Open Access Article
Open Access ArticleSynthesis and bioactivity of psilocybin analogues containing a stable carbon–phosphorus bond†
Marthe
Vandevelde
 ,
Andreas
Simoens
,
Andreas
Simoens
 ,
Bavo
Vandekerckhove
,
Bavo
Vandekerckhove
 and
Christian
Stevens
and
Christian
Stevens
 *
*
Department of Green Chemistry and Technology, Synthesis, Bioresources and Bioorganic Chemistry Research Group, Ghent University, Coupure Links 653, 9000 Ghent, Belgium. E-mail: chris.stevens@ugent.be
First published on 21st February 2024
Abstract
Psilocybin analogues have been synthesized comprising a non-hydrolysable P–C bond to evaluate the biological activity and the selectivity towards 5-HT2AR, 5-HT2BR and the TNAP receptor. No activity was observed towards the phosphatase, however all compounds showed good binding affinity for 5-HT2AR and 5-HT2BR and one compound showed a higher selectivity towards 5-HT2AR than psilocin.
The precipitous escalation, quantified at 25%, in the global prevalence of anxiety and depression in the wake of the COVID-19 pandemic serves as an alarming indicator of a profound and pervasive mental health crisis.1,2 Despite the diverse array of treatments available, the well-known first-line antidepressants exhibit limited efficacy. The treatment of major depressive disorder (MDD) with conventional drugs does not only grapple with a therapeutic delay of at least two weeks, thereby increasing the vulnerability to self-harm and suicide, but also with adverse effects such as sexual dysfunction.3,4 Another factor that amplifies the socio-economic burden of MDD is treatment resistant depression (TRD) in which remission subsequent to treatment has failed. Approximately 30% of adults suffering from MDD experience inadequate response to the administration of at least two different antidepressants of adequate dose and duration.5,6 The aforementioned deficiencies emphasize the urgency with which the scientific community must redirect its focus towards alternatives to the commonly used, yet suboptimal first-line antidepressants.
In this context, psychedelic drugs such as psilocybin, LSD, ketamine and MDMA have gained prominence as potential candidates for the treatment of psychiatric disorders.7,8 Notably, the regulatory approvals in March and December 2019 for intranasal administration of esketamine in conjunction with a conventional antidepressant for adults suffering from TRD are evidence of the evolving landscape.9 Completed phase 2 clinical trials show that psilocybin-assisted therapies produce large, rapid and long-lasting therapeutic effects among patients with MDD due to increased spine density and brain network integration.10–12 Despite all promising insights, the hallucinogenic nature of psychedelic drugs necessitates supervised administration, thereby hampering its widespread use.13 Four recent studies exploring the design and synthesis of non-hallucinogenic, non-toxic compounds offer potential solutions to this constraint.14–17 Another challenge in the search for new leads is the selectivity over off-targets such as 5-HT2BR where its activation is associated with valvular heart disease.18
Recent investigations identified a second binding mode of psilocin in the orthosteric site of 5-HT2AR which triggers only a modest activation of β-arrestin signaling. This nuanced mechanism results in antidepressive and anxiolytic effects devoid of hallucinogenic manifestations.15,19 Moreover, psilocybin's lower addiction potential and reduced toxicity compared to ketamine underscore its compelling potential as alternative antidepressant.20–22
Psilocybin (O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine) (Chart 1), a tryptamine alkaloid found in psilocybe mushrooms species,23 undergoes dephosphorylation in the human body primarily facilitated by an alkaline phosphatase. This process yields its active metabolite psilocin, which is active on 5-HT2AR and 5-HT2BR in our brain, among others.24
Considering the significance of the alkaline phosphatase in drug design and delivery,25,26 coupled with recent insights into the application of phosphonates in medicinal chemistry,27 our present work endeavors to ascertain the activity of phosphonate analogues of psilocybin on the 5-HT2A and 5-HT2B receptors. Notable, investigations into the inhibition of alkaline phosphatase by phosphonates remain absent in the existing literature. Hence, we aim to elucidate their interactions with and potential inhibition of the alkaline phosphatase, thereby contributing to the understanding and development of novel therapeutic strategies for mental health disorders. In alignment with this objective, we have innovatively developed a new synthesis pathway and optimized reaction conditions for phosphonoindoles and phosphonotryptamines.
The introduction of a CH2 group adjacent the aromatic ring results in a more isosteric analogue of psilocybin. However, this modification increases the count of rotational bonds. Conversely, the phosphonate and phosphonic acid analogues devised in this study feature few rotational bonds allowing for a conformation in the receptors of interests which more closely mirrors that of psilocin. Moreover, the synthesis of the variant at the 4-position would prove challenging owing to the lack of stereoselectivity inherent in the Fischer-indole reaction.
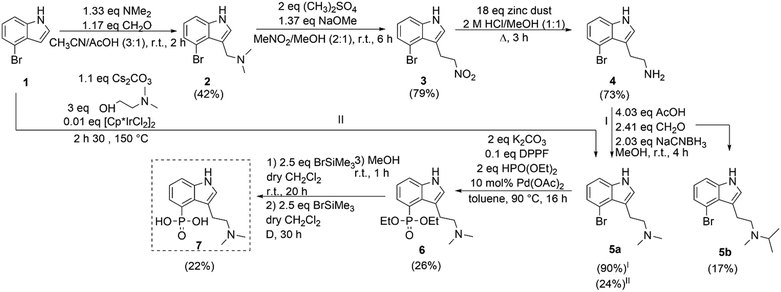
To obtain the target structure 7, a new pathway starting from 4-bromo indole 1 (Scheme 1) was investigated. The first step included a Mannich transformation into compound 2, which was used in the subsequent introduction of a nitromethane anion to obtain compound 3. The latter was further reduced using zinc dust in a sulfuric acid/methanol mixture. 1H-NMR analysis revealed low purity of the product 4 which necessitated the use of less harsh reaction conditions such as a hydrochloric acid/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. An initial yield of 34% was obtained under these conditions, which could be further increased to 73% by minimizing the use of water in the work-up. Next, a variant of the Eschweiler–Clarke reaction which entails the methylation of a primary or secondary amine, gives access to compound 5a. In this particular case, sodium cyanoborohydride was used for reductive amination. Due to a contamination by acetone of the solvent used in the reaction, side product 5b with an isopropyl group instead of a methyl group was formed. Optimization of the reaction led to the formation of compound 5a, which was obtained with an average yield of 90%. Method I, characterized by its simplicity, employs readily accessible, cheaper as well as less hazardous reagents and gives higher yields compared to preceding synthesis pathways of 5a.28,29 Recently, we developed a second pathway (Scheme 1) towards 5a, which consists of only one step and makes use of the borrowing hydrogen strategy. Method II reduces the time required to synthesize compound 5a but requires the use of a more expensive [Cp*IrCl2]2 catalyst.
1) mixture. An initial yield of 34% was obtained under these conditions, which could be further increased to 73% by minimizing the use of water in the work-up. Next, a variant of the Eschweiler–Clarke reaction which entails the methylation of a primary or secondary amine, gives access to compound 5a. In this particular case, sodium cyanoborohydride was used for reductive amination. Due to a contamination by acetone of the solvent used in the reaction, side product 5b with an isopropyl group instead of a methyl group was formed. Optimization of the reaction led to the formation of compound 5a, which was obtained with an average yield of 90%. Method I, characterized by its simplicity, employs readily accessible, cheaper as well as less hazardous reagents and gives higher yields compared to preceding synthesis pathways of 5a.28,29 Recently, we developed a second pathway (Scheme 1) towards 5a, which consists of only one step and makes use of the borrowing hydrogen strategy. Method II reduces the time required to synthesize compound 5a but requires the use of a more expensive [Cp*IrCl2]2 catalyst.
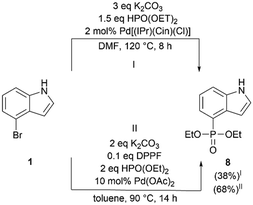
During pathway optimization, different reaction conditions were tested in parallel on the more accessible 5-bromo-N,N-dimethyltryptamine (Table S1†). First, the Michaelis-Arbuzov reaction was evaluated under mild reaction conditions using BF3·Et2O. After 4 h, 31P-NMR revealed the presence of triethyl phosphite, the product of its hydrolysis and its oxidation (triethyl phosphate). However, no evidence of product formation was observed. Rasheed et al. demonstrated that the phosphonylation of 5-bromo indole is feasible with a Michaelis–Arbuzov reaction in an ionic liquid (bbim[Br]).30 Reactions on 4-bromo indole and 5-bromo tryptamine in the ionic liquid failed to form the product, leaving only triethyl phosphite, diethyl phosphite and triethyl phosphate according to 31P-NMR. Additionally, we evaluated a nickel-catalyzed Tavs reaction according to the procedure of Shearan et al.31 Again, no evidence of product formation was found after 54 h of stirring at 160 °C. Another option to introduce a phosphonate group is the Hirao coupling.32 Literature research revealed the lack of Hirao couplings performed on indole containing compounds. To evaluate the feasibility of phosphonotryptamine synthesis, we initially investigated the synthesis of compound 8 (Table S2,†Scheme 2). Starting from 4-bromo indole, the desired compound was synthesized using a non-commercial NHC catalyst (Pd[(IPr)(Cin)(Cl)]))33 under the reaction conditions described by Xu et al. with an isolated yield of 38%.
These reaction conditions were then evaluated for 5-bromo-N,N-dimethyltryptamine, however only 29% conversion was achieved according to 31P-NMR. In experiment 5 and 6 (Table S1†), Pd(OAc)2 and K2CO3 were evaluated. The reaction mixture was stirred under a continuous nitrogen flow, but no full conversion was observed. Changing the base to Et3N in a THF solution, as described by Francesconi et al., resulted in an even lower conversion (12%).34 In experiment 8, similar reaction conditions as in experiment 6 were applied but the flask was carefully flushed with nitrogen and sealed, resulting in full conversion and isolation of compound 9 in a high yield (95%, Table S1†). It is worth mentioning that the same approach is applicable to the synthesis of compound 8. Moreover, these reaction conditions provided a cleaner reaction mixture and a higher isolated yield of 68%. A Mannich reaction was also evaluated on compound 8 but this resulted in a complex reaction mixture. Hence, this path was not further investigated.
With the optimized reaction conditions established, we expanded the library from indoles to tryptamines. First, compound 6, which was only one step away from the target structure, was synthesized following the same procedure as mentioned before. To evaluate the effect of the length and bulkiness of the alkyl side chain, compounds 2 and 5b were phosphonylated as well to obtain compounds 10 and 11 in isolated yields of 9% and 10%, respectively.
Subsequently, phosphonates were dealkylated to their corresponding phosphonic acids. The mild McKenna method was favored for the compounds of interest. The hydrolysis of compound 9 was achieved after 20 h of stirring at room temperature, followed by stirring in methanol for 1 h. No further purification was required, and 12 was isolated in 82% yield. Hydrolysis of compound 6 went less smoothly as 50% of the start product was partially silylated and 50% fully silylated after 20 h of stirring at room temperature according to 31P-NMR. After five days, no further changes were detected. Hence, the reaction mixture was refluxed at 55 °C. The solvent and bromotrimethylsilane were then periodically reintroduced into the mixture. After an additional 30 h, the conversion was complete and methanol was added.
After the chemical synthesis of the target compound 6 and five other compounds (Chart 2), we wanted to verify our two hypotheses. To evaluate the affinity of the phosphonate analogs towards 5-HT2A and 5-HT2BR, a competitive radioligand binding assay was conducted. Additionally, we wanted to determine whether these compounds could influence the metabolism, possibly as inhibitors of alkaline phosphatase. To answer this question, a tissue-nonspecific alkaline phosphatase (TNAP) assay was performed. The results of the initial screening are presented in Table S3.†
Surprisingly, the results of the in vitro assays revealed that all synthesized compounds lacked affinity for TNAP. This refutes our initial hypothesis regarding the inhibitory effects of psilocybin phosphonate analogs on the enzyme, but gives us more information regarding the metabolic stability of the compounds.
However, the results for 5-HT2AR and 5-HT2BR are promising and show a clear structure–activity relationship. For 5-HT2BR, the phosphonate esters of N,N-dimethyltryptamine (DMT) 9 and 6 exhibited the highest inhibitory activity. The phosphonic acids show a significantly lower affinity for 5-HT2BR and the phosphonate ester of gramine 10 displays the lowest affinity of all compounds. Thus, the synthesized compounds can be ranked in terms of increasing affinity for 5-HT2BR as follows: phosphonate esters of DMT > phosphonate ester of N-methyl-N-isopropyltryptamine (MIPT) > phosphonic acids of DMT > phosphonate esters of gramine (9 > 6 > 11 > 7 > 12 > 10).
A similar trend is observed for the activity at 5-HT2AR: 9 > 6 > 12 > 11 > 7 > 10. The phosphonate esters of DMT continue to exhibit the highest affinity and the phosphonate ester of gramine has the lowest affinity for this receptor. Interestingly, unlike the 5-HT2B receptor, the phosphonic acid at the 5-position demonstrates a higher affinity compared to the phosphonic acid at the 4-position. In general, the inhibition percentages of all compounds are lower for 5-HT2AR compared to 5-HT2BR. Chadeayne et al. reported that psilocybin (with a phosphoryloxy group) displays a higher selectivity towards 5-HT2BR compared to psilocin.35 While the synthesized compounds contain a phosphorus group similar to psilocybin, their selectivity for 5-HT2BR is relatively less prominent. Furthermore, the observed lower affinity of compound 11 for both 5-HT2AR and 5-HT2BR is consistent with the findings by McKenna et al. elaborating the comparison between 4-OH-DMT and 4-OH-MIPT.36 For compound 9, further analysis was performed to determine IC50 and inhibition constants (Ki) on 5-HT2AR and 5-HT2BR. The dose–response curves and a comparison of the inhibition constants of compound 9 with values for psilocin and psilocybin found in literature, are represented in Fig. S10† and Table 1.35
Compound 9 shows a higher binding affinity than psilocybin for 5-HT2AR, but a lower although comparable binding affinity for 5-HT2BR. Psilocin is two orders of magnitude more potent than compound 9 for 5-HT2BR, but the difference is smaller for 5-HT2AR. Consequently, the selectivity of compound 9 for 5-HT2BR is circa 5 times lower than is the case for psilocin which addresses the previously mentioned concerns regarding valvular diseases.18
In the present work, we describe the successful and innovative synthesis of seven phosphonate analogs of indoles via a Hirao coupling. Six analogs were subjected to activity testing on three receptors, 5-HT2AR, 5-HT2BR, and TNAP, to investigate the influence of the compounds on metabolism and serotonergic receptors in the brain. Interestingly, none of the compounds showed affinity for TNAP, thereby disproving a possible theory of inhibition of the alkaline phosphatase enzyme by the compounds. Results at the serotonergic receptors showed a clear structure–activity relationship, which provides insights for future selective drug design. Compound 9 showed the highest potency of all the tested compounds. Despite its lower potency compared to psilocin, the selectivity for 5-HT2BR is significantly lower which is favorable with regards to valvular heart diseases.
Leveraging the information from the abovementioned results, the search for candidates with a higher selectivity regarding G protein and β-arrestin signaling and other off-targets can become more oriented. Further in vivo tests can provide us with more insights into the effects of the synthesized compounds.
Author contributions
Marthe Vandevelde: data curation, formal analysis, investigation, methodology, visualization, writing – original draft, writing – review & editing, conceptualization. Andreas Simoens: data curation, formal analysis, conceptualization, project qdministration, writing – original draft, writing – review & editing, supervision. Bavo Vandekerckhove: writing – review & editing, investigation, methodology. Christian Stevens: conceptualization, project administration, supervision, validation, resources, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are indebted to the Ghent University Special Research Fund (BOF, Grant BOF.DOC.2023.0026.02) for financial support.Notes and references
- M. Daly and E. Robinson, Lancet, 2022, 399, 518 CrossRef CAS PubMed.
- Z. Li, M. Ruan, J. Chen and Y. Fang, Neurosci. Bull., 2021, 37, 863–880 CrossRef PubMed.
- E. Jing and K. Straw-Wilson, Ment. Health Clin., 2016, 6, 191–196 CrossRef PubMed.
- J. M. Witkin, A. E. Martin, L. K. Golani, N. Z. Xu and J. L. Smith, in Advances in Pharmacology, ed. J. M. Witkin, Academic Press, 2019, vol. 86, pp. 47–96 Search PubMed.
- S. Karkare, M. Zhdanava, D. Pilon, A. I. Nash, L. Morrison, A. Shah, P. Lefebvre and K. Joshi, Clin. Ther., 2022, 44, 1432–1448 CrossRef PubMed.
- M. Zhdanava, D. Pilon, I. Ghelerter, W. Chow, K. Joshi, P. Lefebvre and J. J. Sheehan, J. Clin. Psychiatry, 2021, 82(2) DOI:10.4088/JCP.20m13699.
- J. M. Mitchell, G. M. Ot'alora, B. van der Kolk, S. Shannon, M. Bogenschutz, Y. Gelfand, C. Paleos, C. R. Nicholas, S. Quevedo, B. Balliett, S. Hamilton, M. Mithoefer, S. Kleiman, K. Parker-Guilbert, K. Tzarfaty, C. Harrison, A. de Boer, R. Doblin, B. Yazar-Klosinski and M. S. C. Group, Nat. Med., 2023, 29, 2473–2480 CrossRef CAS PubMed.
- W. Duan, D. Cao, S. Wang and J. Cheng, Chem. Rev., 2024, 124(1), 124–163 CrossRef CAS PubMed.
- R. S. McIntyre, J. D. Rosenblat, C. B. Nemeroff, G. Sanacora, J. W. Murrough, M. Berk, E. Brietzke, S. Dodd, P. Gorwood, R. Ho, D. V. Iosifescu, C. Lopez Jaramillo, S. Kasper, K. Kratiuk, J. G. Lee, Y. Lee, L. M. W. Lui, R. B. Mansur, G. I. Papakostas, M. Subramaniapillai, M. Thase, E. Vieta, A. H. Young, C. A. Zarate, Jr. and S. Stahl, Am. J. Psychiatry, 2021, 178, 383–399 CrossRef PubMed.
- A. K. Davis, F. S. Barrett, D. G. May, M. P. Cosimano, N. D. Sepeda, M. W. Johnson, P. H. Finan and R. R. Griffiths, JAMA Psychiatry, 2021, 78, 481–489 CrossRef PubMed.
- R. E. Daws, C. Timmermann, B. Giribaldi, J. D. Sexton, M. B. Wall, D. Erritzoe, L. Roseman, D. Nutt and R. Carhart-Harris, Nat. Med., 2022, 28, 844–851 CrossRef CAS PubMed.
- L. X. Shao, C. Liao, I. Gregg, P. A. Davoudian, N. K. Savalia, K. Delagarza and A. C. Kwan, Neuron, 2021, 109, 2535–2544 CrossRef CAS PubMed e2534.
- R. F. Service, Science, 2022, 375, 370 CrossRef CAS PubMed.
- L. P. Cameron, R. J. Tombari, J. Lu, A. J. Pell, Z. Q. Hurley, Y. Ehinger, M. V. Vargas, M. N. McCarroll, J. C. Taylor, D. Myers-Turnbull, T. Liu, B. Yaghoobi, L. J. Laskowski, E. I. Anderson, G. Zhang, J. Viswanathan, B. M. Brown, M. Tjia, L. E. Dunlap, Z. T. Rabow, O. Fiehn, H. Wulff, J. D. McCorvy, P. J. Lein, D. Kokel, D. Ron, J. Peters, Y. Zuo and D. E. Olson, Nature, 2021, 589, 474–479 CrossRef CAS PubMed.
- D. Cao, J. Yu, H. Wang, Z. Luo, X. Liu, L. He, J. Qi, L. Fan, L. Tang, Z. Chen, J. Li, J. Cheng and S. Wang, Science, 2022, 375, 403–411 CrossRef CAS PubMed.
- A. L. Kaplan, D. N. Confair, K. Kim, X. Barros-Álvarez, R. M. Rodriguiz, Y. Yang, O. S. Kweon, T. Che, J. D. McCorvy, D. N. Kamber, J. P. Phelan, L. C. Martins, V. M. Pogorelov, J. F. DiBerto, S. T. Slocum, X.-P. Huang, J. M. Kumar, M. J. Robertson, O. Panova, A. B. Seven, A. Q. Wetsel, W. C. Wetsel, J. J. Irwin, G. Skiniotis, B. K. Shoichet, B. L. Roth and J. A. Ellman, Nature, 2022, 610, 582–591 CrossRef CAS PubMed.
- C. Dong, C. Ly, L. E. Dunlap, M. V. Vargas, J. Sun, I.-W. Hwang, A. Azinfar, W. C. Oh, W. C. Wetsel, D. E. Olson and L. Tian, Cell, 2021, 184, 2779–2792.e2718 CrossRef CAS PubMed.
- B. L. Roth, N. Engl. J. Med., 2007, 356, 6–9 CrossRef CAS PubMed.
- Y. N. Yin and T. M. Gao, Neurosci. Bull., 2023, 39, 170–172 CrossRef PubMed.
- M. W. Johnson, R. R. Griffiths, P. S. Hendricks and J. E. Henningfield, Neuropharmacology, 2018, 142, 143–166 CrossRef CAS PubMed.
- R. S. Gable, Am. J. Drug Alcohol Abuse, 1993, 19, 263–281 CrossRef CAS PubMed.
- L. Orsolini, V. Salvi and U. Volpe, Expert Opin. Drug Saf., 2022, 21, 803–812 CrossRef PubMed.
- M. Coppola, F. Bevione and R. Mondola, J. Xenobiot., 2022, 12, 41–52 CrossRef CAS PubMed.
- H. A. Geiger, M. G. Wurst and R. N. Daniels, ACS Chem. Neurosci., 2018, 9, 2438–2447 CrossRef CAS PubMed.
- K. Moonen, I. Laureyn and C. V. Stevens, Chem. Rev., 2004, 104, 6177–6216 CrossRef CAS PubMed.
- B. Le-Vinh, Z. B. Akkuş-Dağdeviren, N. M. N. Le, I. Nazir and A. Bernkop-Schnürch, Adv. Ther., 2022, 5, 2100219 CrossRef CAS.
- M. Krecmerova, P. Majer, R. Rais and B. S. Slusher, Front. Chem., 2022, 10, 889737 CrossRef CAS PubMed.
- M. Alzweiri, A. S. Alsegiani, E. Karaj, D. A. Almarghalani, Y. Tabaza, Z. A. Shah and L. M. V. Tillekeratne, Bioorg. Med. Chem. Lett., 2021, 47, 128205 CrossRef CAS PubMed.
- E. K. Olsen, E. Hansen, L. W. K. Moodie, J. Isaksson, K. Sepčić, M. Cergolj, J. Svenson and J. H. Andersen, Org. Biomol. Chem., 2016, 14, 1629–1640 RSC.
- S. Rasheed, D. D. Subba Rao, S. Chennamsetty, T. Basha and C. Raju, ChemInform, 2015, 46, no-no Search PubMed.
- S. J. I. Shearan, E. Andreoli and M. Taddei, Beilstein J. Org. Chem., 2022, 18, 1518–1523 CrossRef CAS PubMed.
- S. Van der Jeught and C. V. Stevens, Chem. Rev., 2009, 109, 2672–2702 CrossRef CAS PubMed.
- A. Simoens, T. Scattolin, T. Cauwenbergh, G. Pisano, C. S. J. Cazin, C. V. Stevens and S. P. Nolan, Chem. – Eur. J., 2021, 27, 5653–5657 CrossRef CAS PubMed.
- O. Francesconi, M. Martinucci, L. Badii, C. Nativi and S. Roelens, Chem. – Eur. J., 2018, 24, 6828–6836 CrossRef CAS PubMed.
- A. R. Chadeayne, D. N. K. Pham, B. G. Reid, J. A. Golen and D. R. Manke, ACS Omega, 2020, 5, 16940–16943 CrossRef CAS PubMed.
- D. J. McKenna, D. B. Repke, L. Lo and S. J. Peroutka, Neuropharmacology, 1990, 29, 193–198 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00043a |
| This journal is © The Royal Society of Chemistry 2024 |