars)95 | Lower Cv at any given volume (74% vs. 58% at 20 μl)96 |
| Higher hydration number (4.598 vs. 4.127 at 66% w/v)88 | Greater solubility (68.1% vs. 52.3% at 30 °C)97 |
| Larger hydrated volume (62.5% vs. 87% water for a 1.5 M solution)21 | Low concentration required to vitrify at a given cooling rate96 |
| Superior ice growth inhibiter (“approximately twice as effective”)90 | — |
| Displace water bound to the carbonyl groups of phospholipid membranes91 | — |
| Causes more dynamic slowing of the surrounding water network93 | — |
| More effective for protein stabilisation92 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, at a total concentration 112.5 mM. This gave a post-thaw survival of 51%.105 Inositol is a non-reducing monosaccharide that can confer freeze tolerance to cricket cells, but not to crickets, while trehalose can confer freeze tolerance to both cricket cells and to whole crickets.106 Inositol is inferior to trehalose for protection during lyophilisation.107
1 ratio, at a total concentration 112.5 mM. This gave a post-thaw survival of 51%.105 Inositol is a non-reducing monosaccharide that can confer freeze tolerance to cricket cells, but not to crickets, while trehalose can confer freeze tolerance to both cricket cells and to whole crickets.106 Inositol is inferior to trehalose for protection during lyophilisation.107
Trehalose as an intracellular cryoprotectant
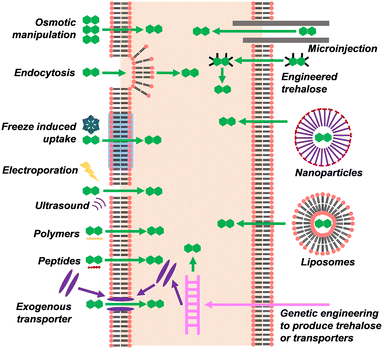
As discussed above in the context of cryopreservation performance, trehalose is most effective when it is in both the intracellular and extracellular space.116–118 In the intracellular space, it is able to inhibit intracellular ice, prevent cellular dehydration caused by both extracellular ice and extracellular cryoprotectants, and stabilise intracellular proteins. However, trehalose is a polar molecule, and does not cross the cell membrane by diffusion, nor are there any trehalose-specific transporters in most mammalian cells (e.g. a non-penetrating cryoprotectant). Various methods have been used to load trehalose into the intracellular space of cells which do not naturally produce it namely; electroporation, membrane permeabilising polymers, genetic engineering, microinjection, nanoparticles, liposomes, freeze-induced uptake, molecular engineering, and natural endocytosis through preincubation (Fig. 5). For an in-depth review of the topic, see Stewart and He.119 The ideal method for trehalose permeation would have low cytotoxicity, not be time-consuming or labour-intensive, and would allow for a high enough concertation of trehalose to be loaded that it could entirely replace penetrating cryoprotectants.Preincubation, fluid-phase endocytosis, and osmotic manipulation
Fluid-phase endocytosis, or “cellular drinking” is where cells internalise the surrounding fluid using vesicles. This allows for the non-specific uptake of molecules from the surrounding environment. In this way, cells can be slowly loaded with trehalose by incubating them in a trehalose solution for a number of hours. Trehalose pre-incubation was first used in carrot and tobacco cells where it allowed for trehalose to be used as the sole cryoprotectant.120,121 This process is slow – cells have to be incubated for up to 24 hours before freezing – and the maximum concentration of trehalose that can be loaded is limited. Preincubation with trehalose has been used to improve the cryosurvival of mesenchymal stem cells,122 and is more effective than simply adding trehalose to the cryopreservation media without preincubation.123 In Stokich et al.124 human hepatocyte cell monolayer recovery was improved by a 24-hour preincubation with 100 mM trehalose in addition to the standard 10% DMSO, resulting in 39% and 10% viability respectively. In Campbell et al.125 preincubation with 200 mM trehalose before preservation with 400 mM trehalose greatly increased metabolic activity measured 7 days after thawing in bovine endothelial cells. In Hara et al.126 1.3 M trehalose in the absence of solvent increased proliferation compared to glycerol for human embryonic kidney cells (36% vs. 10%). For cattle ovarian granulosa, a 30-minute preincubation with 200–400 mM trehalose, before flash freezing with trehalose + 5% DMSO and 5% EG, greatly improved viability (70% vs. 15%).127 It is possible to force trehalose to cross the cell membrane by creating large osmotic pressure differences between the intracellular and extracellular spaces. However, it only allows for small amounts of trehalose to be loaded and it can damage cells. This has been attempted in red blood cells, allowing for up to 43 mM trehalose to be loaded.128,129 This has also been attempted in T-lymphocytes, resulting in 2.2 mM being loaded.130Freeze-induced uptake
When trehalose is simply added to the extracellular space immediately prior to freezing, it can be mistakenly considered to only be an extracellular cryoprotectant. However, the phase change in the cell membrane during freezing, combined with freezing-induced osmotic effects and membrane damage, allows trehalose and other small molecules to enter the cell. The amount of trehalose loaded by freeze-induced uptake increases with freezing rate, resulting in a faster optimal cooling rate for cryoprotection from freeze-induced uptake than with DMSO.131 Beattie et al.132 was one of the earliest studies using intracellular trehalose to preserve mammalian cells. Here, human pancreatic islets were cryopreserved with 2 M DMSO + 300 mM trehalose, which resulted in improved cell recovery compared to 2 M DMSO + 11.1 mM glucose (94% vs. 58%). Insulin production was also greatly improved. Intracellular trehalose was measured and was found to increase as temperature was lowered. The threshold temperature for freeze-induced uptake appears to be 15 °C, as only very small amounts of intracellular trehalose were detected before this temperature was reached. In mouse neuroblastoma cells, both preincubation and freezing with trehalose contribute to increased cell recovery. Trehalose positively combines with L-proline at equal total concentration, even when L-proline was simply added to the extracellular freezing media, suggesting that L-proline protects cells through a mechanism other than inducing freeze tolerant metabolism.105 In Zhang et al.133 a 500 mM extracellular trehalose concentration was optimal for mouse embryonic fibroblasts, resulting in 50% survival. 160 mM trehalose could be detected inside cells after preservation. Preincubation with 25 mM trehalose had no positive effect. Freeze-induced uptake may also occur in platelets134 and spermatozoa.135Electroporation
Electroporation is the application of an electric current to cells which causes the formation of pores, resulting in a temporary increase in membrane permeability. In Shirakashi et al.136 mouse myeloma cells were loaded with 100 mM trehalose from an extracellular trehalose concertation of 290 mM. Higher intracellular concentrations were achievable but required damaging voltages to be used. In Dovgan et al.137 human umbilical stem cells were electroporated and incubated with 200 mM trehalose for 25 minutes, which resulted in improved cell recovery relative to 10% DMSO (86% vs. 60%). For human adipose-derived stem cells, electroporation with trehalose increased recovery compared to trehalose incubation, as measured by trypan blue excision but not when measured by MTT assay.138Polymers
A polymer known as ‘PP-50’ (poly(L-lysine iso-phthalamide) grafted with L-phenylalanine) has been used to increase membrane permeability in a reversible pH-responsive manner.139 It can be used to permeabilise human red blood cells to trehalose resulting in 251 mM trehalose uptake from 700 mM solution after a 9 h incubation period. In ovine red blood cells, PP-50 allowed 123 mM trehalose loading from a 360 mM extracellular solution, with a 9-hour incubation. This resulted in 83% survival between the end of incubation and thawing. However, PP-50 causes some haemolysis, and this was not accounted for in the post-thaw haemolysis lysis assay, as RBCs were washed after incubation, which removed free haemoglobin. Elsewhere in the paper, it was found that 22% haemolysis occurs during loading, resulting in a probable final survival of around 61%. So, while this shows that the level of trehalose loading archivable with PP-50 greatly improves survival, it does not present PP-50 as a good way of achieving it. Nonetheless, PP-50 can be used to increase cryosurvival with trehalose uptake.116 The PP-50 trehalose combination was also used to improve the cryosurvival of osteosarcoma cells, although does not result in better cryosurvival than that with DMSO.140,141Carrier peptides
A cargo peptide was developed by Wei et al. that non-covalently binds to trehalose and carries it across the cell membrane. The peptide was non-toxic, but only very small amounts of trehalose could be loaded into the cell.142Gene expression for trehalose synthesis
Trehalose can be synthesised from glucose using trehalose 6-phosphate synthase and trehalose 6-phosphate phosphatase. Cells can be transfected with genes that encode these enzymes, but the intracellular trehalose concentration that can be achieved is low.143,144Transporter proteins and channels
Most cell types have an endogenous receptor called P2Z (or P2X7) which forms pores when ATP is present extracellularly. In human hematopoietic stem cells, it allowed for 200 mM trehalose to be loaded from 200 mM extracellular trehalose after a 60-minute incubation period, resulting in 91% post-thaw viability as measured by proliferation. This is compared to 11% viability after freezing with 200 mM extracellular trehalose alone.117 Other pores and channels can be added artificially. For example, cells can be genetically engineered to express the trehalose transporter TRET1. In Chinese hamster ovary cells, this allowed 23 mM trehalose uptake after incubation in 400 mM trehalose for 4 hours. This did not affect the cells' proliferation.145 This technique was later used to protect Chinese hamster ovary cells during cryopreservation.146 H5 is a pore-forming agent which has been engineered from α-haemolysin. H5 forms pores in the cell membrane which can be opened and closed by altering zinc ion concentration. This allowed for 80% cryosurvival in murine fibroblasts, 70% cryosurvival for human keratinocytes,147 and 92% post-thaw viability for human hemopoietic stem cells.148 This approach allows for high concentrations of trehalose to be loaded quickly, but the potential of H5 to cause an immune response may limit its use in cells that are to be used clinically.Microinjection
Microinjection allows for small volumes of liquid to be directly introduced into the intracellular space. It allows very high concentrations of trehalose to be loaded but it poses challenges when used on cells which are smaller than oocytes. In Eroglu et al.149 human oocytes were microinjected with 150 mM trehalose and frozen in 500 mM trehalose, resulting in 66% survival. In a later study, mouse oocytes were injected with 500 mM trehalose and frozen in 500 mM trehalose solution, giving 80% survival. Using 500 mM extracellular trehalose only gave just 15% survival. Reducing intracellular trehalose concentration to 80 mM and adding 500 mM DMSO (4% w/v) resulted in 97% survival. These oocytes were able to develop into embryos at the highest rate. One explanation for the increased cryosurvival in the presence of DMSO, is that DMSO crosses intracellular membranes and enter the organelles.150Nanoparticles
Nanoparticles can encapsulate trehalose and deliver it into the cell. Thermally responsive nanoparticles can be used to load up to 300 mM trehalose into murine fibroblasts, from a 40 minute incubation followed by cold shock.151 In human adipose-derived stem cells, 24-hour incubation with pH-responsive nanoparticles and 200 mM extracellular trehalose allowed for a post-thaw viability equivalent to that given by 10% DMSO.152 Nanoparticles that increase membrane permeability have been used to load 51 mM trehalose into red blood cells from 350 mM extracellular trehalose, after a 7 hour incubation period, resulting in 91% post-thaw survival.153Liposomes
Holovati and Acker154 developed liposomes that can be used to deliver trehalose into RBCs. These liposomes were used to carry a small volume of 300 mM trehalose and were incubated with red blood cells for 4 hours (for an estimated total of 15 mM intracellular trehalose), then frozen with 300 mM trehalose. This resulted in 67% post-thaw recovery, compared to 27% recovery when incubated in extracellular trehalose alone. The concentration of intracellular trehalose alone provided minimal cryoprotection, but surprisingly the liposomes themselves provided a very large degree of cryoprotection; cells incubated with liposomes containing no trehalose and frozen in 300 mM extracellular trehalose gave 60% recovery.155 The ability of liposomes to improve cryopreservation could cause misleading results when using them to deliver cryoprotectants. For example, Motta et al.156 used liposomes to assess the effect of intracellular and extracellular trehalose on umbilical cord blood stem cell preservation but did not control for the effects of the liposomes themselves. However, this cryoprotective ability is useful in its own right, and could be used as part of a multi-component cryoprotectant solution to deliver low concentrations of non-penetrating cryoprotectants such as antifreeze proteins. In Stoll et al.157 liposomes allowed for up to 80 mM trehalose to be loaded into human RBCs, dependent on liposome concentration. This slightly increased post-thaw recovery, but post-thaw recovery did not increase with increasing liposome concentration above 1 mM, which was the lowest liposome concentration used.Ultrasound
In Zhang et al.158 ultrasound was used to induce non-specific permeability in human platelets. This technique allowed only 30 mM of trehalose to enter the cell after 30 minutes of treatment, which is insufficient for cryoprotection. This method also altered platelet morphology.Modified trehalose
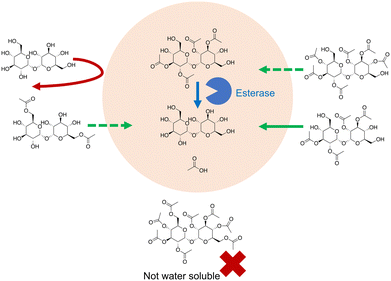
Trehalose itself can be chemically modified so that it can penetrate the cell membrane (Fig. 6). In Abazari et al.159 trehalose was acetylated at six of its hydroxyl groups, greatly increasing its hydrophobicity. Rat hepatocytes were incubated with 30 mM acetylated trehalose, resulting in an intracellular acetylated trehalose concentration of 80 mM within 1 hour, and of up to 300 mM after 8 hours. Trehalose concentration lagged behind this with a concentration of around 100 mM after 12 hours.Trehalose removal
While trehalose has almost no direct toxicity at concentrations required for cryopreservation, trapped intracellular trehalose can cause osmotic damage when trehalose is removed from the extracellular solution.131 However, after thawing, trehalose will be eliminated from cells due to cell division, and possibly by exocytosis and/or conversion to glucose.160–162 Therefore, to maximise cell survival, the osmolarity of the recovery media can be progressively lowered to maintain osmotic balance, although this strategy is labour-intensive. Alternatively, using a lower intracellular concentration of trehalose in conjunction with a penetrating cryoprotectant can mitigate osmotic damage.150Conclusions
This review has summarised the broad scope of trehalose in cryopreservation, highlighting specific areas where there were successes and also where the benefits were limited. We have also gathered the literature on solutions to overcome the limitations of trehalose's low membrane permeability, to show where chemistry can be deployed to delivery this inside cells: adapting trehalose to become a penetrating cryoprotectant. Whilst trehalose clearly shows benefits when added to the extracellular media, the delivery question challenge remains open to innovative solutions which may enable the reduction or removal of the need for conventional penetrating cryoprotectants (such as DMSO) whilst ensuring that post-thaw cells are viable and functional. With the rapid growth of cell-based therapies and biotechnology process, the need for tools to bank, transport and deliver cryopreserved cells (and other biological components) has never been higher. Any emerging tools will all need critical evaluation of the price, biocompatibility, and accessibility, compared to e.g. DMSO which is low cost, widely available and provides consistent results.Data availability
This is a review article and no new data was generated.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program grant agreement 866056. M. I. G. thanks the Royal Society for an Industry Fellowship (191037) joint with Cytiva. The Biotechnology and Biological Sciences Research Council (BBSRC) and the University of Warwick funded Midlands Integrative Biosciences Training Partnership (MIBTP) [grant number BB/M01116X/1] and Cytiva are thanked for support to A. M. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author-Accepted Manuscript version arising from this submission.Notes and references
- J. Deng, D. R. Davies, G. Wisedchaisri, M. Wu, W. G. J. Hol and C. Mehlin, An Improved Protocol for Rapid Freezing of Protein Samples for Long-Term Storage, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2004, 60(1), 203–204 CrossRef PubMed
.
- Y. Miyamoto, M. Ikeuchi, H. Noguchi and S. Hayashi, Long-Term Cryopreservation of Human and Other Mammalian Cells at −80 °C for 8, Cell Med., 2018, 10, 215517901773314 CrossRef
.
- A. Arav, O. Friedman, Y. Natan, E. Gur and N. Shani, Rat Hindlimb Cryopreservation and Transplantation: A Step Toward “Organ Banking”, Am. J. Transplant., 2017, 17(11), 2820–2828 CrossRef CAS PubMed
.
- V. Isachenko, R. Dittrich, G. Keck, E. Isachenko, G. Rahimi, H. Van Der Ven, M. Montag, I. Hoffmann, A. Müller, W. Distler, M. W. Beckmann and P. Mallmann, Cryopreservation of Ovarian Tissue: Detailed Description of Methods for Transport, Freezing and Thawing, Geburtshilfe Frauenheilkd., 2012, 72(10), 927–932 CrossRef CAS PubMed
.
- D. J. A. Crommelin, T. J. Anchordoquy, D. B. Volkin, W. Jiskoot and E. Mastrobattista, Addressing the Cold Reality of MRNA Vaccine Stability, J. Pharm. Sci., 2021, 110(3), 997–1001 CrossRef CAS PubMed
.
- Z. Gavish, M. Ben-Haim and A. Arav, Cryopreservation of Whole Murine and Porcine Livers, Rejuvenation Res., 2008, 11(4), 765–772 CrossRef PubMed
.
- G. M. Fahy, B. Wowk, R. Pagotan, A. Chang, J. Phan, B. Thomson and L. Phan, Physical and Biological Aspects of Renal Vitrification, Organogenesis, 2009, 5(3), 167 CrossRef PubMed
.
- D. Berz, E. M. McCormack, E. S. Winer, G. A. Colvin and P. J. Quesenberry, Cryopreservation of Hematopoietic Stem Cells, Am. J. Hematol., 2007, 82(6), 463 CrossRef
.
- J. W. Lagerberg, Cryopreservation of Red Blood Cells, Methods Mol. Biol., 2015, 1257, 353–367 CrossRef CAS PubMed
.
- Z. Shu, S. Heimfeld and D. Gao, Hematopoietic SCT with Cryopreserved Grafts: Adverse Reactions after Transplantation and Cryoprotectant Removal before Infusion, Bone Marrow Transplant., 2014, 49(4), 469–476 CrossRef CAS PubMed
.
- N. Teramoto, N. D. Sachinvala and M. Shibata, Trehalose and Trehalose-Based Polymers for Environmentally Benign, Biocompatible and Bioactive Materials, Molecules, 2008, 13(8), 1773–1816 CrossRef CAS PubMed
.
- K. Y. Lee, Y. D. Chang and Y. G. Kim, Trehalose, a Major Sugar Cryoprotectant of the Overwintering Rice Water Weevil, Lissorhoptrus Oryzophilus (Coleoptera: Curculionidae), J. Asia-Pac. Entomol., 2002, 5(1), 35–41 CrossRef
.
- J. Rozsypal, V. Koštál, H. Zahradníčková and P. Šimek, Overwintering Strategy and Mechanisms of Cold Tolerance in the Codling Moth (Cydia Pomonella), PLoS One, 2013, 8(4), e61745 CrossRef CAS
.
- S. Hengherr, A. G. Heyer, H. R. Köhler and R. O. Schill, Trehalose and Anhydrobiosis in Tardigrades – Evidence for Divergence in Responses to Dehydration, FEBS J., 2008, 275(2), 281–288 CrossRef CAS
.
- A. B. Richards, S. Krakowka, L. B. Dexter, H. Schmid, A. P. M. Wolterbeek, D. H. Waalkens-Berendsen, A. Shigoyuki and M. Kurimoto, Trehalose: A Review of Properties, History of Use and Human Tolerance, and Results of Multiple Safety Studies, Food Chem. Toxicol., 2002, 40(7), 871–898 CrossRef CAS PubMed
.
- P. Mazur and K. W. Cole, Roles of Unfrozen Fraction, Salt Concentration, and Changes in Cell Volume in the Survival of Frozen Human Erythrocytes, Cryobiology, 1989, 26(1), 1–29 CrossRef CAS
.
- L. M. Crowe, D. S. Reid and J. H. Crowe, Is Trehalose Special for Preserving Dry Biomaterials?, Biophys. J., 1996, 71(4), 2087 CrossRef CAS PubMed
.
- C. Branca, S. MacCarrone, S. Magazu, G. Maisano, S. M. Bennington and J. Taylor, Tetrahedral Order in Homologous Disaccharide-Water Mixtures, J. Chem. Phys., 2005, 122, 174513 CrossRef CAS
.
- C. Branca, S. Magazù, G. Maisano and P. Migliardo, α,α-Trehalose−Water Solutions. 3. Vibrational Dynamics Studies by Inelastic Light Scattering, J. Phys. Chem. B, 1999, 103(8), 1347–1353 CrossRef CAS
.
- Q. Liu and J. W. Brady, Anisotropic Solvent Structuring in Aqueous Sugar Solutions, J. Am. Chem. Soc., 1996, 118(49), 12276–12286 CrossRef CAS
.
- N. K. Jain and I. Roy, Effect of Trehalose on Protein Structure, Protein Sci., 2009, 18(1), 24 CrossRef CAS
.
- J. Solocinski, Q. Osgood, M. Wang, A. Connolly, M. A. Menze and N. Chakraborty, Effect of Trehalose as an Additive to Dimethyl Sulfoxide Solutions on Ice Formation, Cellular Viability, and Metabolism, Cryobiology, 2017, 75, 134–143 CrossRef CAS
.
- R. D. Lins, C. S. Pereira and P. H. Hünenberger, Trehalose-Protein Interaction in Aqueous Solution, Proteins, 2004, 55(1), 177–186 CrossRef CAS
.
-
J. H. Crowe, J. S. Clegg and L. M. Crowe, Anhydrobiosis: The Water Replacement Hypothesis, The Properties of Water in Foods ISOPOW, 1998, vol. 6, pp. 440–455 Search PubMed
.
- X. Tang and M. J. Pikal, The Effect of Stabilizers and Denaturants on the Cold Denaturation Temperatures of Proteins and Implications for Freeze-Drying, Pharm. Res., 2005, 22(7), 1167–1175 CrossRef CAS
.
- P. Boutron and J. F. Peyridieu, Reduction in Toxicity for Red Blood Cells in Buffered Solutions Containing High Concentrations of 2,3-Butanediol by Trehalose, Sucrose, Sorbitol, or Mannitol, Cryobiology, 1994, 31(4), 367–373 CrossRef CAS PubMed
.
- S. B. Leslie, S. A. Teter, L. M. Crowe and J. H. Crowe, Trehalose Lowers Membrane Phase Transitions in Dry Yeast Cells, Biochim. Biophys. Acta, Biomembr., 1994, 1192(1), 7–13 CrossRef CAS PubMed
.
- O. Kandror, A. DeLeon and A. L. Goldberg, Trehalose Synthesis Is Induced upon Exposure of Escherichia Coli to Cold and Is Essential for Viability at Low Temperatures, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(15), 9727–9732 CrossRef CAS PubMed
.
- A. S. Rudolph, J. H. Crowe and L. M. Crowe, Effects of Three Stabilizing Agents—Proline, Betaine, and Trehalose—on Membrane Phospholipids, Arch. Biochem. Biophys., 1986, 245(1), 134–143 CrossRef CAS PubMed
.
- G. Chen, A. Yue, Z. Ruan, Y. Yin, R. Wang, Y. Ren and L. Zhu, Comparison of the Effects of Different Cryoprotectants on Stem Cells from Umbilical Cord Blood, Stem Cells Int., 2016, 2016, 1396783 Search PubMed
.
- J. P. Rodrigues, F. H. Paraguassú-Braga, L. Carvalho, E. Abdelhay, L. F. Bouzas and L. C. Porto, Evaluation of Trehalose and Sucrose as Cryoprotectants for Hematopoietic Stem Cells of Umbilical Cord Blood, Cryobiology, 2008, 56(2), 144–151 CrossRef CAS
.
- L. L. Q. Pu, X. Cui, B. F. Fink, M. L. Cibull and D. Gao, Long-Term Preservation of Adipose Aspirates after Conventional Lipoplasty, Aesthetic Surg. J., 2004, 24(6), 536–541 CrossRef
.
- L. M. D. F. Cardoso, M. A. Pinto, A. Henriques Pons and L. A. Alves, Cryopreservation of Rat Hepatocytes with Disaccharides for Cell Therapy, Cryobiology, 2017, 78, 15–21 CrossRef CAS PubMed
.
- Y. Z. Wen, B. X. Su, S. S. Lyu, G. Hide, Z. R. Lun and D. H. Lai, Trehalose, an Easy, Safe and Efficient Cryoprotectant for the Parasitic Protozoan Trypanosoma Brucei, Acta Trop., 2016, 164, 297–302 CrossRef CAS PubMed
.
- D. Martinetti, C. Colarossi, S. Buccheri, G. Denti, L. Memeo and L. Vicari, Effect of Trehalose on Cryopreservation of Pure Peripheral Blood Stem Cells, Biomed. Rep., 2017, 6(3), 314 CrossRef CAS
.
- Y.-A. Lee, Y.-H. Kim, B.-J. Kim, B.-G. Kim, K.-J. Kim, J.-H. Auh, J. A. Schmidt and B.-Y. Ryu, Cryopreservation in Trehalose Preserves Functional Capacity of Murine Spermatogonial Stem Cells, PLoS One, 2013, 8(1), e54889 CrossRef CAS
.
- H. Gurruchaga, J. Ciriza, L. Saenz Del Burgo, J. R. Rodriguez-Madoz, E. Santos, F. Prosper, R. M. Hernández, G. Orive and J. L. Pedraz, Cryopreservation of Microencapsulated Murine Mesenchymal Stem Cells Genetically Engineered to Secrete Erythropoietin, Int. J. Pharm., 2015, 485(1–2), 15–24 CrossRef CAS
.
- Y. Liu, X. Xu, X. Ma, J. Liu and Z. Cui, Effect of Various Freezing Solutions on Cryopreservation of Mesenchymal Stem Cells from Different Animal Species, CryoLetters, 2011, 32(5), 425–435 CAS
.
- A. Ntai, A. La Spada, P. De Blasio and I. Biunno, Trehalose to Cryopreserve Human Pluripotent Stem Cells, Stem Cell Res., 2018, 31, 102–112 CrossRef CAS PubMed
.
- X. B. Zhang, K. Li, K. H. Yau, K. S. Tsang, T. F. Fok, C. K. Li, S. M. Lee and P. M. P. Yuen, Trehalose Ameliorates the Cryopreservation of Cord Blood in a Preclinical System and Increases the Recovery of CFUs, Long-Term Culture-Initiating Cells, and Nonobese Diabetic-SCID Repopulating Cells, Transfusion, 2003, 43(2), 265–272 CrossRef CAS PubMed
.
- L. S. Limaye and V. P. Kale, Cryopreservation of Human Hematopoietic Cells with Membrane Stabilizers and Bioantioxidants as Additives in the Conventional Freezing Medium, J. Hematother. Stem Cell Res., 2001, 10(5), 709–718 CrossRef CAS
.
- C. Scheinkönig, S. Kappicht, H. J. Kolb and M. Schleuning, Adoption of Long-Term Cultures to Evaluate the Cryoprotective Potential of Trehalose for Freezing Hematopoietic Stem Cells, Bone Marrow Transplant., 2004, 34(6), 531–536 CrossRef
.
- K. W. Yong, B. Pingguan-Murphy, F. Xu, W. A. B. W. Abas, J. R. Choi, S. Z. Omar, M. A. N. Azmi, K. H. Chua and W. K. Z. W. Safwani, Phenotypic and Functional Characterization of Long-Term Cryopreserved Human Adipose-Derived Stem Cells, Sci. Rep., 2015, 5, 9596 CrossRef PubMed
.
- G. J. Morris, Rapidly Cooled Human Sperm: No Evidence of Intracellular Ice Formation, Hum. Reprod., 2006, 21(8), 2075–2083 CrossRef CAS
.
- E. Isachenko, V. Isachenko, I. I. Katkov, S. Dessole and F. Nawroth, Vitrification of Mammalian Spermatozoa in the Absence of Cryoprotectants: From Past Practical Difficulties to Present Success, Reprod. BioMed. Online, 2003, 6(2), 191–200 CrossRef
.
- Z. Zhu, X. Fan, Y. Pan, Y. Lu and W. Zeng, Trehalose Improves Rabbit Sperm Quality during Cryopreservation, Cryobiology, 2017, 75, 45–51 CrossRef CAS
.
- Ü. Cirit, H. Baǧiş, K. Demir, C. Agca, S. Pabuccuoǧlu, Ö. Varişli, C. Clifford-Rathert and Y. Agca, Comparison of Cryoprotective Effects of Iodixanol, Trehalose and Cysteamine on Ram Semen, Anim. Reprod. Sci., 2013, 139(1–4), 38–44 CrossRef PubMed
.
- E. G. Aisen, V. H. Medina and A. Venturino, Cryopreservation and Post-Thawed Fertility of Ram Semen Frozen in Different Trehalose Concentrations, Theriogenology, 2002, 57(7), 1801–1808 CrossRef CAS
.
- E. G. Aisen, V. H. Medina and A. Venturino, Cryopreservation and Post-Thawed Fertility of Ram Semen Frozen in Different Trehalose Concentrations, Theriogenology, 2002, 57(7), 1801–1808 CrossRef CAS PubMed
.
- R. F. Bittencourt, E. Oba, C. E. de Almeida Biscarde, H. C. Azevedo, M. V. Bittencourt, G. F. O. de Menezes, A. da Silva Lima, K. da Mata Fuchs and A. de Lisboa Ribeiro Filho, Dimethylacetamide and Trehalose for Ram Semen Cryopreservation, Cryobiology, 2018, 85, 1–6 CrossRef CAS PubMed
.
- F. Berlinguer, G. G. Leoni, S. Succu, F. Mossa, M. Galioto, M. Madeddu and S. Naitana, Cryopreservation of European Mouflon (Ovis Gmelini Musimon) Semen during the Non-Breeding Season Is Enhanced by the Use of Trehalose, Reprod. Domest. Anim., 2007, 42(2), 202–207 CrossRef CAS PubMed
.
- E. Aisen, M. Quintana, V. Medina, H. Morello and A. Venturino, Ultramicroscopic and Biochemical Changes in Ram Spermatozoa Cryopreserved with Trehalose-Based Hypertonic Extenders, Cryobiology, 2005, 50(3), 239–249 CrossRef CAS
.
- M. N. Bucak, A. Ateşşahin, Ö. Varişli, A. Yüce, N. Tekin and A. Akçay, The Influence of Trehalose, Taurine, Cysteamine and Hyaluronan on Ram Semen Microscopic and Oxidative Stress Parameters after Freeze-Thawing Process, Theriogenology, 2007, 67(5), 1060–1067 CrossRef CAS PubMed
.
- E. G. Aisen, H. L. Alvarez, A. Venturino and J. J. Garde, Effect of Trehalose and EDTA on Cryoprotective Action of Ram Semen Diluents, Theriogenology, 2000, 53(5), 1053–1061 CrossRef CAS PubMed
.
- S. S. Valente, R. M. Pereira, M. C. Baptista, C. C. Marques, M. I. Vasques, M. V. C. S. Pereira, A. E. M. Horta and J. P. Barbas, In Vitro and in Vivo Fertility of Ram Semen Cryopreserved in Different Extenders, Anim. Reprod. Sci., 2010, 117(1–2), 74–77 CrossRef CAS PubMed
.
- T. Matsuoka, H. Imai, H. Kohno and Y. Fukui, Effects of Bovine Serum Albumin and Trehalose in Semen Diluents for Improvement of Frozen-Thawed Ram Spermatozoa, J. Reprod. Dev., 2006, 52(5), 675–683 CrossRef CAS
.
- B. Akhtarshenas, H. Karami Shabankareh, H. Hajarian, M. N. Bucak, A. R. Abdolmohammadi and M. Dashtizad, The Protease Inhibitor Antipain Has a Beneficial Synergistic Effect with Trehalose for Ram Semen Cryopreservation, Reprod. Domest. Anim., 2018, 53(6), 1359–1366 CrossRef CAS PubMed
.
- R. A. Tonieto, K. L. Goularte, G. D. A. Gastal, R. S. Schiavon, J. C. Deschamps and T. Lucia, Cryoprotectant Effect of Trehalose and Low-Density Lipoprotein in Extenders for Frozen Ram Semen, Small Rumin. Res., 2010, 93(2–3), 206–209 CrossRef
.
- C. C. Pérez-Marín, F. D. Requena, A. Arando, S. Ortiz-Villalón, F. Requena and E. I. Agüera, Effect of Trehalose- and Sucrose-Based Extenders on Equine Sperm Quality after Vitrification: Preliminary Results, Cryobiology, 2018, 80, 62–69 CrossRef
.
- F. Vafaei, H. Kohram, A. Zareh-Shahne, E. Ahmad and A. Seifi-Jamadi, Influence of Different Combinations of Permeable and Nonpermeable Cryoprotectants on the Freezing Capacity of Equine Sperm, J. Equine Vet. Sci., 2019, 75, 69–73 CrossRef PubMed
.
- R. A. De Oliveira, S. Budik and C. Aurich, Influence of Partial or Total Replacement of Glycerol by Alternative Cryoprotectants in Ghent Freezing Extender on Post-Thaw Sperm Quality in Stallions, Reprod. Domest. Anim., 2017, 52(5), 715–721 CrossRef CAS
.
- E. L. Squires, S. L. Keith and J. K. Graham, Evaluation of Alternative Cryoprotectants for Preserving Stallion Spermatozoa, Theriogenology, 2004, 62(6), 1056–1065 CrossRef CAS
.
- B. Khalili, A. Farshad, M. J. Zamiri, A. Rashidi and P. Fazeli, Effects of Sucrose and Trehalose on the Freezability of Markhoz Goat Spermatozoa, Asian-Australas. J. Anim. Sci., 2009, 22(12), 1614–1619 CrossRef CAS
.
- E. M. E. Aboagla and T. Terada, Trehalose-Enhanced Fluidity of the Goat Sperm Membrane and Its Protection During Freezing, Biol. Reprod., 2003, 69(4), 1245–1250 CrossRef CAS PubMed
.
- R. Franěk, Z. Marinović, J. Lujić, B. Urbányi, M. Fučíková, V. Kašpar, M. Pšenička and Á. Horváth, Cryopreservation and Transplantation of Common Carp Spermatogonia, PLoS One, 2019, 14(4), e0205481 CrossRef PubMed
.
- L. Lyons, R. Jerry and P. C. Southgate, Cryopreservation of Black-Lip Pearl Oyster (Pinctada Margaritifera, L.) Spermatozoa: Effects of Cryoprotectants on Spermatozoa Motility, J. Shellfish Res., 2005, 24(4), 1187–1190 Search PubMed
.
- R. Kozdrowski, The Effect of Trehalose on Post-Thaw Viability and Fertility of European Brown Hare (Lepus Europaeus Pallas, 1778) Spermatozoa, Anim. Reprod. Sci., 2009, 116(3–4), 326–334 CrossRef CAS PubMed
.
- L. L. Q. Pu, X. Cui, B. F. Fink, M. L. Cibull and D. Gao, Cryopreservation of Adipose Tissues: The Role of Trehalose, Aesthetic Surg. J., 2005, 25(2), 126–131 CrossRef CAS
.
- G. Erdag, A. Eroglu, J. R. Morgan and M. Toner, Cryopreservation of Fetal Skin Is Improved by Extracellular Trehalose, Cryobiology, 2002, 44(3), 218–228 CrossRef CAS PubMed
.
- X. L. Kang and H. Shen, Pigmentation of Skin Graft Is Improved by Cryopreservation of Human Skin with Trehalose, Kouqiang Hemian Waike Zazhi, 2012, 70(6), 1464–1472 Search PubMed
.
- H. Yokomise, K. Inui, H. Wada, S. Hasegawa, N. Ohno and S. Hitomi, Reliable Cryopreservation of Trachea for One Month in a New Trehalose Solution, J. Thorac. Cardiovasc. Surg., 1995, 110(2), 382–385 CrossRef CAS PubMed
.
- X. G. Zhang, Y. H. Wang, C. Han, S. Hu, L. Q. Wang and J. H. Hu, Effects of Trehalose Supplementation on Cell Viability and Oxidative Stress Variables in Frozen-Thawed Bovine Calf Testicular Tissue, Cryobiology, 2015, 70(3), 246–252 CrossRef CAS PubMed
.
- E. Katenz, F. W. R. Vondran, R. Schwartlander, G. Pless, X. Gong, X. Cheng, P. Neuhaus and I. M. Sauer, Cryopreservation of Primary Human Hepatocytes: The Benefit of Trehalose as an Additional Cryoprotective Agent, Liver Transpl., 2007, 13(1), 38–45 CrossRef
.
- L. M. Sasnoor, V. P. Kale and L. S. Limaye, Supplementation of Conventional Freezing Medium with a Combination of Catalase and Trehalose Results in Better Protection of Surface Molecules and Functionality of Hematopoietic Cells, J. Hematother. Stem Cell Res., 2003, 12(5), 553–564 CrossRef CAS PubMed
.
- T. E. Honadel and G. J. Killian, Cryopreservation of Murine Embryos with Trehalose and Glycerol, Cryobiology, 1988, 25(4), 331–337 CrossRef CAS
.
- H. Y. Wang, Y. Z. Wen, Z. R. Lun and S. S. Lu, Visual Observation of African Trypanosomes during Cryopreservation, Biopreserv. Biobanking, 2014, 12(4), 265–268 CrossRef CAS
.
- Y. Z. Wen, B. X. Su, S. S. Lyu, G. Hide, Z. R. Lun and D. H. Lai, Trehalose, an Easy, Safe and Efficient Cryoprotectant for the Parasitic Protozoan Trypanosoma Brucei, Acta Trop., 2016, 164, 297–302 CrossRef CAS PubMed
.
- Y. A. Lee, Y. H. Kim, S. J. Ha, K. J. Kim, B. J. Kim, B. G. Kim, S. H. Choi, I. C. Kim, J. A. Schmidt and B. Y. Ryu, Cryopreservation of Porcine Spermatogonial Stem Cells by Slow-Freezing Testis Tissue in Trehalose, J. Anim. Sci., 2014, 92(3), 984–995 CrossRef CAS PubMed
.
- M. Sato, S. Inaba, J. Sukenobe, T. Sasaki, R. Inoue, M. Noguchi and A. Nakagiri, A Modified Perlite Protocol with a Mixed Dimethyl Sulfoxide and Trehalose Cryoprotectant Improves the Viability of Frozen Cultures of Ectomycorrhizal Basidiomycetes, Mycologia, 2019, 111(1), 161–176 CrossRef CAS PubMed
.
- H. Huang, G. Zhao, Y. Zhang, J. Xu, T. L. Toth and X. He, Predehydration and Ice Seeding in the Presence of Trehalose Enable Cell Cryopreservation, ACS Biomater. Sci. Eng., 2017, 3(8), 1758–1768 CrossRef CAS
.
- J. O'Brien, Stability of Trehalose, Sucrose and Glucose to Nonenzymatic Browning in Model Systems, J. Food Sci., 1996, 61(4), 679–682 CrossRef
.
- Y. A. Lee, Y. H. Kim, S. J. Ha, B. J. Kim, K. J. Kim, M. S. Jung, B. G. Kim and B. Y. Ryu, Effect of Sugar Molecules on the Cryopreservation of Mouse Spermatogonial Stem Cells, Fertil. Steril., 2014, 101(4), 1165–1175 CrossRef CAS
.
- J. Gómez-Fernández, E. Gómez-Izquierdo, C. Tomás, E. Mocé and E. De Mercado, Effect of Different Monosaccharides and Disaccharides on Boar Sperm Quality after Cryopreservation, Anim. Reprod. Sci., 2012, 133(1–2), 109–116 CrossRef
.
- J. L. Chaytor, J. M. Tokarew, L. K. Wu, M. Leclre, R. Y. Tam, C. J. Capicciotti, L. Guolla, E. Von Moos, C. S. Findlay, D. S. Allan and R. N. Ben, Inhibiting Ice Recrystallization and Optimization of Cell Viability after Cryopreservation, Glycobiology, 2012, 22(1), 123–133 CrossRef CAS PubMed
.
- M. A. Bender, P. T. Tran and L. H. Smith, Preservation of Viable Bone Marrow Cells by Freezing, J. Appl. Physiol., 1960, 15, 520–524 CrossRef CAS PubMed
.
- R. C. Deller, T. Congdon, M. A. Sahid, M. Morgan, M. Vatish, D. A. Mitchell, R. Notman and M. I. Gibson, Ice Recrystallisation Inhibition by Polyols: Comparison of Molecular and Macromolecular Inhibitors and Role of Hydrophobic Units, Biomater. Sci., 2013, 1(5), 478–485 RSC
.
- S. Magazu, V. Villari, P. Migliardo, G. Maisano and M. T. F. Telling, Diffusive Dynamics of Water in the Presence of Homologous Disaccharides: A Comparative Study by Quasi Elastic Neutron Scattering. IV, J. Phys. Chem. B, 2001, 105(9), 1851–1855 CrossRef CAS
.
- A. Lerbret, P. Bordat, F. Affouard, Y. Guinet, A. Hédoux, L. Paccou, D. Prévost and M. Descamps, Influence of Homologous Disaccharides on the Hydrogen-Bond Network of Water: Complementary Raman Scattering Experiments and Molecular Dynamics Simulations, Carbohydr. Res., 2005, 340(5), 881–887 CrossRef CAS PubMed
.
- H. Kawai, M. Sakurai, Y. Inoue, R. Chûjô and S. Kobayashi, Hydration of Oligosaccharides: Anomalous Hydration Ability of Trehalose, Cryobiology, 1992, 29(5), 599–606 CrossRef CAS
.
- T. Sei, T. Gonda and Y. Arima, Growth Rate and Morphology of Ice Crystals Growing in a Solution of Trehalose and Water, J. Cryst. Growth, 2002, 240(1–2), 218–229 CrossRef CAS
.
- M. Del, F. Amalfa, A. M. Nuñez, S. Díaz, A. C. Biondi De Lopez and E. A. Disalvo, Effect of Trehalose and Sucrose on the Hydration and Dipole Potential of Lipid Bilayers, Biophys. J., 2000, 78(5), 2452 CrossRef
.
- M. Sola-Penna and J. R. Meyer-Fernandes, Stabilization against Thermal Inactivation Promoted by Sugars on Enzyme Structure and Function: Why Is Trehalose More Effective than Other Sugars?, Arch. Biochem. Biophys., 1998, 360(1), 10–14 CrossRef CAS
.
- K. Shiraga, A. Adachi, M. Nakamura, T. Tajima, K. Ajito and Y. Ogawa, Characterization of the Hydrogen-Bond Network of Water around Sucrose and Trehalose: Microwave and Terahertz Spectroscopic Study, J. Chem. Phys., 2017, 146(10), 105102 CrossRef
.
- L. L. Kuleshova, D. R. MacFarlane, A. O. Trounson and J. M. Shaw, Sugars Exert a Major Influence on the Vitrification Properties of Ethylene Glycol-Based Solutions and Have Low Toxicity to Embryos and Oocytes, Cryobiology, 1999, 38(2), 119–130 CrossRef CAS
.
- K. D. Roe and T. P. Labuza, Glass Transition and Crystallization of Amorphous Trehalose-Sucrose Mixtures, Int. J. Food Prop., 2005, 8(3), 559–574 CrossRef CAS
.
- V. Berejnov, N. S. Husseini, O. A. Alsaied and R. E. Thorne, Effects of Cryoprotectant Concentration and Cooling Rate on Vitrification of Aqueous Solutions, J. Appl. Crystallogr., 2006, 39(2), 244–251 CrossRef CAS
.
- A. M. Lammert, S. J. Schmidt and G. A. Day, Water Activity and Solubility of Trehalose, Food Chem., 1998, 61(1–2), 139–144 CrossRef CAS
.
- X. Wen, S. Wang, J. G. Duman, J. Fnu Arifin, V. Juwita, W. A. Goddard, A. Rios, F. Liu, S. K. Kim, R. Abrol, A. L. DeVries and L. M. Henling, Antifreeze Proteins Govern the Precipitation of Trehalose in a Freezing-Avoiding Insect at Low Temperature, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(24), 6683–6688 CrossRef CAS PubMed
.
- C. F. Wu, H. C. Tsung, W. J. Zhang, Y. Wang, J. H. Lu, Z. Y. Tang, Y. P. Kuang, W. Jin, L. Cui, W. Liu and Y. L. Cao, Improved Cryopreservation of Human Embryonic Stem Cells with Trehalose, Reprod. BioMed. Online, 2005, 11(6), 733–739 CrossRef PubMed
.
- F. De Lara Janz, A. De Aguiar Debes, R. De Cássia Cavaglieri, S. A. Duarte, C. M. Romão, A. F. Morón, M. Zugaib and S. P. Bydlowski, Evaluation of Distinct Freezing Methods and Cryoprotectants for Human Amniotic Fluid Stem Cells Cryopreservation, J. Biomed. Biotechnol., 2012, 2012, 649353 Search PubMed
.
- K. J. Kim, Y. A. Lee, B. J. Kim, Y. H. Kim, B. G. Kim, H. G. Kang, S. E. Jung, S. H. Choi, J. A. Schmidt and B. Y. Ryu, Cryopreservation of Putative Pre-Pubertal Bovine Spermatogonial Stem Cells by Slow Freezing, Cryobiology, 2015, 70(2), 175–183 CrossRef
.
- S. W. Lestari, K. F. Ilato, M. I. A. Pratama, N. N. Fitriyah, M. Pangestu, G. Pratama and R. Margiana, Sucrose “versus” Trehalose Cryoprotectant Modification in Oocyte Vitrification: A Study of Embryo Development, Biomed. Pharmacol. J., 2018, 11(1), 97–104 CAS
.
- M. Schulz, J. Risopatrón, G. Matus, E. Pineda, C. Rojas, V. Isachenko, E. Isachenko and R. Sánchez, Trehalose Sustains a Higher Post-Thaw Sperm Motility than Sucrose in Vitrified Human Sperm, Andrologia, 2017, 49(9), e12757 CrossRef PubMed
.
- M. Jafaroghli, B. Khalili, A. Farshad and M. J. Zamiri, The Effect of Supplementation of Cryopreservation Diluents with Sugars on the Post-Thawing Fertility of Ram Semen, Small Rumin. Res., 2011, 96(1), 58–63 CrossRef
.
- T. L. Bailey, M. Wang, J. Solocinski, B. P. Nathan, N. Chakraborty and M. A. Menze, Protective Effects of Osmolytes in Cryopreserving Adherent Neuroblastoma (Neuro-2a) Cells, Cryobiology, 2015, 71(3), 472–480 CrossRef CAS
.
- J. Toxopeus, V. Koštál and B. J. Sinclair, Evidence for Non-Colligative Function of Small Cryoprotectants in a Freeze-Tolerant Insect, Proc. R. Soc. B, 2019, 286(1899), 20190050 CrossRef CAS
.
- S. C. Groan, Lyophilization of Hypha-Forming Tropical Wood-Inhabiting Basidiomycotina, Mycologia, 2019, 92(4), 810–817 Search PubMed
.
- T. H. H. Chen, K. K. Kartha, N. L. Leung, W. G. W. Kurz, K. B. Chatson and F. Constabel, Cryopreservation of Alkaloid-Producing Cell Cultures of Periwinkle (Catharanthus Roseus), Plant Physiol., 1984, 75(3), 726–731 CrossRef CAS PubMed
.
- S. D. Johnston, E. Qualischefski, J. Cooper, R. McLeod, J. Lever, B. Nixon, A. L. Anderson, R. Hobbs, J. Gosálvez, C. López-Fernández and T. Keeley, Cryopreservation of Saltwater Crocodile (Crocodylus Porosus) Spermatozoa, Reprod., Fertil. Dev., 2017, 29(11), 2235–2244 CrossRef CAS
.
- J. Nynca, S. Judycka, E. Liszewska, S. Dobosz, J. Grudniewska, K. Arai, T. Fujimoto and A. Ciereszko, Utility of Different Sugar Extenders for Cryopreservation and Post-Thaw Storage of Sperm from Salmonidae
Species, Aquaculture, 2016, 464, 340–348 CrossRef CAS
.
- C. G. Silva, E. R. Cunha, G. R. Blume, J. V. Malaquias, S. N. Báo and C. F. Martins, Cryopreservation of Boar Sperm Comparing Different Cryoprotectants Associated in Media Based on Powdered Coconut Water, Lactose and Trehalose, Cryobiology, 2015, 70(2), 90–94 CrossRef CAS PubMed
.
- H. Woelders, A. Matthijs and B. Engel, Effects of Trehalose and Sucrose, Osmolality of the Freezing Medium, and Cooling Rate on Viability and Intactness of Bull Sperm after Freezing and Thawing, Cryobiology, 1997, 35(2), 93–105 CrossRef CAS PubMed
.
- Y. Chen, R. H. Foote and C. C. Brockett, Effect of Sucrose, Trehalose, Hypotaurine, Taurine, and Blood Serum on Survival of Frozen Bull Sperm, Cryobiology, 1993, 30(4), 423–431 CrossRef CAS PubMed
.
- J. Liu, C. Tanrikut, D. L. Wright, G. Y. Lee, M. Toner, J. D. Biggers and T. L. Toth, Cryopreservation of Human Spermatozoa with Minimal Non-Permeable Cryoprotectant, Cryobiology, 2016, 73(2), 162–167 CrossRef CAS PubMed
.
- E. Caturla-Sánchez, M. J. Sánchez-Calabuig, J. F. Pérez-Gutiérrez, J. Cerdeira, C. Castaño and J. Santiago-Moreno, Vitrification of Dog Spermatozoa: Effects of Two Cryoprotectants (Sucrose or Trehalose) and Two Warming Procedures, Cryobiology, 2018, 80, 126–129 CrossRef
.
- A. L. Lynch, R. Chen and N. K. H. Slater, PH-Responsive Polymers for Trehalose Loading and Desiccation Protection of Human Red Blood Cells, Biomaterials, 2011, 32(19), 4443–4449 CrossRef CAS PubMed
.
- S. S. Buchanan, M. A. Menze, S. C. Hand, D. W. Pyatt and J. F. Carpenter, Cryopreservation of Human Hematopoietic Stem and Progenitor Cells Loaded with Trehalose: Transient Permeabilization via the Adenosine Triphosphate-Dependent P2Z Receptor Channel, Cell Preserv. Technol., 2006, 3(4), 212–222 CrossRef
.
- L. Diniz-Mendes, E. Bernardes, P. S. Araujo, A. Panek and V. Paschoalin, Preservation of Frozen Yeast Cells by Trehalose, Biotechnol. Bioeng., 1999, 65(5), 572–578 CrossRef CAS
.
- S. Stewart and X. He, Intracellular Delivery of Trehalose for Cell Banking, Langmuir, 2019, 35(23), 7414–7422 CrossRef CAS
.
- I. S. Bhandal, R. M. Hauptmann and J. M. Widholm, Trehalose as Cryoprotectant for the Freeze Preservation of Carrot and Tobacco Cells, Plant Physiol., 1985, 78(2), 430 CrossRef CAS
.
- A. E. Oliver, K. Jamil, J. H. Crowe and F. Tablin, Loading Human Mesenchymal Stem Cells with Trehalose by Fluid-Phase Endocytosis, Cell Preserv. Technol., 2004, 2(1), 35–49 CrossRef CAS
.
- I. Kusuma, R. S. Hadi, B. Kiranadi and A. Boediono, Trehalose Preincubation Increases Mesenchymal (CD271+) Stem Cells Post-Cryopreservation Viability, Med. J. Indones., 2016, 25(3), 128–135 CrossRef
.
- S. Kamalifar, N. Azarpira, L. Sadeghi, S. Ghorbani-Dalini, S. M. Nekoei, M. H. Aghdaie, E. Esfandiari and M. R. Azarpira, ROCK Y-27632 Inhibitor, Ascorbic Acid, and Trehalose Increase Survival of Human Wharton Jelly Mesenchymal Stem Cells After Cryopreservation, Exp. Clin. Transplant., 2018, 18(4), 505–511 CrossRef
.
- B. Stokich, Q. Osgood, D. Grimm, S. Moorthy, N. Chakraborty and M. A. Menze, Cryopreservation of Hepatocyte (HepG2) Cell Monolayers: Impact of Trehalose, Cryobiology, 2014, 69(2), 281–290 CrossRef CAS
.
- L. H. Campbell and K. G. M. Brockbank, Culturing with Trehalose Produces Viable Endothelial Cells after Cryopreservation, Cryobiology, 2012, 64(3), 240–244 CrossRef CAS PubMed
.
- J. Hara, J. Tottori, M. Anders, S. Dadhwal, P. Asuri and M. Mobed-Miremadi, Trehalose Effectiveness as a Cryoprotectant in 2D and 3D Cell Cultures of Human Embryonic Kidney Cells, Artif. Cells, Nanomed., Biotechnol., 2017, 45(3), 609–616 CrossRef CAS PubMed
.
- Y. X. Zheng, L. Z. Ma, S. J. Liu, C. T. Zhang, R. Meng, Y. Z. Chen and Z. L. Jiang, Protective Effects of Trehalose on Frozen-Thawed Ovarian Granulosa Cells of Cattle, Anim. Reprod. Sci., 2019, 200, 14–21 CrossRef CAS PubMed
.
- G. R. Satpathy, Z. Török, R. Bali, D. M. Dwyre, E. Little, N. J. Walker, F. Tablin, J. H. Crowe and N. M. Tsvetkova, Loading Red Blood Cells with Trehalose: A Step towards Biostabilization, Cryobiology, 2004, 49(2), 123–136 CrossRef CAS PubMed
.
- X. Zhou, H. He, B. Liu and T. Hua, Loading Trehalose into Red Blood Cells by Improved Hypotonic Method, Cell Preserv. Technol., 2008, 6(2), 119–122 CrossRef CAS
.
- R. Reuss, J. Ludwig, R. Shirakashi, F. Ehrhart, H. Zimmermann, S. Schneider, M. M. Weber, U. Zimmermann, H. Schneider and V. L. Sukhorukov, Intracellular Delivery of Carbohydrates into Mammalian Cells through Swelling-Activated Pathways, J. Membr. Biol., 2004, 200(2), 67–81 CrossRef CAS
.
- M. Zhang, H. Oldenhof, H. Sieme and W. F. Wolkers, Freezing-Induced Uptake of Trehalose into Mammalian Cells Facilitates Cryopreservation, Biochim. Biophys. Acta, 2016, 1858(6), 1400–1409 CrossRef CAS
.
- G. M. Beattie, J. H. Crowe, A. D. Lopez, V. Cirulli, C. Ricordi and A. Hayek, Trehalose: A Cryoprotectant That Enhances Recovery and Preserves Function of Human Pancreatic Islets after Long-Term Storage, Diabetes, 1997, 46(3), 519–523 CrossRef CAS
.
- M. Zhang, H. Oldenhof, H. Sieme and W. F. Wolkers, Combining Endocytic and Freezing-Induced Trehalose Uptake for Cryopreservation of Mammalian Cells, Biotechnol. Prog., 2017, 33(1), 229–235 CrossRef CAS PubMed
.
- C. Gläfke, M. Akhoondi, H. Oldenhof, H. Sieme and W. F. Wolkers, Cryopreservation of Platelets Using Trehalose: The Role of Membrane Phase Behavior during Freezing, Biotechnol. Prog., 2012, 28(5), 1347–1354 CrossRef
.
- H. Oldenhof, M. Zhang, K. Narten, J. Bigalk, B. Sydykov, W. F. Wolkers and H. Sieme, Freezing-Induced Uptake of Disaccharides for Preservation of Chromatin in Freeze-Dried Stallion Sperm during Accelerated Aging, Biol. Reprod., 2017, 97(6), 892–901 CrossRef PubMed
.
- R. Shirakashi, C. M. Köstner, K. J. Müller, M. Kürschner, U. Zimmermann and V. L. Sukhorukov, Intracellular Delivery of Trehalose into Mammalian Cells by Electropermeabilization, J. Membr. Biol., 2002, 189(1), 45–54 CrossRef CAS
.
-
B. Dovgan, J. Dermol, A. Barlič, M. Knežević and D. Miklavčič, Cryopreservation of Human Umbilical Stem Cells in Combination with Trehalose and Reversible Electroporation, IFMBE Proc., 2016, vol. 53, pp. 307–310 Search PubMed
.
- B. Dovgan, A. Barlič, M. Knežević and D. Miklavčič, Cryopreservation of Human Adipose-Derived Stem Cells in Combination with Trehalose and Reversible Electroporation, J. Membr. Biol., 2017, 250(1), 1–9 CrossRef CAS
.
- A. L. Lynch, R. Chen, P. J. Dominowski, E. Y. Shalaev, R. J. Yancey and N. K. H. Slater, Biopolymer Mediated Trehalose Uptake for Enhanced Erythrocyte Cryosurvival, Biomaterials, 2010, 31(23), 6096–6103 CrossRef CAS
.
- S. A. Mercado and N. K. H. Slater, Increased Cryosurvival of Osteosarcoma Cells Using an Amphipathic PH-Responsive Polymer for Trehalose Uptake, Cryobiology, 2016, 73(2), 175–180 CrossRef CAS
.
- D. M. C. Sharp, A. Picken, T. J. Morris, C. J. Hewitt, K. Coopman and N. K. H. Slater, Amphipathic Polymer-Mediated Uptake of Trehalose for Dimethyl Sulfoxide-Free Human Cell Cryopreservation, Cryobiology, 2013, 67(3), 305 CrossRef CAS PubMed
.
- Y. Wei, C. Li, L. Zhang and X. Xu, Design of Novel Cell Penetrating Peptides for the Delivery of Trehalose into Mammalian Cells, Biochim. Biophys. Acta, 2014, 1838(7), 1911–1920 CrossRef CAS PubMed
.
- N. Guo, I. Puhlev, D. R. Brown, J. Mansbridge and F. Levine, Trehalose Expression Confers Desiccation Tolerance on Human Cells, Nat. Biotechnol., 2000, 18(2), 168–171 CrossRef CAS PubMed
.
- A. García de Castro and A. Tunnacliffe, Intracellular Trehalose Improves Osmotolerance but Not Desiccation Tolerance in Mammalian Cells, FEBS Lett., 2000, 487(2), 199–202 CrossRef PubMed
.
- N. Chakraborty, M. A. Menze, J. Malsam, A. Aksan, S. C. Hand and M. Toner, Cryopreservation of Spin-Dried Mammalian Cells, PLoS One, 2011, 6(9), e24916 CrossRef CAS
.
- T. Uchida, M. Furukawa, T. Kikawada, K. Yamazaki and K. Gohara, Intracellular Trehalose via Transporter TRET1 as a Method to Cryoprotect CHO-K1 Cells, Cryobiology, 2017, 77, 50–57 CrossRef CAS PubMed
.
- A. Eroglu, M. J. Russo, R. Bieganski, A. Fowler, S. Cheley, H. Bayley and M. Toner, Intracellular Trehalose Improves the Survival of Cryopreserved Mammalian Cells, Nat. Biotechnol., 2000, 18(2), 163–167 CrossRef CAS PubMed
.
- S. S. Buchanan, S. A. Gross, J. P. Acker, M. Toner, J. F. Carpenter and D. W. Pyatt, Cryopreservation of Stem Cells Using Trehalose: Evaluation of the Method Using a Human Hematopoietic Cell Line, Stem Cells Dev., 2004, 13(3), 295–305 CrossRef CAS
.
- A. Eroglu, M. Toner and T. L. Toth, Beneficial Effect of Microinjected Trehalose on the Cryosurvival of Human Oocytes, Fertil. Steril., 2002, 77(1), 152–158 CrossRef
.
- A. Eroglu, S. E. Bailey, M. Toner and T. L. Toth, Successful Cryopreservation of Mouse Oocytes by Using Low Concentrations of Trehalose and Dimethylsulfoxide, Biol. Reprod., 2009, 80(1), 70 CrossRef CAS
.
- W. Zhang, J. Rong, Q. Wang and X. He, The Encapsulation and Intracellular Delivery of Trehalose Using a Thermally Responsive Nanocapsule, Nanotechnology, 2009, 20(27), 275101 CrossRef
.
- W. Rao, H. Huang, H. Wang, S. Zhao, J. Dumbleton, G. Zhao and X. He, Nanoparticle-Mediated Intracellular Delivery Enables Cryopreservation of Human Adipose-Derived Stem Cells Using Trehalose as the Sole Cryoprotectant, ACS Appl. Mater. Interfaces, 2015, 7(8), 5017–5028 CrossRef CAS PubMed
.
- M. Stefanic, K. Ward, H. Tawfik, R. Seemann, V. Baulin, Y. Guo, J. B. Fleury and C. Drouet, Apatite Nanoparticles Strongly Improve Red Blood Cell Cryopreservation by Mediating Trehalose Delivery via Enhanced Membrane Permeation, Biomaterials, 2017, 140, 138–149 CrossRef CAS
.
- J. L. Holovati and J. P. Acker, Spectrophotometric Measurement of Intraliposomal Trehalose, Cryobiology, 2007, 55(2), 98–107 CrossRef CAS
.
- J. L. Holovati, M. I. C. Gyongyossy-Issa and J. P. Acker, Effects of Trehalose-Loaded Liposomes on Red Blood Cell Response to Freezing and Post-Thaw Membrane Quality, Cryobiology, 2009, 58(1), 75–83 CrossRef CAS
.
- J. P. R. Motta, F. H. Paraguassú-Braga, L. F. Bouzas and L. C. Porto, Evaluation of Intracellular and Extracellular Trehalose as a Cryoprotectant of Stem Cells Obtained from Umbilical Cord Blood, Cryobiology, 2014, 68(3), 343–348 CrossRef
.
- C. Stoll, J. L. Holovati, J. P. Acker and W. F. Wolkers, Synergistic Effects of Liposomes, Trehalose, and Hydroxyethyl Starch for Cryopreservation of Human Erythrocytes, Biotechnol. Prog., 2012, 28(2), 364–371 CrossRef CAS
.
- S. Z. Zhang, J. L. Fan, X. G. Xu, G. M. Chen, F. M. Zhu and L. X. Yan, An Experimental Study of the Use of Ultrasound to Facilitate the Loading of Trehalose into Platelets, Cryobiology, 2009, 59(2), 135–140 CrossRef CAS PubMed
.
- A. Abazari, L. G. Meimetis, G. Budin, S. S. Bale, R. Weissleder and M. Toner, Engineered Trehalose Permeable to Mammalian Cells, PLoS One, 2015, 10(6), e0130323 CrossRef PubMed
.
- A. Eroglu, G. Elliott, D. L. Wright, M. Toner and T. L. Toth, Progressive Elimination of Microinjected Trehalose during Mouse Embryonic Development, Reprod. BioMed. Online, 2005, 10(4), 503–510 CrossRef CAS PubMed
.
- G. M. Fahy, Analysis of “Solution Effects” Injury. Equations for Calculating Phase Diagram Information for the Ternary Systems NaCl-Dimethylsulfoxide-Water and NaCl-Glycerol-Water, Biophys. J., 1980, 32(2), 837 CrossRef CAS PubMed
.
- R. R. Alfieri and P. G. Petronini, Hyperosmotic Stress Response: Comparison with Other Cellular Stresses, Pfluegers Arch., 2007, 454(2), 173–185 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |