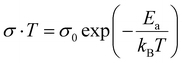Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemically-assisted synthesis of 3D, 2D and quasi 2D lead halide perovskites for supercapacitor applications†
Apurba
Mahapatra
 *a,
Manoranjan
Mandal
b,
Ayon
Das Mahapatra
c,
Vishnu
Anilkumar
*a,
Manoranjan
Mandal
b,
Ayon
Das Mahapatra
c,
Vishnu
Anilkumar
 a,
Jan
Nawrocki
a,
Jan
Nawrocki
 a,
Rohit D.
Chavan
a,
Rohit D.
Chavan
 a,
Pankaj
Yadav
*d and
Daniel
Prochowicz
a,
Pankaj
Yadav
*d and
Daniel
Prochowicz
 *a
*a
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, Warsaw 01-224, Poland. E-mail: amahapatra@ichf.edu.pl; pankaj.yadav@sse.pdpu.ac.in; dprochowicz@ichf.edu.pl
bDepartment of Physics, School of Science, GITAM University, Bengaluru, 561203, India
cInstitute of Chemistry, Hebrew University of Jerusalem, Jerusalem, 9190401, Israel
dDepartment of Solar Energy, School of Energy Technology, Pandit Deendayal Energy University, Gandhinagar, 382 007, Gujarat, India
First published on 1st March 2024
Abstract
Lead halide perovskite (LHP) thin-film-based energy storage devices have gained considerable attention due to their favourable electronic and ionic conductivity. Here, we have fabricated electrodes for electrolyte-based supercapacitors using mechanochemically synthesized LHPs and demonstrated that the type of starting material and dimensionality of the perovskite strongly influence the device performance. First, we have compared the electrochemical performance of electrodes obtained from the single crystalline (SCP) and mechanosynthesized (MSP) methylammonium lead tribromide (MAPbBr3), and ∼2 times improvement was observed in the MAPbBr3 MSP-based energy storage device due to higher average microstrain induced by ball milling, which modulates ion migration. Next, we fabricated electrodes based on mechanochemically synthesized MAPbBr3, 2D MA2PbBr4 and quasi-2D BA2MAPb2Br7, and demonstrated that the structure and ion migration could play a vital role in the overall performance of the LHP-based electrochemical supercapacitor. The areal capacitance (∼209 mF cm−2) and specific capacitance (∼138.35 F g−1) calculated at a scan rate of 5 mV s−1 with the maximum power density and cycle stability were found to be highest in the 2D MA2PbBr4 perovskite-based supercapacitor. This result can be attributed to its smallest particle size, which helps to improve the interaction between electrolyte ions and the perovskite electrode interface at a lower scan rate.
1. Introduction
The utilisation of green energy generated from solar cells may be limited due to its intermittent nature and weather-dependent performance. Thus, energy storage is essential to store the energy generated from solar cells at their peak performance and utilise the stored energy during the night or in bad weather. On the other hand, innovative energy storage devices with low cost and efficient performance are also vastly desirable to fulfil the growing demand for renewable power grids and electric vehicles. Electrochemical batteries are majorly used energy storage devices for uninterrupted energy supply. Supercapacitors (SUCs) are an attractive alternative to electrochemical batteries because of their high power density and long cycle life.1,2 Therefore, many efforts have been made to solve the relatively low energy density problem of SUCs to reach the industrial benchmark standards for a wide-range of applications such as electric vehicles, various electronic devices, and pulse power systems.1Lead halide perovskites (LHPs) are widely used for the fabrication of high-efficiency photovoltaic cells and other optoelectronic devices such as LEDs, sensors, and photodetectors.3–7 Recently, LHPs have been extensively explored as SUC working materials due to their high ion diffusion and mixed conductivity properties.8–11 Ions in the LHPs have low activation energies with significant ionic diffusion coefficients, which are favourable properties for efficient SUCs. In addition, easy and cost-effective fabrication of LHP materials with desired high ionic conductivity attracts the research community as emerging candidates for SUC application.11–13 Electrochemical supercapacitors (ESCs) typically consist of two narrow distanced electrodes filled with the electrolyte.13,14 The porosity of the electrodes increases the effective surface area with the electrolyte, and increases the ionic diffusion at the electrode/electrolyte interface. For this reason., the ion migration issue of LHP materials is a reward for ESC application.15
LHPs can be used as either a cathode or an anode, or both in the ESCs. Zhou et al. first explored the use of an LHP as an electrode material in an electrochemical capacitor by utilising the ionic conduction of methylammonium lead triiodide (MAPbI3).16 Generally, the electrochemical performance of the electrode materials is directly related to the morphology and their synthesis technique. For instance, Bag et al. reported porous electrodes from MAPbBr3 single crystals (SCs) with an areal capacitance of 81.5 mF cm−2 at a fixed current density of 5 mA cm−2 as compared to 39.8 μF cm−2 for MAPbBr3 thin-films.9 On the other hand, the spark plasma sintering chamber processed MAPbI3 powder-based solid-state pellet SUC devices show ultrahigh energy density (34.2 W h kg−1 at 100 Hz). Therefore, the synthesis protocol of LHPs strongly influenced the performance of LHP-based ESCs. Recently, photorechargeable SUCs have been demonstrated by using MAPbBr3 SC-based porous electrodes that can simultaneously harvest solar energy and store it.17
In this work, we reported for the first time the fabrication process of electrodes for supercapacitors from the mechanochemically synthesized LHPs. First, we compared the structural and morphological properties of MAPbBr3 synthesized by high-energy ball milling of perovskite precursors (denoted as MAPbBr3 MSP) and grinding MAPbBr3 single crystals (denoted as MAPbBr3 SCP). The XRD structural analysis of MAPbBr3 MSP shows the presence of higher microstrains compared to the MAPbBr3 SC. These microstrains and applied synthesis protocol for electrode formation highly influence the ion migration in these materials. We have utilized both materials as potential active electrodes. The electrochemical capacitor measurements show a maximum specific capacitance of ∼159 mF cm−2/98.36 F g−1 at a scan rate of 5 mV s−1 for the MAPbBr3 MSP-based electrode, which is much higher than that of all reported MAPbBr3-based ESCs, and surpass all existing MAPbBr3-based electrode's capacitance with the same electrolyte and device structure.9,18 Next, we investigated the effect of structural evolution on the total capacitance of LHP-based supercapacitors by testing 3D MAPbBr3, 2D BA2PbBr4 and quasi-2D BA2MAPb2Br7. It is found that the 2D MA2PbBr4 MSP-based electrode shows maximum areal capacitance (∼209 mF cm−2) and specific capacitance (∼138.35 F g−1) at a scan rate of 5 mV s−1, besides having relatively slower and lower ion kinetics compared to the other samples. This is due to the smallest particle size of the BA2PbBr4 MSP as compared to the other MSPs, which improves the interaction between electrolyte ions and increases the effective surface area in the perovskite electrode interface. Stability analysis of these devices was also carried out over 2000 cycles of charging and discharging. Among the studied materials, the 2D BA2PbBr4 MSP based supercapacitor retains over 97% capacitance and shows maximum stability due to the slowest ion kinetics.
2. Experimental section
2.1. Synthesis of single crystalline and mechanosynthesized LHP powders (MSPs)
MAPbBr3 single crystals (SCs) were synthesised using the inverse temperature crystallisation (ITC) method.19,20 1.2 M MAPbBr3 solution was prepared by dissolving equimolar amount of lead(II) bromide (PbBr2) and methylammonium bromide (MABr) in dimethylformamide (DMF) and stirred for 4 h at room temperature. Next, the solution was filtered using a 0.2 μm PTFE filter and kept at 85 °C in a sealed bottle for 3 h, which resulted in the growth of MAPbBr3 SCs. The MAPbBr3 SCs were ground for 40 min in a Retsch MM200 mill at 30 Hz to prepare MAPbBr3 SC based powder (SCP). All LHP MSPs were synthesised in a solid-state high-energy ball mill in a similar way (ground for 40 min at 30 Hz). For MAPbBr3, MABr and PbBr2 and for BA2PbBr4, benzylammonium bromide (BABr) and PbBr2 were taken as starting precursors. Stochiometric proportions of these starting precursors were taken in an airtight vial and mixed in a Retsch MM200 mill at 30 Hz for 40 min. For the mechanosynthesis of BA2MAPb2Br7, the as-prepared MAPbBr3 and BA2PbBr4 were mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and ground using a similar procedure.
1 molar ratio and ground using a similar procedure.
2.2. Material characterization
All obtained halide perovskite powders were characterized using an Empyrean X-ray powder diffractometer (PANalytical) with Cu Kα radiation (λ = 1.5406 Å) over a 2θ range of 10° to 50° at room temperature. The operating current and voltage were kept at 40 kV and 40 mA, respectively. Morphological analysis of perovskite powders was performed using an FEI Nova NanoSEM 450 scanning electron microscope under high vacuum.2.3. Electrode preparation and electrochemical characterization
The electrochemical experiments were characterized with a BioLogic SP-150 potentiostat by using the standard three-electrode technique in a small-volume analytical cell. For supercapacitor measurements, LHP powders were used as the working electrodes, the Pt metal was used as the counter electrode and the Ag/AgCl was used as the reference electrode. 0.1 M tetrabutylammonium tetrafluoroborate (C16H36BF4N) in dichloromethane solvent is used as the liquid electrolyte.9 LHP based electrodes were prepared by drop casting a LHP slurry on a glassy carbon electrode (diameter = 3 mm) and dried at 70 °C for 12 h in an air oven. The homogeneous slurry of LHPs was prepared by mixing 80 wt% of the synthesized perovskite powder, 10 wt% of polyvinylidene fluoride (PVDF) binder, and 10 wt% of activated carbon in an appropriate amount of NMP solvent for 24 h.3. Results and discussion
A schematic illustration of the fabrication method of electrodes is shown in Fig. 1. This method involves the use of LHPs obtained from the crystallization process and ball milling of solid starting reagents. Previously, we have shown that the mechanochemically synthesized perovskite powders can be effectively used as an absorber layer in solar cells.21–24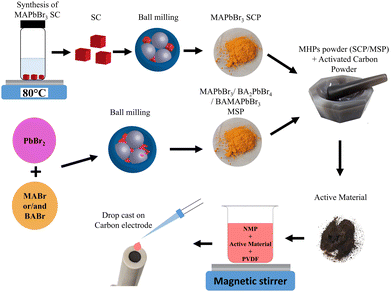 | ||
| Fig. 1 A schematic illustration of the electrode fabrication based on single crystalline (SCP) and mechanosynthesized MAPbBr3 powders (MSP) for supercapacitor application. | ||
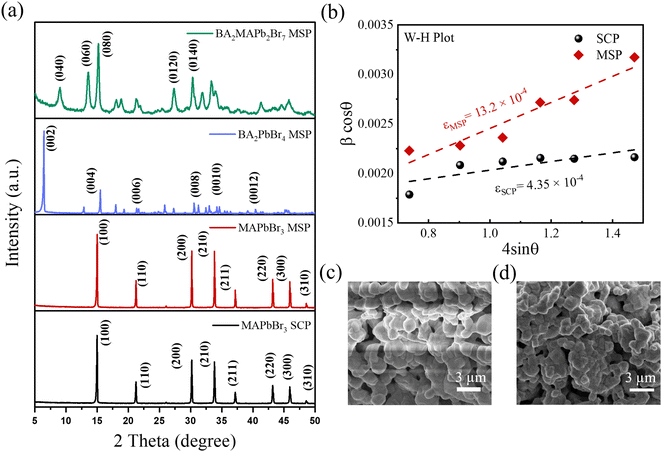
Fig. 2a shows pXRD patterns of all the synthesised LHPs by ball milling. The pXRD patterns of the MAPbBr3 SCP and MAPbBr3 MSP show similar diffraction peaks at 2θ = 14.99, 21.21, 30.18, 33.85, and 45.94° assigned to (100), (110), (200), (210) and (300) planes, which confirmed the formation of the pure cubic MAPbBr3 phase.25 For 2D BA2PbBr4, the presence of peaks at 6.44, 12.91, 19.36, 25.86, 32.50, and 39.18° assigned to (002), (004), (006), (008), (0010), and (0012) planes confirmed the formation of the pure 2D orthorhombic crystal phase.26 The XRD pattern of the quasi-2D BA2MAPb2Br7 (n = 2) powder shows distinct peaks at a regular interval with a periodicity and preferential orientation along the (0k0) plane confirmed the formation of the pure quasi-2D Ruddlesden–Popper Perovskite (RPP) phase.27,28 As was previously reported,9,29 the lattice strain and crystallite size can affect the ion migration in LHP materials. Therefore, we have calculated the microstrain induced on the identical crystallography planes for MAPbBr3 SCP and MSP using the Williamson–Hall (W–H) equation:
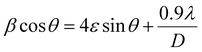 | (1) |
The electrochemical performance of the fabricated bromide-based materials (MAPbBr3 SCP, MAPbBr3 MSP, BA2PbBr4 MSP, and BA2MAPb2Br7 MSP) was investigated by measuring their specific capacitance. Cyclic voltammetry (CV), galvanometric charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) were performed using three electrode systems in 0.1 M C16H36BF4N electrolyte solution. The specific capacitance of the prepared material is evaluated using the following equations:
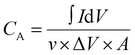 | (2) |
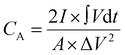 | (3) |
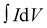 is the integrated area under the CV curve, A is defined as sample deposited area (cm2), I is the current density, v is the scan rate, ΔV is the potential window (V) and dt is defined as change in discharge time.
is the integrated area under the CV curve, A is defined as sample deposited area (cm2), I is the current density, v is the scan rate, ΔV is the potential window (V) and dt is defined as change in discharge time.
The corresponding coulombic efficiency (η, %) was calculated using charging (tc) and discharging (td) times of the GCD using the following relationship:
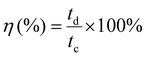 | (4) |
Moreover, areal energy density (EA, W h cm−2) was evaluated using the equation:
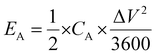 | (5) |
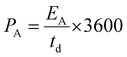 | (6) |
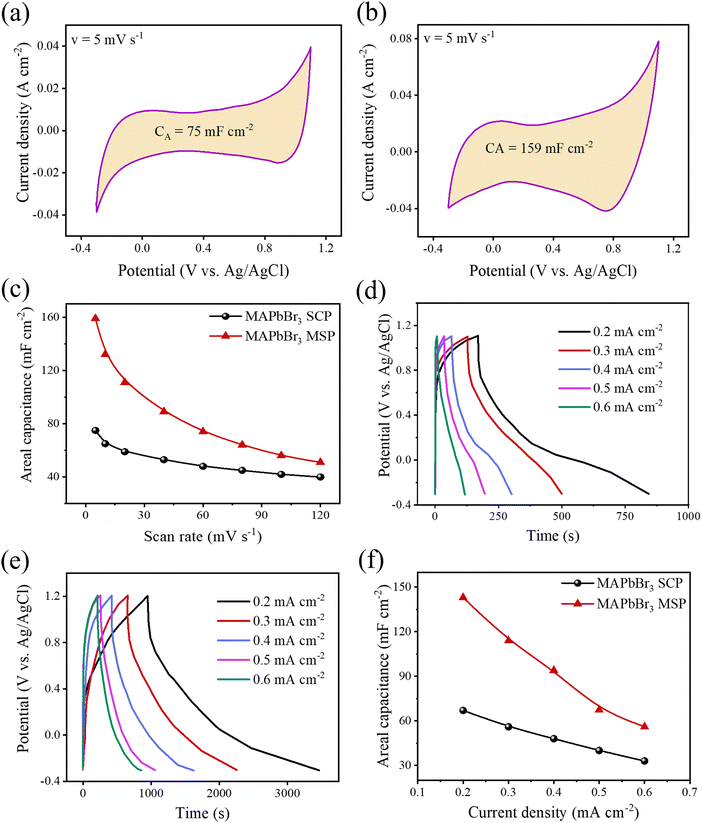
The CV measurements of all the electrodes were conducted at various scan rates ranging from 5 to 120 mV s−1 (Fig. S2, ESI†). All the CV spectra exhibit a high degree of symmetry between the charging and discharging sweep without any noticeable redox peak at each scan rate. Therefore, all the electrochemical cells behave as electric double-layer capacitance, and symmetry between charging and discharging sweep suggests fast and efficient electrolytic ion transport into the active pores of all the LHP based electrodes.8,9,30 The quasi-rectangular shaped CVs of all the ESCs indicate that there is charge contribution from the faradaic mechanism, which is attributed to the diffusion-limited intercalation/de-intercalation process of ions of the liquid electrolyte.31Fig. 3a and b show the CV spectra of MAPbBr3 SCP and MAPbBr3 MSP at a scan rate of 5 mV s−1. The MAPbBr3 MSP-based electrode offers a broader integrated CV area than that of the MAPbBr3 SCP electrode, which can lead to a better storage capacity. The maximum areal/specific capacitance values for MAPbBr3 SCP is 75 mF cm−2/50.22 F g−1 at a scan rate of 5 mV s−1, which are in the same order as the previously reported work (Table S1, ESI†).9 In turn, the maximum areal/specific capacitance values for the MAPbBr3 MSP based electrode is 159 mF cm−2/98.36 F g−1 at a scan rate of 5 mV s−1, which is almost two times higher than that of the all reported MAPbBr3 based ESCs. The areal capacitance of the electrodes at different scan rates has been evaluated from the CV curves and is plotted as shown in Fig. 3c. The plot shows that the areal capacitance gradually decreases with an increase in the scan rate from 5 to 120 mV s−1. The decrease in capacitance values of respective electrodes is due to ineffective interaction at the interface between the electrolyte ions and perovskite electrode. At a fast scan rate, electrolyte ions do not have sufficient time to diffuse inside the electrode, and only a few ions can access the electrode surface leading to low values in capacitance. The charge storage mechanism of the electrode was further investigated using the Dunn power law: ip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aυb. Here, ip is the cathodic peak current density at a particular potential (vs. Ag/AgCl), υ is the scan rate, and the b value signifies the exponent adjustment parameter. If the b value is 1 then it signifies contributions from fast near-surface activities like surface redox reactions and charging/discharging of electric double layer capacitance at the electrode–electrolyte interface and/or faradaic charge transfer at the surface-bound atoms (pseudocapacitance), while the b value of 0.5 represents slow diffusion-controlled faradaic processes (intercalated pseudocapacitance), which involves bulk properties also like a battery (Fig. S3, ESI†).8 The values of b for MAPbBr3 SCP vary between ∼0.98 and 0.77 in the applied potential range of 0.1 to 0.9 V, indicative of a capacitive dominant charge storage mechanism, whereas above 0.9 V and up to 1.1 V, the diffusion contribution surpasses the capacitive contribution part. However, for MAPbBr3 MSP, the b value mostly lies close to ∼0.75 in the applied potential range of 0.2 to 0.8 V, which signifies the similar contribution of diffusion and capacitive behaviour of charge storage mechanism. Elsewhere, in the potential window of 0.1–1.1 V, the lower b value (<0.7 V) is related to the diffusion mechanism in the corresponding charge storage process. Both MAPbBr3 SCP and MAPbBr3 MSP show transitional behaviour of energy storage (as the b value varies in between 0.5 and 1), where the energy storage process of MAPbBr3 SCP is mostly representative of a surface dominated capacitive process but equal contribution from diffusion-controlled intercalation and surface-capacitive processes is observed for MAPbBr3 MSP. Around a low applied potential range of 0.2–0.3 V, MAPbBr3 SCP acts as a pseudocapacitor and at high potential (∼1.1 V) MAPbBr3 MSP acts like a battery type material.32Fig. 3d and e show the GCD graphs of MAPbBr3 SCP and MSP-based electrodes at various scan rates ranging from 0.2 to 0.6 mA cm−2, respectively. The areal/specific capacitances are 67 mF cm−2/44.86 F g−1 for MAPbBr3 SCP and 143 mF cm−2/86.66 F g−1 for MAPbBr3 MSP at 0.2 mA cm−2. The capacitance values extracted from the GCD are consistent with the CV findings, which reveals the excellent storage capacity of the respective electrode and validate our results. The variation of areal and specific capacitances with different current densities is shown in Fig. 3f. The areal and specific capacitances decrease with an increase in the current density due to the change in the internal resistance of both electrodes. Long-term charging discharging cycling stability is one of the key parameters for energy storage applications. The long-term cycling stability of both electrodes was evaluated by repeating the GCD measurement for 2000 cycles over a current density of 0.6 A cm−2 (Fig. S4, ESI†) within the potential window of 1.5 V. The stability performance of the MAPbBr3 SCP electrode is found to be ∼95% capacitance retention, whereas the capacitance retention is around ∼93% of its initial capacitance value for the MAPbBr3 MSP electrode. The MAPbBr3 SCP based electrode is more stable compared to the MAPbBr3 MSP based electrode, which can be attributed to the ion migration in metal halide perovskite materials.8,33 The lattice defects and crystallite size affect the ion migration in metal halide perovskite materials. From the W–H plot (Fig. 1b), we clearly see that MAPbBr3 SCP and MAPbBr3 MSP have different crystallite sizes and microstrain, which can influence the ion migration. Corresponding changes in EA with PA for MAPbBr3 SCP and MAPbBr3 MSP are shown in Fig. S5 (ESI†), where the decrement of EA (from ∼0.015 to ∼0.009 mW h cm−2 for SCP and from ∼0.05 to ∼0.035 mW h cm−2 for MSP) occurs with increment in PA (from ∼0.077 to ∼0.274 mW cm−2 for SCP and from ∼0.074 to 0.196 mW cm−2 for MSP), which is in agreement with previous reports.34,35 At a particular PA value of ∼0.17 mW cm−2, the EA value is higher (∼0.038 mW h cm−2) for MAPbBr3 MSP than the corresponding value of ∼0.011 mW h cm−2 for MAPbBr3 SCP, which signifies better electrochemical performance for MAPbBr3 MSP.36 The air stability of the MAPbBr3 MSP based electrode was investigated over time by monitoring changes in the XRD patterns of the thick film on a glass slide. Fig. S6 (ESI†) shows the XRD patterns of the fresh and aged (7 days and 14 days) MAPbBr3 MSP based electrodes stored under ambient conditions. The negligible intensity drop and broadening of the full width at half maxima (FWHM) in the aged MAPbBr3 MSP based electrodes indicate high air stability of these electrodes.
aυb. Here, ip is the cathodic peak current density at a particular potential (vs. Ag/AgCl), υ is the scan rate, and the b value signifies the exponent adjustment parameter. If the b value is 1 then it signifies contributions from fast near-surface activities like surface redox reactions and charging/discharging of electric double layer capacitance at the electrode–electrolyte interface and/or faradaic charge transfer at the surface-bound atoms (pseudocapacitance), while the b value of 0.5 represents slow diffusion-controlled faradaic processes (intercalated pseudocapacitance), which involves bulk properties also like a battery (Fig. S3, ESI†).8 The values of b for MAPbBr3 SCP vary between ∼0.98 and 0.77 in the applied potential range of 0.1 to 0.9 V, indicative of a capacitive dominant charge storage mechanism, whereas above 0.9 V and up to 1.1 V, the diffusion contribution surpasses the capacitive contribution part. However, for MAPbBr3 MSP, the b value mostly lies close to ∼0.75 in the applied potential range of 0.2 to 0.8 V, which signifies the similar contribution of diffusion and capacitive behaviour of charge storage mechanism. Elsewhere, in the potential window of 0.1–1.1 V, the lower b value (<0.7 V) is related to the diffusion mechanism in the corresponding charge storage process. Both MAPbBr3 SCP and MAPbBr3 MSP show transitional behaviour of energy storage (as the b value varies in between 0.5 and 1), where the energy storage process of MAPbBr3 SCP is mostly representative of a surface dominated capacitive process but equal contribution from diffusion-controlled intercalation and surface-capacitive processes is observed for MAPbBr3 MSP. Around a low applied potential range of 0.2–0.3 V, MAPbBr3 SCP acts as a pseudocapacitor and at high potential (∼1.1 V) MAPbBr3 MSP acts like a battery type material.32Fig. 3d and e show the GCD graphs of MAPbBr3 SCP and MSP-based electrodes at various scan rates ranging from 0.2 to 0.6 mA cm−2, respectively. The areal/specific capacitances are 67 mF cm−2/44.86 F g−1 for MAPbBr3 SCP and 143 mF cm−2/86.66 F g−1 for MAPbBr3 MSP at 0.2 mA cm−2. The capacitance values extracted from the GCD are consistent with the CV findings, which reveals the excellent storage capacity of the respective electrode and validate our results. The variation of areal and specific capacitances with different current densities is shown in Fig. 3f. The areal and specific capacitances decrease with an increase in the current density due to the change in the internal resistance of both electrodes. Long-term charging discharging cycling stability is one of the key parameters for energy storage applications. The long-term cycling stability of both electrodes was evaluated by repeating the GCD measurement for 2000 cycles over a current density of 0.6 A cm−2 (Fig. S4, ESI†) within the potential window of 1.5 V. The stability performance of the MAPbBr3 SCP electrode is found to be ∼95% capacitance retention, whereas the capacitance retention is around ∼93% of its initial capacitance value for the MAPbBr3 MSP electrode. The MAPbBr3 SCP based electrode is more stable compared to the MAPbBr3 MSP based electrode, which can be attributed to the ion migration in metal halide perovskite materials.8,33 The lattice defects and crystallite size affect the ion migration in metal halide perovskite materials. From the W–H plot (Fig. 1b), we clearly see that MAPbBr3 SCP and MAPbBr3 MSP have different crystallite sizes and microstrain, which can influence the ion migration. Corresponding changes in EA with PA for MAPbBr3 SCP and MAPbBr3 MSP are shown in Fig. S5 (ESI†), where the decrement of EA (from ∼0.015 to ∼0.009 mW h cm−2 for SCP and from ∼0.05 to ∼0.035 mW h cm−2 for MSP) occurs with increment in PA (from ∼0.077 to ∼0.274 mW cm−2 for SCP and from ∼0.074 to 0.196 mW cm−2 for MSP), which is in agreement with previous reports.34,35 At a particular PA value of ∼0.17 mW cm−2, the EA value is higher (∼0.038 mW h cm−2) for MAPbBr3 MSP than the corresponding value of ∼0.011 mW h cm−2 for MAPbBr3 SCP, which signifies better electrochemical performance for MAPbBr3 MSP.36 The air stability of the MAPbBr3 MSP based electrode was investigated over time by monitoring changes in the XRD patterns of the thick film on a glass slide. Fig. S6 (ESI†) shows the XRD patterns of the fresh and aged (7 days and 14 days) MAPbBr3 MSP based electrodes stored under ambient conditions. The negligible intensity drop and broadening of the full width at half maxima (FWHM) in the aged MAPbBr3 MSP based electrodes indicate high air stability of these electrodes.
Electrochemical impedance spectroscopy (EIS) was performed under 50 mV amplitude of AC voltage. A Bode modulus plot is shown in Fig. 4a where the total impedance variation with respect to frequency has been shown. At a low frequency of 0.1 Hz, the impedance reduces from 0.9 kΩ for MAPbBr3 SCP to 0.66 kΩ for MAPbBr3 MSP, which again confirms the reduction in the charge transfer resistances offered by the electrode–electrolyte system in MSP. The significant improvement in charge storage capacity for MAPbBr3 MSP is ascribed to its higher ion-migration. Enhanced charge storage performance is observed in MAPbBr3 MSP, which is evident from the frequency dependent imaginary and real capacitance plots shown in Fig. 4b and c, respectively. Frequency dependent capacitance C(f) is classified into real (CReal) and imaginary (CImaginary) counterparts revealed by the following equations:
| C(f) = CReal − jCImaginary | (7) |
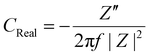 | (8) |
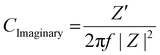 | (9) |
where kB is the Boltzman constant, T is the absolute temperature and σ0 is a constant. From the Arrhenius plots shown in Fig. S7 (ESI†) the activation energies (Ea) of the ions are calculated to be 0.23 ± 0.07 eV and 0.21± 0.07 eV for the MAPbBr3 SCP and MAPbBr3 MSP pellets, respectively. The lower Ea of MAPbBr3 MSP signifies higher ion migration in comparison with the MAPbBr3 SCP. In MAPbBr3 MSP, the higher ion migration can accelerate the decomposition of the MSP active electrode and generate nonradiative recombination defect sites in comparison with the MAPbBr3 SCP.3,8,11 As a result, the SCP electrode shows longer stability compared to the MAPbBr3 MSP electrode. On the other hand, higher ion migration of MAPbBr3 MSP signifies better electrochemical performance of the MAPbBr3 MSP based electrode.
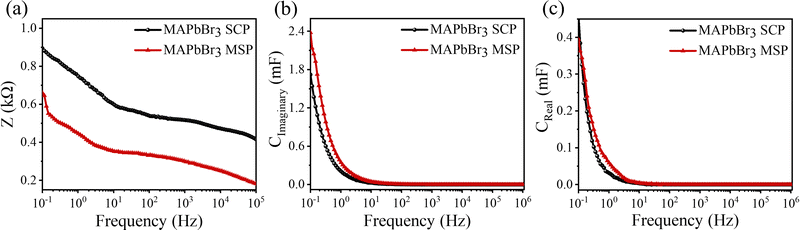 | ||
| Fig. 4 (a) Total impedance, (b) imaginary part and (c) real part of capacitance are plotted with applied frequency for MAPbBr3 SCP and MAPbBr3 MSP electrodes. | ||
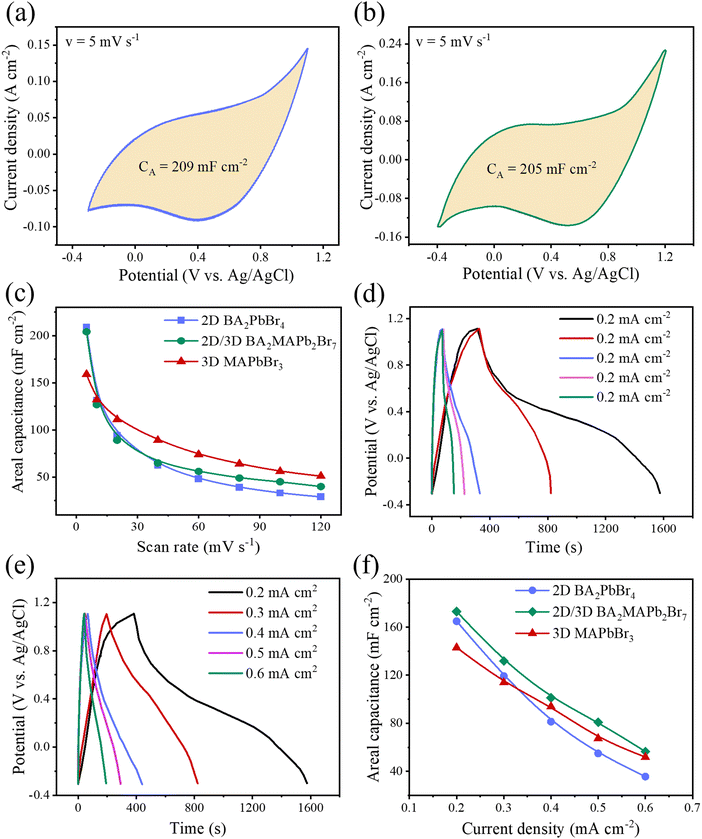
Next, to understand in-depth the effect of structural evolution and layered structure on the total capacitance of LHP-based supercapacitors, three different LHPs such as 3D MAPbBr3, 2D BA2PbBr4 and quasi-2D BA2MAPb2Br7 were investigated. Fig. S2c and d (ESI†) show the CV spectra of BA2PbBr4 and BA2MAPb2Br7 MSP-based electrodes at different scan rates. The maximum areal/specific capacitance values for BA2PbBr4 and BA2MAPb2Br7 MSP based electrodes are 209 mF cm−2/148.25 F g−1, and 205 mF cm−2/138.35 F g−1 at a scan rate of 5 mV s−1, which are much higher than those of all reported similar LHP based ESCs (Fig. 5a and b). The 2D BA2PbBr4 MSP-based electrode shows maximum areal/specific capacitance values as compared to all other electrodes at a low scan rate (Fig. 5c). This can be attributed to the possible effect of highest surface area (smallest particle size) as compared to the other MSPs, which improves the interaction between electrolyte ions and the perovskite electrode interface. From Fig. 5c, we clearly show that the areal capacitance of 2D BA2PbBr4 decreases gradually with an increase in the scan rate compared to the other MSP-based electrode. The faster reduction in the capacitance value of the BA2PbBr4 electrode is due to the slow ion migration and ineffective interaction between electrolyte ions and the perovskite electrode interface at a high scan rate.8 On the other hand, the quasi-2D MSP-based electrode shows higher capacitance at a higher scan rate as compared to the other MSPs. These results can be correlated to the faster interaction of the quasi-2D MSP-based electrode due to its square bar type morphological structure (Fig. S1b, ESI†). Fig. 5d and e show the GCD graphs of BA2PbBr4 and BA2MAPb2Br7 MSP-based electrodes at different current densities of 0.2–0.6 mA cm−2. The calculated areal/specific capacitances are 181 mF cm−2/118.75 F g−1 for BA2PbBr4 MSP and 171 mF cm−2/107.5 F g−1 for BA2MAPb2Br7 MSP at 0.2 mA cm−2, which are almost comparable. Furthermore, the variation of areal capacitance values of 2D, 3D and quasi-2D MSP electrodes with different current densities (varies from 0.2–0.6 mA cm−2) is shown in Fig. 5f. All MSP electrodes show a similar trend of decreasing areal capacitance values with an increase in current density. However, the decreasing areal capacitance value with the scan rate is faster for the 2D MSP electrode due to slower charge transfer and/or ion migration with ineffective interaction at the electrolyte–electrode interface. The long-term cycling stability of BA2PbBr4 and BA2MAPb2Br7 MSP based electrodes is shown in Fig. S8 (ESI†). The stability performance of the BA2PbBr4 SCP electrode is found to be ∼98% capacitance retention, whereas the capacitance retention is around ∼96% of its initial capacitance value for the BA2MAPb2Br7 MSP electrode. However, quasi-2D MSP shows excellent capacitance values with acceptable stability among LHP MSP based electrodes, indicating the most promising alternatives for LHP based energy storage devices.
4. Conclusion
In conclusion, we have fabricated supercapacitors from powdered 2D and 3D LHPs. First, we demonstrated that the electrochemical properties of the fabricated working electrode from MAPbBr3 MSP lead to the maximum specific capacitance of ∼ 159 mF cm−2/98.36 F g−1 at a scan rate of 5 mV s−1, which is much higher than that of the MAPbBr3 SCP and MAPbBr3 based electrodes. This enhancement was attributed to the induced macrostrain, higher ionic migration and lower partial size of the MAPbBr3 MSP electrode. The charge transfer resistance of the MAPbBr3 MSP electrode drops to 0.66 kΩ from 0.9 kΩ for the MAPbBr3 MSP electrode due to the increased ionic conductivity and specific surface area in MAPbBr3 MSP. Next, three different mechanochemically synthesized bromide-based LHP (3D, 2D, and quasi-2D) based electrodes have been studied. It was found that the 2D BA2PbBr4 MSP based electrode shows the best performance at a low scan rate due to having the highest surface area among the electrodes, which helps to improve the interaction between electrolyte ions and perovskite electrode interface. In addition, the 2D BA2PbBr4 MSP based electrode has retained ∼98% of its initial specific capacitance value even after 2000 GCD cycles and shows superior cyclic stability to other electrodes due to lower level of ion migration and better stability of the 2D perovskite structure. Therefore, low dimensional LHPs could be potential alternatives for developing thin-film perovskite-based supercapacitors with improved performance and stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. P. acknowledges the National Centre for Research and Development (V4-JAPAN/2/14/PeDET/2022) for financial support.References
- Poonam, K. Sharma, A. Arora and S. K. Tripathi, J. Energy Storage, 2019, 21, 801–825 CrossRef
.
- P. Naskar, A. Maiti, P. Chakraborty, D. Kundu, B. Biswas and A. Banerjee, J. Mater. Chem. A, 2021, 9, 1970–2017 RSC
.
- A. Mahapatra, D. Prochowicz, J. Kruszyńska, S. Satapathi, S. Akin, H. Kumari, P. Kumar, Z. Fazel, M. M. Tavakoli and P. Yadav, J. Mater. Chem. C, 2021, 9, 15189–15200 RSC
.
- A. Mahapatra, D. Prochowicz, M. M. Tavakoli, S. Trivedi, P. Kumar and P. Yadav, J. Mater. Chem. A, 2020, 8, 27–54 RSC
.
- G. Pacchioni, Nat. Rev. Mater., 2021, 6, 108 CrossRef CAS
.
- H. Wei and J. Huang, Nat. Commun., 2019, 10, 1066 CrossRef PubMed
.
- A. Mahapatra, V. Anilkumar, R. D. Chavan, P. Yadav and D. Prochowicz, ACS Photonics, 2023, 10, 1424–1433 CrossRef CAS
.
- R. Kumar and M. Bag, J. Phys. Chem. C, 2021, 125, 16946–16954 CrossRef CAS
.
- R. Kumar, P. S. Shukla, G. D. Varma and M. Bag, Electrochim. Acta, 2021, 398, 139344 CrossRef CAS
.
- C. H. Ng, H. N. Lim, S. Hayase, Z. Zainal, S. Shafie, H. W. Lee and N. M. Huang, ACS Appl. Energy Mater., 2018, 1, 692–699 CrossRef CAS
.
- L. Zhang, J. Miao, J. Li, Q. Li, L. Zhang, J. Miao, J. Li and Q. Li, Adv. Funct. Mater., 2020, 30, 2003653 CrossRef CAS
.
- T. Y. Yang, G. Gregori, N. Pellet, M. Grätzel and J. Maier, Angew. Chem., Int. Ed., 2015, 54, 7905–7910 CrossRef CAS PubMed
.
- W. Zhang, G. E. Eperon and H. J. Snaith, Nat. Energy, 2016, 1, 16048 CrossRef CAS
.
- A. Halder, M. Ghosh, A. M. Khayum, S. Bera, M. Addicoat, H. S. Sasmal, S. Karak, S. Kurungot and R. Banerjee, J. Am. Chem. Soc., 2018, 140, 10941–10945 CrossRef CAS PubMed
.
- R. Kumar and M. Bag, Energy Technol., 2022, 10, 2100889 CrossRef CAS
.
- S. Zhou, L. Li, H. Yu, J. Chen, C.-P. Wong, N. Zhao, S. Zhou, L. Li, H. Yu, J. Chen, C. Wong and N. Zhao, Adv. Electron. Mater., 2016, 2, 1600114 CrossRef
.
- R. Kumar, A. Kumar, P. S. Shukla, G. Das Varma, D. Venkataraman and M. Bag, ACS Appl. Mater. Interfaces, 2022, 14, 35592–35599 CrossRef CAS PubMed
.
- S. Narayanan, N. Parikh, M. M. Tavakoli, M. Pandey, M. Kumar, A. Kalam, S. Trivedi, D. Prochowicz and P. Yadav, Eur. J. Inorg. Chem., 2021, 1201–1212 CrossRef CAS
.
- W.-G. G. Li, H.-S. S. Rao, B.-X. X. Chen, X.-D. D. Wang and D.-B. Bin Kuang, J. Mater. Chem. A, 2017, 5, 19431–19438 RSC
.
- A. Mahapatra, V. Anilkumar, J. Nawrocki, S. V. Pandey, R. D. Chavan, P. Yadav and D. Prochowicz, Adv. Electron. Mater., 2023, 9, 2300226 CrossRef CAS
.
- D. Prochowicz, M. Franckevičius, A. M. Cieślak, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, J. Mater. Chem. A, 2015, 3, 20772–20777 RSC
.
- D. Prochowicz, P. Yadav, M. Saliba, M. Saski, S. M. Zakeeruddin, J. Lewiński and M. Grätzel, ACS Appl. Mater. Interfaces, 2017, 9, 28418–28425 CrossRef CAS PubMed
.
- D. Prochowicz, P. Yadav, M. Saliba, D. J. Kubicki, M. M. Tavakoli, S. M. Zakeeruddin, J. Lewiński, L. Emsley and M. Grätzel, Nano Energy, 2018, 49, 523–528 CrossRef CAS
.
- D. Prochowicz, M. Saski, P. Yadav, M. Grätzel and J. Lewiński, Acc. Chem. Res., 2019, 3233–3243 CrossRef CAS PubMed
.
- A. Mahapatra, N. Parikh, H. Kumari, M. K. Pandey, M. Kumar, D. Prochowicz, A. Kalam, M. M. Tavakoli and P. Yadav, J. Appl. Phys., 2020, 127, 185501 CrossRef CAS
.
- Y. Li, L. Chen, B. Liu, P. Jin, R. Gao, L. Zhou, P. Wan, Q. Xu and X. Ouyang, J. Mater. Chem. C, 2021, 9, 17124–17128 RSC
.
- Y. H. Chang, J. C. Lin, Y. C. Chen, T. R. Kuo and D. Y. Wang, Nanoscale Res. Lett., 2018, 13, 1–7 CrossRef CAS PubMed
.
- D. Ghosh, K. Marjit, G. Ghosh, S. Ghosh and A. Patra, J. Phys. Chem. C, 2022, 34, 14590–14597 CrossRef
.
- P. Muhammed Shafi and A. Chandra Bose, AIP Adv., 2015, 5, 57137 CrossRef
.
- K. Jayaramulu, M. Horn, A. Schneemann, H. Saini, A. Bakandritsos, V. Ranc, M. Petr, V. Stavila, C. Narayana, B. Scheibe, Š. Kment, M. Otyepka, N. Motta, D. Dubal, R. Zbořil and R. A. Fischer, Adv. Mater., 2021, 33, 2004560 CrossRef CAS PubMed
.
- K. Vijaya Sankar, S. Surendran, K. Pandi, A. M. Allin, V. D. Nithya, Y. S. Lee and R. Kalai Selvan, RSC Adv., 2015, 5, 27649–27656 RSC
.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. X. Shen, Adv. Sci., 2018, 5, 1700322 CrossRef PubMed
.
- A. Kalam, R. Runjhun, A. Mahapatra, M. M. Tavakoli, S. Trivedi, H. Tavakoli Dastjerdi, P. Kumar, J. Lewiński, M. Pandey, D. Prochowicz and P. Yadav, J. Phys. Chem. C, 2020, 124, 3496–3502 CrossRef CAS
.
- M. Baptista, G. Gaspar, K. G. U. Wijayantha and K. Lobato, Appl. Surf. Sci. Adv., 2022, 10, 100262 CrossRef
.
- P. S. Chauhan, S. Kumar, A. Mondal, P. Sharma, M. N. Parekh, V. Panwar, A. M. Rao and A. Misra, J. Mater. Chem. A, 2022, 11, 95–107 RSC
.
- M. I. Pratheepa and M. Lawrence, SN Appl. Sci., 2020, 2, 1–12 Search PubMed
.
- M. Arunkumar and A. Paul, ACS Omega, 2017, 2, 8039–8050 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma01144e |
| This journal is © The Royal Society of Chemistry 2024 |