 Open Access Article
Open Access ArticleEffects of the cross-linked structures of polymer gels containing iron porphyrins on photoreduction of carbon dioxide†
Shota
Furusawa
,
Masanori
Nagao
 *,
Hikaru
Matsumoto
*,
Hikaru
Matsumoto
 and
Yoshiko
Miura
and
Yoshiko
Miura
 *
*
Department of Chemical Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: nagaom@chem-eng.kyushu-u.ac.jp
First published on 20th September 2024
Abstract
We prepared polystyrene-based polymer gels containing iron porphyrin and evaluated the effects of the cross-linked structures on the photoreduction of carbon dioxide to carbon monoxide. The amount of generated carbon monoxide was influenced by the diffusion of the substrates into the gel structures.
As industrial development progresses, the concentration of CO2 emissions in the atmosphere continues to rise, thereby contradicting efforts toward carbon neutrality.1 This has led to a growing focus on utilizing CO2 as a raw material for C1 compounds like CH4,2,3 CH3OH,4,5 and HCOOH,6,7 as well as for petrochemicals. Utilizing CO2 in this way could help address the urgent challenges of global warming and fossil fuel depletion simultaneously.8 However, obtaining these raw materials directly from CO2 is challenging, so they are typically used in reactions after being converted to CO through reduction process involving two-electrons.9,10 While electrochemical reduction is effective for CO2 reduction,11–13 photochemical reduction plays a crucial role in advancing toward a carbon-neutral society by harnessing cleaner energy sources like sunlight (visible light).14,15
Metal porphyrins with transition metals (e.g., Fe, Co and Ni) have been used for CO2 photoreduction.15–18 Metal porphyrins can store and supply many electrons to the metal site, making it possible to achieve two-electron reduction of CO2.19 Many studies have been focusing on catalyst design in homogeneous systems. Aukauloo and co-workers designed iron-coordinated tetraphenyl porphyrin (FeTPP) catalysts with urea functional groups as scaffolds for multi-point hydrogen bonding to CO2.20 Furthermore, heterogeneous systems using metal porphyrins as constituent units, such as metal organic frameworks,17,21,22 covalent organic frameworks,23–25 and hydrogen-bonded organic framework26 have been reported. The use of heterogeneous catalysts enables easy recovery of the catalysts from reaction solution, which encourages their practical applications.27
Polymers are used as supports for immobilized catalysts due to their ease of preparation, achieved by polymerizing the catalytic functional group as a monomer.28–32 However, heterogeneous reactions with immobilized catalysts often exhibit lower efficiency compared to homogeneous reactions due to the reduced frequency of collisions between the catalysts and substrates. To address this challenge, our group has previously focused on the polymer gel structures. The polymer gels supporting the catalysts have demonstrated excellent substrate diffusion into the cross-linked polymer structures, leading to high reaction efficiency.32–34 However, to the best of our knowledge, the influence of the polymer structures as heterogeneous catalysts on the CO2 photoreduction remains unclear.
Herein, we synthesized polystyrene-based polymer gels incorporating FeTPP catalyst and evaluated the effects of cross-linked structures on CO2 photoreduction (Fig. 1). To introduce the catalyst porphyrin into the polymer structures, a porphyrin monomer with two vinyl groups was synthesized (TPP-M, Fig. S1†). The synthesis was conducted using a Rothemund synthesis approach with 5-phenyldipyrromethane and benzaldehyde. Subsequently, iron porphyrin monomer (FeTPP-M) was obtained through coordination with iron chloride at a high temperature of 120 °C. Porphyrin derivatives exhibit characteristic absorbance peaks within the visible light range in UV-vis spectrum. Following the coordination reaction, the absorbance peaks at Q-bands shifted, indicating successful iron coordination into the porphyrin rings (Fig. S2†).
 | ||
| Fig. 1 Schematic illustration of the preparation of polymer gels containing FeTPP with different cross-linking ratios. | ||
Polymer gels incorporating FeTPP (PgFeTPP) was obtained by copolymerizing styrene, divinylbenzene (DVB, cross-linker), and FeTPP-M. Three types of PgFeTPP were prepared by varying the amount of DVB, resulting in ratios of DVB to total monomer weight were 10, 50 and 100 wt% (Table 1, PgFeTPP-DVB10, PgFeTPP-DVB50, and PgFeTPP-DVB100, respectively). In addition, a polymer gel without porphyrin (Pg) and that containing TPP-M (PgTPP) were also prepared for comparison (Table 1). After washing the polymer gels, the weighed yields were over 80% for all polymer gels. To confirm the gel structures of the synthesized polymers, swollen bulks of PgFeTPP series in DMF were cut into 10 mm squares (Fig. 2a–c). These PgFeTPP cubes were then immersed sequentially in THF and MeOH to exchange the solvent inside the polymer cubes. After drying MeOH in vacuo, the length of one side of the cubes measured 6, 7, and 9 mm for PgFeTPP-DVB10, -DVB50, and -DVB100, respectively (Fig. 2d–f). To further demonstrate the effect of cross-linking ratios on the gel structures, compression test was conducted using PgFeTPP series swollen with DMF. In Fig. 3, the maximum strain values indicate the rates of height changes of the polymer gels until they were broken by the compression. The maximum strain values decreased with the cross-linking ratios, indicating that the higher cross-linking ratio rendered the polymer gels more rigid. These demonstrated that the prepared PgFeTPP series exhibited swelling with organic solvents, which is a typical feature of polymer gels, and that the higher cross-linking ratios resulted in reduced swelling ratios.
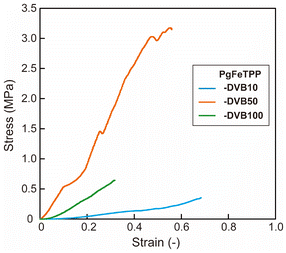 | ||
| Fig. 3 Stress–strain curves for DMF-swollen PgFeTPP series under uniaxial compression. Blue, orange, and green lines represent PgFeTPP with DVB contents of 10, 50, and 100 wt%, respectively. | ||
| Entry | Monomer feed ratio (wt%) | Shrinking ratio (vol%)a | |||
|---|---|---|---|---|---|
| Styrene | DVB | TPP-M | FeTPP-M | ||
| a Shrinking ratio was calculated following formula: shrinking ratio (vol%) = 100 × Volume (dried state)/Volume (swollen state). | |||||
| Pg | 90 | 10 | — | — | — |
| PgTPP | 89 | 10 | 1 | — | — |
| PgFeTPP-DVB10 | 89 | 10 | — | 1 | 22 |
| PgFeTPP-DVB50 | 49 | 50 | — | 1 | 34 |
| PgFeTPP-DVB100 | — | 99 | — | 1 | 73 |
For each characterization, the polymer gels were crushed in a dry state using a mortar. The colours of the polymer powders were white, dark purple, and green for Pg, PgTPP, and PgFeTPP, respectively (Fig. S3†). In FT-IR spectra, the characteristic peaks of polystyrene were shown at 695–750 cm−1 (Fig. S4†). However, there is no significant difference among the polymer gels because the feed ratio of the porphyrin monomers was small (1 wt%). Absorbance spectra of these polymer powders were obtained through diffuse reflectance UV–vis measurements. While the polystyrene-based polymer (Pg) did not exhibit any absorbance peak in the range from 400 to 800 nm, the polymer containing TPP (PgTPP) showed characteristic absorption peaks in the Soret-band (422 nm) and Q-band (519, 551, 599, and 655 nm, Fig. 4a). Furthermore, these peak-top wavelengths were consistent with those of TPP-M (Fig. S5a†), demonstrating the incorporation of TPP units into PgTPP while maintaining the porphyrin ring structure. A distinct peak at 750 nm observed in the PgTPP spectrum was also derived from TPP-M (Fig. 4a and S5a†). In diffuse reflectance UV–vis measurements, peaks in the longer wavelength range appear more pronounced due to light scattering. Although the peak at 750 nm was minimal in TPP monomer, the particle morphology of PgTPP enhanced the light scattering, thereby making the peak at longer wavelength more prominent. The polymer containing FeTPP (PgFeTPP-DVB10) exhibited absorption peaks of the Soret-band (415 nm) and Q-band (572 and 613 nm), which were distinct from those of PgTPP (Fig. 4a). The absorbance spectra of PgFeTPP-DVB10, -DVB50, and -DVB100 showed the same peak-top wavelengths (Fig. 4b). This suggested that porphyrin rings in PgFeTPP had distinct features from those in PgTPP. However, diffuse reflectance UV-vis measurements of FeTPP-M showed peak-top wavelengths in the Soret band (428 nm) and Q band (514, 569, 658 and 693 nm), which did not match the spectrum of PgFeTPP (Fig. S5b†). Considering that the peak-top wavelengths of the PgTPP spectrum were consistent with those of TPP-M (Fig. S5a†), a possible reason for the lack of match in PgFeTPP is the reaction of radical species generated during radical polymerization with the coordinated iron atoms, changing its valence. Next, we evaluated the amount of iron atoms in PgFeTPP polymers through X-ray fluorescence measurement. The powders of PgFeTPP showed weight fraction of iron atoms over 0.05 wt%, which values were more than 10 times larger than those of Pg and PgTPP (Fig. 5). This result supports the presence of iron atoms derived from FeTPP within the PgFeTPP series. The characterization through UV-vis measurement and XRF analysis indicates the successful preparation of three types of polystyrene-based polymer gels with different cross-linking ratios. Importantly, iron porphyrins were incorporated into these polymers even without a perfect match of chemical structures to its monomer (FeTPP-M).
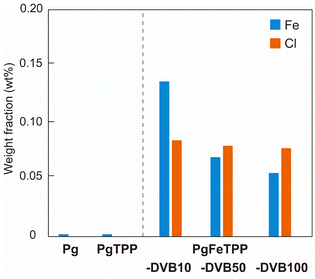 | ||
| Fig. 5 Weight fraction of Fe and Cl in polymer powders measured by X-ray fluorescence analysis. Blue and orange bars indicate Fe and Cl, respectively. | ||
The obtained polymer powders were classified using sieves to minimize the influence of particle size distribution in photoreduction experiments. The particle sizes were categorized as ∼53 μm, 53–100 μm, 100–150 μm and 150–200 μm, respectively. Scanning electron microscope (SEM) images of the particles confirmed the accuracy of the particle size classification by the sieves (Fig. 6).
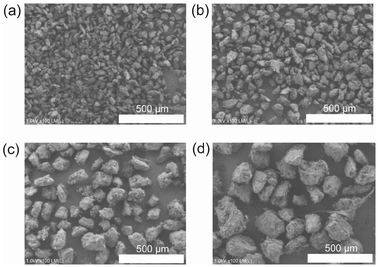 | ||
| Fig. 6 SEM images of crushed PgFeTPP-DVB10 particles classified using sieves. (a) ∼53 μm, (b) 53–100 μm, (c) 100–150 μm, and (d) 150–250 μm. | ||
Photoreduction of CO2 was performed by irradiating a CO2-saturated solution of DMF/PhOH (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PhOH = 9.4 mL
PhOH = 9.4 mL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 mL) containing PgFeTPP (polymer catalyst, 10 mg, 13 μM of FeTPP unit), 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzimidazole (BIH, electron doner; 50 mM) and IrBPY (photosensitizer, 0.1 mM) with an Xe light source (λ > 400 nm). The amount of the porphyrin unit was calculated based on the feed amount of FeTPP-M in the polymerization step. After irradiation for 6 hours, PgFeTPP-DVB10 with a particle size of 53–100 μm produced 4.9 μmol of CO and 0.20 μmol of H2 (entry 4 in Table 2 and Fig. 7). The selectivity for CO production was 96%. The turnover number (TON) for CO was 37. In contrast, PgTPP, which contained no iron atoms, did not exhibit CO generation after 6 hours of light irradiation (entry 2 in Table 2). When the amount of PgFeTPP-DVB10 in the reaction solution was decreased (2 mg) or increased (50 mg), the amount of CO generated correspondingly changed (Fig. 7a). On the other hand, no correlation was observed between the amount of H2 generated and the amount of polymer catalyst (Fig. 7b). These results confirm that the iron porphyrins incorporated into the polymer gels acted as the catalyst for the CO2 reduction. Although the TONCO of PgFeTPP-DVB10 was smaller than that of commercial iron porphyrin molecule (TONCO = 79, entry 1 in Table 2), a decrease in catalytic activity is a general tendency of heterogeneous catalysts. These results indicate that the synthesized polystyrene-based polymer gel incorporating FeTPP units functioned as a heterogeneous catalyst for CO2 photoreduction.
0.6 mL) containing PgFeTPP (polymer catalyst, 10 mg, 13 μM of FeTPP unit), 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzimidazole (BIH, electron doner; 50 mM) and IrBPY (photosensitizer, 0.1 mM) with an Xe light source (λ > 400 nm). The amount of the porphyrin unit was calculated based on the feed amount of FeTPP-M in the polymerization step. After irradiation for 6 hours, PgFeTPP-DVB10 with a particle size of 53–100 μm produced 4.9 μmol of CO and 0.20 μmol of H2 (entry 4 in Table 2 and Fig. 7). The selectivity for CO production was 96%. The turnover number (TON) for CO was 37. In contrast, PgTPP, which contained no iron atoms, did not exhibit CO generation after 6 hours of light irradiation (entry 2 in Table 2). When the amount of PgFeTPP-DVB10 in the reaction solution was decreased (2 mg) or increased (50 mg), the amount of CO generated correspondingly changed (Fig. 7a). On the other hand, no correlation was observed between the amount of H2 generated and the amount of polymer catalyst (Fig. 7b). These results confirm that the iron porphyrins incorporated into the polymer gels acted as the catalyst for the CO2 reduction. Although the TONCO of PgFeTPP-DVB10 was smaller than that of commercial iron porphyrin molecule (TONCO = 79, entry 1 in Table 2), a decrease in catalytic activity is a general tendency of heterogeneous catalysts. These results indicate that the synthesized polystyrene-based polymer gel incorporating FeTPP units functioned as a heterogeneous catalyst for CO2 photoreduction.
| Entry | Catalyst | Particle size (μm) | CO (μmol) | H2 (μmol) | Selectivity (CO%) | TONCO | TOFCO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (h−1) b (h−1) |
|---|---|---|---|---|---|---|---|
| a A reaction solution was prepared by adding DMF (9.4 mL), PhOH (600 μL), IrBPY (0.1 mM), BIH (50 mM), and the catalyst. The concentration of FeTPP unit was 13 μM. The reaction started with irradiation of a white Xenon light (λ > 400 nm). b TOFCO was calculated using TONCO values after 1 and 2 hours of light irradiation (Tables S2 and S3†). c Small molecule of FeTPP was used. | |||||||
| 1c | FeTPP | — | 10.3 | 0.75 | 95 | 79 | 24 |
| 2 | PgTPP | 53–100 | 0 | 0.20 | — | — | — |
| 3 | PgFeTPP-DVB10 | ∼53 | 4.89 ± 0.18 | 0.69 ± 0.12 | 88 | 38 | 8.5 |
| 4 | PgFeTPP-DVB10 | 53–100 | 4.89 ± 0.04 | 0.24 ± 0.07 | 95 | 38 | 7.2 |
| 5 | PgFeTPP-DVB10 | 100–150 | 5.37 ± 0.20 | 0.65 ± 0.17 | 89 | 41 | 8.2 |
| 6 | PgFeTPP-DVB10 | 150–200 | 5.62 ± 0.67 | 0.34 ± 0.09 | 94 | 43 | 9.4 |
| 7 | PgFeTPP-DVB50 | 53–100 | 1.30 ± 0.10 | 0.32 ± 0.15 | 80 | 10 | 1.8 |
| 8 | PgFeTPP-DVB100 | 53–100 | 0.83 ± 0.06 | 0.23 ± 0.07 | 78 | 6.4 | 1.8 |
Next, the effect of the particle sizes of the polymer gels were evaluated. After 6 hours of light irradiation, the CO production remained around 5.0 μmol for all particle sizes (entries 3–6 in Table 2 and Fig. 8a). The TONCO and TOFCO were also consistent across different particle sizes. Although a slight amount of H2 was observed as a by-product (<0.82 μmol), the CO selectivity was consistently over 86% for all particle sizes. This indicates that the particle size, which corresponds to the surface area, did not impact CO2 photoreduction in our system. If the catalytic reaction occurred solely on the particle surface, we would expect higher CO production from smaller particles due to their larger surface area. However, the lack of correlation between CO amount and particle size suggests that the catalytic reaction occurs not only on the particle surfaces but also within the particles.
For the catalytic reaction within the polymer gel particles, the substrate, and additives (CO2, photosensitiser, proton donor, and electron donor) must diffuse into the polymer gel structures. To investigate the effect of cross-linking ratios of the polymer gels, we evaluated the photocatalytic reactivity of PgFeTPP-DVB10, -DVB50, and -DVB100 (particle size: 53–100 μm). After 6 hours of light irradiation, PgFeTPP-DVB10, -DVB50, and -DVB100 produced 4.9, 1.4, and 0.89 μmol of CO, respectively (entry 4, 7, and 8 in Table 2 and Fig. 8b). As the cross-linking ratio of PgFeTPP increased, CO generation tended to decrease. This decrease can be attributed to the suppressed diffusion of CO2 or other additives into the more cross-linked polymer gel structures. These results highlight the unique property of polymer gel structures as scaffolds for heterogeneous photocatalysts, particularly in terms of internal substrate diffusion.
Conclusions
In this study, we prepared polystyrene-based polymer gels incorporating iron porphyrin (FeTPP) units and evaluated their catalytic activity for CO2 photoreduction. The polymer gels were synthesized by adjusting the feed ratio of the cross-linker (divinylbenzene). The successful incorporation of iron porphyrin units into the polymer structures were confirmed through diffuse reflectance UV-vis measurements and XRF analysis. The polymer gels containing FeTPP exhibited photocatalytic activity in converting CO2 to CO under visible light irradiation. Interestingly, the particle sizes of the crushed polymer gels with the same cross-linking ratios did not correlate with the amount of CO produced. However, higher cross-linking ratio in the polymers led to a decreased in the amount of CO produced. These findings suggest that the observed reaction occurred within the polymer gel structures, facilitated by the internal diffusion of CO2 and other additives. This work highlights the potential of polymer gels as effective scaffolds for heterogeneous catalysts in photoreactions and contributes to the development of practical and effective CO2 photoreduction systems using principles of polymer chemistry.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Tobe Maki foundation, the Iwatani Naoji foundation, and JSPS Grants-in-Aid (JP23H02015). We also thank the support of Prof. Shimakoshi for diffuse reflectance UV–vis measurements.References
- A. Huovila, H. Siikavirta, C. A. Rozado, J. Rökman, P. Tuominen, S. Paiho, Å. Hedman and P. Ylén, J. Cleaner Prod., 2022, 341, 130912 CrossRef CAS.
- H. Rao, C.-H. Lim, J. Bonin, G. M. Miyake and M. Robert, J. Am. Chem. Soc., 2018, 140, 17830–17834 CrossRef CAS PubMed.
- Q. Huang, J. Liu, L. Feng, Q. Wang, W. Guan, L.-Z. Dong, L. Zhang, L.-K. Yan, Y.-Q. Lan and H.-C. Zhou, Natl. Sci. Rev., 2020, 7, 53–63 CrossRef CAS PubMed.
- S. Navarro-Jaén, M. Virginie, J. Bonin, M. Robert, R. Wojcieszak and A. Y. Khodakov, Nat. Rev. Chem., 2021, 5, 564–579 CrossRef PubMed.
- C. Shen, X.-Y. Meng, R. Zou, K. Sun, Q. Wu, Y.-X. Pan and C.-J. Liu, Angew. Chem., Int. Ed., 2024, e202402369 CAS.
- T.-C. Zhuo, Y. Song, G.-L. Zhuang, L.-P. Chang, S. Yao, W. Zhang, Y. Wang, P. Wang, W. Lin, T.-B. Lu and Z.-M. Zhang, J. Am. Chem. Soc., 2021, 143, 6114–6122 CrossRef CAS PubMed.
- A. Nakada, K. Koike, T. Nakashima, T. Morimoto and O. Ishitani, Inorg. Chem., 2015, 54, 1800–1807 CrossRef CAS PubMed.
- C. Mesters, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 223–238 CrossRef PubMed.
- Y. Yamazaki, H. Takeda and O. Ishitani, J. Photochem. Photobiol., C, 2015, 25, 106–137 CrossRef CAS.
- R. Bonetto, F. Crisanti and A. Sartorel, ACS Omega, 2020, 5, 21309–21319 CrossRef CAS PubMed.
- R. Francke, B. Schille and M. Roemelt, Chem. Rev., 2018, 118, 4631–4701 CrossRef CAS PubMed.
- C. Costentin, M. Robert and J.-M. Savéant, Chem. Soc. Rev., 2013, 42, 2423–2436 RSC.
- S. Zhang, Q. Fan, R. Xia and T. J. Meyer, Acc. Chem. Res., 2020, 53, 255–264 CrossRef CAS PubMed.
- C. Wang, Z. Sun, Y. Zheng and Y. H. Hu, J. Mater. Chem. A, 2019, 7, 865–887 RSC.
- L. Zou, R. Sa, H. Lv, H. Zhong and R. Wang, ChemSusChem, 2020, 13, 6124–6140 CrossRef CAS PubMed.
- E. Boutin, L. Merakeb, B. Ma, B. Boudy, M. Wang, J. Bonin, E. Anxolabéhère-Mallart and M. Robert, Chem. Soc. Rev., 2020, 49, 5772–5809 RSC.
- V. N. Gopalakrishnan, J. Becerra, E. F. Pena, M. Sakar, F. Béland and T.-O. Do, Green Chem., 2021, 23, 8332–8360 RSC.
- E. Nikoloudakis, I. López-Duarte, G. Charalambidis, K. Ladomenou, M. Ince and A. G. Coutsolelos, Chem. Soc. Rev., 2022, 51, 6965–7045 RSC.
- J. Bonin, M. Chaussemier, M. Robert and M. Routier, ChemCatChem, 2014, 6, 3200–3207 CrossRef CAS.
- E. Pugliese, P. Gotico, I. Wehrung, B. Boitrel, A. Quaranta, M.-H. Ha-Thi, T. Pino, M. Sircoglou, W. Leibl, Z. Halime and A. Aukauloo, Angew. Chem., Int. Ed., 2022, 61, e202117530 CrossRef CAS PubMed.
- J. Jin, New J. Chem., 2020, 44, 15362–15368 RSC.
- Z.-B. Fang, T.-T. Liu, J. Liu, S. Jin, X.-P. Wu, X.-Q. Gong, K. Wang, Q. Yin, T.-F. Liu, R. Cao and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 12515–12523 CrossRef CAS PubMed.
- H. L. Nguyen and A. Alzamly, ACS Catal., 2021, 11, 9809–9824 CrossRef CAS.
- X. Ding, B. Yu, B. Han, H. Wang, T. Zheng, B. Chen, J. Wang, Z. Yu, T. Sun, X. Fu, D. Qi and J. Jiang, ACS Appl. Mater. Interfaces, 2022, 14, 8048–8057 CrossRef CAS PubMed.
- P. L. Cheung, S. K. Lee and C. P. Kubiak, Chem. Mater., 2019, 31, 1908–1919 CrossRef CAS.
- A.-A. Zhang, D. Si, H. Huang, L. Xie, Z.-B. Fang, T.-F. Liu and R. Cao, Angew. Chem., Int. Ed., 2022, 61, e202203955 CrossRef CAS PubMed.
- Z. Dai, Q. Sun, X. Liu, C. Bian, Q. Wu, S. Pan, L. Wang, X. Meng, F. Deng and F.-S. Xiao, J. Catal., 2016, 338, 202–209 CrossRef CAS.
- J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815–838 CrossRef CAS PubMed.
- Q. Sun, Z. Dai, X. Meng and F.-S. Xiao, Chem. Soc. Rev., 2015, 44, 6018–6034 RSC.
- M. Debruyne, V. V. Speybroeck, P. V. D. Voort and C. V. Stevens, Green Chem., 2021, 23, 7361–7434 RSC.
- B. Altava, M. I. Burguete, E. García-Verdugo and S. V. Luis, Chem. Soc. Rev., 2018, 47, 2722–2771 RSC.
- H. Matsumoto, T. Iwai, M. Sawamura and Y. Miura, ChemPlusChem, 2024, e202400039 CrossRef PubMed.
- H. Matsumoto, H. Seto, T. Akiyoshi, M. Shibuya, Y. Hoshino and Y. Miura, ACS Omega, 2017, 2, 8796–8802 CrossRef CAS PubMed.
- H. Matsumoto, Y. Hoshino, T. Iwai, M. Sawamura and Y. Miura, ChemCatChem, 2020, 12, 4034–4037 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, characterization, synthetic procedure of FeTPP monomer, detailed information of the polymerization, and results of CO2 photoreduction. See DOI: https://doi.org/10.1039/d4lp00135d |
| This journal is © The Royal Society of Chemistry 2024 |




