Open Access Article
Open Access ArticleHydrothermal synthesis of an MoS2/MnO2 nanocomposite: a unique 3D-nanoflower/1D-nanorod structure for high-performance energy storage applications†
Md. Roxy
Islam
a,
Mizanur
Rahaman
b,
Md. Muktadir
Billah
*a and
Muhammad Rakibul
Islam
 *b
*b
aDepartment of Materials and Metallurgical Engineering, Bangladesh University of Engineering and Technology, Dhaka, Bangladesh. E-mail: mbillah@mme.buet.ac.bd
bDepartment of Physics, Bangladesh University of Engineering and Technology, Dhaka, Bangladesh. E-mail: rakibul@phy.but.ac.bd
First published on 9th May 2024
Abstract
In this study, an MoS2/MnO2 nanocomposite electrode with a novel 3D nanoflower/1D nanorod architecture is effectively synthesized using a straightforward, cost-effective hydrothermal process. The addition of the 1D MnO2 nanorod offers a structural backbone, while the 3D MoS2 nanoflower generates additional reactive active sites. The hybrid structure makes fast electron and ion movement possible, which also increases the capacity for charge collection. This leads to 199.12 F g−1 specific capacitance at 40 mA g−1 in a designed 3D-1D MoS2/MnO2(6 wt%) electrode and exceptional rate capability with a tremendous cycling life (95% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). This discovery paves the way for the low-cost and straightforward construction of a hierarchical nanocomposite electrode with improved charge storage and electrical conductivity in energy storage applications like supercapacitors.
000 cycles). This discovery paves the way for the low-cost and straightforward construction of a hierarchical nanocomposite electrode with improved charge storage and electrical conductivity in energy storage applications like supercapacitors.
1. Introduction
Energy from renewable sources is becoming a major topic due to the fast expansion of the world economy, rapid consumption of fossil fuels, and global climate change. Using renewable energy sources, such as wind, water, sun, and others, is a path to reduce current pollution and energy problems.1–4 However, a continuous energy supply from renewable sources is not accessible due to the unavailability of sunlight throughout the entire time period and the unreliability of wind and tides. An alternative way to maintain a sustainable energy future by using energy from renewable sources is to use advanced energy storage devices, such as fuel cells, batteries, and supercapacitors (SCs).1,5–9 Among the available energy storage alternatives, SCs are now in the limelight because of their low price, longer cycle life, high density of energy, etc. and are suitable for applications that require rapid and higher-power energy.10–12 Because of their increased power density, safety, benefits of high charge–discharge rate, outstanding cycle stability, extended cycle life, low-temperature operation, low maintenance cost, etc., supercapacitors may replace batteries thus piquing the scientific and technological attention of many.11,13–18 For such advantages, SCs find their applications in smart door locks, hybrid forklifts, cranes, smart grids, televisions, UPS (uninterruptible power supply) systems, electric vehicles, and other electronic devices.19,20A transition metal dichalcogenide called molybdenum disulfide (MoS2) can be employed as an active component for designing energy storage devices, due to its electrical conductivity, surface area, and intrinsic ion conductivity which suggests good electrochemical properties.21–25 The two-dimensional planar layered structure of MoS2 is interconnected with sulfur–molybdenum–sulfur bonds. Mo atoms have different valence states (+2 to +6) and the interlayered space conducive to the electrolytic cation intercalation gives MoS2 electrochemically pseudo-capacitance properties. But MoS2 has moderate interfacial active sites to react with electrolyte ions, poor cycling stability, low energy density, and no stability in the interphase layer of electrolyte–solid, and it shows structural destruction especially aggregation during charge/discharge cycles, which are the drawbacks for supercapacitors.26–28
Metal oxide nanoparticles such as Co3O4, NiO, V2O5, TiO2, and MnO2 have been added to the MoS2 structure to enhance its capacitive properties.29–35 Kanaujiya et al.29 synthesized mesoporous MnO2@MoS2 nanocomposites using a versatile hydrothermal approach. Wang et al.32 have synthesized hierarchical MoS2/Mn3O4 hybrid architectures where nanoparticles of Mn3O4 are uniformly incorporated into MoS2 thin layers and achieved two times more capacitance than MoS2 with 69.3% capacitance retention. Liao et al.33 fabricated a MoS2/MnO2 (nanosheet/nanowire) hybrid using the lithography technique to analyze the interfacial impact on the storage of energy. But, MoS2 nanosheet preparation methods take time and the yield is low. Those nanosheets have a restacking tendency at the time of charging–discharging that produces high surface energy and as a result, the electrical conductivity reduces.36–40 To solve those drawbacks, a cost-effective procedure providing a higher yield, for example, a simple hydrothermal synthesis route, can play a vital role in producing a flower-like 3D-MoS2 nanostructure. The MoS2 nanoflower can give a high specific surface area, where a lot of interfacial active sites are present to react with electrolyte ions.29 Due to the presence of a larger surface area and greater electrolytic ion migration active spots, the flower-shaped nanostructure has the ability to store more energy. Moreover, a longer cycle life can be maintained when it is used as the active material of electrodes.41 Additionally, the incorporation of nanoparticles into the flower-shaped structure may generate an increased number of active spots for the electrolytic ion diffusion, greater stability of the structure, and defect-rich surface.42–45 However, as far as we know, the electrochemical behavior of nanocomposites made of a 3D MoS2 nanoflower (NF) and 1D MnO2 nanorods produced with various MoS2 and MnO2 weight ratios has not been thoroughly investigated.
In this study, an MoS2 nanoflower/MnO2 nanorod, a distinctive structurally combined nanocomposite, has been prepared to improve the capacitive performance of an MoS2 nanoflower. Here, a hydrothermal method has been used to prepare MoS2 nanoflowers and MnO2 nanorods and to incorporate the rod-shaped MnO2 nanoparticles into the MoS2 nanoflowers in varying concentrations (2, 4, and 6 wt%). Manganese oxide (MnO2) is a metal oxide semiconductor and has its own high theoretical capacitance, nontoxic nature, high cycling stability, electro-catalytic activity, lower cost, and abundance advantages.46,47 MnO2 has different polymorphs, and among them, our synthesized MnO2 contains double chains of edge-sharing MnO6 octahedral which exhibits high catalytic activities.48 The addition of MnO2 nanorods into MoS2 nanoflowers may give more stability to the structure, rapid transportation of ions, and effective surface interactions with electrolyte ions; therefore, cycling stability and capacitance of MoS2 nanoflowers may increase.45
In this experiment, the physical and structural characteristics of synthesized samples were studied by FE-SEM (field emission scanning electron microscopy), HR-TEM (high-resolution transmission electron microscopy), and XRD (X-ray diffraction), respectively. FE-SEM results show that the as-synthesized MnO2 has a 1D nanorod structure. The synthesized MoS2/MnO2 nanocomposites and pure MoS2 have a 3D flower-shape morphology consisting of many nanolayered petals. HR-TEM results show that MnO2 nanorods are present between the MoS2 nanosheets in MoS2/MnO2 nanocomposites, and the interlayer spacing of MoS2 increases with the MnO2 concentration. XRD analysis reveals that the crystallite size decreases and the dislocation density increases for the MoS2 NF when increased amounts of MnO2 nanorod are added. Using a system of three electrodes, the electrochemical behavior of the as-synthesized samples was examined in 0.5 M Na2SO4 electrolyte at room temperature. Among all synthesized MoS2/MnO2 nanocomposites (NCs), MoS2/MnO2 (6 wt%) NC can store 199.12 F g−1 at a 40 mA g−1 current density. Here the density of energy was found to be 6.91 W h kg−1 at a 10 W kg−1 power density. At 170 mA g−1 current density, the MoS2/MnO2 (6 wt%) NC undergoes 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles and provides 95% capacitance retention which confirms its tremendous cycling stability. The MoS2/MnO2 nanocomposites show a higher capacitance than the bare MoS2 electrode denoting that the 3D MoS2 nanoflower incorporated with the MnO2 nanorod gives a larger specific surface area, greater interlayer spacing, higher wettability, faster intrinsic electrical conductivity, improved electrolytic cation intercalation at the interface, and an overall defect-rich structure. The novelty of this work is the synthesis of 3D-nanoflower/1D-nanorod structural nanocomposites of MoS2/MnO2, and when compared (Table 5) to the capacitance of MoS2 based supercapacitors that have been published in the different literature, our synthesized MoS2/MnO2 (6 wt%) NC shows good capacitance alongside capacitance retention. So, to prepare a high-performance energy storage device, this experiment can provide significant insights.
000 charge/discharge cycles and provides 95% capacitance retention which confirms its tremendous cycling stability. The MoS2/MnO2 nanocomposites show a higher capacitance than the bare MoS2 electrode denoting that the 3D MoS2 nanoflower incorporated with the MnO2 nanorod gives a larger specific surface area, greater interlayer spacing, higher wettability, faster intrinsic electrical conductivity, improved electrolytic cation intercalation at the interface, and an overall defect-rich structure. The novelty of this work is the synthesis of 3D-nanoflower/1D-nanorod structural nanocomposites of MoS2/MnO2, and when compared (Table 5) to the capacitance of MoS2 based supercapacitors that have been published in the different literature, our synthesized MoS2/MnO2 (6 wt%) NC shows good capacitance alongside capacitance retention. So, to prepare a high-performance energy storage device, this experiment can provide significant insights.
2. Section of experimentation
2.1. Chemical reagents
Sodium molybdate dihydrate (Na2MoO4·2H2O), manganese sulfate monohydrate (MnSO4·H2O), potassium permanganate (KMnO4), and dimethyl sulfoxide (C2H6OS) were obtained from Merck, Darmstadt, Germany. From Research Lab, India, thiourea (CH4N2S), sodium sulfate (Na2SO4) and polyvinyl alcohol (PVA) (C2H4O)x were collected. Without any purification, analytical grade chemicals were used.2.2. MoS2 NF preparation
A clear precursor solution was prepared by dissolving 0.14 M sodium molybdate dihydrate and 0.65 M thiourea in 120 ml of deionized water. Then the solution was kept in a Teflon-lined autoclave for 24 hours at 200 °C. The resultant black precipitate was then collected from the autoclave and after drying at 70 °C for several hours the MoS2 nanoflower was obtained.2.3. MnO2 nanorod preparation
5.6878 g of MnSO4·H2O and 2.3657 g of KMnO4 were taken in 140 mL of deionized water. After stirring for 30 minutes, the solution was kept in a Teflon-lined autoclave for 12 h in an oven at 140°. After collecting the resultant brown precipitate, it was dried for a few hours at 80 °C yielding the MnO2 nanorod.2.4. Preparation of the MnO2 incorporated MoS2NF
Fig. 1(a) shows the schematic representation of nanocomposite preparation. At first, a desired amount of MnO2 nanorod was added to 50 mL of deionized water and sonicated for 1 hour. After dissolving 0.14 M sodium molybdate dihydrate and 0.65 M thiourea in 70 mL of deionized water, the solution was transferred to the sonicated 50 mL MnO2 solution. After stirring for 1 hour, the whole solution was kept in a Teflon-lined autoclave for 24 h at 200 °C. The desired black precipitate was collected and dried for a few hours at 80 °C. Various amounts of MnO2 nanorods such as 2 wt%, 4 wt%, and 6 wt% were incorporated. The NCs are entitled as MoS2/MnO2 (2 wt%), MoS2/MnO2 (4 wt%) and MoS2/MnO2 (6 wt%) respectively.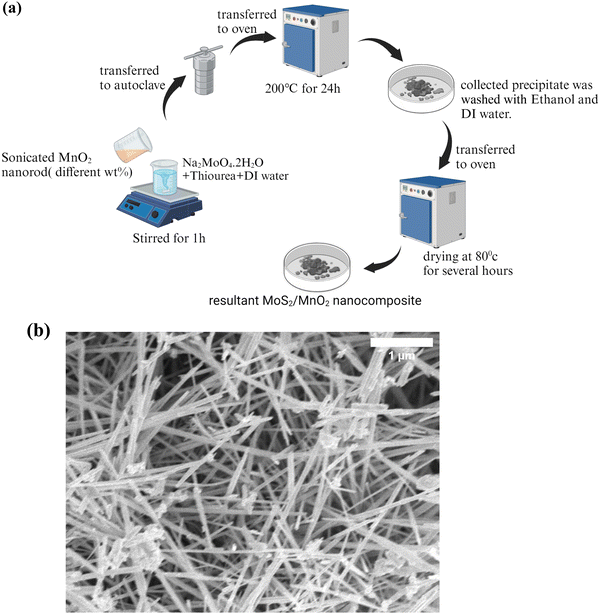 | ||
| Fig. 1 (a) Schematic representation of the synthesis of MoS2/MnO2 nanocomposites. (b) FE-SEM image of the MnO2 nanorod at a low magnification. | ||
2.5. Preparation of electrodes
To prepare working electrodes, a 100 μL slurry of the as-synthesized material was deposited on a cross-sectional surface (0.3 cm2) of a modified graphite electrode. To prepare the slurry, PVA (4% of the as-synthesized material) and dimethyl sulfoxide were added to the as-synthesized material. After sonicating for 1 h, the slurry was deposited and for drying, the working electrodes were kept at 70 °C. Here PVA acts as a binder and dimethyl sulfoxide works as a polar aprotic solvent.49,502.6. Characterization
To get the microscopic information about the surface of the as-synthesized samples, FE-SEM (JSM 7600, Jeol) images were taken. Microstructural and morphological properties of the synthesized materials were also observed using HR-TEM (JEOL, JEM 2100 F) images. Using the radiation of CuKα (λ = 1.5406 Å) of the X-ray diffractometer of 3040XPert PRO, Philips, the XRD data were obtained. The electrochemical capabilities of the as-synthesized samples were investigated by using a CS310 electrochemical workstation (Corrtest, China) in 0.5 M Na2SO4 electrolyte using a three-electrode system. Here the working electrode is a modified graphite electrode, the reference electrode is Ag/AgCl, and the counter electrode is a platinum plate (1 cm × 1 cm).3. Results and discussion
3.1. Scanning electron microscopy
Fig. 1(b) presents the FESEM image of MnO2 consisting of densely aligned disorderly arranged nanorods with several micrometers of length and a width of 47–98 nm. Some fragmented nanorods were observed and the formation of bundled nanorods could be seen. Under high magnification (Fig. S1, ESI†), the surface of the nanorods appears to be smooth.45,51From the FESEM image of MoS2 (Fig. 2(a)), flower-like spherical 3D nanostructures were observed that confirm the successful synthesis of MoS2 nanoflowers having a diameter between 5 and 6 μm. Those nanoflowers consist of plenty of aligned curved petals, which are assembled by several MoS2 nanosheets, through a common inner center. The petal thickness is observed to be varied between 2 and 5 nm.52Fig. 2(b–d) shows FESEM images of MoS2/MnO2 nanocomposites for different concentrations of MnO2, where the curved petals of MoS2 are more uniform and smoothly aligned. These nanoflowers with observed diameters in the range of 4–6 μm are in the cluster form or not dependent on each other.53 When the MnO2 nanorod was taken in the precursor solution of MoS2, Mo4+ ions from the cationic precursor could be attracted on the surface of the MnO2 nanorod because of van der Waals, cohesive or electrostatic, or other chemical forces.45 S2− ions from the anionic precursor combine with Mo4+ ions on the surface of the MnO2 nanorod to form flower-shaped nanostructures.
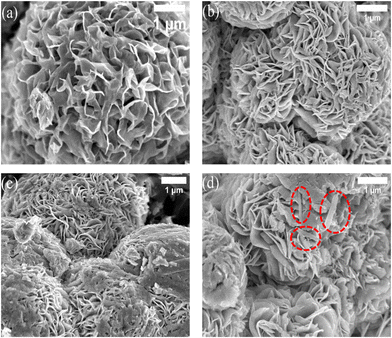 | ||
| Fig. 2 (a)–(d) FE-SEM images of MoS2 NF, MoS2/MnO2 (2 wt%), MoS2/MnO2(4 wt%), and MoS2/MnO2 (6 wt%) nanocomposites, respectively. | ||
From Fig. 2(b–d) we observe that the diameter of the MoS2 nanoflower reduces with the addition of MnO2 and the thickness of the petal for MoS2/MnO2(2 wt%) is in the range of 10–20 nm but for MoS2/MnO2(4 wt%) and MoS2/MnO2(6 wt%), the thickness got increased (20–40 nm) may be due to agglomeration of multiple layers of nanosheets.54 Due to the increase of petal thickness, porosity and specific surface area would increase and as a result more electrical contact with the current collector would provide an excellent charge transfer rate and improvement in electrochemical capacity. As shown in Fig. 2(b and c), the existence of MnO2 nanorods is not clearly observed which may be due to the low concentration of MnO2. For higher concentrations of MnO2, nanorods are clearly observed as shown in Fig. 2(d).
From EDX mapping shown in Fig. S2(a–d) (ESI†), the compositional information shown in Table 1 reveals that the surface of the MoS2 NF is enriched with S atoms. In addition, in Fig. 2(b–d) the presence of dispersed fragmented particles on the surface of petals with Mo, S, Mn, and O elemental distribution from EDX mapping confirms that the petals consist of Mo and S and the fragmented particle has Mn and O. From the EDX results, we observe that the %atomic ratio of S and Mo was greater than 4, and this excess amount of sulfur atoms appeared as a result of many defects on the ultrathin MoS2 nanosheets. The defective structures can provide more stability to the nanosheets by reducing the surface energy and generating more reactive sites.55,56
| MnO2 concentration (wt%) in the prepared samples | Mo (atom%) | S (atom%) | Mn (atom%) | O (atom%) | Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S |
|---|---|---|---|---|---|
| 0 | 6.62 | 93.38 | 0 | 0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 2 | 11.82 | 70.30 | 0.63 | 17.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 4 | 8.34 | 63.74 | 0.78 | 27.09 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 6 | 8.64 | 77.29 | 0.98 | 13.09 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
3.2. Transmission electron microscopy
TEM images of MoS2 NF and MoS2/MnO2 nanocomposites are shown in Fig. 3(a–d). From Fig. 3(a) it can be observed that the MoS2 NF consists of many thin petals and those petals are composed of several MoS2 nanosheets. As shown in Fig. 3(b–d), the curved and wrinkled nanosheets of MoS2 are very thin and partially transparent. Light contrasts in various areas indicate the ultra-thin nature of the nanosheets. In the TEM image (Fig. 3(b–d)) of MoS2/MnO2(2, 4, 6 wt%) nanocomposites, the presence of MnO2 nanorods between MoS2 nanosheets is clearly visible. The transfer of electrons between MoS2 and MnO2 is facilitated due to the close integration between MnO2 and MoS2. Fig. 4(a–d) displays HR-TEM images along with the associated SAED (selected area electron diffraction) patterns. From the HRTEM image, in Fig. 4(a), we observed disorderly arranged lattice fringes and weak diffraction rings as shown in the inset SAED pattern confirming the poor crystallinity of the MoS2 NF.57 | ||
| Fig. 3 (a)–(d) TEM images of MoS2 NF, MoS2/MnO2 (2 wt%), MoS2/MnO2 (4 wt%) and MoS2/MnO2 (6 wt%) nanocomposites, respectively. | ||
 | ||
| Fig. 4 (a)–(d) HR-TEM images of MoS2 NF, MoS2/MnO2 (2 wt%), MoS2/MnO2 (4 wt%), and MoS2/MnO2 (6 wt%) nanocomposites, respectively. The insets of figures (a)–(d) show the corresponding SAED patterns. | ||
The magnified HRTEM image (Fig. 4(a)) provides an interlayer spacing of 0.63 nm that agrees with the hexagonal MoS2 (002) plane indicating the growth of hexagonal nanosheets along the vertical [002] crystal axis.58 The interlayer spacings for MoS2/MnO2(2 wt%), MoS2/MnO2(4 wt%), and MoS2/MnO2(6 wt%) were found to be 0.64 nm, 0.65 nm, and 0.67 nm, respectively indicating a lattice expansion due to the addition of MnO2.28 Such an increase of interlayer spacing may be attributed to the diffusion of MnO2 between MoS2 layers.59 The enlargement of interlayer spacing and unique nanoflower-nanorod morphology may provide better ions intercalation, more electroactive sites, and enhancement of electrolyte access that gives the desired electrochemical property with improved efficiency in attaining higher capacitance.29,45,59
The SAED patterns for all the samples (inset of Fig. 4(a–d)) show bright concentric rings of diffraction that ensure the polycrystalline nature of the as-synthesized samples. Also, diffraction rings (inset of Fig. 4(a–d)) of the (100), (103), (105), and (110) lattice planes of MoS2 are observed in all as-synthesized samples. Due to the low concentration of MnO2, only lattice plane (521) is observed in (inset of Fig. 4(b and c)) MoS2/MnO2 (2 wt%) and MoS2/MnO2 (4 wt%). When the concentration of MnO2 is increased, (400), (411), (541), and (600) lattice planes of MnO2 nanorods are found in MoS2/MnO2 (6 wt%) nanocomposites.
The findings from the TEM analysis demonstrate that the MoS2/MnO2 nanocomposites were successfully prepared and confirm intimate contact between the MnO2 nanorods and the MoS2 NF. From the TEM images of the nanocomposite, discontinuity in the lattice fringes of the curled edges suggests that the crystals possess dislocations and defects.53,56
3.3. X-ray diffraction
The XRD patterns were studied to confirm the phase, structure, and crystallinity of the MoS2 nanoflower and MoS2/MnO2 nanocomposites, as shown in Fig. 5. As illustrated in Fig. 5(a), the hexagonal phase of MoS2 (JCPDS No. 37-1492), which is a member of the space group P63/mmc, agrees well with the diffraction peaks of the pristine MoS2. The peaks at 2θ = 14.04°, 33.49°, 39.46°, 48.57°, and 59.14° are attributed to the (002), (100), (103), (105) and (110) lattice planes, respectively.53 Each reflection in the XRD pattern seen in Fig. S3 (ESI†) for the MnO2 nanorod may easily be attributed to a pure tetragonal phase of the space group I4/m (no. 87), with a = 0.985 nm and c = 0.285 nm (for the lattice constants). The absence of peaks for any other manganese oxides suggests that the produced MnO2 nanorods have a pure phase.60 In Fig. 5(a) the sharp peak of pure MoS2 nanoflower at 14.04°, which has a 0.63 nm d-spacing corresponding to the (002) plane, confirms that MoS2 layers stacked toward the c axis during synthesis.61For MoS2/MnO2 nanocomposites, all the primary diffraction peaks of pure MoS2 are present. Due to the low concentration of MnO2 in MoS2/MnO2 (2 wt%), no diffraction peak of MnO2 is observed. But the primary characteristic peaks of MnO2 can be only clearly observed for MoS2/MnO2(4 wt%) and MoS2/MnO2(6 wt%) nanocomposites.44,62,63 For MoS2/MnO2 NCs the broadened and weak diffraction of the (002) plane imply low crystallinity, indicating that the pristine MoS2 NF has a larger average crystallite size than that of the MoS2/MnO2 nanocomposites.64 A lower angle shift of the initial XRD peak occurs, which may be due to lattice distortion, indicating an increase in the MoS2 interlayer distance in the nanocomposites.64,65
The crystallite size (L), the density of dislocation (δ), and the microstrain (ε) of all samples were determined from the (002) peak using eqn (1)–(3), respectively.66,67
 | (1) |
| δ = 1/L2 | (2) |
 | (3) |
At the (002) peak, the structural parameters were measured. Table 2 and Fig. 6 present the variation of structural parameters of the MoS2 NF and MoS2/MnO2 NCs.68–70 The crystallite size was found to be decreased from 7.51 nm to 5.59 nm when the concentration of MnO2 increased from 2 wt% to 6 wt%.68–70 Because the presence of MnO2 nanorods helps to grow cross-linked MoS2 nanosheets.71,72 Microstrain and dislocation density increase with the concentration of MnO2 suggesting increased defect states like dislocation generation, imperfect crystal structure, and vacancies in the MoS2 NF.73,74 Using the following equations, the values of lattice constants were calculated:
where a, b, and c denote lattice constants, d = atomic plane spacing, λ = wavelength (1.5406 Å), (h, k, l) denotes miller indices, and θ = incident angle. The calculated values (in Table 1) of lattice constants are less than standard values which represent less volume of lattice cell.75 Increased dislocation density, smaller crystallite size, and difference in d spacing create lattice distortion. As a result, defects in lattice occur which gives profound active sites with an improved surface area, and at the time of charging/discharging, the charge transfer rate increases between active materials and solution.76–79
| Synthesized particles | β (radian) | L (nm) | d (Å) (002) | d (Å) (100) | a = b (Å) | c (Å) | ε × 10−3 | δ × 10−3 (nm−2) |
|---|---|---|---|---|---|---|---|---|
| MoS2 | 1.12 | 7.51 | 6.30 | 2.672 | 3.08 | 12.60 | 39.41 | 17.71 |
| MoS2/MnO2(2 wt%) | 1.30 | 6.40 | 6.35 | 2.675 | 3.09 | 12.70 | 46.65 | 20.07 |
| MoS2/MnO2(4 wt%) | 1.34 | 6.23 | 6.34 | 2.671 | 3.08 | 12.68 | 47.85 | 20.61 |
| MoS2/MnO2(6 wt%) | 1.49 | 5.59 | 6.37 | 2.674 | 3.09 | 12.75 | 53.58 | 23.01 |
3.4. Electrochemical performance analysis
Na+ cation has a great impact on the charge–discharge electrochemistry of the MoS2 NF and MoS2/MnO2 nanocomposites. A mechanism could be suggested as follows:57,80–83
| MoS2 + Na+ + e− ↔ (MoS2) − Na+ (surface) |
| MnO2 + Na+ + e− ↔ (MnO2 − Na+) (surface) |
| (MoS2/MnO2)surface + Na+ + e− ↔ ((MoS2/MnO2) − Na+) (surface) |
| MoS2 + Na+ + e− ↔ Mo S − S Na (intercalation) |
| MnO2 + Na+ + e− ↔ MnO − ONa (intercalation) |
| MoS2/MnO2 + Na+ + e− ↔ (MoS2/MnO2)Na (intercalation) |
For a higher concentration of MnO2 (6 wt%), the shape of the CV curve deviates from the rectangle shape. Such leaf shape of CV curves indicates the presence of electrical double-layer capacitance (EDLC).84–86
where m = mass of active materials, Δt = discharge time, i = discharge current, and ΔV = potential window width. Fig. 9 shows the specific capacitance of the synthesized materials as a function of current densities. At 40 mA g−1 current density, the specific capacitance (Fig. 9) obtained from the GCD curves was 199.12 F g−1, 57.74 F g−1, 48.11 F g−1, for MoS2/MnO2 (6 wt%), MoS2/MnO2 (4 wt%), MoS2/MnO2 (2 wt%), respectively, while only 12.73 F g−1 for the MoS2 NF. Here, the MoS2/MnO2(6 wt%) NC shows the greatest specific capacitance of all the synthesized materials.
 | ||
| Fig. 8 (a)–(d) Galvanostatic charging–discharging curves of MoS2 NF and MoS2/MnO2 NCs at various current densities. € GCD measurements at 0.05 A g−1. | ||
In the GCD curve, iR drop during discharging gives the intrinsic resistance characteristics of the samples.87 At a high discharge current density of 140 mA g−1(Fig. 10), for MoS2/MnO2 NCs short discharge occurs because of electrical double-layer capacitance. For longer discharge electric double-layer capacitance and faradaic capacitance are responsible.87 The MoS2/MnO2 (6 wt%) nanocomposite showed the least iR drop of all the nanocomposites, suggesting less internal resistance of the active materials. During charging–discharging, energy dissipation was eliminated for lower internal resistance materials, and as a result, the energy storage performance improved.87,91 So, to fabricate power-saving supercapacitors, the MoS2/MnO2 (6 wt%) nanocomposite is preferable.
The energy density (E) and power density (P) were determined from the GCD curves of the synthesized samples by using eqn (4) and (5), respectively92
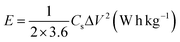 | (4) |
 | (5) |
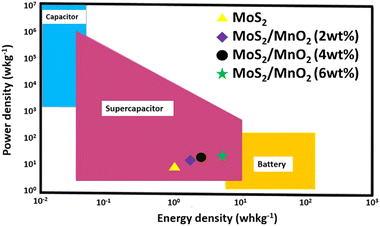
Fig. 11 illustrates the Ragone curve for the MoS2 NF and MoS2/MnO2 NCs and the values are presented in the supercapacitor region.93
 | ||
| Fig. 12 (a) Nyquist curves of the synthesized samples. (b) High frequency zone Nyquist curves of the synthesized samples. The inset shows the equivalent circuit design of Randle's model. | ||
| Sample | R ct (Ω) | CPE-T (μF) | CPE-P | W (Ω) |
|---|---|---|---|---|
| MoS2 | 2.6 | 0.0835 | 0.99 | 10.16 |
| MoS2/MnO2(2 wt%) | 2.5 | 0.2520 | 0.95 | 6.55 |
| MoS2/MnO2(4 wt%) | 2.7 | 0.2600 | 0.98 | 6.39 |
| MoS2/MnO2(6 wt%) | 2.5 | 0.3309 | 1.00 | 5.93 |
Table 3 shows that the Rct (Ω) value has decreased and the CPE-T (μF) value has increased for high concentrations of MnO2 in the MoS2 NF indicating good electrical conductivity, high wettability, higher rate capability, increased reaction rates of the NC electrode, and better efficiency.96,97 Quasi-semicircles and low Rct are the results of double-layer capacitance as well as faradaic reactions (redox) on the electrode surface.95 Due to enlarging interlayer distance the electrolyte gets easy access to the active material; as a result charge transfer resistance decreases when the concentration of MnO2 increases in the MoS2 NF.98 Here CPE is utilized instead of a capacitor because of the compensation of the lack of uniformity in the process and represents the nonideal capacitive property, as, at the microscopic level the electrode surface is porous and rough.98 In fact, the porosity and roughness of the surface may create a capacitance of double-layer type where the value of CPE-P in the range of 0.9 and 1 can show up as a constant phase element, and from Table 3 it can be seen that the CPE-P value of our NC samples satisfies the range. Also, when the MnO2 concentration increases in the MoS2 NF, the value of W (Ω) decreases. It denotes a shorter diffusion path and reduced diffusive resistance for MoS2/MnO2 NCs giving better electrochemical performance.99
From the XRD data (Table 1) the MoS2/MnO2 (6 wt%) nanocomposite has the highest amount of dislocation density. Improvement in the double-layer capacitive property occurs due to higher dislocation density. Increased dislocation density reduces charge transfer resistance because of the smaller phase angle, so faster electron movement occurs between the active material and the electrolyte.98,100
So, from the EIS data, we get that MoS2/MnO2 (6 wt%) NC shows better capacitive performance and this finding is compatible with CV and GCD tests.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles at a current density of 170 mA g−1. From Fig. 13(a) we observed that after 10
000 charge/discharge cycles at a current density of 170 mA g−1. From Fig. 13(a) we observed that after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles retention was around 95%. The nanocomposite also gives an excellent coulombic efficiency of 106% as shown in Fig. 13(a). This superior coulombic efficiency as well as cycling stability can be attributed to the increased wettability, stability in structure, and faster ion diffusion.101 The Nyquist plots for the 1st cycle and 10
000 cycles retention was around 95%. The nanocomposite also gives an excellent coulombic efficiency of 106% as shown in Fig. 13(a). This superior coulombic efficiency as well as cycling stability can be attributed to the increased wettability, stability in structure, and faster ion diffusion.101 The Nyquist plots for the 1st cycle and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th cycles are presented in Fig. 13(b) which demonstrates that after 10
000th cycles are presented in Fig. 13(b) which demonstrates that after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles the radius of the semi-circular portion of the EIS spectrum becomes lower. This indicates reduced charge transfer resistance of the active materials after 10
000 cycles the radius of the semi-circular portion of the EIS spectrum becomes lower. This indicates reduced charge transfer resistance of the active materials after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.96,97 The insets of Fig. 13(b) show the fitted result and the values of the corresponding equivalent circuit components are presented in Table 4. Rct and W values were found to be reduced after 10
000 cycles.96,97 The insets of Fig. 13(b) show the fitted result and the values of the corresponding equivalent circuit components are presented in Table 4. Rct and W values were found to be reduced after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of operation which indicates the generation of more active sites and faster electron transport between the electrode and solution.102,103
000 cycles of operation which indicates the generation of more active sites and faster electron transport between the electrode and solution.102,103
| Cycle | R ct (Ω) | CPE-T (μF) | CPE-P | W (Ω) |
|---|---|---|---|---|
| 1 | 2.50 | 0.33 | 1.00 | 5.93 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.90 | 0.33 | 0.98 | 5.90 |
Table 5 presents the statistics of a MoS2 based electrode.24,34,104–106 Among all the reported statistics, the MoS2/MnO2 (6%) provides better capacitive performance. This work yields better specific capacitance with superior stability. The collaborative effect of the MoS2 NF and MnO2 nanorod, reduced crystallite size, defect-mediated structure, and improved electrical conductivity are responsible for this amazing outcome. So, this composite has a great deal to offer as a contender for high-performance electrode material for capacitors.
| Materials of electrode | Electrolyte/electrode composition (mass ratio) | Current density (A g−1) | Specific capacitance (F g−1) | Cycle number | Retained specific capacitance % | Ref. |
|---|---|---|---|---|---|---|
| MoS2/Mn3O4 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1 | 172 | 2000 | 69.3 | 35 |
| MoS2 nanospheres//Active Carbon (AC) | 1 | 65.33 | 104 | |||
| MoS2NF | 1 M KCl | 1 | 168 | 3000 | 92.6 | 105 |
| MoS2-G | 1 M Na2SO4 | 5 | 130 | 1000 | 92.3 | 106 |
| MoS2 nanowires/NiCo2O4//Active Carbon (AC) | 1.5 | 51.7 | 8000 | 98.2 | 34 | |
| MoS2-CNT | 1 M Na2SO4 | 2 | 74 | 1000 | 80.8 | 24 |
| MoS2/MnO2 | 0.5Na2SO4 | .04 | 199.12 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
95 | This work |
4. Conclusion
The incorporation of MnO2 nanorods into an MoS2 NF provides an increased surface area that is electrochemically active for charge transfer and reduces the ion diffusion length at the time of charging–discharging. The synergistic effect of a 3D-1D structure reduces the inactive volume, opening the opportunity for ions of electrolyte to access the active material. The pseudocapacitive performance alongside the electrical double-layer capacitance of the MoS2 NF can be considerably increased by the MnO2 nanorod. The MoS2/MnO2(6 wt%) nanocomposite shows a higher specific capacitance (199.12 F g−1 at 40 mA g−1), improved capacitance retention, and cycle stability. High-performance energy storage devices can be made with this 3D-1D architectural electrode material that has exceptional electrochemical properties, enhanced stability, and coulombic efficiency.Conflicts of interest
There are no conflicts to declare.References
- P. Simon and Y. Gogotsi, Materials for electrochemical capacitors, Nat. Mater., 2010, 320–329, DOI:10.1038/nmat2297.
- P. Y. Liu, J. J. Zhao, Z. P. Dong, Z. L. Liu and Y. Q. Wang, Interwoving polyaniline and a metal-organic framework grown in situ for enhanced supercapacitor behavior, J. Alloys Compd., 2021, 854, 157181, DOI:10.1016/j.jallcom.2020.157181.
- Y. Zhao, H. Li, R. Tang, X. Wang, Y. Wu, S. Yan and Y. Zhang, Photo-assisted asymmetric supercapacitors based on dual photoelectrodes for enhanced photoelectric energy storage, J. Mater. Chem. A, 2023, 11(29), 15844–15854, 10.1039/D3TA01461D.
- T. Zhu, J. Pan, Z. An, R. Zhe, Q. Ou and H. E. Wang, Bifunctional NiCuOx photoelectrodes to promote pseudocapacitive charge storage by in situ photocharging, J. Mater. Chem. A, 2022, 10(38), 20375–20385, 10.1039/D2TA04966J.
- H. Lu and X. S. Zhao, Biomass-derived carbon electrode materials for supercapacitors, Sustainable Energy Fuels, 2017, 1, 1265–1281, 10.1039/C7SE00099E.
- J. Zhang and X. S. Zhao, On the Configuration of Supercapacitors for Maximizing Electrochemical Performance, ChemSusChem, 2012, 5(5), 818–841, DOI:10.1002/cssc.201100571.
- K. Namsheer and C. S. Rout, Photo-powered integrated supercapacitors: a review on recent developments, challenges and future perspectives, J. Mater. Chem. A, 2021, 9(13), 8248–8278, 10.1039/D1TA00444A.
- C. Xu, B. Xu, Y. Gu, Z. Xiong, J. Sun and X. S. Zhao, Graphene-based electrodes for electrochemical energy storage, Energy Environ. Sci., 2013, 6(5), 1388–1414, 10.1039/C3EE23870A.
- Y. Zhong, X. Xia, F. Shi, J. Zhan, J. Tu and H. J. Fan, Transition metal carbides and nitrides in energy storage and conversion, Adv. Sci., 2016, 3(5), 1500286, DOI:10.1002/advs.201500286.
- Y. X. Chen, K. J. Huang and K. X. Niu, Recent advances in signal amplification strategy based on oligonucleotide and nanomaterials for microRNA detection-a review, Biosens. Bioelectron., 2018, 99, 612–624, DOI:10.1016/j.bios.2017.08.036.
- W. M. Huang, C. Y. Hsu and D. H. Chen, Sodium tungsten oxide nanowires-based all-solid-state flexible transparent supercapacitors with solar thermal enhanced performance, Chem. Eng. J., 2022, 431, 134086, DOI:10.1016/j.cej.2021.134086.
- Y. F. Ren, Z. L. He, H. Z. Zhao and T. Zhu, Fabrication of MOF-derived mixed metal oxides with carbon residues for pseudocapacitors with long cycle life, Rare Met., 2022, 1–6, DOI:10.1007/s12598-021-01836-8.
- Y. H. Wang, K. J. Huang and X. Wu, Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review, Biosens. Bioelectron., 2017, 97, 305–316, DOI:10.1016/j.bios.2017.06.011.
- D. Lan, Y. Chen, P. Chen, X. Chen, X. Wu, X. Pu, Y. Zeng and Z. Zhu, Mesoporous CoO nanocubes@ continuous 3D porous carbon skeleton of rose-based electrode for high-performance supercapacitor, ACS Appl. Mater. Interfaces, 2014, 6(15), 11839–11845, DOI:10.1021/am503378n.
- T. Wang, S. Chen, H. Pang, H. Xue and Y. Yu, MoS2-based nanocomposites for electrochemical energy storage, Adv. Sci., 2017, 4(2), 1600289, DOI:10.1002/advs.201600289.
- Y. Ren, T. Zhu, Y. Liu, Q. Liu and Q. Yan, Direct utilization of photoinduced charge carriers to promote electrochemical energy storage, Small, 2021, 17(21), 2008047, DOI:10.1002/smll.202008047.
- T. Zhu, Z. He, Z. An, R. Xu, Y. Li, R. Zhe, H. E. Wang and H. Pang, Enhancing solar energy harvest by Cu2S/CuCl heteroarrays with enriched sulfur vacancies for photo-rechargeable pseudocapacitors, Sci. China Mater., 2023, 66(6), 2216–2226, DOI:10.1007/s40843-022-2341-2.
- J. H. Kim, S. J. Koo, J. Y. Cheon, Y. Jung, S. Cho, D. Lee, J. W. Choi, T. Kim and M. Song, Self-powered and flexible integrated solid-state fiber-shaped energy conversion and storage based on CNT yarn with efficiency of 5.5%, Nano Energy, 2022, 96, 107054, DOI:10.1016/j.nanoen.2022.107054.
- W. Gu and G. Yushin, Review of nanostructured carbon materials for electrochemical capacitor applications: advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene, Wiley Interdiscip. Rev.: Energy Environ., 2014, 3(5), 424–473 CAS.
- P. Thounthong, V. Chunkag, P. Sethakul, S. Sikkabut, S. Pierfederici and B. Davat, Energy management of fuel cell/solar cell/supercapacitor hybrid power source, J. Power Sources, 2011, 196(1), 313–324, DOI:10.1016/j.jpowsour.2010.01.051.
- X. Chia, A. Y. S. Eng, A. Ambrosi, S. M. Tan and M. Pumera, Electrochemistry of nanostructured layered transition-metal dichalcogenides, Chem. Rev., 2015, 115(21), 11941–11966, DOI:10.1021/acs.chemrev.5b00287.
- R. N. Bulakhe and J. J. Shim, Layer-structured nanohybrid MoS2@ rGO on 3D nickel foam for high performance energy storage applications, New J. Chem., 2017, 41(4), 1473–1482, 10.1039/C6NJ02590K.
- N. Choudhary, M. R. Islam, N. Kang, L. Tetard, Y. Jung and S. I. Khondaker, Two-dimensional lateral heterojunction through bandgap engineering of MoS2 via oxygen plasma, J. Phys.: Condens. Matter, 2016, 28(36), 364002, DOI:10.1088/0953-8984%2F28%2F36%2F364002.
- M. Chen, Y. Dai, J. Wang, Q. Wang, Y. Wang, X. Cheng and X. Yan, Smart combination of three-dimensional-flower-like MoS2 nanospheres/interconnected carbon nanotubes for application in supercapacitor with enhanced electrochemical performance, J. Alloys Compd., 2017, 696, 900–906 CrossRef CAS.
- F. Ghasemi, M. Jalali, A. Abdollahi, S. Mohammadi, Z. Sanaee and S. Mohajerzadeh, A high performance supercapacitor based on decoration of MoS2/reduced graphene oxide with NiO nanoparticles, RSC Adv., 2017, 7(83), 52772–52781, 10.1039/C7RA09060A.
- Y. Zhang, T. He, G. Liu, L. Zu and J. Yang, One-pot mass preparation of MoS2/C aerogels for high-performance supercapacitors and lithium-ion batteries, Nanoscale, 2017, 9(28), 10059–10066, 10.1039/C7NR03187D.
- X. Zhou, L. J. Wan and Y. G. Guo, Facile synthesis of MoS2@ CMK-3 nanocomposite as an improved anode material for lithium-ion batteries, Nanoscale, 2012, 4(19), 5868–5871, 10.1039/C2NR31822A.
- J. B. Cook, H. S. Kim, Y. Yan, J. S. Ko, S. Robbennolt, B. Dunn and S. H. Tolbert, Mesoporous MoS2 as a transition metal dichalcogenide exhibiting pseudocapacitive Li and Na-ion charge storage, Adv. Energy Mater., 2016, 6(9), 1501937, DOI:10.1002/aenm.201501937.
- N. Kanaujiya, N. Kumar, A. K. Srivastava, Y. Sharma and G. D. Varma, One-step synthesized mesoporous MnO2@ MoS2 nanocomposite for high performance energy storage devices, J. Electroanal. Chem., 2018, 824, 226–237, DOI:10.1016/j.jelechem.2018.07.046.
- Y. Shen, Z. Li, Z. Cui, K. Zhang, R. Zou, F. Yang and K. Xu, Boosting the interface reaction activity and kinetics of cobalt molybdate by phosphating treatment for aqueous zinc-ion batteries with high energy density and long cycle life, J. Mater. Chem. A, 2020, 8(40), 21044–21052, 10.1039/D0TA07746A.
- S. Ramesh, K. Karuppasamy, A. Sivasamy, H. S. Kim, H. M. Yadav and H. S. Kim, Core shell nanostructured of Co3O4@ RuO2 assembled on nitrogen-doped graphene sheets electrode for an efficient supercapacitor application, J. Alloys Compd., 2021, 877, 160297, DOI:10.1016/j.jallcom.2021.160297.
- M. Wang, H. Fei, P. Zhang and L. Yin, Hierarchically layered MoS2/Mn3O4 hybrid architectures for electrochemical supercapacitors with enhanced performance, Electrochim. Acta, 2016, 209, 389–398, DOI:10.1016/j.electacta.2016.05.078.
- X. Liao, Y. Zhao, J. Wang, W. Yang, L. Xu, X. Tian, Y. Shuang, K. A. Owusu, M. Yan and L. Mai, MoS2/MnO2 heterostructured nanodevices for electrochemical energy storage, Nano Res., 2018, 11, 2083–2092 CrossRef CAS.
- S. Wen, Y. Liu, F. Zhu, R. Shao and W. Xu, Hierarchical MoS2 nanowires/NiCo2O4 nanosheets supported on Ni foam for high-performance asymmetric supercapacitors, Appl. Surf. Sci., 2018, 428, 616e22, DOI:10.1016/j.apsusc.2017.09.189.
- J. Zhou, M. Guo, L. Wang, Y. Ding, Z. Zhang, Y. Tang, C. Liu and S. Luo, 1T-MoS2 nanosheets confined among TiO2 nanotube arrays for high performance supercapacitor, Chem. Eng. J., 2019, 366, 163–171, DOI:10.1016/j.cej.2019.02.079.
- Y. Zhang, T. He, G. Liu, L. Zu and J. Yang, One-pot mass preparation of MoS2/C aerogels for high-performance supercapacitors and lithium-ion batteries, Nanoscale, 2017, 9(28), 10059–10066, 10.1039/c7nr03187d.
- N. Feng, et al., A polymer-direct-intercalation strategy for MoS2/carbon-derived heteroaerogels with ultrahigh pseudocapacitance, Nat. Commun., 2019, 10, 1372, DOI:10.1038/s41467-019-09384-7.
- T. Stephenson, Z. Li, B. Olsen and D. Mitlin, Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites, Energy Environ. Sci., 2014, 7(1), 209–231, 10.1039/c3ee42591f.
- E. Pomerantseva and Y. Gogotsi, Two-dimensional heterostructures for energy storage, Nat. Energy, 2017, 2(7), 1–6, DOI:10.1038/nenergy.2017.89.
- X. Zhang, Z. Lai, C. Tan and H. Zhang, Solution-processed two-dimensional MoS2 nanosheets: preparation, hybridization, and applications, Angew. Chem., Int. Ed., 2016, 55(31), 8816–8838, DOI:10.1002/anie.201509933.
- X. Wang, et al., High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures, J. Mater. Chem. A, 2014, 2(38), 15958–15963, 10.1039/C4TA03044C.
- Z. Wu, B. Li, Y. Xue, J. Li, Y. Zhang and F. Gao, Fabrication of defect-rich MoS2 ultrathin nanosheets for application in lithium-ion batteries and supercapacitors, J. Mater. Chem. A, 2015, 3(38), 19445–19454, 10.1039/C5TA04549E.
- M. H. Ahmad, R. B. Alam, A. Ul-Hamid, S. F. U. Farhad and M. R. Islam, Hydrothermal synthesis of Co3O4 nanoparticles decorated three dimensional MoS2 nanoflower for exceptionally stable supercapacitor electrode with improved capacitive performance, J. Energy Storage, 2022, 47, 103551, DOI:10.1016/j.est.2021.103551.
- P. He, X. Zhao, Y. Zhang, J. Wu, N. Chen, J. Wei and T. Xu, Remove elemental mercury from simulated flue gas by flower-like MoS2 modified with nanoparticles MnO2, Chem. Eng. J., 2021, 412, 128588, DOI:10.1016/j.cej.2021.128588.
- R. Sha, A. Gopalakrishnan, K. V. Sreenivasulu, V. V. Srikanth and S. Badhulika, Template-cum-catalysis free synthesis of α-MnO2 nanorods-hierarchical MoS2 microspheres composite for ultra-sensitive and selective determination of nitrite, J. Alloys Compd., 2019, 794, 26–34, DOI:10.1016/j.jallcom.2019.04.251.
- J. G. Wang, F. Kang and B. Wei, Engineering of MnO2-based nanocomposites for high-performance supercapacitors, Prog. Mater. Sci., 2015, 74, 51–124, 10.1039/C5TA05523G.
- M. Huang, F. Li, F. Dong, Y. X. Zhang and L. L. Zhang, MnO2-based nanostructures for high-performance supercapacitors, J. Mater. Chem. A, 2015, 3(43), 21380–21423, 10.1039/C5TA05523G.
- X. Duan, J. Yang, H. Gao, J. Ma, L. Jiao and W. Zheng, Controllable hydrothermal synthesis of manganese dioxide nanostructures: shape evolution, growth mechanism and electrochemical properties, CrystEngComm, 2012, 14(12), 4196–4204 RSC.
- B. H. Park and J. H. Choi, Improvement in the capacitance of a carbon electrode prepared using water-soluble polymer binder for a capacitive deionization application, Electrochim. Acta, 2010, 55(8), 2888–2893, DOI:10.1016/j.electacta.2009.12.084.
- H. K. Park, B. S. Kong and E. S. Oh, Effect of high adhesive polyvinyl alcohol binder on the anodes of lithium ion batteries, Electrochem. Commun., 2011, 13(10), 1051–1053, DOI:10.1016/j.elecom.2011.06.034.
- X. Wang and Y. Li, Rational synthesis of α-MnO2 single-crystal nanorods, Chem. Commun., 2002, 764–765, 10.1039/b111723h.
- X. Zhang, X. Huang, M. Xue, X. Ye, W. Lei, H. Tang and C. Li, Hydrothermal synthesis and characterization of 3D flower-like MoS2 microspheres, Mater. Lett., 2015, 148, 67–70, DOI:10.1166/nnl.2017.2321.
- L. Hu, Y. Ren, H. Yang and Q. Xu, Fabrication of 3D hierarchical MoS2/polyaniline and MoS2/C architectures for lithium-ion battery applications, ACS Appl. Mater. Interfaces, 2014, 6(16), 14644–14652, DOI:10.1021/am503995s.
- H. Fu, K. Yu, H. Li, J. Li, B. Guo, Y. Tan, C. Song and Z. Zhu, Enhanced field emission and photocatalytic performance of MoS2 titania nanoheterojunctions via two synthetic approaches, Dalton Trans., 2015, 44(4), 1664–1672, 10.1039/C4DT03035D.
- X. Bai, X. Tong, Y. Gao, W. Zhu, C. Fu, J. Ma, T. Tan, C. Wang, Y. Luo and H. Sun, Hierarchical multidimensional MnO2 via hydrothermal synthesis for high performance supercapacitors, Electrochim. Acta, 2018, 281, 525–533, DOI:10.1016/j.electacta.2018.06.003.
- Z. Wu, B. Li, Y. Xue, J. Li, Y. Zhang and F. Gao, Fabrication of defect-rich MoS2 ultrathin nanosheets for application in lithium-ion batteries and supercapacitors, J. Mater. Chem. A, 2015, 3(38), 19445–19454, 10.1039/C5TA04549E.
- C. Chai, A. Liu, Y. Wang, Y. Lu and H. Che, A MoS2-templated oxidation-etching strategy to synthesize hollow δ-MnO2 nanospheres as a high-performance electrode for supercapacitor, Ceram. Int., 2018, 44(14), 16923–16930, DOI:10.1016/j.ceramint.2018.06.132.
- F. Xiong, Z. Cai, L. Qu, P. Zhang, Z. Yuan, O. K. Asare, W. Xu, C. Lin and L. Mai, Three-dimensional crumpled reduced graphene oxide/MoS2 nanoflowers: a stable anode for lithium-ion batteries, ACS Appl. Mater. Interfaces, 2015, 7(23), 12625–12630, DOI:10.1021/acsami.5b02978.
- J. Wang, H. Zhou, M. Zhu, A. Yuan and X. Shen, Metal-organic framework-derived Co3O4 covered by MoS2 nanosheets for high-performance lithium-ion batteries, J. Alloys Compd., 2018, 744, 220–227, DOI:10.1016/j.jallcom.2018.02.086.
- H. Wang, Z. Lu, D. Qian, Y. Li and W. Zhang, Single-crystal α-MnO2 nanorods: synthesis and electrochemical properties, Nanotechnology, 2007, 18(11), 115616, DOI:10.1088/0957-4484/18/11/115616.
- H. Song, A. Tang, G. Xu, L. Liu, Y. Pan and M. Yin, Hydrothermal synthesis and electrochemical properties of MoS2/C nanocomposite, Int. J. Electrochem. Sci., 2018, 13, 6708–6716 CrossRef CAS.
- H. Fu, K. Yu, H. Li, J. Li, B. Guo, Y. Tan, C. Song and Z. Zhu, Enhanced field emission and photocatalytic performance of MoS2 titania nanoheterojunctions via two synthetic approaches, Dalton Trans., 2015, 44(4), 1664–1672, 10.1039/C4DT03035D.
- X. Lin, X. Wang, Q. Zhou, C. Wen, S. Su, J. Xiang, P. Cheng, X. Hu, Y. Li, X. Wang and X. Gao, Magnetically recyclable MoS2/Fe3O4 hybrid composite as visible light responsive photocatalyst with enhanced photocatalytic performance, ACS Sustainable Chem. Eng., 2018, 7(1), 1673–1682, DOI:10.1021/acssuschemeng.8b05440.
- H. Li, K. Yu, X. Lei, B. Guo, H. Fu and Z. Zhu, Hydrothermal synthesis of novel MoS2/BiVO4 hetero-nanoflowers with enhanced photocatalytic activity and a mechanism investigation, J. Phys. Chem. C, 2015, 119(39), 22681–22689, DOI:10.1021/acs.jpcc.5b06729.
- G. Du, Z. Guo, S. Wang, R. Zeng, Z. Chen and H. Liu, Superior stability and high capacity of restacked molybdenum disulfide as anode material for lithium ion batteries, Chem. Commun., 2010, 46(7), 1106–1108, 10.1039/B920277C.
- V. Radmilovic, H. A. Gasteiger and P. N. Ross, Structure and chemical composition of a supported Pt-Ru electrocatalyst for methanol oxidation, J. Catal., 1995, 154(1), 98–106, DOI:10.1006/jcat.1995.1151.
- W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80(6), 1339, DOI:10.1021/ja01539a017.
- X. Zhang, et al., Facile synthesis of yolk–shell MoO2 microspheres with excellent electrochemical performance as a Li-ion battery anode, J. Mater. Chem. A, 2013, 1(23), 6858–6864, 10.1039/C3TA10399D.
- X. Zhang, et al., Hydrothermal synthesis and characterization of 3D flower-like MoS2 microspheres, Mater. Lett., 2015, 148, 67–70, DOI:10.1016/j.matlet.2015.02.027.
- X. Wang, Z. Zhang, Y. Chen, Y. Qu, Y. Lai and J. Li, Morphology-controlled synthesis of MoS2 nanostructures with different lithium storage properties, J. Alloys Compd., 2014, 600, 84–90, DOI:10.1016/j.jallcom.2014.02.127.
- K. K. Paul, N. Sreekanth, R. K. Biroju, T. N. Narayanan and P. K. Giri, Solar light driven photoelectrocatalytic hydrogen evolution and dye degradation by metal-free fewlayer MoS2 nanoflower/TiO2(B) nanobelts heterostructure, Sol. Energy Mater. Sol. Cells, 2018, 185, 364–374, DOI:10.1016/j.solmat.2018.05.056.
- G. Du, Z. Guo, S. Wang, R. Zeng, Z. Chen and H. Liu, Superior stability and high capacity of restacked molybdenum disulfide as anode material for lithium ion batteries, Chem. Commun., 2010, 46(7), 1106–1108, 10.1039/b920277c.
- A. A. Akl and A. S. Hassanien, Microstructure and crystal imperfections of nanosized CdSxSe1-x thermally evaporated thin films, Superlattices Microstruct., 2015, 85, 67–81, DOI:10.1016/j.spmi.2015.05.011.
- L. Ma, L. Xu, X. Xu, X. Zhou, J. Luo and L. Zhang, Cobalt-doped edge-rich MoS2/nitrogenated graphene composite as an electrocatalyst for hydrogen evolution reaction, Mater. Sci. Eng., B, 2016, 212, 30–38, DOI:10.1016/j.mseb.2016.07.014.
- Q. Fan, A new method of calculating interplanar spacing: the position-factor method, J. Appl. Crystallogr., 2012, 45(6), 1303–1308, DOI:10.1107/S0021889812037764.
- G. Tang, Y. Wang, W. Chen, H. Tang and C. Li, Hydrothermal synthesis and characterization of novel flowerlike MoS2 hollow microspheres, Mater. Lett., 2013, 100, 15–18, DOI:10.1016/j.matlet.2013.02.103.
- R. Zhou, C. Jie Han and X. Min Wang, Hierarchical MoS2-coated three-dimensional graphene network for enhanced supercapacitor performances, J. Power Sources, 2017, 352, 99–110, DOI:10.1016/j.jpowsour.2017.03.134.
- X. Li, L. Zhang, X. Zang, X. Li and H. Zhu, Photo-promoted platinum nanoparticles decorated MoS2@graphene woven fabric catalyst for efficient hydrogen generation, ACS Appl. Mater. Interfaces, 2016, 8(17), 10866–10873, DOI:10.1021/acsami.6b01903.
- A. Gigot, et al., Mixed 1T-2H phase MoS2/reduced graphene oxide as active electrode for enhanced supercapacitive performance, ACS Appl. Mater. Interfaces, 2016, 8(48), 32842–32852, DOI:10.1021/acsami.6b11290.
- S. Patil, A. Harle, S. Sathaye and K. Patil, Development of a novel method to grow mono-/few-layered MoS2 films and MoS2–graphene hybrid films for supercapacitor applications, CrystEngComm, 2014, 16(47), 10845–10855, 10.1039/C4CE01595A.
- M. Huang, F. Li, F. Dong, Y. X. Zhang and L. L. Zhang, MnO2-based nanostructures for high-performance supercapacitors, J. Mater. Chem. A, 2015, 3(43), 21380–21423 RSC.
- T. Nguyen, M. Boudard, M. J. Carmezim and M. F. Montemor, Hydrogen bubbling-induced micro/nano porous MnO2 films prepared by electrodeposition for pseudocapacitor electrodes, Electrochim. Acta, 2016, 202, 166–174 CrossRef CAS.
- X. Tao, J. Du, Y. Sun, S. Zhou, Y. Xia, H. Huang, Y. Gan, W. Zhang and X. Li, Exploring the energy storage mechanism of high performance MnO2 electrochemical capacitor electrodes: an in situ atomic force microscopy study in aqueous electrolyte, Adv. Funct. Mater., 2013, 23(37), 4745–4751, DOI:10.1002/adfm.201300359.
- A. K. Thakur, A. B. Deshmukh, R. B. Choudhary, I. Karbhal, M. Majumder and M. V. Shelke, Facile synthesis and electrochemical evaluation of PANI/CNT/MoS2 ternary composite as an electrode material for high performance supercapacitor, Mater. Sci. Eng., B, 2017, 223, 24–34, DOI:10.1016/j.mseb.2017.05.001.
- J. M. Soon and K. P. Loh, Electrochemical double-layer capacitance of MoS2 nanowall films, Electrochem. Solid-State Lett., 2007, 10(11), A250, DOI:10.1149/1.2778851.
- H. Zhang, J. Wei, Y. Yan, Q. Guo, L. Xie, Z. Yang, J. He, W. Qi, Z. Cao, X. Zhao and P. Pan, Facile and scalable fabrication of MnO2 nanocrystallines and enhanced electrochemical performance of MnO2/MoS2 inner heterojunction structure for supercapacitor application, J. Power Sources, 2020, 450, 227616, DOI:10.1016/j.jpowsour.2019.227616.
- Y. Xie and H. Du, Electrochemical capacitance of a carbon quantum dots–polypyrrole/titania nanotube hybrid, RSC Adv., 2015, 5(109), 89689–89697, 10.1039/C5RA16538E.
- M. Majumder, R. B. Choudhary, A. K. Thakur and I. Karbhal, Impact of rare-earth metal oxide (Eu2O3) on the electrochemical properties of a polypyrrole/CuO polymeric composite for supercapacitor applications, RSC Adv., 2017, 7(32), 20037–20048, 10.1039/C7RA01438D.
- V. K. A. Muniraj, C. K. Kamaja and M. V. Shelke, RuO2· nH2O nanoparticles anchored on carbon nano-onions: an efficient electrode for solid state flexible electrochemical supercapacitor, ACS Sustainable Chem. Eng., 2016, 4(5), 2528–2534, DOI:10.1021/acssuschemeng.5b01627.
- Y. X. Chen, W. J. Ma, K. F. Cai, X. W. Yang and C. J. Huang, In situ growth of polypyrrole onto three-dimensional tubular MoS2 as an advanced negative electrode material for supercapacitor, Electrochim. Acta, 2017, 246, 615–624 CrossRef CAS.
- C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang and J. Zhang, A review of electrolyte materials and compositions for electrochemical supercapacitors, Chem. Soc. Rev., 2015, 44(21), 7484–7539, 10.1039/C5CS00303B.
- J. Bhagwan, A. Sahoo, K. L. Yadav and Y. Sharma, Porous, one dimensional and high aspect ratio Mn3O4 nanofibers: fabrication and optimization for enhanced supercapacitive properties, Electrochim. Acta, 2015, 174, 992–1001, DOI:10.1016/j.electacta.2015.06.073.
- Y. Liu, Q. Wu, L. Liu, P. Manasa, L. Kang and F. Ran, Vanadium nitride for aqueous supercapacitors: a topic review, J. Mater. Chem. A, 2020, 8(17), 8218–8233, 10.1039/d0ta01490g.
- C. Portet, P. L. Taberna, P. Simon and C. Laberty-Robert, Modification of Al current collector surface by sol–gel deposit for carbon–carbon supercapacitor applications, Electrochim. Acta, 2004, 49(6), 905–912, DOI:10.1016/j.electacta.2003.09.043.
- Y. Chen, X. Zhu, D. Yang, P. Wangyang, B. Zeng and H. Sun, A novel design of poly (3, 4-ethylenedioxythiophene): poly (styrenesulfonate)/molybdenum disulfide/poly (3, 4-ethylenedioxythiophene) nanocomposites for fabric micro-supercapacitors with favourable performances, Electrochim. Acta, 2019, 298, 297–304, DOI:10.1016/j.electacta.2018.12.083.
- Y. Chen, W. Ma, K. Cai, X. Yang and C. Huang, In situ growth of polypyrrole onto three-dimensional tubular MoS2 as an advanced negative electrode material for supercapacitor, Electrochim. Acta, 2017, 246, 615–624, DOI:10.1016/j.electacta.2017.06.102.
- L. Ma, L. Xu, X. Xu, X. Zhou, J. Luo and L. Zhang, Cobalt-doped edge-rich MoS2/nitrogenated graphene composite as an electrocatalyst for hydrogen evolution reaction, Mater. Sci. Eng., B, 2016, 212, 30–38 CrossRef CAS.
- E. Rafiee, M. Farzam, M. A. Golozar and A. Ashrafi, An investigation on dislocation density in cold-rolled copper using electrochemical impedance spectroscopy, Int. Scholarly Res. Not., 2013, 2013, 921825, DOI:10.1155/2013/921825.
- C. Lamiel and J. J. Shim, Hierarchical mesoporous graphene@ Ni-Co-S arrays on nickel foam for high-performance supercapacitors, Electrochim. Acta, 2015, 161, 351–357, DOI:10.1016/j.matlet.2016.02.017.
- Y. Jia, Y. Lin, Y. Ma and W. Shi, Hierarchical MnS2-MoS2 nanotubes with efficient electrochemical performance for energy storage, Mater. Des., 2018, 160, 1071–1079, DOI:10.1016/j.matdes.2018.10.031.
- D. Li, et al., Transparent 1T-MoS2 nanofilm robustly anchored on substrate by layer-by-layer self-assembly and its ultra-high cycling stability as supercapacitors, Nanotechnology, 2017, 28(39), 395401, DOI:10.1088/1361-6528/aa7ee3.
- M. R. Islam, S. N. S. Pias, R. B. Alam and S. I. Khondaker, Enhanced electrochemical performance of solution-processed single-wall carbon nanotube reinforced polyvinyl alcohol nanocomposite synthesized via solution-cast method, Nano Exp., 2020, 1(3), 030013, DOI:10.1088/2632-959X/abc050.
- M. R. Islam and S. I. Mollik, Enhanced electrochemical performance of flexible and eco-friendly starch/graphene oxide nanocomposite, Heliyon, 2020, 6(10), e05292, DOI:10.1016/j.heliyon.2020.e05292.
- Y.-P. Gao, K.-J. Huang, X. Wu, Z.-Q. Hou and Y.-Y. Liu, MoS2 nanosheets assembling three-dimensional nanospheres for enhanced-performance supercapacitor, J. Alloys Compd., 2018, 741, 174e81, DOI:10.1016/j.jallcom.2018.01.110.
- X. Wang, J. Ding, S. Yao, X. Wu, Q. Feng, Z. Wang and B. Geng, High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures, J. Mater. Chem. A, 2014, 2(38), 15958–15963 RSC.
- K.-J. Huang, L. Wang, Y.-J. Liu, Y.-M. Liu, H.-B. Wang and T. Gan, et al., Layered MoS2egraphene composites for supercapacitor applications with enhanced capacitive performance, Int. J. Hydrogen Energy, 2013, 38, 14027e34, DOI:10.1016/J.IJHYDENE.2013.08.112.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00065j |
| This journal is © The Royal Society of Chemistry 2024 |